Summary
While precious metals are available to a very limited extent, there is an increasing demand to use them as catalyst. This is also true for palladium (Pd) catalysts and their sustainable recycling and production are required. Since Pd catalysts exist nowadays mostly under the form of nanoparticles, these particles need to be produced in an environment‐friendly way. Biological synthesis of Pd nanoparticles (‘bio‐Pd’) is an innovative method for both metal recovery and nanocatalyst synthesis. This review will discuss the different bio‐Pd precipitating microorganisms, the applications of the catalyst (both for environmental purposes and in organic chemistry) and the state of the art of the reactors based on the bio‐Pd concept. In addition, some main challenges are discussed, which need to be overcome in order to create a sustainable nanocatalyst. Finally, some outlooks for bio‐Pd in environmental technology are presented.
Introduction
In the last decades, there has been a considerable expansion in the use of precious metals in medicine, optical devices, electronics and catalysis (Das, 2010). Among the precious metals, platinum group metals [PGM: e.g. platinum (Pt), rhodium (Rh), ruthenium (Ru) and palladium (Pd)] are widely applied as catalysts. Due to their increasing use, for example in automotive catalytic converters to reduce harmful gaseous emissions, their availability has become more and more limited (Hoffmann, 1988). Especially Pd has become a very widespread catalyst. Its limited supply has caused extreme price volatility (its price raised from about €7300 per kg in November 2005 to about €19 000 per kg in November 2010). Therefore, recovery of palladium from waste is required. Conventionally, these recycling techniques are hydro‐ and pyrometallurgical processes. These processes require significant investments, labour and time. Moreover, considerable amounts of chemicals are involved, thus generating a secondary waste stream (Jacobsen, 2005).
There is a trend to switch from the use of bulk material towards nanoparticles (NPs) of palladium, since NPs are more active and thus less catalyst is needed. Conventional production methods of these NPs require the use of a series of toxic and expensive chemical agents (Hennebel et al., 2009a). Strong reductants (e.g. NaBH4) are required to reduce metal salts into their zerovalent metallic form. This reduction is often performed in solvents. Stabilizers (e.g. polyvinylpyrrolidone) and carrier materials (e.g. Al2O3) prevent the particles from aggregation and release into the environment. A promising alternative for chemical synthesis is to exploit the bioreductive deposition of metals by bacteria (Hennebel et al., 2009a). This biotechnological approach can serve both as a sustainable production method of nanomaterials as well as an efficient recovery technique for palladium and other metals.
This review aims at giving a summary of different bacteria that are able to perform the reduction of Pd(II) to Pd(0) and the mechanisms behind these bioreductive depositions. Subsequently, an overview is given of the demonstrated applications using these microbially synthesized Pd nanoparticles (‘bio‐Pd’), both as metal recovery technique and as nanoscale catalyst for several reduction reactions. Subsequently, the state of the art of the reactor set‐ups with bio‐Pd is discussed, together with some new challenges, which need to be fulfilled in order to create a stable, sustainable and applicable biocatalyst. Finally, a few outlooks for applications of bio‐Pd are discussed.
Bioreductive deposition of Pd(II) as Pd(0) nanoparticles
Different bacterial species are able to reduce Pd(II) to Pd(0) and each of them has specific properties that make them attractive for metal reduction (Table 1). The first reported species with Pd‐reducing capacities was the sulfate‐reducing bacterium Desulfovibrio desulfuricans (Lloyd et al., 1998). After incubation under anaerobic conditions, Pd(II) was added to the culture together with H2 or formate as electron donor. Subsequently, nanoscale deposits of Pd(0) at the cell surface were observed. When hydrogenases were inhibited with Cu, this reduction did not occur. This strongly indicated that a hydrogenase (possibly in combination with cytochrome c3) was responsible for the reduction of Pd(II) to Pd(0). A more detailed study on the involvement of hydrogenases in the reduction of Pd(II) by Desulfovibrio fructosivorans, a species with very well‐characterized periplasmic enzyme systems, was performed by Mikheenko and colleagues (2008). The role of different hydrogenases in the reduction process of Pd(II) was confirmed, since no depositions in the periplasmic space but only some Pd(0) clusters in mutants lacking these enzymes were observed on the cytoplasmatic membrane. The authors suggested that the enzyme probably supplied the electrons for the reduction process and served as a nucleation site for particle growth.
Table 1.
Overview of de different Pd‐reducing species, their Gram staining, oxygen tolerance and properties attractive for metal reduction.
| Species or genus | Gram staining | Aerobe/anaerobe | Attractive properties for Pd reduction | Reference |
|---|---|---|---|---|
| Desulfovibrio desulfuricans | G − | Anaerobe | Sulfate reducing, metal reducing | Lloyd et al. (1998); Yong et al. (2002b) |
| Desulfovibrio vulgaris | G − | Anaerobe | Sulfate reducing | Baxter‐Plant et al. (2003); Humphries and Macaskie (2005) |
| Desulfovibrio fructosivorans | G − | Anaerobe | Sulfate reducing | Mikheenko et al. (2008) |
| Shewanella oneidensis | G − | Facultative anaerobe | Metal reducing | De Windt et al. (2005) |
| Paracoccus denitrificans | G − | Facultative anaerobe | Nitrate reducing | Bunge et al. (2010) |
| Pseudomonas putida | G − | Aerobe | Bunge et al. (2010) | |
| Cupriavidus necator | G − | Facultative aerobe | Resistance to heavy metals | Bunge et al. (2010) |
| Cupriavidus metallidurans | G − | Facultative aerobe | Resistance to heavy metals | Gauthier et al. (2010) |
| Rhodobacter sphaeroides | G − | Facultative aerobe | Photosynthetic, metal resistant | Redwood et al. (2008) |
| Bacillus sphaericus | G + | Aerobe | Creamer et al. (2007) | |
| Plectonema boryanum | G − | Aerobe | Cyanobacterium | Lengke et al. (2007) |
| Calothrix | G − | Aerobe | N‐fixing Cyanobacterium | Brayner et al. (2007) |
| Anabaena | G − | Aerobe | N‐fixing Cyanobacterium | Brayner et al. (2007) |
| Clostridium pasterianum | G + | Anaerobe | Metal reducing, H2 producing through fermentation | Chidambaram et al. (2010) |
| Citrobacter braakii | G − | Facultative anaerobe | H2 producing through fermentation | Hennebel et al. (2011c) |
| Clostridium butyricum | G + | Anaerobe | H2 producing through fermentation | Hennebel et al. (2011c) |
| Bacteroides vulgatus | G − | Anaerobe | As reducing, H2 producing through fermentation | Hennebel et al. (2011c) |
| Klebsiella pneumoniae | G − | Facultative anaerobe | H2 producing through fermentation | Hennebel et al. (2011c) |
| Escherichia coli | G − | Facultative anaerobe | H2 producing through fermentation | Deplanche et al. (2010); Hennebel et al. (2011c) |
| Enterococcus faecium | G + | Facultative anaerobe | H2 producing through fermentation | Hennebel et al. (2011c) |
Another bacterial species that has been extensively studied in this context is the iron‐reducing bacterium Shewanella oneidensis (Fig. 1A). The bioreductive deposition of Pd(0) on the cell wall and in the periplasmic space of S. oneidensis has been described in presence of a series of electron donors (H2, formate, lactate, pyruvate, ethanol) with H2 and formate being the most efficient donors (De Windt et al., 2005). Interestingly, the presence of O2 did not significantly inhibit the reduction process. A similar reduction mechanism as for D. desulfuricans, based on the involvement of hydrogenases and cytochrome c3, was proposed. In addition, the authors suggested a role for the formate dehydrogenase enzyme in the reduction process.
Figure 1.
