Abstract
The distinction between sensory inputs that are a consequence of our own actions from those that result from changes in the external world is essential for perceptual stability and accurate motor control. In this study, we investigated whether linear translations are encoded similarly during active and passive translations by the otolith system. Vestibular nerve afferents innervating the saccule or utricle were recorded in alert macaques. Single unit responses were compared during passive whole body, passive head-on-body, and active head-on-body translations (vertical, fore-aft, or lateral) to assess the relative influence of neck proprioceptive and efference copy-related signals on translational coding. The response dynamics of utricular and saccular afferents were comparable and similarly encoded head translation during passive whole body versus head-on-body translations. Furthermore, when monkeys produced active head-on-body translations with comparable dynamics, the responses of both regular and irregular afferents remained comparable to those recorded during passive movements. Our findings refute the proposal that neck proprioceptive and/or efference copy inputs coded by the efferent system function to modulate the responses of the otolith afferents during active movements. We conclude that the vestibular periphery provides faithful information about linear movements of the head in the space coordinates, regardless of whether they are self- or externally generated.
INTRODUCTION
During everyday life, the vestibular system encodes the motion of our head relative to the world. Linear motion is sensed by the two otolithic organs (the utricle and the saccule), and rotational motion is sensed by three roughly orthogonal semicircular canals. The combined activation of receptor cells in the otoliths provide a three-dimensional estimate of linear acceleration; hair cells in the utricle, which lies roughly along a plane delineated by the fore-aft and interaural axes, detect horizontal translations, whereas hair cells in the saccule, which is oriented approximately in a plane delineated by the fore-aft and vertical axes, detect vertical translations (Fernandez and Goldberg 1976a; Lindeman 1969). Similarly, by combining the activation of the receptor cells of the three canals, the brain creates a three-dimensional representation of instantaneous head rotation. Linear and rotational motion information from the vestibular periphery is relayed to neurons in the vestibular nuclei via the afferent fibers innervating the otoliths and canals, respectively. This information, in turn, is used for a wide range of functions that are crucial for our daily activities. For example, vestibular information is required to produce reflexes required to maintain head and body posture (Peterson and Richmond 1988) and stabilize gaze during orienting head movements, walking, and running (Grossman et al. 1988; Huterer and Cullen 2002). In addition, vestibular sensory information is critical for higher level functions such as self-motion perception and spatial orientation (Gu et al. 2007; Harris et al. 2000; Ohmi 1996; Telford et al. 1995; Tribukait and Eiken 2005).
To date, the processing of linear motion information by the vestibular system has been characterized exclusively during passive whole body motion. Previous studies have shown that primary otolith afferents detect net linear acceleration but do not distinguish translational from gravitational components (Angelaki and Dickman 2000; Purcell et al. 2003; Si et al. 1997). In contrast, many central neurons selectively encode translational motion and remain relatively insensitive to changes in head orientation relative to gravity (Angelaki et al. 2004; Shaikh et al. 2005). During daily activities, however, the otoliths are simultaneously stimulated by both the motion of the head resulting from passively applied movements as well as that which arises from our own actions. The ability to distinguish sensory inputs that are a consequence of our own actions from those that result from changes in the external world is crucial for postural and perceptual stability and accurate motor control (Cullen 2004). For example, vestibulospinal reflexes are essential for postural stability. Nevertheless, despite the importance of such innate reflexes for responding to externally applied perturbations, they can be counterproductive when the behavioral goal is to make an active movement.
Whether and how the vestibular system distinguishes active from passive linear motion has yet to be explored. Recent studies have shown that, in response to rotational motion, neurons at the first central stage of vestibular processing (i.e., vestibular nucleus) can distinguish between self-generated and passive movements (reviewed in Cullen 2004), whereas vestibular afferents do not (Cullen and Minor 2002; Sadeghi et al. 2007c). Notably, during active head rotations, a cancellation signal is generated when the activation of proprioceptors matches the motor-related expectation (Roy and Cullen 2004). This mechanism eliminates information about self-generated rotations from subsequent computation of angular motion for the estimation of orientation and postural control. It remains to be determined, however, whether the ability to distinguish actively generated and passive stimuli is a general feature of vestibular processing; no previous study has explicitly characterized the coding of active versus passive linear motion at comparable stages of processing.
Vestibular receptors in both the otoliths and canals receive bilateral innervation from centrifugally projecting efferent fibers (Dickman and Correia 1993; Gacek and Lyon 1974; Myers et al. 1997; Plotnik et al. 2002; Rasmussen and Gacek 1958). The role of this efferent innervation, however, remains a mystery. The finding that stimulation of the vestibular efferent system increases the resting discharge and decreases the sensitivities of afferents in several species (i.e., monkey: Goldberg and Fernandez 1980; toadfish: Highstein and Baker 1985) has given rise to the idea that activation of the vestibular efferent system could be used to increase the afferent response range during active head motion (Goldberg et al. 2000; Purcell and Perachio 1997). Although this proposal has been refuted for semicircular canal afferents (Cullen and Minor 2002; Sadeghi et al. 2007c), it has not yet been tested for otolith afferents. It is possible that a different strategy is used for encoding linear and rotation acceleration at the level of the vestibular periphery. First, although previous studies have shown that individual efferent neurons innervate multiple end organs (Birinyi et al. 2001; Gleisner and Henriksson 1963), it is possible that some efferent neurons more strongly target otolith organs and receptors. For example, in toadfish (Highstein and Baker 1986) and frog (Birinyi et al. 2001), separate groups of efferent vestibular neurons seem to innervate the semicircular canals and otoliths, albeit with overlap. Second, electrical stimulation of the cerebellum in frog has been shown to preferentially affect the background activity of otoliths afferents (Llinas and Precht 1969). The cerebellum is known to play a critical role in predicting the sensory consequences of voluntary actions (Crapse and Sommer 2008; Cullen 2004), and although it does not project directly to the vestibular periphery, such an effect could potentially be mediated via a multisynaptic pathway (e.g., through the vestibular nuclei) to the periphery.
Accordingly, in this study, we tested the hypothesis that the vestibular efferent system functions to selectively modify the linear motion sensitivities and/or resting discharges of otolith afferents during active head motion. We recorded from single otolith afferents during passively applied and self-generated movements. Responses were compared during passive whole body, passive head-on-body, and active head-on-body translations to assess the relative influence of neck proprioceptive and efference copy-related signals on translational coding.
