Abstract
The outcome of children and adults with acute lymphoblastic leukemia is markedly different. Since there is limited information on the distribution of clinico-biological variables in different age cohorts, we analyzed 5202 patients with acute lymphoblastic leukemia enrolled in the Italian multicenter AIEOP and GIMEMA protocols and stratified them in nine age cohorts. The highest prevalence of acute lymphoblastic leukemia was observed in children, although a second peak was recorded from the 4th decade onwards. Interestingly, the lowest incidence was found in females between 14–40 years. Immunophenotypic characterization showed a B-lineage in 85.8% of patients: a pro-B stage, associated with MLL/AF4 positivity, was more frequent in patients between 10–50 years. T-lineage leukemia (14.2%) was rare among small children and increased in patients aged 10–40 years. The prevalence of the BCR/ABL1 rearrangement increased progressively with age starting from the cohort of patients 10–14 years old and was present in 52.7% of cases in the 6th decade. Similarly, the MLL/AF4 rearrangement constantly increased up to the 5th decade, while the ETV6/RUNX1 rearrangement disappeared from the age of 30 onwards. This study shows that acute lymphoblastic leukemia in adolescents and young adults is characterized by a male prevalence, higher percentage of T-lineage cases, an increase of poor prognostic molecular markers with aging compared to cases in children, and conclusively quantified the progressive increase of BCR/ABL+ cases with age, which are potentially manageable by targeted therapies.
Introduction
Acute lymphoblastic leukemia (ALL) is the most frequent neoplasm in children, whereas it is relatively rare in adults. Over the last decades, there has been a considerable improvement in the outcome of children, with complete remission and long-term survival rates reaching 95% and 80%, respectively.1,2 Contrariwise, in adults the survival rates generally do not exceed 40%.1,4–6 Several factors can explain this marked difference, including the more intensive regimens used in children, comprising high-dose methotrexate, asparaginase and reinduction therapy, fewer toxic effects, greater compliance to high doses of chemotherapy, and increased “physician and parent compliance” in pediatric oncology/hematology wards.7 As a proof of principle, several studies8–13 showed a superior outcome for adolescents and young adults treated with pediatric-like regimens. In adults, the most important risk factors are age, white blood cell (WBC) count, a pro-B ALL and poor prognosis molecular markers.6
Some biological characteristics are partly responsible for the different clinical scenarios, such as the increased incidence of the BCR/ABL114,15 and MLL/AF4 transcripts, negatively affecting prognosis, and a decreased incidence of ETV6/RUNX1 rearrangement, associated with a favorable outcome, in older patients.16 Only few studies have focused on a detailed analysis of clinico-biological features among various age cohorts and those that have done so usually took into account only few age groups in heterogeneous populations.
In this study, we retrospectively evaluated the clinical-biological features of 5202 ALL patients, enrolled in Italian multicenter protocols by AIEOP (Associazione Italiana di Ematologia ed Oncologia Pediatrica) and GIMEMA (Gruppo Italiano Malattie EMatologiche dell’Adulto) for the treatment of pediatric and adult ALL, respectively. All patients were uniformly characterized at presentation. Although comparison of outcomes in the various age cohorts considered was not a purpose of this study, event-free survival was not different from that previously reported:2–5 it decreased proportionally with age and was below 50% in patients >18 years, who, at the time of data collection, were treated with non-intensive regimens (Online Supplementary Figure S1).
Methods
Patients’ enrollment
Between April 1995 and June 2009, 5202 patients were included in the Italian pediatric (AIEOP) and adult (GIMEMA) multicenter protocols, and evaluated for clinico-biological features at presentation. Of 3753 children, 1711 were enrolled in the ALL 95 and 2042 in the ALL 2000 AIEOP trials - designed for children aged between 1–18 years. Of 1449 adults, 457 were enrolled in the GIMEMA LAL 0496 (age 14–60 years), 565 in the GIMEMA LAL 2000 (age 14–60 years), 385 in the GIMEMA LAL 0904 (age 15–60 years), and 42 in the GIMEMA LAL 1205 protocols; the last protocol, enrolling patients ≥18 years harboring the BCR/ABL1 rearrangement, was included because partly simultaneous with the LAL 0904 trial; these trials were previously approved by local ethical committees. All patients, parents or guardians gave their informed consent to blood/marrow collection and to biological analyses, in agreement with the Declaration of Helsinki.
For this study, patients were stratified into nine age cohorts: 1–5, 5–10, 10–14, 14–18, 18–25, 25–30, 30–40, 40–50, and 50–60 years. Infants were excluded from the analysis.
Clinico-biological features
Clinical parameters included gender, WBC count, platelet count and hemoglobin (Hb) levels, mediastinal, spleen and liver enlargement, and central nervous system (CNS) involvement. For WBC and platelet counts and Hb levels, the following cut-points were considered: 50×109/L, 100×109/L and 10 g/dL, respectively. The mediastinum, spleen and liver were considered enlarged if >3 cm. CNS involvement was defined as described previously.17
The diagnosis of ALL was based on May-Grünwald-Giemsa smears and immunophenotyping: the latter allowed definition of the lineage derivation and degree of differentiation of the leukemic cells. The cut-off for positivity was ≥20% for surface antigens and 10% for intracytoplasmatic antigens.
Cases of B-lineage ALL (B-ALL) were subdivided into B1 (pro-B ALL, CD10−), B2 [common-ALL, CD10+ and intracytoplasmic (cy) Igμ−] and B3 (pre-B ALL, CD10+ and cyIgμ+), and NC if not further classified (cyIgμ not tested).18 T-lineage ALL (T-ALL) cases were subdivided into T1 (pro-T and pre-T ALL, cyCD3+ and CD7+, CD2+ and/or CD5+ and/or CD8+, respectively); T2 (cortical T-ALL, CD1a+) and T3 (mature T-ALL, surface CD3+ and CD1a−).18
Molecular analysis of adults19 included BCR/ABL1, E2A/PBX1, ETV6/RUNX1 and MLL rearrangements (i.e. MLL/AF4 and MLL/ENL) screening for B-ALL patients, and BCR/ABL1 and MLL rearrangements for T-ALL cases; SIL/TAL1, NUP298/RAP and NUP214/ABL1 were investigated only in more recent trials, and since these data were not consistently available, they were not considered. Children were screened for BCR/ABL1, ETV6/RUNX1, MLL/AF4 and partly for E2A/PBX1.20
Since karyotyping data were not uniformly available, this parameter was not considered. Flow-cytometry and molecular analyses were centrally performed in two laboratories, one for pediatric cases and the other for adult cases.
