Abstract
Tissue regeneration is a complex trait with few genetic models available. Mouse strains LG/J and MRL are exceptional healers. Using recombinant inbred strains from a large (LG/J, healer) and small (SM/J, nonhealer) intercross, we have previously shown a positive genetic correlation between ear wound healing, knee cartilage regeneration, and protection from osteoarthritis. We hypothesize that a common set of genes operates in tissue healing and articular cartilage regeneration. Taking advantage of archived histological sections from recombinant inbred strains, we analyzed expression of candidate genes through branched-chain DNA technology directly from tissue lysates. We determined broad-sense heritability of candidates, Pearson correlation of candidates with healing phenotypes, and Ward minimum variance cluster analysis for strains. A bioinformatic assessment of allelic polymorphisms within and near candidate genes was also performed. The expression of several candidates was significantly heritable among strains. Although several genes correlated with both ear wound healing and cartilage healing at a marginal level, the expression of four genes representing DNA repair (Xrcc2, Pcna) and Wnt signaling (Axin2, Wnt16) pathways was significantly positively correlated with both phenotypes. Cluster analysis accurately classified healers and nonhealers for seven out of eight strains based on gene expression. Specific sequence differences between LG/J and SM/J were identified as potential causal polymorphisms. Our study suggests a common genetic basis between tissue healing and osteoarthritis susceptibility. Mapping genetic variations causing differences in diverse healing responses in multiple tissues may reveal generic healing processes in pursuit of new therapeutic targets designed to induce or enhance regeneration and, potentially, protection from osteoarthritis.
Keywords: tissue regeneration, articular cartilage, QuantiGene Plex assay, recombinant inbred lines, osteoarthritis
Regeneration potential is phylogenetically dispersed among animal taxa and is a basal trait in vertebrates, including fishes, amphibians, and mammalian fetal tissues (Gierer et al. 1972; Stocum 1984; Fini 1999; Harty et al. 2003; Tanaka 2003; Bely 2010; Cuervo et al. 2012; Seifert et al. 2012). A consensus is that the regenerative capability in adult mammals is extremely limited (ten Koppel et al. 2001; Harty et al. 2003; Carlson 2005; Colwell et al. 2005; Sanchez Alvarado and Tsonis 2006; Kierdorf and Kierdorf 2011). However, at the end of 20th century, the MRL/MpJ mouse emerged as a classical example of mammalian regeneration because it can heal ear wounds (Clark et al. 1998; Metcalfe et al. 2006; Fitzgerald et al. 2008; Rai et al. 2012), surgical wounds (Colwell et al. 2006; Heydemann et al. 2012), and articular cartilage lesions (Fitzgerald et al. 2008; Rai et al. 2012). In addition, DBA/1 (Kench et al. 1999; Li et al. 2001; Masinde et al. 2006) and LG/J (Kench et al. 1999; Li et al. 2001; Masinde et al. 2006; Rai et al. 2012) carry similar regenerative abilities to adulthood. This is in contrast to all other mouse strains tested so far (C57BL/6J, SJL/J, BALB/cByJ, SM/J), in which scar tissue forms at wound sites (Clark et al. 1998; Metcalfe et al. 2006; Fitzgerald et al. 2008; Rai et al. 2012).
Failure to regenerate injured articular cartilage poses a great challenge to musculoskeletal research because it is associated with osteoarthritis. Osteoarthritis is a complex heterogeneous disease in which cartilage degenerates because of aging, trauma, genetics or other unknown etiological agents. We have previously shown that an inverse relationship exists between cartilage healing and osteoarthritis susceptibility (Hashimoto et al. 2012). The super-healer MRL mouse is protected from posttraumatic osteoarthritis as well (Ward et al. 2008). Therefore, study of the genes associated with cartilage regeneration bears great relevance to osteoarthritis research.
A genome-wide microsatellite mapping study has shown that ear wound healing is a complex multigenic trait that is controlled by five to six loci segregating in F2 and backcrosses between MRL/MpJ and C57BL/6J (McBrearty et al. 1998; Heber-Katz 1999). The LG/J inbred strain has a similar healing ability and is one of the founding lines used to breed the MRL/MpJ strain. Both strains share 75% of their genome (Murphy and Roths 1979). Another mapping study in the F2 population derived from LG/J and nonhealing SM/J parental lines detected segregation at four loci that affect ear wound healing, three replicating loci mapped in the MRL/MpJ study plus one novel locus (Blankenhorn et al. 2009).
The complex process of regeneration is characterized by spontaneous initiation of several genes and biological pathways (Gurtner et al. 2008). Several studies have compared the gene expression in ear wound tissues between healers and nonhealers (McBrearty et al. 1998; Li et al. 2001; Masinde et al. 2006; Blankenhorn et al. 2009), generating important information regarding molecular biology of regeneration. The gene expression differences in ear tissues from LGXSM intercross have shown a differential regulation of ∼600 genes within the mapped loci affecting healing (Blankenhorn et al. 2009). However, no strong evidence is yet available for the genes that control cartilage regeneration in these strains.
We have recently studied ear wound healing and cartilage regeneration simultaneously in a set of recombinant inbred (RI) lines generated from LG/J (healer) and SM/J (nonhealer) intercross (Rai et al. 2012). We found a strong correlation between the two phenotypes, indicating a common genetic basis of healing and indicating that the underlying mechanisms might not be tissue-specific. A recent study has shown that distinct differences in cell-cycle properties exist between healer and nonhealer strains (Bedelbaeva et al. 2010). These data suggest that highly regenerative cells are both hyperproliferative and highly apoptotic in nature. The combined effects of increased proliferation and apoptosis might allow the organism to eliminate old cells and keep the cell turnover rate high, as seen in some organ development. However, the molecular and cellular basis for differences in regeneration is not completely understood.
In this study, we harvested injured knee joint tissues from formalin-fixed paraffin-embedded (FFPE) sections archived from common inbred and RI strains and measured the expression signatures of mRNAs and microRNAs (miRNAs) using a novel QuantiGene Plex assay directly from tissue lysates. We hypothesize that the healing responses have a common genetic basis, implying a common suite of mechanisms. We believe that identification of biological processes common to regeneration/healing in articular cartilage has novel implications for osteoarthritis pathogenesis and therapeutic interventions.
Materials and Methods
Mice source and housing
All procedures in this study were approved by the Animal Studies Committee of Washington University. Eight mouse strains (three to four mice per strain) were used based on previous screening (Rai et al. 2012), representing the following three healing categories: good (MRL/MpJ, LG/J, LGXSM-6); intermediate (LGXSM-5, LGXSM-35); and poor (C57BL/6J, SM/J, LGXSM-33). Detailed information and history of RI lines are documented elsewhere (Hrbek et al. 2006).
Phenotyping: ear pinna wound
A 2-mm bilateral hole was produced in the cartilaginous part of the external ear in 6-wk-old mice (McBrearty et al. 1998; Rai et al. 2012). The hole diameter was read 30 d after the punch and the healing area was calculated by subtracting the diameter of the residual hole from the original 2-mm hole. The phenotypic data for ear wound scores are provided in Supporting Information, Table S1.
Phenotyping: articular cartilage defect
Full-thickness articular cartilage defect was created through microsurgery on the trochlear groove of 8-wk-old mice (Fitzgerald et al. 2008; Rai et al. 2012). Harvested knee joints were processed for histology 16 wk postsurgery. FFPE samples were mounted in paraffin blocks, sagittally sectioned at 5-μm intervals, mounted on polylysine-coated slides (Fischer Scientific, Pittsburg, PA), and stained with toluidine blue. The degree of cartilage regeneration was assessed as described previously (Fitzgerald et al. 2008; Rai et al. 2012). The phenotypic data for articular cartilage regeneration scores are provided in Table S2 (only data from the 16-wk postsurgery time point are related to this study).
Archived histological sections
The archived unstained FFPE sections (25 sections per strain, three to four mice per strain) from articular cartilage were used to prepare tissue homogenates (“lyse ‘n’ go”) instead of isolating RNA. Previous reports have suggested that the quality of RNA extracted from FFPE tissues is inferior compared to fresh or frozen tissues or cells. Several factors contribute to this compromised quality of RNA. These include formalin fixation (Bresters et al. 1994; Macabeo-Ong et al. 2002), RNA cross-linking with proteins (Finke et al. 1993; Masuda et al. 1999), and addition of mono-methyl on all four bases (AUGC) (Park et al. 1996; Masuda et al. 1999). To overcome these problems and to make use of our valuable archival FFPE tissues, the Affymetrix QuantiGene Plex assay (Panomics Inc., Fremont, CA) was used. This method is based on a sandwich nucleic acid hybridization assay (branch DNA technology) and provides a novel approach for gene expression analysis and quantification (Yang et al. 2006) by analyzing the reporter genes rather than target sequences (Urdea et al. 1991). There are several advantages associated with the measurement of gene signals directly from FFPE tissue homogenates, including avoidance of variations or errors inherent to RNA extraction and amplification of target sequences. In addition, the sensitivity of branched DNA assay in tissue homogenates has been reported to be 10-fold higher than that of purified RNA (Knudsen et al. 2008).
