Invasive pulmonary aspergillosis (IPA) is the leading invasive fungal disease in high-risk hematologic patients, including those with acute myeloid leukemia (AML) receiving chemotherapy for induction of remission, and allogeneic hematopoietic cell transplant (HCT) recipients.1,2 Studies conducted in the 1990s and early 2000s reported crude mortality rates of 60–80%.3 However, recent data suggest that the mortality rate of IPA has decreased.4,5 Although the exact reasons for the improvement in the outcome are not clear, it is possible that this is partly due to the fact that IPA has been diagnosed at an earlier disease stage, after the introduction of routine chest computed tomography (CT) scans, and the use of serum biomarkers, such as galactomannan (GMI).
The concept of early diagnosis and improved outcomes is very familiar to hematologists since the outcome of hematologic malignancies is usually influenced by the tumor burden when treatment is started. For example, high white blood cell count at diagnosis of acute leukemia is associated with poor outcome,6 disease stage strongly influences the outcome in Hodgkin’s lymphoma,7 and high tumor burden is associated with poor prognosis in multiple myeloma.8 Therefore, it is tempting to speculate that fungal burden may influence the outcome of IPA, with poorer responses to treatment as long as the fungal burden increases.
The halo sign is the radiological representation of lung infarction that follows angioinvasion by hyphae. The nodule represents the coagulation necrosis, and the halo is the edema and hemorrhage that surrounds the zone of infarction. Although not specific, its presence in persistently febrile neutropenic patients must be interpreted as suggestive of an invasive mold disease. An important contribution to the management of IPA was made by studies showing the importance of the halo sign as the earliest detectable sign of disease. Caillot et al. analyzed the diagnosis of IPA in two periods. In the first, high-resolution chest computed tomography (CT) was obtained at the discretion of clinicians, based on clinical signs of infection, whereas in the second period CT scans were systematically performed, regardless of clinical signs. When CT scans were obtained upon clinical suspicion of IPA, the halo sign was detected in 13% of patients, compared with 92% when CT scans were systematically performed. This resulted in a reduction in the time to diagnosis, from seven to 1.9 days, and a marked improvement in the outcome.9 More recently, the importance of the halo sign as an early sign of IPA was demonstrated in a study in which base-line chest CT scans of 235 patients who participated in a randomized clinical trial were reviewed. The halo sign was observed in 61% of cases, and patients with the halo sign had significantly better responses to treatment and greater survival than did patients with other images.10 A similar finding was also observed in a smaller number of patients.11 In these studies, the most reasonable explanation for the improved outcome was that the halo sign represented an earlier stage of IPA, allowing the initiation of antifungal therapy with a lower fungal burden.
Although the outcome of IPA has improved, the prognosis is still poor, particularly in some groups of patients, such as allogeneic HCT recipients. Therefore, attempts to further improve the outcome are needed. In order to do this, clinicians should be able to recognize IPA before the appearance of the halo sign. A detailed description of the events that occur early in the course of IPA was recently provided by an animal model of IPA in persistently neutropenic rabbits, which focused on the first 96 h of IPA.12 In that study, rabbits were immunosuppressed with cytarabine and methylprednisolone, and challenged with 3×108 conidia of Aspergillus fumigatus via endobronchial instillation. The histopathology of the lung was examined at different time points after inoculation of conidia, and showed various alterations in the lung parenchyma before the occurrence of angioinvasion and lung infarction. These changes included the phagocytosis of conidia by alveolar macrophages as early as 4 h after inoculation, and inflammatory infiltration, conidia germination and hyphae formation after 8 h. Lung infarction occurred after 24 h, with the peak infarct score occurring by 30 h after inoculation. Lung infarction was preceded by the detection of galactomannan in the bronchoalveolar lavage, which appeared as early as 8 h after inoculation, and in the serum, which became positive about 12 h after inoculation.
All these inflammatory changes that occur before hyphae invade the blood vessels and cause lung infarction correspond to the “bronchoalveolar” phase of IPA. This phase is not recognized by clinicians because IPA is only diagnosed when the halo sign appears. The bronchoalveolar phase of IPA has been described in non-neutropenic patients, and presents different and usually ‘non-specific’ radiological findings, including areas of peribronchial consolidations, micro (<1 cm) centrilobular nodular opacities, ground-glass infiltrates, branching linear or nodular opacities with a ‘tree-in-bud’ appearance, and focal areas of bronchiectasis.5,13
The impact of neutrophil counts in determining the predominant form of IPA was described in a recently published paper by Bergeron et al. that analyzed the radiological picture of 55 patients with proven or probable IPA.14 Patients were divided into three groups: allogeneic HCT recipients (23 patients), acute leukemia (22 patients) and others (10 patients, all with other hematologic diseases). An angioinvasive pattern was present in 45% of acute leukemia patients and in 13% of HCT recipients. By contrast, an ‘airway-invasive’ (or bronchoalveolar) pattern (with centrilobular micronodules and tree-in-bud infiltrates without large nodules with the halo sign) was present in 44% of allogeneic HCT recipients and in 14% of patients with acute leukemia. Significant differences between these two groups were a higher proportion of prior corticosteroid exposure in HCT recipients, and of neutropenia in acute leukemia patients.
