Abstract
Objective:
To assess the association of race with clinical outcomes in HIV-positive women on continuous HAART.
Design:
Prospective study that enrolled women from 1994 to 1995 and 2001 to 2002.
Setting:
Women's Interagency HIV Study, a community-based cohort in five US cities.
Participants:
One thousand, four hundred and seventy-one HIV-positive continuous HAART users.
Main outcome measures:
Times to AIDS and non-AIDS death and incident AIDS-defining illness (ADI) after HAART initiation.
Results:
In adjusted analyses, black vs. white women had higher rates of AIDS death [adjusted hazard ratio (aHR) 2.14, 95% confidence interval (CI) 1.30, 3.50; P = 0.003] and incident ADI (aHR 1.58, 95% CI 1.08, 2.32; P = 0.02), but not non-AIDS death (aHR 0.91, 95% CI 0.59, 1.39; P = 0.65). Cumulative AIDS death incidence at 10 years was 17.3 and 8.3% for black and white women, respectively. Other significant independent pre-HAART predictors of AIDS death included peak viral load (aHR 1.70 per log10, 95% CI 1.34, 2.16; P < 0.001), nadir CD4+ cell count (aHR 0.65 per 100 cells/μl, 95% CI 0.56, 0.76; P < 0.001), depressive symptoms by Center for Epidemiology Studies Depression score at least 16 (aHR 2.10, 95% CI 1.51, 2.92; P < 0.001), hepatitis C virus infection (aHR 1.57, 95% CI 1.02, 2.40; P = 0.04), and HIV acquisition via transfusion (aHR 2.33, 95% CI 1.21, 4.49; P = 0.01). In models with time-updated HAART adherence, association of race with AIDS death remained statistically significant (aHR 3.09, 95% CI 1.38, 6.93; P = 0.006).
Conclusion:
In continuous HAART-using women, black women more rapidly died from AIDS or experienced incident ADI than their white counterparts after adjusting for confounders. Future studies examining behavioral and biologic factors in these women may further the understanding of HAART prognosis.
Keywords: AIDS, HAART, HIV, race, survival, women
Introduction
Globally, women represent more than 50% of persons living with HIV infection (PLWH), and HIV/AIDS is the leading cause of death worldwide for women of reproductive age [1,2]. In the United States, African American women are 19-fold more likely than white women to be diagnosed with HIV [3], and 2007 United States Centers for Disease Control and Prevention data ranked HIV in the top 10 causes of death for African American and Hispanic women aged 20–54 years [4,5]. Declines in AIDS-related mortality have been less steep in black women than in most other groups [6,7]. Many studies of safety and efficacy of antiretroviral therapy (ART) have been conducted in men of European descent; it is unclear if there is a survival difference by race in PLWH treated with HAART. Whereas some studies have found no differences by ancestry in risk of death or AIDS-defining illness (ADI) in HAART users [8–10], others have observed higher rates of all-cause mortality or decreased rates of virologic suppression in people of African descent, but did not assess or find any difference in AIDS-specific mortality or adherence [11–16].
Postulated explanations for higher AIDS mortality rates among blacks, if present, include decreased access to care, lower HAART adherence, and socioeconomic, behavioral, genetic, or other biologic factors [17–20]. In a previous study from the Women's Interagency HIV Study (WIHS), we found a clinically but not statistically significant survival advantage in white compared to black women on continuous HAART: adjusted hazard ratio (aHR) 0.49 (P = 0.19) for death from AIDS [21]. We extended the WIHS study into the era of modern HAART using follow-up and events from 1995 to 2009, hypothesizing that black women would have higher rates of AIDS death and ADI compared to white women.
Methods
Study population
Women's Interagency HIV Study is a multicenter prospective observational cohort study of 2843 HIV-infected and 975 HIV-uninfected women enrolled in 1994–1995 and 2001–2002. Informed consent was obtained from participants, and the Institutional Review Boards of the six collaborating institutions approved the study. Study participants were interviewed and examined semiannually; numerous laboratory tests and studies were performed [22]. This analysis included all WIHS participants who initiated HAART after WIHS enrollment and reported HAART use at 70% or more of subsequent visits. Although individual women were allowed to have up to four consecutive missed visits or visits off HAART (i.e. in cases of otherwise long-term consistent use), less than 5% of the total semiannual person-visits were off HAART. HAART was defined as any three-drug antiretroviral regimen, one of which must have been a protease inhibitor, a non-nucleoside reverse transcriptase inhibitor, one of the nucleoside reverse transcriptase inhibitors abacavir or tenofovir, an integrase inhibitor (raltegravir), or an entry inhibitor (maraviroc or enfuvirtide) [23].
Exposure variables
The primary exposure was self-categorized race: white (Hispanic and non-Hispanic), black (Hispanic and non-Hispanic), and other, who were predominantly women who self-identified as Hispanic, but not white or black. Women self-categorized their race and ethnicity by responding to interviewer-administered questions: one querying whether they were Hispanic or not, and another asking if they were black/African American, white/Caucasian, American Indian/Alaskan Native, Asian, Native Hawaiian/Other Pacific Islander, or other. Other variables examined included date of HAART initiation defined as the mid-date between the visit at which consecutive HAART use was first reported and the previous visit; age at HAART initiation; time of enrollment in WIHS (1994–1995 or 2001–2002); risk category for HIV acquisition [injection drug use (IDU), heterosexual exposure, transfusion]; and pre-HAART initiation variables of illicit drug use, smoking tobacco, both in the past 6 months, annual income $12 000 or less, having graduated from high school, self-reported prior ADI; hepatitis C virus (HCV) infection defined as positive antibody with viremia; nadir CD4+ cell count and peak log10 HIV-1 RNA, which reflected the lowest CD4+ cell count; and highest HIV viral load measured at any pre-HAART visit. We also included pre-HAART clinical depressive symptom burden defined as Center for Epidemiology Studies Depression score (CES-D) at least 16. As others have done [24–27], we divided the 20 CES-D items into depression component subscores: somatic, positive, negative, and interpersonal. Each of these subscores was dichotomized about the closest value to the sample median to obtain somatic, negative, positive, and interpersonal depression subscales of at least 7, 5, 4, and 1, respectively. We adjusted for WIHS site in all analyses. Starting from October 2002, WIHS included measures for assessment of self-reported adherence (defined as ≥95% use of all prescribed antiretrovirals), which were included in a separate analysis.
Outcome variables
Primary outcomes were time from date of first visit after HAART initiation to AIDS death, non-AIDS death, and incident ADI. Ascertainment and classification of deaths in WIHS have been described previously [28,29]. For this analysis, deaths from infection were included as AIDS deaths. ADIs were self-reported and classified as incident as previously described [30]. To account for loss to follow-up, women without study outcomes were censored at the earlier of 2 years after their last HAART use visit or 31 December 2009 as ascertainment of death beyond that date may not have been complete. We analyzed self-reported HAART adherence at each 6-month visit.
Laboratory methods
Plasma HIV-1 RNA was measured by isothermal nucleic acid sequence-based amplification (NASBA/Nuclisens; Organon Teknika Corp., Durham, North Carolina, USA) with a lower limit of detection of 80 copies/ml. Lymphocyte subsets were quantified using standard flow cytometric methods in laboratories participating in the NIH/NIAID Flow Cytometry Quality Assessment Program [31]. HCV RNA was measured on frozen specimens from HCV antibody-positive women using either the COBAS Amplicor Monitor 2.0 assay or the COBAS Taqman assay (Roche Diagnostics, Branchburg, New Jersey, USA) [32].