Preparation of FFPE tissue homogenates
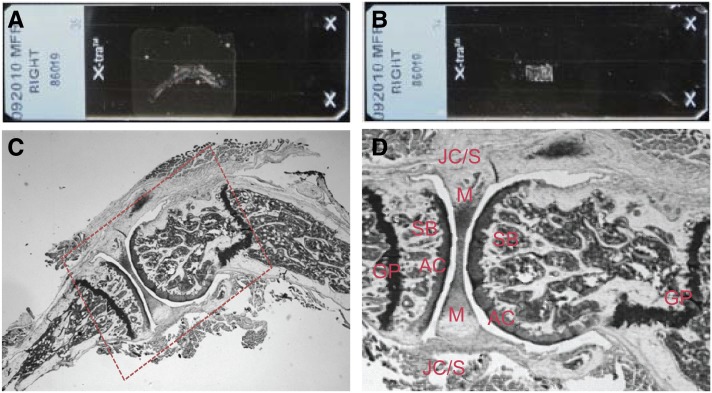
The area of interest was selectively macrodissected from FFPE sections to collect the composite tissues (subchondral bone, cartilage, meniscus, growth plate, joint capsule, and synovium) (Figure 1). Tissue homogenates were prepared according to the procedure described in Affymetrix QuantiGene Plex sample processing kit for FFPE tissues (Panomics Inc.). All the surfaces and instruments were treated with RNaseZAP (Sigma Aldrich, St. Louis, MO). The excess paraffin was scraped off, along with any tissues around the area of interest. The area of interest on the 5-μm slide section was selectively macrodissected using a clean razor blade and transferred to a 1.5-ml microfuge tube. These crude tissues were incubated at 65° for 6 hr with 600 μl homogenizing solution and 6 μl proteinase K (50 μg/ml). To achieve complete dissociation of the tissues, samples were vortexed several times at 60-min intervals at maximum speed during incubation. The tissue homogenate was separated from debris and paraffin by centrifugation at 14,000 rpm for 5 min at room temperature. The residual paraffin appeared as a solid residuum above the homogenate and debris pelleted at the bottom of the tube. The solid paraffin layer was pierced with a pipette tip to transfer the clear homogenate to a fresh microfuge tube, avoiding contamination with residual paraffin or tissue debris. The resulting homogenate was stored at −80° until being used for QuantiGene Plex and QuantiGene miRNA assays.
Figure 1.
Histological landmarks for tissue collection. The archived FFPE sections were selected from each strain, the surface around the sections was treated with RNaseZAP, and the tibiofemoral joint was visually selected (A, C; dotted area). The excess paraffin was removed around the joint tissues (B, D) and the remaining tissues of interest were collected from 25 sections in a microfuge tube. GP, growth plate; SB, subchondral bone; AC, articular cartilage; M, meniscus; JC/S, joint capsule/synovium.
Selection of candidate genes and miRNAs
Sixty-seven mRNAs (Table S3) and six miRNAs (Table S4) were selected for analysis. Individual bead-based oligonucleotide probe sets specific for each mouse target gene were developed by Panomics Inc. using previously published NCBI (National Center for Biotechnology Information) gene accession numbers. The selection of these genes was based on their known role in tissue healing, cartilage homeostasis, joint development, and osteoarthritis. Three reference genes (Gapdh, Hprt1, and Actb) were used to normalize target gene expression. Two panels of genes were submitted for QuantiGene Plex assay, panel 321347 and panel 321389 (http://www.panomics.com).
Quantification of mRNA expression
The quantification of selected mRNAs in tissue lysates was performed in biological and technical replicates using the Affymetrix QuantiGene 2.0 Plex Assay kit (Panomics Inc.). The assays were performed as described previously with the exception that previously we used RNA instead of tissue lysates (Rai et al. 2013). An appropriate volume of working bead mix was prepared by combining the following reagents per well in a microfuge tube and scaled for 96-well plate format: 18.5 μl nuclease-free water; 33.3 μl lysis mixture; 2 μl blocking reagent; 0.2 μl proteinase K; 1 μl capture beads; and 5 μl probe set. The working bead mix was vortexed for 10 sec and 60 μl of it was dispensed into each well of the hybridization plate, followed by addition of 40 μl of tissue homogenate to each well. The plate was sealed using a pressure seal and placed on an inverted plate lid placed onto the VorTemp 56 shaking incubator and incubated for 18−22 hr at 54° while shaking at 600 rpm. Postincubation, the contents of the hybridization plate were transferred to a magnetic separation plate after centrifugation at 240g for 60 sec and mixing the contents a few times with a pipette. The magnetic separation plate was inserted into the hand-held magnetic plate washer and the plate was washed three times using 100 μl wash buffer (wash buffer components 1 and 2 in nuclease-free water) per well. Then, 100 μl preamplifier working reagent (36 μl 2.0 preamplifier in 12 ml amplifier diluent) was dispensed into each well of the magnetic separation plate, sealed, and incubated at 50° for 1 hr while shaking at 600 rpm. After incubation, the plate was washed as described, 100 μl amplifier working reagent (36 μL 2.0 amplifier in 12 ml amplifier diluent) was added per well, and incubation was again performed at 50° for 60 min at 600 rpm. Then, the plate was washed again, 100 μL label probe working reagent (36 μl label probe in 12 ml label probe diluent) was added to each well, and the sealed plate was placed into VorTemp and incubated at 50° for 60 min while shaking at 600 rpm. After 60 min, the washing procedure was repeated as described and 100 μl SAPE (Streptavidin conjugated Phycoerythrin) working reagent (36 μl SAPE to 12 ml SAPE diluent) was pipetted into each well. The plate was foiled, removed from the plate washer, and placed on a shaking platform at room temperature while shaking at 800 rpm for 60 sec, followed by shaking at 600 rpm for 30 min. The plate was washed with 130 μl SAPE wash buffer in each assay well and the plate was sealed, removed from the plate washer, wrapped in aluminum foil, and allowed to shake at 800 rpm for 3 min at room temperature. Finally, the plate was read on Luminex set for specified bead regions with the following parameters: 100-μl sample size; 5000–25,000 DD gate; 45-sec timeout; and 100 bead events per bead region. The average signal values were noted, subtracted from the background signal, and finally normalized to housekeeping genes.
Quantification of miRNA expression
Selected miRNAs were quantified using QuantiGene 2.0 miRNA assay kit (Panomics Inc.) as detailed. Working probe sets for each reaction were prepared by combining the following reagents in a microfuge tube: 25.1 μl nuclease-free water; 33.3 μl lysis mixture; 1 μl blocking reagent; 0.3 μl capture extender; and 0.3 μl label extender. The reaction mixture (60 μl/well) was pipetted into each assay well of the capture plate with conjugated oligonucleotides to the surface of the well. Then, 40 μl sample was dispensed to each assay well, avoiding introduction of bubbles, and 40 μl homogenizing solution was added to the assay background controls. The plate was sealed with adhesive seal and centrifuged at 240g for 20 sec at room temperature to ensure the contents would contact the bottom of the well. Finally, the plate was incubated at 46° for 16–20 hr for hybridization. The next day, the plate was first washed three times using 200–300 μl/well of wash buffer (wash components 1 and 2 in distilled water), and then the inverted plate was centrifuged at 240g for 60 sec to remove traces of wash buffer. A total of 100 μl/well of freshly prepared PreAmp working reagent (11 μl 2.0 PreAmp in 11 ml amplifier/label diluent) were added to each well of the plate. The plate was sealed and set for PreAmp hybridization at 46° for 60 min. After the incubation period, the plate was washed and centrifuged as described. To each well of the plate, 100 μl Amp working reagent (11 μl 2.0 Amp in 11 ml amplifier/label probe) was added, sealed, and again incubated at 46° for 60 min for Amp hybridization. After the incubation period and washing steps, 100 μl/well label probe was dispensed and kept for hybridization at 46° for 60 min. Finally, the plate was washed after incubation and 100 μl substrate was added to each well of the capture plate, which was sealed and incubated at room temperature for 5 min. The plate was read on a luminometer after removing the seal within 15 min. Average signal from the three replicates was calculated and average background signals were deducted from samples, and the data were normalized to Snord68.
Statistical analyses
All statistical analyses were performed using Systat-12 software (Systat Software Inc., Chicago, IL).
Gene expression measurements:
The raw values of mRNA expression levels were normalized using Gapdh, Hprt1, and Actb, whereas those of miRNAs were normalized with Snord68 as the independent variables in a multiple regression for each of the target genes. The residuals from this regression were obtained for each gene and were used to calculate an average for each individual. The more conventional ΔΔCt method measures differential expression of samples relative to a single reference sample and then compares candidate genes to control genes. However, restating measurements of candidate genes relative to control genes as ratios results in amplification of measurement errors and introduces an artifactual correlation between the ratio plotted on the Y-axis and its denominator plotted on the X-axis.
Correlation of gene expression with phenotypic traits:
Pearson correlation was used for examining the associations between gene expression (mRNA or miRNA) values with phenotypic (articular cartilage regeneration or ear wound healing) scores.
Heritability of gene expression:
We used ANOVA to test for significant strain differences in gene expression:
with “Sex” as a fixed effect and “Strain” as a random effect. Sex was removed from the model because there were no significant sex differences. Post hoc pairwise significance tests comparing specific mouse strain pairs were performed using Tukey's honestly significant difference test to control for multiple comparisons.
Because these are fully inbred mouse strains, the broad-sense heritability (H2) of the traits was calculated using the following equation:
where the variance among strains is divided by the sum of the between-strain and within-strain variances. This includes all sources of genetic variation between mouse strains. Standard errors for heritabilities were calculated using the intraclass correlation (Falconer and Mackay 1996) and were used to generate one-sided 95% confidence intervals (Table 1).