The neutrophil count seems to drive the predominant pattern of IPA. The neutrophils are key elements for containing the invasion of conidia into the bloodstream.15 Therefore, the more severely neutropenic the host is, the faster and more likely is the angioinvasion, making the early bronchoalveolar phase very short. In the persistently neutropenic rabbit animal model,12 the bronchoalveolar phase lasted approximately 24 h. In patients with various degrees of neutropenia and T-cell mediated immunodeficiency, the bronchoalveolar phase may be of a longer duration, opening a window of opportunity for early diagnosis of IPA.
The speed with which the angioinvasive phase evolves also explains the performance of diagnostic tests in neutropenic and non-neutropenic patients (Figure 1). With regards to this, the performance of the tests may be seen as a function of the duration of the bronchoalveolar phase, with a higher proportion of patients having positive cultures of respiratory secretions in the non-neutropenic setting (because the bronchoalveolar phase is long enough to spill out fungal elements) and a higher proportion of neutropenic patients having positive serum galactomannan as the main mycological criterion for the diagnosis. This was shown in the paper by Bergeron et al.14 who reported that positive cultures from respiratory secretions occurred in 83% of patients with radiological signs of bronchoalveolar IPA and in 17% of patients with the angioinvasive form of IPA. On the other hand, since the angioinvasive phase occurs earlier the more severely neutropenic the patient is, serum galactomannan is more likely to be positive. By contrast, in patients with higher neutrophil counts the angioinvasive phase is delayed (or even does not occur at all) and serum galactomannan is less likely to be positive.16
Figure 1.
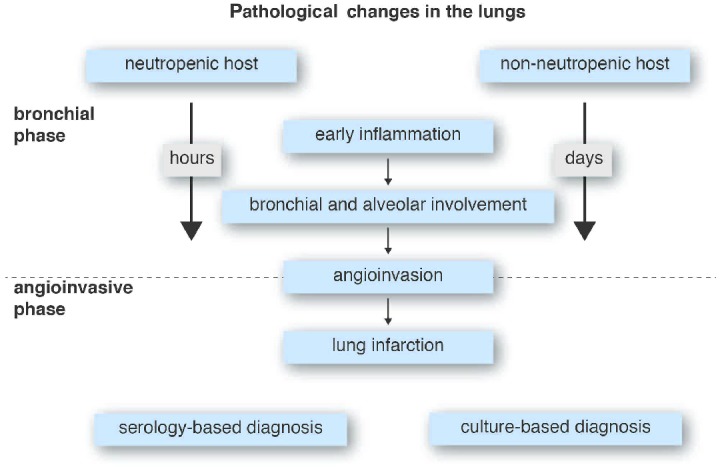
Evolution of invasive pulmonary aspergillosis in the bronchoalveolar and the angioinvasive phases in neutropenic and non-neutropenic patients.
We have recently proposed the inclusion of a new category of invasive aspergillosis which we called probable invasive aspergillosis without pre-specified radiological findings.5 We analyzed 125 episodes of invasive aspergillosis in patients with hematologic malignancies (mostly multiple myeloma). Patients were monitored after the start of antineoplastic therapy with serial (typically 3 times/week) serum galactomannan. Patients in the category of probable aspergillosis without pre-specified radiological findings were similar to those with probable invasive aspergillosis, with the same host, mycological and clinical features. The only exception was in the radiological appearance: none of them presented with the images that qualify for the EORTC/MSG diagnostic criteria of probable aspergillosis (i.e. well circumscribed lesions with or without the halo sign, air crescent or cavity).17 Instead, radiological findings consisted mostly of ill-defined consolidations and ground-glass infiltrates. Interestingly, 26 of 53 cases originally classified as probable aspergillosis without pre-specified radiological findings had repeated imaging about two weeks after the first CT examination. Eleven of these 26 patients (42%) were reclassified as probable aspergillosis by EORTC/MSG definitions because repeated images showed well-circumscribed consolidations and / or marconodules, suggesting that this category may represent an earlier stage of aspergillosis.
