Abstract
The effects of low-activity versus high-activity radioiodine regimens in thyroid remnant ablation for patients with differentiated thyroid carcinoma (DTC) under recombinant human thyrotropin (rhTSH) stimulation have been widely quoted but there has been no systematic review of the evidence. We undertook a systematic review of randomized controlled trials to assess the effects of low-activity radioiodine in thyroid remnant ablation in patients with DTC under rhTSH stimulation compared with high-activity radioiodine. Studies were obtained from computerized searches of MEDLINE, EMBASE, and the Cochrane Library (all until September 2012). Randomized controlled trials were included. Altogether, 637 patients with DTC who participated in three trials for residual ablation were included. Overall, studies had a low risk of bias. We found no statistically significant differences between low-activity (1.11/1.85 GBq) and high-activity (3.7 GBq) radioiodine treatment aided by rhTSH in terms of successful ablation rates on the basis of diagnostic scans [odds ratio (OR) 0.85, 95% confidence interval (CI) 0.49–1.47, P=0.56], thyroglobulin levels (OR 0.66, 95% CI 0.38–1.15, P=0.14), and health-related quality of life (mean difference 0.07, 95% CI −0.96 to 1.09, P=0.9). In addition, the subgroup analysis of 1.11 versus 3.7 GBq (OR 0.83, 95% CI 0.46–1.49, P=0.53) and 1.85 versus 3.7 GBq (OR 1, 95% CI 0.23–4.35, P=1) also showed no significant differences. The lower activity of 1.11 GBq showed significant benefit in terms of reduction in adverse events including neck pain, radiation gastritis, and salivary dysfunction during and after ablation (OR 0.63, 95% CI 0.42–0.93, P=0.02). Limited data from three randomized controlled trials suggested that an rhTSH-aided low radioiodine activity level of as low as 1.115 GBq may be sufficient for thyroid remnant ablation when compared with 3.7 GBq, with fewer common adverse effects in patients with metastasis-free DTC. Further evidence is needed to confirm the effects of low-activity radioiodine for thyroid remnant ablation. Radioiodine treatment of 1.11 GBq showed significant benefit in terms of reduction in adverse events including neck pain, radiation gastritis, and salivary dysfunction during and after ablation (OR 0.63, 95% CI 0.42–0.93, P=0.02). rhTSH-aided low radioiodine activity levels of 1.11 and 1.85 GBq are sufficient for thyroid remnant ablation as compared with 3.7 GBq, with fewer common adverse effects in patients with metastasis-free DTC. A well-designed study that compares low-activity with high-activity radioiodine ablation is needed to fully understand the long-term adverse effects and relapse or metastases.
Keywords: differentiated thyroid cancer, radioiodine, recombinant human thyrotropin
Introduction
Thyroid cancer is the most frequently occurring type of endocrine cancer, and its incidence has been increasing worldwide in recent decades. The principal standard treatment modality for these patients includes total or near-total thyroidectomy, followed by iodine-131 (131I) therapy and lifelong thyroid hormone suppressive therapy. 131I treatment with an activity level sufficient to remove residual thyroid tissue after thyroidectomy is called ‘remnant ablation’. Eradication of normal-thyroid remnants can result in an undetectable level of serum thyroglobulin (Tg), which can facilitate biochemical follow-up. Traditionally, thyroid hormone withdrawal (THW) for 4–6 weeks has been used to attain the increase in serum thyroid-stimulating hormone concentrations that is believed to optimize the trapping and retention of radioiodine for diagnostic procedures, such as thyroid remnant ablation for patients with differentiated thyroid carcinoma (DTC). Exogenous stimulation with recombinant human thyrotropin (rhTSH) is approved for Tg testing or for diagnostic radioiodine scintigraphy in patients on thyroid hormone suppressive therapy in the USA and Europe. This offers an alternative to THW by avoiding the morbidity of hypothyroidism. We previously reported that rhTSH is as effective as THW in radioiodine thyroid remnant ablation with significant benefits on health-related quality of life (QOL) and adverse effects 1. It is still uncertain whether both low activity (1.11 and 1.85 GBq) and high activity (3.7 GBq) of 131I are equally effective for remnant ablation under rhTSH stimulation. Therefore, we evaluated the effects of low-activity versus high-activity regimens of radioiodine in thyroid remnant ablation for patients with DTC under rhTSH stimulation.