Table 1. Heritability and correlations of mRNAs with ear and articular cartilage healing phenotypes.
| Gene Symbol | Heritability |
Correlation with Ear Wound |
Correlation with Articular Cartilage |
Gene Symbol | Heritability |
Correlation with Ear Wound |
Correlation with Articular Cartilage |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vb | Vw | Vt | H2 | 95% C.I. (One-Tailed) | P | r | P | R | P | Vb | Vw | Vt | H2 | 95% C.I. (One-Tailed) | P | r | P | r | P | ||
| Acan | 0.013 | 0.046 | 0.059 | 0.219 | 0.598 | 0.126 | 0.414 | 0.175 | 0.488 | 0.139 | Lef1 | 0.000 | 0.009 | 0.009 | −0.003 | 0.325 | 0.466 | 0.185 | 0.333 | 0.375 | 0.197 |
| Adamts4 | 0.001 | 0.007 | 0.008 | 0.099 | 0.457 | 0.282 | 0.018 | 0.483 | −0.169 | 0.346 | Lep | 0.000 | 0.010 | 0.010 | −0.009 | 0.325 | 0.481 | −0.661 | 0.078 | −0.694 | 0.070 |
| Adamts5 | 0.003 | 0.007 | 0.010 | 0.279 | 0.661 | 0.078 | 0.163 | 0.352 | 0.110 | 0.398 | Map1lc3a | 0.001 | 0.003 | 0.004 | 0.251 | 0.632 | 0.082 | −0.770 | 0.054 | −0.636 | 0.085 |
| Adipoq | 0.012 | 0.021 | 0.033 | 0.375 | 0.750 | 0.022 | −0.530 | 0.121 | −0.714 | 0.066 | Mia1 | 0.016 | 0.012 | 0.028 | 0.575 | 0.892 | 0.002 | 0.407 | 0.179 | 0.492 | 0.137 |
| Apln | −0.001 | 0.006 | 0.005 | −0.118 | 0.325 | 0.725 | 0.148 | 0.364 | 0.155 | 0.359 | Mmp13 | 0.013 | 0.017 | 0.030 | 0.446 | 0.964 | 0.013 | 0.570 | 0.106 | 0.562 | 0.109 |
| Atg7 | 0.016 | 0.003 | 0.020 | 0.824 | 0.987 | <0.001 | −0.223 | 0.302 | −0.196 | 0.324 | Mmp1a | 0.000 | 0.007 | 0.006 | −0.013 | 0.807 | 0.489 | −0.371 | 0.199 | −0.353 | 0.210 |
| Axin2 | 0.003 | 0.008 | 0.011 | 0.258 | 0.639 | 0.077 | 0.802 | 0.049 | 0.814 | 0.047 | Mmp3 | 0.028 | 0.091 | 0.119 | 0.234 | 0.325 | 0.113 | 0.444 | 0.159 | 0.294 | 0.249 |
| Bcl3 | 0.007 | 0.011 | 0.018 | 0.377 | 0.751 | 0.030 | −0.353 | 0.210 | −0.566 | 0.107 | Mmp9 | 0.020 | 0.007 | 0.027 | 0.737 | 0.614 | <0.001 | −0.157 | 0.357 | −0.039 | 0.463 |
| Becn1 | 0.002 | 0.004 | 0.006 | 0.356 | 0.733 | 0.028 | −0.127 | 0.383 | −0.033 | 0.469 | Nampt | 0.000 | 0.002 | 0.002 | −0.114 | 0.325 | 0.716 | 0.335 | 0.222 | 0.108 | 0.400 |
| Bglap1 | 0.027 | 0.016 | 0.043 | 0.636 | 0.924 | <0.001 | −0.127 | 0.383 | −0.033 | 0.469 | Nfkb1 | 0.001 | 0.002 | 0.002 | 0.256 | 0.637 | 0.094 | −0.446 | 0.158 | −0.535 | 0.119 |
| Bmp6 | 0.000 | 0.003 | 0.003 | −0.133 | 0.325 | 0.758 | −0.195 | 0.325 | −0.114 | 0.395 | Pcna | 0.002 | 0.007 | 0.009 | 0.209 | 0.587 | 0.119 | 0.893 | 0.036 | 0.925 | 0.032 |
| Casp3 | 0.003 | 0.004 | 0.008 | 0.441 | 0.803 | 0.014 | 0.712 | 0.066 | 0.740 | 0.060 | Polq | 0.008 | 0.008 | 0.016 | 0.477 | 0.830 | 0.005 | 0.104 | 0.404 | 0.126 | 0.384 |
| Ccl3 | 0.003 | 0.065 | 0.068 | 0.044 | 0.386 | 0.378 | −0.335 | 0.222 | −0.285 | 0.256 | Pthlh | −0.001 | 0.013 | 0.012 | −0.077 | 0.325 | 0.634 | 0.250 | 0.281 | 0.424 | 0.170 |
| Cdkn1a | −0.004 | 0.016 | 0.012 | −0.318 | 0.325 | 0.991 | −0.294 | 0.250 | −0.173 | 0.343 | Retn | 0.000 | 0.004 | 0.003 | −0.144 | 0.325 | 0.779 | −0.325 | 0.228 | −0.450 | 0.156 |
| Cdkn2a | 0.000 | 0.009 | 0.009 | 0.019 | 0.352 | 0.419 | 0.398 | 0.184 | 0.383 | 0.192 | Runx2 | 0.003 | 0.004 | 0.007 | 0.412 | 0.780 | 0.020 | 0.654 | 0.080 | 0.714 | 0.066 |
| Cebpb | 0.002 | 0.015 | 0.017 | 0.138 | 0.505 | 0.222 | −0.673 | 0.075 | −0.786 | 0.051 | Smad1 | 0.002 | 0.006 | 0.008 | 0.217 | 0.596 | 0.111 | 0.411 | 0.177 | 0.416 | 0.174 |
| Chrdl1 | 0.000 | 0.006 | 0.007 | 0.068 | 0.417 | 0.321 | 0.417 | 0.173 | 0.385 | 0.191 | Smad2 | 0.000 | 0.002 | 0.002 | 0.131 | 0.497 | 0.215 | 0.155 | 0.359 | 0.120 | 0.389 |
| Col10a1 | 0.004 | 0.080 | 0.084 | 0.043 | 0.384 | 0.380 | 0.014 | 0.487 | 0.218 | 0.306 | Smad4 | 0.000 | 0.002 | 0.002 | 0.095 | 0.452 | 0.272 | 0.375 | 0.197 | 0.449 | 0.157 |
| Col1a1 | 0.000 | 0.009 | 0.009 | −0.044 | 0.325 | 0.553 | −0.342 | 0.217 | −0.208 | 0.314 | Sox5 | 0.001 | 0.013 | 0.014 | 0.058 | 0.404 | 0.339 | 0.635 | 0.085 | 0.683 | 0.073 |
| Col2a1 | 0.019 | 0.044 | 0.062 | 0.299 | 0.680 | 0.065 | 0.358 | 0.207 | 0.417 | 0.173 | Sox9 | −0.001 | 0.027 | 0.026 | −0.036 | 0.325 | 0.536 | 0.204 | 0.318 | 0.271 | 0.266 |
| Col6a6 | −0.001 | 0.020 | 0.018 | −0.067 | 0.325 | 0.601 | 0.380 | 0.194 | 0.269 | 0.267 | Stat3 | 0.000 | 0.003 | 0.003 | 0.129 | 0.494 | 0.235 | 0.044 | 0.459 | −0.152 | 0.361 |
| Comp | 0.004 | 0.042 | 0.045 | 0.078 | 0.430 | 0.317 | 0.255 | 0.278 | 0.317 | 0.234 | Stat6 | 0.000 | 0.004 | 0.004 | 0.075 | 0.427 | 0.323 | 0.362 | 0.204 | 0.389 | 0.189 |
| Csnk2a1 | 0.000 | 0.002 | 0.002 | 0.046 | 0.388 | 0.364 | −0.586 | 0.101 | −0.473 | 0.145 | Tfap2a | 0.002 | 0.007 | 0.010 | 0.250 | 0.631 | 0.099 | 0.112 | 0.396 | 0.075 | 0.431 |
| Cxcl12 | 0.000 | 0.003 | 0.004 | 0.112 | 0.474 | 0.261 | 0.574 | 0.105 | 0.580 | 0.103 | Tgfb1 | 0.002 | 0.003 | 0.005 | 0.437 | 0.800 | 0.015 | −0.416 | 0.174 | −0.466 | 0.148 |
| Ddr2 | 0.005 | 0.012 | 0.017 | 0.293 | 0.674 | 0.068 | 0.124 | 0.386 | 0.157 | 0.357 | Tgfbr1 | 0.001 | 0.003 | 0.003 | 0.209 | 0.587 | 0.137 | −0.199 | 0.322 | −0.196 | 0.324 |
| Epas1 | 0.001 | 0.002 | 0.004 | 0.375 | 0.750 | 0.031 | −0.067 | 0.438 | −0.063 | 0.441 | Trp53 | 0.000 | 0.003 | 0.003 | 0.123 | 0.487 | 0.226 | −0.103 | 0.404 | −0.338 | 0.220 |
| Fancc | 0.000 | 0.003 | 0.003 | −0.036 | 0.325 | 0.541 | 0.641 | 0.084 | 0.689 | 0.071 | Ulk1 | 0.003 | 0.003 | 0.006 | 0.475 | 0.828 | 0.006 | 0.643 | 0.083 | 0.773 | 0.054 |
| Fgf18 | 0.015 | 0.023 | 0.039 | 0.396 | 0.767 | 0.024 | −0.196 | 0.324 | −0.265 | 0.270 | Vegfa | 0.001 | 0.006 | 0.007 | 0.132 | 0.498 | 0.214 | −0.141 | 0.370 | −0.128 | 0.383 |
| Fzd2 | 0.000 | 0.005 | 0.004 | −0.101 | 0.325 | 0.687 | 0.561 | 0.109 | 0.643 | 0.083 | Wisp1 | 0.008 | 0.011 | 0.019 | 0.433 | 0.797 | 0.010 | 0.320 | 0.232 | 0.356 | 0.208 |
| Gdf5 | 0.008 | 0.024 | 0.032 | 0.247 | 0.628 | 0.102 | −0.112 | 0.397 | −0.198 | 0.322 | Wnt16 | −0.002 | 0.019 | 0.016 | −0.138 | 0.325 | 0.743 | 0.776 | 0.053 | 0.868 | 0.039 |
| Igf1 | 0.017 | 0.017 | 0.034 | 0.505 | 0.849 | 0.006 | −0.184 | 0.334 | −0.215 | 0.308 | Wnt3a | 0.000 | 0.008 | 0.008 | 0.059 | 0.406 | 0.349 | 0.052 | 0.451 | −0.196 | 0.324 |
| Ihh | 0.011 | 0.010 | 0.022 | 0.529 | 0.864 | 0.002 | 0.325 | 0.228 | 0.448 | 0.157 | Xpa | −0.001 | 0.004 | 0.003 | −0.171 | 0.325 | 0.832 | 0.714 | 0.065 | 0.526 | 0.123 |
| Il4 | 0.002 | 0.005 | 0.007 | 0.247 | 0.628 | 0.102 | 0.090 | 0.416 | −0.085 | 0.421 | Xrcc2 | 0.006 | 0.007 | 0.013 | 0.493 | 0.841 | 0.004 | 0.894 | 0.035 | 0.818 | 0.046 |
| Il6 | −0.001 | 0.009 | 0.008 | −0.087 | 0.325 | 0.643 | 0.820 | 0.046 | 0.755 | 0.057 | |||||||||||
Vb, between-strain variation; Vw, within-strain variation; Vt, total phenotypic variation; H2 = heritability; C.I., confidence interval. Bold values indicate significance at the 5% level.