METHODS
Surgical preparation
Two macaque monkeys (Macaca fascicularis) were prepared for chronic extracellular recording under aseptic conditions. All procedures were approved by the McGill University Animal Care Committee and were in compliance with the guidelines of the Canadian Council on Animal Care. The surgical preparation was previously described elsewhere (Sylvestre and Cullen 1999). Briefly, using aseptic techniques and isoflurane anesthesia (2–3%, to effect), a dental acrylic implant was attached to animal's skull using stainless steel screws. Within the implant were embedded a stainless steel post that was used to restrain the animal's head during the experiment and two stainless steel recording chambers that were positioned stereotaxically on the skull to allow recording from the vestibular nerve where it emerges from the internal auditory meatus. In the same procedure, an 18- to 19-mm-diam eye coil (3 loops of Teflon-coated stainless steel wire) was implanted in the right eye behind the conjunctiva. After the surgery, the animals were administered buprenorphine (0.01 mg/kg, IM) for postoperative analgesia and the antibiotic cephazolin (Ancef; 25 mg/kg, IM, for 5 days). Animals were given ≥2 wk to recover from the surgery before experiments began.
Data acquisition
During experiments, the monkey was comfortably seated in a primate chair mounted on a servomotor. The monkey's head was initially restrained during each experiment, and the room was dimly lit. The vestibular nerve was approached through the floccular lobe of the cerebellum, as identified by its eye movement–related activity (Cullen and Minor 2002; Lisberger and Pavelko 1986); entry to the nerve was preceded by a silence, indicating that the electrode had left the cerebellum. As has been described previously (Sadeghi et al. 2007a), extracellular single-unit activity of otolith afferents was recorded using glass microelectrodes (24–27 MΩ), the depth of which was controlled using a light weight precision hydraulic microdrive (Narishige, Tokyo, Japan). Head acceleration was measured in three dimensions using a 3-D linear accelerometer (ADXL330Z, Analog Devices, Norwood, MA) that was firmly attached to the animal's head post. During experimental sessions, unit activity, horizontal and vertical eye positions, and head acceleration signals were recorded on digital audiotape for later playback. The isolation of each unit was later carefully re-evaluated off-line. During playback, action potentials from extracellular recordings were discriminated using a windowing circuit (BAK Electronics, Mount Airy, MD). Eye position and head acceleration signals were low-pass filtered at 250 Hz (8-pole Bessel filter) and sampled at 1 kHz.
Experimental design
Monkeys were trained to generate voluntary head translational movements to track a food target, which was presented before them. A small linear head sled mounted on top of the monkey's head post allowed the animal to translate its head along one of three possible directions during each trial (i.e., lateral, fore-aft, or vertical). After several weeks of training, monkeys learned to actively move their head in each of the three permitted directions to receive a food reward. To study the responses of each afferent during passive head translations, two different methods were used to produce passive translations with comparable profiles (i.e., peak head accelerations of 0.1–0.4 G and predominant frequencies of ≤8 Hz) to those that were generated during voluntary head movements: 1) typical acceleration profiles of the animal's active head movements were integrated and fed into the vestibular stimulator's controller to produce natural passive whole body translations and 2) the experimenter manually translated the animal's head along the small linear sled, which was mounted above the monkey, to produce natural passive head-on-body translations.
All afferents described in this study were activated by translational head movements along at least one of the three major axes tested [i.e., fore-aft (0°), lateral (90°), or vertical] using passive whole body translation (5 Hz, 0.2 G). None of the afferents responded to yaw rotations. Because saccular neurons are most sensitive to vertical translations, whereas utricular units are especially sensitive to translations in the horizontal plane (Fernandez and Goldberg 1976a; Fernandez et al. 1972; Purcell et al. 2003), we classified an afferent as utricular/saccular if it was maximally stimulated by translation in horizontal/vertical axis. Consistent with Fernandez et al. (1972), all the units classified as saccular units were encountered in close association with afferents innervating the posterior canals (i.e., excitatory responses for nose up pitch rotations), whereas those considered as innervating the utricle were in the same track with afferents innervating horizontal (i.e., excitatory responses for ipsilateral yaw rotations) or anterior canals (i.e., excitatory responses for nose down pitch rotations).
During the experiments, once the preferred translational direction of a given afferent was established (i.e., lateral, fore-aft, or vertical), 8–10 natural passive whole body translations were also applied along that axis. We next carefully released the monkey's head to allow freedom of motion along the preferred direction and manually applied 10–15 cycles of passive head-on-body sinusoidal translations (∼5 Hz, ∼0.2 G), as well as, 10–15 natural passive head-on-body translations. Finally, the neuron's activity was recorded while the monkey made voluntary head movements along the preferred direction. Each unit's spontaneous activity was also recorded in the absence of vestibular stimulation so that its regularity of discharge could be computed. For the subpopulation of utricular afferents that remained well isolated (n = 15), we again restrained the head and applied passive whole body translation (5 Hz, 0.2 G) along two intermediate axes (30 and 60°). During off-line analysis, we calculated the optimal axis for maximum sensitivity of these afferents using a cosine fit (Angelaki and Dickman 2000; Purcell et al. 2003). On average, the calculated maximum sensitivity was only 13% larger than the average sensitivity measured in response to stimulation along preferred fore-aft/interaural axes. Notably, this does not affect the main conclusions of this study, where the main purpose was to compare afferent responses during passive versus active translations along the preferred axis (i.e., 1 of the 3 axes along which the animal could make voluntary translations).
Data analysis
Data were imported into the Matlab (The MathWorks, Natick, MA) programming environment for analysis. Head acceleration signals were digitally filtered at 20 Hz. The neural discharge was represented using a spike density function in which a Kaiser window was convolved with the spike train (Cherif et al. 2008). The resting discharge of each unit (defined as the mean firing rate when stationary with the head in the stereotaxic position) and CV of the interspike interval were determined. A normalized CV (CV*) was calculated using the method described by Goldberg et al. (1984) in the squirrel monkey. The distribution of CV* was bimodal and similar to that reported in previous studies of otolith and canal afferents (Angelaki et al. 1992; Goldberg et al. 1984; Hirvonen et al. 2005; Marlinski et al. 2004; Ramachandran and Lisberger 2006; Sadeghi et al. 2007a, c). Accordingly, neurons with a CV* <0.15 were classified as regular, whereas those with a CV* ≥0.15 were classified as irregular (Haque et al. 2004; Sadeghi et al. 2007a).
A least-squares regression analysis was used to determine each afferent's bias discharge (spikes/s), phase shift of each unit relative to head acceleration, and head acceleration sensitivity [(spikes/s)/G with G = 9.81 m/s2] in response to passive sinusoidal translation (Roy and Cullen 2001; Sylvestre and Cullen 1999), using ≥10 cycles of the stimulus. The bias, sensitivity, and phase shift of each neuron in response to sinusoidal translations (5 Hz, 0.2 G) were calculated by estimating the coefficients for the following model
| (1) |
where FR is firing rate, Sa is the sensitivity to head acceleration, θ is the phase shift, and Ḧ is head acceleration. In addition, neuronal sensitivities to active translations and passive translations with comparable trajectories (i.e., the natural passive stimuli) were calculated by estimating the coefficients of the following equation
| (2) |
where FR is firing rate,  is head acceleration, Sa is the sensitivity to head acceleration,
is head acceleration, Sa is the sensitivity to head acceleration, 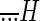 is head jerk, and Sj is the sensitivity to head jerk. To compare a model's ability to predict an afferent's firing rate, the variance-accounted-for {VAF = 1 − [var (mod − fr)/var (fr)]}, where mod represents the modeled firing rate and fr represents the actual firing rate) was computed (Cullen et al. 1996). Values are expressed as means ± SE, and a Student's t-test was used to determine whether the average of two measured parameters differed significantly from each other. The power spectrums of the passively and actively generated translations were computed using multitaper estimation techniques with eight Slepian functions (Jarvis and Mitra 2001) as previously described (Sadeghi et al. 2007a).
