Abstract:
Rollout of routine HIV-1 viral load monitoring is hampered by high costs and logistical difficulties associated with sample collection and transport. New strategies are needed to overcome these constraints. Dried blood spots from finger pricks have been shown to be more practical than the use of plasma specimens, and pooling strategies using plasma specimens have been demonstrated to be an efficient method to reduce costs. This study found that combination of finger-prick dried blood spots and a pooling strategy is a feasible and efficient option to reduce costs, while maintaining accuracy in the context of a district hospital in Malawi.
Key Words: HIV, viral load, pooling, DBS, resource-limited setting, finger prick, operational research
INTRODUCTION
The World Health Organization recommends routine HIV viral load (VL) monitoring of patients on antiretroviral therapy (ART).1 As clinical and immunologic criteria for treatment failure have comparatively low sensitivity and positive predictive values (PPVs),2–5 VL monitoring enables earlier detection of ART failure, preventing emergence of drug resistance, enabling timely switching to second-line regimens (if indicated), and preventing inappropriate switching to second-line regimens.6–8
In most resource-limited settings, VL monitoring is not currently available because of logistic and cost constraints.9 Logistic constraints include the need for an efficient sample transport system, a reliable cold chain, and phlebotomists to draw venous blood (VB) for plasma, the standard sample type for VL testing. Use of dried blood spots (DBS) prepared from either finger prick (FP) or ethylenediamine tetraacetic acid–anticoagulated VB provide a practical alternative to plasma testing.10,11 DBS samples can be stored at room temperature for at least 1 month.10 Using FP DBS does away with the need for phlebotomy, enabling task shifting to lower staff cadres, where skilled human resources are scarce. A recent study conducted in Malawi found good correlation between VL results from plasma and DBS using the NucliSENS EasyQ HIV-1 v2.0 assay (bioMérieux, Marcy l'Etoile, France).11
Other recent studies have shown that pooled testing methods can achieve substantial cost reductions without compromising test accuracy.12–17 Testing VL on pooled DBS samples could reduce logistic and cost constraints, restricting access to VL testing, if shown to have satisfactory accuracy compared with individual plasma testing.
This study aimed to determine the efficiency, cost savings, and accuracy of pooled VL testing compared with individual plasma testing using 2 mini pooling methods, using pooled FP DBS, VB DBS, and plasma samples.
METHODS
Setting
The study was carried out from August to November 2012 at the district hospital in Thyolo, a rural district in southern Malawi. The district has a population of approximately 620,000 and an HIV prevalence of 14.5%.18 At the end of 2012, 35,000 patients were on ART, of whom 0.2% were on a second-line regimen.18
The VL testing laboratory at Thyolo District Hospital became operational in April 2011. VL monitoring replaced CD4 monitoring of patients attending the hospital ART clinic and was later extended to patients attending other health facilities in the district. VL monitoring coverage was approximately 15% by the end of 2012. Patients with a VL ≥1000 copies per milliliter receive an adherence intervention and a repeat VL test 3 months later. Patients with a repeat VL ≥5000 copies per milliliter are considered for second-line ART.
Study Population
Three hundred fifty patients participated in the study. All HIV-positive patients older than 18 years, on ART for at least 6 months, and having VB drawn as part of their clinical management were eligible to participate. Ethics approval was obtained from the Malawi National Health Sciences Research Committee and the Médecins Sans Frontières Ethics Review Board. All participants provided written informed consent.
Sample Collection and Preparation
Each participant provided an FP DBS sample and a tube of VB. A laboratory technician drew blood in to a BD Vacutainer EDTA tube (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ); this was used to prepare VB DBS and plasma samples. Laboratory technicians prepared 5 FP DBS and 5 VB DBS samples per participant by applying 50 μL of blood on Whatman 903 filter paper cards (Whatman, Maidstone, United Kingdom). DBS samples were dried overnight and stored at room temperature in separate plastic bags with desiccants and humidity indicator cards until tested. Within 3 hours of collection, after preparing VB DBS samples, the remainder of the EDTA blood was centrifuged at 3000g for 10 minutes. Plasma was transferred to sterile polypropylene tubes and stored at −20°C until testing.