Single nucleotide polymorphisms analysis
Utilizing whole-genome sequences for LG/J at 21-times coverage and SM/J at 14-times coverage (H. A. Lawson, I. Nikolskiy, S. Chun, M. McLellan, J. Fay, E. Mardis, J. M. Cheverud, unpublished data), we evaluated candidate genes for single nucleotide polymorphisms (SNPs). For each gene, SNPs were identified in the 5′ and 3′ UTRs, exons, introns, and the regions 2500 base pairs upstream and downstream of the UTRs. The sequences of genic elements were according to the UCSC genome browser annotations, NCBI37/mm9 assembly (http://genome.ucsc.edu/). Conservation scores for each SNP were obtained from the PhastCons30Placental table of the UCSC browser. Nonsynonymous SNPs with a conservation score of 0.90 or greater were evaluated for potential functional significance using the algorithms PolyPhen-2 (Adzhubei et al. 2010, 2013), SIFT (Kumar et al. 2009), and LRT (Chun and Fay 2009). PolyPhen-2 uses sequence-based predictive features in light of tertiary structures of proteins to predict the functional significance of nonsynonymous amino acid substitutions. SIFT makes use of sequence homology and median evolutionary conservation scores to predict conserved protein function. LRT is a likelihood ratio test comparing the probability that a codon has evolved under negative selection to a model in which the codon has evolved neutrally, where rates of synonymous and nonsynonymous substitutions are equal. Genetic maps with the locations of SNPs and microsatellite markers are available for the RI lines (Hrbek et al. 2006). For each gene, the genotype in each RI line was inferred by finding the genotypes of the closest set of flanking markers in the appropriate RI line genetic map. When a gene was between proximal and distal RI map markers that differ in genotype, a genotype was not assigned.
Fold-change differences in gene expression and cluster analysis
We used t test and probabilities of obtaining the observed results given the null hypothesis of less than 5% is accepted as statistically significant. The fold-changes in gene expression were calculated using the least square means for each strain against C57BL/6J and heat maps were generated to visualize the magnitude of fold-change variations. We used Ward minimum variance method (cluster analysis) to determine strain inter-relationships of gene expression correlation strengths.
Results
Findings of the broad-sense heritabilities
H2 showed that the expression levels of 19 mRNAs (Table 1) and six miRNAs (Table 2) were significantly heritable, indicating that the variation in gene expression corresponded to genetic differences among strains. The heritabilities of mRNAs ranged from 35.6% to 82.4%, whereas those of miRNAs ranged from 60.0% to 83.9%. There were six genes (Igf1, Ihh, Mia1, Bglap, Mmp9, and Atg7) that showed a statistically significant heritability of 50% or more. The highest heritability was found to be for Atg7 (82.4%; P < 0.001), followed by Mmp9 (73.3%; P < 0.001) and Bglap1 (63.6%; P < 0.001). For miRNAs, the heritability was more than 50%, with miR-17 being the most significantly heritable (H2 = 83.9%; P < 0.001). Among the rest, miR-224 (H2 = 81.8%; P < 0.001), miR-146b–5p (H2 = 80.4%; P < 0.001), miR-27b–3p (H2 = 64.0%; P = 0.001), miR-675–5p (H2 = 62.5%; P = 0.001), and miR-140–5p (H2 = 60.0%; P = 0.002) were also significantly heritable.
Table 2. Heritability and correlations of miRNAs with ear and articular cartilage healing phenotypes.
| miRNA | Heritability |
Correlation with Ear Wound |
Correlation with Articular Cartilage |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vb | Vw | Vt | H2 | 95% C.I. (One-Tailed) | P | r | P | r | P | |
| miR-17 | 0.41 | 0.08 | 0.49 | 0.84 | 0.990 | <0.001 | 0.083 | 0.422 | −0.128 | 0.382 |
| miR-27b-3p | 0.49 | 0.28 | 0.77 | 0.64 | 0.926 | 0.001 | 0.258 | 0.276 | −0.288 | 0.253 |
| miR-140-5p | 0.62 | 0.41 | 1.03 | 0.6 | 0.906 | 0.002 | 0.016 | 0.485 | −0.169 | 0.347 |
| miR-146b-5p | 0.37 | 0.09 | 0.45 | 0.8 | 0.983 | <0.001 | 0.197 | 0.324 | −0.148 | 0.364 |
| miR-224 | 0.41 | 0.09 | 0.5 | 0.82 | 0.986 | <0.001 | −0.013 | 0.487 | −0.147 | 0.366 |
| miR-675 | 0.3 | 0.18 | 0.48 | 0.63 | 0.918 | 0.001 | 0.601 | 0.096 | −0.580 | 0.102 |
miRNA, microRNA; Vb, between-strain variation; Vw, within-strain variation; Vt, total phenotypic variation; H2, heritability; C.I., confidence interval. Bold values indicate significance at the 5% level.
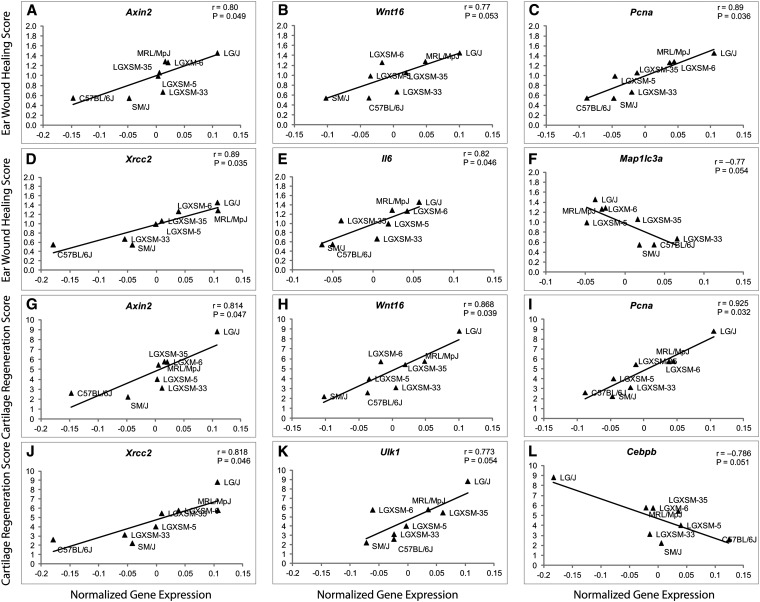
Findings of the association between gene expression signatures and healing scores
Ear wound and cartilage healing scores (Y-axis of respective graphs) were plotted against gene expression levels (X-axis of each graph) (Figure 2) to determine significance of correlation. Six gene expression traits were significantly correlated with both ear wound and articular cartilage phenotypes, indicating that regulation of these genes plausibly orchestrates tissue healing. The genes that were strongly correlated with ear wound healing were Xrcc2 (r = 0.894; P = 0.035), Pcna (r =0.893; P = 0.036), Axin2 (r = 0.802; P = 0.049), Wnt16 (r = 0.776; P = 0.053), Il6 (r = 0.820; P = 0.046), and Map1lc3a (r = −0.770; P = 0.054). The genes that were most strongly associated with articular cartilage regeneration were Pcna (r = 0.925; P = 0.032), followed by Wnt16 (r = 0.868; P = 0.039), Xrcc2 (r = 0.818; P = 0.046), Axin2 (r = 0.814; P = 0.047), Cebpb (r = −0.786; P = 0.051), and Ulk1 (r = 0.773; P = 0.054). Interestingly, four of these genes were common to both ear wound healing and articular cartilage regeneration (Xrcc2, Pcna, Axin2, and Wnt16). Il6 and Map1lc3a correlated with ear wound healing and correlated in the same direction with articular cartilage regeneration with borderline significance (P = 0.057 and P = 0.085, respectively). Likewise, the Cebpb and Ulk1, which were significantly correlated with articular cartilage regeneration, were also correlated with ear wound healing with borderline significance (P = 0.083 and P = 0.075, respectively). In addition, there were several other genes that were correlated with both phenotypes at a probability of 10%, including Adipoq, Casp3, Mmp13, Runx2, Csnk2a1, Cxcl12, Fancc, Fzd2, and Sox5. The association of gene expression with each phenotype and levels of significance are presented in Table 1. None of the miRNAs was significantly associated with healing (Table 2).
