Summary
Thirty endophytic bacteria were isolated from various plant species growing near Saint‐Petersburg, Russia. Based on a screening for various traits, including plant‐beneficial properties and DNA fragment patterns, potential siblings were removed. The remaining isolates were taxonomically identified using 16S rDNA sequences and potential human and plant pathogens were removed. The remaining strains were tested for their ability to promote radish root growth and to protect tomato plants against tomato foot and root rot. One strain, Bacillus subtilis HC8, isolated from the giant hogweed Heracleum sosnowskyi Manden, significantly promoted plant growth and protected tomato against tomato foot and root rot. Metabolites possibly responsible for these plant‐beneficial properties were identified as the hormone gibberellin and (lipo)peptide antibiotics respectively. The antibiotic properties of strain HC8 are similar to those of the commercially available plant‐beneficial strain Bacillus amyloliquefaciens FZB42. However, thin layer chromatography profiles of the two strains differ. It is speculated that endophytes such as B. subtilis HC8 contribute to the fast growth of giant hogweed.
Introduction
Bacteria that associate with plants include rhizobacteria, epiphytic bacteria and endophytic bacteria. Endophytic bacteria are defined as bacteria that can be detected within the tissues of apparently healthy plants (Schulz and Boyle, 2006). Although the majority of research on plant‐associated bacteria has been focused on rhizobacteria, interest in the diversity and role of endophytic bacteria is increasing. The main reason for the interest in endophytes is the realization that, if these bacteria can be reintroduced in the endophytic stage, a more stable relationship can be established between plant‐beneficial endophytic bacteria and plants than for rhizospheric or epiphytic bacteria and plants. Therefore, endophytes with the plant‐beneficial traits are potentially excellent plant growth promoters and/or biological control agents for sustainable crop production (Di Fiore and Del Gallo, 1995; Strobel, 2006).
The best studied host plants of bacterial endophytes are species of agricultural importance, such as rice (Baldani et al., 2000; Okunishi et al., 2005), maize (McInroy and Kloepper, 1995; Rijavec et al., 2007), cotton (Misaghi and Donndelinger, 1990; McInroy and Kloepper, 1995), potato (Sturz et al., 1998; Krechel et al., 2002) and sugar cane (Rennie et al., 1982; James and Olivares, 1997). The most common taxa of isolated heterotrophic endophytes include Bacillus (Bai et al., 2003), Enterobacter (Torres et al., 2008), Pseudomonas (Reiter et al., 2003; Rai et al., 2007), Serratia (Gyaneshwar et al., 2001; Berg et al., 2005) and Streptomyces (Sessitsch et al., 2002; Coombs and Franco, 2003).
It is assumed that bacterial endophytes use the same mechanisms of biological control and plant growth promotion as their rhizospheric counterparts (Berg and Hallmann, 2006). Widely recognized mechanisms of biocontrol mediated by plant growth‐promoting microbes are antibiosis (Thomashow and Weller, 1995; Chin‐A‐Woeng et al., 1998; Haas and Défago 2005; Lugtenberg and Kamilova, 2009), induced systemic resistance (Van Peer et al., 1991; Kloepper et al., 2004; Van Loon, 2007), competition for niches and nutrients (Kamilova et al., 2005; Validov, 2007) and predation and parasitism (Ordentlich et al., 1998; Harman et al., 2004).
Beneficial bacterial endophytes that use the above‐mentioned mechanisms of biocontrol include: (i) Bacillus sp. CY22, which produces the antibiotic iturin A and suppresses root rot of balloon flower caused by Rhizoctonia solani (Cho et al., 2003), (ii) Bacillus pumilus SE 34, which induces systemic resistance against Fusarium wilt of tomato (Benhamou et al., 1998) and (iii) Pseudomonas fluorescens, carrying the chitinase‐encoding gene chiA, which is able to control the phytopathogenic fungus R. solani on bean seedlings (Downing and Thomson, 2000). In addition to protecting against pathogens, a number of endophytic bacteria is supposed to promote plant growth directly by the production and/or modulation of plant hormones (Bastian et al., 1998; Spaepen et al., 2009), by fixing atmospheric nitrogen (Baldani et al., 2000; Oliveira et al., 2002) and by solubilization of bound phosphates (Verma et al., 2001; Kuklinsky‐Sobral et al., 2004). Using these mechanisms, some endophytic bacteria can significantly contribute to the growth of plants on low‐fertility soils (Sevilla et al., 2001).
The main aims of this study are: (i) to collect different endophytic bacteria from different plants, (ii) to screen these bacteria for a number of plant‐beneficial traits, such as secretion of the exo‐enzymes chitinase, cellulase, β‐glucanase and protease, and production of hormones and antifungal metabolites, (iii) to test the selected potentially beneficial strains for their abilities to promote growth of radish and to control tomato foot and root rot (TFRR) caused by the fungus Fusarium oxysporum f. sp. radicis‐lycopersici (Forl) and (iv) to evaluate the putative compounds responsible for the plant growth promotion and antifungal activities of (a) selected endophytic strain(s). The results are reported in this paper.
Results and discussion
Isolation and preliminary characterization of endophytic bacteria
Procedures of chemical sterilization of plant parts from different plants were developed (Table 1) to kill non‐endophytic microorganisms. Validation of the surface sterilization procedure was done by culturing aliquots of water from the last rinsing onto nutrient media. Bacterial growth was never detected on such control plates, indicating the efficiency of the developed sterilization protocols.
Table 1.
Origin of endophytes and protocols for surface sterilization of plant samples.a
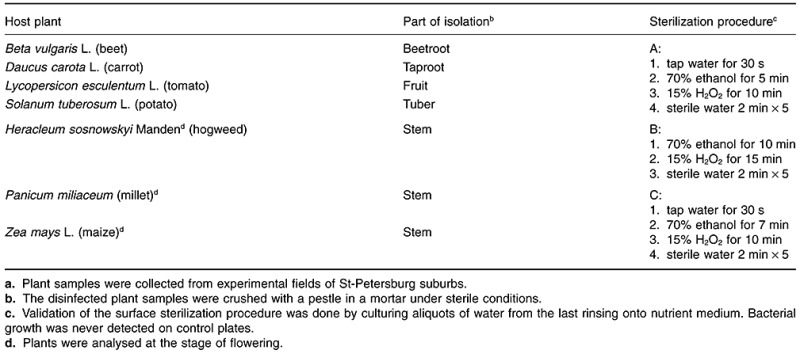
A total of 30 morphologically different strains was chosen from a larger collection of isolates obtained after plating plant juices on 1/20 TSA. The strategy described by Validov and colleagues (2007) was used for the elimination of siblings and potential pathogens. To eliminate siblings, the 30 strains were compared for their motility, their amplified ribosomal DNA restriction analysis patterns and production of the exo‐enzymes chitinase, cellulase, β‐glucanase and protease. Strains originating from the same sample that were indistinguishable with respect to these mentioned traits were considered as likely siblings. Eighteen isolates were removed from the collection as possible siblings. This left us with 12 strains for further analysis.