In another paper, Girmenia et al. analyzed 109 episodes of IPA (56 with AML, 31 with lymphoproliferative diseases, and 22 allogeneic HCT recipients).18 Seventy-six episodes were classified as proven or probable IPA, and 33 were probable aspergillosis without pre-specified radiological findings. All clinical and mycological criteria were similar between the two groups, with the exception of the radiological findings. Marconodules with halo sign were more likely to be present in neutropenic than in non-neutropenic patients (32.9% vs. 5.4%). Repeat imaging showed an evolution from the early images to the EORTC/MSG-defined images in 42.9% of AML patients, 50% of patients with lymphoproliferative diseases, and in 63.6% of allogeneic HCT recipients, confirming that the non-specific images represent an earlier stage of IPA.
More recently, Kyo et al. analyzed 30 cases of probable IPA in patients with acute leukemia.19 All patients were neutropenic, and had repeated CT scans performed every three days if fever persisted after treatment initiation, or if galactomannan serum was more than 0.4 while on treatment. Each patient had a median of eight CT scans. All patients exhibited a bronchoalveolar pattern of IPA preceding the angioinvasive phase. All patients survived. The authors concluded that the bronchoalveolar pattern seems to be the first CT abnormality of IPA, and that prevention of the angioinvasive phase may have contributed to the recent decrease in mortality of IPA.
How can we make a diagnosis of early IPA? The first step is to apply serial serum serology monitoring with galactomannan (3 times/week), and establish aggressive criteria for ordering a chest CT scan. The second step is to recognize the images that precede the halo sign and to interpret them as early IPA in the context of positive serum galactomannan (Figure 2). In non-neutropenic patients, since serum galactomannan may be negative, a bronchoalveolar lavage should be obtained and the diagnosis of IPA established on the basis of direct exam, culture and / or positive galactomannan.
Figure 2.
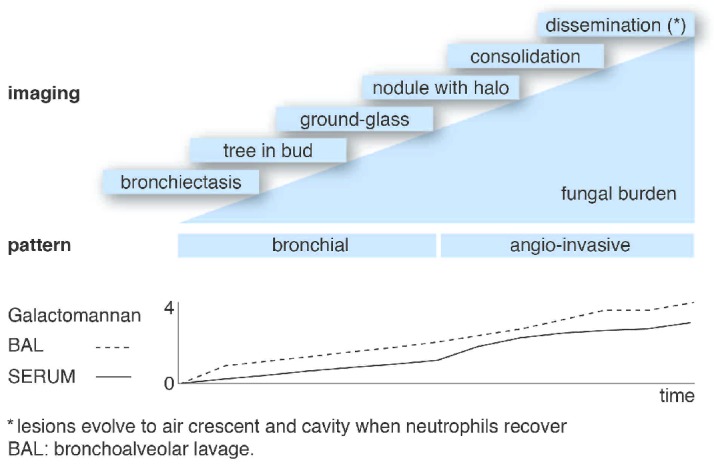
The clinical evolution of invasive pulmonary aspergillosis. *Lesions evolve to air crescent and cavity when neutrophils recover. BAL: bronchoalveolar lavage.
As stated above, diagnosing IPA at an earlier stage will result in the initiation of appropriate antifungal therapy when the fungal burden is still low. This may potentially further improve the outcome. In addition, by starting treatment earlier and preventing the occurrence of the angioinvasive phase of IPA it is possible that a shorter period of treatment will be needed. For example, in a cohort of 115 hematologic patients with invasive aspergillosis (32% with probable aspergillosis without pre-specified radiological findings), 37% received treatment for 14 days or under, with a 69% 6-week probability of survival in the entire cohort.20
In summary, the application of serial serum galactomannan and an aggressive strategy of early chest CT scan in neutropenic patients may allow clinicians to diagnose IPA with a low fungal burden, before the appearance of the halo sign, and thus prevent the occurrence of lung infarction. The potential benefits and costs of such an approach should be evaluated by prospective studies.