Statistical analysis
Patients’ characteristics were summarized by cross-tabulation (categorical variables) and quantiles (ordinal factors). Non-parametric tests were applied, as appropriate, for comparisons between groups (Pearson's χ2, Mantel-Haenszel χ2 or Fisher’s exact test for categorical variables, Mann-Whitney or Kruskal-Wallis test for continuous variables). Analyses were performed using SAS v9.2 software. All tests were two-sided, accepting P values ≤0.05 as statistically significant.
Results
Incidence of acute lymphoblastic leukemia
The distribution of ALL among the different age cohorts is illustrated in Figure 1A. The majority of ALL cases was included within the 1–5 year age cohort (37% of the whole cohort) and the prevalence progressively decreased up to the 3rd decade; however, a slight increase in ALL was again recorded starting from the 4th decade onwards (>5% in the 30–40, 40–50 and 50–60 age groups).
Figure 1.
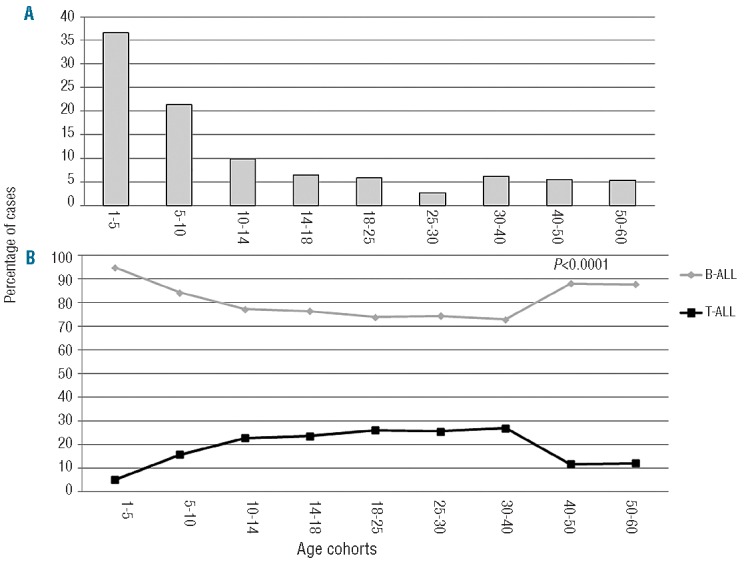
(A) Percentage of the distribution of ALL among various age groups; (B) Lineage derivation in the various age cohorts. BALL: gray line; T-ALL: black line.
Immunophenotye
Flow cytometry analysis revealed an overall predominance of B-ALL in the whole cohort (85.8%), while T-ALL was much less frequent (14.2%). The distribution of Band T-ALL was of interest (Table 1A, Figure 1B). The incidence of T-ALL increased from the 10–14 age cohort up to the 4th decade of life, with a tendency to decline thereafter. Conversely, B-ALL was less frequent in the same age framework (P<0.0001).
Table 1.
Immunophenotypic features (A), clinical parameters (B) and molecular features (C) among different age cohorts. Median counts and P-values are also indicated.
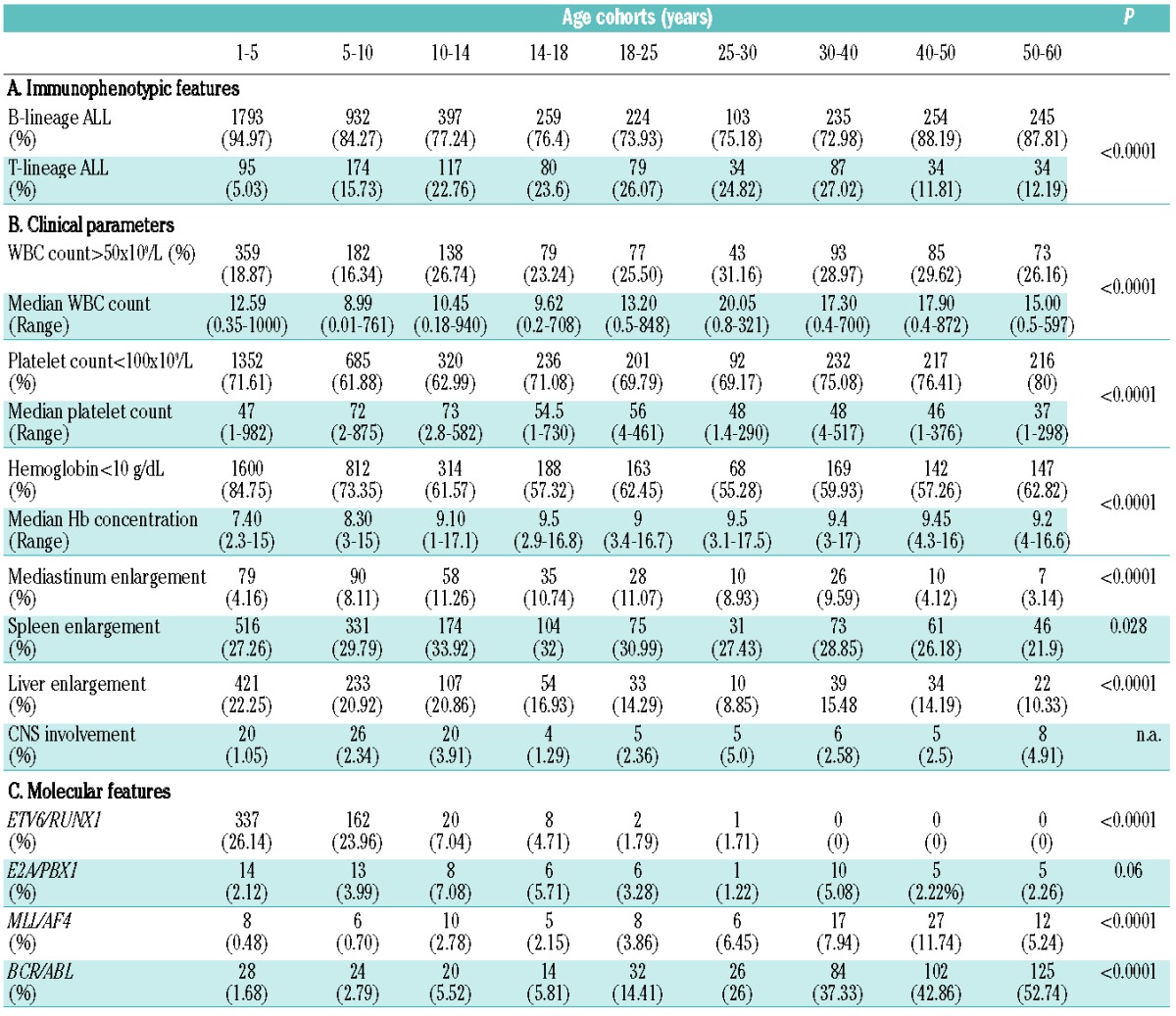
When looking at the differentiation stage of B-ALL, the most significant (P<0.0001) finding was a constant and significant increase in the percentage of pro-B up to the 5th decade of life: in small children (age cohorts 1–5 and 5–10 years) the incidence of pro-B ALL was 3.27% and 3.88%, in older children (10–14 years) and adolescents (14–18 years) it was 8.9% and 10.5%, while in adults it was 17.58%, 13.92%, 17.89% and 18.78% for the 18–25, 25–30, 30–40, 40–50 age cohorts, respectively (Online Supplementary Figure S2).
In T-ALL, a peak of pro-T/ pre-T ALL cases was recorded in the 5th and 6th decades, with an incidence of 68.18% and 58.33% of cases, respectively; consistently, the percentage of cortical T-ALL was very low in adults and young elderly (Online Supplementary Figure S3).
Gender distribution
