Abstract
Carfilzomib, a selective proteasome inhibitor, was approved in 2012 for the treatment of relapsed and refractory multiple myeloma. Safety data for single-agent carfilzomib have been analyzed for 526 patients with advanced multiple myeloma who took part in one of 4 phase II studies (PX-171-003-A0, PX-171-003-A1, PX-171-004, and PX-171-005). Overall analyses of adverse events and treatment modifications are presented, as well as specific analyses of adverse events by organ system. Overall, the most common adverse events of any grade included fatigue (55.5%), anemia (46.8%), and nausea (44.9%). In the grouped analyses, any grade adverse events were reported in 22.1% for any cardiac (7.2% cardiac failure), 69.0% for any respiratory (42.2% dyspnea), and 33.1% for any grouped renal impairment adverse event (24.1% increased serum creatinine). The most common non-hematologic adverse events were generally Grade 1 or 2 in severity, while Grade 3/4 adverse events were primarily hematologic and mostly reversible. There was no evidence of cumulative bone marrow suppression, either neutropenia or thrombocytopenia, and febrile neutropenia occurred infrequently (1.1%). Notably, the incidence of peripheral neuropathy was low overall (13.9%), including patients with baseline peripheral neuropathy (12.7%). Additionally, the incidence of discontinuations or dose reductions attributable to adverse events was low. These data demonstrate that single-agent carfilzomib has an acceptable safety profile in heavily pre-treated patients with relapsed/refractory multiple myeloma. The tolerable safety profile allows for administration of full-dose carfilzomib, both for extended periods and in a wide spectrum of patients with advanced multiple myeloma, including those with pre-existing comorbidities.
Introduction
The American Cancer Society estimates that there will be approximately 22,350 new cases of and 10,710 deaths from multiple myeloma (MM) in the United States in 2013.1 Despite multiple treatment options, MM remains largely incurable with median survival of approximately seven years2 and a poor outcome for patients who have become refractory to current treatments, with median event free and overall survival of five and nine months, respectively.3 Existing agents, including the proteasome inhibitor bortezomib and the immunomodulatory agents thalidomide and lenalidomide, have improved outcomes in patients with relapsed and refractory (RR) MM4,5 including increased survival rates.6 As MM remains incurable and there is a lack of adequate treatment for patients who have failed these agents, MM treatments with greater efficacy and improved safety profiles are needed, especially for patients with advanced disease.3 Carfilzomib, a selective proteasome inhibitor, was granted approval in 2012 in the United States for RRMM based on efficacy results from the single-arm trial PX-171-003-A1787,8 and combined safety data from 4 phase II studies (PX-171-003-A0 [003-A0], PX-171-003-A1 [003-A1], PX-171-004 [004], and PX-171-005 [005])8 and was followed by the approval of pomalidomide, an immunomodulatory agent, in 2013.9 Symptoms in MM are diverse, and patients can range from asymptomatic to severely disabled with multiple complications.10 Clinical features of active MM include hypercalcemia, renal insufficiency, anemia, and bone lesions, as well as hyperviscosity, amyloidosis, and recurrent bacterial infections,11 particularly respiratory infections, including pneumonia, one of the most common causes of death in patients with MM.12 Symptoms and comorbidities are increased in patients with relapsed and refractory disease, and treatment of these late-stage patients is particularly complicated due to the heterogeneity of the disease and patients’ characteristics.5,13
An important consideration in RRMM is the collective side effect profile associated with MM treatments. In particular, bortezomib and thalidomide are each associated with toxicities that may limit long-term use and/or use in selected patients.4,5,9,10,14 Thrombosis, for example, can be observed in all stages of MM but there is an increased risk with immunomodulatory drugs when combined with dexamethasone, as well as with the combination of melphalan and prednisone.15 Additionally, immune-mediated inflammatory diseases are associated with myelosuppression.9,16–18 Bortezomib is commonly associated with gastrointestinal (GI) adverse events (AEs), reactivation of herpes zoster, bone marrow suppression including thrombocytopenia, and dose-limiting peripheral neuropathy (PN) (up to 30% Grade 1/2 and 7–15% Grade 3/4). The PN often leads to discontinuation, and can be debilitating and occasionally irreversible.19–21 Thalidomide is even more strongly implicated in PN,22 and a recent analysis of patients with newly diagnosed MM revealed that although thalidomide improved efficacy when added to melphalan-prednisone, it negatively impacted safety.23 Maintenance therapy, as well as consolidation strategies, with many of these drugs are being investigated as important ways to improve and prolong responses in patients with MM,24 and these extended treatment periods may draw increased attention to tolerability and cumulative toxicities when considering long-term treatment options.
Carfilzomib was initially evaluated in 2 phase I studies (PX-171-00125 and PX-171-00226) investigating two different dosing schedules: 5 consecutive days of a 14-day cycle and 2 consecutive days/week for 3 weeks of a 28-day cycle). Consecutive day dosing demonstrated promising antitumor activity. However, single-agent carfilzomib administered using the 2 consecutive day dosing schedule (PX-171-002) was better tolerated and was chosen for further exploration in phase II studies. Here we present combined safety data for single-agent carfilzomib in 526 patients with advanced MM who took part in one of the 4 phase II studies (Figure 1).
Figure 1.
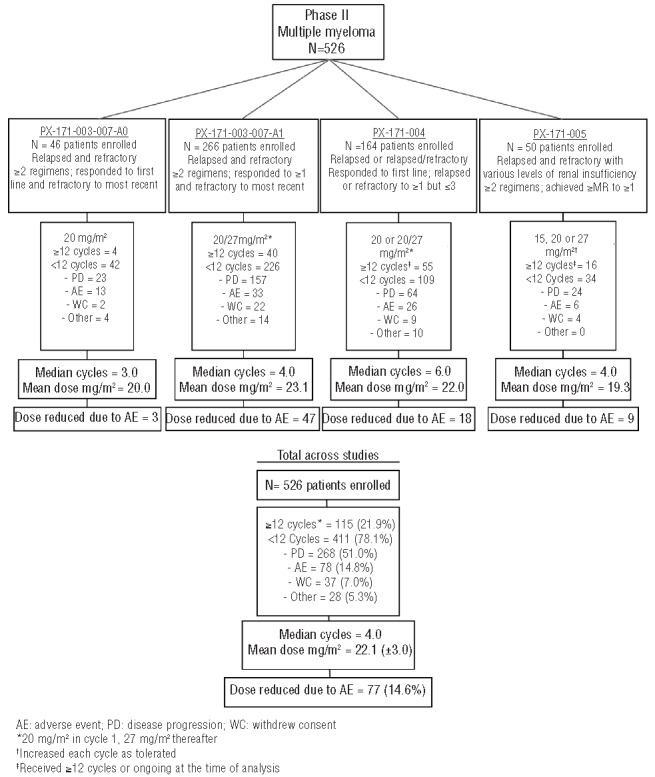
Overview of phase II carfilzomib safety studies.