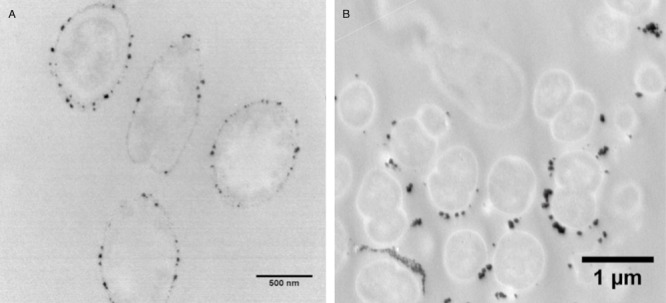
A. Nanoparticles of Pd(0) in and on the outer cell parts of Shewanella oneidensis. B. Nanoparticles of Pd(0) precipitated by the fermentative bacterium Citrobacter braakii.
Bunge and colleagues (2010) stated that the bacterial reduction of Pd(II) is not restricted to bacteria that reduce metals in a dissimilatory way (D. desulfuricans and S. oneidensis), but that a broader spectrum of Gram‐negative bacteria can sorb and subsequently reduce Pd(II). Pd(0) NPs were formed both in the periplasm and on the cell surface of Paracoccus denitrificans, Pseudomonas putida and Cupriavidus necator after 14 h incubation with formate. Pd(0) was also formed with autoclaved cells, where all hydrogenases had been inactivated and thus excluding the necessity of hydrogenase for the reduction process.
Cyanobacteria are another interesting group of bacteria that are able to form Pd(0) NPs out of Pd(II) (Brayner et al., 2007; Lengke et al., 2007). Deposits of Pd(0) were found in the medium, on the cell surface and intracellular. Brayner and colleagues (2007) suggested the nitrogenase enzyme to be responsible for the reduction.
A new approach was to produce Pd(0) by fermentative bacteria (Chidambaram et al., 2010; Hennebel et al., 2011c). Several fermentative species produce H2 during fermentation processes and can subsequently reduce Pd(II) to Pd(0) (Table 1). These bacteria were cultivated under fermentative conditions in an anaerobic minimal medium in which glucose was supplied as a carbon source before Pd(II) was added. A picture of bio‐Pd produced by the fermentative Citrobacter braakii is shown in Fig. 1B. This fermentatively produced H2 (referred to as ‘biohydrogen’) can subsequently be used as a reductant for Pd(II) reduction. Addition of Pd(II) to a fermenting culture of Clostridium pasteurianum resulted in the formation of NPs of Pd(0) on the cell wall and in the cytoplasm of the bacteria (Chidambaram et al., 2010). Moreover, this biohydrogen could further serve as hydrogen donor for the catalytic activity of the Pd(0). A reactive catalyst could thus be obtained in one step. Deplanche and colleagues (2010) attributed the reduction of Pd(II) by Escherichia coli to three types of hydrogenases.
Desulfovibrio desulfuricans and S. oneidensis are the most studied organisms in the context of the bioreductive synthesis of Pd(0) NPs. The NPs are formed at the outer surface of the bacterial cells (Lloyd et al., 1998; De Windt et al., 2005), which makes them available for applications as catalyst. This is in contrast with for example cyanobacteria. Moreover, the particles synthesized by D. desulfuricans and S. oneidensis are small and show a narrow size distribution (De Windt et al., 2006; Bennett et al., 2010) and the precipitation occurs within minutes, which is not really the case for the particles produced by the Gram‐negative species described by Bunge and colleagues (2010) and Sobjerg and colleagues (2009). Still, to increase the catalytic activity of bio‐Pd, the size of the nanoparticles should be decreased to 1–10 nm. Application of fermentative bacteria can be promising due to in situ production of H2. However, the particles show poor cell adhesion and a large particle size distribution (Hennebel et al., 2011c). Due to the location on the outer parts of the cells, the narrow size distribution and the fast reduction, bio‐Pd produced by D. desulfuricans and S. oneidensis have shown their applicability in catalysis. Nevertheless, it is possible that the conditions for deposition of Pd(0) by other strains can be optimized so that also other forms of bio‐Pd become more interesting for applications as catalyst.
Pd recovery from waste streams
Due to the limited availability of Pd and consequently its price volatility, the recovery and recycling of Pd has become of particular interest. Waste streams from spent automotive catalysts and printed circuit boards contain substantial amounts of Pd and other precious metals. For these waste streams, bacterial recovery of Pd(II) is a promising recovery technique since it is less labour intensive and requires less waste‐generating chemicals than conventional recovery techniques (Jacobsen, 2005). However, significant amounts of Cu(II) [up to 25 weight % and more (Creamer et al., 2006)], a known hydrogenase inhibitor, are present in the leachates of these scraps. For this reason, native biomass of D. desulfuricans was not able to remove Pd(II) from a printed board circuit leachate in presence of H2 (Creamer et al., 2006). To enable the recovery of Pd(II), cells which reduced Pd(II) to Pd(0) in absence of Cu(II) could be used. Indeed, a ‘pre‐palladized’ culture of D. desulfuricans was capable of removing Pd(II) from the leachate. The recovery is in that case not enzymatic anymore but based on the autocatalytic growth of Pd(0) clusters on the cells. The same findings were valid for E. coli (Mabbett et al., 2006). The observations for D. desulfuricans and E. coli were in contrast to the observations of Gauthier and colleagues (2010), who showed that this pre‐palladization was not required for recovery of Pd from a scrap leachate by C. necator and Cupriavidus metallidurans.
Several reactor types were built for the purpose of recovering Pd(II) from metal‐rich waste streams. An electrobioreactor for Pd recovery was developed by Yong and colleagues (2002a). In this system, H2 was produced electrochemically and could be delivered efficiently to a biofilm of D. desulfuricans on a Pd‐Ag membrane electrode, which allowed efficient transfer of H2. Creamer and colleagues (2006) applied a similar system to bio‐recover Au(0), Pd(0) and Cu(II) but this time from real waste scrap leachates. The presence of Cu(II) played a crucial role since it largely influenced the enzymatic reduction of Pd(II) but not of Au(III). This allowed for a stepwise separation of these three metals: Au(III) was first reduced by native biomass of D. desulfuricans, followed by the reduction of Pd(II) by pre‐palladized biomass and finally, Cu(II) could be precipitated as hydroxides and sulfates using the off gas of a bacterial culture.
These studies showed that the concept of Pd biorecovery is feasible when a waste stream containing pure Pd(II) is treated. When a mixture of different metals is present, the efficiency of the recovery process can be much lower. Especially Cu(II) appears to have an inhibiting effect on the process. Moreover, the concentrations of the metals in the considered solutions and scraps were quite high (several mg l−1), the efficiency of biorecovery should also be evaluated at lower metal concentrations.
Use of bio‐Pd as catalyst
Bio‐Pd catalysed removal of environmental contaminants
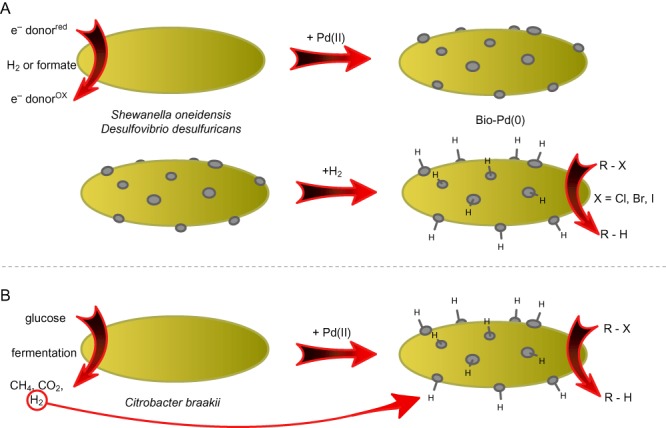
Bio‐Pd was successfully applied to remove a wide range of environmental contaminants (Table 2). The reactions that are described in this context are reduction of inorganic contaminants and dehalogenation of organic molecules. These contaminants result from different industrial processes and from the use of pesticides, pharmaceuticals, solvents etc. In all cases, a hydrogen donor (H2 or formate) was used as the reductive agent to charge the bio‐Pd catalyst (Fig. 2A). Noteworthy is that the particle size and the reactivity can be steered by altering the Pd/cell dry weight ratio (De Windt et al., 2006) and that the external addition of a hydrogen donor can be omitted when fermentatively cultivated species are used which produce biohydrogen (Fig. 2B) (Chidambaram et al., 2010; Hennebel et al., 2011c).