Statistical analysis
Racial groups were compared by means or proportions of potential confounding covariates. We performed unadjusted competing risk Kaplan–Meier and adjusted proportional hazards survival models of time to AIDS death, non-AIDS death, and incident ADI by racial group [33]. In models restricted to 1255 women with adherence data, adherence to HAART (available from October 2002 forward) was included as a time-dependent variable. Women without study outcomes were censored at the earlier of 2 years after their last HAART use visit or 31 December 2009. Multivariate proportional hazards analyses were performed with backwards stepwise selection among the following variables assessed at the pre-HAART visit: race, age, HIV exposure category, income, education, date of WIHS enrollment, WIHS site, year of HAART initiation, illicit drug use, smoking status, depressive symptoms, nadir CD4+, peak HIV-1 RNA, pre-HAART ADI, HCV status, and adherence. As the primary exposure of interest, race was forced into the stepwise models, as were age and smoking tobacco. Logistic regression models of adherence from October 2002 onwards were fit using the same predictor variables, and generalized estimation equations (GEEs) to adjust for within-person collinearity of repeated visits [34]. Multivariate models of adherence were fit using the same stepwise approach (without adherence as a predictor) described above. A sensitivity analysis assessing collinearity of HCV and IDU was also performed.
Results
Of the 2090 HIV-positive WIHS participants who reported HAART use, 1471 met inclusion criteria for continuous HAART use: 823 (55.9%) were black, 334 (22.7%) white, and 314 (21.3%) reported their race as other. During the study follow-up, 167 women died of AIDS, 136 died of non-AIDS causes, and 11 with unknown cause of death. Most deaths of unknown cause were recent without enough information available to allow adjudication for cause. Clinical and sociodemographic characteristics of the study population are shown in Table 1. Black compared to white women were older at HAART initiation, more likely to have used illicit drugs, have HCV, and smoke tobacco. Women reporting their race as other initiated HAART earlier in calendar time and had lower income and educational attainment. There was no statistical difference in the prevalence of depressive symptoms at the pre-HAART visit across racial groups. HAART adherence was significantly lower in black women.
Table 1.
Participant characteristics in percentages (%) or means (SDs).
| White (N = 334) | Black (N = 823) | Other (N = 314) | P-value (N = 1471) | |
| Demographics | ||||
| Age at last pre-HAART visit (years) | 40.0 (8.4) | 41.3 (8.5) | 39.3 (8.0) | <0.001 |
| Baseline risk category | ||||
| IDU | 28.8% | 28.9% | 24.4% | 0.60 |
| Heterosexual risk | 68.5% | 67.9% | 72.1% | |
| Transfusion risk | 2.7% | 3.2% | 3.6% | |
| Pre-HAART annual income ≤$12 000 | 40.8% | 58.9% | 59.9% | <0.001 |
| Education level (% graduating from high school) | 72.2% | 65.5% | 49.2% | <0.001 |
| WIHS year of enrollment (1994–1995 vs. 2001–2002) | ||||
| 1994–1995 | 77.8% | 80.9% | 91.7% | <0.001 |
| 2001–2002 | 22.2% | 19.1% | 8.3% | |
| Calendar year HAART initiated | 2001.4 (4.6) | 2001.4 (4.2) | 2000.5 (4.1) | 0.002 |
| Behaviors at pre-HAART visit | ||||
| Pre-HAART illicit drug use | 9.9% | 13.7% | 8.0% | 0.01 |
| Pre-HAART smoking | 39.2% | 54.8% | 34.7% | <0.001 |
| Pre-HAART depressive symptoms CES-D ≥16 (%) | 41.6% | 46.1% | 47.5% | 0.27 |
| Pre-HAART CES-D subscale depression | ||||
| Negative subscale ≤4 vs. >4 | 42.5% | 47.2% | 51.0% | 0.10 |
| Somatic subscale ≤6 vs. >6 | 39.8% | 44.6% | 44.2% | 0.31 |
| Positive subscale ≤3 vs. >3 | 42.5% | 40.5% | 49.4% | 0.03 |
| Interpersonal subscale 0 vs. >0 | 32.0% | 38.8% | 41.4% | 0.03 |
| Clinical characteristics pre-HAART | ||||
| Pre-HAART nadir CD4+ | 221 (154) | 210 (158) | 206 (143) | 0.38 |
| Pre-HAART peak HIV-1 RNA (log base 10) | 4.7 (0.9) | 4.8 (0.8) | 4.8 (0.8) | 0.37 |
| Pre-HAART AIDS-defining illness | 46.1% | 49.0% | 47.1% | 0.64 |
| Pre-HAART HCV infection | 27.0% | 31.8% | 22.6% | 0.006 |
| Post-HAART events/outcomes | ||||
| Adherence ≥95% vs. <95%a | 80.9% | 73.1% | 80.1% | <0.001 |
| Vital status at end of study | ||||
| Alive | 84.4% | 75.6% | 80.6% | NAb |
| AIDS death | 6.0% | 13.4% | 11.8% | NAb |
| Non-AIDS death | 9.3% | 10.1% | 7.0% | NAb |
| Unknown/indeterminate | 0.3% | 1.0% | 0.6% | NAb |
CES-D, Center for Epidemiology Studies Depression score; HCV, hepatitis C virus; IDU, injection drug use; WIHS, Women's Interagency HIV Study.
aAll person-visits from October 2002 onwards for which adherence was self-reported in 1255 women. P-values are from generalized estimation models.
bSee Table 2 for statistical comparison of times to AIDS and non-AIDS deaths.
Cumulative incidence of death
Figures 1 and 2 show cumulative incidence of AIDS and non-AIDS death, respectively, stratified by race. Ten years after HAART initiation, black women had the highest cumulative incidence of AIDS death, 17.3 vs. 8.3% for whites and 14.2% for others (Fig. 1), whereas cumulative incidence of non-AIDS death was similar in blacks and whites (13.1 and 12.3%, respectively) and lower (7.7%) in women reporting their race as other (Fig. 2).
Fig. 1.
Cumulative incidence of AIDS death over time.
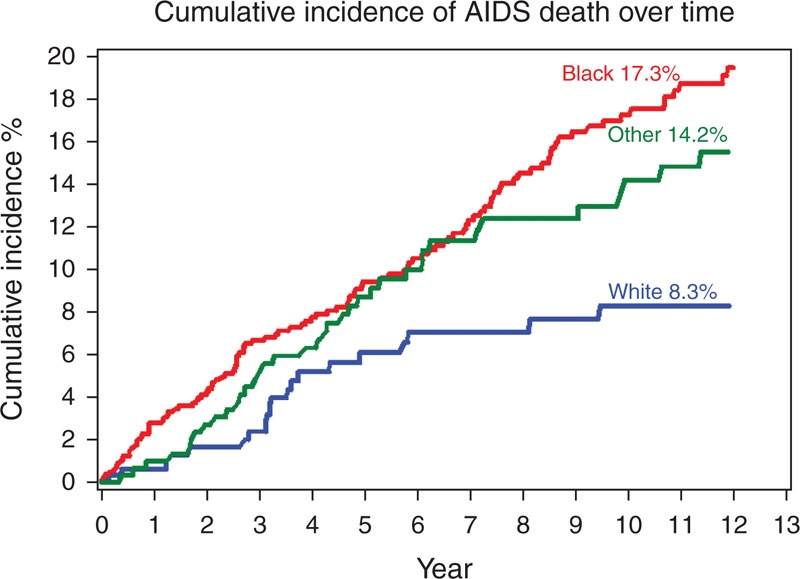
Lines correspond to the cumulative incidence of AIDS death stratified by race. The red line represents black women, the blue line represents white women and the green line represents women who reported their race as other.
Fig. 2.