Methods
This analysis was based on 4 phase II studies, the methods of which have been presented elsewhere: 003-A0,27 003-A1 (NCT00511238),7 004 (NCT00530816),28,29 and 005 (NCT00721734).30
Patients
Patients 18 years of age or over with histologically confirmed MM by serum M-protein (≥1 g/dL) or urine M-protein (≥200 mg/24 h) were eligible. An Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 and adequate organ and bone marrow function (including WBC ≥2 × 109/L, ANC ≥1 × 109/L, hemoglobin ≥8.0 g/dL, platelets ≥50 × 109/L, and creatinine clearance (CrCl) ≥30 mL/min) were required. Patients enrolled in study 005 could have hemoglobin ≥7.0 g/dL, platelets ≥30 × 109/L; varying degrees of renal insufficiency; or be undergoing chronic hemodialysis. Patients with pre-existing Grade 1 or 2 (without pain) neuropathy were permitted to enroll. Patients with congestive heart failure (CHF, New York Heart Association class III to IV), symptomatic cardiac ischemia, conduction abnormalities uncontrolled by conventional intervention, or myocardial infarction within the previous six months (3 months for 005) were excluded.
Drug administration
Carfilzomib was administered intravenously (IV) over 2–10 minutes on Days (D) 1, 2, 8, 9, 15, and 16 in 28-day cycles. To ameliorate fever, chills, shortness of breath, and/or rigors observed in phase I studies in a small number of patients,26 dexamethasone (4 mg) PO or IV was administered prior to all doses of carfilzomib in Cycle 1, prior to all doses during the first dose escalation, and at the discretion of the investigator in later cycles. The planned dose regimen was 20/27 mg/m2 (starting dose of 20 mg/m2 in Cycle 1 escalating to 27 mg/m2 in Cycle 2) for all studies except 005 (15/20/27 mg/m2 in Cycles 1–3 n=50). Dose reduction guidelines were provided for prolonged hematologic AEs (Grade 3 neutropenia, Grade 4 thrombocytopenia, and lymphopenia) and for Grade 3 or over non-hematologic toxicities. Conditions that did not require dose reduction were: Grade 3 nausea, vomiting, and diarrhea; Grade 3 fatigue; alopecia of any grade; Grade 3 or higher hyperglycemia attributed to dexamethasone (003-A1, 004, and 005).
Overall analysis
All patients who received one dose or more of carfilzomib were included in this safety analysis. Carfilzomib exposure was analyzed using summary statistics; summaries of dose modifications, patients’ demographics and disease characteristics, and patient disposition were tabulated. Safety data included incidence, severity, duration, and outcome of AEs. AEs were coded using Medical Dictionary for Regulatory Activities (MedDRA) version 8.1 terminology and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v.3.0.31 Treatment-emergent AEs were defined as AEs that started on or after the first day of carfilzomib administration or conditions that were present at baseline but worsened in severity following treatment. Treatment-related AEs included those that were possibly or probably related to treatment.
Analysis of adverse events by organ system
Analyses were performed using grouped terms for hematologic, cardiac, renal, GI, PN, pulmonary, and hepatic events based on frequency and severity of AEs (Online Supplementary Methods). All reported events of tumor lysis syndrome (TLS) were analyzed (Online Supplementary Methods). Treatment-related AEs were not investigated for these analyses.
Results
Overall analysis
Patients’ characteristics
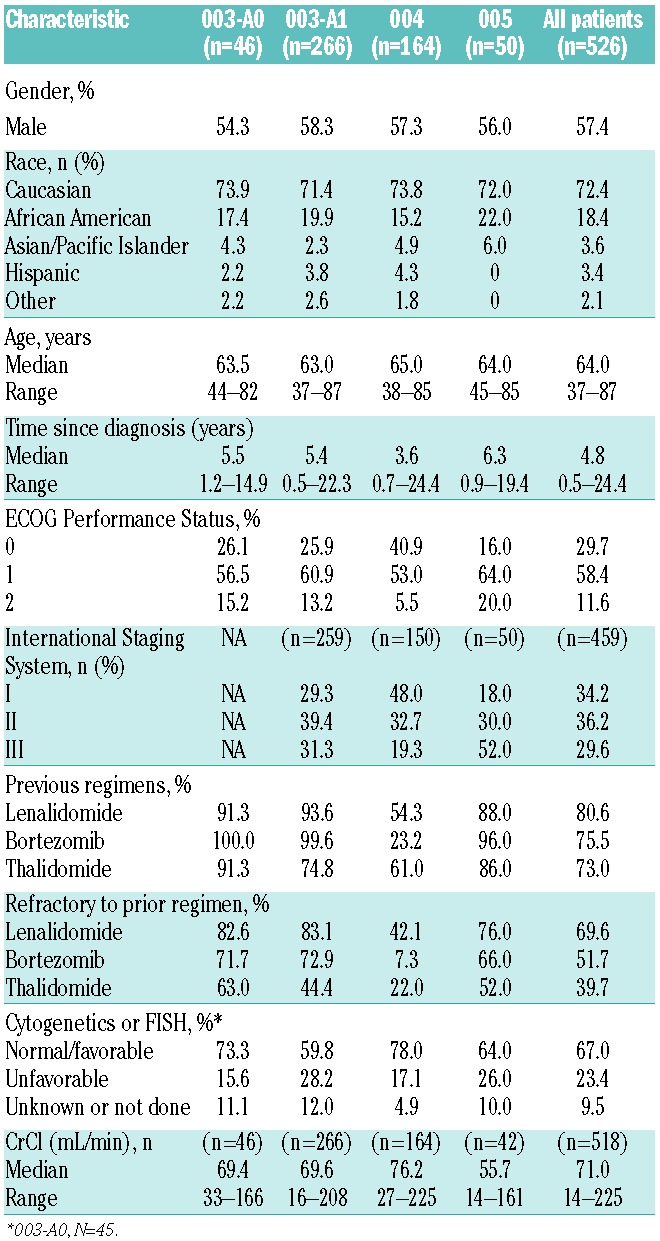
A total of 526 patients across all 4 phase II studies were included in the analysis; 72.4% were Caucasian, 57.4% were male, and the median age was 64 years (range 37–87; Table 1). Median time from MM diagnosis to study treatment was 4.8 years. Of the total population, 23.4% of patients had known unfavorable characteristics as defined by either cytogenetic or fluorescence in situ hybridization (FISH) analysis (including either t(4;14), t(14;16), deletion (17p;13) by cytogenetics/FISH or deletion (13q;14) by cytogenetics) based on the mSMART criteria,32 and 29.6% had stage III disease as according to the International Staging System. The patient population was heavily pre-treated with a median of 4 prior regimens. Greater than 75% of the population had received prior treatment with both lenalidomide and bortezomib; 51.7% and 69.6% of the population was refractory to bortezomib and lenalidomide, respectively.