Table 2.
Overview of the different environmental contaminants that were successfully degraded with a bio‐Pd catalyst, together with the reaction mechanism and the Pd‐reducing species used in the study.
| Compound | Type of reaction | Polluted environmental compartment | Pd‐reducing species | Reference |
|---|---|---|---|---|
| Cr(VI) | Reduction | Industrial wastewaters | D. desulfuricans D. vulgaris E. coli | Humphries and Macaskie (2005); Mabbett et al. (2006) |
| C. pasteurianum | Chidambaram et al. (2010) | |||
| ClO4‐ | Reduction | Groundwater and drinking water | S. oneidensis | De Windt et al. (2006) |
| Polychlorobifenyls (PCBs) | Dechlorination (1–10 Cl) | Air, water, soil, sediments | D. desulfuricans D. vulgaris | Baxter‐Plant et al. (2003) |
| S. oneidensis | De Windt et al. (2005) | |||
| Chlorophenols | Dechlorination (1 Cl) | D. desulfuricans D. vulgaris | Baxter‐Plant et al. (2003) | |
| Lindane | Dechlorination (6 Cl) | Soil and groundwater | S. oneidensis | Mertens et al. (2007) |
| Trichloroethylene (TCE) | Dechlorination (3 Cl) | Groundwater | S. oneidensis | Hennebel et al. (2009b) |
| Polybrominated diphenyl ethers (PBDE) | Debromination (1–10 Br) | Indoor air and dust | D. desulfuricans | Harrad et al. (2007); Deplanche et al. (2009) |
| Iodinated contrast media (ICM) | Deiodination (3 I) | Wastewaters and surface waters | S. oneidensis | Hennebel et al. (2010); Forrez et al. (2011) |
| C. braakii | Hennebel et al. (2011c) |
Figure 2.
A. Two‐step dehalogenation of halogenated substances with a bio‐Pd catalyst and an external hydrogen donor. B. One‐step dehalogenation of halogenated substances with a bio‐Pd catalyst produced by fermentative species.
Bio‐Pd as a catalyst in organic chemistry
Bio‐Pd (precipitated by D. desulfuricans and Bacillus sphaericus) was applied as catalyst in the hydrogenation of organic molecules, e.g. the hydrogenation of itaconic acid (Creamer et al., 2007), 2‐pentyne (Bennett et al., 2010) and a wide variety of other organic molecules (Deplanche et al., 2009). The reaction rates were dependent on the solvent in which the reaction was performed and extremely dependent on the molecule that is to be hydrogenated. Molecules with very high structural similarity showed large differences in hydrogenation efficiency. For example, 3,4‐dihydroisoquinoline could be hydrogenated to 1,2,3,4‐tetrahydroisoquinoline with a yield of 47%, whereas 3,4‐dihydro‐1‐methlisoquinoline, differing from the previous product by only one methyl group, could not be hydrogenated at all. In general, bio‐Pd gave more consistent results in terms of selectivity compared with Pd/Al2O3 (Bennett et al., 2010). The applicability of bio‐Pd catalysts for coupling reactions in synthetic organic chemistry was reported by Sobjerg and colleagues (2009). Two bacteria (P. putida and C. necator) were used to precipitate Pd(0). Both forms of bio‐Pd could successfully catalyse the Suzuki‐Miyaura coupling of an aryl halide with phenylboronic acid and the Mizoroki‐Heck coupling of an aryl halide with n‐butylacrylate. Gauthier and colleagues (2010) applied bio‐Pd recovered from a metal‐containing leachate by two different Cupriavidus species for the Mizoroki‐Heck coupling.
Reactor set‐ups using a bio‐Pd catalyst: state of the art
Bio‐Pd for in situ groundwater remediation
Chidambaram and colleagues (2010) showed that Pd(II) could be reduced by a biofilm of C. pasteurianum grown on sand grains. Aquifer microcosms were developed for in situ remediation of groundwater contaminated with Cr(VI). In one of these microcosms, Pd(II) was added and subsequently reduced to Pd(0). In this microcosm, Cr(VI) could be reduced to Cr(III), which was confirmed by µXANES. In a microcosm with viable cells without added Pd(II), no Cr(III) was detected.
Use of bio‐Pd in an aqueous suspension
The attachment of the nanoscale Pd(0) catalysts to a microscale bacterial scaffold allowed separation of the catalysts from the reaction medium by relatively simple techniques like microfiltration in membrane reactors. Several membrane systems were used to keep the bio‐Pd catalyst in the reaction medium and to prevent leaching of the catalyst in the effluent. The use of hollow fibre membranes for the retention of bio‐Pd was demonstrated for the removal of the groundwater contaminant lindane and the removal of iodinated contrast media (ICM) from wastewaters (Mertens et al., 2007; Forrez et al., 2011). Lindane was removed for 83% using 100 mg Pd l−1 and the ICM iohexol, iomeprol, iopromide and diatrizoate were removed for more than 90% using 141 mg Pd l−1. These hollow fibres allow a high membrane area per unit of volume but are very vulnerable to clogging. A more robust alternative are plate membranes (Fig. 3B). A plate membrane reactor filled with bio‐Pd (produced by S. oneidensis) was developed by Hennebel and colleagues (2009b) for the removal of the chlorinated solvent TCE from groundwater. The highest removal rates (2340 ± 144 mg TCE day−1 g−1 Pd) were obtained using H2 as electron donor and 100 mg Pd l−1. Almost no Pd leaching to the effluent took place (a maximum effluent concentration of 180 µg Pd l−1).
Figure 3.
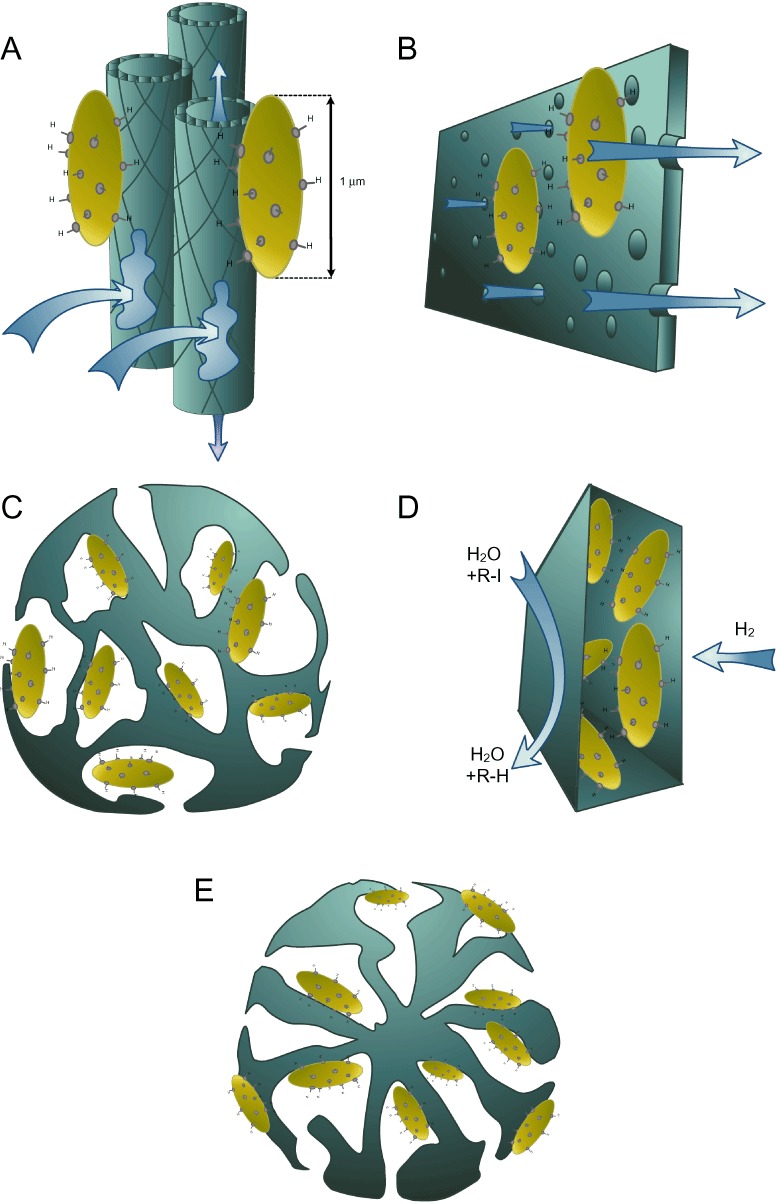
Retention mechanisms of bio‐Pd in reactor set‐ups. A. Retention by hollow fibre membranes. B. Retention by plate membranes. C. Encapsulation in alginate beads. D. Encapsulation in polymeric membranes. E. Coating on zeolites.