Characterization of potential plant‐beneficial traits
The 12 remaining strains were screened for their antagonistic activity towards four phytopathogens, their ability to produce auxin, their growth on ACC as the sole N‐source and their ability to solubilize bound phosphates (Table 2).
Table 2.
Overview of potential plant‐beneficial traits of the selected endophytic strains.a
| Strain | Host plant | Antifungal activityb | Exo‐enzymesc | Auxind/e | ACCf/PO4g |
|---|---|---|---|---|---|
| BT18 | Beta vulgaris L. (beet) | A, Forl, Fs, Pu | C, βG, P | −/− | −/− |
| CAR2 | Daucus carota L.(carrot) | − | C, βG | +++/− | −/− |
| HC2 | Heracleum sp. (hogweed) | − | − | +/+ | −/+ |
| HC8 | Heracleum sp. (hogweed) | A, Forl, Fs, Pu | C, βG, P | −/− | −/− |
| ML15 | Panicum miliaceum (millet) | Pu | P | +/− | −/− |
| ML16 | Panicum miliaceum (millet) | − | C, βG | ++/− | −/− |
| TM1 | L. esculentum L. (tomato) | − | − | +++/+ | −/− |
| TM2 | L. esculentum L. (tomato) | − | − | −/− | −/− |
| PT19 | Solanum tuberosum L. (potato) | − | P | −/− | −/− |
| PT20 | Solanum tuberosum L. (potato) | − | − | −/− | −/− |
| MZ3 | Zea mays L.(maize) | A, Forl, Fs, Pu | C, βG, P | −/− | −/− |
| MZ4 | Zea mays L.(maize) | − | − | −/− | −/+ |
After elimination of siblings.
A, Aspergillus niger; Forl, Fusarium oxysporum f.sp. radicis‐lycopersici; Fs, Fusarium solani; Pu, Pythium ultimum.
C, cellulase; βG, β‐glucanase; P, protease.
Auxin level after growth in medium supplemented with tryptophan: +++ > 60 µg ml−1, ++ > 30 µg ml−1, + > 10 µg ml−1, – < 10 µg ml−1.
Auxin level after growth in medium without tryptophan: + > 10 µg ml−1, − < 10 µg ml−1.
ACC, 1‐aminocyclopropane‐1‐carboxylate.
Solubilization of bound phosphates.
Three strains, namely BT18, HC8 and MZ3, show strong antifungal activity against all four tested pathogens. These strains also have cellulase, glucanase and protease activity. Strain ML15 is antagonistic only towards Pythium ultimum and does not secrete cellulases and glucanases. None of the strains showed chitinase activity.
Two strains, HC2 and TM1, produce detectable amounts of auxins in the presence and absence of tryptophan in the medium. The level of auxin secreted by strain HC2 is less than 30 µg ml−1. In the case of TM1, the auxin level in the media without and with tryptophan is less than 30 µg ml−1 and higher than 60 µg ml−1 respectively. Three strains, namely CAR2, ML15 and ML16, produce different auxin levels and only in the medium supplemented with tryptophan.
None of the 12 strains was able to utilize ACC as the sole nitrogen source. However, all of them, except ML15 and TM2, showed a poor to good growth on N‐free medium. Two strains, namely HC2 and MZ4, were able to solubilize hydroxyapatite in an in vitro plate assay.
Molecular identification of endophytic strains
BLAST searches in the GenBank database using 16S rDNA sequences revealed that the strains belong to different bacterial species (Table 3).
Table 3.
Molecular identification of endophytic strains and risk group classification.a
| Strain | Bacterial species and accession numberb | Phylum | Risk groupc |
|---|---|---|---|
| BT18 | Bacillus subtilis HQ667318 | Firmicutes | 1 |
| CAR2 | Enterobacter agglomerans HQ667319 | γ‐Proteobacteria | 2 |
| HC2 | Rahnella aquatilis HQ667320 | γ‐Proteobacteria | 1 |
| HC8 | Bacillus subtilis HM441224 | Firmicutes | 1 |
| ML15 | Bacillus cereus HQ667321 | Firmicutes | 2 |
| ML16 | Enterobacter agglomerans HQ667322 | γ‐Proteobacteria | 2 |
| MZ3 | Bacillus subtilis HQ667323 | Firmicutes | 1 |
| MZ4 | Acinetobacter baumannii HQ667324 | γ‐Proteobacteria | 1 |
| PT19 | Serratia sp. HQ667325 | γ‐Proteobacteria | 1 |
| PT20 | Enterobacter amnigenus HQ667326 | γ‐Proteobacteria | 2 |
| TM1 | Enterobacter agglomerans HQ667327 | γ‐Proteobacteria | 2 |
| TM2 | Kocuria sp. HQ667328 | Actinobacteria | 2 |
Based on comparison of their 16S rDNA sequences with those in the GenBank database sharing at least 99% homology.
All sequences have been submitted to GenBank. Sequences were obtained by sequencing the 5′ end using primer 27 fm for HC8 and the 3′ end using primer R1522 for all other strains. Sequences are between 600 and 800 bp long.
Risk group 1 includes bacteria that are safe to be applied in the field; risk group 2 includes potential human and plant pathogens.
To see whether these strains are safe to be applied in the field as biocontrol and/or plant‐growth‐promoting strains, we evaluated to which risk group (Anonymous, 1998) they belong. Of the 12 remaining strains as many as six strains belong to risk group 2 (Table 3), indicating a high percentage of potential human and/or plant pathogens among these endophytes. Therefore, they were excluded from further experiments. High levels of potential pathogens have been found earlier for rhizosphere bacteria (Berg et al., 2005; Egamberdiyeva et al., 2008).
The remaining six endophytic strains were BT18, HC8 and MZ3, identified as Bacillus subtilis, HC2 (Rahnella aquatilis), MZ4 (Acinetobacter baumanii), and PT19 (Serratia sp.). All of them have been found earlier as endophytes (Bai et al., 2003; Berg et al., 2005; Torres et al., 2008). Of these, strains BT18, HC8 and MZ3, which possess strong antifungal activity in vitro against Aspergillus niger, Forl, Fusarium solani and P. ultimum as well as strain HC2, which produces auxin, can be considered as potential beneficial strains.
Plant growth promotion by B. subtilis HC8 and possible mechanism of action
Four endophytic strains, namely BT18, HC2, HC8 and MZ3, were tested for their ability to promote the growth of radish plants in non‐sterile potting soil (Fig. 1). Radish was chosen as the model plant because its roots secrete a high level of tryptophan (Kamilova et al., 2006), which can be used by many beneficial bacteria as the precursor of auxin. The only tested strain that was able to increase the root weight of radish plants was B. subtilis HC8 (Fig. 1). The root weight was chosen because this is the commercially interesting plant part. Strain HC8 significantly enhanced fresh root biomass, with as much as 46% compared with uninoculated control plants. Inoculation with the auxin‐producing strain R. aquatilis HC2 and with B. subtilis BT18 did not show a significant increase of root growth. B. subtilis strain MZ3 decreased the root biomass, but not significantly.