Methods
Criteria for considering studies for this review
Patients with DTC participating in randomized controlled clinical trials in any language were included after total or near-total thyroidectomy followed by rhTSH-aided radioiodine treatment for residual DTC.
We considered the ablation rate of postsurgical thyroid residues, health-related QOL, and death from any cause as primary outcomes; adverse effects, secondary malignancy, costs, relapse, and metastases of DTC iodine were considered as secondary outcomes. The follow-up had to be at least 6 months after radioiodine ablation.
Search strategy for identification of studies
We identified studies regardless of language or publication status by searching The Cochrane Library (issue 3, 2012), MEDLINE (until September 2012), and EMBASE (until 2012). We contacted authors of published trials, where appropriate, for further information. The search terms used were thyroid neoplasm/differentiated thyroid cancer, recombinant human thyrotropin, radioiodine, and/or randomized controlled trials.
Selection of studies
We included all published and unpublished randomized controlled trials (RCTs) that involved patients of any age who were receiving 131I for thyroid remnant ablation and in which DTC stage had been adequately defined (TNM). All potentially relevant articles were investigated as full text. Inter-rater agreement for study selection was measured using the κ statistic 2. We prespecified a minimum, mean, or median follow-up of 6 months from the time of 131I treatment for prespecified outcomes. We also prespecified the following ablation rates for comparison purposes: 1.11 and 1.85 versus 3.7 GBq; 1.11 versus 3.7 GBq; and 1.85 versus 3.7 GBq. Additional outcome measures were health-related QOL and adverse effects.
Quality assessment
Quality assessment of RCTs included allocation concealment, whether intention-to-treat analysis had been carried out, comparability of groups at baseline, and blinding of outcome assessors.
Data abstraction
Two reviewers independently abstracted data and assessed the methodological quality of the studies. Any differences were resolved by discussion between reviewers.
Data synthesis and statistical analysis
We used Review Manager (RevMan), version 5.2. from the Cochrane collaboration for data analysis. Where appropriate, the results of comparable groups of trials were combined for odds ratios (ORs) using random-effect models in view of study heterogeneity. Results were presented with 95% confidence intervals (CIs). Heterogeneity was identified by visual inspection of the forest plots by using a standard χ2-test and a significance level of α=0.1, in view of the low power of such tests. Heterogeneity was specifically examined with I2, where I2 values of 50% or higher indicate a substantial level of heterogeneity 3. When heterogeneity was found, we attempted to determine potential reasons for it by examining individual study and subgroup characteristics.
Results
Results of the search
The electronic searches revealed 135 studies. Of these references, we excluded 116 citations. After reading the titles and abstracts, 19 potential controlled clinical trials were retrieved for further assessment. Three randomized controlled clinical trials 4–6 were included. Trial durations were from December 2001 to July 2010 in two trials 5–6; the duration was not mentioned in one trial 4. Four RCTs comparing rhTSH-aided versus THW-aided radioiodine remnant ablation 7–10, eight historical case–control studies 11–18, and four nonrandomized prospective controlled clinical trials 19–22 on rhTSH -aided 131I thyroid remnant ablation were excluded.
