Summary
Fire blight caused by the Gram‐negative bacterium Erwinia amylovora can be controlled by antagonistic microorganisms. We characterized epiphytic bacteria isolated from healthy apple and pear trees in Australia, named Erwinia tasmaniensis, and the epiphytic bacterium Erwinia billingiae from England for physiological properties, interaction with plants and interference with growth of E. amylovora. They reduced symptom formation by the fire blight pathogen on immature pears and the colonization of apple flowers. In contrast to E. billingiae, E. tasmaniensis strains induced a hypersensitive response in tobacco leaves and synthesized levan in the presence of sucrose. With consensus primers deduced from lsc as well as hrpL, hrcC and hrcR of the hrp region of E. amylovora and of related bacteria, these genes were successfully amplified from E. tasmaniensis DNA and alignment of the encoded proteins to other Erwinia species supported a role for environmental fitness of the epiphytic bacterium. Unlike E. tasmaniensis, the epiphytic bacterium E. billingiae produced an acyl‐homoserine lactone for bacterial cell‐to‐cell communication. Their competition with the growth of E. amylovora may be involved in controlling fire blight.
Introduction
Fire blight is a devastating disease of apple and pear fruit trees, and is caused by the Gram‐negative bacterium Erwinia amylovora. The pathogen affects apples and pears as well as other rosaceous plants, including some ornamentals. Fire blight is responsible for severe losses in apple and pear production in North America, Europe, the Mediterranean region and New Zealand. Strategies to control fire blight include chemical control, breeding of resistant plants and biological control. None of the proposed strategies reviewed in details elsewhere is completely satisfactory (Vanneste, 2000).
Colonization of flowers by E. amylovora is considered as a primary step in the development of most fire blight infections of apple and pear trees (Stockwell et al., 1999). Blossoms remain susceptible to bacterial colonization for few days after opening and the presence of antagonistic bacteria in this period can suppress colonization by E. amylovora (Stockwell et al., 2002; Temple et al., 2004; Stockwell and Stack, 2007).
The non‐pathogenic epiphytic bacterium Pseudomonas fluorescens strain A506 has been used as a fire blight biocontrol agent, and it is commercially available (BlightBan A506). The epiphyte Pantoea agglomerans (syn. Erwinia herbicola) has been investigated as a potential antagonist (Vanneste et al., 1992; Wright et al., 2001; Giddens et al., 2003), and strain C9‐1 has been applied experimentally in the USA (Johnson and Stockwell, 1998). Bacillus megaterium and Bacillus pumilus strains have been reported to inhibit growth of E. amylovora (Jock et al., 2002). Isolation of Enterobacter agglomerans from clinical specimens and its classification together with E. herbicola (Gavini et al., 1989) has restricted the use of P. agglomerans (E. herbicola ) strains as a control agent in most countries with fire blight. There are also reports that P. fluorescens can bind to human nerve cells and fibronectin (Picot et al., 2001; de Lima Pimenta, 2003). Obviously, it is of great importance to look for additional antagonists to control fire blight preferentially with bacteria of the genus Erwinia. Here, we investigate properties of two novel Erwinia species to act as antagonists against fire blight.
Results
Growth inhibition of E. amylovora
The levan‐positive epiphytic strains Et1/99 and Et2/99 from Australia were classified into the novel species Erwinia tasmaniensis and did not affect apple seedlings nor immature pear slices to produce symptoms resembling fire blight (Geider et al., 2006). The E. tasmaniensis strains and the recently classified epiphytic bacterium Erwinia billingiae (Mergaert et al., 1999) were tested for their antagonistic effects against E. amylovora in assays with immature pear. Pear slices, which were soaked in a suspension of E. tasmaniensis or E. billingiae cells and then inoculated with E. amylovora (500 cfu ml−1), showed absence or a significant reduction of ooze production and necrosis compared with controls with water (Fig. 1A). The E. tasmaniensis strains showed often enhanced effects in 5% sucrose solution while E. billingiae Eb660 and Eb661 were as efficient in symptom reduction when pear slices were soaked with bacteria in water. Erwinia billingiae still showed strong antagonistic effects when high levels (up to 50 000 cfu ml−1) of E. amylovora were applied. The German isolate FLA03, classified as E. tasmaniensis, weakly interfered with growth of E. amylovora.
Figure 1.