Figure 1.

Plant growth promotion mediated by endophytic bacteria. Seedlings of radish were inoculated with a suspension of bacterial cells except for the control (C) and planted in soil. Each variant consisted of four replicates with five seedlings each. Numbers inside the columns represent the mean fresh weight of the root system scored 31 days after inoculation. Bars indicate confidence interval (P = 0.05). The asterisk indicates a significantly different value.
One of the mechanisms of stimulation of plant growth by bacteria involves the production of phytohormones, such as auxins, gibberellins and cytokinins. Auxins are known to be essential for plant physiology directly affecting the root and shoot architecture (Spaepen et al., 2009). Because HC8 did not produce auxin in the tested laboratory media (Table 2), its ability to produce the plant hormones cytokinin and gibberellin was tested. Indeed, gibberellin but not cytokinin was found to be produced by HC8 (150 ng per 109 cells). Previously, microbial production of similar amounts of gibberellins (approximately 200 ng per 109 cells) has been reported for Bacillus lichenoformis and B. pumilus (Gutierrez‐Manero et al., 2001). Gibberellin is not known to enhance root growth directly (Spaepen et al., 2009). A possible explanation of the results is that gibberellin acts synergistically with another, unknown compound.
Biocontrol of TFRR by B. subtilis HC8 and possible mechanism of action
The three B. subtilis strains, BT18, HC8 and MZ3, were selected as the best antagonists (Table 2). Therefore, their ability to control TFRR was evaluated. Seed bacterization with only HC8 significantly decreased disease symptoms, from 91% to 42% (Fig. 2A). Significant biocontrol activity of HC8 was also found in a second experiment (Fig. 2B). Although the two other antagonistic strains, MZ3 and BT18, did not show significant biocontrol of TFRR (Fig. 2A) plants bacterized with these strains did show reduced disease severity (results not shown).
Figure 2.
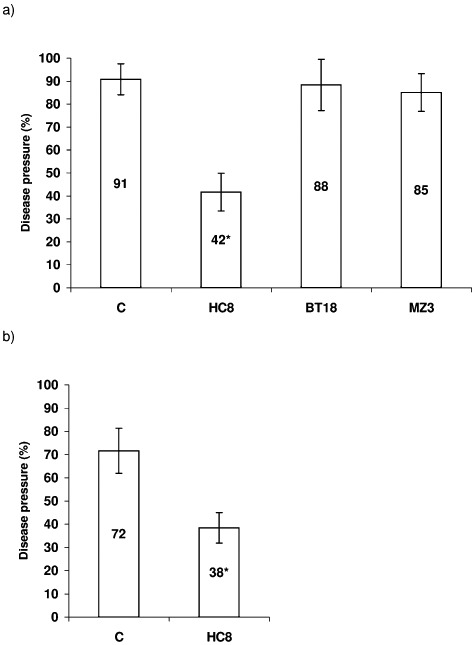
Biocontrol of TFRR in stone wool substrate by endophytic bacteria. Tomato seeds were inoculated with a suspension of bacterial cells except for the control (C) and grown in stone wool plugs with added spores. Each variant consisted of 4 replicas with 30 plants each. Numbers inside the columns present the percentage of sick plants scored 2 weeks after inoculation. Bars indicate confidence interval (P < 0.05). Statistically different values are indicated with asterisks. (A) and (B) represent different experiments.
For the detection of one or more compounds responsible for the antifungal activity, and therefore probably for biocontrol, the crude methanolic extracts from the dried and acid precipitated supernatant fluid of B. subtilis HC8 were profiled on thin layer chromatography (TLC) plates, using iturin A as a reference antibiotic (Fig. 3A). We have also profiled Bacillus amyloliquefaciens FZB42 to evaluate the similarity/difference between two beneficial strains.
Figure 3.
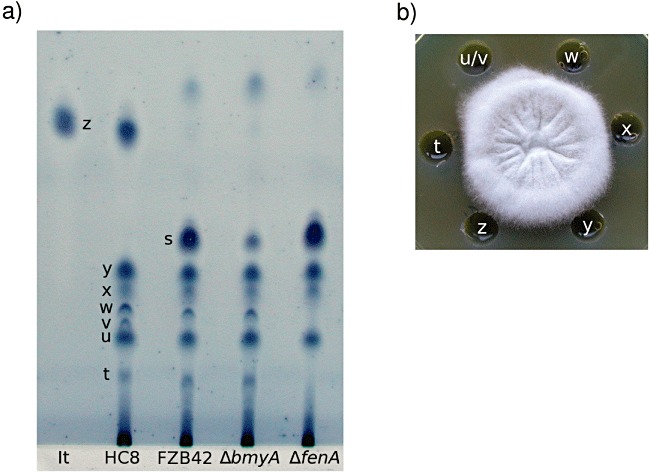
Evaluation of antifungal metabolites produced by B. subtilis HC8 and B. amyloliquefaciens FZB42. A. TLC analysis of methanol extract of the supernatant fluids of Bacillus strains. The plate was developed in chloroform/methanol/water 65:25:4 (v/v/v) for 2.5 h. For visualization, the developed plate was stained in iodine followed by dipping in 1% aqueous starch. Pure iturin A (It) was used as a reference. HC8, endophytic strain B. subtilis HC8; FZB42, Bacillus amyloliquefaciens FZB42; ΔbmyA, mutant of FZB42 unable to produce bacillomycin D; ΔfenA, mutant of FZB42 unable to produce fengycin; t‐z, major spots of the HC8 crude extract; z, likely correspond to iturin; s, fraction likely to contain bacillomycin D; w and t likely to contain fengycin. B. Antifungal activity of individual fractions of crude extract from B. subtilis HC8 towards Forl in vitro. t‐z, major fractions corresponding to spots in A).
The iodine‐starch pattern of supernatant fluids of FZB42 and HC8 are very similar. Dried supernatant fluids and acid precipitated supernatant fluids had indistinguishable patterns (results not shown). We found for both HC8 and FZB42 major spots in positions t, u, w, x and y. The Retention factor (Rf) values of these spots shown in Fig. 3A are t, 0.10; u, 0.16; w, 0.21; x, 0.23; and y, 0.26. Although the two strains produce very similar antibiotic patterns, there are also clear differences, not only in taxonomy. Spot v (Rf = 0.18) is present in HC8 but always missing in FZB42. In addition, FZB42 lacks spot z with the Rf value similar to that of iturin A (Rf = 0.47). Spot s (Rf = 0.31) of strain FZB42 is not visible in HC8 material.