Figure 2.
Genes significantly correlated with ear and articular cartilage healing phenotypes. Knee joint tissues from FFPE sections were macrodissected and tissue homogenates were prepared. The gene expression levels were quantified through QuantiGene Plex assay and were correlated with ear and articular cartilage healing scores. Five genes, namely Axin2, Wnt16, Pcna, Xrcc2, and Il6, were found to be significantly positively correlated with ear wound healing, whereas Map1lc3a was significantly negatively correlated with ear wound healing (A–F). Similarly, five genes, namely Axin2, Wnt16, Pcna, Xrcc2, and Ulk1, were found to be significantly positively correlated, whereas Cebpb was significantly negatively correlated with articular cartilage healing (G–L). r = Pearson correlation coefficient.
Notably, of the majority of genes most strongly correlated with both phenotypes, the heritability estimates are nonsignificant (Table 1). However, given the relatively small number of strains assessed, the standard error for an estimate of 0% heritability approaches 30%, so that heritability estimates must be greater than 50% to be significant.
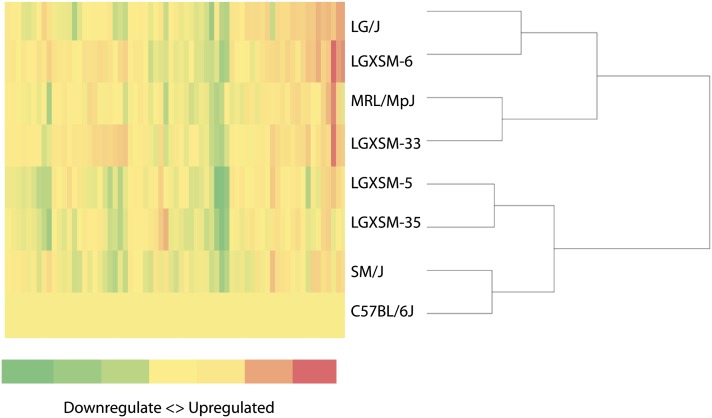
Findings of the inter-relation of strains based on gene expression signatures
The most interesting finding from the cluster analysis was the classification of strains into two distinct clusters largely (but not exclusively) corresponding to their healing ability (Rai et al. 2012) with 100% bootstrap support (1000 iterations) (Figure 3). One cluster represented nonhealers and moderate healers, including SM/J, C57BL/6J, LGXSM-5, and LGXSM-35, whereas the other cluster represented predominately classical healers LG/J, LGXSM-6, and MRL/MpJ. Surprisingly, strain LGXSM-33, a poor healer, was clustered with MRL/MpJ, a super-healer.
Figure 3.
Cluster analysis of normalized gene expression levels. Knee joint tissues from FFPE sections were macrodissected and tissue homogenates were prepared to quantify candidate genes through QuantiGene Plex assay. The gene expression levels were used to calculate fold difference among strains compared to C57BL/6J (a nonhealer control strain) as shown in a seven-tiered categorical scale. Hierarchical clustering analysis was performed using Ward minimum variance method to produce a dendrogram. As shown here, the healer strains (except for LGXSM-33) clustered together exclusive of other nonhealers and intermediate healers, indicating an association between gene expression levels and healing potential.
Findings of the SNPs analysis
We assessed composition and distribution of SNPs in the genes showing correlation at a significant (P < 0.05) or marginally significant (P < 0.1) level (Table 3). We found a few SNPs occupying evolutionary conserved positions between alleles of LG/J and SM/J parental lines as discussed.
Table 3. SNPs in genes correlated with ear and articular cartilage healing phenotypes.
| Gene Symbol | Chromosome | Genotype |
Total SNPs | Highly Conserved Site | Total Noncoding | Highly Conserved Site | Noncoding DNA |
Coding DNA |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LGXSM-6 | LGXSM-35 | LGXSM-5 | LGXSM-33 | Upstream 2500 bp | Highly Conserved Site | 5′ UTR | Highly Conserved Site | Intronic SNPs | Highly Conserved Site | 3′ UTR | Highly Conserved Site | Downstream 2500 bp | Highly Conserved Site | Exonic SNPs | Highly Conserved Site | Synonymous | Nonsynonymous | Significancea | Position | Exon | Substitution | ||||||
| Axin2 | 11 | SS | SS | LL | SS | 37 | 4 | 32 | 1 | 1 | 0 | 0 | 0 | 30 | 1 | 1 | 0 | 0 | 0 | 5 | 3 | 3 | 2 | Np; PolyPhen-2 | 108,805,010; 108,803,724 | 5; 7 | Arg:648:Cys; Tyr:474:His |
| Wnt16 | 6 | LL | LL | ?b | SS | 81 | 5 | 79 | 5 | 5 | 0 | 3 | 1 | 47 | 3 | 3 | 0 | 21 | 1 | 2 | 0 | 2 | 0 | Np | — | — | — |
| Pcna | 2 | LL | SS | ? | SS | 6 | 1 | 5 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 1 | 0 | Np | — | — | — |
| Xrcc2 | 5 | LL | LL | LL | SS | 100 | 4 | 98 | 2 | 30 | 0 | 0 | 0 | 59 | 2 | 2 | 0 | 7 | 0 | 2 | 2 | 1 | 1 | Np | 25,204,113 | 2 | Gly:26:Ala |
| Il6 | 5 | LL | LL | LL | SS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | — | — | — | — |
| Cebpb | 2 | ? | LL | LL | SS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | —c | — | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | — | — | — | — |
| Map1lc3a | 2 | LL | LL | LL | ? | 32 | 1 | 32 | 1 | 16 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 14 | 1 | 0 | 0 | 0 | 0 | — | — | — | — |
| Ulk1 | 5 | LL | SS | SS | LL | 4 | 0 | 3 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | Np | — | — | — |
| Adipoq | 16 | LL | SS | LL | ? | 23 | 0 | 23 | 0 | 4 | 0 | 0 | 0 | 12 | 0 | 2 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | — | — | — | — |
| Casp3 | 8 | LL | ? | LL | LL | 59 | 0 | 59 | 0 | 0 | 0 | 0 | 0 | 58 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | — | — | — | — |
| Csnk2a1 | 2 | LL | ? | LL | SS | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | — | — | — | — |
| Cxcl12 | 6 | LL | SS | LL | SS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | — | — | — | — |
| Fancc | 13 | SS | LL | LL | LS | 23 | 0 | 23 | 0 | 1 | 0 | 0 | 0 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | — | — | — | — |
| Fzd2 | 11 | SS | LL | ? | SS | 13 | 2 | 13 | 2 | 5 | 1 | 1 | 1 | –c | — | 1 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | — | — | — | — |
| Mmp13 | 9 | LL | SS | LL | LL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | — | — | — | — |
| Runx2 | 17 | SS | LL | SS | LL | 578 | 15 | 578 | 15 | 0 | 0 | 0 | 0 | 578 | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | — | — | — | — |
| Sox5 | 6 | LL | LL | SS | LL | 3,226 | 115 | 3,221 | 111 | 10 | 3 | 1 | 0 | 3,175 | 104 | 21 | 4 | 14 | 0 | 5 | 4 | 5 | 0 | Np | 143,783,835 | 14 | — |
Np, no prediction.
Predicted significance rated by PolyPhen-2, SIFT, and LRT.
Genotype unassignable.
Single exon.
Discussion
This study combines molecular, biological, and genetic approaches to gain insight into genes and physiological pathways involved in the tissue healing, including knee cartilage. The most intriguing finding is that four genes were significantly correlated to both ear wound and articular cartilage healing. These genes represented two important functional classifications: DNA repair (Pcna, Xrcc2) and Wnt signaling (Wnt16, Axin2).
