Summary
We propose antimicrobial photodynamic therapy (aPDT) as an alternative strategy to reduce the use of antibiotics in shrimp larviculture systems. The growth of a multiple antibiotic resistant Vibrio harveyi strain was effectively controlled by treating the cells with Rose Bengal and photosensitizing for 30 min using a halogen lamp. This resulted in the death of > 50% of the cells within the first 10 min of exposure and the 50% reduction in the cell wall integrity after 30 min could be attributed to the destruction of outer membrane protein of V. harveyi by reactive oxygen intermediates produced during the photosensitization. Further, mesocosm experiments with V. harveyi and Artemia nauplii demonstrated that in 30 min, the aPDT could kill 78.9% and 91.2% of heterotrophic bacterial and Vibrio population respectively. In conclusion, the study demonstrated that aPDT with its rapid action and as yet unreported resistance development possibilities could be a propitious strategy to reduce the use of antibiotics in shrimp larviculture systems and thereby, avoid their hazardous effects on human health and the ecosystem at large.
Introduction
Antimicrobial photodynamic therapy (aPDT) is an antimicrobial strategy emerging as a propitious alternative to antibiotics in recent years (Wainwright, 1998; 2009; 2010; Lee et al., 2004; Konopka and Goslinski, 2007; Donnelly et al., 2008; Almeida et al., 2009; Garland et al., 2009; Nakonechny et al., 2010). aPDT is hypothesized to target microorganisms via photosensitized production of reactive oxygen species (Wainwright, 1998; 2010). It has been shown effective, in killing bacterial pathogens isolated from clinical settings (Komerik et al., 2003; O'Riordan et al., 2005; Nakonechny et al., 2010), fishes (Wong et al., 2005; Magaraggia et al., 2006; Almeida et al., 2009) and environmental samples (Wainwright et al., 1998; Jemli et al., 2002; Spesia et al., 2005; Caminos and Durantini, 2006). Various viral, fungal and protozoan pathogens are reportedly amenable to the reactive oxygen species generated by photosensitizers (Casteel et al., 2004; Jori and Brown, 2004; Costa et al., 2008). Reactive oxygen intermediates (ROI) can initiate a cascade of toxic reactions in microorganisms ranging from alterations of cell wall properties by lipid peroxidation and disruption of membrane bound proteins (Humpries and Sweda, 1998; Cabiscol et al., 2000; Komerik et al., 2003) to single‐ and double‐stranded breakages of DNA (Sies and Menck, 1992; Sies, 1993). Because aPDT is mediated through ROI that attack a wide range of cellular targets rapidly, the likelihood of developing resistance becomes plausibly remote. However, the type of photosensitizer used and bacterial cell wall structure strongly influences aPDT targets (Ergaieg and Seux, 2009) like poly‐l‐lysine‐chlorine (e6) (pL‐ce6) conjugate (Demidova and Hamblin, 2005). The pL‐ce6 conjugate replaces the cations in the LPS of Gram‐negative bacteria, distorting the outer membrane structure forming a channel. But the same photosensitizer because of its high molecular weight and the resulting inability to penetrate the peptidoglycan layer becomes less effective against Gram‐positive bacteria. On the contrary, Rose Bengal (RB) was found to target the cellular constituents via a diffusion controlled process rather than by active binding to the cell (Demidova and Hamblin, 2005).
Broad spectrum activity of aPDT is obligatory in aquaculture, as the aquatic environment is replete with diverse microflora both beneficial and otherwise and, rampant antibiotic application in this sector has raised serious environmental and human health concerns (Spanggaard et al., 2001; Alminov, 2009). Multiple antibiotic resistant (MAR) Vibrio harveyi has been isolated from shrimp culture systems across Asia and Latin America (Karunasagar et al., 1994; Abraham et al., 1997; Roque et al., 2001). Sixty percent of Vibrio isolated from Artemia nauplii reared in penaeid hatchery in India were resistant to erythromycin, nitrofurazone and oxytetracycline (Hameed and Balasubramanian, 2000). Besides, there exists a strong evidence towards the transfer of antibiotic resistance genes from the fish pathogen Aeromonas to human pathogen Escherichia coli (Rhodes et al., 2000a,b; Cabello, 2006; Sorum, 2006) and quinolone resistant gene from aquatic bacteria Shewanella algeae and Vibrio to human Gram‐negative pathogens (Jacoby, 2005; Poirel et al., 2005; Robicsek et al., 2005; 2006; Cabello, 2006). Ostensibly, the antibiotic resistant genes of Vibrio cholerae associated with the 1992 Latin American cholera epidemic were passed on from antibiotic resistant bacteria that emerged from large‐scale antibiotic use by the Ecuadorian shrimp industry (Weber et al., 1994; Angulo et al., 2004).