Retention of bio‐Pd by encapsulation and by coating on surfaces
An alternative for the retention of bio‐Pd by membranes is the encapsulation in polymeric matrices. Bio‐Pd was encapsulated in several materials in order to prevent leaching of Pd in the effluent of the reactors and to facilitate separation and recycling of the catalyst.
Hennebel and colleagues (2009c) incorporated bio‐Pd, produced by S. oneidensis, in a series of encapsulation materials: polyurethane, polyacrylamide, alginate and silica. Encapsulation in alginate beads is illustrated in Fig. 3C. The encapsulated bio‐Pd had at least a six times lower dechlorination rate for TCE to ethane compared with a bio‐Pd suspension. Polyurethane cubes were used in a fixed bed reactor. In a flow‐through system, the maximum TCE removal rate was 865 ± 151 mg TCE g−1 Pd day−1. This removal rate is significantly lower compared with a bio‐Pd suspension in a membrane reactor (Hennebel et al., 2009b).
Polymeric membranes have also been used as encapsulation matrix for bio‐Pd. Hennebel and colleagues (2010) made catalytically active membranes by incorporating bio‐Pd (produced by S. oneidensis) in PVDF (polyvinylidene fluoride) and PSf (polysulfone) membranes. These membranes were prepared by the immersion‐precipitation method. The deiodination rates of diatrizoate obtained with bio‐Pd membranes were only slightly lower than the ones with a suspension of bio‐Pd. The PVDF membranes containing bio‐Pd were used in a membrane contactor set‐up. In this contactor, water spiked with diatrizoate was circulated on one side of the membrane and H2 was dosed in a controlled way at the other side of the membrane. A scheme of this set‐up is shown in Fig. 3D. The aim of this set‐up was to dose H2 in a more controlled way and directly to the catalyst with less limitations due to its limited water solubility. In this system diatrizoate was removed for 77% after 48 h at pH 10.
Next to encapsulation in polymeric matrices, Hennebel and colleagues (2009c) coated bio‐Pd on zeolites. This coated bio‐Pd was five times less active for the dechlorination of TCE compared with a bio‐Pd suspension. This coating on porous zeolites is illustrated in Fig. 3E. Another coating surface is graphite (Hennebel et al., 2011a). This graphite can be further used as a cathode of a bio‐electrochemical system (see Efficient delivery of the hydrogen donor). Also the anode of microbial fuel cells (MFCs) can be coated with bio‐Pd, for example coated on a Teflon‐coated carbon paper (Ogi et al., 2011). The aim for the use of bio‐Pd was to maximize the power output of the cell. The maximum power output of a bio‐Pd‐containing cell was 90% of the power of a cell containing a chemical Pd catalyst.
In a recent study, a biofilm of Serratia was grown on polyurethane foam, which could reduce Pd(II). The obtained Pd(0) was successfully used for reduction of Cr(VI). However, reactors containing this biofilm‐Pd showed heterogeneities in reaction rates and dead spots (Beauregard et al., 2010).
Comparison of the different set‐ups
The different set‐ups, as discussed above, differ in two important aspects: the production of Pd (production in situ or pre‐production) and the retention of bio‐Pd (filtering by membranes, encapsulation in a matrix or coating on a surface). The production in situ offers the advantage of a cheaper production requiring no pure cultures, no complex growth media and especially no external hydrogen (when working with H2‐producing species). Still, addition of an external carbon source can be needed. However, when the in situ production of bio‐Pd is used for groundwater remediation (as described by Chidambaram et al., 2010), the injection of a heavy metal like Pd into the soil might raise questions. The presence of Pd in groundwater should definitely be avoided. Looking at the different retention mechanisms, the size of the bacteria (about 1 µm) makes the retention of bio‐Pd by microfiltration membranes very straightforward. Of course, this requires a strong attachment of the nanoparticles to the cell wall in order to avoid leaching of nanoparticles into the effluent. Moreover, the membranes can be sensitive to clogging and regular backwash can decrease the efficiency of the reactor. Encapsulation could be an option to assure better retention of the nanoparticles in the system. These reactors are insensible to clogging but this encapsulation will cause a decrease of the reaction rates. The challenge is to find a matrix which is porous enough to limit the loss of catalytic activity but still assures the good retention of the nanoparticles inside the matrix. Another challenge is to evaluate the long‐term stability of these reactor systems. Conclusively, the optimal reaction configuration will be very dependent on the type of application.
Challenges for the usage of bio‐Pd as catalyst
Catalytic application of biorecovered Pd
Ideally, bio‐Pd is synthesized from a Pd‐containing scrap leachate and subsequently applied as a catalyst in order to obtain a closed cycle of Pd. This was demonstrated by Gauthier and colleagues (2010), who biorecovered Pd from a heavy metal scrap leachate using C. necator and C. metallidurans. The obtained bio‐Pd was subsequently successfully applied as a catalyst for the Mizoroki‐Heck coupling of n‐butylacrylate with 4‐iodoanisole. However, it is probable that the success of both the biorecovery and the catalytic application are very dependent on the composition of the leachate. The scrap can contain compounds which inhibit the biorecovery process and other metals present on the bacteria after the recovery process might decrease the catalytic activity of Pd.
Doping of bio‐Pd with other metals to enhance activity
In order to increase reaction rates or to enable new reactions, there is a growing interest in the use of bimetallic catalysts. In the case of Pd, a whole range of elements has been used as promoting element which can improve either the geometry of the catalyst or the transfer of electrons. For example, the combination of chemically produced Pd and Fe is a very well‐documented bimetallic catalyst for reduction reactions (Zhang et al., 1998; Crabb and Marshall, 2001). Also other transition metals like Cu, Ni and Ag have been used as promoting element for Pd catalysts (Tzitzios and Georgakilas, 2005; Massard et al., 2007; Kim et al., 2008). The combination of Pd and Au is also widely applied for reductive reactions, for example dechlorination of TCE (Nutt et al., 2005). These bimetallic catalysts can have different structures, such as alloys (Venezia et al., 2003) or core‐shell structures (Nutt et al., 2005). Doping of bio‐Pd, produced by S. oneidensis, with Au(0) is currently under investigation. First results indicate that a bio‐Pd/Au catalyst, synthesized by co‐precipitation of Pd and Au, can significantly increase the reaction rates of dechlorination reactions (S. De Corte, T. Hennebel, J.P. Fitts, T. Sabbe, W. Verstraete and N. Boon, unpubl. results).
Assuring long‐term stability of the product
A topic that urgently requires more in‐depth studies is the long‐term stability of bio‐Pd as a product. Three phenomena can occur when preserving bio‐Pd for longer periods of time: (i) leaching of the NPs from the biomass, (ii) leaching of Pd(II) from the metallic nanoparticle and (iii) poisoning of the catalyst.
Leaching of Pd nanoparticles can be due to detachment from the cell wall or desintegration of the bacterial biomass. Degradation of the bacterial support will cause release of NPs and thus needs to be avoided. The cell structures need to stay intact for longer periods of time by an appropriate preservation technique. Little is known about the fate of nanoparticles in the environment and potential adverse health effects, but due to the precautionary principle, their release needs to be prevented. The leaching of ions is commonly reported for catalysts based on transition metals (Calvo et al., 2010). Leaching of Pd(II) depends mostly on the type of solvent and the pH of the reaction medium. In the case of chemical synthesis reactions, such as the Pd catalysed Suzuki‐Miyaura coupling reactions, also the presence of additives (base, TBAB) and substrates (aryl halides, phenyl boronic acid) play a crucial role.