The genes involved in DNA repair serve a key function during G2 phase of the cell cycle, including that of chondrocytes (Kim et al. 2010). Both Pcna and Xrcc2 play key roles in maintaining chromosome stability, homologous recombination, and cell proliferation at wound sites (Matullo et al. 2005). Both Pcna and Xrcc2 have also been implicated in DNA repair deficits in certain cancers (Matullo et al. 2005; Moreno et al. 2006; Zienolddiny et al. 2006). It is plausible that higher expression of these genes in healing strains indicates expedited proliferation of cells (Tchetina 2011), implicating an elevated repair response. Comparing sequence differences between LG/J and SM/J alleles, we noted that Xrcc2 contains a nonsynonymous change in exon 2 Gly:26:Ala at a highly conserved position, implicating this particular SNP as a functional variant relevant to healing in the LGXSM intercross. In contrast, Pcna harbors six SNPs, with only one exonic SNP sitting in a highly conserved site where it produces a synonymous amino acid substitution. Identification of likely functional SNP candidates within Pcna will require additional criteria beyond that used here.
The other genes significantly correlated with both phenotypes represented the Wnt signaling pathway. Canonical Wnt signaling is important for embryogenesis, bone metabolism (Baron and Rawadi 2007), and tissue regeneration (Stoick-Cooper et al. 2007). Wnt/β-catenin signaling regulates progenitor cell fate and proliferation through embryonic development and stem cell functions (Logan and Nusse 2004). An upregulation of Wnt16 has been observed in osteoarthritis (Dell’accio et al. 2008; Velasco et al. 2010) and injured cartilage (Dell’accio et al. 2008). Axin2, a known inhibitor of the Wnt signaling pathway (Yu et al. 2005), suppresses β-catenin and bone remodeling. Knockout of Axin2 has been shown to result in an age-dependent high bone mass phenotype (Yan et al. 2009). In addition, Axin2 is expressed in developing cartilage and plays an important role in regulating chondrocyte maturation and axial skeletal development (Dao et al. 2010). Upregulation of Axin2 in healers in our study may indicate a differential response of progenitor cells in the bone marrow toward the regenerative process. SNP analysis revealed a SNP occupying a highly conserved position immediately downstream of the Wnt16 3′ UTR, and the 5′ UTR contains one highly conserved SNP. Two nonconserved exonic SNPs yield synonymous substitutions in the resulting proteins. The two Axin2 alleles carried in our RI lines contain 37 SNPs. Out of 30 intronic SNPs, one is highly conserved; however, out of five exonic SNPs, three are highly conserved. A nonsynonymous exonic SNP sits in exon 7 (Arg:648:Cys) and occupies a poorly conserved site. One other exonic SNP produces a nonsynonymous substitution in exon 5 (Tyr:474:His), which is predicted as potentially damaging by the PolyPhen-2 algorithm.
Additionally, we identified four genes correlating with both healing phenotypes, albeit strongly with one yet weakly with the other (Figure 2, Table 1). Expression of Ulk1 and Cebpb correlated significantly positively with cartilage healing, but at a marginal level of significance with ear wound healing. Il6 and Map1lc3a were significantly correlated with ear wound healing in both directions, respectively, yet marginally so with cartilage healing in the positive direction. Ulk1 is an important regulator of autophagy (Chan et al. 2007) and plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation (Kundu et al. 2008). Another important marker and effector of autophagy is Map1lc3a (Choi et al. 2012). Autophagy genes are repressed in cartilage after aging, injury, osteoarthritis, or mechanical loading (Carames et al. 2010, 2012). The upregulation of autophagy genes, especially Ulk1, might be associated with increased cell turnover in cartilage and protection from osteoarthritis.
Il6 is an important player in the development of chronic joint inflammation and is thought to be involved in osteoarthritis-associated joint inflammation (Ohshima et al. 1998; de Hooge et al. 2000). It has been reported that Il6 expression can be regulated by Cebpb (Natsuka et al. 1992). Our laboratory has previously shown that Cebpb is associated with cytokine-induced downregulation of extracellular matrix genes in chondrocytes and the repression of cartilage gene expression in noncartilaginous tissues (Okazaki et al. 2002, 2006), and is associated with upregulation of chemokines (Zhang et al. 2010) and MMP-13 (Okazaki et al. 2002). These results indicate a more plausible positive role of Il6 in wound healing than in cartilage regeneration. Conversely, the role of Cebpb appears to be more specific in cartilage repair and osteoarthritis. LG/J and SM/J alleles for both Il6 and Cebpb are identical in their sequences, implying that variations beyond the immediate vicinity, including possible trans-polymorphisms, are actually responsible for their variation in expression level among strains. In our RI panel, Ulk1 harbors four nonconserved SNPs: one synonymous exonic; one intronic; and two within the first 2500 base pairs upstream of the 5′ UTR. Map1lc3a harbors 32 SNPs, most of which occur upstream (16 SNPs) and downstream (14 SNPs) of its 5′ UTR and 3′ UTR, respectively. One of the 14 SNPs located downstream of the 3′ UTR is highly conserved. Map1lc3a has a single nonconserved intronic SNP but none in exonic regions.
Another nine genes were correlated with both phenotypes at marginal significance (Table 3). Of particular note, Sox5 harbors an abundance of 3226 SNPs, 115 of which sit in highly conserved sites. The majority of SNPs are intronic. We observed no SNPs between LG/J and SM/J alleles for four genes among those correlating at least marginally with both wound healing phenotypes (Il6, Cebpb, Cxcl12, and Mmp13). Strain expression differences with respect to such genes must be attributable to genetic variations outside of the gene proper or trans-polymorphism in other genes influencing its expression level, function, or both. Another gene of interest was p21 (aka Cdkn1a), since it has been found that the downregulation of p21 plays a most striking role in repair as its deletion alone confers regenerative ability (Bedelbaeva et al. 2010). In our panel of genes, the expression of p21 was found to be negatively correlated with both ear wound and articular cartilage repair phenotypes, although it did not reach a statistically significant level. Our findings of its negative correlation with regenerative phenotype and a nonsignificant trend in its expression are in line with the findings of Bedelbaeva. However, there could be two main reasons why our study did not detect a significant relationship between the expression of p21 and healing potential. First, the activity of p21 relevant to healing might occur specifically in one of the several tissue types in the knee joint. Because we assayed expression levels from the total knee joint, any tissue-specific effect of p21 that might have been working toward regeneration could have been diluted out, making it more difficult to detect a statistically significant relationship between overall p21 expression in the knee joint and healing potential. Second, and more importantly, there is very little genotypic variation in our samples. Although LG/J and SM/J alleles do vary at 54 bases within the p21 sequence, all the RI lines used in our study are homozygous for the same SM/J allele, except for LGXSM-5, which is heterozygous with one LG/J and one SM/J p21 allele. Potential functional variation in p21 is poorly represented in our study, therefore masking its detectable effect on healing.
Another important finding was that the strains can be classified into two main clusters that largely (but not exclusively) correspond to their healing ability (Rai et al. 2012). The three top healer strains (except for LGXSM-33) clustered together exclusive of other nonhealers and intermediate healers. This clustering was based on the gene expression variations among RI lines, the basis of which might arise because of functional differences between alleles arising from sequence variations, genetic background, or both.
We have shown that healing responses in both LGXSM-33 and LGXSM-6 closely parallel one another subsequent to injury, but with LGXSM-33 failing to keep pace and halting its progression in the final weeks of healing, during which LGXSM-6 completely regenerates its articular cartilage (Rai et al. 2012). Perhaps LGXSM-33 exhibits an expression profile that is quantitatively similar to that of healers, yet it fails to surpass a minimum threshold toward a state attributed with a healing ability.
Our analysis of the broad-sense heritabilities showed that the expressions of one-third of the genes plus all six of the miRNAs were significantly heritable. These results indicate that the gene expression differences observed across the strains are attributable to their genetic differences and are not attributable to environmental, nongenetic factors.
Based on the available genetic map for the LGXSM RI panel (Hrbek et al. 2006), we assigned the genotypes for each RI line for each gene correlating (at least marginally) with one or both healing phenotypes. Among the RI lines examined, LGXSM-6 heals the best and LGXSM-33 heals the worst, whereas the other two, LGXSM-5 and LGXSM-35, are intermediate healers. Across genes correlating with healing, we note that LGXSM-6 carries the LG/J allele most often among RI lines. Similarly, the nonhealing LGXSM-33 carries the SM/J allele more often. We surmise that healing ability as a function of the expression of these genes at ear and knee wound sites is conferred mostly by the LG/J allele relative to the SM/J allele.
A limiting aspect of this study was that the tissue lysates for gene expression analyses were prepared from the multiple joint tissues (including cartilage, subchondral bone, meniscus, synovium, joint capsule, and growth plate) instead of just from the articular cartilage. From the tiny mouse knee sections, it was not possible to restrict the analysis only to articular cartilage. Laser capture microdissection (LCM) would have been an alternative strategy, but because we did not have the sections on the LCM-compatible slides, we used whole knee joint tissues, as has been suggested previously (Loeser et al. 2012). Although this approach appears to be less sensitive in detecting genes that were differentially regulated exclusively in articular cartilage, and although it might limit the ability to determine which particular tissue contributed to expression of a specific gene, it has the advantage of allowing discovery of genes that are more globally involved in the regenerative process in the knee joint. Another potential limitation of this study is the number of mice (i.e., three to four for each strain) analyzed for gene expression. Because the sample size was not large, we computed the repeatability of the assays. Repeatability is the variance between individuals as a proportion of the variance within individuals (between replicates) plus the variance between individuals. Our repeatability analysis compared the differences among individuals across the whole sample relative to the differences between repeat measures for single individuals (Falconer and Mackay 1996). A higher repeatability value (0.8–0.9) adds confidence to the data for each replicate. Here, we achieved high repeatability values, even to the extent that a single measure would suffice instead of three replicates. Although a few genes had low repeatability values, repeating these measures three times did not provide much improvement (only from 0.63 to 0.73). It is quite possible that these genes were not really expressed at a high enough level to detect differences. Thus, high repeatability of the assay covers the limitation of a low number of mice.