Cumulative incidence of non-AIDS death over time.
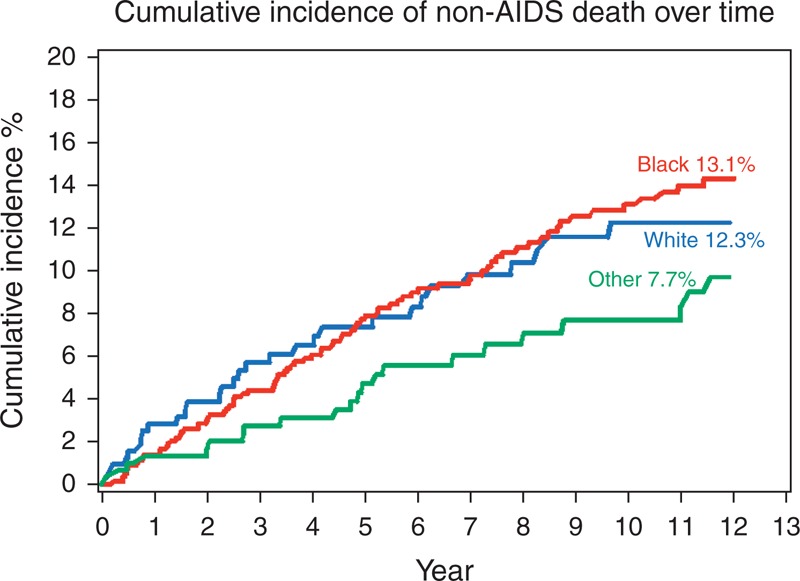
Lines correspond to the cumulative incidence of non-AIDS death stratified by race. The red line represents black women, the blue line represents white women and the green line represents women who reported their race as other.
Associations with death from AIDS
In univariate and multivariate analyses, black women experienced more rapid death from AIDS than white women. In multivariate models not adjusting for adherence (middle column, Table 2) the aHR for AIDS death in black vs. white women was 2.14 (95% CI 1.30, 3.50; P = 0.003). Other significant independent predictors of AIDS death were higher peak pre-HAART viral load (aHR 1.70 per log10 HIV RNA increase, 95% CI 1.34, 2.16; P < 0.001), HCV status (aHR 1.57, 95% CI 1.02, 2.40; P = 0.04), and depressive symptoms (aHR 2.10, 95% CI 1.51, 2.92; P < 0.001). As shown in univariate models (left column, Table 2), negative, positive, interpersonal, and somatic depressive symptoms qualitatively had equivalent associations with time to AIDS death; thus only CES-D at least 16 was included in the multivariate model because of issues of collinearity and multiple comparisons. Women who acquired HIV via transfusion vs. heterosexual exposure were more likely to die from AIDS (aHR 2.33, 95% CI 1.21, 4.49; P = 0.01). Higher pre-HAART nadir CD4+ cell count (aHR 0.65 per 100 cells/μl increment, 95% CI 0.56, 0.76; P < 0.001) and later year of HAART initiation (aHR 0.91 per year, 95% CI 0.85, 0.97; P = 0.005) protected against AIDS death.
Table 2.
Predictors of AIDS death hazards ratios (HRs) or adjusted hazard ratios (aHRs).a
| Univariate Model (N = 1471) | Multivariate model without adherenceb (N = 1471) | Multivariate model with time-dependent adherenceb,c (N = 1255) | ||||
| HR (95% CI) | P-value | aHR (95% CI) | P-value | aHR (95% CI) | P-value | |
| Demographics | ||||||
| Race | ||||||
| Black vs. white | 2.36 (1.45, 3.85) | <0.001 | 2.14 (1.30, 3.50) | 0.003 | 3.09 (1.38, 6.93) | 0.006 |
| Other vs. white | 1.76 (1.01, 3.07) | 0.048 | 1.56 (0.88, 2.77) | 0.132 | 2.09 (0.83, 5.29) | 0.120 |
| Age at visit per 5 years | 1.09 (0.99, 1.19) | 0.086 | 1.04 (0.93, 1.17) | 0.471 | 1.03 (0.87, 1.22) | 0.735 |
| Mode of HIV acquisition | ||||||
| IDU vs. heterosexual | 1.60 (1.14, 2.25) | 0.007 | 0.93 (0.60, 1.43) | 0.728 | 0.58 (0.31, 1.06) | 0.076 |
| Transfusion vs. heterosexual | 2.56 (1.36, 4.81) | 0.003 | 2.33 (1.21, 4.49) | 0.011 | 3.51 (1.32, 9.31) | 0.012 |
| Pre-HAART annual income ≤$12 000 | 1.81 (1.30, 2.52) | <0.001 | ||||
| Education: high school graduation or beyond | 0.84 (0.61, 1.15) | 0.27 | ||||
| WIHS year of enrollment (1994–1995 vs. 2001–2002) | 0.24 (0.10, 0.60) | 0.002 | ||||
| Calendar year HAART initiated | 0.89 (0.84, 0.95) | <0.001 | 0.91 (0.85, 0.97) | 0.005 | 1.15 (1.05, 1.26) | <0.002 |
| Behaviors pre-HAART | ||||||
| Pre-HAART illicit drug use | 2.29 (1.55, 3.37) | <0.001 | 1.51 (1.00, 2.30) | 0.053 | ||
| Pre-HAART smoking | 1.64 (1.20, 2.24) | 0.002 | 1.28 (0.88, 1.87) | 0.195 | 1.61 (0.95, 2.74) | 0.078 |
| Pre-HAART depressive symptoms CES-D 16+ vs. <16 | 2.31 (1.68, 3.17) | <0.001 | 2.10 (1.51, 2.92) | <0.001 | 2.85 (1.75, 4.66) | <0.001 |
| Pre-HAART CES-D subscale depression | ||||||
| Negative subscale ≤4 vs. >4 | 1.80 (1.32, 2.46) | <0.001 | NAd | NAd | NAd | NAd |
| Somatic subscale ≤6 vs. >6 | 1.89 (1.39, 2.57) | <0.001 | NAd | NAd | NAd | NAd |
| Positive subscale ≤3 vs. >3 | 1.90 (1.40, 2.58) | <0.001 | NAd | NAd | NAd | NAd |
| Interpersonal subscale 0 vs. >0 | 1.62 (1.20, 2.20) | 0.002 | NAd | NAd | NAd | NAd |
| Clinical characteristics pre-HAART | ||||||
| Pre-HAART nadir CD4+ per 100 cells/μl | 0.54 (0.47, 0.63) | <0.001 | 0.65 (0.56, 0.76) | <0.001 | 0.79 (0.65, 0.96) | 0.016 |
| Pre-HAART log10 peak VL | 2.36 (1.89, 2.95) | <0.001 | 1.70 (1.34, 2.16) | <0.001 | 1.64 (1.17, 2.30) | 0.004 |
| Pre-HAART AIDS-defining illness | 2.38 (1.72, 3.31) | <0.001 | ||||
| Pre-HAART HCV infection | 2.01 (1.46, 2.76) | <0.001 | 1.57 (1.02, 2.40) | 0.040 | 2.72 (1.49, 4.99) | 0.001 |
| Post-HAART outcomes | ||||||
| Adherence to HAART ≥95% vs. <95%b,c | 0.30 ( 0.19, 0.46) | <0.001 | 0.33 (0.21, 0.53) | <0.001 | ||
CES-D, Center for Epidemiology Studies Depression score; CI, confidence interval; HCV, hepatitis C virus; IDU, injection drug use; WIHS, Women's Interagency HIV Study; VL, viral load.
aFrom proportional hazards models.
bFrom stepwise selection proportional hazards models with race, WIHS site, age, and smoking forced in.
cRestricted to survival from October 2002 to December 2009 for which self-reported adherence was obtained.
dNA – only CES-D and not the specific subscales was considered for the multivariate model.