is head jerk, and Sj is the sensitivity to head jerk. To compare a model's ability to predict an afferent's firing rate, the variance-accounted-for {VAF = 1 − [var (mod − fr)/var (fr)]}, where mod represents the modeled firing rate and fr represents the actual firing rate) was computed (Cullen et al. 1996). Values are expressed as means ± SE, and a Student's t-test was used to determine whether the average of two measured parameters differed significantly from each other. The power spectrums of the passively and actively generated translations were computed using multitaper estimation techniques with eight Slepian functions (Jarvis and Mitra 2001) as previously described (Sadeghi et al. 2007a).
RESULTS
We recorded from 48 units during active and passive translations. When classified based on the direction of stimulation that led to maximum activation during passive sinusoidal translations (5 Hz and 0.2 G; see methods), there were 20, 15, and 13 units sensitive to lateral, fore-aft, and vertical translations, respectively. The average head acceleration sensitivity (i.e., Sa in Eq. 1) of afferents innervating the saccule (n = 13) was 143.9 ± 64.7 (spikes/s)/G, which was not significantly different from that of the units innervating the utricle [141.6 ± 22.5 (spikes/s)/G; P = 0.97, n = 35]. When classified based on regularity of discharge, there were 31 regular (CV* = 0.03–0.12), and 17 irregular (CV* = 0.17–0.42) afferents. For both regular and irregular units, the sensitivities were similar for utricular and saccular afferents (P > 0.5). Thus we pooled the results obtained for afferents innervating the two otolith organs. The mean resting discharge rates for the two groups of afferents were 83 ± 5 and 69 ± 5 spikes/s for regular and irregular units, respectively.
Modulation during passive sinusoidal translation
Figure 1 shows the relationship between head acceleration sensitivity (i.e., Sa in Eq. 1) and phase and CV* in response to sinusoidal translations at 5 Hz (0.2 G). Acceleration sensitivity increased as a function of CV* for regular afferents (n = 31, r2 = 0.8, P < 0.0005), whereas for irregular units, it remained relatively constant as a function of CV* (n = 17, r2 = 0.06, P > 0.05). In contrast, phase lead increased as a function of CV* for both regular and irregular afferents (r2 = 0.7, P < 0.0005). These relationships are consistent with those reported for otolith afferents in chinchilla (Goldberg et al. 1990b). A polar plot showing the average acceleration sensitivity and response phase for the population of the regular (black arrow) and irregular (gray arrow) units is shown in the inset. The average sensitivity of regular units was 57.1 ± 7.9 (spikes/s)/G. Moreover, although the modulation of individual regular afferents could either lag or lead acceleration (Fig. 1B, squares), on average their responses lagged linear acceleration by 5.9 ± 1.8°. For the population of the irregular units, the average acceleration sensitivity was 249.2 ± 28.0 (spikes/s)/G, which was higher than sensitivity of regular units (Fig. 1B, inset). In contrast to the regular afferents, the modulation of each irregular afferent in our sample led head acceleration (Fig. 1B, triangles), and the average phase lead across the population was 34.1 ± 4.6°. Thus overall, both acceleration sensitivity and phase were significantly larger for irregular units compared with regular units.
FIG. 1.

Comparison of responses of regular and irregular otolith afferents during passive sinusoidal (5 Hz, 0.2 G) translations. A: acceleration sensitivity plotted as a function of normalized CV (CV*) for regular (squares) and irregular (triangles) afferents. A power law was fit to regular units (CV* < 0.15). Horizontal line is the mean sensitivity for irregular afferents (CV* ⩾ 0.15). B: response phase relative to peak linear acceleration plotted as a function of CV* for regular (squares) and irregular (triangles) afferents. A semilogarithmic relation was fit to all units. Inset: polar plot showing the average gain and phase of the response of regular (black arrow) and irregular (gray arrow) afferents innervating otoliths. The length of the arrows represents the response sensitivity [(spikes/s)/G] of each neuron.
Afferent responses during natural passive and active head movements
The responses of an example regular (CV* = 0.04) and irregular (CV* = 0.24) otolith afferent are shown in Fig. 2. These afferents were typical in that their response sensitivities and phases were comparable during passive head-on-body (Fig. 2B) and passive whole body translations (data not shown) along the interaural axis (paired t-test, P > 0.1). Figure 2C shows the responses of the same two afferents during actively generated head-on-body translations along the same axis. The corresponding power spectra of head acceleration profiles shown in Fig. 2A show comparable frequency content (i.e., low-pass up to ∼8 Hz) when the monkey's head was passively translated relative to its body and during actively generated head-on-body movements (cf. head acceleration traces for each afferent between Fig. 2, B and C).
FIG. 2.
Activities of an example regular (left; CV* = 0.04) and irregular (right; CV* = 0.24) otolith afferent during passive and active interaural translations with comparable acceleration profiles. A: power spectra of head acceleration during passive (blue) and comparable active (red) translations. Both movements had similar power for the range of frequencies of 0–10 Hz. B: response of afferents to passive translations. Superimposed on the firing rate (shaded trace) is the model fit (black trace) based on the bias discharge, the acceleration sensitivity, and jerk sensitivity. C: response of afferents to active translations. The estimated response (black trace) based on the bias discharge, acceleration sensitivity, and jerk sensitivity is superimposed on the firing rate. To obtain the prediction fit (dashed green trace), the bias and the sensitivity values of the passive model were applied. The variance-accounted-for (VAF) of the estimation and prediction were similar.
Equation 2 was first used to estimate each afferent's bias and sensitivity to passive head-on-body translations. For the example regular afferent, this equation provided an excellent fit to the response (VAF = 95%) with an estimated bias discharge rate of 55 spikes/s, acceleration sensitivity of 27.7 (spikes/s)/G, and jerk sensitivity of 0.26 (spikes/s)/(G/s). The estimated response profile is shown by the heavy black trace superimposed on the firing rate (gray shaded area) in Fig. 2B (left). As expected based on the response dynamics of regular afferents (Fig. 1), the jerk sensitivity of this unit was negligible. Notably, when the response was estimated using only the bias and acceleration terms of Eq. 2, the decrease in VAF was only 3.8%. In contrast, the irregular unit had higher sensitivities to both jerk and acceleration [2.4 (spikes/s)/(G/s) and 114.1 (spikes/s)/G, respectively], as well as a bias of 103 spikes/s. Accordingly, exclusion of the jerk term from Eq. 2 resulted in 16% decrease in VAF [i.e., 87 (Fig. 2B, black trace in right panel) vs. 71%]. This difference in the relative weight of a jerk term for fitting the responses of irregular and regular afferents was consistent across our populations of otolith afferents (22.0 ± 3.4 vs. 1.8 ± 0.4% decrease in VAF).