Another limitation for the use of bio‐Pd might imply the poisoning of the catalyst. For example, sulfides are known to have a strong affinity for the Pd metal and may block the active sites of the catalyst via formation of strong Pd–S bonds and layers of sulfide around the Pd clusters (Gravil and Toulhoat, 1999; Alfonso et al., 2003). Since sulfide is a natural water constituent under reducing conditions produced by microbial sulfate reduction, sulfide induced catalyst deactivation is a crucial issue which hinders the full exploitation of the catalyst potential as a treatment technology for groundwater remediation (Angeles‐Wedler et al., 2009). A possible approach to prevent sulfide poisoning is the oxidative removal of sulfide prior to any contact with the noble metal (Angeles‐Wedler et al., 2008).
High temperature applications in organic chemistry
In advanced synthetic organic chemistry and petrochemistry, homogeneous and heterogeneous Pd catalysts are very frequently used. Heterogeneous catalysts allow an easy separation of the catalyst from the reaction medium. Due to the attachment of the nanoparticles to the bacterial cell wall structures, the use of bio‐Pd could prevent the leaching of Pd NPs in the synthesized product. Therefore, bio‐Pd could be an interesting heterogeneous Pd catalyst. However, these reactions are often performed at high temperatures. Under these circumstances, the bacterial cells will disintegrate. By doing so, sulfur will be released and will consequently poison the catalyst. Preliminary tests in dehydrogenation reactions at 250°C indicated a very fast loss of catalytic activity due to sulfur poisoning (S. De Corte, T. Hennebel, W. Verstraete and N. Boon, unpubl. results). This phenomenon will be difficult to overcome because sulfur is an essential constituent of microbial biomass.
Efficient delivery of the hydrogen donor
Several studies showed that multiple hydrogen donors can be used for Pd driven reactions. Indeed, not only hydrogen gas but also formate, formic acid, isopropanol and ethanol can effectively donate reactive hydrogen species for the reduction and dehalogenation of pollutants (Kopinke et al., 2004; De Windt et al., 2005; Hennebel et al., 2009b). In the case of bio‐Pd, only H2, formate and formic acid were effective to charge the nanoparticles and it was demonstrated that H2 was more effective than formate (Hennebel et al., 2009b). However, a major challenge is the efficient supply of the hydrogen donor to the catalyst. Yet, the external supply of H2 gas can give rise to large costs and technical difficulties because of the high safety risks for gas storage (Hennebel et al., 2011b). As discussed in Reactor set‐ups using a bio‐Pd catalyst: state of the art, several reactor types were constructed to limit the hydrogen supply, for example by a membrane contactor (Hennebel et al., 2010), or to produce hydrogen gas in a sustainable way, for example by using microbially produced H2 (Humphries et al., 2007), or by using a H2‐producing species for Pd reduction, e.g. C. pasteurianum (Chidambaram et al., 2010).
A promising configuration for the gentle delivery of a hydrogen donor to a catalyst is a microbial electrolysis cell (MEC). In MEC systems, electrons travel through an external circuit to a cathode, where they can be consumed for H2 formation (Mu et al., 2009). Yet, electrical energy needs to be supplied to the electrical circuit by means of a power source in order to provide the correct potential for H2 generation (−0.414 V). Hennebel and colleagues (2011a) have demonstrated the opportunities of such a system by the development of a MEC with bio‐Pd coated on graphite at the cathode for catalytic dehalogenation of diatrizoate and TCE. Applying −0.8 V with a power source, 93% removal of the contaminant was achieved after 1 h of recirculation at neutral pH and complete removal after 3 h, whereas only 48% removal was observed using the same MEC with non‐Pd coated graphite granules. Biocatalysed electrolysis required a quarter (Rozendal et al., 2006) to half (Hennebel et al., 2011a) of the energy needed for conventional water electrolysis (Rasten et al., 2003). The price of hydrogen produced through water electrolysis is strongly dependent on the electricity price (Turner, 2004). As this can be expected for biocatalysed electrolysis as well, the reduced consumption of electrical energy per unit of hydrogen is a strong advantage of biocatalysed electrolysis.
Outlooks
Design of a cost‐effective technology
The economical feasibility is the main determining factor for pilot‐scale and full‐scale bio‐Pd applications. Several costs need to be taken into account. A first cost is attributed to the production of the bio‐Pd. The culturing and subsequent handlings of the bacteria can amount to €1000 kg−1 cell dry weight. The cost of the palladium salt can be approximately €20 000 kg−1 and is dependent on the extremely volatile price of Pd. This cost can be 70% lower if the Pd is recovered from a Pd(II) containing waste stream or leachate (personal communications with Umicore, Belgium). However, some synthetic Pd salt might still be required for pre‐palladization of the biomass. When Pd nanoparticles are purely chemically produced, the cost for culturing of the bacteria is omitted, but extra costs are attributed to the use of chemicals: reducing agents (e.g. NaBH4), stabilizers (e.g. polyvinylpyrrolidone) and carrier materials (mostly activated carbon or Al2O3).
In order to prevent leaching of the Pd in reactor effluents, one can choose either for membrane filtration or for encapsulation. The cost for microfiltration membranes can be estimated on about €100 m−2. Hennebel and colleagues (2009c) estimated the cost of encapsulation of bio‐Pd in several materials. For a reactor with 100 mg bio‐Pd l−1, the price for bio‐Pd coated on zeolites was about €75 m−3 whereas for polyacrylamide beads this cost amounted to €2250 m−3. Moreover, the loss of activity due to encapsulation needs to be taken into account. Also the cost of the hydrogen donor needs to be included. Since H2 is mostly produced electrochemically, its cost is very dependent on the energy price. A less expensive alternative for H2 production is a MEC (see Efficient delivery of the hydrogen donor). When fermenting bacteria are used, H2 can be delivered in situ and this cost can be omitted. However, the cost for culturing these strains can be higher due to the continuous need for anaerobization and the continuous dosage of a carbon source.
Application of bio‐Pd to treat groundwater, soils and sludges
The largest environmental market for the technologies presented in this work is soil remediation. Indeed, halogenated pollutants are ubiquitous in groundwater and soils. The suggested technologies can be applied as a part of a ‘pump‐and‐treat’ strategy for the sanitation of groundwater or as in situ remediation technology by supplying the Pd catalyst in the soil. Conventional pump‐and‐treat methods involve pumping contaminated water to the surface followed by treatment with for example air stripping and/or adsorption on activated carbon. A successful pump‐and‐treat‐based field‐study using chemical Pd coated on egg shells in a fixed bed reactor was performed at Edward Air Force Base (California, USA; Davie et al., 2008). Until now, only one field‐study explored the applicability of bio‐Pd at larger scale. Groundwater contaminated with a mixture of hexachlorohexanes and chlorobenzenes was treated in a fluidized bed reactor with 20 g of bio‐Pd encapsulated in alginate at a flow rate and contact time of 50 l h−1 and 4 h respectively (Hennebel et al., 2011b). Further up‐scaling projects will need to examine the feasibility of full‐scale bio‐Pd treatments. Biocatalytical treatment can economically compete with currently used other pump‐and‐treat technologies. However, it needs to be shown that bio‐Pd offers advantages, such as price or sustainability of the production process, over chemically produced Pd catalysts.