The characteristics and quality of the included studies
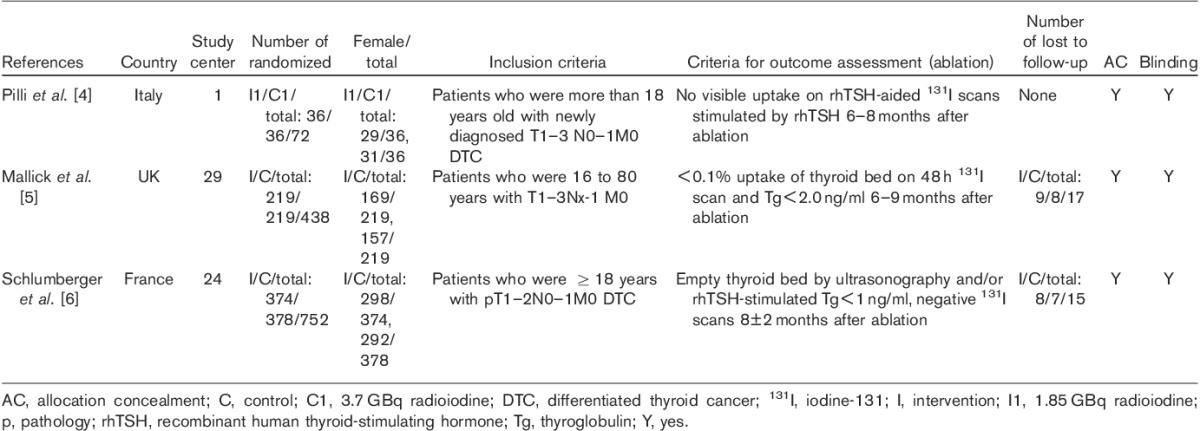
Altogether, 637 DTC patients participated in three trials. The characteristics and quality of the included studies are listed in Table 1. The risk of bias in the included trials was considered low. Summary data on age, sex, tumor pathology, and staging were reported for all participants. No significant differences were found between comparison groups. All included patients had undergone a total or near-total thyroidectomy before residual ablation. The diagnostic activity of radioiodine for assessment of ablation was between 140 and 185 MBq in all the included studies. The QOL was assessed using the Billewicz scale and Short Form-36 (SF-36) scores 5,6. Inclusion and exclusion criteria were specified in all the included trials. Apart from L-T4 replacement, comedications and comorbidities were not mentioned in all included trials.
Table 1.
Quality and characteristics of included studies for residual ablation
Effects of recombinant human thyrotropin-aided low and high activity of iodine-131 thyroid remnant ablation
Ablation rate
Two RCTs compared ablation rates between 1.11 and 3.7 GBq, and one trial compared ablation rates between 1.85 and 3.7 GBq. The lower versus higher activity of 131I aided by rhTSH showed no significant difference in successful ablation rate on the basis of either diagnostic scans (OR 0.85, 95% CI 0.49–1.47, P=0.56) or Tg levels (OR 0.66, 95% CI 0.38–1.15, P=0.14) (Figs 1 and 2, respectively). In addition, the subgroup analysis of 1.11 versus 3.7 GBq (OR 0.83, 95% CI 0.46–1.49, P=0.53) and 1.85 versus 3.7 GBq (OR 1, 95% CI 0.23–4.35, P=1) also showed no significant differences (Figs 3 and 4, respectively).
Fig. 1.
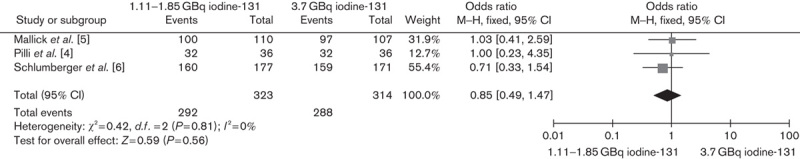
Effect of 1.11/1.85 versus 3.7 GBq iodine-131 under rhTSH stimulation on thyroid remnant ablation rate according to diagnostic scans. CI, confidence interval; rhTSH, recombinant human thyrotropin.
Fig. 2.
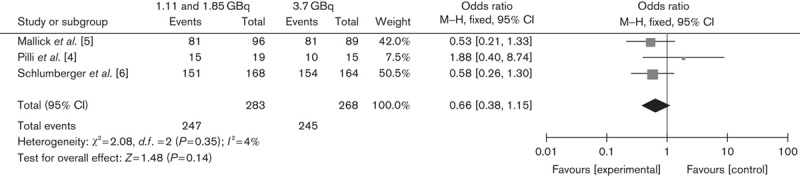
Effect of 1.11/1.85 versus 3.7 GBq iodine-131 under rhTSH stimulation on thyroid remnant ablation rate according to thyroglobulin levels. CI, confidence interval; rhTSH, recombinant human thyrotropin.
Fig. 3.
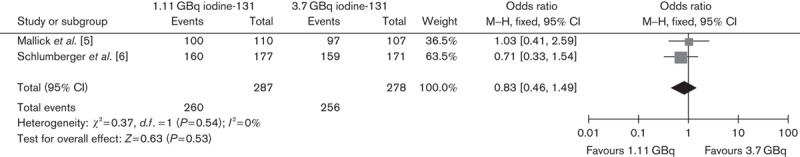
Effect of 1.11 versus 3.7 GBq iodine-131 under rhTSH stimulation on thyroid remnant ablation rate. CI, confidence interval; rhTSH, recombinant human thyrotropin.