Next, to address whether afferents responded differently to active head translations, we used this same model (i.e., Eq. 2, with parameter estimates taken form the passive condition in Fig. 2B) to predict each afferent's response in the active condition (Fig. 2C, dashed green traces). The example afferents were typical of the regular and irregular afferents in our sample in that their modulation was similar during passive and active head-on-body translations. This was verified by the good prediction of the passive model for the responses to active translations (VAF = 91.2 and 83.1% for the regular and irregular afferent, respectively).
To further assess whether there were any differences in the bias and/or translational sensitivities of otolith afferents between passive and active movements, we estimated the parameters of Eq. 2 to obtain the best fit of each afferent's modulation during active head translations. The best fit to each example afferents' modulation during active translations is shown by the black trace in Fig. 2C. For the regular afferent, we estimated (VAF = 91.4%) a bias discharge rate, acceleration sensitivity, and jerk sensitivity of 56 spikes/s, 28.6 (spikes/s)/G, and 0.21 (spikes/s)/(G/s), respectively. These coefficients corresponded well to those estimated for the passive translation condition (Fig. 2B). Moreover, there was little improvement in VAF for this optimal estimation as compared with the passive-based prediction. Similar findings were obtained for the example irregular afferent [bias = 102 spikes/s, acceleration sensitivity = 120 (spikes/s)/G, jerk sensitivity = 2.1 (spikes/s)/(G/s), and VAF = 83.5%]. Taken together, these results confirmed that the responses of our two example afferents were similar during active and passive movements.
Population analysis: responses to passive and active translations are comparable
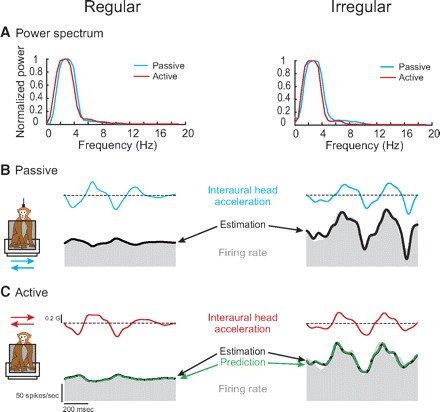
Figure 3 compares the bias discharge rate and acceleration sensitivity in both conditions for the entire population of afferents. The mean bias discharge measured across afferents from the fits to the passive and active translations was 84 ± 4 and 85 ± 4 spikes/s, respectively. The similarity of the bias between the two conditions is shown by the slope of the line fitted to the data in Fig. 3A (regression slope = 1.01), which was not different from 1 (P = 0.65). Similarly, the acceleration sensitivity for passive and active translations [mean of 80.6 ± 10.1 and 79.4 ± 10.3 (spikes/s)/G, respectively] were identical as shown by the slope of the line fitted to the data points in Fig. 3B (slope = 1.02), which was not different from 1 (P = 0.29). Thus taken as a population, the response dynamics of otolith afferents were similar during active and passive head translations. Furthermore, as is shown in Table 1, this observation could be extended when different functional groups of afferents were separately considered. Overall, regardless of discharge regularity, presumed organ of innervation, and/or direction of stimulation, response biases and sensitivities (i.e., bias and Sa in Eq. 2, respectively) were comparable during active versus passive translations (paired t-test, P > 0.05).
FIG. 3.
Comparison of parameters of estimations for responses to active and passive translations for the population of utricular (empty symbols) and saccular (filled symbols) otolith afferents. A: estimated bias for responses in the 2 conditions. There was no significant difference between active and passive conditions for regular (squares) and irregular (triangles) afferents. B: estimated acceleration sensitivities were not different between active and passive translations across otolith afferents. The unity lines (dashed lines) were superimposed on each plot to facilitate the comparison.
TABLE 1.
Comparison of bias and head acceleration sensitivities (Sa) during passive and active translations
| N | Passive Bias, (spikes/s) | Active Bias, (spikes/s) | P Value | Passive Sa, [(spikes/s)/G] | Active Sa, [(spikes/s)/G] | P Value | |
|---|---|---|---|---|---|---|---|
| Regular | |||||||
| Utricular | |||||||
| Lateral | 13 | 77 ± 9 | 78 ± 9 | 0.06 | 58.8 ± 13.8 | 55.2 ± 13.8 | 0.05 |
| Fore-aft | 7 | 78 ± 9 | 78 ± 9 | 0.90 | 37.4 ± 8.3 | 38.5 ± 8.7 | 0.37 |
| Saccular | 11 | 95 ± 8 | 95 ± 8 | 0.89 | 51.0 ± 9.4 | 49.1 ± 9.3 | 0.07 |
| Irregular | |||||||
| Utricular | |||||||
| Lateral | 7 | 87 ± 9 | 89 ± 9 | 0.06 | 183.6 ± 29.7 | 188.0 ± 32.2 | 0.52 |
| Fore-aft | 8 | 75 ± 6 | 76 ± 7 | 0.19 | 157.1 ± 28.1 | 161.7 ± 32.2 | 0.52 |
| Saccular | 2 | 109 ± 9 | 109 ± 6 | —* | 136.1 ± 16.2 | 129.7 ± 2.0 | —* |
Values are means ± SE.
Comparison not made because of the small data set.
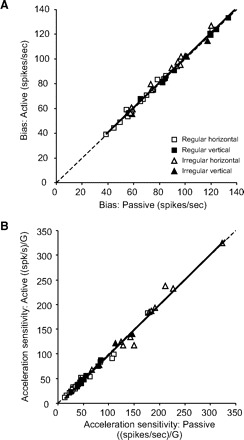
Finally, we evaluated whether differences in discharge bias (or possibly sensitivities) might be observed in afferents with more irregular resting rate. Prior work had reported an increase in the resting discharge and a decrease in sensitivity of afferent responses following electrical stimulation of the brain stem efferents in squirrel monkey (Goldberg and Fernandez 1980). Moreover, such effects were greater for irregularly than regularly discharging afferents. Figure 4A shows the bias and sensitivities of regular and irregular afferents during passive and active translations. Comparison of the bias discharge rates and translational acceleration and jerk sensitivities showed no differences between the two conditions for each group of afferents. This conclusion was further supported by the finding that the coefficients estimated for Eq. 2 from the data from passive translations provided an excellent predictive fit to the data from the active translations regardless of the afferents regularity. In fact, the difference between the predicted VAF (coefficients from the fit to passive translation data applied to active translation condition) and the VAF calculated from the best fit to the active translation data were only 1.3 ± 0.2 (2.65 ± 0.52% of VAF for the optimal fit). Notably, this difference in VAF was comparable across neurons regardless of CV* and background discharge rate (Fig. 4, B and C, respectively; VAF diff vs. CV*, r2 = 0.04; P = 0.16; VAF diff vs. background discharge rate, r2 = 0.002, P = 0.76).