The pump‐and‐treat technology will not be effective for contaminants which strongly interact with the soil matrix. For example, PCBs tend to adsorb on soil particles due to their hydrophobicity. Also metals are sometimes difficult to pump due to their affinity to soil components. In these cases, the pump‐and‐treat with bio‐Pd will be ineffective. Therefore, an alternative technology was developed consisting of the supply of a Pd(II) salt and a carbon source (e.g. glucose) to a soil. It was shown that Pd NPs were formed by biofilms of an inoculated culture of C. pasteurianum on sand grains in aquifer mesocosms (Chidambaram et al., 2010). Subsequently, an almost complete reduction of the toxic Cr(VI) was obtained. However, the inoculation is very likely not required in a remediation programme, as many bacteria that reduce Pd and form Pd NPs will belong to the indigenous microbial community. The advantage of such a technology would be the good contact between catalyst, pollutant and soil matrix. A drawback of this technology might be the low efficiency of Pd reduction and hence the environmental implications and costs related to the unreduced Pd(II). An example of a niche market for this bio‐Pd‐based technology might be the clean‐up of river and lake sludges contaminated with PCBs. One could mix the bio‐Pd and a slowly degradable organic carbon source with these sludges at extremely low concentrations approaching the background values of metals. Autochthonous bacteria would degrade the carbon source and form hydrogen gas in these anaerobic environments. The latter could be used as electron donor to charge the bio‐Pd. A final and more controlled way of bio‐Pd application in soils could be in reactive barriers or wells. It is clear that bioremediation strategies are not yet elaborated in comparison with the pump‐and‐treat strategy. Many fundamental aspects such as the durability of the dehalogenation process, the sensitivity for poisoning and the in situ hydrogen production need to be addressed in batch and pilot scale experiments. Moreover, technological aspects such as a homogeneous dosing of the catalyst and the electron donor to soils or sludges need to be studied.
Application of bio‐Pd for wastewater treatment
A major issue of conventional wastewater treatment is the presence of micropollutants such as pharmaceuticals and pesticides at concentration levels of µg l−1 in the effluents of WWTPs. The use of bio‐Pd‐based reactors holds promising opportunities for reductive post‐treatment of WWTP effluents and hospital wastewaters in order to decrease the environmental load of halogenated micropollutants from these point sources. Catalytic dechlorination and deiodination using biogenic palladium NPs should be further explored for these pharmaceuticals that cannot be treated by other advanced treatment methods. As proof of principle, the removal of ICM was demonstrated (see Bio‐Pd catalysed removal of environmental contaminants). These ICM are not effectively removed in WWTPs and because of their high degree of substitution of the benzene ring, they are even resistant to ozonation and other advanced oxidation techniques (Ternes et al., 2003). Another recalcitrant compound of recent interest is diclofenac, a non‐steroidal anti‐inflammatory drug. It is one of the pharmaceuticals most detected in WWTP effluents worldwide (Zhang et al., 2008; Okuda et al., 2009; Sodre et al., 2010). According to the precautionary principle, the environmental input of such persistent and hence long‐living, mobile compounds should be limited. Moreover, these contaminants considerably contribute to the absorbable organic halogen (AOX) content in wastewater, especially in hospital wastewaters and effluents from radiographic practices (Putschew et al., 2000; Schittko et al., 2004). The AOX will become an important parameter of wastewater regulation. Consequently, the development of innovative post‐treatment methods is of utmost importance in the coming decade.
Application of bio‐Pd for drinking water production
In drinking water production, the application of reductive treatment methods should be considered since pharmaceutical residues can be introduced into groundwater through surface water filtration, leakage and groundwater recharge. Because the sorption affinity of pharmaceuticals to sludge and suspended matter is limited, their retention by bank filtration is insufficient, which makes that they were detected in drinking water supplies (Seitz et al., 2006). For example, trace concentrations of diclofenac were detected in Berlin tap water (Heberer, 2002). Therefore, we suggest the implementation of a bio‐Pd‐based reactor system at the inlet of drinking water facilities that make use of groundwater resources. Immediately after pumping the water out of the subsoil, the water is anoxic, which makes it possible to maintain a reducing environment for catalytic dehalogenation in the reactor tank. After aeration, the dehalogenated metabolites can be further degraded/metabolized by oxidative metabolic or cometabolic processes (De Gusseme et al., 2009; 2011), for example during sand filtration.
Conclusions
In the past decade, a wide range of microorganisms were reported to be capable of precipitating Pd(0) NPs (‘bio‐Pd’) from solutions containing Pd(II). It was shown that bio‐Pd can be a potentially powerful tool as metal recovery technique and in environmental applications such as reduction of inorganic contaminants and dehalogenation of organic pollutants. The microbial scaffold allows a green synthesis of NPs and offers some advantages and perspectives for the development of reactor technologies for metal recovery and soil and water treatment (groundwater, wastewater and drinking water). However, some main issues need to be addressed. For example, the hydrogen donor should be delivered to the catalyst in a sustainable way. In addition, up‐scaling of the production process needs to be optimized and the long‐term stability of the catalyst needs to be assured. If these conditions are fulfilled, an economically feasible and effective full scale technology can be developed.
Acknowledgments
S. De Corte and B. De Gusseme (aspirant) are supported by a PhD grant of the Research Foundation Flanders (FWO). T. Hennebel is supported by Ghent University Multidisciplinary Research Partnership (MRP) – Biotechnology for a sustainable economy (01 MRA 510W) and a project grant (7741‐02) of the Research Foundation Flanders (FWO). This work was part of a research project obtained from the EU Commission within the Program of the Seventh Framework (FP7‐KBBE‐2010‐4): EU ULIXES project (266473). The authors thank Tim Lacoere for assistance with figures.
References
- Alfonso D.R., Cugini A.V., Sholl D.S. Density functional theory studies of sulfur binding on Pd, Cu and Ag and their alloys. Surf Sci. 2003;546:12–26. [Google Scholar]
- Angeles‐Wedler D., Mackenzie K., Kopinke F.‐D. Permanganate oxidation of sulfur compounds to prevent poisoning of Pd catalysts in water treatment processes. Environ Sci Technol. 2008;42:5734–5739. doi: 10.1021/es800330s. [DOI] [PubMed] [Google Scholar]
- Angeles‐Wedler D., Mackenzie K., Kopinke F.D. Sulphide‐induced deactivation of Pd/Al2O3 as hydrodechlorination catalyst and its oxidative regeneration with permanganate. Appl Catal B. 2009;90:613–617. [Google Scholar]