To test which spots are active against Forl, we extracted the whole HC8 and FZB42 strips (major and minor spots as well as the regions without visible spots) and checked their antibiotic activity against Forl in vitro (Fig. 3B). Also for biological activity dried supernatant fluids and acid precipitated supernatant fluids have indistinguishable patterns. We found for both HC8 and FZB42 that four spots, namely t, u (in case of HC8 it was u/v because spot v sometimes migrates very close to spot u, which makes it difficult to analyse them separately), w and x, clearly inhibit the growth of Forl. Spot y does not show any antibiotic activity. Interestingly, both spot z of HC8 and spot s of FZB42 had very low antibiotic activities when re‐extracted from TLC plate.
Based on Rf values and activity against Forl, spot z could be iturin. We used mutant strains of FZB42, which do not produce bacillomycin D and fengycin, to see which FZB42 spots, and possibly HC8 spots, correspond with these antibiotics. The results (Fig. 3A) showed that the fengycin‐deficient mutant lacks two spots, t and w, both of which are present in both HC8 and FZB42. These spots are also still present in the fengycin producing mutant strain ΔbmyA indicating that compounds in positions t and w represent (derivatives of) fengycin. The bacillomycin D lacking mutant ΔbmyA does produce spot s but in lower amounts than its wild‐type strain FZB42, therefore this spot probably contains bacillomycin D. No information on the identity of spots u, x and y from HC8 was generated.
The antibiotics iturin and bacillomycin D belong to the same family of cyclic lipopeptides, which comprises iturins A, C, D and E, bacillomycin D, F and L, bacillopeptin and mycosubtilin (Moyne et al., 2004). Iturins interact with the cytoplasmic membrane of the target cells forming ion‐conducting pores (Magnet‐Dana and Peypoux, 1994). These antifungals appeared to work synergistically with other lipopeptides, such as surfactins and fengycins. For example, Chen and colleagues (2009) report that the fungicidal activity of FZB42 is due to synergistic action of bacillomycin D and fengycin because without fengycin the antifungal effect of this strain is less profound. This may explain why the iturin and bacillomycin D fractions almost lack biological activity in our experiments.
Taking together all these data suggest that B. subtilis HC8 produces several (lipo)peptide antibiotics, some of them are different from FZB42 and may be important for antifungal and biocontrol activity of HC8.
Do endophytes play a role in the growth of the giant hogweed?
In this study we have isolated the novel biocontrol and plant growth‐promoting strain B. subtilis HC8 from the giant hogweed Heracleum sosnowskyi. This plant can grow in low nutritional environments while reaching a high biomass. This observation has led us to speculate that microbes colonizing the inner plant tissues of Heracleum have beneficial traits, which may contribute to its enormous growth. Strain HC8 appears to have the ability to produce a large variety of bioactive compounds that might play a role in biocontrol and plant growth promotion mediated by this strain. Although it was isolated from H. sosnowskyi, it is able to promote the growth of radish and reduce TFRR in tomato plants. This will facilitate its application as a bioinoculant. Endophytes from the plant Heracleum have never been isolated previously. It may be interesting to evaluate the entire endophytic microbial content of Heracleum in more detail.
Experimental procedures
Isolation of endophytic bacteria
Endophytic bacteria were isolated from several plant species. These include four vegetable plants [beet (Beta vulgaris L.), carrot (Daucus carota L.), potato (Solanum tuberosum L.) and tomato (Lycopersicon esculentum L.)], two grasses [maize (Zea mays L.) and millet (Panicum miliaceum)], and the weed plant Heracleum sosnowskyi Manden. Plants were collected from experimental fields of the All‐Russia Research Institute for Agricultural Microbiology (ARRIAM), which is located near Pushkin, Saint‐Petersburg, Russia (Table 1).
To isolate endophytes, different surface sterilization procedures were developed (detailed in Table 1), which are modifications of previously published ones (Misaghi and Donndelinger, 1990). Briefly, plant samples were disinfected and subsequently crushed with a pestle in a mortar under sterile conditions. Aliquots of 100 µl of the resulting plant juices were plated on 1/20 tryptic soy agar (TSA, Difco Laboratories, MI, USA) plates. The sterility check consisted of aliquots of water from the last rinsing that were plated on 1/20 TSA. Plates were incubated at 28°C for 3 days. Colonies derived from plant juice were further analysed.
Microbial strains and growth conditions
All isolated bacterial strains were grown in, and maintained on, full strength TSA. Strain B. amyloliquefaciens FZB42 (Idriss et al., 2002), and its mutants AK1 and AK2 (Koumoutsi et al., 2004) were purchased from the Bacillus Genetic Stock Center (BGSC, http://www.bgsc.org/). Strain FZB42 was used for comparison studies as a known antibiotic‐producing Bacillus strain. This strain is also commercialized as a biofertilizer, biocontrol and plant growth‐promoting agent (RhizoVital, ABiTEP, Berlin, Germany). Its two mutants, AK1 (ΔbmyA, defective in the production of bacillomycin D) and AK2 (ΔfenA, defective in the production of fengycin), were used to attempt to localize these antibiotics on TLC plates.
The fungi A. niger, Forl, F. solani and the oomycete P. ultimum were routinely cultivated on potato‐dextrose agar (PDA, Difco Laboratories). To obtain spores of Forl to be used in biocontrol experiments, the fungus was grown in Czapek‐Dox liquid medium (Difco Laboratories) for 4 days at 28°C at 150 r.p.m.
For the extraction of antibiotics and gibberellins, strains were grown in Brain Heart Infusion broth (BHI, Difco Laboratories, MI, USA). To extract cytokinins and to check the ability of strains to sulubilize phosphates, bacteria were grown in minimal medium (MM) containing per litre of distilled water: NH4Cl, 0.4 g; MgSO4·7H2O, 0.5 g; CaCl2·2H2O, 0.1 g; glucose, 10 g; yeast extract, 50 mg, and agar, 18 g. For the evaluation of ACC (1‐aminocyclopropane‐1‐carboxylate) utilization, bacteria were grown in sucrose‐malt extract‐yeast extract medium, which has the following composition (weight l−1): glucose, 1.2 g; KH2PO4, 0.4 g; K2HPO4, 2.0 g; MgSO4, 0.2 g; CaCl2, 0.1 g; FeSO4, 5.0 mg; H3BO3, 2 mg; ZnSO4, 5.0 mg; Na2MoO4, 1.0 mg; MnSO4, 3.0 mg; CoSO4, 1.0 mg; CuSO4, 1.0 mg; NiSO4, 1.0 mg; yeast extract, 50 mg; pH 6.4.
Characterization of exo‐enzymes produced by endophytic bacteria
Production of the exo‐enzymes cellulase, chitinase, β‐glucanase and protease was judged as the appearance of clear zones around the growth of a bacterium on the following solid media. Cellulase activity was tested on 1/20 TSA agar plates supplemented with 1% carboxymethylcellulose (Hankin and Anagnostakis, 1977). Production of chitinase was evaluated on 1/20 TSA agar plates supplemented with 1% colloidal chitin (Wirth and Wolf, 1990). β‐glucanase activity was detected on 1/20 TSA agar plates supplemented with 0.1% lichenan (Walsh et al., 1995). Protease secretion was evaluated after growing the strains for 48 h on 1/20 TSA supplemented with 5% skimmed milk according to Brown and Foster (1970).