FIG. 4.
Comparison of responses during active and passive translations. A: average values of bias and sensitivity for the population of regular and irregular afferents during active (gray bars) and passive (black bars) movements. B: difference in VAF between predicted and estimated responses during active translations as a function of CV*. VAF difference was <4% (dashed line) for regular (squares) and irregular (triangles) afferents. C: difference in VAF between predicted and estimated responses during active translations as a function of background discharge. VAF difference was <4% (dashed line) for most of the afferents.
DISCUSSION
Centrifugally projecting efferent fibers innervate the vestibular receptors of the otoliths as well as semicircular canals (Gacek and Lyon 1974; Rasmussen and Gacek 1958). However, the role of this efferent innervation remains unknown. Here we studied the proposal that the vestibular efferent system functions to change the background firing rate and response sensitivity of vestibular afferents during actively generated translations (Goldberg et al. 2000; Purcell and Perachio 1997). We found that the background activity and response sensitivities of the primary otolith afferents, innervating both the saccule and utricle, are identical during comparable passively and actively generated linear acceleration. Taken together, our findings provide evidence to refute the hypothesis that, in primates, the activation of the vestibular efferent system functions to selectively modulate afferent responses during active head motion.
Response to passive sinusoidal translations: comparison with previous studies
Most previous studies that have investigated the response dynamics of otolith afferents have applied horizontal centrifugal force or translations in the horizontal plane and accordingly have focused on characterizing the afferents innervating the utricle (Angelaki and Dickman 2000; Goldberg et al. 1990a; Purcell et al. 2003; Si et al. 1997). Prior studies have, however, compared the resting rates and steady state (i.e., static) responses of utricular and saccular afferents evoked by the application of static tilts (Fernandez and Goldberg 1976a; Fernandez et al. 1972; Tomko et al. 1981). Although the resting discharge rates and static responses were slightly lower for saccular than utricular afferents the in cat (Tomko et al. 1981), they were typically comparable in primate (Fernandez and Goldberg 1976a). In addition, off-axis centrifugal forces have been applied to characterize the dynamic responses of saccular afferents to sinusoidal linear force in anesthetized squirrel monkeys (Fernandez and Goldberg 1976c). In this study, we applied comparable pure translations in both the horizontal and vertical planes and thus were able to compare afferent response dynamics of both end organs.
Unlike utricle receptors, the receptors in the saccule are biased at rest because they are stimulated by the constant force exerted by gravity. As a result, saccule afferent responses will modulate around the 1 or −1 G point of their parabolic force–response curve (Fernandez and Goldberg 1976b) during vertical linear motion, depending on whether they innervate receptors that are sensitive to downward or upward translations, respectively. However, although their force–response curve is inherently nonlinear, relatively vigorous stimulation (i.e., more than ∼2 G) is required to drive afferents out of their linear range (Fernandez and Goldberg 1976b). In this study, our stimuli were designed to test neurons only in the linear portion of their stimulus–response curves. Accordingly, comparison of the response sensitivity of regular saccular afferents that were preferentially excited by upward translations (i.e., haircells exposed to downward linear acceleration) with those excited by downward translations showed comparable sensitivities. Note, this comparison was not done for irregular saccular afferents because of our limited sample. Moreover, we found that, on average, the sensitivity and phase of saccular afferents were comparable to those of afferents innervating the utricle. Thus the two groups are discussed as a pooled population.
Previous studies of utricular afferents have reported significantly greater sensitivities for irregular than regular units in response to passively applied translations. Difference in sensitivity range from four- to fivefold [47–78 vs. 255–281 (spikes/s)/G for regular and irregular afferents, respectively], across species (squirrel monkey: Fernandez and Goldberg 1976c; chinchilla: Goldberg et al. 1990a; gerbil: Purcell et al. 2003). In this study, we found a comparable difference [66 vs. 286 (spikes/s)/G for regular and irregular afferents, respectively] when we estimated their maximal sensitivity (see methods). Overall, the design of our study was most comparable to that of Angelaki and Dickman (2000). Both studies describe the activity of otolith afferents in response to passive translations (with frequencies of ≤5 Hz) in alert macaque monkeys. Because only normalized sensitivities were reported in this prior study, it is difficult to directly compare values. Nevertheless, the relative differences between the average sensitivities of regular and irregular afferents are comparable across both studies. In addition, consistent with findings of these previous studies, we found that, on average, the responses of regular units lagged acceleration by ∼6°, whereas those of irregular afferents led acceleration by ∼30°.
Implications regarding the role of the efferent vestibular system
The principal goal of this study was to establish whether the vestibular afferents that innervate the otoliths differentially encode active and passive translations. Stimulation of the efferent vestibular system results in an increase in the resting discharge rate (Boyle and Highstein 1990; Goldberg and Fernandez 1980; Marlinski et al. 2004; Plotnik et al. 2002, 2005) and a reduction of the sensitivity of afferents (Goldberg and Fernandez 1980). These findings led to the proposal that activation of the efferent system during active head movements could be used to decrease the probability of inhibitory cut-off or excitatory saturation of afferents, thereby effectively increasing the dynamic range available (Goldberg and Fernandez 1980). Indeed, there are several lines of evidence that support a role for the efferent vestibular system in contributing to the differential processing of active and passive movements. First, experiments in alert toadfish have shown that efferent activation in this species accompanies the responses leading up to an escape reaction (Boyle and Highstein 1990; Highstein and Baker 1985). The behaviorally induced excitation of efferents, in turn, led to an increase in the discharge rate and a decrease in the rotational sensitivity of afferents when tested using passive head rotation. These findings led to the suggestion that the vestibular efferent system carries a motor efference copy signal that modulates the responses of vestibular afferents during self-generated movements in toadfish. Second, the convergence of somatosensory and proprioceptive information with vestibular signals via efferent projections has been reported in the periphery of frog and fish (Boyle and Highstein 1990; Caston and Bricout-Berthout 1984; Hartmann and Klinke 1980). These extravestibular signals could also be used to modulate the sensitivity of afferents during active movements (Cullen and Minor 2002; Goldberg and Fernandez 1980; Klinke 1970). Third, it has been shown recently that activation of the vestibular efferent system in alert macaque monkeys significantly increases background firing rate of afferent fibers (Sadeghi et al. 2007b). Previous studies, however, have shown that the vestibular afferents innervating semicircular canals similarly encode active and passive head movements in normal macaques and following contralateral labyrinthectomy (Cullen and Minor 2002; Sadeghi et al. 2007c). Thus in the case of active rotational movements, activation of the primate efferent system is not used to increase the available dynamic range.