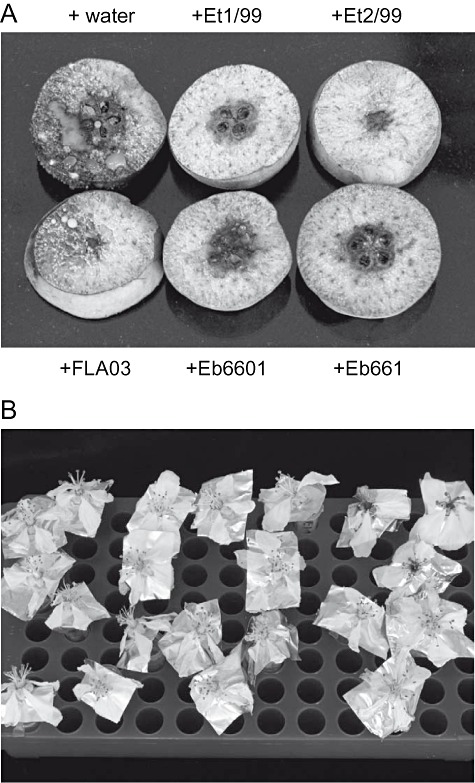
Antagonistic effects of E. billingiae, E. tasmaniensis on pear slices and apple flowers inoculated with E. amylovora. A. Symptoms after treatment of pear slices with Eb660, Eb661, FLA03, Et1/99 and Et2/99. One slice from a set of four similar slices with the same treatment is shown. B. Fire blight symptoms on apple flowers sprayed with Eb660, Eb661, Et1/99 and Et2/99, and then inoculated with 500 cells of E. amylovora Ea1/79Sm. Control was treatment with water before inoculation with E. amylovora (three flowers top right). Evaluation for growth of Ea1/79Sm is given in Table 1. The experiments were repeated at least twice producing similar results.
In similar growth competition assays with apple flowers, the antagonistic strain was diluted in water to 1 × 108 cfu ml−1 and sprayed on detached apple flowers about 1 or 2 days after opening with cells of several E. billingiae and E. tasmaniensis strains. After drying at room temperature, 5000 E. amylovora in 10 µl of water were applied to the pistil and the flowers then incubated for 5 days (Fig. 1B). The number of recovered E. amylovora cells (Smr) was quantified by plating serial dilutions on agar plates (Table 1). In contrast to control flowers sprayed with water, the number of E. amylovora cells diminished in flowers treated with suspensions of E. billingiae Eb660 and Eb661. The E. tasmaniensis strains Et1/99 and Et2/99 also largely reduced growth of E. amylovora in apple flowers. It should be emphasized that flowers are not equally protected after application of antagonistic bacteria. In two of three flowers, Et2/99 reduced growth of E. amylovora to a low level, whereas another flower showed an atypical intermediate colonization by the pathogen (Table 1).
Table 1.
Effect of E. billingiae and E. tasmaniensis on colonization of apple flowers with E. amylovora.
| Inoculation with E. amylovora Ea1/79Sm | |
|---|---|
| Flowers treated with | Ea1/78Sm recovered (cfu) |
| E. billingiae Eb660 | 0 |
| E. billingiae Eb661 | 2 × 102 |
| E. tasmaniensis Et1/99 | 1 × 103 |
| E. tasmaniensis Et2/99 | 3 × 103a |
| Water control | 4 × 107 |
Mean of three flowers, single flowers: 0/1 × 104/0.
The numbers refer to the average of Ea1/79Sm cells recovered on StI agar with streptomycin and cycloheximide from three flowers at 5 days. The antagonistic bacteria were sprayed at 1 × 108 cfu ml−1 in water. Inoculation was done with 10 µl droplets containing 5000 cfu of Ea1/79Sm.
The E. tasmaniensis strains Et1/99 and Et2/99 as well as the E. billingiae strains Eb660 and Eb661 did not produce growth inhibition zones on a lawn of Ea1/79. Although weak effects of culture supernatants of E. billingiae on growth of E. amylovora were occasionally observed, interference with E. amylovora in flowers should mainly be due to a dominant population of an antagonistic bacterial species.
The levansucrase gene and PCR detection of E. tasmaniensis
The epiphytic E. tasmaniensis strains from Australia produce large amounts of levan. To amplify the lsc gene, the PCR primers LSC1 and LSC2c were designed from the lsc gene of E. amylovora (Accession Number X75079). They amplified a 565 bp fragment from E. tasmaniensis, which was sequenced for strains Et1/99, Et2/99 and Et4/99. Alignment with the corresponding part of lsc from E. amylovora showed significant differences for the structural fragment, but the E. tasmaniensis sequences were identical among each other (Fig. 2). These primers could therefore be applied for detection of E. tasmaniensis in bacterial populations on plant surfaces. From parts of E. tasmaniensis lsc, which are divergent to E. amylovora, PCR primers were designed, and produced a PCR band of 0.3 kb. The primers LSCa1 and LSCa2c for amplification of DNA from several strains or bacterial species only amplified DNA from E. tasmaniensis. No signal was obtained for DNA from Brenneria rubrifaciens, B. nigrifluens, B. quercina, B. salicis, E. amylovora CFBP1232T, E. amylovora Ea1/79, E. amylovora Ea273, E. billingiae Eb661, Erwinia mallotivora, Erwinia papayae, Erwinia persicina including an isolate from apple of our laboratory, Erwinia psidii, Erwinia pyrifoliae Ejp557 (Japan), E. pyrifoliae Ep1/96 (Korea), Erwinia rhapontici, Erwinia toletana, Erwinia tracheiphila, Pantoea stewartii DC283 and Pectobacterium cypripedii (data not shown; the species name only indicates a type strain). We therefore conclude a specific amplification of the lsc gene from E. tasmaniensis strains with these primers in respect to other Erwinia species, and they are therefore useful to detect this species among other bacteria.