Fig. 4.
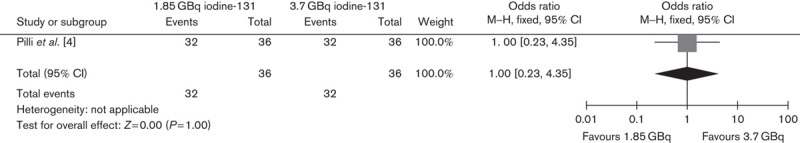
Effect of 1.85 versus 3.7 GBq iodine-131 thyroid remnant ablation on adverse effects. CI, confidence interval.
Health-related quality of life
Two included trials compared different 131I activity levels in terms of health-related QOL. The lower versus higher activity of 131I aided by rhTSH showed no significant difference in QOL (Fig. 5) (mean difference 0.07, 95% CI −0.96 to 1.09, P=0.9).
Fig. 5.
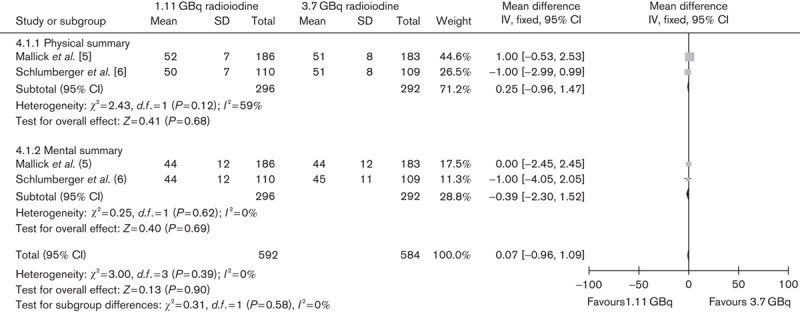
Effect of 1.11 versus 3.7 GBq iodine-131 under rhTSH stimulation on health-related quality of life. CI, confidence interval; rhTSH, recombinant human thyrotropin.
Adverse effects
An activity of 1.11 versus 3.7 GBq of radioiodine treatment aided by rhTSH showed significant benefit in terms of reduction in adverse events including neck pain, radiation gastritis, and salivary dysfunction during and after ablation (OR 0.63, 95% CI 0.42–0.93, P=0.02) (Fig. 6).
Fig. 6.
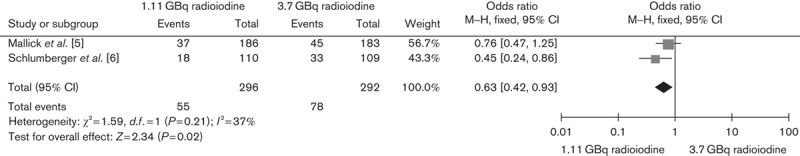
Effect of 1.11 versus 3.7 GBq iodine-131 thyroid remnant ablation on adverse effects. CI, confidence interval.
Discussion
Radioiodine ablation after total thyroidectomy is indicated generally for DTC patients to ablate normal thyroid tissue, eliminate any suspected but unproven metastases, and treat known persistent disease. We previously reported that rhTSH is as effective as THW in radioiodine thyroid remnant ablation with significant benefits on health-related QOL and adverse effects 1. Unfortunately, there is uncertainty with regard to the activity of 131I required for thyroid remnant ablation.
Decreased uptake of 131I at 24 h after the administration of rhTSH was observed in two trials 3,18. Therefore, rhTSH does not appear potent enough to induce 131I uptake for therapeutic purpose when a small activity of 131I is administered. Possible explanations for the reduced 131I uptake may be the following: interference due to administration of T4, a low activity of 1.85 MBq 19 and 18.5 MBq of 131I 7 24 h after a single injection of rhTSH, accelerated iodine clearance 19, or faster renal clearance of iodine observed in euthyroid patients compared with patients treated for hypothyroidism 8. However, no significant differences were found in thyroid uptake and in the effective half-life of 131I in the remnant thyroid, which supported the idea that the pretherapy 131I uptake does not correlate with the rate of successful ablation 7. Similarly, one study indicates that rhTSH-aided 1.11 GBq radioiodine is not sufficient for a satisfactory thyroid ablation rate 19. In 2004, the European Agency licensed rhTSH for use in thyroid remnant ablation with 3.7 GBq 131I (European Medicines Agency, 2005). Therefore, we included three RCTs 4–6 to evaluate the effects of low-activity versus high-activity regimens of radioiodine in thyroid remnant ablation for patients with DTC under rhTSH stimulation.