The cohort evaluated included 2889 males and 2313 females, with a male-to-female ratio of 1.25. Gender distribution was of interest: in fact, while there was an overall prevalence of the male gender in almost all age cohorts, this phenomenon was particularly evident between the age of 14 and 40, started to revert in the 5th decade of life and females were prevalent over the age of 50 (P<0.0001; Figure 2A). Furthermore, a striking association was found between lineage derivation and gender, T-ALL being more frequently diagnosed in male subjects: this association was statistically significant up to the 4th decade of life (Figures 1B, 2A and 2B).
Figure 2.
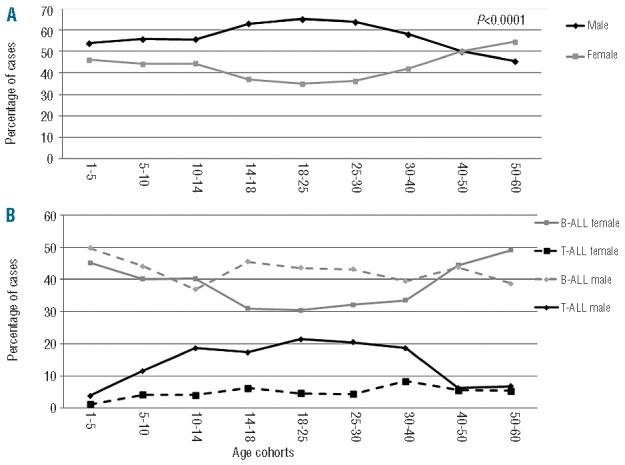
Gender distribution. (A) Gender distribution analysis among age cohorts. The analysis reveals a decreased incidence of females between 14 and 50 years. The gray line represents females, the black line males. (B) Gender distribution and lineage derivation among different age groups. The gray continuous line represents B-ALL females, the gray dotted line B-ALL males, the black continuous line T-ALL males and the black dotted line T-ALL females.
Hematologic parameters and correlation with immunophenotype
A WBC count >50×109/L was significantly less frequent in children, being recorded in 18.87% and 16.34% of patients aged 1–5 and 5–10, while it progressively increased from pre-adolescence onwards (P<0.0001); a platelet count <100×109/L was more frequently detected in patients older than the age of 30 years (P<0.0001); finally, Hb levels <10g/dL were more frequently detected in younger patients and even more in small children (1–5 years, P<0.0001) (Online Supplementary Figure S4A, Table 1B).
A WBC count >50×109/L was more frequently recorded in T-ALL than in B-ALL patients (53.41% versus 16.56%, P<0.0001), regardless of the age cohorts considered. However, in B-ALL a WBC count >50×109/L was relatively rare up to 25 years, while it was more recurrent form 25 years onwards. At variance, in T-ALL the scenario was almost opposite, with a WBC count >50×109/L detected in >45% of patients aged <30 years, and tended to decrease thereafter (P<0.0001, Online Supplementary Figure S4B).
Finally, a significantly higher percentage of cases with platelet count <100×109/L and Hb levels <10g/dL was observed in B-ALL compared to T-ALL (platelet count 70.12% versus 64.94% and Hb levels 77.14% versus 42.94%; P=0.0053 and P<0.0001, respectively; Online Supplementary Figure S4C,D).
Organ involvement
Organ involvement was considered; since reactive adenopathies are a frequent event in healthy children, this parameter was not taken into account.