Table 1.
Baseline patients’ and disease characteristics.
Drug exposure, dose modifications, and discontinuations
Across the studies, patients received the following carfilzomib doses: 1.7% 15 mg/m2, 2.7% 15/20 mg/m2, 5.1% 15/20/27 mg/m2, 37.6% 20 mg/m2, and 52.9% 20/27 mg/m2. The approved dose of 20/27 mg/m2 was received by 82.7% of the patients assigned to receive that dose. A median of 4 cycles (range 1–21) were given, and 19.0% of patients started 12 cycles. Dose modifications due to an AE were low with 77 of 526 (14.6%) patients requiring a dose reduction and 119 of 526 (22.6%) requiring a dose delay. Of patients who discontinued, 14.8% cited an AE as the reason for discontinuation. The most common AEs associated with discontinuation in 1% or more of patients were CHF (1.5%), dyspnea (1.3%), acute renal failure or increased blood creatinine (both 1.1%), and cardiac arrest (1.0%).
General safety and tolerability
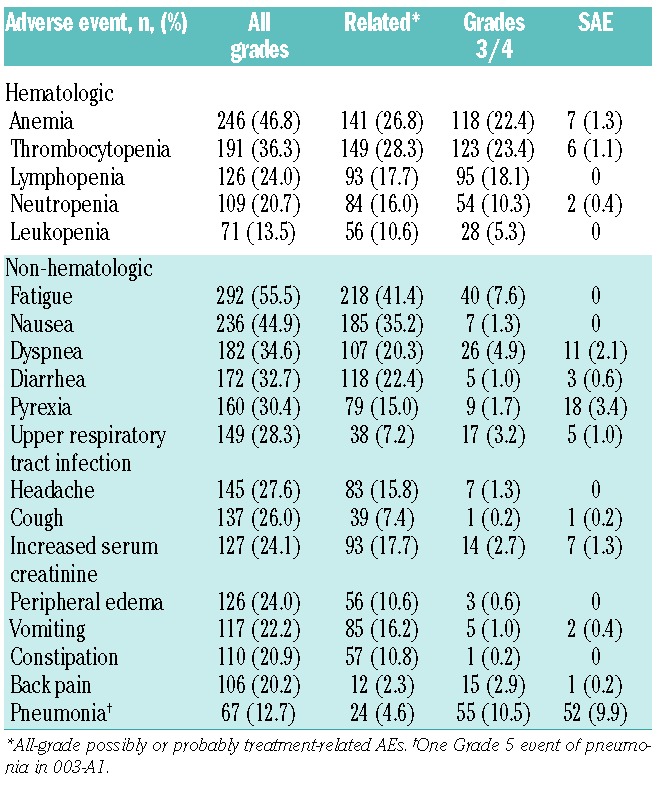
Most patients experienced a treatment-emergent AE, regardless of relationship to study drug; the most common AEs of any grade were fatigue (55.5%), anemia (46.8%), and nausea (44.9%) (Table 2). The most common Grade 3 or over AEs were thrombocytopenia (23.4%), anemia (22.4%), lymphopenia (18.1%), and pneumonia (10.5%). Overall, Grade 3/4 non-hematologic AEs were uncommon (<10% Grade 3 and <1% Grade 4). Excluding disease progression reported as an AE, the most common serious AEs (SAEs) were pneumonia (9.9%), acute renal failure (4.2%), pyrexia (3.4%), CHF (3.4%), dyspnea (2.1%), hypercalcemia (2.1%), and pathological fracture (2.1%). Many of these events are also representative of progressive disease, which confounds attribution to use of the drug or to advanced MM. The most common AEs that were possibly or probably related to study treatment included fatigue (41.4%), nausea (35.2%), thrombocytopenia (28.3%), anemia (26.8%), diarrhea (22.4%), and dyspnea (20.3%). Across the 4 studies, there were 37 deaths on study or within 30 days of last dose. Disease progression was the primary cause of death in 24 of the 37 patients. Seven deaths were deemed by the Investigator to be at least possibly related to carfilzomib and included: cardiac arrest (n=2), hepatic failure (n=1), dyspnea (n=1), multi-organ failure (n=1), cardiac disorder (n=1), and unknown (n=1). By sponsor assessment, 5 patients (1.0%) died due to a cardiac AE and an additional 3 patients with a primary cause of death due to disease progression had a cardiac component associated with their death, for a total of 8 (1.5%) cardiac-related deaths, all of which were possibly related to carfilzomib.
Table 2.
Treatment-emergent adverse events and serious adverse events regardless of causality and treatment-related adverse events.