Here we have specifically addressed the possibility that the vestibular efferent system serves different functional roles regarding the modulation of canal versus otolith afferent responses. This proposal is consistent with the results of electrical stimulation studies suggesting that cerebellar stimulation might preferentially alter (via a multisynaptic pathway) the background activity of otolith afferents while leaving the discharges of afferents innervating the semicircular canals largely unaffected (Llinas and Precht 1969). Preliminary single unit studies in the vestibular system (Brooks and Cullen 2007) and electrosensory system of electric fish (Bell et al. 1999; Mohr et al. 2003; Sawtell et al. 2007), as well as functional MRI (fMRI) studies of tactile processing in humans (Blakemore et al. 1998, 1999), have shown that the cerebellum is involved in predicting the sensory consequences of voluntary actions. Double-labeling experiments have further shown that vestibular efferent cells send extensive projections both to the labyrinth and the vestibulo-cerebellum (Shinder et al. 2001). Accordingly, reciprocal connections between the cerebellum and efferent neurons could be used during intentional head movements to reduce the likelihood of saturation or silencing (Goldberg et al. 2000) of otolith afferents.
Our findings, however, provide firm evidence that primary otolith afferents are not differentially influenced by the efferent pathway during active and passive head translations. Responses during active head translations were well predicted based on response during passive head translations. Moreover, responses were comparable during passive whole body and head-on-body head translations, also refuting the proposal that the efferent system modulates otolith afferents by encoding neck somatosensory and proprioceptive information. Overall, our results extend previous findings regarding canal afferents (Cullen and Minor 2002; Sadeghi et al. 2007c) and show that, in alert macaque monkeys, afferent nerve fibers innervating all of the end organs respond similarly during self-generated and passive motion.
Notably, vestibular input resulting from active rotational movements (reafference) is suppressed at the next stage of processing: the vestibular nuclei (McCrea et al. 1999; Roy and Cullen 2001, 2004). Given that the majority of the neurons in the vestibular nuclei receive convergent inputs from multiple vestibular end organs (Curthoys and Markham 1971; Dickman and Angelaki 2002; Kaufman et al. 2000; Markham and Curthoys 1972; McConville et al. 1996; Straka et al. 2002; Tomlinson et al. 1996; Uchino et al. 2005; Yakushin et al. 2006; Zakir et al. 2000; Zhang et al. 2001), it seems logical that a comparable strategy is used for encoding both linear and rotation acceleration at the level of the vestibular periphery. As such, otolith-derived reafference can be suppressed at the level of vestibular nuclei in a manner comparable to canal-derived inputs (McCrea et al. 1999; Roy and Cullen 2001, 2004). Further experiments will be needed to determine whether this hypothesis is true. In addition, further studies will be needed to understand whether the connectivity between the vestibular efferent system and cerebellum observed is present in primates and what role it might play in the processing of vestibular inputs.
GRANTS
This work was supported by the Canadian Institutes of Health Research, National Institute of Deafness and Other Communication Disorders Grant DC-02390, and Canadian Space Agency.
Acknowledgments
We thank W. Kucharski, S. Nuara, and J. Knowles for excellent technical assistance and C. Massot, J. Brooks, and M. Van Horn for critically reading the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Angelaki 2000.Angelaki DE, Dickman JD. Spatiotemporal processing of linear acceleration: primary afferent and central vestibular neuron responses. J Neurophysiol 84: 2113–2132, 2000 [DOI] [PubMed] [Google Scholar]
- Angelaki 1992.Angelaki DE, Perachio AA, Mustari MJ, Strunk CL. Role of irregular otolith afferents in the steady-state nystagmus during off-vertical axis rotation. J Neurophysiol 68: 1895–1900, 1992 [DOI] [PubMed] [Google Scholar]
- Angelaki 2004.Angelaki DE, Shaikh AG, Green AM, Dickman JD. Neurons compute internal models of the physical laws of motion. Nature 430: 560–564, 2004 [DOI] [PubMed] [Google Scholar]
- Bell 1999.Bell CC, Han VZ, Sugawara Y, Grant K. Synaptic plasticity in the mormyrid electrosensory lobe. J Exp Biol 202: 1339–1347, 1999 [DOI] [PubMed] [Google Scholar]
- Birinyi 2001.Birinyi A, Straka H, Matesz C, Dieringer N. Location of dye-coupled second order and of efferent vestibular neurons labeled from individual semicircular canal or otolith organs in the frog. Brain Res 921: 44–59, 2001 [DOI] [PubMed] [Google Scholar]
- Blakemore 1998.Blakemore SJ, Wolpert DM, Frith CD. Central cancellation of self-produced tickle sensation. Nat Neurosci 1: 635–640, 1998 [DOI] [PubMed] [Google Scholar]
- Blakemore 1999.Blakemore SJ, Wolpert DM, Frith CD. The cerebellum contributes to somatosensory cortical activity during self-produced tactile stimulation. NeuroImage 10: 448–459, 1999 [DOI] [PubMed] [Google Scholar]
- Boyle 1990.Boyle R, Highstein SM. Efferent vestibular system in the toadfish: action upon horizontal semicircular canal afferents. J Neurosci 10: 1570–1582, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks 2007.Brooks J, Cullen KE. Reference frames and reafference in the rostral fastigial nucleus. Soc Neurosci Abstr 37: 861.2, 2007 [Google Scholar]
- Caston 1984.Caston J, Bricout-Berthout A. Responses to somatosensory input by afferent and efferent neurons in the vestibular nerve of the frog. Brain Behav Evol 24: 135–143, 1984 [DOI] [PubMed] [Google Scholar]
- Cherif 2008.Cherif S, Cullen KE, Galiana HL. An improved method for the estimation of firing rate dynamics using an optimal digital filter. J Neurosci Methods 173: 165–181, 2008 [DOI] [PubMed] [Google Scholar]
- Crapse 2008.Crapse TB, Sommer MA. Corollary discharge across the animal kingdom. Nat Rev Neurosci 9: 587–600, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen 2004.Cullen KE. Sensory signals during active versus passive movement. Curr Opin Neurobiol 14: 698–706, 2004 [DOI] [PubMed] [Google Scholar]
- Cullen 2002.Cullen KE, Minor LB. Semicircular canal afferents similarly encode active and passive head-on-body rotations: implications for the role of vestibular efference. J Neurosci 22: RC226, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen 1996.Cullen KE, Rey CG, Guitton D, Galiana HL. The use of system identification techniques in the analysis of oculomotor burst neuron spike train dynamics. J Comput Neurosci 3: 347–368, 1996 [DOI] [PubMed] [Google Scholar]
- Curthoys 1971.Curthoys IS, Markham CH. Convergence of labyrinthine influences on units in the vestibular nuclei of the cat. I. Natural stimulation. Brain Res 35: 469–490, 1971 [DOI] [PubMed] [Google Scholar]
- Dickman 2002.Dickman JD, Angelaki DE. Vestibular convergence patterns in vestibular nuclei neurons of alert primates. J Neurophysiol 88: 3518–3533, 2002 [DOI] [PubMed] [Google Scholar]
- Dickman 1993.Dickman JD, Correia MJ. Bilateral communication between vestibular labyrinths in pigeons. Neuroscience 57: 1097–1108, 1993 [DOI] [PubMed] [Google Scholar]
- Fernandez 1976a.Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long-duration centrifugal force. J Neurophysiol 39: 970–984, 1976a [DOI] [PubMed] [Google Scholar]
- Fernandez 1976b.Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. II. Directional selectivity and force-response relations. J Neurophysiol 39: 985–995, 1976b [DOI] [PubMed] [Google Scholar]
- Fernandez 1976c.Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. III. Response dynamics. J Neurophysiol 39: 996–1008, 1976c [DOI] [PubMed] [Google Scholar]
- Fernandez 1972.Fernandez C, Goldberg JM, Abend WK. Response to static tilts of peripheral neurons innervating otolith organs of the squirrel monkey. J Neurophysiol 35: 978–987, 1972 [DOI] [PubMed] [Google Scholar]
- Gacek 1974.Gacek RR, Lyon M. The localization of vestibular efferent neurons in the kitten with horseradish peroxidase. Acta Otolaryngol 77: 92–101, 1974 [DOI] [PubMed] [Google Scholar]
- Gleisner 1963.Gleisner L, Henriksson NG. Efferent and afferent activity pattern in the vestibular nerve of the frog. Acta Otolaryngol Suppl 192: 190, 1963 [DOI] [PubMed] [Google Scholar]
- Goldberg 2000.Goldberg JM, Brichta AM, Wackym PA. Efferent vestibular system: anatomy, physiology and neurochemistry. In: Neurochemistry of the Vestibular System, edited by Beitz AJ and Anderson JH. Boca Raton, FL: CRC, 2000, p. 61–94.