Mediastinal, spleen and liver enlargements were prevalently recorded in T-ALL (P<0.0001). The first two were more frequently detected between 10 and 25 years (P<0.0001 and P=0.028, respectively; Table 1B, Online Supplementary Table S1, Figure 3A,B), while liver enlargement decreased with age (P<0.0001; Table 1B, Online Supplementary Table S1 and Figure 3C).
Figure 3.
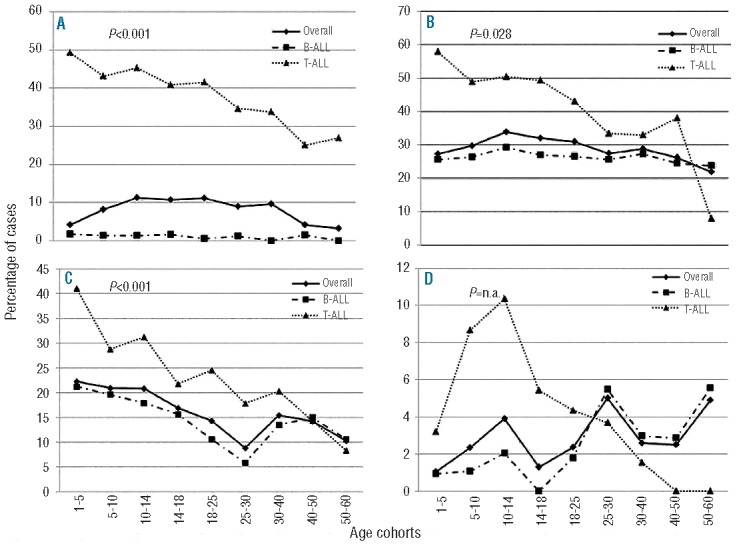
Organ involvement among age cohorts in the whole cohort (continuous line) and stratified according to the lineage derivation (B-ALL: dashed line, T-ALL: dotted line). (A) Mediastinal involvement; (B) spleen involvement; (C) liver involvement; (D) CNS involvement.
Finally, CNS involvement was a rare event (2%) and was more frequently detected in T-ALL than in B-ALL (5.95% versus 1.47%, P<0.0001); furthermore, in T-ALL, it was associated with hyperleukocytosis (P<0.0001). When patients were stratified according to age, a peak incidence of CNS positivity was recorded in the groups aged 10–14, 25–30 and 50–60 (3.91%, 5% and 4.91%, respectively). Interestingly, if patients were stratified for both lineage derivation and age, in T-ALL CNS positivity was a more frequent event in children, particularly in the age groups 5–10, 10–14 and 14–18 (8.67%, 10.34% and 5.41%, respectively), and then progressively decreased, whereas in BALL meningosis was more frequent in older patients, particularly in the 25–30 (5.48%) and 50–60 age groups (5.56%) (Table 1B, Online Supplementary Table S1 and Figure 3D): notably, in the latter age group, a significant association was found with the BCR/ABL1 rearrangement (10% of patients with BCR/ABL1 had CNS involvement, P=0.028).
Molecular analysis in B-lineage acute lymphoblastic leukemia
The overall frequency of molecular aberrations is reported in Table 1C and Figure 4.
Figure 4.
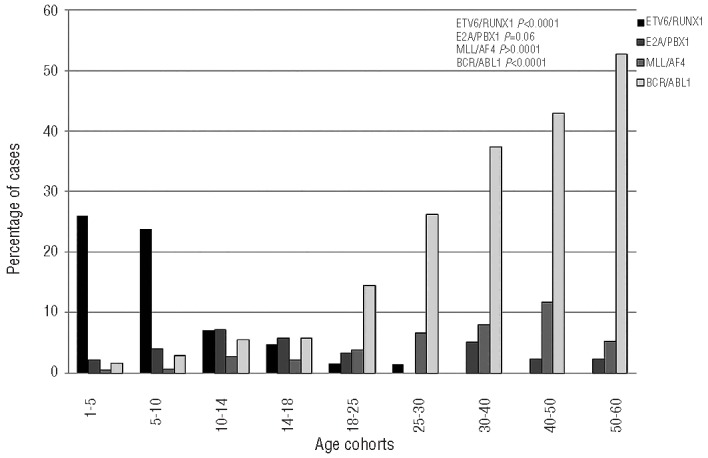
Incidence of the molecular aberrations in different age cohorts of B-ALL patients. A significant decrease of ETV6/RUNX1 is observed with age progression, while BCR/ABL and MLL1/AF4 rearrangements are more frequent in adults.
ETV6/RUNX1 was the most frequent alteration in small children (age cohorts 1–5: 26.14%; 5–10: 23.96%), being detected in more than 20% of cases; its incidence decreased progressively in pre-adolescents, adolescents and young adults (age cohorts 10–14: 7.04%; 14–18: 4.71%; 18–25: 1.49%; 25–30: 1.41%), and it disappeared in adults from 30 years onwards (P<0.0001).
The E2A/PBX1 aberration was relatively infrequent, being detected in ≤7% of cases in all age cohorts, without a specific trend; it was slightly more frequent in the 10–14 and 14–18 groups, reaching incidences of 7% and 5.7% respectively, but showed no significant variation (P=0.06).
The MLL/AF4 rearrangement was virtually absent in the 1–5 and 5–10 age groups (0.48% and 0.7%, respectively), while it progressively increased up to the 5th decade of life, being detected in 2.78%, 2.15%, 3.86%, 6.45%, 7.94% and 11.74% of patients in the 10–14, 14–18, 18–25, 25–30, 30–40, and 40–50 age cohorts, respectively, and decreased again to 5.24% in the 50–60 age cohort (P<0.0001).
Finally, the BCR/ABL1 rearrangement showed a striking behavior: in fact, it was very rare in small children (1.68% and 2.79% in the 1–5 and 5–10 age groups, respectively), while its frequency progressively increased with age (age cohorts 10–14: 5.52%; 14–18: 5.81%; 18–25: 14.41%, 25–30: 26%; 30–40: 37.33%; 40–50: 42.86%) reaching a frequency of 52.74% in patients of the 50–60 age group (P<0.0001).