Hematologic analysis
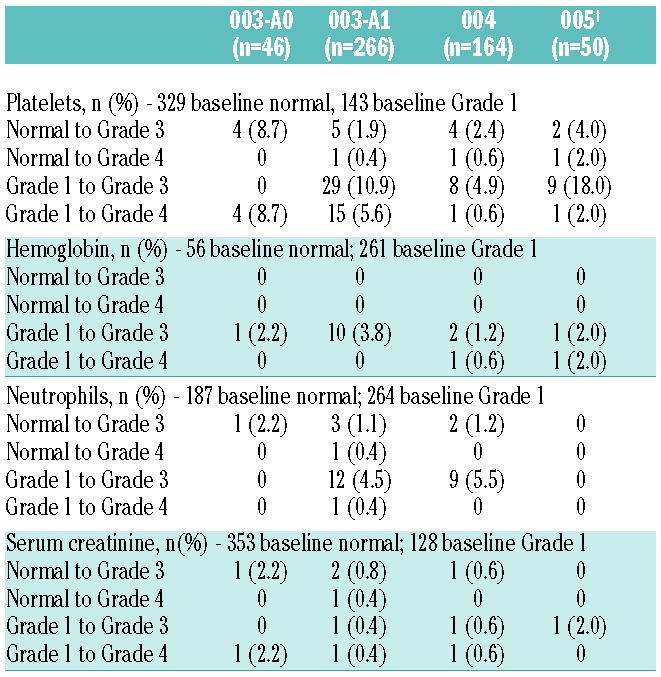
Overall, 370 patients (70.3%) experienced a hematologic AE. Across the studies, incidences of hematologic AEs of any grade were 37.8% for thrombocytopenia, 25.9% for lymphopenia, 22.6% for neutropenia, and 46.8% for anemia. Less than or equal to 1.1% of patients required a dose reduction or discontinuation for any of the 4 hematologic AE groupings analyzed. Median platelet counts decreased, reaching a Grade less than 1 nadir at Day 8, and returned to normal by Day 1 of the next cycle. There was no evidence of cumulative thrombocytopenia (Online Supplementary Table S1), and clinically significant episodes of bleeding associated with concurrent thrombocytopenia were rare being 33 incidences in 28 patients: 25 Grade 1, 4 Grade 2, and 4 Grade 3. It was uncommon (7 of 187 patients, 3.7%) for patients with normal neutrophil counts at baseline to shift to Grade 3/4 neutropenia (Table 3). Febrile neutropenia occurred infrequently: 6 patients (1.1%) reported febrile neutropenia. Hemoglobin remained stable throughout the cycle, with mean and median nadirs remaining at Grade 1.
Table 3.
Shift of laboratory values from baseline normal or baseline Grade 1 to Grade 3 or Grade 4.
Non-hematologic analysis by organ system Peripheral neuropathy
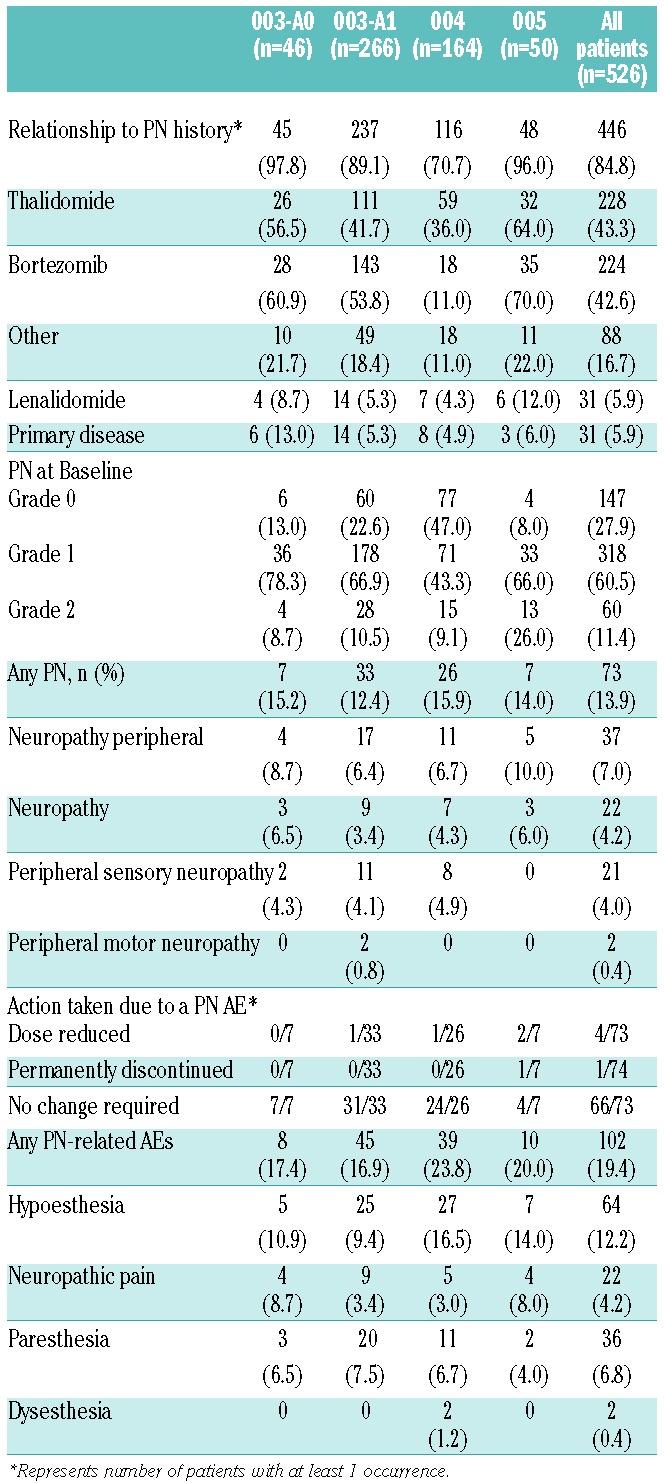
The majority of patients (84.8%) had a prior history of PN (Table 4). Of these, 42.6% were attributed to bortezomib and 43.3% to thalidomide, with 25.9% and 21.1% resulting in prior therapy discontinuations, respectively. Overall, 47.1% of patients had discontinued a previous treatment due to PN. At baseline in the carfilzomib studies, 378 (71.9%) patients had active PN (all Grades 1 or 2). In spite of this, PN aggregate grouping AEs across all studies were reported infrequently (13.9% overall); 41 patients (7.8%) experienced Grade 1 PN, 25 (4.8%) Grade 2 PN, and 7 (1.3%) Grade 3 PN. No Grade 4 or over PN was reported and all of the Grade 3 PN occurred in patients with Grade 1 or 2 at baseline. Moreover, the majority of the 378 patients with Grade 1 or 2 baseline PN (330 of 378, 87.3%) did not report AEs related to PN at any time on study. One patient (0.2%) discontinued treatment due to neuropathic pain and 4 (0.8%) required a dose reduction due to a neuropathy AE. In addition, the majority of PN AEs occurred before Cycle 6 (calculated as a percentage of the actual number of patients treated in a given cycle), suggesting a lack of cumulative toxicity (Online Supplementary Table S1).
Table 4.
Peripheral neuropathy adverse event summary.