The quantity of evidence from three included trials is acceptable for rhTSH-aided radioiodine treatment. No significant heterogeneity was found between the trials. No serious adverse events were observed in the included trials; no data on costs and secondary malignancies were reported. All included patients in the three trials were at low risk for relapse. The first randomized trial reported that increasing the initial activity of 131I from 1.11 GBq to more than 1.85 GBq resulted in a plateau of the dose–response curve when radiation-absorbed dose was calculated under THW. On the basis of dosimetry results, one should aim to deliver about 30 000 cGy to the thyroid remnant, as higher doses do not appear to yield a higher ablation rate 23. In agreement with the original study 23, our result suggested that low activity (1.11 and 1.85 GBq) is as effective as high activity (3.7 GBq) in radioiodine ablation, with significant benefits in terms of reduction in adverse effects. There were no significant differences in health-related QOL scores on the Short Form-36 between patients receiving low-activity 131I and those receiving high-activity 131I on the day of ablation and 3 months after ablation. Therefore, low-activity (1.11 GBq) radioiodine was recommended for thyroid remnant ablation in patients with low risk for relapse.
Because there is currently no accepted standard of diagnostic criteria for successful thyroid remnant ablation, the definition of successful ablation is different between the studies. Many studies used a visual inspection of the follow-up scan, others used a cutoff level associated with a quantitative measurement of neck uptake, and some studies used Tg measurements in addition to the scan result. To reduce the impact of this difference on the results, we performed a subgroup analysis on trials that evaluated the successful ablation rate on the basis of stimulated Tg levels. The results (OR=0.66, 95% CI 0.38–1.15, P=0.14) indicated that both 1.11 and 1.85 GBq are as effective as 3.7 MBq activity in achieving a successful ablation rate.
In our analysis, the assessment time of successful remnant ablation was between 6 and 12 months. We did not address future recurrences because no randomized trials on long-term adverse effects were found between low-activity and high-activity radioiodine ablation. Long-term follow-up is required to examine the recurrence rate and the risk for second primary cancer. A recent study with at least 10 years of follow-up reported that the long-term outcomes are similar in DTC patients treated with 1.1 GBq of 131I and prepared either with rhTSH or l-thyroxine (LT4) withdrawal 24. The irradiation of 131I to patients is an important factor for secondary malignancy. The absolute risk for radioiodine-induced second primary cancer had not been well established, but the risk for any second primary cancer after initial diagnosis of thyroid cancer was increased ∼30% over that of the general population, and the risk appeared to increase with increasing cumulative administered activity 25,26. A meta-analysis also indicated that the risk for second primary malignancies in thyroid cancer survivors treated with radioiodine is slightly higher compared with that of thyroid cancer survivors not treated with radioiodine 27. Therefore, we again recommend low activity of radioiodine for residual ablation in patients with low risk for relapse. With respect to patients with high risk for relapse and metastases, the activity of radioiodine for residual ablation should be individualized. Future studies should pay more attention to secondary malignancy, relapse, and metastases of DTC on administration of low-activity compared with high-activity radioiodine ablation.
Conclusion
Limited data from three randomized controlled clinical trials suggest that rhTSH-aided low activity (1.11 and 1.85 GBq) radioiodine may be sufficient for thyroid remnant ablation as compared with 3.7 GBq, with fewer common adverse effects in patients with metastasis-free DTC. Further evidence is needed to confirm the effects of low-activity radioiodine for thyroid remnant ablation. A well-designed study comparing low-activity with high-activity radioiodine ablation is needed in order to fully understand the long-term adverse effects and relapse or metastases.
Acknowledgements
The authors thank Dr Ujjal Mallick and Dr Allan Hackshaw for providing valuable missing data. They also thank Gudrun Paletta and Karla Bergerhoff in the Cochrane Metabolic and Endocrine Disorders group for their comments and advice on the review.
This study was supported by the National Natural Science Fund (grant 81271612), Shanghai Pujiang Program (13PJD022), and the Shanghai Health Bureau Fund (grant 20124016).