Pooling Strategies
The “minipool” and “minipool + algorithm” strategies used pools of 5 samples, as previously described.13 Seventy sets of minipools were constituted from the 350 sets of samples. Plasma pools were constituted by pooling 100 μL of plasma from 5 patients, for a total volume of 500 μL. DBS pools were constituted using 2 circles of 50-μL of blood per patient eluted in 2-mL lysis buffer and pooling 400-μL eluates from 5 patients. Pooled samples were analyzed according to standard operating procedures.
As each pool contained 5 samples, a pool VL threshold of 1000 copies per milliliter was used to assess the accuracy and efficiency of pooling for identifying patients with a VL ≥ 5000 copies per milliliter and a pool VL threshold of 200 copies per milliliter was used to assess the accuracy and efficiency of pooling for identifying patients with a VL ≥1000 copies per milliliter. These thresholds were chosen because they were in clinical use.
Minipool Strategy
Using the minipool strategy, pools with a VL result above the threshold were deconvoluted by testing individual samples from each patient represented in the pool.
Minipool + Algorithm Strategy
Using the minipool + algorithm strategy, pools with a VL result above the threshold were deconvoluted by serial testing of individual samples from patients represented in the pool, in random order, and subtracting individual test results from the pool result until the VL of the pool had been accounted for.
For both pooling strategies, pools with a VL result less than the threshold were not deconvoluted, and all patients with samples in these pools were considered to have a VL lesser than the individual threshold.
VL Assay
Quantitative HIV-1 RNA VL testing was performed with the NucliSENS EasyQ v2.0 assay (bioMérieux). The assay has a lower limit of detection of 50 copies per milliliter using a plasma input volume of 0.5 mL, and 800 copies per milliliter using a DBS input volume of 0.1 mL.
Statistical Analyses
VL in individual plasma samples served as the reference for all calculations of accuracy and efficiency. Accuracy was determined by calculating sensitivity, specificity, PPV, and negative predictive value (NPV). Relative efficiency was defined as 1 minus the average number of tests performed divided by the number of samples. Cost savings were calculated with a reagent price of $30 per test, the local price at the time of the study. Exact binomial 95% confidence intervals were calculated. P values were calculated using 2-sample tests of proportion. Statistical tests were 2 sided at alpha = 0.05. All analyses were performed using Stata version 11 (StataCorp, College Station, TX).
RESULTS
Of the 350 participants in the study, 70.5% were women and 16.3% had suspected treatment failure identified on clinical grounds. Their median age was 38 years [interquartile range (IQR): 30–46], and median time on ART was 37 months (IQR: 18–65).
Of the participants, 82.0% had an undetectable individual plasma VL, 10.0% had a detectable VL <1000 copies per milliliter and 8.0% had a VL ≥1000 copies per milliliter, including 6.6% with a VL ≥5000 copies per milliliter. Compared with patients having routine VL monitoring, participants with clinically suspected treatment failure were less likely to have a plasma VL <1000 copies per milliliter (86.0% vs. 93.2%; P = 0.066), and more likely to have a plasma VL ≥5000 copies per milliliter (12.3% vs. 5.5%; P = 0.057).
Efficiency of Pooled Testing
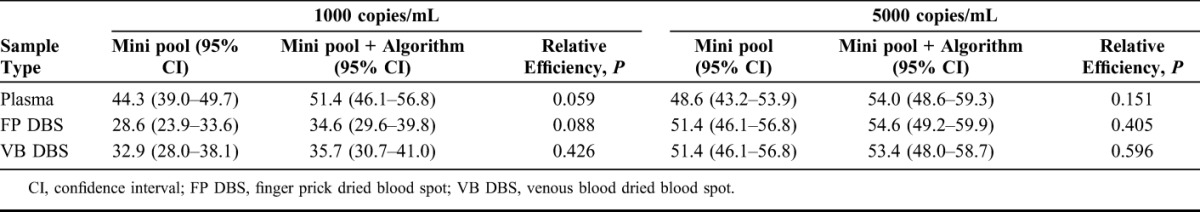
The efficiencies of the 2 minipooling strategies with each sample type are shown in Table 1. For example, with FP DBS and a 5000 copies per milliliter threshold, mini pooling would reduce the number of tests required by 51.4% compared with testing individual plasma samples. There were no statistically significant differences in the efficiency of the 2 algorithms (P > 0.05), although the minipool + algorithm was slightly more efficient than the minipool strategy at a 1000 copies per milliliter threshold with pooled plasma and FP DBS samples. Pooling plasma was more efficient than pooling DBS at the 1000 copies per milliliter threshold (P < 0.002), but similar at the 5000 copies per milliliter threshold (P ≥ 0.45). There were no statistically significant differences in efficiency between FP DBS and VB DBS samples (P ≥ 0.22).