Cardiac
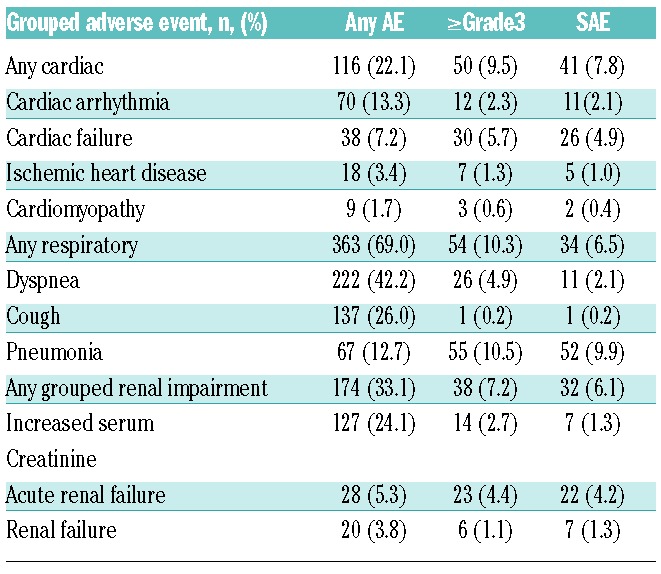
Overall, 73.6% of patients had a past medical history of cardiovascular events and 70.0% had baseline cardiac risk factors (the latter defined as a patient who reported use of at least one cardiovascular or anti-diabetic medication prior to study entry). Aggregated cardiac failure events (including CHF, pulmonary edema, and decreased ejection fraction) were reported in 38 patients (7.2%), regardless of causality (Table 5). The overall mortality rate, including due to disease progression, was the same (7%) in patients who had baseline cardiac risk factors as it was for patients without these risk factors. Any cardiac disorder AE was reported by 22.1% of patients. Hypertension (mainly Grade 1–2) was reported in 14.3% of patients, more than half of whom had a history of hypertension. In response to a cardiac-related AE, 6 patients (1.1%) had a carfilzomib dose reduction. Cardiac events leading to treatment discontinuation were noted in 23 patients (4.4%) and included CHF (1.5%), cardiac arrest (1.0%), and myocardial ischemia (0.6%). The rate of cardiac AEs did not increase in later cycles (Online Supplementary Table S1) and cardiac disorder AEs within one day of dosing occurred in 62 patients (11.8%).
Table 5.
Special analysis of grouped-term organ system adverse events.
Pulmonary
The most commonly reported respiratory AEs were dyspnea (42.2%) and cough (26.0%). The majority of dyspnea events were Grade 1 or Grade 2; Grade 3 events were reported by 25 patients (4.8%), and no Grade 4 dyspnea events were reported. One patient (0.2%) died with a Grade 5 event (in the setting of concurrent congestive heart failure). Dyspnea resolved for 67.9% of patients; 60.7% did not require any change in carfilzomib therapy, 6 patients (1.1%) required dose reductions, and 7 patients (1.3%) discontinued treatment. The rate of dyspnea did not increase in later cycles (Online Supplementary Table S1). Overall, 269 patients (51.1%) experienced a respiratory AE within one day of dosing, approximately half (139 patients, 25.9%) were dyspnea. Overall, AEs in the respiratory system organ class led to dose reduction in 10 patients (1.9%). Other pulmonary AEs of clinical importance included pleural effusion (4%), pulmonary hypertension (2%), pulmonary embolism (1%), hemoptysis (0.6%), and pneumonitis (0.4%). No interstitial lung disease or pulmonary fibrosis was reported. The most commonly reported respiratory SAE was dyspnea in 11 patients (2.1%), which resolved or stabilized in all but one patient. At least one respiratory infection AE was reported for 18.8% of patients, with pneumonia being the most common: 67 patients (12.7%) as well as the most commonly reported SAE (52 patients, 9.9%). Respiratory infection AEs were reported as the primary cause of death in 2 patients.
Renal
At baseline, 23.8% of patients had moderate to severe renal dysfunction (CrCl <50 mL/min) and 39.4% had mild renal dysfunction (CrCl ≥50 to <80 mL/min). Overall, 86.8% of the 515 patients evaluable for creatinine values did not have worsening of renal function during the course of treatment. At least one episode of worsening renal function was reported in 68 patients (13.2%). The worsening renal function was considered transient in 31 patients, and median duration of the worsening was 1.4 weeks. The remaining 37 patients (7.2% of the total population) experienced non-transient worsening, for which 8 of 37 patients discontinued treatment due to an AE related to renal dysfunction. For all patients with worsening renal function, the median time to first episode was 44.5 days (approximately Cycle 2) and the incidence of first episodes of worsening was evenly distributed over time across earlier and later time points, suggesting a lack of cumulative toxicity. Overall, 174 patients (33.1%) had at least one grouped renal impairment AE and the most common AEs included: increased blood creatinine (24.1%), acute renal failure (5.3%), renal failure (3.8%), increased blood urea (2.7%), and decreased renal CrCl (1.1%). Nearly half of these patients (48%) experienced a renal AE in association with disease progression. Overall, 38 patients (7.2%) experienced Grade 3/4 grouped renal impairment AEs; 31 of those were Grade 3. Included in the Grade 3/4 grouped renal AEs were renal failure (1.1%), acute renal failure (4.4%), and renal impairment (0.2%). Shifts of serum creatinine from normal or Grade 1 to Grade 3 or 4 ranged from 0 to 2.2% across the phase II studies (Table 3). Of the 174 patients who reported any renal event, 50% required no change in carfilzomib therapy, 12.1% discontinued carfilzomib, and 10.9% required a dose reduction. Patients discontinuing treatment included 6 patients (1.1%) each due to a renal AE or increased serum creatinine.
Data concerning GI and hepatic AEs, TLS, and herpes virus infection are included in the Online Supplementary Results.