Conflicts of interest
There are no conflicts of interest.
Footnotes
*Chao Ma, Limin Tang, and Hongliang Fu contributed equally to the writing of this article.
Correspondence to Hui Wang, MD, Department of Nuclear Medicine, Affiliated Xinhua Hospital of Medical School Shanghai Jiaotong University, Shanghai 200092, China Tel: +86 021 25078593; fax: +86 021 25076986; e-mail: wanghuishanghai@hotmail.com
References
- 1.Ma C, Xie J, Liu W, Wang G, Zuo S, Wang X, Wu F.Recombinant human thyrotropin (rhTSH) aided radioiodine treatment for residual or metastatic differentiated thyroid cancer.Cochrane Database Syst Rev 2010;11:CD008302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen J.A coefficient of agreement for nominal scales.Educ Psychol Meas 1960;20:37–46 [Google Scholar]
- 3.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0. The Cochrane Collaboration. Available at: http://www.cochrane-handbook.org [Accessed August 2012]
- 4.Pilli T, Brianzoni E, Capoccetti F, Castagna MG, Fattori S, Poggiu A, et al. A comparison of 1850 (50 mCi) and 3700 MBq (100 mCi) 131-iodine administered doses for recombinant thyrotropin-stimulated postoperative thyroid remnant ablation in differentiated thyroid cancer.J Clin Endocrinol Metab 2007;92:3542–3546 [DOI] [PubMed] [Google Scholar]
- 5.Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer.N Engl J Med 2012;366:1674–1685 [DOI] [PubMed] [Google Scholar]
- 6.Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer.N Engl J Med 2012;366:1663–1673 [DOI] [PubMed] [Google Scholar]
- 7.Chianelli M, Todino V, Graziano FM, Panunzi C, Pace D, Guglielmi R, et al. Low-activity (2.0 GBq; 54 mCi) radioiodine post-surgical remnant ablation in thyroid cancer: comparison between hormone withdrawal and use of rhTSH in low-risk patients.Eur J Endocrinol 2009;160:431–436 [DOI] [PubMed] [Google Scholar]
- 8.Hänscheid H, Lassmann M, Luster M, Thomas SR, Pacini F, Ceccarelli C, et al. Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal.J Nucl Med 2006;47:648–654 [PubMed] [Google Scholar]
- 9.Pacini F, Ladenson PW, Schlumberger M, Driedger A, Luster M, Kloos RT, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study.J Clin Endocrinol Metab 2006;91:926–932 [DOI] [PubMed] [Google Scholar]
- 10.Vaiano A, Claudio Traino A, Boni G, Grosso M, Lazzeri P, Colato C, et al. Comparison between remnant and red-marrow absorbed dose in thyroid cancer patients submitted to 131I ablative therapy after rh-TSH stimulation versus hypothyroidism induced by l-thyroxine withdrawal.Nucl Med Commun 2007;28:215–223 [DOI] [PubMed] [Google Scholar]
- 11.Barbaro D, Boni G, Meucci G, Simi U, Lapi P, Orsini P, et al. Recombinant human thyroid-stimulating hormone is effective for radioiodine ablation of post-surgical thyroid remnants.Nucl Med Commun 2006;27:627–632 [DOI] [PubMed] [Google Scholar]
- 12.Borget I, Remy H, Chevalier J, Ricard M, Allyn M, Schlumberger M, De Pouvourville G.Length and cost of hospital stay of radioiodine ablation in thyroid cancer patients: comparison between preparation with thyroid hormone withdrawal and thyrogen.Eur J Nucl Med Mol Imaging 2008;35:1457–1463 [DOI] [PubMed] [Google Scholar]
- 13.Duntas LH, Biondi B.Short-term hypothyroidism after levothyroxine-withdrawal in patients with differentiated thyroid cancer: clinical and quality of life consequences.Eur J Endocrinol 2007;156:13–19 [DOI] [PubMed] [Google Scholar]
- 14.Luster M, Felbinger R, Dietlein M, Reiners C.Thyroid hormone withdrawal in patients with differentiated thyroid carcinoma: a one hundred thirty-patient pilot survey on consequences of hypothyroidism and a pharmacoeconomic comparison to recombinant thyrotropin administration.Thyroid 2005;15:1147–1155 [DOI] [PubMed] [Google Scholar]