Figure 2.

Sequence comparison of parts from lsc genes of the E. tasmaniensis strains Et1/99, Et2/99 and Et4/99 with lsc of E. amylovora strain Ea1/79.
Hypersensitive response and hrp genes of E. tasmaniensis
The production of a hypersensitive response (HR) is a typical property of most plant‐pathogenic bacteria. When the cells of the three E. tasmaniensis strains Et1/99, Et2/99 and Et4/99 were infiltrated into tobacco leaves (cv. ‘SR1’) at a density of 1 × 108 cfu ml−1, they caused HR in leaves (cv. ‘SR1’) only after conditioning in an inducing medium (IM) (Fig. 3). No HR was observed for the E. billingiae strain Eb661. After cultivation in nutrient broth, the E. tasmaniensis strains induced HR in leaves of other tobacco cultivars such as ‘Samsun’.
Figure 3.

Induction of HR on tobacco leaves. Ea1/79, Et1/99, Et2/99 and Et4/99 were cultured in inducing medium and produced local necrotic HR lesions (right) in a leaf of cultivar ‘SR1’. No hypersensitive response was found for E. billingiae Eb661 or with the E. tasmaniensis strains Et1/99, Et2/99 and Et4/99 without conditioning in IM (left).
In order to increase the capacity for HR induction, the hrpL gene of E. amylovora was introduced into an E. tasmaniensis strain. When Et1/99(pGThrpL‐Ea1) or Et4/99(pGThrpL‐Ea1) were infiltrated into tobacco leaves (cv. ‘SR1’), a reliable HR was observed without conditioning of the bacteria in IM. It is assumed that the IM medium increases expression of hrp genes.
Genes in the hrp cluster of E. tasmaniensis were analysed with primers from the two conserved genes hrcC/hrcR by comparison with sequences of E. amylovora and E. pyrifoliae. PCR bands of 0.7 and 0.5 kb were obtained with DNA of Et1/99, Et2/99 and Et4/99 for hrcC and hrcR fragments respectively. These products were cloned into pGEM‐T, sequenced and aligned to the corresponding genes from other pathogenic bacteria. The alignment of 187 amino acids of HrcC showed 90% similarity to E. amylovora and 91% to the related Asian pear pathogen. Alignments of 146 amino acids of HrcR showed 91% similarity to E. amylovora and 95% to the proteins of the Asian pear pathogen (Fig. 4). Amino acid similarities greater that 70% were also found for HrcC proteins of other plant‐pathogenic bacteria, such as Dickeya (Erwinia) chrysanthemi, Pectobacterium carotovorum and Pseudomonas syringae. Conserved domains homologous to the proteins of the flagella biosynthesis and type III secretion pathways were found, which is a common feature of Hrp/Hrc proteins.
Figure 4.

Alignment of partial amino acid sequences HrcC, HrcR and HrpL from E. tasmaniensis, E. amylovora and E. pyrifoliae strains. HrcC (187 aa): E. tasmaniensis Et2/99, E. amylovora Ea1/79 and E. pyrifoliae Ep4/96; HrcR (146 aa): same but Et4/99; HrpL (182 aa): same but Et1/99.
The hrpL gene of Et1/99 was amplified by PCR applying primers HRPL1 and HRPL2c derived from the hrpL sequence of E. amylovora. Gene comparison on the protein level revealed high similarity to the HrpL proteins of E. amylovora and E. pyrifoliae (Fig. 4). HrpL of P. stewartii (Accession Number AF282857) was only 75% similar to HrpL of E. tasmaniensis. Nevertheless, the presence of hrp genes in the genome of E. tasmaniensis strain Et1/99 supports its ability to induce HR in tobacco leaves and may add to its epiphytic fitness.
Acyl‐homoserine lactone production by E. billingiae
The E. tasmaniensis and E. billingiae strains were investigated for production of acyl‐homoserine lactones (AHLs) involved in cell‐to‐cell communication of many bacteria. The screening was performed by using different sensor strains Chromobacterium violaceum CV026 and E. coli MT102 (pJBA132) as described in Experimental procedures. Synthesis of an AHL was detected for the plant‐pathogenic bacterium E. rhapontici, for three E. billingiae strains assayed (Fig. 5), Eb660, Eb661 and Eb1261, for P. stewartii (DC283) and for D. chrysanthemi, but not for E. amylovora, the Asian pear pathogen E. pyrifoliae, nor for E. tasmaniensis and E. mallotivora. In particular, a complementation of C. violaceum CV026 was not observed for strains Ea1/79, Ep1/96, nor for Et1/99, Et2/99 and Et4/99 (Table 2). The genus Erwinia is apparently heterogeneous for AHL production.
Figure 5.