In both univariate and multivariate subanalyses of the 1255 women with adherence data, at least 95% self-reported adherence to HAART strongly protected against AIDS death (aHR 0.33, 95% CI 0.21, 0.53; P < 0.001) (right column, Table 2). When time-updated adherence was included in the model, the association of African American race with AIDS death persisted (aHR 3.09, 95% CI 1.38, 6.93; P = 0.006). Adjustment for adherence did not statistically or qualitatively attenuate the previously described association of other variables with time to AIDS death.
Associations with non-AIDS death
There was no significant association of race with non-AIDS death; aHR 0.91, 95% CI 0.59, 1.39; P = 0.65 and aHR 0.75, 95% CI 0.42, 1.34; P = 0.34 for black and other vs. white, respectively (right column, Table 3) in multivariate analyses. Significant independent predictors of the incidence of non-AIDS death were: HCV status (aHR 2.45, 95% CI 1.67, 3.60; P < 0.001), older age at HAART initiation (aHR 1.19 per 5 years, 95% CI 1.06, 1.33; P = 0.004), smoking (aHR 1.61, 95% CI 1.06, 2.44; P = 0.03), and annual income $12 000 or less (aHR 1.57, 95% CI 1.05, 2.35; P = 0.03). There was no association of HAART adherence or depressive symptoms with non-AIDS death in univariate or multivariate models, respectively; thus the multivariate model of time to non-AIDS death adjusting for time-dependent adherence to HAART is not presented.
Table 3.
Predictors of non-AIDS death hazards ratios (HRs) or adjusted hazard ratios (aHRs).a
| Univariate model (N = 1471) | Multivariate modelb,c (N = 1471) | |||
| HR (95% CI) | P-value | aHR (95% CI) | P-value | |
| Demographics | ||||
| Race | ||||
| Black vs. white | 1.25 (0.81, 1.91) | 0.31 | 0.91 (0.59, 1.39) | 0.65 |
| Other vs. white | 0.79 (0.45, 1.40) | 0.42 | 0.75 (0.42, 1.34) | 0.34 |
| Age at visit per 5 years | 1.24 (1.12, 1.36) | <0.001 | 1.19 (1.06, 1.33) | 0.004 |
| Mode of HIV acquisition | ||||
| IDU vs. heterosexual | 3.15 (2.18, 4.55) | <0.001 | ||
| Transfusion vs. heterosexual | 2.66 (1.14, 6.22) | 0.02 | ||
| Pre-HAART annual income ≤$12 000 | 2.20 (1.50, 3.21) | <0.001 | 1.57 (1.05, 2.35) | 0.03 |
| Education: high school graduation or beyond | 0.61 (0.43, 0.86) | 0.005 | ||
| WIHS year of enrollment (1994–1995 vs. 2001–2002) | 0.48 (0.23, 0.98) | 0.044 | ||
| Calendar year of HAART initiated | 0.95 (0.90, 1.01) | 0.10 | ||
| Behaviors pre-HAART | ||||
| Pre-HAART illicit drug use | 1.82 (1.20, 2.78) | 0.005 | ||
| Pre-HAART smoking | 2.63 (1.81, 3.84) | <0.001 | 1.61 (1.06, 2.44) | 0.03 |
| Pre-HAART depressive symptoms CES-D 16+ vs. <16 | 1.73 (1.23, 2.44) | 0.002 | 1.40 (0.98, 2.00) | 0.06 |
| Pre-HAART CES-D subscale depression | ||||
| Negative subscale ≤4 vs. >4 | 1.65 (1.17, 2.33) | 0.004 | NAd | NAd |
| Somatic subscale ≤6 vs. >6 | 1.60 (1.14, 2.25) | 0.007 | NAd | NAd |
| Positive subscale ≤3 vs. >3 | 1.75 (1.24, 2.46) | 0.001 | NAd | NAd |
| Interpersonal subscale 0 vs. >0 | 1.46 (1.04, 2.05) | 0.03 | NAd | NAd |
| Clinical characteristics pre-HAART | ||||
| Pre-HAART nadir CD4+ per 100 cells/μl | 0.88 (0.78, 1.00) | 0.051 | ||
| Pre-HAART log10 peak VL | 1.17 (0.94, 1.45) | 0.16 | ||
| Pre-HAART AIDS-defining illness | 1.68 (1.18, 2.38) | 0.004 | ||
| Pre-HAART HCV infection | 3.58 (2.51, 5.11) | <0.001 | 2.45 (1.67, 3.60) | <0.001 |
| Post-HAART events/outcomes | ||||
| Adherence to HAART ≥95% vs. <95%b,e | 0.80 (0.49, 1.29) | 0.36 | ||
CES-D, Center for Epidemiology Studies Depression score; CI, confidence interval; HCV, hepatitis C virus; IDU, injection drug use; WIHS, Women's Interagency HIV Study; VL, viral load.
aFrom proportional hazards model.
bFrom stepwise selection proportional hazards models with race, WIHS site, age, and smoking forced in
cNote: As adherence was not associated with non-AIDS death in unadjusted analysis or multivariate analysis, the multivariate model with adherence is not reported here as was done in Table 2.
dNA – only CES-D and not the specific subscales was considered for the multivariate model.
eRestricted to survival from October 2002 through December 2009 for which self-reported adherence was obtained.
Incident AIDS-defining illness
The proportion of women who reported an incident ADI at 10 years was 21.3, 13.9, and 16.6% for black, white, and other women, respectively (see Supplemental Digital Content 1, Figure of Cumulative Incidence of Incident AIDS-Defining Illness). In multivariate analyses, black women (aHR 1.58, 95% CI 1.08, 2.32; P = 0.02) and women who reported their race as other (aHR 1.60, 95% CI 1.01, 2.54; P = 0.045) were more likely than white women to report an incident ADI. Incident ADIs were also more common in women with a higher pre-HAART peak viral load (aHR 1.51 per log10 increment, 95% CI 1.26, 1.81; P < 0.001), prior history of ADI (aHR 2.19, 95% CI 1.63, 2.96; P < 0.001), and current smokers (aHR 1.65, 95% CI 1.24, 2.20; P < 0.001), and less common in women who initiated HAART in more recent calendar time (aHR 0.75 per year, 95% CI 0.70, 0.80; P < 0.001).
Analyses excluding illicit drug users
To eliminate possible confounding by drug use, we performed analyses excluding women who reported any history of using crack, cocaine or heroin, or a history of IDU at the pre-HAART visit. With drug users (33.1% of participants) excluded, the association of black (vs. white) race with AIDS death was larger (aHR 3.10, 95% CI 1.47, 6.53; P = 0.003); black women again more rapidly experienced an incident ADI (aHR 1.90, 95% CI 1.12, 3.24; P = 0.02) in this subset. With drug users excluded, there was again no association of race with non-AIDS death (aHR for African American race 1.32, 95% CI 0.65, 2.66; P = 0.43) and little change in the association of other predictors with AIDS deaths (see Supplemental Digital Content 2, Supplemental Table 1 Predictors of AIDS Death with Illicit Drug Users Excluded and Supplemental Digital Content 3, Supplemental Table 2 Predictors of Non-AIDS Death with Illicit Drug Users Excluded).
Sensitivity analysis of injection drug use and hepatitis C virus
There was some association between HIV acquisition via IDU and HCV infection; 73% of IDU vs. 25% of women who acquired HIV via heterosexual exposure were infected with HCV. A sensitivity analysis using three different models of predictors of AIDS death (HCV infection alone, IDU alone, and both) indicated that HCV infection and IDU were not collinear in their effect on AIDS deaths (data not shown).