Over two decades of research has yielded a plethora of alternative methods to replace antibiotic use in aquaculture systems (Verschuere et al., 2000a; Defoirdt et al., 2007). These strategies include pre‐empting the host immune system using immunostimulants and interfering with the pathogen directly employing probiotics (Merchie et al., 1997; Verschuere et al., 2000a,b; Defoirdt et al., 2007; Anas et al., 2009; Pai et al., 2010). Despite in vitro successes with these strategies, their widespread application is limited by their inconsistency in the field and more importantly their handicap to provide protection during emergencies (Spanggaard et al., 2001; Pai et al., 2010). Emergency situations warrant strategies that directly target and reduce pathogen concentration in the system within a short time. Therefore, despite negative impacts, aquaculturists continue to use antibiotics owing to the lack of viable alternatives with rapid action to combat sudden disease outbreaks/emergencies. To address this issue, we explored the suitability of using aPDT in shrimp culture systems, as a non‐toxic and rapid alternative to antibiotics in controlling the Vibrio population. The efficiency of photodynamic therapy in controlling the growth of multiple antibiotic resistant V. harveyi strain was investigated under both in vitro and in vivo conditions using RB as the photosensitizer in an Artemia nauplii model. Also the targets of aPDT in V. harveyi were investigated using SDS and comet assay.
Results
Photosensitized killing of V. harveyi by RB
We observed a time‐dependent increase in the death rate of V. harveyi at increasing concentration of RB (Fig. 1A). Interestingly, > 50% death occurred within the first 10 min of photo excitation at 20–50 µM concentrations RB, peaking to 80–100% over 30 min. Figure 1B shows the representative images of the responses of V. harveyi to photoexcitation in the presence of 30 µM RB. Complete elimination of V. harveyi was achieved at 30 µM concentration of RB after 30 min photoexcitation. Less than 20% death of V. harveyi was observed at the highest concentration (50 µM) of RB alone (Fig. S1) or when exposed to light without photosensitizer.
Figure 1.
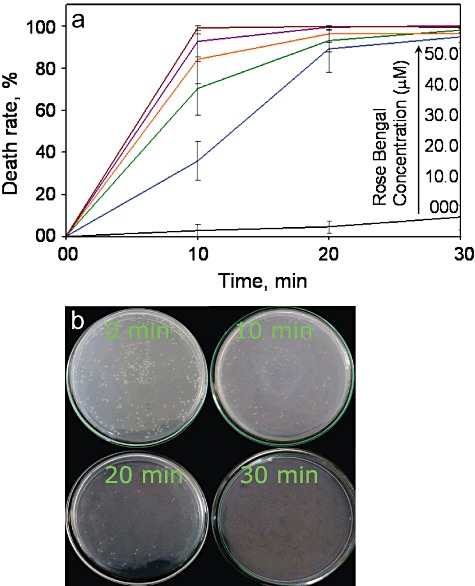
A. Death rate of V. harveyi at increasing concentrations of photosensitizer and exposure time. Values expressed as average ± SE. No significant differences between the death rates at various concentrations (P >0.05). B. Growth of V. harveyi after photoexcitation in the presence of 30 µM RB for an incubation period of 0, 10, 20 and 30 min respectively (representative plates).
Effect of aPDT on cell wall integrity and genetic stability of V. harveyi
aPDT affected both cell wall integrity and outer membrane protein profile (Omp) of V. harveyi following photosensitization. We observed a 50% decrease in the cell wall integrity of V. harveyi after 30 min of photosensitization (Fig. 2). A complete disintegration was observed in the outer membrane protein profile of V. harveyi after photosensitization. While there were three distinct bands between 29–43 kDa typical of V. harveyi (Abdallah et al., 2009) in the SDS‐PAGE of Omps extracted from V. harveyi before photosensitization, there were none after (Fig. 3A). Biochemical measurements further supported the disruption in the outer membrane integrity by showing a drastic decline in Omp (Fig. 3B).
Figure 2.
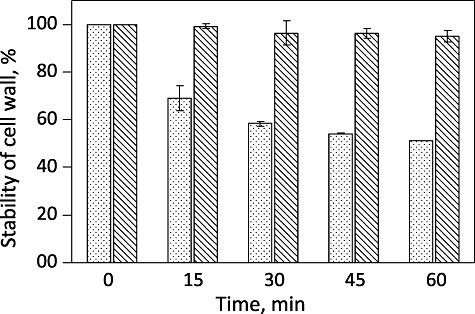
Cell wall integrity of V. harveyi upon photoexcitation in the absence (bar with line) and presence (bar filled dots) of RB. Values expressed as average ± SD.
Figure 3.