Correlation between molecular aberrations, white blood cell count and flow cytometry in B-lineage acute lymphoblastic leukemia
To investigate whether specific aberrations might influence the WBC count at presentation, this parameter was correlated with the presence of ETV6/RUNX1, E2A/PBX1, MLL/AF4 and BCR/ABL1 rearrangements in the different age cohorts.
ETV6/RUNX1 was significantly associated with a lower WBC count when the whole cohort was considered (P=0.0001); the same trend was also observed in the 1–5, 5–10, 10–14 and 14–18 age groups, although it did not reach statistical significance. For the remaining groups, a statistical analysis was not feasible because of the small number/absence of positive cases (Online Supplementary Table S2).
No significant association was found between the E2A/PBX1 rearrangement and WBC count, either in the global series or in the various age cohorts (Online Supplementary Table S3). At variance, the MLL/AF4 rearrangement was associated with a higher WBC count in almost all age groups and in the whole cohort (P<0.0001, Online Supplementary Table S4).
Finally, the BCR/ABL1 rearrangement displayed a peculiar behavior (Online Supplementary Table S5): this aberration was associated with a higher percentage of patients with a WBC count >50×109/L in the whole cohort (P<0.0001) and in younger children (1–5 years), adolescents and young adults (10–30 years) and older patients (>50 years). There was no statistical association between BCR/ABL positivity and WBC count >50×109/L in the remaining age groups. The overall results are shown in Figure 5A.
Figure 5.
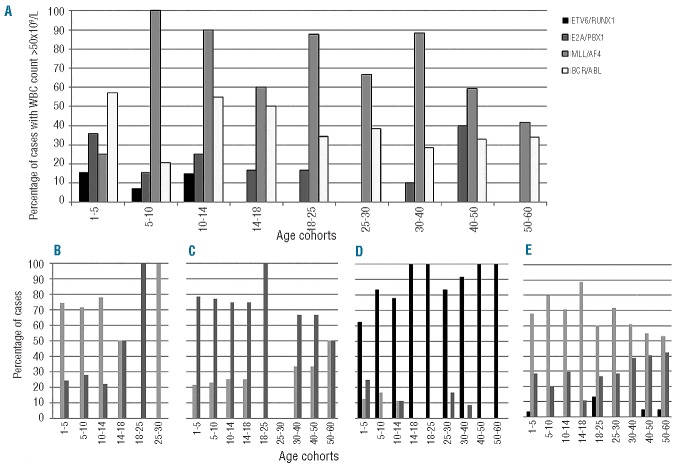
Hyperleukocytosis and stage of differentiation of B-ALL analyzed on the basis of the molecular aberrations. (A) Percentage of cases with hyperleukocytosis (WBC count >50×109/L) in cases with ETV6/RUNX1+ (black bars), E2A/PBX1+ (dark gray bars), MLL1/AF4+ (light gray bars) and BCR/ABL+ (white bars). (B–E) Stage of differentiation within the different molecular aberrations [(B) ETV6/RUNX1; (C) E2A/PBX1+; (D) MLL1/AF4; (E) BCR/ABL+]; the black bars indicate a pro-B ALL, the light gray bars a common ALL and the dark gray bars a pre-B ALL. Detailed P-values are provided in Online Supplementary Tables S2–S9.
Next, a correlation between molecular aberrations and flow cytometry was carried out (Online Supplementary Tables S6–S9). The ETV6/RUNX1 rearrangement was more frequently identified in common ALL (Figure 5B): this association reached statistical significance when the whole cohort was considered (P<0.0001) and in the age cohort 1–5 years old (P=0.025; Online Supplementary Table S6). The E2A/PBX1 aberration was never detected in proB ALL cases, while it was more frequent in pre-B ALL cases (Figure 5C); nevertheless, this finding was significant only when the whole cohort was considered (P<0.0001), and in the 1–5, 5–10 and 18–25 year old age cohorts (P=0.013, P=0.018 and P=0.012, respectively), probably because of the small number of positive cases in the other subgroups (Online Supplementary Table S7).
Contrariwise, the MLL/AF4 rearrangement was detected almost exclusively in pro-B ALL cases (Figure 5D) with this finding being highly significant in every age cohort (Online Supplementary Table S8).
Finally, the BCR/ABL1 fusion product was rarely detected in pro-B ALL cases (Figure 5E): the association between a non-pro-B stage and the presence of BCR/ABL transcript was statistically significant when the whole cohort was considered (P=0.0017) and starting from the 4th decade of life onwards, (P≤0.0001, Online Supplementary Table S9).
Discussion
ALL is a heterogeneous disease affecting children and adults with distinct incidences. According to the National Cancer Institute, Surveillance, Epidemiology and End Results (US-SEER) Program21 approximately 60.3% of ALL cases are diagnosed in patients under the age of 20; 10.3% between the ages of 20 and 34, 5.9% between 35 and 44 years old, 6.7% between 45 and 54, 6.1% between 55 and 64, 5.0% between 65 and 74, 4.0% between 75 and 84 and 1.7% in people over 85 years of age.
Since the clinical scenarios differ profoundly among age groups, particularly in terms of outcome,1–6 we considered data from a series of children and adults with ALL to evaluate the clinical, hematologic and biological features at diagnosis among the patients subdivided into nine age cohorts.
Few studies have looked at these features in a prospective, uniform manner. In children it was found that a hyperdyploid karyotype and the ETV6/RUNX1 aberration decrease with age, whereas the presence of BCR/ABL1 and an increased incidence of T-lineage ALL are more frequent in older children and young adults.7,22 A study on a relatively small cohort of adults with ALL23 found that the male/female ratio is decreased in older patients (>60 years) and that they have a decreased incidence of mediastinal involvement, adenopathy, and splenomegaly, a reduced median WBC count, and a lower percentage of T-lineage ALL.