- Baxter‐Plant V., Mikheenko I.P., Macaskie L.E. Sulphate‐reducing bacteria, palladium and the reductive dehalogenation of chlorinated aromatic compounds. Biodegradation. 2003;14:83–90. doi: 10.1023/a:1024084611555. [DOI] [PubMed] [Google Scholar]
- Beauregard D.A., Yong P., Macaskie L.E., Johns M.L. Using non‐invasive magnetic resonance imaging (MRI) to assess the reduction of Cr(VI) using a biofilm‐palladium catalyst. Biotechnol Bioeng. 2010;107:11–20. doi: 10.1002/bit.22791. [DOI] [PubMed] [Google Scholar]
- Bennett J.A., Creamer N.J., Deplanche K., Macaskie L.E., Shannon I.J., Wood J. Palladium supported on bacterial biomass as a novel heterogeneous catalyst: a comparison of Pd/Al2O3 and bio‐Pd in the hydrogenation of 2‐pentyne. Chem Eng Sci. 2010;65:282–290. [Google Scholar]
- Brayner R., Barberousse H., Hernadi M., Djedjat C., Yepremian C., Coradin T. Cyanobacteria as bioreactors for the synthesis of Au, Ag, Pd, and Pt nanoparticles via an enzyme‐mediated route. J Nanosci Nanotechnol. 2007;7:2696–2708. doi: 10.1166/jnn.2007.600. et al. [DOI] [PubMed] [Google Scholar]
- Bunge M., Sobjerg L.S., Rotaru A.E., Gauthier D., Lindhardt A.T., Hause G. Formation of palladium(0) nanoparticles at microbial surfaces. Biotechnol Bioeng. 2010;107:206–215. doi: 10.1002/bit.22801. et al. [DOI] [PubMed] [Google Scholar]
- Calvo L., Gilarranz M.A., Casas J.A., Mohedano A.F., Rodriguez J.J. Hydrodechlorination of diuron in aqueous solution with Pd, Cu and Ni on activated carbon catalysts. Chem Eng J. 2010;163:212–218. [Google Scholar]
- Chidambaram D., Hennebel T., Taghavi S., Mast J., Boon N., Verstraete W. Concomitant microbial generation of palladium nanoparticles and hydrogen to immobilize chromate. Environ Sci Technol. 2010;44:7635–7640. doi: 10.1021/es101559r. et al. [DOI] [PubMed] [Google Scholar]
- Crabb E.M., Marshall R. Properties of alumina supported Pd‐Fe and Pt‐Fe catalysts prepared using surface organometallic chemistry. Appl Catal A. 2001;217:41–53. [Google Scholar]
- Creamer N.J., Baxter‐Plant V.S., Henderson J., Potter M., Macaskie L.E. Palladium and gold removal and recovery from precious metal solutions and electronic scrap leachates by Desulfovibrio desulfuricans. Biotechnol Lett. 2006;28:1475–1484. doi: 10.1007/s10529-006-9120-9. [DOI] [PubMed] [Google Scholar]
- Creamer N.J., Mikheenko I.P., Yong P., Deplanche K., Sanyahumbi D., Wood J. Novel supported Pd hydrogenation bionanocatalyst for hybrid homogeneous/heterogeneous catalysis. Catal Today. 2007;128:80–87. et al. [Google Scholar]
- Das N. Recovery of precious metals through biosorption – a review. Hydrometallurgy. 2010;103:180–189. [Google Scholar]
- Davie M.G., Cheng H., Hopkins G.D., Lebron C.A., Reinhard M. Implementing heterogeneous catalytic dechlorination technology for remediating TCE‐contaminated groundwater. Environ Sci Technol. 2008;42:8908–8915. doi: 10.1021/es8014919. [DOI] [PubMed] [Google Scholar]
- De Gusseme B., Pycke B., Hennebel T., Marcoen A., Vlaeminck S.E., Noppe H. Biological removal of 17 alpha‐ethinylestradiol by a nitrifier enrichment culture in a membrane bioreactor. Water Res. 2009;43:2493–2503. doi: 10.1016/j.watres.2009.02.028. et al. [DOI] [PubMed] [Google Scholar]
- De Gusseme B., Vanhaecke L., Verstraete W., Boon N. Degradation of acetaminophen by Delftia tsuruhatensis and Pseudomonas aeruginosa in a membrane bioreactor. Water Res. 2011;45:1856–1864. doi: 10.1016/j.watres.2010.11.040. [DOI] [PubMed] [Google Scholar]
- De Windt W., Aelterman P., Verstraete W. Bioreductive deposition of palladium (0) nanoparticles on Shewanella oneidensis with catalytic activity towards reductive dechlorination of polychlorinated biphenyls. Environ Microbiol. 2005;7:314–325. doi: 10.1111/j.1462-2920.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- De Windt W., Boon N., Van den Bulcke J., Rubberecht L., Prata F., Mast J. Biological control of the size and reactivity of catalytic Pd(0) produced by Shewanella oneidensis. Antonie Van Leeuwenhoek Int J Gen Mol Microbiol. 2006;90:377–389. doi: 10.1007/s10482-006-9088-4. et al. [DOI] [PubMed] [Google Scholar]
- Deplanche K., Snape T.J., Hazrati S., Harrad S., Macaskie L.E. Versatility of a new bioinorganic catalyst: palladized cells of Desulfovibrio desulfuricans and application to dehalogenation of flame retardant materials. Environ Technol. 2009;30:681–692. doi: 10.1080/09593330902860712. [DOI] [PubMed] [Google Scholar]
- Deplanche K., Caldelari I., Mikheenko I.P., Sargent F., Macaskie L.E. Involvement of hydrogenases in the formation of highly catalytic Pd(0) nanoparticles by bioreduction of Pd(II) using Escherichia coli mutant strains. Microbiology-Sgm. 2010;156:2630–2640. doi: 10.1099/mic.0.036681-0. [DOI] [PubMed] [Google Scholar]
- Forrez I., Carballa M., Fink G., Wick A., Hennebel T., Vanhaecke L. Biogenic metals for the oxidative and reductive removal of pharmaceuticals, biocides and iodinated contrast media in a polishing membrane bioreactor. Water Res. 2011;45:1763–1773. doi: 10.1016/j.watres.2010.11.031. et al. [DOI] [PubMed] [Google Scholar]
- Gauthier D., Sobjerg L.S., Jensen K.M., Lindhardt A.T., Bunge M., Finster K. Environmentally benign recovery and reactivation of palladium from industrial waste by using gram‐negative bacteria. ChemSusChem. 2010;3:1036–1039. doi: 10.1002/cssc.201000091. et al. [DOI] [PubMed] [Google Scholar]
- Gravil P.A., Toulhoat H. Hydrogen, sulphur and chlorine coadsorption on Pd(111): a theoretical study of poisoning and promotion. Surf Sci. 1999;430:176–191. [Google Scholar]
- Harrad S., Robson M., Hazrati S., Baxter‐Plant V.S., Deplanche K., Redwood M.D., Macaskie L.E. Dehalogenation of polychlorinated biphenyls and polybrominated diphenyl ethers using a hybrid bioinorganic catalyst. J Environ Monit. 2007;9:314–318. doi: 10.1039/b616567b. [DOI] [PubMed] [Google Scholar]
- Heberer T. Tracking persistent pharmaceutical residues from municipal sewage to drinking water. J Hydrol. 2002;266:175–189. [PubMed] [Google Scholar]
- Hennebel T., De Gusseme B., Boon N., Verstraete W. Biogenic metals in advanced water treatment. Trends Biotechnol. 2009a;27:90–98. doi: 10.1016/j.tibtech.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Hennebel T., Simoen H., De Windt W., Verloo M., Boon N., Verstraete W. Biocatalytic dechlorination of trichloroethylene with bio‐palladium in a pilot‐scale membrane reactor. Biotechnol Bioeng. 2009b;102:995–1002. doi: 10.1002/bit.22138. [DOI] [PubMed] [Google Scholar]
- Hennebel T., Verhagen P., Simoen H., De Gusseme B., Vlaeminck S.E., Boon N., Verstraete W. Remediation of trichloroethylene by bio‐precipitated and encapsulated palladium nanoparticles in a fixed bed reactor. Chemosphere. 2009c;76:1221–1225. doi: 10.1016/j.chemosphere.2009.05.046. [DOI] [PubMed] [Google Scholar]
- Hennebel T., De Corte S., Vanhaecke L., Vanherck K., Forrez I., De Gusseme B. Removal of diatrizoate with catalytically active membranes incorporating microbially produced palladium nanoparticles. Water Res. 2010;44:1498–1506. doi: 10.1016/j.watres.2009.10.041. et al. [DOI] [PubMed] [Google Scholar]
- Hennebel T., Benner J., Clauwaert P., Vanhaecke L., Aelterman P., Callebaut R. Dehalogenation of environmental pollutants in microbial electrolysis cells with biogenic palladium nanoparticles. Biotechnol Lett. 2011a;33:89–95. doi: 10.1007/s10529-010-0393-7. et al. [DOI] [PubMed] [Google Scholar]
- Hennebel T., Simoen H., Verhagen P., De Windt W., Dick J., Weise C. Biocatalytic dechlorination of hexachlorocyclohexane by immobilized bio‐Pd in a pilot scale fluidized bed reactor. Environ Chem Lett. 2011b et al (in press): doi:10.1007/s10311‐010‐0295‐x. [Google Scholar]