Footnotes
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.Pagano L, Caira M, Candoni A, Offidani M, Martino B, Specchia G, et al. Invasive aspergillosis in patients with acute myeloid leukemia: a SEIFEM-2008 registry study. Haematologica. 2010;95(4):644–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nucci M, Garnica M, Gloria AB, Lehugeur DS, Dias VC, Palma LC, et al. Invasive fungal diseases in haematopoietic cell transplant recipients and in patients with acute myeloid leukaemia or myelodysplasia in Brazil. Clin Microbiol Infect. 2013;19(8):745–51 [DOI] [PubMed] [Google Scholar]
- 3.Denning DW. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis. 1996;23(3):608–15 [DOI] [PubMed] [Google Scholar]
- 4.Maertens J, Buve K, Theunissen K, Meersseman W, Verbeken E, Verhoef G, et al. Galactomannan serves as a surrogate endpoint for outcome of pulmonary invasive aspergillosis in neutropenic hematology patients. Cancer 2009;115(2):355–62 [DOI] [PubMed] [Google Scholar]
- 5.Nucci M, Nouer SA, Grazziutti M, Kumar NS, Barlogie B, Anaissie E. Probable invasive aspergillosis without prespecified radiologic findings: proposal for inclusion of a new category of aspergillosis and implications for studying novel therapies. Clin Infect Dis. 2010;51(11):1273–80 [DOI] [PubMed] [Google Scholar]
- 6.Ganzel C, Rowe JM. Prognostic factors in adult acute leukemia. Hematol Oncol Clin North Am. 2011;25(6):1163–87 [DOI] [PubMed] [Google Scholar]
- 7.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med. 1998;339(21):1506–14 [DOI] [PubMed] [Google Scholar]
- 8.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;(15)23:3412–20 [DOI] [PubMed] [Google Scholar]
- 9.Caillot D, Casasnovas O, Bernard A, Couaillier JF, Durand C, Cuisenier B, et al. Improved management of invasive pulmonary aspergillosis in neutropenic patients using early thoracic computed tomographic scan and surgery. J Clin Oncol. 1997;15(1):139–47 [DOI] [PubMed] [Google Scholar]
- 10.Greene RE, Schlamm HT, Oestmann JW, Stark P, Durand C, Lortholary O, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007;44(3):373–9 [DOI] [PubMed] [Google Scholar]
- 11.Horger M, Hebart H, Einsele H, Lengerke C, Claussen CD, Vonthein R, et al. Initial CT manifestations of invasive pulmonary aspergillosis in 45 non-HIV immunocompromised patients: association with patient outcome? Eur J Radiol. 2005;55(3):437–44 [DOI] [PubMed] [Google Scholar]
- 12.Hope WW, Petraitis V, Petraitiene R, Aghamolla T, Bacher J, Walsh TJ. The initial 96 hours of invasive pulmonary aspergillosis: histopathology, comparative kinetics of galactomannan and (1–>3) beta-d-glucan and consequences of delayed antifungal therapy. Antimicrob Agents Chemother. 2010;54(11):4879–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kojima R, Tateishi U, Kami M, Murashige N, Nannya Y, Kusumi E, et al. Chest computed tomography of late invasive aspergillosis after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(7):506–11 [DOI] [PubMed] [Google Scholar]
- 14.Bergeron A, Porcher R, Sulahian A, De Bazelaire C, Chagnon K, Raffoux E, et al. The strategy for the diagnosis of invasive pulmonary aspergillosis should depend on both the underlying condition and the leukocyte count of patients with hematologic malignancies. Blood. 2012;119(8):1831–7 [DOI] [PubMed] [Google Scholar]
- 15.Mircescu MM, Lipuma L, van Rooijen N, Pamer EG, Hohl TM. Essential role for neutrophils but not alveolar macrophages at early time points following Aspergillus fumigatus infection. J Infect Dis 2009;200(4):647–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cordonnier C, Botterel F, Ben Anour R, Pautas C, Maury S, Kuentz M, et al. Correlation between galactomannan antigen levels in serum and neutrophil counts in haematological patients with invasive aspergillosis. Clin Microbiol Infect. 2009;15(1):81–6 [DOI] [PubMed] [Google Scholar]
- 17.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girmenia C, Guerrisi P, Frustaci AM, Fama A, Finolezzi E, Perrone S, et al. New category of probable invasive pulmonary aspergillosis in haematological patients. Clin Microbiol Infect. 2012;18(10):990–6 [DOI] [PubMed] [Google Scholar]
- 19.Kyo K, Ochi T, Okatani T, Itagaki M, Imanaka R, Katayama Y, et al. Is halo sign the earliest sign of pulmonary invasive aspergillosis (PIA)? Program and Abstracts of the 52th Interscience Conference on Antimicrobial Agents and Chemotherapy 2012;Abstract M-1226. [Google Scholar]
- 20.Nouer SA, Nucci M, Kumar NS, Grazziutti M, Barlogie B, Anaissie E. Earlier response assessment in invasive aspergillosis based on the kinetics of serum Aspergillus galactomannan: proposal for a new definition. Clin Infect Dis. 2011;53(7):671–6 [DOI] [PMC free article] [PubMed] [Google Scholar]