The current study included 3753 children and 1449 adults enrolled between 1995 and 2009 in, respectively, two and four consecutive multicenter Italian protocols for children and adults. All cases were prospectively and uniformly characterized at the time of diagnosis.
The highest peak of incidence was detected in children between 1 and 5 years old; the occurrence of the disease increased again from the 4th decade, reaching a steady percentage of about 5.5%, as reported elsewhere23 and similar to the US-SEER data; in fact, in our cohort, the overall percentage of ALL cases was 74% in patients aged <18 years, 8.5% in cases between 18 and 30, and 6.2%, 5.5% and 5.3% in patients aged 30–40, 40–50 and 50–60 years, respectively. Patients ≥60 years were, at that time, eligible for only one protocol enrolling exclusively BCR/ABL1+ patients; we cannot, therefore, determine the incidence of ALL in these individuals.
Gender distribution was remarkable: starting from the 10–14 age cohort there was a striking decrease of the ALL rate in females; this tendency was evident up to the 4th decade of life, and disappeared in the cohort aged 50–60 years. This phenomenon seems to be associated with lineage derivation: in fact, the incidence of ALL is higher in male adolescents and young adults with T-ALL and only to a lesser extent with B-ALL. Although these data were partly known,24,25 the analysis on this broad cohort strongly suggests that age-related sex hormone levels may be a “protective factor” in ALL initiation in females and prompts further, prospective investigations of the contribution of the hormonal changes occurring during menarche and menopause.
Similarly, organ involvement was widely distributed: besides mediastinal enlargement, frequently detected in older children and young adults (age 10–25 years) and significantly associated with a T-lineage phenotype, spleen and liver enlargement, also associated with a T-lineage, were less frequent in older patients, in line with earlier reports,24,26 thus suggesting a physiological atrophy of lymphoid organs with aging. As previously reported,17,27 CNS involvement was rare, being found more frequently in adults than in children. In children, it was more frequently detected in T-ALL patients aged 5–18 years. The reason for the preferential contamination of CNS by leukemic T cells in children is not clear, but it might be postulated that adhesion and/or metastatic molecules are more expressed in pediatric leukemic T cells than in adult ones. This hypothesis is corroborated by the high levels of inter-leukin-15, a pro-inflammatory cytokine that promotes T-cell proliferation, detected in childhood ALL with CNS involvement.28 Otherwise, this behavior might be sustained by more invasive properties of pediatric T-ALL, as also suggested by the association with a higher WBC count, and organ enlargement observed in pediatric, but not in adult T-ALL.
Immunophenotypic analysis confirmed the overall higher incidence of B-ALL versus T-ALL, but also showed that T-ALL is significantly more frequent in patients aged between 14 and 40 years, thus confirming that this immunophenotypic subset is more represented in adolescents and young adults. Moreover, hyperleukocytosis (WBC >50×109/L) was infrequent in pediatric B-ALL and progressively increased with age in this group, whereas in T-ALL hyperleukocytosis was less frequent in patients >30 years. The study also showed that, among B-ALL, there is a progressive increase of pro-B cases up to the 5th decade of life, a finding significantly associated with the presence of the MLL/AF4 rearrangement. As far as concerns T-ALL, T1 cases were more frequently detected in the 5th and 6th decades; this finding is of interest, since early T-ALL cases tend to have a more unfavorable prognosis.29
Molecular screening showed the disappearance of the ETV6/RUNX1 rearrangement and the progressive increase of the BCR/ABL1 and MLL/AF4 fusion transcripts with age progression. The disappearance of ETV6/RUNX1 with aging further supports the notion that this subset of ALL has a prenatal origin, as indicated by neonatal blood spots or Guthrie card screening.30 Moreover, it is in agreement with the finding that a “second leukemogenic hit” must occur during childhood.31,32 It could be speculated that, upon puberty, the pre-leukemic clone harboring the ETV6/RUNX1 rearrangement is physiologically cleared out by other factors, such as hormonal changes or a normal degeneration of lymphoid development.
In our analysis the MLL/AF4 rearrangement was virtually absent in the cohort of patients 1–5 years old and was detected more frequently with increasing age, suggesting that the rearrangement detected in these patients is probably different from that of infant ALL. To validate this hypothesis, the 1–5 age cohort was further subdivided into single years and we did not detect an increased incidence of the transcript in the 1–2 years’ age group (data not shown).
It is, therefore, unlikely that the leukemogenic clone originates from in utero conditions, as opposed to infant MLL-rearranged leukemias:33 it is intriguing to speculate that infant MLL-rearranged leukemias require the concomitant presence of residual cord blood and/or maternal cells to occur, whereas in pediatric and adult MLL-rearranged leukemias these “nurturing” cells are no longer required. Indeed, previous gene expression profiling studies showed that infant MLL-rearranged leukemias share a common expression pattern with MLL-germline infant leukemias.34
Finally, the constant and progressive increase of BCR/ABL1 rearrangements in the elderly indicates that the genomic instability associated with aging leads to the appearance of the leukemic clone and the accumulation of additional oncogenic hits.
Overall, the variable incidence of these lesions suggests that in children harboring ETV6/RUNX1 and, to a lesser extent, MLL/AF4 the driver event is prenatal, whereas in BCR/ABL1+ ALL, the driver event occurs during life-time and induces an overt leukemia only when several additional hits occur.
While ETV6/RUNX1+ cases were characterized by a low WBC count in the whole cohort, a striking association between WBC count >50×109/L was found in all MLL/AF4+ patients, but not in all patients harboring the BCR/ABL1 rearrangement. This finding, particularly evident in patients aged 30 and 50 years, did not change when a different cut-off point was used (i.e. 30×109/L, data not shown). The fact that BCR/ABL1+ patients have a different clinical presentation is in line with the well-documented biological heterogeneity of this subset at the transcriptional level35 and with the presence of additional molecular lesions, such as IKZF1 deletions.36
Overall, this study – involving the largest cohort of ALL cases uniformly characterized across ages – confirms and extends previous results on the incidence and distribution of clinico-biological characteristics.7,14–16,22–26 Furthermore, it provides three important pieces of information. First, it conclusively shows that the subset of adolescents and young adults has a higher frequency of hyperleukocytosis and low platelet count, a predominance of males, a greater percentage of T-ALL, and an increase of unfavorable prognosis aberrations. Altogether, these data help to understand the worse outcome of this age group and support the use of more intensive regimens in it.8–13
Secondly, the study shows that the incidence of molecular aberrations varies with age. Nevertheless, the associated clinical characteristics do not change among the different age cohorts, indicating that the most important factor for leukemia initiation is the biological mechanism/s responsible for the emergence of the molecular lesion/s.