- 15.Montesano T, Durante C, Attard M, Crocetti U, Meringolo D, Bruno R, et al. Age influences TSH serum levels after withdrawal of l-thyroxine or rhTSH stimulation in patients affected by differentiated thyroid cancer.Biomed Pharmacother 2007;61:468–471 [DOI] [PubMed] [Google Scholar]
- 16.Robbins RJ, Larson SM, Sinha N, Shaha A, Divgi C, Pentlow KS, et al. A retrospective review of the effectiveness of recombinant human TSH as a preparation for radioiodine thyroid remnant ablation.J Nucl Med 2002;43:1482–1488 [PubMed] [Google Scholar]
- 17.Tuttle RM, Brokhin M, Omry G, Martorella AJ, Larson SM.Recombinant human TSH-assisted radioactive iodine remnant ablation achieves short-term clinical recurrence rates similar to those of traditional thyroid hormone withdrawal.J Nucl Med 2008;49:764–770 [DOI] [PubMed] [Google Scholar]
- 18.Wong R, Topliss DJ, Bach LA, Hamblin PS, Kalff V, Long F, et al. Recombinant human thyroid-stimulating hormone (Thyrogen) in thyroid cancer follow up: experience at a single institution.Intern Med J 2009;39:156–163 [DOI] [PubMed] [Google Scholar]
- 19.Wong R, Topliss DJ, Bach LA, Hamblin PS, Kalff V, Long F, Stockigt JR.Ablation of thyroid residues with 30 mCi (131)I: a comparison in thyroid cancer patients prepared with recombinant human TSH or thyroid hormone withdrawal.J Clin Endocrinol Metab 2002;87:4063–4068 [DOI] [PubMed] [Google Scholar]
- 20.Papadimitriou D, Kottou S, Oros L, Ilias I, Molfetas M, Tsapaki V, et al. Differentiated thyroid cancer: comparison of therapeutic iodine 131 biological elimination after discontinuation of levothyroxine versus administration of recombinant human thyrotropin.Ann Nucl Med 2006;20:63–67 [DOI] [PubMed] [Google Scholar]
- 21.Rosário PW, Borges MAR, Purisch S.Preparation with recombinant human thyroid-stimulating hormone for thyroid remnant ablation with 131I is associated with lowered radiotoxicity.J Nucl Med 2008;49:1776–1782 [DOI] [PubMed] [Google Scholar]
- 22.Mernagh P, Campbell S, Dietlein M, Luster M, Mazzaferri E, Weston AR.Cost-effectiveness of using recombinant human TSH prior to radioiodine ablation for thyroid cancer, compared with treating patients in a hypothyroid state: the German perspective.Eur J Endocrinol 2006;155:405–414 [DOI] [PubMed] [Google Scholar]
- 23.Bal C, Padhy AK, Jana S, Pant GS, Basu AK.Prospective randomized clinical trial to evaluate the optimal dose of 131I for remnant ablation in patients with differentiated thyroid carcinoma.Cancer 1996;77:2574–2580 [DOI] [PubMed] [Google Scholar]
- 24.Molinaro E, Giani C, Agate L, Biagini A, Pieruzzi L, Bianchi F, et al. Patients with differentiated thyroid cancer who underwent radioiodine thyroid remnant ablation with low-activity 131I after either recombinant human TSH or thyroid hormone therapy withdrawal showed the same outcome after a 10-year follow-up.J Clin Endocrinol Metab 2013;98:2693–2700 [DOI] [PubMed] [Google Scholar]
- 25.Sandeep TC, Strachan MW, Reynolds RM, Brewster DH, Scélo G, Pukkala E, et al. Second primary cancers in thyroid cancer patients: a multinational record linkage study.J Clin Endocrinol Metab 2006;91:1819–1825 [DOI] [PubMed] [Google Scholar]
- 26.Rubino C, de Vathaire F, Dottorini ME, Hall P, Schvartz C, Couette JE, et al. Second primary malignancies in thyroid cancer patients.Br J Cancer 2003;89:1638–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawka AM, Thabane L, Parlea L, Ibrahim-Zada I, Tsang RW, Brierley JD, et al. Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: a systematic review and meta-analysis.Thyroid 2009;19:451–457 [DOI] [PubMed] [Google Scholar]