Associations of race and other covariates with adherence
We fit GEE models assessing predictors of adherence at all visits at which HAART use was reported. In univariate and multivariate models, black women were less likely than white women to report at least 95% HAART adherence [adjusted odds ratio (aOR) 0.66, 95% CI 0.53, 0.83; P < 0.001] (Table 4). Women with depressive symptoms were also less likely to adhere to HAART (aOR 0.77, 95% CI 0.65, 0.92; P = 0.004); each of the depression subscales (negative, somatic, positive, and interpersonal) was qualitatively associated with nonadherence with similar strength. Other independent predictors of lower adherence were illicit drug use (aOR 0.75, 95% CI 0.58, 0.98; P = 0.03), HCV status (aOR 0.77, 95% CI 0.63, 0.95; P = 0.02), and smoking (aOR 0.73, 95% CI 0.61, 0.88; P < 0.001). There was no association between duration of HAART use and adherence.
Table 4.
Predictors of at least 95% adherence to HAART odds ratios (ORs) or adjusted odds ratios (aORs).a
| Univariate model (N = 1255) | Multivariate modelb (N = 1255) | |||
| OR (95% CI) | P-value | aOR (95% CI) | P-value | |
| Demographics | ||||
| Race | ||||
| Black vs. white | 0.63 (0.51, 0.80) | <0.001 | 0.66 (0.53, 0.83) | <0.001 |
| Other vs. white | 0.80 (0.61, 1.05) | 0.10 | 0.76 (0.58, 1.00) | 0.051 |
| Age at visit per 5 years | 1.06 (1.00, 1.11) | 0.04 | 1.10 (1.04, 1.17) | 0.001 |
| Mode of HIV acquisition | ||||
| IDU vs. heterosexual | 0.75 (0.62, 0.90) | 0.002 | ||
| Transfusion vs. heterosexual | 1.38 (0.83, 2.29) | 0.22 | ||
| Pre-HAART annual income ≤$12 000 | 0.85 (0.71, 1.01) | 0.06 | ||
| Education: high school graduation or beyond | 1.00 (0.84, 1.20) | 0.96 | 0.86 (0.73, 1.02) | 0.09 |
| WIHS year of enrollment (1994–1995 vs. 2001–2002) | 1.04 (0.84, 1.28) | 0.72 | ||
| Calendar year HAART initiated | 1.00 (0.98, 1.02) | 0.96 | ||
| Behaviors pre-HAART | ||||
| Pre-HAART illicit drug use | 0.57 (0.44, 0.73) | <0.001 | 0.75 (0.58, 0.98) | 0.03 |
| Pre-HAART smoking | 0.63 (0.53, 0.74) | <0.001 | 0.73 (0.61, 0.88) | <0.001 |
| Pre-HAART depressive symptoms CES-D 16+ vs. <16 | 0.72 (0.61, 0.86) | <0.001 | 0.77 (0.65, 0.92) | 0.004 |
| Pre-HAART CES-D subscale depression | ||||
| Negative subscale ≤4 vs. >4 | 0.73 (0.61, 0.86) | <0.001 | NAc | NAc |
| Somatic subscale ≤6 vs. >6 | 0.65 (0.55, 0.77) | <0.001 | NAc | NAc |
| Positive subscale ≤3 vs. >3 | 0.77 (0.65, 0.91) | 0.002 | NAc | NAc |
| Interpersonal subscale 0 vs. >0 | 0.82 (0.69, 0.98) | 0.03 | NAc | NAc |
| Clinical characteristics pre-HAART | ||||
| Pre-HAART nadir CD4+ per 100 cells/μl | 1.08 (1.02, 1.15) | 0.01 | 1.07 (1.01, 1.14) | 0.02 |
| Pre-HAART log10 peak VL | 0.91 (0.82, 1.01) | 0.07 | ||
| Pre-HAART AIDS-defining illness | 0.77 (0.65, 0.91) | 0.002 | ||
| Pre-HAART HCV infection | 0.72 (0.60, 0.87) | <0.001 | 0.77 (0.63, 0.95) | 0.02 |
CES-D, Center for Epidemiology Studies Depression score; CI, confidence interval; HCV, hepatitis C virus; IDU, injection drug use; WIHS, Women's Interagency HIV Study; VL, viral load.
aBased on GEE logistic regression. Study visit was also considered but was not statistically associated with adherence.
bFrom backwards selection GEE models with race, WIHS site, WIHS visit, age, and smoking forced in.
cNA – only CES-D and not the specific subscales was considered for the multivariate model.
Discussion
In this large study of continuous HAART users with varied ancestry, we found that black women were twice as likely as white women to experience adverse HIV clinical outcomes, specifically death from AIDS and incident ADI, even after adjusting for multiple potential confounding characteristics including illicit drug use, depressive symptoms, and adherence. These findings of strong independent associations of African American race with AIDS death and incident ADI after controlling for important potential confounders raise the possibility that both genetic and biologic factors, or either of them, could be substantially influencing HAART efficacy. These differences seem specific to HIV as there were no differences by race in non-AIDS deaths.
Collection of self-reported HAART adherence data in WIHS after 2002 allowed us to assess the contribution of adherence to higher death rates in black women. Although black women were less likely to adhere to HAART (aOR 0.66) than white women, multivariate models including time-updated adherence did not mitigate this group's faster rate of death from AIDS. Further, in models excluding illicit drug users, the association of AIDS deaths with ancestry was even larger (hazard ratio 3.09 vs. 2.14 for analyses excluding and including drug users, respectively). These findings suggest that factors other than modifiable behaviors may be contributing to racial disparities in AIDS clinical outcomes.
The finding of a strong independent association of African American race with AIDS-specific clinical outcomes after accounting for numerous potential confounding factors raises the question of what other potential mediators may be contributing. There is the possibility of unmeasured confounders that we did not capture through our chosen covariables. For example, we analyzed annual income and education level to assess socioeconomic status, but perhaps variables such as neighborhood, family structure, and employment status would have provided additional measures. Similarly, unmeasured behavioral factors may have varied by race and could influence mortality. Because all participants by definition were accessing healthcare to obtain HAART, lack of access to care is substantially obviated as a confounder; however, measurement of health insurance status (public vs. private) and patient–provider relationships may be informative.
The study results suggest that factors other than demographics, socioeconomic status, and modifiable behaviors may be contributing to racial disparities in AIDS clinical outcomes. Multiple sources of evidence suggest that genetically determined diversity in immunogenetic and pharmacokinetic parameters is associated with clinical findings in HIV-infected persons using HAART and may be contributing to our findings of a two-fold higher risk in black vs. white women of death from AIDS, the single most important clinical outcome.
Host immunogenetic and pharmacogenetic variability are both known to influence clinical outcomes in HIV disease. Several studies have shown that specific human leukocyte antigen complex (HLA) class 1 alleles, which are known to vary by ancestry, are associated with HIV disease progression [35–38], including elite suppression [39]. Further, recent studies have indicated that some alleles (e.g. HLA B∗5701 and Bw4), which are protective for progression in untreated HIV disease, may have deleterious effects in patients taking HAART, with a lower likelihood of virologic suppression or CD4 response [40–43]. Chemokine receptor gene polymorphisms have also been associated with differential clinical outcomes on HAART based on ancestry (e.g. CCR5 2459G allele) [44]. Pharmacogenetic factors that can influence drug metabolism [45–48] and transport [49–51] are known to vary by ancestry, but their effects on response to HAART are not clear [52–63].