AHL assays with C. violaceum CV026 as a sensor (vertical streak). a, upper streak; b, lower streak; 1a, E. persicina; 1b, E. rhapontici; 2a, E. billingiae Eb1261; 2b, E. billingiae Eb661; 3a, P. stewartii DC283; 3b, D. chrysanthemi 3937; 4a, E. billingiae Eb660; 4b, E. mallotivora; 5a, E. amylovora Ea1/79; 5b, E. pyrifoliae Ep1/96.
Table 2.
AHL synthesis of various Erwinia strains.
| Strain | AHL signala |
|---|---|
| D. chrysanthemi Ech3937 | + |
| D. chrysanthemi Ech540 | + |
| E. amylovora Ea1/79 | − |
| E. billingiae Eb660 | + |
| E. billingiae Eb661 | + |
| E. billingiae Eb1261 | + |
| E. mallotivora CFBP2503 | − |
| E. persicina CFBP3622 | − |
| E. pyrifoliae Ep1/96 | − |
| E. rhapontici CFBP3618 | + |
| E. tasmaniensis Et1/99 | − |
| E. tasmaniensis Et2/99 | − |
| E. tasmaniensis Et4/99 | − |
| P. stewartii DC283 | + |
Induced colour change of CV26 and fluorescence of E. coli MT102(pJBA132).
We introduced an internal AHL reporter into cells of E. amylovora Ea1/79 and P. stewartii DC283, which is plasmid pJB132, where the gfp gene is fused to a luxI promoter. No fluorescence was observed for Ea1/79(pJBA132). Cells of DC283 (pJBA132) showed intensive green fluorescence due to AHL activation of gfp gene in pJBA132. When Ea1/79(pJBA132) was cross‐streaked with DC283 or other AHL‐producing bacteria, they produced fluorescence proofing the ability of E. amylovora to respond to exogenous AHL. Others such as E. tasmaniensis did not restore fluorescence. In addition, the esaR gene of P. stewartii was cloned with and without esaI into plasmid pSU23 and expressed under control of the lacZ promoter. When strains Ea1/79(pesaR) and Ea1/79(pesaIR) were cross‐streaked with CV026, colour formation was restored with Ea1/79 (pesaIR), but not with Ea1/79 (pesaR). These experiments show the ability of E. amylovora to synthesize AHL, when the luxI (esaI) gene is provided in trans.
Discussion
Bacteria have developed several strategies to face competitive conditions of their environment. They may release toxic compounds described for B. pumilus, some B. megaterium strains (Jock et al., 2002) and also for other Gram‐positive bacteria (Emmert and Handelsman, 1999). For sensing cell density, they often secrete small compounds, such as AHLs (von Bodman et al., 2003) or autoinducer 2 (AI‐2), a furanosyl diborate from Gram‐negative bacteria or oligopeptides from Gram‐positive bacteria (Federle and Bassler, 2003). These are assumed to affect gene regulation during cell growth. The AHLs can be partially selective for a species while AI‐2 compounds might be more general. Erwinia billingiae synthesizes AI‐2 (Mohammadi and Geider, 2007), but these bacteria also produce AHL. The AI‐2 was detected for many Erwinia species, but not for Pseudomonas strains. The role of AI‐2 in bacterial gene regulation is still open, but its accumulation in the environment as in apple flowers could interfere with cell growth of other bacteria such as E. amylovora.
Erwinia amylovora was described as an AHL producer (Venturi et al., 2004; Molina et al., 2005). We could not confirm release of AHL from E. amylovora with the sensor C. violaceum, nor by applying other AHL‐sensor strains such as Escherichia coli with the gfp‐reporter plasmid pJBA132. Also E. coli with plasmids pSB403 and pSB406 carrying lux genes from P. aeruginosa with the corresponding promoter fusions to the lux operon of Vibrio fischeri and V. harveyi BB170 did not respond to culture supernatants of E. amylovora (Mohammadi and Geider, 2007). On the other hand, heterologous expression of a luxI gene in E. amylovora resulted in AHL synthesis.
The virulence of plant‐associated bacteria often depends on their ability to produce an HR on non‐host plants (Alfano and Collmer, 1997). The HR induction is only one of the many factors required for virulence. The conversion of P. fluorescens into an HR producer by overexpression of the rspL gene (Preston et al., 2001) has been an example for latent hrp genes. HrpL activates hrp genes of E. amylovora (Wei and Beer, 1995), and E. tasmaniensis also increases the formation of HR lesions at high HrpL levels. Its ability of HR induction and even levan synthesis may add to the epiphytic fitness of E. tasmaniensis. On the other hand, the lack of both properties in E. billingiae is obviously not obstructive to interference with growth of E. amylovora. Interference of autoinducers with gene expression of E. amylovora may contribute to antagonism of E. billingiae.