Finally, it shows that MLL/AF4 and BCR/ABL1 rearrangements constantly increase with age. This is particularly relevant for the BCR/ABL1 fusion, detected in 52.7% of the 50–60 year-old subgroup. Given the profound changes in the management of Philadelphia-positive ALL following the advent of tyrosine kinase inhibitors,37–39 less fit patients can also be successfully treated nowadays. Indeed, it is now possible to treat patients effectively with tyrosine kinase inhibitors plus steroids without systemic chemotherapy,37,39 which is a viable option for elderly ALL patients.
In conclusion, this large retrospective study on adult and pediatric ALL characterized at presentation has allowed better definition of clinico-biological features in the various age subgroups, helps to unravel the differences in clinical behavior and outcome, and provides therapeutic indications for specific subgroups of patients.
Footnotes
The online version of this article has a Supplementary Appenix.
Funding
This work was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC) Special Program Molecular Clinical Oncology, 5 × 1000, Milan, Italy; Ministero dell’Università e Ricerca (MIUR), Fondo per gli Investimenti della Ricerca di Base (FIRB), Rome, Italy; Associazione Italiana per la Ricerca sulla leucemia (AIL), Italy; Compagnia di San Paolo, Turin, Italy; Progetto “Oncologia”, Ministero della Salute, Rome, Italy;Fondazione Cariplo, Milano, Italy, project ENCCA (European network of Cancer in Children and Adolescents, grant agreement HEALTH-F2-2011-261474 (EU-FP7/2007-2013).
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Silverman LB, Gelber RD, Dalton VK, Asselin BL, Barr RD, Clavell LA, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97(5):1211–8 [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371 (9617):1030–43 [DOI] [PubMed] [Google Scholar]
- 3.Annino L, Vegna ML, Camera A, Specchia G, Visani G, Fioritoni G, et al. Treatment of adult acute lymphoblastic leukemia (ALL): long-term follow-up of the GIMEMA ALL 0288 randomized study. Blood. 2002;99 (3):863–71 [DOI] [PubMed] [Google Scholar]
- 4.Rowe JM, Buck G, Burnett AK, Chopra R, Wiernik PH, Richards SM, et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106 (12):3760–7 [DOI] [PubMed] [Google Scholar]
- 5.Thomas X, Boiron JM, Huguet F, Dombret H, Bradstock K, Vey N, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol. 2004;22(20):4075–8 [DOI] [PubMed] [Google Scholar]
- 6.Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29(5):532–43 [DOI] [PubMed] [Google Scholar]
- 7.Nachman J. Clinical characteristics, biologic features and outcome for young adult patients with acute lymphoblastic leukaemia. Br J Haematol. 2005;130(2):166–73 [DOI] [PubMed] [Google Scholar]
- 8.Boissel N, Auclerc MF, Lhéritier V, Perel Y, Thomas X, Leblanc T, Rousselot P, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol. 2003; 21(5):774–80 [DOI] [PubMed] [Google Scholar]
- 9.de Bont JM, Holt B, Dekker AW, van der Does-van den Berg A, Sonneveld P, Pieters R, et al. Significant difference in outcome for adolescents with acute lymphoblastic leukemia treated on pediatric vs adult protocols in the Netherlands. Leukemia. 2004;18 (12):2032–5 [DOI] [PubMed] [Google Scholar]
- 10.Barry E, DeAngelo DJ, Neuberg D, Stevenson K, Loh ML, Asselin BL, et al. Favorable outcome for adolescents with acute lymphoblastic leukemia treated on Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium Protocols. J Clin Oncol. 2007;25 (7):813–9 [DOI] [PubMed] [Google Scholar]
- 11.Ribera JM, Oriol A, Sanz MA, Tormo M, Fernández-Abellán P, del Potro E, et al. Comparison of the results of the treatment of adolescents and young adults with standard-risk acute lymphoblastic leukemia with the Programa Español de Tratamiento en Hematología pediatric-based protocol ALL-96. J Clin Oncol. 2008;26(11):1843–9 [DOI] [PubMed] [Google Scholar]
- 12.Stock W, La M, Sanford B, Bloomfield CD, Vardiman JW, Gaynon P, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children’s Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112(5):1646–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huguet F, Leguay T, Raffoux E, Thomas X, Beldjord K, Delabesse E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. 2009;27(6):911–8 [DOI] [PubMed] [Google Scholar]
- 14.Burmeister T, Schwartz S, Bartram CR, Gökbuget N, Hoelzer D, Thiel E, et al. for the GMALL Study Group Patients’ age and BCR-ABL frequency in adult B-precursor ALL: a retrospective analysis from the GMALL study group. Blood. 2008;112(3):918–9 [DOI] [PubMed] [Google Scholar]
- 15.Gleissner B, Gökbuget N, Bartram CR, Janssen B, Rieder H, Janssen JW, et al. Leading prognostic relevance of the BCR-ABL translocation in adult acute B-lineage lymphoblastic leukemia: a prospective study of the German Multicenter Trial Group and confirmed polymerase chain reaction analysis. Blood. 2002;99(5):1536–43 [DOI] [PubMed] [Google Scholar]
- 16.Harrison CJ. Cytogenetics of paediatric and adolescent acute lymphoblastic leukaemia. Br J Haematol. 2009;144(2):147–56 [DOI] [PubMed] [Google Scholar]
- 17.Bürger B, Zimmermann M, Mann G, Kühl J, Löning L, Riehm H, et al. Diagnostic cere-brospinal fluid examination in children with acute lymphoblastic leukemia: significance of low leukocyte counts with blasts or traumatic lumbar puncture. J Clin Oncol. 2003;21(2): 184–8 [DOI] [PubMed] [Google Scholar]
- 18.Bene MC, Castoldi G, Knapp W, Ludwig WD, Matutes E, Orfao A, et al. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL) Leukemia. 1995;9(10): 1783–6 [PubMed] [Google Scholar]
- 19.Elia L, Mancini M, Moleti L, Meloni G, Buffolino S, Krampera M, et al. A multiplex reverse transcriptase-polymerase chain reaction strategy for the diagnostic molecular screening of chimeric genes: a clinical evaluation on 170 patients with acute lymphoblastic leukemia. Haematologica. 2003;88(3):275–9 [PubMed] [Google Scholar]
- 20.van Dongen JJ, Macintyre EA, Gabert JA, Delabesse E, Rossi V, Saglio G, et al. Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease. Report of the BIOMED-1 Concerted Action: investigation of minimal residual disease in acute leukemia. Leukemia. 1999;13(12):1901–28 [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institue, Surveillance, Epidemiology and End Results Program. http://seer.cancer.gov/csr/gov/1975_2008 Visited on date 06/06/2012.