We found that higher depressive symptom scores were associated with AIDS death after adjusting for other covariates. The significant overlap of items of the CES-D with somatic symptoms and advanced disease (fatigue, anorexia, lower energy, and lack of mental acuity) could be confounding the association of depression with poor clinical outcomes, as has been found in prior studies [64]. However, we found that all components of the CES-D score including somatic, negative, positive, and interpersonal subscales were significantly associated with AIDS death at qualitatively similar levels (left column, Table 2). Thus the strong association of depressive symptoms with AIDS death did not result just from the somatic components within the CES-D score. This finding is clinically significant as depression is potentially modifiable through treatment [65–67].
Although the black women were more likely to smoke tobacco, and tobacco use was associated with AIDS death in univariate analysis, its use was not associated with AIDS death in the multivariate models. Tobacco use was, however, associated with non-AIDS death as expected.
Strengths of our study include its large sample size, representativeness of the WIHS cohort, and the long follow-up. This is one of the largest studies to date to assess clinical outcomes by ancestry and adjusts for confounding behaviors and characteristics that are not typically measured in clinical settings. The WIHS dataset includes measurements of most of the postulated and observed behavioral (e.g. adherence) and clinical (e.g. depressive symptoms) confounders that may influence mortality. Deaths within WIHS are actively ascertained. Whereas most prior studies investigating the association of race with AIDS death in HAART users have not shown a positive association [8–16], in the case of our previous WIHS study, we believe we did not meet statistical significance as a result of underpowering [21].
Study limitations include the potential for unmeasured confounders that may also influence AIDS-specific clinical outcomes. An additional limitation is the inclusion of some self-reported covariates, for example, adherence and prior ADI, as well as self-categorized race. Self-reported adherence measured on a biannual basis is limited by recall bias and the potential for misreporting. Additional measures of adherence could include hair levels of antiretrovirals [68,69], frequency of filling prescriptions, and pill counts, which have their own limitations. A previous WIHS study compared self-report of AIDS-specific diagnoses with AIDS diagnoses documented by county AIDS surveillance registries and found a fair degree of accuracy with some variation by specific condition [70]. Self-categorization of race may not accurately reflect genetic background. Categorization of race with ancestry informative markers in WIHS may provide a more accurate assessment of race from a genetic perspective and is planned for the future. There is also the potential for misclassification of death [71–74].
In summary, our results provide evidence that African American compared to white American women experience higher rates of the most important HIV-related outcomes, specifically AIDS death and incident ADI. This association was specific to AIDS-related outcomes and was not explained by many measured potential confounding factors including HAART adherence. There is growing evidence supporting a differential response to HAART possibly based on host genetic variation, including genes that influence pharmacokinetics. Future studies replicating these associations, and examining specific pharmaco-genetic and immuno-genetic variations, as well as behaviours, are important and may inform both our understanding of HIV pathogenesis and the selection of HAART regimens for people of African descent.
Acknowledgements
Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, New York (Howard Minkoff); Washington, District of Columbia Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange).
Author contributions: K.M. was the lead author and responsible for the overall coordination and execution of the study including study conception and design, data analysis and interpretation, and writing, review and revision of the manuscript.
D.R.H. and Q.S. led the statistical team and were responsible for overall statistical analysis and data interpretation, and also contributed to the writing, critical appraisal, and editing of the manuscript.
M.C. and M.Y. were involved in obtaining funding for the study, the process of data collection and data interpretation, as well as the critical appraisal and editing of the manuscript.
M.G., E.T.G., D.R.G., and C.L.P. were involved in data interpretation, as well as the critical appraisal and editing of the manuscript.
K.A. was the Principal Investigator and senior author on the study and as such was responsible for obtaining funding, collecting data, study conception and design, data analysis and interpretation, writing, review, and revision of the manuscript.
The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). K.M. acknowledges support from the Institutional AIDS training grant, ‘HIV, AIDS and Opportunistic Infections’ (T32 AI007501). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Correspondence to Kerry Murphy, MD, Albert Einstein College of Medicine, Montefiore Medical Center, Department of Medicine, Division of Infectious Diseases, 1300 Morris Park Avenue, Belfer Building, Rm 610, Bronx, NY 10461, USA. Tel: +1 718 515-2593; fax: +1 718 547-0584; e-mail: kerry.murphy@einstein.yu.edu
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global report: UNAIDS report on the global AIDS epidemic. WHO Library Cataloguing-in-Publication Data; 2010 [Google Scholar]
- 2.WHO Report Women and Health: today's evidence tomorrow's agenda. WHO Library Cataloguing-in-Publication Data; 2009 [Google Scholar]
- 3.Hall HI, An Q, Hutchinson A, Sansom S. Estimating the lifetime risk of a diagnosis of the HIV infection in 33 states, 2004–2005. J Acquir Immune Defic Syndr 2008; 49:294–297 [DOI] [PubMed] [Google Scholar]
- 4.Prejean J, Song R, Hernandez A, Ziebell R, Green T, Walker F, et al. for the HIV Incidence Surveillance Group Estimated HIV incidence in the United States, 2006–2009. PLoS One 2011; 6:e17502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu JQ, Kochanek KD, Murphy SL, Tejada-Vera B. Deaths: final data for 2007. National vital statistics reports. Vol 58. Hyattsville, MD:National Center for Health Statistics; 2010 [PubMed] [Google Scholar]
- 6.Simard EP, Fransua M, Naishadham D, Jemal A. The influence of sex, race/ethnicity, and educational attainment on human immunodeficiency virus death rates among adults, 1993–2007. Arch Intern Med 2012; 172:1591–1598 [DOI] [PubMed] [Google Scholar]
- 7.Cunningham W. HIV racial disparities time to close the gaps comment on ‘the influence of sex, race/ethnicity, and educational attainment on human immunodeficiency virus death rates among adults, 1993–2007’. Arch Intern Med 2012; 172:1599–1600 [DOI] [PubMed] [Google Scholar]
- 8.Jensen-Fangel S, Pedersen L, Pedersen C, Larsen CS, Tauris P, Mοller A, et al. The effect of race/ethnicity on the outcome of highly active antiretroviral therapy for human immunodeficiency virus type 1-infected patients. Clin Infect Dis 2002; 35:1541–1548 [DOI] [PubMed] [Google Scholar]