TABLE 1.
Relative Efficiencies of Different Pooling Methods, With Different Sample Types, at Thresholds of 1000 and 5000 copies per milliliters
Accuracy of Pooled Testing
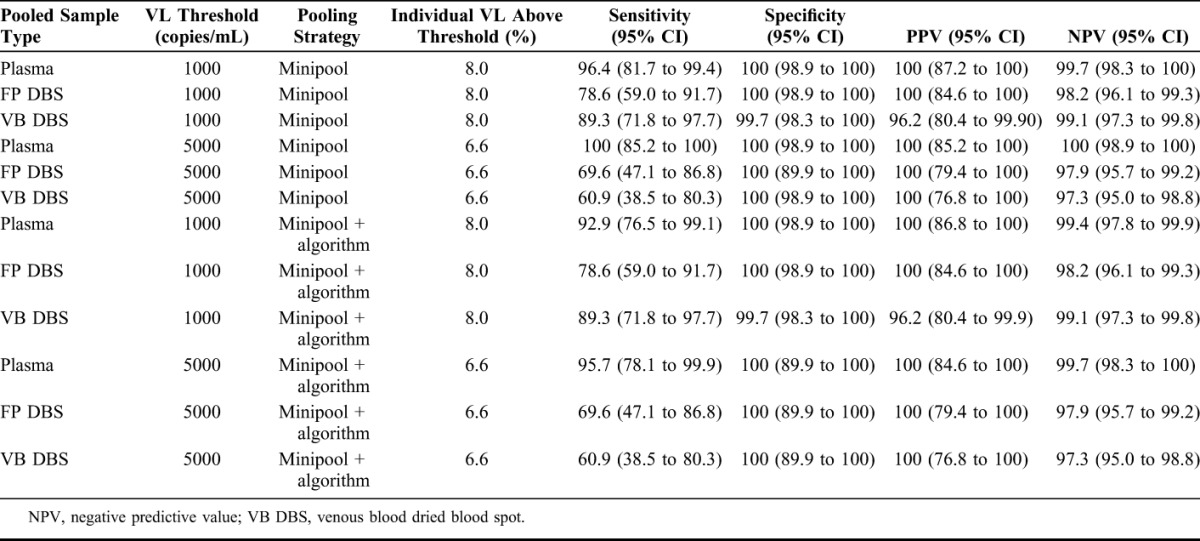
The accuracies of the 2 minipool strategies in combination with each sample type are shown in Table 2. NPV ranged from 97.3% to 100%, and PPV ranged from 96.2% to 100%, despite the limited sensitivity (range, 60.9%–69.6%) of pooled DBS samples at the 5000 copies per milliliter threshold.
TABLE 2.