Characterization of antifungal metabolites produced by bacteria
To test production of antifungal metabolites in vitro, a plug of mycelium, 5 mm in diameter, was taken from an actively growing culture on solid medium and stabbed in the centre of a PDA agar plate, which was subsequently inoculated with up to six individual bacterial strains at a distance of 3 cm from the fungus. All plates were incubated at 28°C for 1 week and subsequently scored for inhibition of fungal growth.
The method of Chitarra and colleagues (2002) was used with some modifications to extract antibiotics produced by B. subtilis HC8. Briefly, the strain was grown in BHI medium for 60 h. Subsequently cells were removed by centrifugation at 13 000 r.p.m. for 10 min. The supernatant fluid was divided into two equal parts, one part (100 ml) was freeze‐dried and the other was acidified to pH 2.0 with concentrated HCl. The resulting dry biomass and precipitate, respectively, were extracted twice with methanol. The methanolic extract was concentrated by vacuum evaporation, dissolved in methanol and stored at −20°C.
The methanolic extracts were analysed by TLC on silica gel 60 F254 plates with a 20 × 2.5 cm concentrating zone (Merck, Darmstadt, Germany). Plates were developed in chloroform/methanol/water 65:25:4 (v/v/v) for 1.5 h at room temperature. After drying, the pattern of compounds on the developed plate was visualized using UV254 and stained in an iodine chamber for 5 min at room temperature followed by dipping in 1% aqueous starch. The putative antifungals were preliminarily characterized by their Rf values. Pure iturin A from B. subtilis (Sigma‐Aldrich, Steinheim, Germany) was used as a reference.
To analyse the antifungal activity of the different spots, TLC plates were run in duplicate, one was used for staining and the other one to recover the fractions by extraction. To extract the spots, silica regions were scratched off the plate and were extracted with methanol. The activity of the individual extracts was tested against Forl in in vitro assay as follows. A plug of mycelium was placed in the centre of a PDA plate and pre‐grown for 2 days. Subsequently six wells, 8 mm in diameter, were made in the agar plate at a distance of 1.0 cm from the growing fungus. The bottom of the wells was sealed using melted agar, and each of the wells was filled with 80 µl of an individual extract. Methanol was used as a control. The plates were incubated for 2 days at 28°C and the inhibition of the Forl growth was judged. All experiments were carried out at least three times.
To compare the activity and Rf values of the HC8 extract with those of a known antibiotic‐producing strain, methanolic extracts of B. amyloliquefaciens FZB42 were prepared and profiled on TLC plates as described for strain HC8.
Characterization of bacterial phytohormone production
The production of auxin (IAA, indole‐3‐acetic acid) was determined as described by Kamilova and colleagues (2005) using Salkowski reagent (Gordon and Weber, 1951). A modified method of Gutierrez‐Manero and colleagues (2001) was used for the extraction of gibberellins from the supernatant fluid of B. subtilis HC8. Bacteria were grown in 100 ml BHI medium for 60 h at 28°C at 150 r.p.m. Bacterial cells were removed by centrifugation for 15 min at 5 000 r.p.m. and the supernatant fluid was subsequently filtered through a 0.22 µm Millipore filter. Bacteria‐free supernatant was then acidified to pH 2.5 with concentrated HCl and partitioned four times with water‐saturated ethyl acetate (v/v). The organic phase, containing the gibberellins, was dried by vacuum evaporation and subsequently dissolved in water‐saturated ethyl acetate and stored at −20°C. A modified method of Jones and Varner (1967) was used for the evaluation of the biological activity of the crude extract. Briefly, seeds of barley cv. Triumph, 1989 harvest, were transversely cut in half and the embryo part was removed. The embryo‐free halves were then surface‐sterilized with 70% ethanol for 2 min followed by 4% sodium hypochlorite for 2 min and several rinses with sterile water. The disinfected half seeds were stored in sterile water at +4°C for 2 days. For gibberellin assays, 10 half seeds were transferred to a 100 ml Erlenmeyer flask with 6 ml of test solution containing: (i) 20 mM sodium succinate buffer, pH 5.3, (ii) 20 mM CaCl2 and (iii) the sample to be assayed. Chloramphenicol at a final concentration 10 µg ml−1 was added to each flask to prevent bacterial growth. After incubation for 27 h at 25°C in the dark, 1.0 ml of the solution was added to a tube containing 1.0 ml of starch reagent (Jones and Varner, 1967) and incubated for 10 min at room temperature. The reaction was stopped by adding 1.0 ml of iodine reagent (Jones and Varner, 1967). A volume of 2.0 ml of distilled water was added and, after mixing, the intensity of blue colour was measured at 620 nm. The gibberellin concentration in the crude extract was determined by using a calibration curve with pure gibberellic acid (GA3) as a standard. The experiment was performed three times.
A modified method of D. Vereecke (pers. comm.) was used for the extraction of cytokinins secreted by B. subtilis HC8. Briefly, bacteria were grown in 50 ml MM for 96 h at 28°C and 150 r.p.m. Subsequently, the bacterial cells were removed by centrifugation for 20 min at 10 000 r.p.m. and subsequently filtering the supernatant through a 0.22 µm Millipore filter. The cell free supernatant fluid was transferred to a Sep – PakPlus C18 column (Waters, USA), which had previously been activated with 5 ml 100% methanol and equilibrated with 0.1% acetic acid. Subsequently, the cytokinins were eluted with 3 ml of 80% methanol‐2% acetic acid, concentrated in vacuo and resuspended in water before further use. The method of Biddington and Thomas (1973) was used for the evaluation of the biological activity of the eluate. Briefly, seeds of Amaranthus caudatus L. (purchased from Sluis Garden http://www.gardenseeds.nl/) were allowed to germinate on wet filter paper at 25°C in the dark for 72 h. The seed coats and the roots were subsequently removed and 10 explants consisting of cotyledons and the upper part of the hypocotyl were placed on filter paper, which had been moistened with 2 ml 0.2 M phosphate buffer (pH 6.3) containing 1 mg ml−1 tyrosine and the sample to be tested. After an incubation period of 18 h at 25°C in the dark the seedlings were placed in 1.0 ml distilled water. Betacyanin was extracted from the samples by three cycles of freezing and thawing and the optical density of the supernatant fluids was measured at 542 nm. The amount of cytokinins was determined by using a calibration curve with pure trans‐zeatin as a standard. The experiment was performed three times.
Utilization of ACC and solubilization of bound phosphates by endophytic bacteria
The ability of bacteria to utilize ACC as the sole nitrogen source was monitored by screening for growth on plates according to Belimov and colleagues (2005).