- 9.Silverberg M, Leyden W, Quesenberry C, Horberg M. Race/ethnicity and risk of AIDS and death among HIV-infected patients with access to care. J Gen Intern Med 2009; 24:1065–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore RD, Keruly JC, Bartlett JG. Improvement in the health of HIV-infected persons in care: reducing disparities. Clin Infect Dis 2012; 55:1242–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemly DC, Shepherd BE, Hulgan T, Rebeiro P, Stinnette S, Blackwell RB, et al. Race and sex differences in antiretroviral therapy use and mortality among HIV-infected persons in care. J Infect Dis 2009; 199:991–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weintrob AC, Grandits GA, Agan BK, Ganesan A, Landrum ML, Crum-Cianflone NF, et al. and the IDCRP HIV Working Group Virologic response differences between African Americans and European Americans initiating highly active antiretroviral therapy with equal access to care. J Acquir Immune Defic Syndr 2009; 52:574–580 [DOI] [PubMed] [Google Scholar]
- 13.Hartzell JD, Spooner K, Howard R, Wegner S, Wortmann G. Race and mental health diagnosis are risk factors for highly active antiretroviral therapy failure in a military cohort despite equal access to care. J Acquir Immune Defic Syndr 2007; 44:411–416 [DOI] [PubMed] [Google Scholar]
- 14.McGinnis KA, Fine MJ, Sharma RK, Skanderson M, Wagner JH, Rodrigues-Barradas MC, et al. Understanding racial disparities in HIV using data from the Veterans Aging Cohort 3-Site Study and VA Administrative Data. Am J Public Health 2003; 93:1728–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans C, et al. Meta-analysis of differences in viral suppression by race in clinical trials. ICAAC 2012; [Abstract H-881] [Google Scholar]
- 16.Giordano TP, Bartsch G, Zhang Y, Tedaldi E, Absalon J, Mannheimer S, et al. Disparities in outcomes for African American and Latino subjects in the Flexible Initial Retrovirus Suppressive Therapies (FIRST) Trial. AIDS Patient Care STDs 2010; 24:287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gebo KA, Fleishman JA, Conviser R, Reilly ED, Korthuis PT, Moore RD, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr 2005; 38:96–103 [DOI] [PubMed] [Google Scholar]
- 18.Cohen MH, Cook JA, Grey D, Young M, Hanau LH, Tien P, et al. Medically eligible women who do not use HAART: the importance of abuse, drug use, and race. Am J Public Health 2004; 94:1147–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thrasher AD, Earp JA, Golin CE, Zimmer CR. Discrimination, distrust, and racial/ethnic disparities in antiretroviral therapy adherence among a national sample of HIV-Infected patients. J Acquir Immune Defic Syndr 2008; 49:84–93 [DOI] [PubMed] [Google Scholar]
- 20.Mannheimer S, Friedland G, Matts J, Child C, Chesney M. for the Terry Beirn Community Programs for Clinical Research on AIDS The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis 2002; 34:1115–1121 [DOI] [PubMed] [Google Scholar]
- 21.Anastos K, Schneider MF, Gange SJ, Minkoff H, Greenblatt RM, Feldman J, et al. for the Women's Interagency HIV Study Collaborative Group The association of race, sociodemographic, and behavioral characteristics with response to highly active antiretroviral therapy in women. J Acquir Immune Defic Syndr 2005; 39:537–544 [PubMed] [Google Scholar]
- 22.Barkan S, Melnick S, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology 1998; 9:117–125 [PubMed] [Google Scholar]
- 23.DHHS/Henry J. Kaiser Family Foundation Panel on clinical practices for the treatment of HIV infection Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents; November 3, 2008 revision. http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf [Google Scholar]
- 24.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measur 1977; 1:385–401 [Google Scholar]
- 25.Berkman LF, Berkman CS, Kasl S, Freeman DH, Jr, Leo L, Ostfeld AM, et al. Depressive symptoms in relation to physical health and functioning in the elderly. Am J Epidemiol 1986; 124:372–388 [DOI] [PubMed] [Google Scholar]
- 26.Hertzog C, Vanalstine J, Usala PD, Hultsch DF, Dixon R. Measurement properties of the Center for Epidemiologic Studies Depression scale (CES-D) in older populations. Psychol Assess 1990; 2:64–72 [Google Scholar]
- 27.Nguyen HT, Kitner-Triolo M, Evans MK, Zonderman AB. Factorial invariance of the CES-D in low socioeconomic status African Americans compared with a nationally representative sample. Psychiatry Res 2004; 126:177–187 [DOI] [PubMed] [Google Scholar]
- 28.Cohen MH, French AL, Benning L, Kovacs A, Anastos K, Young M, et al. Causes of death among women with human immunodeficiency virus infection in the era of combination antiretroviral therapy. Am J Med 2002; 113:91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.French AL, Gawel SH, Hershow R, Benning L, Hessol NA, Levine AM, et al. Trends in mortality and causes of death among women with HIV in the United States: a 10-year study. J Acquir Immune Defic Syndr 2009; 51:399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep 1992; 41:1–19 [PubMed] [Google Scholar]
- 31.Calvelli T, Denny TN, Paxton H, Gelman R, Kagan J. Guidelines for flow cytometric immunophenotyping: a report from the National Institute of Allergy and Infectious Diseases, Division of AIDS. Cytometry 1993; 14:702–715 [DOI] [PubMed] [Google Scholar]
- 32.Al-Harthi L, Voris J, Du W, Nowicki M, Frederick T, Landay A, Kovacs A. Evaluating the impact of hepatitis C virus (HCV) on highly active antiretroviral therapy-mediated immune responses in HCV/HIV-coinfected women: role of HCV on expression of primed/memory T cells. J Infect Dis 2006; 193:1202–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein JP, Moeschberger ML. Survival analysis techniques for censored and truncated data. 2nd ed.New York, NY:Springer-Verlag; 2003 [Google Scholar]
- 34.Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. New York, NY:Oxford University Press; 1994 [Google Scholar]
- 35.Carrington M, O’Brien SJ. The influence of HLA genotype on AIDS. Annu Rev Med 2003; 54:535–551 [DOI] [PubMed] [Google Scholar]
- 36.Goulder PJ, Watkins DI. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat Rev Immunol 2008; 8:619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Telenti A, Zanger UM. Pharmacogenetics of anti-HIV drugs. Annu Rev Pharmacol Toxicol 2008; 48: [DOI] [PubMed] [Google Scholar]
- 38.Carrington M, Martin MP, van Bergen J. KIR-HLA intercourse in HIV disease. Trends Microbiol 2008; 16:620–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.International HIV Controllers Study The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 2011; 330:1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuniholm MH, Gao X, Xue X, Kovacs A, Anastos K, Marti D, et al. Human leukocyte antigen genotype and risk of HIV disease progression before and after initiation of antiretroviral therapy. J Virol 2011; 85:10826–10833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahuja SK, Kulkarni H, Catano G, Agan BK, Camargo JF, He W, et al. CCL3L1-CCR5 genotype influences durability of immune recovery during antiretroviral therapy of HIV-1-infected individuals. Nat Med 2008; 14:413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brumme ZL, Brumme CJ, Chui C, Mo T, Wynhoven B, Woods CK, et al. Effects of human leukocyte antigen class I genetic parameters on clinical outcomes and survival after initiation of highly active antiretroviral therapy. J Infect Dis 2007; 195:1694–1704 [DOI] [PubMed] [Google Scholar]
- 43.Rauch A, Nolan D, Furrer H, McKinnon E, John M, Mallal S, et al. HLA-Bw4 homozygosity is associated with an impaired CD4 T cell recovery after initiation of antiretroviral therapy. Clin Infect Dis 2008; 46:1921–1925 [DOI] [PubMed] [Google Scholar]