Discussion
These results demonstrate that single-agent carfilzomib has a favorable safety profile in heavily pre-treated patients with RRMM.7,27–30 The most common treatment-related AEs were both non-hematologic (fatigue and nausea) and hematologic (thrombocytopenia and anemia), while the most commonly reported non-hematologic AEs were predominantly Grade 1 or 2 in severity. While Grade 3 or 4 treatment-emergent AEs occurred in 80.2% of patients, they were primarily hematologic in nature. Carfilzomib was tolerable as indicated by the lack of cumulative toxicities and the low proportion of patients who had to discontinue or reduce their dose due to an AE. While cross-trial comparisons are inherently flawed, it is notable that 9% of patients completed the planned bortezomib therapy of 8 cycles of 21 days plus 3 cycles of 35 days (1.3 mg/m2) in the APEX trial19 compared with 19.0% of patients who started at least 12 28-day cycles of carfilzomib. Similarly, 37% of patients in the APEX trial19 and 22% in the SUMMIT trial33 discontinued bortezomib due to an AE compared with 14.8% for carfilzomib. Results from the ongoing phase III ENDEAVOR (NCT01568866) and CLARION (NCT01818752) trials, in which patients are being treated with either carfilzomib or bortezomib, will provide more information on any differences between the two proteasome inhibitors.
To further emphasize the long-term tolerability profile of carfilzomib, patients from the phase II studies, along with several patients from phase I studies, were able to enter an extension study (PX-171-010) following completion of their primary study. As of June 2012, 89 patients with MM had enrolled and the total duration of carfilzomib treatment (primary study + 010 study) was a median of 89 weeks with no evidence of unique or late-onset cumulative toxicity.34
Hematologic abnormalities, most notably anemia, are a common comorbidity for patients with advanced-stage MM.10 These AEs, particularly thrombocytopenia and neutropenia, are caused by the disease itself, occur more commonly, and are a more serious concern following MM treatment with other drugs, especially in combination with alkylating agents.35 The hematologic safety profile of carfilzomib, particularly the lack of evidence of cumulative thrombocytopenia and low rates of febrile neutropenia, compares favorably with other MM therapies including pomalidomide9,36 and bortezomib,37 providing further evidence of its acceptable safety profile in heavily pre-treated patients with MM. In addition, the hematologic AEs reported for carfilzomib were infrequently dose limiting, the Grade 3/4 hematologic AEs were generally reversible, and serious clinical sequelae were rare.
As with hematologic abnormalities, PN is common in patients with late-stage MM. PN caused by MM has been considered secondary to plasma cell dyscrasia or following direct compression. PN has become a more worrisome condition because many treatment options cause PN or exacerbate existing PN.22 Pre-clinically, carfilzomib is more selective for the proteasome than bortezomib and, unlike bortezomib, does not induce neurodegeneration in vitro via a proteasome-independent mechanism.38 PN due to off target effects, has been singled out as the most significant dose-limiting toxicity for bortezomib39 and resulted in dose reductions in 12% and discontinuations in 5–8% of patients in an analysis of phase II studies.19,40 While subcutaneous administration of bortezomib appears to somewhat decrease the incidence of new-onset PN, without negatively impacting efficacy, the rates of PN (38%) reported with subcutaneous bortezomib in patients not previously exposed to bortezomib is higher than that noted in the carfilzomib studies presented here.41 In the case of thalidomide, it is estimated that 70% of patients treated with this agent for 12 months will develop PN and approximately 15% of patients will need to interrupt thalidomide treatment due to PN, with incidence rates of 25–83%.22 As noted for carfilzomib, 0.2% of patients discontinued due to PN and dose reductions were required in 0.8% of patients. The frequency of Grade 3 or over PN observed with carfilzomib (1.3%) is lower than rates reported for bortezomib (9–13%).22 In addition, the incidence of PN AEs did not increase in later treatment cycles, supporting long-term tolerability of carfilzomib. Overall, carfilzomib induces a low rate of new-onset PN, does not exacerbate existing PN, and is not dose limiting, allowing for longer duration of treatment for MM. These findings support pre-clinical studies demonstrating selectivity of proteasome inhibition by carfilzomib with minimal neurotoxic off-target effects.38
As with PN and hematologic abnormalities, pulmonary complications and cardiac events are common in patients with late-stage MM. Dyspnea can be a serious complication of both the disease itself as well as from MM treatments, and is common in the treatment of MM.9,42,43 Cardiac disease in patients with MM has been shown to be caused by multiple comorbidities, including age-related cardiovascular risk, chronic anemia, amyloidosis, A-V shunt with bony lesions, hyperviscosity, and prior anthracycline exposure.44,45 While approximately 20% of patients reported treatment-related dyspnea, the majority of events were low grade and transient. In those cases where dyspnea presented on the day of or the day after dosing, it occurred more frequently in earlier cycles. Importantly, most cases resolved without dose reduction or discontinuation. Identifying the etiology of this AE is somewhat complicated due to the non-specific nature of this low-grade, transient symptom observed on study that can be related to a number of underlying disorders. With regard to cardiac events, the data for carfilzomib are comparable to results reported for current MM therapies in the RRMM patient population.18,19 While it is difficult to make cross-trial comparisons, it is important to note that the rate of cardiac failure AEs observed in these studies (7.2%) was similar to the 5% reported for bortezomib in the APEX trial.43 The extent to which the cardiac events reported here were due to patients’ baseline comorbidities, toxicity from prior treatments, effects of MM, carfilzomib itself, or a combination of these factors, cannot be determined in these single-arm trials.
Renal events are another AE commonly seen in patients with MM and are a major manifestation of MM.10 In contrast to some MM therapies, renal events that occurred following carfilzomib administration infrequently led to dose modifications or treatment discontinuation.46 Patients in the carfilzomib studies who had baseline renal dysfunction, including patients on hemodialysis, received the same carfilzomib doses as patients without renal impairment at baseline. Importantly, these results are comparable to what is seen with bortezomib but different from lenalidomide and may also be different from pomalidomide, which is excreted renally like lenalidomide.9,14,46,47 Although the majority of patients entered these studies with renal dysfunction at baseline, Grade 3/4 renal AEs were uncommon. In addition, nearly half of all renal AEs were associated with disease progression. Worsening of renal function from baseline was also uncommon and was transient in almost half of those patients who experienced it.
Adverse events less commonly associated with MM but of clinical interest were also evaluated in this cross-study analysis, including GI events, TLS, herpes virus infections, and hepatic events. A discussion of the findings for these AEs can be found in the Online Supplementary Methods.