These findings bear great significance for osteoarthritis research. Previously, we have shown that LGXSM-6 (healer) is protected from developing posttraumatic osteoarthritis, whereas the nonhealer (LGXSM-33) strain is susceptible to posttraumatic osteoarthritis (Hashimoto et al. 2012). Given the inverse relationship between cartilage healing and osteoarthritis, albeit in a limited set of mouse strains, we suggest that these genes may also be involved in protection from cartilage degeneration as it occurs in osteoarthritis.
In summary, our study has shown that a subset of genes is common to both ear wound and cartilage healing, clustering the variation in gene expression levels classify groups of strains corresponding to healing potential, and the basis of gene expression differences appears to have some genetic component. Given the evidence for common modes of healing in different tissue types, continued genetic and molecular dissection of components of healing will further uncover the mechanism(s) of tissue regeneration.
Supplementary Material
Acknowledgments
We thank the Genome Technology Access Center in the Department of Genetics at Washington University School of Medicine for help with QuantiGene Plex analysis. This project was supported by an ARRA Grand Opportunity Grant, Award Number RC2-AR058978 (L.J.S., J.M.C.), and the histological analysis was supported by The Center for Musculoskeletal Biology and Medicine, Award Number P30AR057235 (L.J.S.) from the National Institute of Arthritis, Musculoskeletal, and Skin Diseases. M.F.R. and E.J.S. were supported by the Ruth L. Kirschstein National Research Service Award fellowship from the National Institutes of Health through grant T32-AR060719. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis, Musculoskeletal, and Skin Diseases or the National Institutes of Health. L.J.S. owns stock or stock options in ISTO Technologies and receives royalties from Merck/Millipore for a type IIA collagen N-propeptide enzyme-linked immunosorbent assay. M.F.R., E.J.S., A.M., and J.M.C. have nothing to disclose. All authors have no conflicts of interest.
Footnotes
Communicating editor: D. W. Threadgill
Literature Cited
- Adzhubei, I., D. M. Jordan, and S. R. Sunyaev, 2013 Predicting functional effect of human missense mutations using PolyPhen-2. Curr. Protoc. Hum. Genet. Chapter 7: Unit7.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adzhubei I. A., Schmidt S., Peshkin L., Ramensky V. E., Gerasimova A., et al. , 2010. A method and server for predicting damaging missense mutations. Nat. Methods 7: 248–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R., Rawadi G., 2007. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 148: 2635–2643 [DOI] [PubMed] [Google Scholar]
- Bedelbaeva K., Snyder A., Gourevitch D., Clark L., Zhang X. M., et al. , 2010. Lack of p21 expression links cell cycle control and appendage regeneration in mice. Proc. Natl. Acad. Sci. USA 107: 5845–5850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bely A. E., 2010. Evolutionary loss of animal regeneration: pattern and process. Integr. Comp. Biol. 50: 515–527 [DOI] [PubMed] [Google Scholar]
- Blankenhorn E. P., Bryan G., Kossenkov A. V., Clark L. D., Zhang X. M., et al. , 2009. Genetic loci that regulate healing and regeneration in LG/J and SM/J mice. Mamm. Genome 20: 720–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresters D., Schipper M. E., Reesink H. W., Boeser-Nunnink B. D., Cuypers H. T., 1994. The duration of fixation influences the yield of HCV cDNA-PCR products from formalin-fixed, paraffin-embedded liver tissue. J. Virol. Methods 48: 267–272 [DOI] [PubMed] [Google Scholar]
- Carames B., Hasegawa A., Taniguchi N., Miyaki S., Blanco F. J., et al. , 2012. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann. Rheum. Dis. 71: 575–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carames B., Taniguchi N., Otsuki S., Blanco F. J., Lotz M., 2010. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 62: 791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson B. M., 2005. Some principles of regeneration in mammalian systems. Anat. Rec. B New Anat. 287: 4–13 [DOI] [PubMed] [Google Scholar]
- Chan E. Y., Kir S., Tooze S. A., 2007. siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J. Biol. Chem. 282: 25464–25474 [DOI] [PubMed] [Google Scholar]
- Choi J., Jo M., Lee E., Oh Y. K., Choi D., 2012. The role of autophagy in human endometrium. Biol. Reprod. 86: 70. [DOI] [PubMed] [Google Scholar]
- Chun S., Fay J. C., 2009. Identification of deleterious mutations within three human genomes. Genome Res. 19: 1553–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L. D., Clark R. K., Heber-Katz E., 1998. A new murine model for mammalian wound repair and regeneration. Clin. Immunol. Immunopathol. 88: 35–45 [DOI] [PubMed] [Google Scholar]
- Colwell A. S., Krummel T. M., Kong W., Longaker M. T., Lorenz H. P., 2006. Skin wounds in the MRL/MPJ mouse heal with scar. Wound Repair Regen. 14: 81–90 [DOI] [PubMed] [Google Scholar]
- Colwell A. S., Longaker M. T., Lorenz H. P., 2005. Mammalian fetal organ regeneration. Adv. Biochem. Eng. Biotechnol. 93: 83–100 [DOI] [PubMed] [Google Scholar]
- Cuervo R., Hernandez-Martinez R., Chimal-Monroy J., Merchant-Larios H., Covarrubias L., 2012. Full regeneration of the tribasal Polypterus fin. Proc. Natl. Acad. Sci. USA 109: 3838–3843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao D. Y., Yang X., Flick L. M., Chen D., Hilton M. J., et al. , 2010. Axin2 regulates chondrocyte maturation and axial skeletal development. J. Orthop. Res. 28: 89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hooge A. S., van De Loo F. A., Arntz O. J., van Den Berg W. B., 2000. Involvement of IL-6, apart from its role in immunity, in mediating a chronic response during experimental arthritis. Am. J. Pathol. 157: 2081–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’accio F., De Bari C., Eltawil N. M., Vanhummelen P., Pitzalis C., 2008. Identification of the molecular response of articular cartilage to injury, by microarray screening: Wnt-16 expression and signaling after injury and in osteoarthritis. Arthritis Rheum. 58: 1410–1421 [DOI] [PubMed] [Google Scholar]
- Falconer D. S., Mackay T. F. C., 1996. Introduction to Quantitative Genetics, Ed. 4 Longmans Green, Harlow, Essex, UK [Google Scholar]
- Fini M. E., 1999. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog. Retin. Eye Res. 18: 529–551 [DOI] [PubMed] [Google Scholar]
- Finke J., Fritzen R., Ternes P., Lange W., Dolken G., 1993. An improved strategy and a useful housekeeping gene for RNA analysis from formalin-fixed, paraffin-embedded tissues by PCR. Biotechniques 14: 448–453 [PubMed] [Google Scholar]
- Fitzgerald J., Rich C., Burkhardt D., Allen J., Herzka A. S., et al. , 2008. Evidence for articular cartilage regeneration in MRL/MpJ mice. Osteoarthritis Cartilage 16: 1319–1326 [DOI] [PubMed] [Google Scholar]
- Gierer A., Berking S., Bode H., David C. N., Flick K., et al. , 1972. Regeneration of hydra from reaggregated cells. Nat. New Biol. 239: 98–101 [DOI] [PubMed] [Google Scholar]
- Gurtner G. C., Werner S., Barrandon Y., Longaker M. T., 2008. Wound repair and regeneration. Nature 453: 314–321 [DOI] [PubMed] [Google Scholar]
- Harty M., Neff A. W., King M. W., Mescher A. L., 2003. Regeneration or scarring: an immunologic perspective. Dev. Dyn. 226: 268–279 [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Rai M. F., Janiszak K. L., Cheverud J. M., Sandell L. J., 2012. Cartilage and bone changes during development of post-traumatic osteoarthritis in selected LGXSM recombinant inbred mice. Osteoarthritis Cartilage 20: 562–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber-Katz E., 1999. The regenerating mouse ear. Semin. Cell Dev. Biol. 10: 415–419 [DOI] [PubMed] [Google Scholar]
- Heydemann A., Swaggart K. A., Kim G. H., Holley-Cuthrell J., Hadhazy M., et al. , 2012. The superhealing MRL background improves muscular dystrophy. Skelet Muscle 2: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrbek T., de Brito R. A., Wang B., Pletscher L. S., Cheverud J. M., 2006. Genetic characterization of a new set of recombinant inbred lines (LGXSM) formed from the inter-cross of SM/J and LG/J inbred mouse strains. Mamm. Genome 17: 417–429 [DOI] [PubMed] [Google Scholar]
- Kench J. A., Russell D. M., Fadok V. A., Young S. K., Worthen G. S., et al. , 1999. Aberrant wound healing and TGF-beta production in the autoimmune-prone MRL/+ mouse. Clin. Immunol. 92: 300–310 [DOI] [PubMed] [Google Scholar]