The ability of bacteria to solubilize phosphates was evaluated on hydroxyapatite medium as described by Kim and colleagues (1997) with some modifications. Briefly, endophytic strains were grown in MM in which the phosphorus was present in the form of hydroxyapatite (Ca5HO13P3, Sigma‐Aldrich, Steinheim, Germany) at 12 g l−1 and the pH was adjusted to 7.0. Plates were incubated at 28°C for 1 week. Phosphorus‐solubilizing activity was judged as the appearance of clear zones around the growth area of a bacterial sample spotted on the plate.
Molecular characterization of endophytic strains
Amplified ribosomal DNA restriction analysis in combination with phenotypic characterization was applied to eliminate putative siblings as described by Validov and colleagues (2007). Briefly, portions of the 16S rRNA genes were obtained via PCR amplification with primers 27 fm (5′‐AGA GTT TGA TCM TGG CTC AG‐3′) and 1522R (5′‐AAG GAG GTG ATC CAG CCG CA‐3′) (Weisburg et al., 1991). The amplified DNA fragments were subsequently digested with the four nucleases TaqI, BsuRI, HinfI and Hin6I. The resulting fragments were subsequently separated on a 2% agarose gel and the profiles of the endophytic strains were compared.
For nucleotide sequence determination, PCR products were separated on a 1% agarose gel, recovered and purified from agarose using a QIAquick PCR Purification Kit (QIAGEN GmbH, Hilden, Germany). Sequencing was performed by ServiceXS (Leiden, The Netherlands). Similarity searches in GenBank were performed using BLAST (http://www.ncbi.nlm.nih.gov/blast/; Altschul et al., 1997).
Plant growth promotion
Endophytic bacteria were tested for their ability to promote the growth of radish plants. To do this, seeds of radish cv. Duro (Russkiy Ogorod – NC, Moscow, Russia) were allowed to germinate for 24 h on moist filter paper at room temperature. The germinated seeds were then soaked in a suspension of bacterial cells in 0.85% NaCl adjusted to 106 cfu ml−1 for 15 min. As a negative control, seedlings were treated with 0.85% NaCl without added bacteria. The treated seedlings were subsequently planted in non‐sterile potting soil (Terravita, Russia) mixed with field podsol soil in the ratio 4:1 and grown under agroindustrial conditions in the summer greenhouse of ARRIAM. Each variant consisted of four replicates with five seedlings each. After 31 days of growth, the fresh weight of the roots was determined.
Biocontrol of TFRR
Biocontrol of TFRR was carried out in stone wool substrate as described by Validov and colleagues (2007). Briefly, 120 stone wool plugs were soaked in 1.0 l of commercial Plant Nutrient Solution (PNS, Wageningen UR Greenhouse Horticulture, Bleiswijk, the Netherlands) supplemented with Forl spores (107 spores l−1) and bacterial cells (106 cfu ml−1). In the negative control PNS was supplemented with spores only. Seeds of tomato cv. Carmello (Syngenta, B.V., Enkhuizen, The Netherlands) were placed in the stone wool plugs (one seed per plug) and grown for 14 days under greenhouse conditions at 80% humidity and 16 h of daylight. The plants were then removed from the stone wool and examined for symptoms of foot and root rot. Only roots without any brown spots or lesions were referred to as healthy. Dead plants, wilting plants or plants with symptoms of foot and root rot were considered as diseased. All experiments were performed twice.
Statistics
Homogeneity of variance and analysis of variance (anova) at P = 0.05 were conducted with the program DIANA (Saint‐Petersburg, Russia) and spss software (Chicago, IL, USA) for the plant growth promotion and biocontrol assays respectively.
Acknowledgments
The research described here was supported by the Netherlands Organization for Scientific research (NWO, project number 047.018.001), by Russian Foundation for Fundamental Investigations (RFFI, project 06.04.89000 NVOC_a ‘Plants interaction with beneficial bacteria’) and by Goskontract Minobrnauki (project number P760 from 20.05.2010 ‘Development of microbial preparations on the basis of endophytes and rhizobacteria for cultivation of agricultural crops in conditions of technogenic disasters’) and represents part of the Russian‐Dutch collaboration project ‘Center of Excellence’. We thank Dr Bernadette Kroon (Syngenta B.V., Enkhuizen, The Netherlands) for providing us with tomato seeds. We thank Prof Dr Bert van Duijn (Fytagoras B.V., Leiden, The Netherlands) for providing us with aged barley seeds. We thank Dr Danny Vereecke for giving us a protocol for cytokinin determination. We thank Gerben Voshol for his help in some experiments and for critically reading the manuscript.
References
- Altschul S.E., Madden T.L., Schaffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSI‐BLAST: a new generation of protein database search programs. Nucleic Acid Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. Jedermann‐Verlag Dr Otto Pfeffer oHG; 1998. [Google Scholar]
- Bai Y., Zhou X., Smith D. Enhanced soybean plant growth resulting from coinoculation of Bacillus strains with Bradyrhizobium japonicum. Crop Sci. 2003;43:1774–1781. [Google Scholar]
- Baldani V., Baldani J., Dobereiner J. Inoculation of rice plants with the endophytic diazotrophs Herbaspirillum seropedicae and Burkholderia spp. Biol Fertil Soils. 2000;30:485–491. [Google Scholar]
- Bastian F., Cohern A., Piccoli P., Luna V., Baraldi R., Bottini R. Production of indole‐3‐acetic acid and gibberellins A1 and A3 by Acetobacter diazotrophicus and Herbaspirillum seropedicae in chemically defined culture media. J Plant Growth Regul. 1998;24:7–11. [Google Scholar]
- Belimov A.A, Hontzeas N., Safronova V.I., Demchinskaya S.V., Piluzza G., Bullitta S., Glick B.R. Cadmium‐tolerant plant growth‐promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.) Soil Biol Biochem. 2005;37:241–250. [Google Scholar]
- Benhamou N., Kloepper J.W., Tuzun S. Induction of resistance against Fusarium wilt of tomato by combination of chitosan with an endophytic bacterial strain: ultrastructure and cytochemistry of the host response. Planta. 1998;204:153–168. [Google Scholar]