The AE profile presented here for carfilzomib was derived from a late-stage, heavily pre-treated patient population with RRMM. The patients in these studies had a median age of 64 years, similar to the median age of patients with MM. Comorbidities in general increase with age, and it is suggested that the severity of comorbidities in MM negatively affect survival outcomes in a progressive manner.48 In addition, it is important to note that aging is associated with reduced organ function, including renal and cardiac function.48 Rates and causes of death were consistent with other studies on causes of death in MM12 and with overall survival analyses3 performed across trials of heavily pre-treated patients with end-stage MM. Although it is difficult to compare studies, it is important to note that the rate of on-study deaths with carfilzomib (7%) is comparable to the rate seen with lenalidomide in the MM-014 study49 (10%; same on-study definition as carfilzomib) as well as the rate seen with bortezomib in the SUMMIT study33 (5%; on study definition of within 20 days of study end). Likewise, the proportion of on-study deaths due to a cardiac cause in these carfilzomib studies is similar to historical data reported in a retrospective review of more than 3000 patients with newly diagnosed MM.12 Moreover, there was no difference in mortality following carfilzomib treatment between patients with cardiac risk factors at baseline and those without cardiac risk factors.
Overall, the results of the phase II safety analyses presented here demonstrate the general tolerability of carfilzomib in a large, well-characterized group of patients with RRMM. The lack of cumulative toxicities observed in patients treated with carfilzomib indicate the potential for full doses of carfilzomib to be used for extended periods in a wide spectrum of patients with advanced MM, including those with pre-existing comorbidities (particularly PN). The safety results presented here combined with the robust and durable responses of carfilzomib, including overall response rate of 23.7% and a clinical benefit rate of 37.0% in the pivotal 003-A1 study with prolonged duration of response of 7.8 and 8.3 months, respectively, and a median overall survival of 15.6 months, indicate that carfilzomib will help meet the current unmet medical need of the patient population with RRMM.7
Acknowledgments
The authors would like to thank all of the patients and their families who contributed to these studies. The authors also acknowledge the statistical support of Sandra Dixon, MS (Onyx Pharmaceuticals, Inc.) and critical review of the manuscript by Thomas Renau, PhD and A. Peter Morello, PhD, CMPP (Onyx Pharmaceuticals, Inc.). Medical writing and editorial assistance was provided by Melissa Kirk, PhD (Fishawack Communications) and funded by Onyx Pharmaceuticals, Inc.
Footnotes
The Online version of this paper contains a Supplementary Appendix.
Funding
These studies were supported by Onyx Pharmaceuticals, Inc. and the Multiple Myeloma Research Consortium.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.American Cancer Society Cancer Facts and Figure 2013. Atlanta: American Cancer Society; 2013 [Google Scholar]
- 2.Eshaghian S, Berenson JR. Multiple myeloma: improved outcomes with new therapeutic approaches. Curr Opin Support Palliat Care. 2012;6(3):330–6 [DOI] [PubMed] [Google Scholar]
- 3.Kumar SK, Lee JH, Lahuerta JJ, Morgan G, Richardson PG, Crowley J, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter International Myeloma Working Group study. Leukemia. 2012;26(1):149–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lonial S, Mitsiades CS, Richardson PG. Treatment options for relapsed and refractory multiple myeloma. Clin Cancer Res. 2011;17(6):1264–77 [DOI] [PubMed] [Google Scholar]
- 5.Laubach J, Mitsiades M, Mahindra A, Luskin MR, Rosenblatt J, Ghobrial IM, et al. Management of relapsed and relapsed/refractory multiple myeloma. J Natl Compr Canc Netw. 2011;9(10):1209–16 [DOI] [PubMed] [Google Scholar]
- 6.Kastritis E, Zervas K, Symeonidis A, Terpos E, Delimbassi S, Anagnostopoulos N, et al. Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): an analysis of the Greek Myeloma Study Group (GMSG). Leukemia. 2009; 23(6):1152–7 [DOI] [PubMed] [Google Scholar]
- 7.Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012; 120(14):2817–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.KYPROLIS™ Prescribing Information. Onyx Pharmaceuticals, Inc; South San Francisco, CA: 2012 [Google Scholar]
- 9.POMALYST® Prescribing Information. Celgene Corportation, Summit, NJ: 2013 [Google Scholar]
- 10.Pingali SR, Haddad RY, Saad A. Current concepts of clinical management of multiple myeloma. Dis Mon. 2012;58(4):195–207 [DOI] [PubMed] [Google Scholar]
- 11.Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374(9686):324–39 [DOI] [PubMed] [Google Scholar]
- 12.Augustson BM, Begum G, Dunn JA, Barth NJ, Davies F, Morgan G, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United kingdom Medical Research Council trials between 1980 and 2002--Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005;23(36):9219–26 [DOI] [PubMed] [Google Scholar]
- 13.Mohty B, El-Cheikh J, Yakoub-Agha I, Avet-Loiseau H, Moreau P, Mohty M. Treatment strategies in relapsed and refractory multiple myeloma: a focus on drug sequencing and ‘retreatment’ approaches in the era of novel agents. Leukemia. 2012;26(1):73–85 [DOI] [PubMed] [Google Scholar]
- 14.van de Donk NW, Lokhorst HM, Dimopoulos M, Cavo M, Morgan G, Einsele H, et al. Treatment of relapsed and refractory multiple myeloma in the era of novel agents. Cancer Treat Rev. 2011;37(4):266–83 [DOI] [PubMed] [Google Scholar]
- 15.Cesarman-Maus G, Braggio E, Fonseca R. Thrombosis in multiple myeloma (MM). Hematology. 2012;17 (1 Suppl):S177–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacy MQ, Allred JB, Gertz MA, Hayman SR, Short KD, Buadi F, et al. Pomalidomide plus low-dose dexamethasone in myeloma refractory to both bortezomib and lenalidomide: comparison of 2 dosing strategies in dual-refractory disease. Blood. 2011;118 (11):2970–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimopoulos MA, Chen C, Spencer A, Niesvizky R, Attal M, Stadtmauer EA, et al. Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 2009;23(11):2147–52 [DOI] [PubMed] [Google Scholar]
- 18.Hazarika M, Rock E, Williams G, Dagher R, Sridhara R, Booth B, et al. Lenalidomide in combination with dexamethasone for the treatment of multiple myeloma after one prior therapy. Oncologist. 2008;13(10):1120–7 [DOI] [PubMed] [Google Scholar]