- Kierdorf U., Kierdorf H., 2011. Deer antlers—a model of mammalian appendage regeneration: an extensive review. Gerontology 57: 53–65 [DOI] [PubMed] [Google Scholar]
- Kim J., Xu M., Xo R., Mates A., Wilson G. L., et al. , 2010. Mitochondrial DNA damage is involved in apoptosis caused by pro-inflammatory cytokines in human OA chondrocytes. Osteoarthritis Cartilage 18: 424–432 [DOI] [PubMed] [Google Scholar]
- Knudsen B. S., Allen A. N., McLerran D. F., Vessella R. L., Karademos J., et al. , 2008. Evaluation of the branched-chain DNA assay for measurement of RNA in formalin-fixed tissues. J. Mol. Diagn. 10: 169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Henikoff S., Ng P. C., 2009. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 4: 1073–1081 [DOI] [PubMed] [Google Scholar]
- Kundu M., Lindsten T., Yang C. Y., Wu J., Zhao F., et al. , 2008. Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood 112: 1493–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Gu W., Masinde G., Hamilton-Ulland M., Xu S., et al. , 2001. Genetic control of the rate of wound healing in mice. Heredity (Edinb) 86: 668–674 [DOI] [PubMed] [Google Scholar]
- Loeser R. F., Olex A. L., McNulty M. A., Carlson C. S., Callahan M. F., et al. , 2012. Microarray analysis reveals age-related differences in gene expression during the development of osteoarthritis in mice. Arthritis Rheum. 64: 705–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan C. Y., Nusse R., 2004. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20: 781–810 [DOI] [PubMed] [Google Scholar]
- Macabeo-Ong M., Ginzinger D. G., Dekker N., McMillan A., Regezi J. A., et al. , 2002. Effect of duration of fixation on quantitative reverse transcription polymerase chain reaction analyses. Mod. Pathol. 15: 979–987 [DOI] [PubMed] [Google Scholar]
- Masinde G. L., Li R., Nguyen B., Yu H., Srivastava A. K., et al. , 2006. New quantitative trait loci that regulate wound healing in an intercross progeny from DBA/1J and 129 x 1/SvJ inbred strains of mice. Funct. Integr. Genomics 6: 157–163 [DOI] [PubMed] [Google Scholar]
- Masuda N., Ohnishi T., Kawamoto S., Monden M., Okubo K., 1999. Analysis of chemical modification of RNA from formalin-fixed samples and optimization of molecular biology applications for such samples. Nucleic Acids Res. 27: 4436–4443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matullo G., Guarrera S., Sacerdote C., Polidoro S., Davico L., et al. , 2005. Polymorphisms/haplotypes in DNA repair genes and smoking: a bladder cancer case-control study. Cancer Epidemiol. Biomarkers Prev. 14: 2569–2578 [DOI] [PubMed] [Google Scholar]
- McBrearty B. A., Clark L. D., Zhang X. M., Blankenhorn E. P., Heber-Katz E., 1998. Genetic analysis of a mammalian wound-healing trait. Proc. Natl. Acad. Sci. USA 95: 11792–11797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe A. D., Willis H., Beare A., Ferguson M. W., 2006. Characterizing regeneration in the vertebrate ear. J. Anat. 209: 439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno V., Gemignani F., Landi S., Gioia-Patricola L., Chabrier A., et al. , 2006. Polymorphisms in genes of nucleotide and base excision repair: risk and prognosis of colorectal cancer. Clin. Cancer Res. 12: 2101–2108 [DOI] [PubMed] [Google Scholar]
- Murphy E. D., Roths J. B., 1979. Autoimmunity and lymphoproliferation: Induction by mutant gene lpr and acceleration by a male-associated factor in strain BXSB, pp. 207–220 in Genetic Control of Autoimmune Disease, edited by Rose N. R., Bigazzi P. E., Warner N. L. Elsevier, New York [Google Scholar]
- Natsuka S., Akira S., Nishio Y., Hashimoto S., Sugita T., et al. , 1992. Macrophage differentiation-specific expression of NF-IL6, a transcription factor for interleukin-6. Blood 79: 460–466 [PubMed] [Google Scholar]
- Ohshima S., Saeki Y., Mima T., Sasai M., Nishioka K., et al. , 1998. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc. Natl. Acad. Sci. USA 95: 8222–8226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki K., Li J., Yu H., Fukui N., Sandell L. J., 2002. CCAAT/enhancer-binding proteins beta and delta mediate the repression of gene transcription of cartilage-derived retinoic acid-sensitive protein induced by interleukin-1 beta. J. Biol. Chem. 277: 31526–31533 [DOI] [PubMed] [Google Scholar]
- Okazaki K., Yu H., Davies S. R., Imamura T., Sandell L. J., 2006. A promoter element of the CD-RAP gene is required for repression of gene expression in non-cartilage tissues in vitro and in vivo. J. Cell. Biochem. 97: 857–868 [DOI] [PubMed] [Google Scholar]
- Park Y. N., Abe K., Li H., Hsuih T., Thung S. N., et al. , 1996. Detection of hepatitis C virus RNA using ligation-dependent polymerase chain reaction in formalin-fixed, paraffin-embedded liver tissues. Am. J. Pathol. 149: 1485–1491 [PMC free article] [PubMed] [Google Scholar]
- Rai M. F., Hashimoto S., Johnson E. E., Janiszak K. L., Fitzgerald J., et al. , 2012. Heritability of articular cartilage regeneration and its association with ear wound healing in mice. Arthritis Rheum. 64: 2300–2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai M. F., Patra D., Sandell L. J., Brophy R. H., 2013. Transcriptome analysis of injured human meniscus reveals a distinct phenotype of meniscus degeneration with aging. Arthritis Rheum. 65: 2090–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Alvarado A., Tsonis P. A., 2006. Bridging the regeneration gap: genetic insights from diverse animal models. Nat. Rev. Genet. 7: 873–884 [DOI] [PubMed] [Google Scholar]
- Seifert A. W., Monaghan J. R., Smith M. D., Pasch B., Stier A. C., et al. , 2012. The influence of fundamental traits on mechanisms controlling appendage regeneration. Biol. Rev. Camb. Philos. Soc. 87: 330–345 [DOI] [PubMed] [Google Scholar]
- Stocum D. L., 1984. The urodele limb regeneration blastema. Determination and organization of the morphogenetic field. Differentiation 27: 13–28 [DOI] [PubMed] [Google Scholar]
- Stoick-Cooper C. L., Weidinger G., Riehle K. J., Hubbert C., Major M. B., et al. , 2007. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development 134: 479–489 [DOI] [PubMed] [Google Scholar]
- Tanaka E. M., 2003. Regeneration: if they can do it, why can’t we? Cell 113: 559–562 [DOI] [PubMed] [Google Scholar]
- Tchetina E. V., 2011. Developmental mechanisms in articular cartilage degradation in osteoarthritis. Arthritis (Egypt) 2011: 683970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Koppel P. G., van Osch G. J., Verwoerd C. D., Verwoerd-Verhoef H. L., 2001. A new in vivo model for testing cartilage grafts and biomaterials: the ’rabbit pinna punch-hole’ model. Biomaterials 22: 1407–1414 [DOI] [PubMed] [Google Scholar]
- Urdea M. S., Horn T., Fultz T. J., Anderson M., Running J. A., et al. , 1991. Branched DNA amplification multimers for the sensitive, direct detection of human hepatitis viruses. Nucleic Acids Symp. Ser. 24: 197–200 [PubMed] [Google Scholar]
- Velasco J., Zarrabeitia M. T., Prieto J. R., Perez-Castrillon J. L., Perez-Aguilar M. D., et al. , 2010. Wnt pathway genes in osteoporosis and osteoarthritis: differential expression and genetic association study. Osteoporos. Int. 21: 109–118 [DOI] [PubMed] [Google Scholar]
- Ward B. D., Furman B. D., Huebner J. L., Kraus V. B., Guilak F., et al. , 2008. Absence of posttraumatic arthritis following intraarticular fracture in the MRL/MpJ mouse. Arthritis Rheum. 58: 744–753 [DOI] [PubMed] [Google Scholar]
- Yan Y., Tang D., Chen M., Huang J., Xie R., et al. , 2009. Axin2 controls bone remodeling through the beta-catenin-BMP signaling pathway in adult mice. J. Cell Sci. 122: 3566–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Maqsodi B., Ma Y., Bui S., Crawford K. L., et al. , 2006. Direct quantification of gene expression in homogenates of formalin-fixed, paraffin-embedded tissues. Biotechniques 40: 481–486 [DOI] [PubMed] [Google Scholar]
- Yu H. M., Jerchow B., Sheu T. J., Liu B., Costantini F., et al. , 2005. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development 132: 1995–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Bryan J. L., DeLassus E., Chang L. W., Liao W., et al. , 2010. CCAAT/Enhancer-binding protein {beta} and NF-{kappa}B mediate high level expression of chemokine genes CCL3 and CCL4 by human chondrocytes in response to IL-1{beta}. J. Biol. Chem. 285: 33092–33103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zienolddiny S., Campa D., Lind H., Ryberg D., Skaug V., et al. , 2006. Polymorphisms of DNA repair genes and risk of non-small cell lung cancer. Carcinogenesis 27: 560–567 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.