The ability of E. tasmaniensis to form levan is advantageous to reduce high sucrose in the environment such as in nectar. The polyfructan can be a protectant against plant cell defence reactions and the released glucose is a convenient carbon source. Erwinia amylovora also produces levan and survives high sucrose concentrations (Geier and Geider, 1993). On the other hand, E. pyrifoliae from Korea and similar isolates from Japan are not able to synthesize levan (Kim et al., 2001). An lsc gene could not be detected with the E. amylovora lsc primers and analysis of the DNA sequence between the pst‐glmS regions of E. pyrifoliae and E. billingiae (Kube et al., unpublished) reveals the absence of the lsc gene. Furthermore, in contrast to E. billingiae, E. tasmaniensis strains do not produce a detectable amount of capsular exopolysaccharide (K. Geider, unpublished), although a gene cluster related to the ams region of E. amylovora exists in the genome of Et1/99 (Kube et al., 2008). This deficiency and a lack of E. billingiae for HR induction may explain their inability to cause disease symptoms in plants.
In summary, E. tasmaniensis and E. billingiae have the common property to synthesize AI‐2, which might interfere with E. amylovora, similar to AHL of E. billingiae. Both lack at least expression of one important virulence factor such as synthesis of capsular EPS or HR induction, but seem to be well‐equipped to colonize plant surfaces and to prevent growth of E. amylovora in a competitive environment.
Experimental procedures
Bacterial strains used in the experiments and diagnostics of E. amylovora and levan producers
The bacterial strains are listed in Table 3. Erwinia amylovora and all other plant‐pathogenic and plant‐associated bacteria and C. violaceum were grown at 28°C, routinely in nutrient broth Standard I (StI, Merck, Darmstadt, Germany). Escherichia coli strains were grown at 37°C overnight. The isolation and taxonomic classification of levan‐producing bacteria from Australia were described previously (Geider et al., 2006). Erwinia amylovora produces mucoid white colonies on MM2C agar, yellow colonies on MM2Cu agar and typical dome‐shaped colonies with levan on Luria–Bertani (LB) agar with 5% sucrose (LB‐sucrose) (Bereswill et al., 1998). LB‐sucrose agar was also used for identification of E. tasmaniensis strains.
Table 3.
Bacteria used in this study.
| Description | Reference | |
|---|---|---|
| Strain | ||
| CFBP2503 | E. mallotivora, Japan | Hauben et al. (1998) |
| CFBP 3618 | E. rhapontici, UK | Hauben et al. (1998) |
| CFBP 3622 | Erwinia persicina, Japan | Hao et al. (1990) |
| CV026 | C. violaceum | McClean et al. (1997) |
| DC283 | P. stewartii, USA | Coplin et al. (2002) |
| Ea1/79 | E. amylovora, Germany | Falkenstein et al. (1988) |
| Ea1/79Sm | spontaneous Smr mutant of Ea1/79 | |
| Eb1261 | NCBP1261, E. billingiae, UK | Mergaert et al. (1999) |
| Eb660 | NCBP660, E. billingiae, UK | Mergaert et al. (1999) |
| Eb661 | NCBP661, E. billingiae, UK | Mergaert et al. (1999) |
| Ech 3937 | D. chrysanthemi | Kazemi‐Pour et al. (2004) |
| Ejp557 | E. pyrifoliae, Japan | Kim et al. (2001) |
| Ep1/96 | E. pyrifoliae, South Korea | Kim et al. (1999) |
| Et1/99 | E. tasmaniensis, Tasmania, Australia | Geider et al. (2006) |
| Et2/99 | E. tasmaniensis, Victoria, Australia | Geider et al. (2006) |
| Et4/99 | E. tasmaniensis, Queensland, Australia | Geider et al. (2006) |
| FLA03 | E. tasmaniensis, Heidelberg, Germany | This work |
| Plasmids | ||
| pJBA132 | TcR, in E. coli MT102, AHL sensor, gfp controlled via luxR/I from V. fischeri | Andersen et al. (2001) |
| pGThrpL‐Ea1 | ApR, hrpL gene from Ea1/79, Plac | Jock et al. (2003) |
HR on tobacco leaves
Overnight cultures of bacteria in StI medium were centrifuged and cells re‐suspended in sterile water to an OD600 of 0.1 (108 cfu ml−1). Tobacco leaves (cv. ‘SR1’) were punctured with a thin needle and 0.1–0.2 ml of bacterial suspension was infiltrated. If indicated, bacteria from overnight cultures were grown for 6 h in IM [2 mM (NH4)2SO4; 1 mM KH2PO4; 1 mM MgSO47H2O; 100 mM MES; 0.1% casamino acids; 1 % sucrose; pH 5.5 adjusted with NaOH; MgSO4 and sucrose autoclaved separately) (Coplin et al., 2002). The necrotic symptoms of HR were evaluated 2 days after infiltration.
Assays with immature pear slices
Immature pears (cv. ‘Bartlett’) were stored at 4°C for a short time after harvest. Pear slices were cut with a sterile knife and placed in a Petri dish. Overnight cultures of bacteria to be tested for the antagonistic effects were diluted 20 times in water, or in 5% sucrose. Immature pear slices were soaked for 10 min in the bacterial suspensions and then air‐dried under a laminar flow hood. Then, dilutions of E. amylovora were applied to each slice in 10 μl aliquots containing 500 cells. Slices were incubated at 28°C in small plastic boxes or Petri dishes sealed with Parafilm, until evaluation after 5 days.