- Berg G., Hallmann J. Control of plant pathogenic fungi with bacterial endophytes. In: Schulz B., Boyle C., Sieber T., editors. Springer‐Verlag; 2006. pp. 53–69. [Google Scholar]
- Berg G., Krechel A., Ditz M., Sikora R.A., Ulrich A., Hallmann J. Endophytic and ectophytic potato‐associated bacterial communities differ in structure and antagonistic function against plant pathogenic fungi. FEMS Microbiol Ecol. 2005;51:215–229. doi: 10.1016/j.femsec.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Biddington N.L., Thomas T.H. A modified Amaranthus betacyanin bioassay for the rapid determination of cytokinins in plant extracts. Planta (Berl.) 1973;111:183–186. doi: 10.1007/BF00386279. [DOI] [PubMed] [Google Scholar]
- Brown M.R., Foster J.H. A simple diagnostic milk medium for Pseudomonas aeruginosa. J Clin Pathol. 1970;23:172–177. doi: 10.1136/jcp.23.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X‐A., Koumoutsi A., Scholz R., Borriss R. More than anticipated – production of antibiotics and other secondary metabolites by Bacillus amyloliquefaciens FZB42. J Mol Microbiol Biotechnol. 2009;16:14–24. doi: 10.1159/000142891. [DOI] [PubMed] [Google Scholar]
- Chin‐A‐Woeng T.F.C., Bloemberg G.V., van der Bij A.J., van der Drift K.M.G.M., Schripsema J., Kroon B. Biocontrol by phenazine‐1‐carboxamide‐producing Pseudomonas chlororaphis PCL1391 of tomato root rot caused by Fusarium oxysporum f. sp. radicis‐lycopersici. Mol Plant Microbe Interact. 1998;11:1069–1077. , and et al. [Google Scholar]
- Chitarra S.C., Breeuwer P., Nout R.J.M., van Aelst C.A., Rombouts M.F., Abee T. An antifungal compound produced by Bacillus subtilis YM 10–20 inhibits germination of Penicillium roqueforti conidiospores. J Appl Microbiol. 2002;96:159–166. doi: 10.1046/j.1365-2672.2003.01819.x. [DOI] [PubMed] [Google Scholar]
- Cho S., Lim W., Hong S., Park S., Yun H. Endophytic colonization of ballon flower by antifungal strain Bacillus sp. CY22. Biosci Biotech Biochem. 2003;67:2132–2138. doi: 10.1271/bbb.67.2132. [DOI] [PubMed] [Google Scholar]
- Coombs J.T, Franco C.M. Isolation and identification of actinobacteria from surface‐sterilized wheat roots. Appl Environ Microbiol. 2003;69:5603–5608. doi: 10.1128/AEM.69.9.5603-5608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore S., Del Gallo M. Endophytic bacteria: their possible role in the host plant. In: Fendrik I., editor. Springer; 1995. pp. 169–187. [Google Scholar]
- Downing K., Thomson J. Introduction of the Serratia marcescens chiA gene into an endophytic Pseudomonas fluorescens for the biocontrol of phytopathogenic fungi. Can J Microbiol. 2000;46:363–369. doi: 10.1139/w99-147. [DOI] [PubMed] [Google Scholar]
- Egamberdiyeva D., Kamilova F., Validov S., Gafurova L., Kucharova Z., Lugtenberg B. High incidence of plant growth‐stimulating bacteria associated with the rhizosphere of wheat grown in salinated soil in Uzbekistan. Environ Microbiol. 2008;10:1–9. doi: 10.1111/j.1462-2920.2007.01424.x. [DOI] [PubMed] [Google Scholar]
- Gordon S.A., Weber R.P. Colorimetric estimation of indole acetic acid. Plant Physiol. 1951;26:192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez‐Manero F.J., Ramos Solano B., Probanza A., Mehouachi J., Tadeo F.R., Talon M. The plant growth‐promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberellins. Physiol Plantarum. 2001;111:206–211. [Google Scholar]
- Gyaneshwar P., James E.K., Mathan N., Reddy P.M., Reinhold‐Hurek B., Ladha J.K. Endophytic colonization of rice by a diazotrophic strain of Serratia marcescens. J Bacteriol. 2001;183:2634–2645. doi: 10.1128/JB.183.8.2634-2645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D., Défago G. Biological control of soil‐borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol. 2005;3:307–319. doi: 10.1038/nrmicro1129. [DOI] [PubMed] [Google Scholar]
- Hankin L., Anagnostakis S.L. Solid media containing carboxymethylcellulose to detect CX cellulase activity of microorganisms. J Gen Microbiol. 1977;98:109–115. doi: 10.1099/00221287-98-1-109. [DOI] [PubMed] [Google Scholar]
- Harman G.E., Howel C.H., Viterbo A., Chet I., Lorito M. Trichoderma species–opportunistic, avirulent plant symbionts. Nat Rev Microbiol. 2004;2:43–56. doi: 10.1038/nrmicro797. [DOI] [PubMed] [Google Scholar]
- Idriss E.E.S., Makarewicz O., Farouk A., Rosner K., Greiner R., Bochow H. Extracellular phytase activity of Bacillus amyloliquefaciens FZB 45 contributes to its plant‐growth‐promoting effect. Microbiology. 2002;148:2097–2109. doi: 10.1099/00221287-148-7-2097. et al. [DOI] [PubMed] [Google Scholar]
- James E.K., Olivares F.L. Infection and colonization of sugar cane and other graminaceous plants by endophytic diazotrophs. Crit Rev Plant Sci. 1997;17:77–119. [Google Scholar]
- Jones R.L., Varner J.E. The bioassay of gibberellins. Planta (Berl.) 1967;72:53–59. doi: 10.1007/BF00387479. [DOI] [PubMed] [Google Scholar]
- Kamilova F., Validov S., Azarova T., Mulders I., Lugtenberg B. Enrichment for enhanced competitive root tip colonizers selects for a new class of biocontrol bacteria. Environ Microbiol. 2005;7:1809–1817. doi: 10.1111/j.1462-2920.2005.00889.x. [DOI] [PubMed] [Google Scholar]
- Kamilova F., Kravchenko L.V., Shaposhnikov A.I., Azarova T., Makarova N., Lugtenberg B.J.J. Organic acids, sugars, and L‐tryptophane in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol Plant Microbe Interact. 2006;19:250–256. doi: 10.1094/MPMI-19-0250. [DOI] [PubMed] [Google Scholar]
- Kim K.Y., McDonald G.A., Jordan D. Solubilization of hydroxyapatite by Enterobacter agglomerans and cloned Escherichia coli in culture medium. Biol Fertil Soils. 1997;24:347–352. [Google Scholar]
- Kloepper J.W., Ryu C‐M., Zhang S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology. 2004;94:1259–1266. doi: 10.1094/PHYTO.2004.94.11.1259. [DOI] [PubMed] [Google Scholar]
- Koumoutsi A., Chen X‐H., Henne A., Liesegang H., Hitzeroth G., Franke P. Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. J Bacteriol. 2004;186:1084–1096. doi: 10.1128/JB.186.4.1084-1096.2004. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krechel A., Faupel A., Hallmann J., Ulrich A., Berg G. Potato‐associated bacteria and their antagonistic potential towards plant‐pathogenic fungi and the plant‐parasitic nematode Meloidogyne incognita (Kofoid & White) Chitwood. Can J Microbiol. 2002;48:772–786. doi: 10.1139/w02-071. [DOI] [PubMed] [Google Scholar]