- Goldberg 1990a.Goldberg JM, Desmadryl G, Baird RA, Fernandez C. The vestibular nerve of the chinchilla. IV. Discharge properties of utricular afferents. J Neurophysiol 63: 781–790, 1990a [DOI] [PubMed] [Google Scholar]
- Goldberg 1990b.Goldberg JM, Desmadryl G, Baird RA, Fernandez C. The vestibular nerve of the chinchilla. V. Relation between afferent discharge properties and peripheral innervation patterns in the utricular macula. J Neurophysiol 63: 791–804, 1990b [DOI] [PubMed] [Google Scholar]
- Goldberg 1980.Goldberg JM, Fernandez C. Efferent vestibular system in the squirrel monkey: anatomical location and influence on afferent activity. J Neurophysiol 43: 986–1025, 1980 [DOI] [PubMed] [Google Scholar]
- Goldberg 1984.Goldberg JM, Smith CE, Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol 51: 1236–1256, 1984 [DOI] [PubMed] [Google Scholar]
- Grossman 1988.Grossman GE, Leigh RJ, Abel LA, Lanska DJ, Thurston SE. Frequency and velocity of rotational head perturbations during locomotion. Exp Brain Res 70: 470–476, 1988 [DOI] [PubMed] [Google Scholar]
- Gu 2007.Gu Y, Deangelis GC, Angelaki DE. A functional link between area MSTd and heading perception based on vestibular signals. Nat Neurosci 10: 1038–1047, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque 2004.Haque A, Angelaki DE, Dickman JD. Spatial tuning and dynamics of vestibular semicircular canal afferents in rhesus monkeys. Exp Brain Res 155: 81–90, 2004 [DOI] [PubMed] [Google Scholar]
- Harris 2000.Harris LR, Jenkin M, Zikovitz DC. Visual and non-visual cues in the perception of linear self-motion. Exp Brain Res 135: 12–21, 2000 [DOI] [PubMed] [Google Scholar]
- Hartmann 1980.Hartmann R, Klinke R. Efferent activity in the goldfish vestibular nerve and its influence on afferent activity. Pfluegers Arch 388: 123–128, 1980 [DOI] [PubMed] [Google Scholar]
- Highstein 1985.Highstein SM, Baker R. Action of the efferent vestibular system on primary afferents in the toadfish, Opsanus tau J Neurophysiol 54: 370–384, 1985 [DOI] [PubMed] [Google Scholar]
- Highstein 1986.Highstein SM, Baker R. Organization of the efferent vestibular nuclei and nerves of the toadfish, Opsanus tau J Comp Neurol 243: 309–325, 1986 [DOI] [PubMed] [Google Scholar]
- Hirvonen 2005.Hirvonen TP, Minor LB, Hullar TE, Carey JP. Effects of intratympanic gentamicin on vestibular afferents and hair cells in the chinchilla. J Neurophysiol 93: 643–655, 2005 [DOI] [PubMed] [Google Scholar]
- Huterer 2002.Huterer M, Cullen KE. Vestibuloocular reflex dynamics during high-frequency and high-acceleration rotations of the head on body in rhesus monkey. J Neurophysiol 88: 13–28, 2002 [DOI] [PubMed] [Google Scholar]
- Jarvis 2001.Jarvis MR, Mitra PP. Sampling properties of the spectrum and coherency of sequences of action potentials. Neural Comput 13: 717–749, 2001 [DOI] [PubMed] [Google Scholar]
- Kaufman 2000.Kaufman GD, Shinder ME, Perachio AA. Convergent properties of vestibular-related brain stem neurons in the gerbil. J Neurophysiol 83: 1958–1971, 2000 [DOI] [PubMed] [Google Scholar]
- Klinke 1970.Klinke R. Efferent influence on the vestibular organ during active movements of the body. Pfluegers Arch 318: 325–332, 1970 [DOI] [PubMed] [Google Scholar]
- Lindeman 1969.Lindeman HH. Studies on the morphology of the sensory regions of the vestibular apparatus with 45 figures. Ergebnisse Anat Entwicklungsgeschichte 42: 1–113, 1969 [PubMed] [Google Scholar]
- Lisberger 1986.Lisberger SG, Pavelko TA. Vestibular signals carried by pathways subserving plasticity of the vestibulo-ocular reflex in monkeys. J Neurosci 6: 346–354, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas 1969.Llinas R, Precht W. The inhibitory vestibular efferent system and its relation to the cerebellum in the frog. Exp Brain Res 9: 16–29, 1969 [DOI] [PubMed] [Google Scholar]
- Markham 1972.Markham CH, Curthoys IS. Convergence of labyrinthine influences on units in the vestibular nuclei of the cat. II. Electrical stimulation. Brain Res 43: 383–396, 1972 [DOI] [PubMed] [Google Scholar]
- Marlinski 2004.Marlinski V, Plotnik M, Goldberg JM. Efferent actions in the chinchilla vestibular labyrinth. J Assoc Res Otolaryngol 5: 126–143, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville 1996.McConville KM, Tomlinson RD, Na EQ. Behavior of eye-movement-related cells in the vestibular nuclei during combined rotational and translational stimuli. J Neurophysiol 76: 3136–3148, 1996 [DOI] [PubMed] [Google Scholar]
- McCrea 1999.McCrea RA, Gdowski GT, Boyle R, Belton T. Firing behavior of vestibular neurons during active and passive head movements: vestibulo-spinal and other non-eye-movement related neurons. J Neurophysiol 82: 416–428, 1999 [DOI] [PubMed] [Google Scholar]
- Mohr 2003.Mohr C, Roberts PD, Bell CC. The mormyromast region of the mormyrid electrosensory lobe. I. Responses to corollary discharge and electrosensory stimuli. J Neurophysiol 90: 1193–1210, 2003 [DOI] [PubMed] [Google Scholar]
- Myers 1997.Myers SF, Salem HH, Kaltenbach JA. Efferent neurons and vestibular cross talk in the frog. J Neurophysiol 77: 2061–2070, 1997 [DOI] [PubMed] [Google Scholar]
- Ohmi 1996.Ohmi M. Egocentric perception through interaction among many sensory systems. Brain Res Cogn Brain Res 5: 87–96, 1996 [DOI] [PubMed] [Google Scholar]