Assays with apple flowers
Flowers were taken at day 2 after opening from young apple trees raised in a climatic chamber for blooming. The bacteria were grown as for the pear assays. Erwinia amylovora strain Ea1/79Sm was diluted in water to 5 × 105 cfu ml−1 and 10 µl pipetted into the pistil area of flowers. These were placed in Eppendorf tubes with water and incubated for 5 days in a climatic cabinet at high humidity with 16 h illumination at 24°C. Flowers were extracted without petioles in 1.5 ml of water in a large Eppendorf tube for 15 min. Ea1/79Sm was selected by plating on StI agar with Sm (500 µg ml−1).
DNA manipulations
The PCR primers (Table 4) were designed by the program Primer designer v.4.0 (Scientific and Educational Software, 1995) and synthesized commercially. The PCR reactions were carried out in a volume of 50 µl with lysed bacteria as DNA template (15 min at 65°C in 0.1% Tween 20), with 10 pmol of each primer and 1 U of Taq DNA polymerase in a buffer system described by Bereswill and colleagues (1992). Amplification was performed with 35 cycles (denaturation 94°C for 30 s, annealing 52°C for 30 s, extension at 72°C of 1 min) after initial denaturation of 5 min at 94°C and final extension of 5 min at 72°C. The PCR products were separated on a 1% agarose gel and stained with ethidium bromide.
Table 4.
PCR primers used in this study.
| PCR primer | Sequence (5′‐3′) | Length |
|---|---|---|
| HRCC1 | TGATGGCGTGGTGCTGGTGA | 20 |
| HRCC2c | CTTCCAGCGCCTGGATATCG | 20 |
| HRCR | GATCACACGCAATGCCATC | 19 |
| HRCR2c | GGTCCAGCCATTGATTAGC | 19 |
| LSC1 | AACCAACGCTGTGGACTC | 18 |
| LSC2c | TGCTCTTCCGTCTGGTAA | 18 |
| LSCa1 | GACGGCGGATCGCAATACGAA | 21 |
| LSCa2c | CACACCCTCATCAGACGTCAC | 21 |
| HRPL1 | GGCACAAGCCTTGCTAA | 17 |
| HRPL2c | CGGCAAGACAGGACACT | 17 |
DNA sequencing was done commercially (Seqlab, Göttingen) and sequence analysis performed with programs Align Plus V. 4.00 and Clone Manager V. 5.20 (Scientific and Educational Software) and with programs nblast and pblast in searches in the Internet (EBI, NIH).
N‐AHL detection assays
The AHL was detected with the C. violaceum mutant CV026 by restoration of the production of the purple pigment violacein in the presence of AHL (McClean et al., 1997), and with the gfp‐based fluorescent reporter strain E. coli MT102(pJBA132) (Andersen et al., 2001). T‐streaks on plates were used for C. violaceum and the gfp reporter strain. Violacein production was estimated visually and the fluorescence caused by gfp was observed under UV light.
Acknowledgments
We thank Annette Wensing for comments on the manuscript.
References
- Alfano J.R., Collmer A. The type III (Hrp) secretion pathway of plant‐pathogenic bacteria: trafficking harpins, Avr proteins, and death. J Bacteriol. 1997;179:5655–5661. doi: 10.1128/jb.179.18.5655-5662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J.B., Heydorn A., Hentzer M., Eberl L., Geisenberger O., Christensen B.B. gfp‐based N‐acyl homoserine‐lactone sensor systems for detection of bacterial communication. Appl Environ Microbiol. 2001;67:575–585. doi: 10.1128/AEM.67.2.575-585.2001. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bodman S.B., Bauer W.D., Coplin D.L. Quorum sensing in plant‐pathogenic bacteria. Annu Rev Phytopathol. 2003;41:455–482. doi: 10.1146/annurev.phyto.41.052002.095652. [DOI] [PubMed] [Google Scholar]
- Bereswill S., Pahl A., Bellemann P., Zeller W., Geider K. Sensitive and species‐specific detection of Erwinia amylovora by PCR‐analysis. Appl Environ Microbiol. 1992;58:3522–3526. doi: 10.1128/aem.58.11.3522-3526.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereswill S., Jock S., Geider K. Identification of Erwinia amylovora by growth morphology on agar containing copper sulfate and by capsule staining with lectin. Plant Dis. 1998;82:158–164. doi: 10.1094/PDIS.1998.82.2.158. [DOI] [PubMed] [Google Scholar]
- Coplin D.L., Majerczak D.R., Zhang Y., Kim W.‐K., Jock S., Geider K. Identification of Pantoea stewartii subsp. stewarti by PCR and strain differentiation by PFGE. Plant Dis. 2002;86:304–311. doi: 10.1094/PDIS.2002.86.3.304. [DOI] [PubMed] [Google Scholar]