- Hennebel T., Van Nevel S., Verschuere S., De Corte S., De Gusseme B., Cuvelier C. Palladium nanoparticles produced by fermentatively cultivated bacteria as catalyst for diatrizoate removal with biogenic hydrogen. Appl Environ Microbiol. 2011c doi: 10.1007/s00253-011-3329-9. et al (in press). [DOI] [PubMed] [Google Scholar]
- Hoffmann J.E. Recovering platinum‐group metals from auto catalysts. J Met. 1988;40:40–44. [Google Scholar]
- Humphries A.C., Macaskie L.E. Reduction of Cr(VI) by palladized biomass of Desulfovibrio vulgaris NCIMB 8303. J Chem Technol Biotechnol. 2005;80:1378–1382. doi: 10.1002/bit.20450. [DOI] [PubMed] [Google Scholar]
- Humphries A.C., Penfold D.W., Macaskie L.E. Cr(VI) reduction by bio and bioinorganic catalysis via use of bio‐H‐2: a sustainable approach for remediation of wastes. J Chem Technol Biotechnol. 2007;82:182–189. [Google Scholar]
- Jacobsen R.T. Catalyst recovery – Part 3: removing contaminants from spent catalysts. Chem Eng Prog. 2005;101:41–43. [Google Scholar]
- Kim S.J., Oh S.D., Lee S., Choi S.H. Radiolytic synthesis of Pd‐M (M = Ag, Ni, and Cu)/C catalyst and their use in Suzuki‐type and Heck‐type reaction. J Ind Eng Chem. 2008;14:449–456. [Google Scholar]
- Kopinke F.D., Mackenzie K., Koehler R., Georgi A. Alternative sources of hydrogen for hydrodechlorination of chlorinated organic compounds in water on Pd catalysts. Appl Catal A Gen. 2004;271:119–128. [Google Scholar]
- Lengke M.F., Fleet M.E., Southarn G. Synthesis of palladium nanoparticles by reaction of filamentous cyanobacterial biomass with a palladium(II) chloride complex. Langmuir. 2007;23:8982–8987. doi: 10.1021/la7012446. [DOI] [PubMed] [Google Scholar]
- Lloyd J.R., Yong P., Macaskie L.E. Enzymatic recovery of elemental palladium by using sulfate‐reducing bacteria. Appl Environ Microbiol. 1998;64:4607–4609. doi: 10.1128/aem.64.11.4607-4609.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabbett A.N., Sanyahumbi D., Yong P., Macaskie L.E. Biorecovered precious metals from industrial wastes: single‐step conversion of a mixed metal liquid waste to a bioinorganic catalyst with environmental application. Environ Sci Technol. 2006;40:1015–1021. doi: 10.1021/es0509836. [DOI] [PubMed] [Google Scholar]
- Massard R., Uzio D., Thomazeau C., Pichon C., Rousset J.L., Bertolini J.C. Strained Pd overlayers on Ni nanoparticles supported on alumina and catalytic activity for buta‐1,3‐diene selective hydrogenation. J Catal. 2007;245:133–143. [Google Scholar]
- Mertens B., Blothe C., Windey K., De Windt W., Verstraete W. Biocatalytic dechlorination of lindane by nano‐scale particles of Pd(0) deposited on Shewanella oneidensis. Chemosphere. 2007;66:99–105. doi: 10.1016/j.chemosphere.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Mikheenko I.P., Rousset M., Dementin S., Macaskie L.E. Bioaccumulation of palladium by Desulfovibrio fructosivorans wild‐type and hydrogenase‐deficient strains. Appl Environ Microbiol. 2008;74:6144–6146. doi: 10.1128/AEM.02538-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y., Rabaey K., Rozendal R.A., Yuan Z.G., Keller J. Decolorization of azo dyes in bioelectrochemical systems. Environ Sci Technol. 2009;43:5137–5143. doi: 10.1021/es900057f. [DOI] [PubMed] [Google Scholar]
- Nutt M.O., Hughes J.B., Wong M.S. Designing Pd‐on‐Au bimetallic nanoparticle catalysts for trichloroethene hydrodechlorination. Environ Sci Technol. 2005;39:1346–1353. doi: 10.1021/es048560b. [DOI] [PubMed] [Google Scholar]
- Ogi T., Honda R., Tamaoki K., Saitoh N., Konishi Y. Direct room‐temperature synthesis of a highly dispersed Pd nanoparticle catalyst and its electrical properties in a fuel cell. Powder Technol. 2011;205:143–148. [Google Scholar]
- Okuda T., Yamashita N., Tanaka H., Matsukawa H., Tanabe K. Development of extraction method of pharmaceuticals and their occurrences found in Japanese wastewater treatment plants. Environ Int. 2009;35:815–820. doi: 10.1016/j.envint.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Putschew A., Wischnack S., Jekel M. Occurrence of triiodinated X‐ray contrast agents in the aquatic environment. Sci Total Environ. 2000;255:129–134. doi: 10.1016/s0048-9697(00)00461-7. [DOI] [PubMed] [Google Scholar]
- Rasten E., Hagen G., Tunold R. Electrocatalysis in water electrolysis with solid polymer electrolyte. Electrochim Acta. 2003;48:3945–3952. [Google Scholar]
- Redwood M.D., Deplanche K., Baxter‐Plant V.S., Macaskie L.E. Biomass‐supported palladium catalysts on Desulfovibrio desulfuricans and Rhodobacter sphaeroides. Biotechnol Bioeng. 2008;99:1045–1054. doi: 10.1002/bit.21689. [DOI] [PubMed] [Google Scholar]
- Rozendal R.A., Hamelers H.V.M., Euverink G.J.W., Metz S.J., Buisman C.J.N. Principle and perspectives of hydrogen production through biocatalyzed electrolysis. Int J Hydrogen Energy. 2006;31:1632–1640. [Google Scholar]
- Schittko S., Putschew A., Jekel M. Bank filtration: a suitable process for the removal of iodinated X‐ray contrast media? Water Sci Technol. 2004;50:261–268. [PubMed] [Google Scholar]
- Seitz W., Weber W.H., Jiang J.Q., Lloyd B.J., Maier M., Maier D., Schulz W. Monitoring of iodinated X‐ray contrast media in surface water. Chemosphere. 2006;64:1318–1324. doi: 10.1016/j.chemosphere.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Sobjerg L.S., Gauthier D., Lindhardt A.T., Bunge M., Finster K., Meyer R.L., Skrydstrup T. Bio‐supported palladium nanoparticles as a catalyst for Suzuki‐Miyaura and Mizoroki‐Heck reactions. Green Chem. 2009;11:2041–2046. [Google Scholar]
- Sodre F.F., Locatelli M.A.F., Jardim W.F. Occurrence of emerging contaminants in Brazilian drinking waters: a sewage‐to‐tap issue. Water Air Soil Pollut. 2010;206:57–67. [Google Scholar]
- Ternes T.A., Stuber J., Herrmann N., McDowell D., Ried A., Kampmann M., Teiser B. Ozonation: a tool for removal of pharmaceuticals, contrast media and musk fragrances from wastewater? Water Res. 2003;37:1976–1982. doi: 10.1016/S0043-1354(02)00570-5. [DOI] [PubMed] [Google Scholar]
- Turner J.A. Sustainable hydrogen production. Science. 2004;305:972–974. doi: 10.1126/science.1103197. [DOI] [PubMed] [Google Scholar]
- Tzitzios V.K., Georgakilas V. Catalytic reduction of N2O over Ag‐Pd/Al2O3 bimetallic catalysts. Chemosphere. 2005;59:887–891. doi: 10.1016/j.chemosphere.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Venezia A.M., Liotta L.F., Pantaleo G., La Parola V., Deganello G., Beck A. Activity of SiO2 supported gold‐palladium catalysts in CO oxidation. Appl Catal A Gen. 2003;251:359–368. et al. [Google Scholar]
- Yong P., Farr J.P.G., Harris I.R., Macaskie L.E. Palladium recovery by immobilized cells of Desulfovibrio desulfuricans using hydrogen as the electron donor in a novel electrobioreactor. Biotechnol Lett. 2002a;24:205–212. [Google Scholar]
- Yong P., Rowson N.A., Farr J.P.G., Harris I.R., Macaskie L.E. Bioreduction and biocrystallization of palladium by Desulfovibrio desulfuricans NCIMB 8307. Biotechnol Bioeng. 2002b;80:369–379. doi: 10.1002/bit.10369. [DOI] [PubMed] [Google Scholar]
- Zhang W.X., Wang C.B., Lien H.L. Treatment of chlorinated organic contaminants with nanoscale bimetallic particles. Catal Today. 1998;40:387–395. [Google Scholar]
- Zhang Y.J., Geissen S.U., Gal C. Carbamazepine and diclofenac: removal in wastewater treatment plants and occurrence in water bodies. Chemosphere. 2008;73:1151–1161. doi: 10.1016/j.chemosphere.2008.07.086. [DOI] [PubMed] [Google Scholar]