Accuracy of Minipooling and Minipooling + Algorithm Strategy for Detecting an Elevated Viral Load (VL) at 1000 and 5000 copies/mL Thresholds as Compared With Individual Plasma VL (N = 350)
DISCUSSION
This study combined the use of DBS and pooling strategies for quantitative HIV-1 VL determination. In Thyolo, approximately 23,000 VL tests are currently required per year to attain full VL monitoring coverage in accordance with the national guidelines, which recommend VL testing 6 and 24 months after starting ART and every 24 months thereafter. At a reagent price of $30 per test and a 5000 copies per milliliter threshold, pooling DBS samples would save $345,000 per year by reducing the number of tests required by approximately 50%. At a 1000 copies per milliliter threshold, pooling DBS samples would reduce the number of tests required by approximately 30%, saving $207,000 per year. As the cost of VL reagents varies across countries in the region, ranging from $11 to $90 per test19 because of differences in failure rates, cost savings would vary according to the setting. For if too many positives “poison” your pools, the saving will also be diminished. In each context, potential costs and benefits need to be balanced when choosing thresholds. Although raising the threshold produces greater savings, it also reduces the sensitivity of pooled VL testing at detecting treatment failure.
Several factors need to be considered when choosing between the minipool and minipool + algorithm strategies. Although the minipool + algorithm had a slightly higher efficiency than the minipool strategy at the 1000 copies per milliliter threshold, this advantage of the minipool + algorithm is offset by several disadvantages, including the greater complexity of the algorithm; a greater probability of false-negative individual VL results in pools with more than 1 sample above the individual threshold because of interassay variability, and a longer average run time because serial testing of individual samples used in deconvolution of positive pools requires up to 5 extra runs compared with 1 extra run using the mini pooling strategy. Despite slightly lower cost-savings, implementation of the minipool strategy may be operationally more feasible than implementing the minipool + strategy in resource-limited settings.
The use of DBS samples may enable task shifting to lower cadres, resulting in human resource allocation efficacies. The prevalence of treatment failure in the patient population also needs to be considered when developing algorithms for pooled testing. Pooling methods become less efficient as the prevalence of treatment failure increases.
Previous pooling studies have found similar efficiencies using plasma or dried plasma spots as the sample type.12–17 One study that used pooled VB DBS found lower efficiencies and NPVs with pooled VB DBS than with plasma.15 In contrast to other studies, our study compared VL results from both FP DBS and VB DBS pools with individual plasma VL results.
Our findings are important as they address 2 of the major obstacles to the rollout of routine VL testing in resource-limited settings, namely the high cost of individual VL testing and the logistic difficulties associated with sample collection and transport.9 Although point-of-care VL tests may play an important role in increasing access to VL monitoring in the future,20 the use of DBS, tested at a district or central laboratory, is a feasible alternative to plasma testing and maybe more suitable and cost efficient than point-of-care VL testing in some settings, particularly those requiring high-throughput testing.
Strengths of this study include the sample size and using the same laboratory technicians to perform all tests in 1 laboratory. In addition, our findings demonstrate that constituting, testing, and resolving of positive pools are feasible in a rural district hospital laboratory. Implementing pooling strategies will help with the scale-up of routine VL monitoring, which in turn will increase costs saved as a result of pooling.
VL monitoring provides a direct measurement of response to ART and is more reliable than CD4 monitoring at detecting treatment failure. As more patients are initiated on ART at higher CD4 thresholds, as a result of changes in ART guidelines, VL monitoring will become the only reliable method of determining response to treatment. This study demonstrates that the use of FP DBS or VB DBS combined with pooled testing using the NucliSENS assay and provides a feasible option to facilitate the rollout of routine VL monitoring in resource-limited settings.
Footnotes
Supported by Médecins Sans Frontières, Brussels Operational Centre.