- 22.Chessels JM, Swansbury GJ, Reeves B, Bailey CC, Richards SM. Cytogenetics and prognosis in childhood lymphoblastic leukaemia: results of MRC UKALL X. Medical Research Council Working Party in Childhood Leukaemia. Br J Haematol. 1997;99(1):93–100 [DOI] [PubMed] [Google Scholar]
- 23.Thomas X, Olteanu N, Charrin C, Lhéritier V, Magaud JP, Fiere D. Acute lymphoblastic leukemia in the elderly: The Edouard Herriot Hospital experience. Am J Hematol. 2001;67 (2):73–83 [DOI] [PubMed] [Google Scholar]
- 24.Larson RA. Acute lymphoblastic leukemia: Older patients and newer drugs. Hematology Am Soc Hematol Educ Program. 2005:131–136 [DOI] [PubMed] [Google Scholar]
- 25.Cartwright RA, Gurney KA, Moorman AV. Sex ratios and the risks of haematological malignancies. Br J Haematol. 2002;118 (4):1071–7 [DOI] [PubMed] [Google Scholar]
- 26.Chessells JM, Hall E, Prentice HG, Durrant J, Bailey CC, Richards SM. The impact of age on outcome in lymphoblastic leukaemia; MRC UKALL X and XA compared: a report from the MRC Paediatric and Adult Working Parties. Leukemia. 1998; 12 (4)463–73 [DOI] [PubMed] [Google Scholar]
- 27.Gökbuget N, Hoelzer D. Meningeosis leukaemica in adult acute lymphoblastic leukaemia. J Neurooncol. 1998;38(2–3):167–80 [DOI] [PubMed] [Google Scholar]
- 28.Cario G, Izraeli S, Teichert A, Rhein P, Skokowa J, Möricke A, et al. High inter-leukin-15 expression characterizes childhood acute lymphoblastic leukemia with involvement of the CNS. J Clin Oncol. 2007;25(30): 4813–20 [DOI] [PubMed] [Google Scholar]
- 29.Coustan-Smith E, Mullighan CG, Onciu M, Behm FG, Raimondi SC, Pei D, et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol. 2009;10(2):147–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greaves M. Childhood leukaemia. BMJ. 2002;324(7332):283–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bateman CM, Colman SM, Chaplin T, Young BD, Eden TO, Bhakta M, et al. Acquisition of genome-wide copy number alterations in monozygotic twins with acute lymphoblastic leukemia. Blood. 2010;115(17):3553–8 [DOI] [PubMed] [Google Scholar]
- 32.Anderson K, Lutz C, van Delft FW, Bateman CM, Guo Y, Colman SM, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469(7330): 356–61 [DOI] [PubMed] [Google Scholar]
- 33.Gale KB, Ford AM, Repp R, et al. Backtracking leukemia to birth: identification of clonotypic gene fusion sequences in neonatal blood spots. Proc Natl Acad Sci USA. 1997;94(25):13950–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stam RW, Schneider P, Hagelstein JA, van der Linden MH, Stumpel DJ, de Menezes RX, et al. Gene expression profiling-based dissection of MLL translocated and MLL germline acute lymphoblastic leukemia in infants. Blood. 2010;115(14):2835–44 [DOI] [PubMed] [Google Scholar]
- 35.Chiaretti S, Li X, Gentleman R, Vitale A, Wang KS, Mandelli F, et al. Gene expression profiles of B-lineage adult acute lymphocytic leukemia reveal genetic patterns that identify lineage derivation and distinct mechanisms of transformation. Clin Cancer Res. 2005;11(20): 7209–19 [DOI] [PubMed] [Google Scholar]
- 36.Mullighan CG, Su X, Zhang J, Radtke I, Phillips LA, Miller CB, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360(5):470–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vignetti M, Fazi P, Cimino G, Martinelli G, Di Raimondo F, Ferrara F, et al. Imatinib plus steroids induces complete remissions and prolonged survival in elderly Philadelphia chromosome-positive patients with acute lymphoblastic leukemia without additional chemotherapy: results of the Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) LAL0201-B protocol. Blood. 2007;109(9):3676–8 [DOI] [PubMed] [Google Scholar]
- 38.Bassan R, Rossi G, Pogliani EM, Di Bona E, Angelucci E, Cavattoni I, et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group. J Clin Oncol. 2010;28(22): 3644–52 [DOI] [PubMed] [Google Scholar]
- 39.Foà R, Vitale A, Vignetti M, Meloni G, Guarini A, De Propris MS, et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118 (25):6521–8 [DOI] [PubMed] [Google Scholar]