- Peterson 1988.Peterson BW, Richmond FJ. Control of Head Movement. New York: Oxford, 1988
- Plotnik 2005.Plotnik M, Marlinski V, Goldberg JM. Efferent-mediated fluctuations in vestibular nerve discharge: a novel, positive-feedback mechanism of efferent control. J Assoc Res Otolaryngol 6: 311–323, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnik 2002.Plotnik M, Marlinski V, Goldberg JM. Reflections of efferent activity in rotational responses of chinchilla vestibular afferents. J Neurophysiol 88: 1234–1244, 2002 [DOI] [PubMed] [Google Scholar]
- Purcell 2003.Purcell IM, Newlands SD, Perachio AA. Responses of gerbil utricular afferents to translational motion. Exp Brain Res 152: 317–322, 2003 [DOI] [PubMed] [Google Scholar]
- Purcell 1997.Purcell IM, Perachio AA. Three-dimensional analysis of vestibular efferent neurons innervating semicircular canals of the gerbil. J Neurophysiol 78: 3234–3248, 1997 [DOI] [PubMed] [Google Scholar]
- Ramachandran 2006.Ramachandran R, Lisberger SG. Transformation of vestibular signals into motor commands in the vestibuloocular reflex pathways of monkeys. J Neurophysiol 96: 1061–1074, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen 1958.Rasmussen GL, Gacek RR. Concerning the question of the efferent fiber component of the vestibular nerve of the cat. Anat Rec 130: 361–362, 1958 [Google Scholar]
- Roy 2004.Roy JE, Cullen KE. Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei. J Neurosci 24: 2102–2111, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy 2001.Roy JE, Cullen KE. Selective processing of vestibular reafference during self-generated head motion. J Neurosci 21: 2131–2142, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi 2007a.Sadeghi SG, Chacron MJ, Taylor MC, Cullen KE. Neural variability, detection thresholds, and information transmission in the vestibular system. J Neurosci 27: 771–781, 2007a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi 2007b.Sadeghi SG, Goldberg JM, Minor LB, and Cullen KE. Vestibular-nerve afferents of alert macaques in normal conditions and following vestibular lesion. Soc Neurosci Abstr 37: 861.9, 2007b [Google Scholar]
- Sadeghi 2007c.Sadeghi SG, Minor LB, Cullen KE. Response of vestibular-nerve afferents to active and passive rotations under normal conditions and after unilateral labyrinthectomy. J Neurophysiol 97: 1503–1514, 2007c [DOI] [PubMed] [Google Scholar]
- Sawtell 2007.Sawtell NB, Williams A, Bell CC. Central control of dendritic spikes shapes the responses of Purkinje-like cells through spike timing-dependent synaptic plasticity. J Neurosci 27: 1552–1565, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh 2005.Shaikh AG, Green AM, Ghasia FF, Newlands SD, Dickman JD, Angelaki DE. Sensory convergence solves a motion ambiguity problem. Curr Biol 15: 1657–1662, 2005 [DOI] [PubMed] [Google Scholar]
- Shinder 2001.Shinder ME, Purcell IM, Kaufman GD, Perachio AA. Vestibular efferent neurons project to the flocculus. Brain Res 889: 288–294, 2001 [DOI] [PubMed] [Google Scholar]
- Si 1997.Si X, Angelaki DE, Dickman JD. Response properties of pigeon otolith afferents to linear acceleration. Exp Brain Res 117: 242–250, 1997 [DOI] [PubMed] [Google Scholar]
- Straka 2002.Straka H, Holler S, Goto F. Patterns of canal and otolith afferent input convergence in frog second-order vestibular neurons. J Neurophysiol 88: 2287–2301, 2002 [DOI] [PubMed] [Google Scholar]
- Sylvestre 1999.Sylvestre PA, Cullen KE. Quantitative analysis of abducens neuron discharge dynamics during saccadic and slow eye movements. J Neurophysiol 82: 2612–2632, 1999 [DOI] [PubMed] [Google Scholar]
- Telford 1995.Telford L, Howard IP, Ohmi M. Heading judgments during active and passive self-motion. Exp Brain Res 104: 502–510, 1995 [DOI] [PubMed] [Google Scholar]
- Tomko 1981.Tomko DL, Peterka RJ, Schor RH. Responses to head tilt in cat eighth nerve afferents. Exp Brain Res 41: 216–221, 1981 [DOI] [PubMed] [Google Scholar]
- Tomlinson 1996.Tomlinson RD, McConville KM, Na EQ. Behavior of cells without eye movement sensitivity in the vestibular nuclei during combined rotational and translational stimuli. J Vestib Res 6: 145–158, 1996 [PubMed] [Google Scholar]
- Tribukait 2005.Tribukait A, Eiken O. On the role of otoliths and semicircular canals in spatial orientation: dynamics of the visually perceived eye level during gondola centrifugation. Percept Psychophys 67: 1242–1251, 2005 [DOI] [PubMed] [Google Scholar]
- Uchino 2005.Uchino Y, Sasaki M, Sato H, Bai R, Kawamoto E. Otolith and canal integration on single vestibular neurons in cats. Exp Brain Res 164: 271–285, 2005 [DOI] [PubMed] [Google Scholar]
- Yakushin 2006.Yakushin SB, Raphan T, Cohen B. Spatial properties of central vestibular neurons. J Neurophysiol 95: 464–478, 2006 [DOI] [PubMed] [Google Scholar]
- Zakir 2000.Zakir M, Kushiro K, Ogawa Y, Sato H, Uchino Y. Convergence patterns of the posterior semicircular canal and utricular inputs in single vestibular neurons in cats. Exp Brain Res 132: 139–148, 2000 [DOI] [PubMed] [Google Scholar]
- Zhang 2001.Zhang X, Zakir M, Meng H, Sato H, Uchino Y. Convergence of the horizontal semicircular canal and otolith afferents on cat single vestibular neurons. Exp Brain Res 140: 1–11, 2001 [DOI] [PubMed] [Google Scholar]