- Kuklinsky‐Sobral J., Araújo W.L., Mendes R., Geraldi I.O., Pizzirani‐Kleiner A.P., Azevedo J.L. Isolation and characterization of soybean‐associated bacteria and their potential for plant growth promotion. Environ Microbiol. 2004;6:1244–1251. doi: 10.1111/j.1462-2920.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Kamilova F. Plant‐growth‐promoting rhizobacteria. Annu Rev Microbiol. 2009;63:541–556. doi: 10.1146/annurev.micro.62.081307.162918. [DOI] [PubMed] [Google Scholar]
- McInroy J., Kloepper J. Survey of indigenous bacterial endophytes from cotton and sweet corn. Plant Soil. 1995;173:337–342. [Google Scholar]
- Magnet‐Dana R., Peypoux F. Iturins, a special class of poreforming lipopeptides: biological and physiological properties. Toxicology. 1994;87:151–174. doi: 10.1016/0300-483x(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Misaghi I.J, Donndelinger C.R. Endophytic bacteria in symptom‐free cotton plants. Phytopathology. 1990;80:808–811. [Google Scholar]
- Moyne A‐L., Cleveland T.E., Tuzun S. Molecular characterization and analysis of the operon encoding the antifungal lipopeptide bacillomycin D. FEMS Microbiol Lett. 2004;234:43–49. doi: 10.1016/j.femsle.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Okunishi S., Sako K., Mano H., Imamura A., Morisaki H. Bacterial flora of endophytes in the maturing seeds of cultivated rice (Oryza sativa. Microbes Environ. 2005;20:168–177. [Google Scholar]
- Oliveira A., Dobereiner J., Baldani J. The effect of inoculating endophytic N2‐fixing bacteria on micropropagated sugarcane plants. Plant Soil. 2002;242:205–215. [Google Scholar]
- Ordentlich A.Y., Elad A.Y., Chet I. The role of chitinase of serratia marcescens in biocontrol of Sclerotium rolfssi. Phytopathology. 1998;78:84–88. [Google Scholar]
- Rai R., Dash P.K., Prasanna B.M., Syngh A. Endophytic bacterial flora in the stem tissue of a tropical maize (Zea mays L.) genotype: isolation, identification and enumeration. World J Microbiol Biotechnol. 2007;23:853–858. [Google Scholar]
- Reiter B., Wermbter N., Gyamfi S., Schwab H., Sessitsch A. Endophytic Pseudomonas spp. populations of pathogen‐infected potato plants analyzed by 16S rDNA‐ and 16S rRNA‐based denaturating gradient gel electrophoresis. Plant Soil. 2003;257:397–405. [Google Scholar]
- Rennie R.J., Freitas J.R.D., Ruschel A.P., Vose P.B. Isolation and identification of N2‐fixing bacteria associated with sugarcane (Saccharum sp.) Can J Microbiol. 1982;28:462–467. [Google Scholar]
- Rijavec T., Lapanje A., Rupnik M. Isolation of bacterial endophytes from germinated maize kernels. Can J Microbiol. 2007;53:802–808. doi: 10.1139/W07-048. [DOI] [PubMed] [Google Scholar]
- Schulz B., Boyle C. What are endophytes? In: Schulz B., Boyle C., Sieber T., editors. Springer‐Verlag; 2006. pp. 1–13. [Google Scholar]
- Sessitsch A., Reiter B., Pfeifer U., Wilhelm P. Cultivation‐independent population analysis of bacterial endophytes in three potato varieties based on eubacterial and Actinomycetes‐specific PCR of 16S rRNA genes. FEMS Microbiol Ecol. 2002;39:23–32. doi: 10.1111/j.1574-6941.2002.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Sevilla M., Gunapala N., Burris R.H., Kennedy C. Comparison of benefit to sugarcane plant growth and 15N2 incorporation followinginoculation of sterile plants with Acetobacter diazotrophicus wild‐type and nif− mutant strains. Mol Plant Microbe Interact. 2001;14:358–366. doi: 10.1094/MPMI.2001.14.3.358. [DOI] [PubMed] [Google Scholar]
- Spaepen S., Vanderleyden J., Okon Y. 2009.
- Strobel G. Muscodor albus and its biological promise. J Ind Microbiol Biotechnol. 2006;33:514–522. doi: 10.1007/s10295-006-0090-7. [DOI] [PubMed] [Google Scholar]
- Sturz A.V., Christie B.R., Matheson B.G. Associations of bacterial endophyte populations from red clover and potato crops with potential foe beneficial allelopathy. Can J Microbiol. 1998;44:162–167. [Google Scholar]
- Thomashow L.S., Weller D.M. Current concepts in the use of introduced bacteria for biological disease control: mechanisms and antifungal metabolites. In: Stacey G., Keen N.T., editors. Chapman and Hall; 1995. pp. 187–235. [Google Scholar]
- Torres A.R., Araujo W.L., Cursino L., Hungria M., Plotegher F., Mostasso F.L., Azevedo J.L. Diversity of endophytic enterobacteria associated with different host plants. J Microbiol. 2008;46:373–379. doi: 10.1007/s12275-007-0165-9. [DOI] [PubMed] [Google Scholar]
- Validov S. 2007. ) Biocontrol of tomato foot and root rot by Pseudomonas bacteria in stonewool. PhD Thesis. Leiden, The Netherlands: Leiden University [WWW document]. URL http://hdl.handle.net/1887/12480.
- Validov S., Kamilova F., Qi S., Stephan D., Wang J.J., Makarova N., Lugtenberg B. Selection of bacteria able to control Fusarium oxysporum f. sp. radicis lycopersici in stonewool substrate. J Appl Microbiol. 2007;102:461–471. doi: 10.1111/j.1365-2672.2006.03083.x. [DOI] [PubMed] [Google Scholar]
- Van Loon L.C. Plant responses to plant growth‐promoting bacteria. Eur J Plant Pathol. 2007;119:243–254. [Google Scholar]
- Van Peer R., Niemann G.J., Schippers B. Induced resistance and phytoalexin accumulation in biological control of Fusarium wilt of carnation by Pseudomonas sp. strain WCS417r. Phytopathology. 1991;81:728–734. [Google Scholar]
- Verma S.C., Ladha J.K., Tripathi A.K. Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. J Biotechnol. 2001;91:127–141. doi: 10.1016/s0168-1656(01)00333-9. [DOI] [PubMed] [Google Scholar]
- Walsh G.A., Murphy R.A., Killeen G.F., Headon D.R., Power R.F. Technical note: detection and quantification of supplemental fungal β‐gluconase activity in animal feed. J Anim Sci. 1995;73:1074–1076. doi: 10.2527/1995.7341074x. [DOI] [PubMed] [Google Scholar]
- Weisburg W.G., Barns S.M., Pelletier D.A., Lane D.J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth S.J., Wolf G.A. Dye labelled substrates for the assay and detection of chitinase and lysozyme activity. J Microbiol Methods. 1990;12:197–205. [Google Scholar]