- 19.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–98 [DOI] [PubMed] [Google Scholar]
- 20.Orlowski RZ, Nagler A, Sonneveld P, Blade J, Hajek R, Spencer A, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25(25):3892–901 [DOI] [PubMed] [Google Scholar]
- 21.Jagannath S, Barlogie B, Berenson J, Siegel D, Irwin D, Richardson PG, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127(2):165–72 [DOI] [PubMed] [Google Scholar]
- 22.Mohty B, El-Cheikh J, Yakoub-Agha I, Moreau P, Harousseau JL, Mohty M. Peripheral neuropathy and new treatments for multiple myeloma: background and practical recommendations. Haematologica. 2010;95(2):311–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palumbo A, Waage A, Hulin C, Beksac M, Zweegman S, Gay F, et al. Safety of thalidomide in newly diagnosed elderly myeloma patients: a meta-analysis of data from individual patients in six randomized trials. Haematologica. 2013;98(1):87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludwig H, Durie BG, McCarthy P, Palumbo A, San Miguel J, Barlogie B, et al. IMWG consensus on maintenance therapy in multiple myeloma. Blood. 2012;119(13):3003–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Connor OA, Stewart AK, Vallone M, Molineaux CJ, Kunkel LA, Gerecitano JF, et al. A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clin Cancer Res. 2009;15(22):7085–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alsina M, Trudel S, Furman RR, Rosen PJ, O’Connor OA, Comenzo RL, et al. A phase 1 single-agent study of twice-weekly consecutive-day dosing of the proteasome inhibitor carfilzomib in patients with relapsed or refractory multiple myeloma or lymphoma. Clin Cancer Res. 2012;18(17):4830–40 [DOI] [PubMed] [Google Scholar]
- 27.Jagannath S, Vij R, Stewart AK, Trudel S, Jakubowiak AJ, Reiman T, et al. An open-label single-arm pilot phase II study (PX-171-003-A0) of low-dose, single-agent carfilzomib in patients with relapsed and refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2012;12(5):310–8 [DOI] [PubMed] [Google Scholar]
- 28.Vij R, Wang M, Kaufman JL, Lonial S, Jakubowiak AJ, Stewart AK, et al. An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood. 2012;119(24):5661–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vij R, Siegel DS, Jagannath S, Jakubowiak AJ, Stewart AK, McDonagh K, et al. An open-label, single-arm, phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib. Br J Haematol. 2012;158(6):739–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badros AZ, Vij R, Martin T, Zonder JA, Kunkel L, Wang Z, et al. Carfilzomib in multiple myeloma patients with renal impairment: pharmacokinetics and safety. Leukemia. 2013;Epub Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Bethesda, MD: U.S. National Institute of Health, National Cancer Institute, Cancer Therapy Evaluation Program; 2006 [Google Scholar]
- 32.Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK, et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23(12):2210–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003; 348(26):2609–17 [DOI] [PubMed] [Google Scholar]
- 34.Siegel DS, Wang M, Martin TG, III, Infante JR, Kaufman JL, Ranjangam K, et al. A Phase 2 Study of Prolonged Carfilzomib Therapy in Patients with Multiple Myeloma Previously Enrolled in Carfilzomib Clinical Trials. Blood. 2012;120(21):Abstract 2962. [Google Scholar]
- 35.Gay F, Palumbo A. Management of older patients with multiple myeloma. Blood Rev. 2011;25(2):65–73 [DOI] [PubMed] [Google Scholar]
- 36.Richardson PG, Siegel DS, Vij R, Hofmeister CC, Jagannath S, Chen C, et al. Randomized, Open Label Phase 1/2 Study of Pomalidomide (POM) Alone or in Combination with Low-Dose Dexamethasone (LoDex) in Patients (Pts) with Relapsed and Refractory Multiple Myeloma Who Have Received Prior Treatment That Includes Lenalidomide (LEN) and Bortezomib (BORT): Phase 2 Results. Blood. 2011;118(21):Abstract 634. [Google Scholar]
- 37.Mikhael JR, Belch AR, Prince HM, Lucio MN, Maiolino A, Corso A, et al. High response rate to bortezomib with or without dexamethasone in patients with relapsed or refractory multiple myeloma: results of a global phase 3b expanded access program. Br J Haematol. 2009;144(2):169–75 [DOI] [PubMed] [Google Scholar]
- 38.Arastu-Kapur S, Anderl JL, Kraus M, Parlati F, Shenk KD, Lee SJ, et al. Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: a link to clinical adverse events. Clin Cancer Res. 2011;17(9): 2734–43 [DOI] [PubMed] [Google Scholar]
- 39.Cavaletti G, Jakubowiak AJ. Peripheral neuropathy during bortezomib treatment of multiple myeloma: a review of recent studies. Leuk Lymphoma. 2010;51(7):1178–87 [DOI] [PubMed] [Google Scholar]
- 40.Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24(19):3113–20 [DOI] [PubMed] [Google Scholar]
- 41.Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12(5):431–40 [DOI] [PubMed] [Google Scholar]
- 42.Revlimid® Prescribing Information. Celgene Corporation, Summit, NJ: 2012 [Google Scholar]
- 43.Velcade™ Prescribing Information. Millennium Pharmaceuticals, Cambridge, MA: 2012 [Google Scholar]
- 44.Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003; 78(1):21–33 [DOI] [PubMed] [Google Scholar]
- 45.Inanir S, Haznedar R, Atavci S, Unlu M. Arteriovenous shunting in patients with multiple myeloma and high-output failure. J Nucl Med. 1998;39(1):1–3 [PubMed] [Google Scholar]
- 46.Dimopoulos MA, Terpos E, Goldschmidt H, Alegre A, Mark T, Niesvizky R. Treatment with lenalidomide and dexamethasone in patients with multiple myeloma and renal impairment. Cancer Treat Rev. 2012;38(8):1012–9 [DOI] [PubMed] [Google Scholar]
- 47.Leal TB, Remick SC, Takimoto CH, Ramanathan RK, Davies A, Egorin MJ, et al. Dose-escalating and pharmacological study of bortezomib in adult cancer patients with impaired renal function: a National Cancer Institute Organ Dysfunction Working Group Study. Cancer Chemother Pharmacol. 2011;68(6):1439–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palumbo A, Bringhen S, Ludwig H, Dimopoulos MA, Blade J, Mateos MV, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN). Blood. 2011;118(17):4519–29 [DOI] [PubMed] [Google Scholar]
- 49.Richardson P, Jagannath S, Hussein M, Berenson J, Singhal S, Irwin D, et al. Safety and efficacy of single-agent lenalidomide in patients with relapsed and refractory multiple myeloma. Blood. 2009;114(4):772–8 [DOI] [PubMed] [Google Scholar]