- 44.Mehlotra RK, Cheruvu VK, Blood Zikursh MJ, Benish RL, Lederman MM, Salata RA, et al. Chemokine (C-C Motif) receptor 5: 2459 genotype in patients receiving highly active antiretroviral therapy: race-specific influence on virologic success. J Infect Dis 2011; 204:291–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ameyaw MM, Regateiro F, Li T, Tariq M, Mobarek A, Thornton N, et al. MDR1 pharmacogenetics: frequency of the C3435T mutation in exon 26 is significantly influenced by ethnicity. Pharmacogenetics 2001; 11:217–221 [DOI] [PubMed] [Google Scholar]
- 46.Haas DW, Ribaudo HJ, Kim RB, Tierney C, Wilkinson GR, Gulick RM, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS 2004; 18:2391–2400 [PubMed] [Google Scholar]
- 47.Xie HG, Kim RB, Wood AJ, Stein CM. Molecular basis of ethnic differences in drug disposition and response. Annu Rev Pharmacol Toxicol 2001; 41:815–850 [DOI] [PubMed] [Google Scholar]
- 48.Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol 2004; 44:499–523 [DOI] [PubMed] [Google Scholar]
- 49.Marzolini C, Paus E, Buclin T, Kim RB. Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical significance. Clin Pharmacol Ther 2004; 75:13–33 [DOI] [PubMed] [Google Scholar]
- 50.Fellay J, Marzolini C, Meaden ER, Back DJ, Buclin T, Chave JP, et al. Response to antiretroviral treatment in HIV-1 infected individuals with allelic variants of the multidrug resistance transporter 1: a pharmacogenetics study. Lancet 2002; 359:30–36 [DOI] [PubMed] [Google Scholar]
- 51.Dey S. Single nucleotide polymorphisms in human P-glycoprotein: its impact on drug delivery and disposition. Expert Opin Drug Deliv 2006; 3:23–35 [DOI] [PubMed] [Google Scholar]
- 52.Haas DW, Smeaton LM, Shafer RW, Robbins GK, Morse GD, Labbe L, et al. Pharmacogenetics of long-term responses to antiretroviral regimens containing efavirenz and/or nelfinavir: an adult AIDS Clinical Trials Group Study. J Infect Dis 2005; 192:1931–1942 [DOI] [PubMed] [Google Scholar]
- 53.Nasi M, Borghi V, Pinti M, Bellodi C, Lugli E, Maffei S, et al. MDR1 C3435T genetic polymorphism does not influence the response to antiretroviral therapy in drug-naive HIV-positive patients. AIDS 2003; 17:1696–1698 [DOI] [PubMed] [Google Scholar]
- 54.Brumme ZL, Dong WW, Chan KJ, Hogg RS, Montaner JS, O'Shaughnessy MV, Harrigan PR. Influence of polymorphisms within the CX3CR1 and MDR-1 genes on initial antiretroviral response. AIDS 2003; 17:201–208 [DOI] [PubMed] [Google Scholar]
- 55.Schackman BR, Ribaudo HJ, Krambrink A, Hughes V, Kuritzkes DR, Gulick RM. Racial differences in virologic failure associated with adherence and quality of life on efavirenz-containing regimens for initial HIV therapy results of ACTG A5095. J Acquir Immune Defic Syndr 2007; 46:547–554 [DOI] [PubMed] [Google Scholar]
- 56.Gulick RM, Ribaudo HJ, Shikuma CM, Lalama C, Shackman BR, Meyer WA, 3rd, et al. for the ACTG A5095 Study Team Three- vs. four-drug antiretroviral regimens for the initial treatment of HIV-1 infection: a randomized control trial. JAMA 2006; 296:769–781 [DOI] [PubMed] [Google Scholar]
- 57.Ribaudo HJ, Liu H, Schwab M, Schaeffeler E, Eichelbaum M, Motsinger-Reif AA, et al. Effect of CYP2B6, ABCB1, and CYP3A5 polymorphisms on efavirenz pharmacokinetics and treatment response: an AIDS Clinical Trials Group Study. J Infect Dis 2010; 202:717–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ribaudo HJ, Haas DW, Tierney C, Kim RB, Wilkinson GR, Gulick RM, et al. Pharmacogenetics of plasma efavirenz exposure after treatment discontinuation: an Adult AIDS Clinical Trials Group Study. Clin Infect Dis 2006; 42:401–407 [DOI] [PubMed] [Google Scholar]
- 59.Haas DW, Gebretsadik T, Mayo G, Menon UN, Acosta EP, Shintani A, et al. Associations between CYP2B6 polymorphisms and pharmacokinetics after a single dose of nevirapine or efavirenz in African Americans. J Infect Dis 2009; 199:872–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gatanaga H, Hayashida T, Tsuchiya K, Yoshino M, Kuwahara T, Tsukada H, et al. Successful efavirenz dose reduction in HIV type-1 infected individuals with cytochrome P450 2B6 ∗6 and ∗26. Clin Infect Dis 2007; 45:1230–1237 [DOI] [PubMed] [Google Scholar]
- 61.Wyen C, Hendra H, Vogel M, Hoffmann C, Knechten H, Brockmeyer NH, et al. on behalf of the German Competence Network for HIV/AIDS Impact of the CYP2B6 983T > C polymorphism on nonnucleoside reverse transcriptase inhibitor plasma concentrations in HIV-infected patients. J Antimicrob Chemother 2008; 61:914–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haas DW, Kuritzkes DR, Ritchie MD, Amur S, Gage BF, Maartens G, et al. on behalf of participants in the workshop ‘Pharmocogenomics: a path towards personalized HIV care’ Pharmacogenomics of HIV therapy: summary of a workshop sponsored by the National Institute of Allergy and Infectious Diseases. HIV Clin Trials 2011; 12:277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gandhi M, Greenblatt RM, Bacchetti P, Jin C, Huang Y, Anastos K, et al. A Single-nucleotide polymorphism in CYP2B6 leads to >3-fold increases in efavirenz concentrations in plasma and hair among HIV-infected women. J Infect Dis 2012; 206:1453–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pettit JW, Lewinsohn PM, Seeley JR, Roberts RE, Hibbard JH, Hurtado AV. Association between the Center for Epidemiologic Studies Depression Scale (CES-D) and mortality in a community sample: an artifact of the somatic complaints factor?. Int J Clin Health Psychol 2008; 8:382–397 [PMC free article] [PubMed] [Google Scholar]
- 65.Cook JA, Cohen MH, Burke J, Grey D, Anastos K, Kirstein L, et al. Effects of depressive symptoms and mental health quality of life on use of highly active antiretroviral therapy among HIV-seropositive women. J Acquir Immune Defic Syndr 2002; 30:401–409 [DOI] [PubMed] [Google Scholar]
- 66.|Cook JA, Grey D, Burke J, Cohen MH, Gurtman AC, Richardson JL, et al. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. Am J Public Health 2004; 94:1133–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cook JA, Grey D, Burke-Miller J, Cohen MH, Anastos K, Gandhi M, et al. Effects of treated and untreated depressive symptoms on highly active antiretroviral therapy use in a US multisite cohort of HIV-positive women. AIDS Care 2006; 18:93–100 [DOI] [PubMed] [Google Scholar]
- 68.Gandhi M, Ameli N, Bacchetti P, Anastos K, Gange SJ, Minkoff H, et al. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis 2011; 52:1267–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gandhi M, Ameli N, Bacchetti P, Gange SJ, Anastos K, Levine A, et al. Protease inhibitor levels in hair samples strongly predict virologic responses to HIV treatment. AIDS 2009; 23:471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hessol NA, Schwarcz S, Ameli N, Oliver G, Greenblatt RM. Accuracy of self-reports of acquired immunodeficiency syndrome and acquired immunodeficiency syndrome: related conditions in women. Am J Epidemiol 2001; 153:1128–1133 [DOI] [PubMed] [Google Scholar]
- 71.Hernando V, Sobrino-Vegas P, Carmen Burriel M, Berenguer J, Navarro G, Santos I, et al. Differences in the causes of death of HIV-positive patients in a cohort study by data sources and coding algorithms. AIDS 2012; 26:1829–1834 [DOI] [PubMed] [Google Scholar]
- 72.Lifson AR. and the INSIGHT Cause of Death Writing Group Determination of the underlying cause of death in three multicenter international HIV clinical trials. HIV Clin Trials 2008; 9:177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Selik RM, Anderson RN, McKenna MT, Rosenberg HM. Increase in deaths caused by HIV infection due to changes in rules for selecting underlying cause of death. J AIDS 2003; 32:62–69 [DOI] [PubMed] [Google Scholar]
- 74.Grigg B, Brooks RG, Lieb S, Grigg M. Coding changes and apparent HIV-AIDS mortality trends in Florida, 1999. JAMA 2001; 286:1839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