- Emmert E.A.B., Handelsman J. Biocontrol of plant disease: a (Gram‐) positive perspective. FEMS Microbiol Lett. 1999;171:1–9. doi: 10.1111/j.1574-6968.1999.tb13405.x. [DOI] [PubMed] [Google Scholar]
- Falkenstein H., Bellemann P., Walter S., Zeller W., Geider K. Identification of Erwinia amylovora, the fireblight pathogen, by colony hybridization with DNA from plasmid pEA29. Appl Enviro Microbiol. 1988;54:2798–2802. doi: 10.1128/aem.54.11.2798-2802.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federle M.J., Bassler B.L. Interspecies communication in bacteria. J Clin Invest. 2003;112:1291–1299. doi: 10.1172/JCI20195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavini F., Mergaert J., Beji A., Mielcarek C., Izard D., Kersters K. Transfer of Enterobacter agglomerans (Beijerinck 1888) Ewing and Fife 1972 to Pantoea gen. nov. as Pantoea agglomerans comb. nov. and description of Pantoea dispersa sp. nov. Int J Syst Bacteriol. 1989;39:337–345. [Google Scholar]
- Geider K., Auling G., Du Z., Jakovljevic V., Jock S., Völksch B. Erwinia tasmaniensis sp. nov., a non‐phytopathogenic bacterium from apple and pear trees. Int J Syst Evol Microbiol. 2006;56:2937–2943. doi: 10.1099/ijs.0.64032-0. [DOI] [PubMed] [Google Scholar]
- Geier G., Geider K. Characterization and influence on virulence of the levansucrase gene from the fireblight pathogen Erwinia amylovora. Physiol Mol Plant Pathol. 1993;42:387–404. [Google Scholar]
- Giddens S.R., Houliston G.J., Mahnty K.H. The influence of antibiotic production and pre‐emptive colinization on the population dynamics of Pantoea agglomeransErwinia herbicola) Eh1087 and Erwinia amylovora in planta. Environ Microbiol. 2003;5:1016–1021. doi: 10.1046/j.1462-2920.2003.00506.x. [DOI] [PubMed] [Google Scholar]
- Hao M.V., Brenner D.J., Steigerwalt A.G., Kosako Y., Komagata K. Erwinia persicinus, a new species isolated from plants. Int J Syst Bacteriol. 1990;40:379–383. doi: 10.1099/00207713-40-4-379. [DOI] [PubMed] [Google Scholar]
- Hauben L., Moore E.R.B., Vaterin L., Steenackers M., Mergaert J., Verdonck L., Swings J. Phylogenetic position of phytopathogens within Enterobacteriaceae. Syst Appl Microbiol. 1998;21:384–397. doi: 10.1016/S0723-2020(98)80048-9. [DOI] [PubMed] [Google Scholar]
- Jock S., Völksch B., Mansvelt L., Geider K. Characterization of Bacillus strains from apple and pear trees in South Africa antagonistic to Erwinia amylovora. FEMS Microbiol Lett. 2002;211:247–252. doi: 10.1111/j.1574-6968.2002.tb11232.x. [DOI] [PubMed] [Google Scholar]
- Jock S., Kim W.‐K., Barny M.‐A., Geider K. Molecular characterization of natural Erwinia pyrifoliae strains deficient in the hypersensitive response. Appl Env Microbiol. 2003;69:679–682. doi: 10.1128/AEM.69.1.679-682.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K.B., Stockwell V.O. Management of fire blight: a case study in microbial ecology. Annu Rev Phytopathol. 1998;36:227–248. doi: 10.1146/annurev.phyto.36.1.227. [DOI] [PubMed] [Google Scholar]
- Kazemi‐Pour N., Condemine G., Hugouvieux‐Cotte‐Pattat N. The secretome of the plant pathogenic bacterium Erwinia chrysanthemi. Proteomics. 2004;4:3177–3186. doi: 10.1002/pmic.200300814. [DOI] [PubMed] [Google Scholar]
- Kim W.‐S., Gardan L., Rhim S.L., Geider K. Erwinia pyrifoliae sp. nov., a novel pathogen that affects Asian pear trees (Pyrus pyrifolia Nakai) Int J Syst Bacteriol. 1999;49:899–906. doi: 10.1099/00207713-49-2-899. [DOI] [PubMed] [Google Scholar]
- Kim W.‐S., Hildebrand M., Jock S., Geider K. Molecular comparison of pathogenic bacteria from pear trees in Japan and the fire blight pathogen Erwinia amylovora. Microbiology. 2001;147:2951–2959. doi: 10.1099/00221287-147-11-2951. [DOI] [PubMed] [Google Scholar]
- Kube M., Migdoll A., Müller I., Kuhl H., Beck A., Reinhardt R., Geider K. 2008;10 doi: 10.1111/j.1462-2920.2008.01639.x. , and ) The genome of Erwinia tasmaniensis strain Et1/99, a novel non‐pathogenic bacterium in the genus Erwinia. Environ Microbiol , (doi: 10.1111/j.1462‐2920.2008.01639.x.) [DOI] [PubMed] [Google Scholar]