Presented as Poster 612 at 20th Conference on Retroviruses and Opportunistic Infections March 3–6, 2013, Atlanta, GA.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.World Health Organization (WHO). Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Recommendations for a Public Health Approach. Geneva, Switzerland: WHO; 2013. Available at: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. Accessed July 17, 2013 [PubMed] [Google Scholar]
- 2.Lynen L, Van Griensven J, Elliott J. Monitoring for treatment failure in patients on first-line antiretroviral treatment in resource-constrained settings. Curr Opin HIV AIDS. 2010;5:1–5 [DOI] [PubMed] [Google Scholar]
- 3.Keiser O, Chi B, Gsponer T, et al. Outcomes of antiretroviral treatment in programmes with and without routine viral load monitoring in Southern Africa. AIDS. 2011;25:1761–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Oosterhout JJ, Brown L, Weigel R, et al. Diagnosis of antiretroviral therapy failure in Malawi: poor performance of clinical and immunological WHO criteria. Trop Med Int Health. 2009;14:856–861 [DOI] [PubMed] [Google Scholar]
- 5.Kantor R, Diero L, Delong A, et al. Misclassification of first-line antiretroviral treatment failure based on immunological monitoring of HIV infection in resource-limited settings. Clin Infect Dis. 2009;49:454–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sigaloff KC, Hamers RL, Wallis CL, et al. Unnecessary antiretroviral treatment switches and accumulation of HIV resistance mutations; two arguments for viral load monitoring in Africa. J Acquir Immune Defic Syndr. 2011;58:23–31 [DOI] [PubMed] [Google Scholar]
- 7.ART-LINC of IeDEA Study Group, Keiser O, Tweya H, Boulle A, et al. Switching to second-line antiretroviral therapy in resource-limited settings: comparison of programmes with and without viral load monitoring. AIDS. 2009;23:1867–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UNAIDS, Médecins Sans Frontières: Speed Up Scale-up: Strategies, Tools and Policies to Get the Best HIV Treatment to More People, Sooner. Geneva, Switzerland: MSF Access Campaign; 2012 [Google Scholar]
- 9.Roberts T, Bygrave H, Fajardo E, et al. Challenges and opportunities for the implementation of virological testing in resource-limited settings. J Int AIDS Soc. 2012;15:17324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johannessen A. Dried blood spots in HIV monitoring: applications in resource-limited settings. Bioanalysis. 2010;2:1893–1908 [DOI] [PubMed] [Google Scholar]
- 11.Metcalf C, Fajardo E, Pannus P, et al. Use of finger-prick dried blood spots for quantifying HIV-1 viral load: a diagnostic accuracy study conducted in Thyolo, Malawi. Paper presented at: 20th Conference on Retroviruses and Opportunistic Infections; March 3–6, 2013; Atlanta, GA. Abstract 608
- 12.Smith D, May S, Perez-Santiago J, et al. The use of pooled viral load testing to identify antiretroviral treatment failure. AIDS. 2009;23:2151–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.May S, Gamst A, Haubrich R, et al. Pooled nucleic acid testing to identify antiretroviral treatment failure during HIV infection. J Acquir Immune Defic Syndr. 2010;53:194–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tilghman MW, Guerena DD, Licea A, et al. Pooled nucleic acid testing to detect antiretroviral treatment failure in Mexico. J Acquir Immune Defic Syndr. 2011;56:70–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Zyl G, Preiser W, Potschka S, et al. Pooling strategies to reduce the cost of HIV-1 RNA load monitoring in a resource-limited setting. Clin Infect Dis. 2011;52:264–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H, Ku N, Kim S, et al. Pooled nucleic acid testing to identify ART failure during HIV infection in a tertiary teaching hospital: Seoul, South Korea. Paper presented at: 20th Conference on Retroviruses and Opportunistic Infections; 2013; Atlanta, GA. Abstract 611
- 17.Chohan B, Tapia K, Merkel M, et al. Pooled HIV-1 RNA viral load testing for detection of antiretroviral treatment failure in Kenyan Children. J Acquir Immune Defic Syndr. 2013;63:e87–e93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malawi Demographic and Health Survey 2010. National Statistical Office, Zomba, Malawi
- 19.Médecins Sans Frontières Access Campaign. Putting HIV Treatment to the Test: A Product Guide for Viral Load and Point-of-Care CD4 Diagnostic Tools. Geneva, Switzerland: MSF; 2013. Available at: http://www.msfaccess.org/content/putting-hiv-treatment-test. Accessed July 17, 2013 [Google Scholar]
- 20.UNITAID. HIV/AIDS Diagnostic Technology Landscape. 2nd ed Geneva, Switzerland: World Health Organisation; 2012. Available at: http://www.unitaid.eu/images/marketdynamics/publications/UNITAIDHIV_Diagnostics_Landscape-2nd_edition.pdf. Accessed March 6, 2013 [Google Scholar]