- De Lima Pimenta A., Di Martino P., Le Bouder E., Hulen C., Blight M.A. In vitro identification of two adherence factors required for in vivo virulence of Pseudomonas fluorescens. Microbes Infect. 2003;5:1177–1187. doi: 10.1016/j.micinf.2003.09.002. [DOI] [PubMed] [Google Scholar]
- McClean K.H., Winson M., Fish L., Adrian T., Chhabr S.R., Camara M. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N‐acylhomoserine lactones. Microbiology. 1997;143:3703–3711. doi: 10.1099/00221287-143-12-3703. et al. [DOI] [PubMed] [Google Scholar]
- Mergaert J., Hauben L., Cnockaert M.C., Swings J. Reclassification of non‐pigmented Erwinia herbicola strains from trees as Erwinia billingiae sp. nov. Int J Syst Bacteriol. 1999;49:377–383. doi: 10.1099/00207713-49-2-377. [DOI] [PubMed] [Google Scholar]
- Mohammadi M., Geider K. Autoinducer‐2 of the fire blight pathogen Erwinia amylovora and other plant‐associated bacteria. FEMS Microbiol Lett. 2007;266:34–41. doi: 10.1111/j.1574-6968.2006.00510.x. [DOI] [PubMed] [Google Scholar]
- Molina L., Rezzonico F., Defago G., Duffy B. Autoinduction in Erwinia amylovora: evidence of an acyl‐homoserine lactone signal in the fire blight pathogen. J Bacteriol. 2005;187:3206–3213. doi: 10.1128/JB.187.9.3206-3213.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picot L., Abdelmoula S.M., Merieau A., Leroux P., Cazin L., Orange N., Feuilloley M.G. Pseudomonas fluorescens as a potential pathogen: adherence to nerve cells. Microbes Infect. 2001;3:985–995. doi: 10.1016/s1286-4579(01)01462-9. [DOI] [PubMed] [Google Scholar]
- Preston G.M., Bertrand N., Rainey P.B. Type III secretion in plant growth‐promoting Pseudomonas fluorescens SBW25. Mol Microbiol. 2001;41:999–1014. doi: 10.1046/j.1365-2958.2001.02560.x. [DOI] [PubMed] [Google Scholar]
- Stockwell V.O., Stack J.P. Using Pseudomonas spp. for integrated biological control. Phytopathology. 2007;97:244–249. doi: 10.1094/PHYTO-97-2-0244. [DOI] [PubMed] [Google Scholar]
- Stockwell V.O., McLaughlin R.J., Henkels M.D., Loper J.E., Sugar D., Roberts R.G. Epiphytic colonization of pear stigmas and hypanthia by bacteria during primary bloom. Phytopathology. 1999;89:1162–1168. doi: 10.1094/PHYTO.1999.89.12.1162. [DOI] [PubMed] [Google Scholar]
- Stockwell V.O., Johnson K.B., Sugar D., Loper J.E. Antibiosis contributes to biological control of fire blight by Pantoea agglomerans strain Eh252 in orchards. Phytopathology. 2002;92:1202–1209. doi: 10.1094/PHYTO.2002.92.11.1202. [DOI] [PubMed] [Google Scholar]
- Temple T.N., Stockwell V.O., Loper J.E., Johnson K.B. Bioavailability of iron to Pseudomonas fluorescens strain A506 on flowers of pear and apple. Phytopathology. 2004;94:1286–1294. doi: 10.1094/PHYTO.2004.94.12.1286. [DOI] [PubMed] [Google Scholar]
- Vanneste J.L. Erwinia amylovora. In: Vanneste J., editor. CABI publishing; 2000. pp. 199–358. [Google Scholar]
- Vanneste J.L., Yu J., Beer S.V. Role of antibiotic production by Erwinia herbicola Eh252 in biological control of Erwinia amylovora. J Bacteriol. 1992;174:2785–2796. doi: 10.1128/jb.174.9.2785-2796.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturi V., Venuti C., Devescovi G., Lucchese C., Friscina A., Degrassi G. The plant pathogen Erwinia amylovora produces acyl‐homoserine lactone signal molecules in vitro and in planta. FEMS Microbiol Lett. 2004;241:179–183. doi: 10.1016/j.femsle.2004.10.015. et al. [DOI] [PubMed] [Google Scholar]
- Wei Z.‐M., Beer S.V. hrpL activates Erwinia amylovora hrp gene transcription and is a member of the ECF subfamily of σ factors. J Bacteriol. 1995;177:6201–6210. doi: 10.1128/jb.177.21.6201-6210.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S.A.I., Zumoff C.H., Schneider L., Beer S.V. Pantoea agglomerans strain Eh318 produces two antibiotics that inhibit Erwinia amylovora in vitro. Appl Environ Microbiol. 2001;67:284–292. doi: 10.1128/AEM.67.1.284-292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