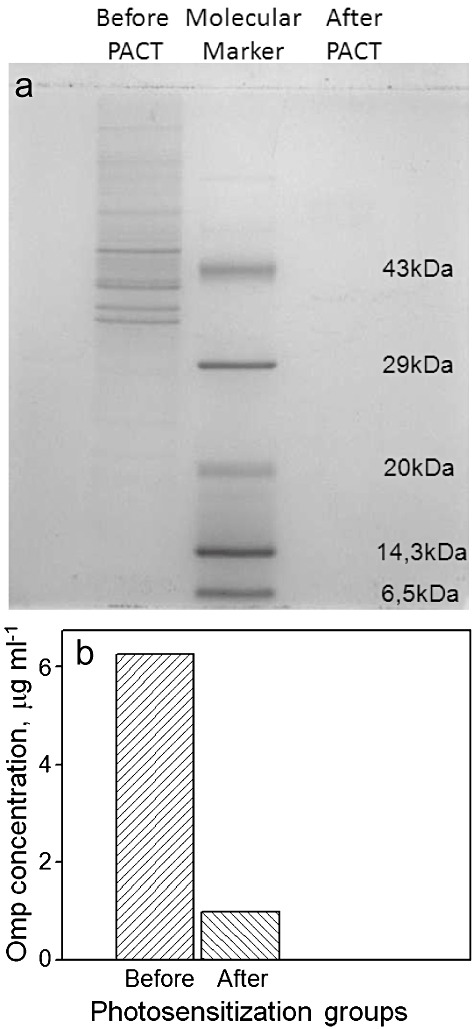
SDS‐PAGE images of profile (A) and concentration (B) of outer membrane proteins of V. harveyi cells before and after photosensitization with RB for 30 min.
We assessed DNA strand integrity of V. harveyi after photosensitization by single cell gel electrophoresis (comet assay). Surprisingly, the aPDT treatment regime employed in the study did not seem to affect DNA integrity of V. harveyi (Fig. 4). The extent of damage was assessed by classifying comets based on their tail lengths: as low or no damage (0–10 mm length), medium (10–20 mm) and heavy (> 20 mm). V. harveyi treated with 50 mM cadmium chloride was used as positive control. We observed that the photosensitization in the presence of 30 µM of RB did not induce any significant adverse affect to the genetic stability V. harveyi.
Figure 4.
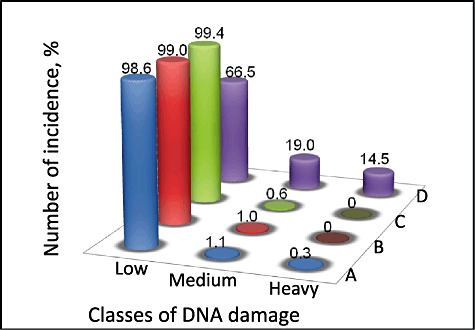
Histogram of comet assay results of V. harveyi exposed to photodynamic antimicrobial chemotherapy. Different treatment groups are V. harveyi with RB incubated under dark (A); V. harveyi exposed to light for 30 min (B); V. harveyi after APDT for 30 min (C) and positive controls (D), V. harveyi treated with 500 mM CdCl2 solution). Comets are classified based on tail length into Low (0–10 mm), Medium (10–20 mm) and Heavy (20−30 mm).
aPDT is non‐toxic to Artemia and accords protection from the pathogen V. harveyi
We first assessed the toxicity of RB with or without photosensitization in Artemia nauplii. The freshly hatched nauplii were exposed to different concentrations of RB for 180 min in the presence and absence of light. The nauplii were considered dead if no appendage movement was observed and survival was calculated from the average number of live nauplii per total count. We found that RB at all concentrations, in the presence or absence of light is not toxic to Artemia nauplii (Fig. 5). Separately, when the nauplii were exposed to the pathogen, treated with RB and photosensitized, total bacterial population significantly reduced, especially those of vibrios (Fig. 6). It was observed that, 78.9% of the total heterotrophic bacterial population was reduced in Artemia nauplii exposed to aPDT. More interestingly, 91.2% of the Vibrio populations were removed from Artemia nauplii on aPDT. This indicated that aPDT was effective in killing the pathogen under in vivo conditions as well, without deleterious effects on Artemia nauplii.
Figure 5.
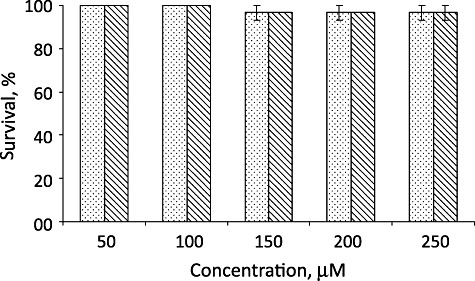
Survival of Artemia nauplii after exposure to different concentration of RB in the absence (bar filled with dots) and presence (Bar with slanting line) of light for 180 min. Values expressed as average ± SE.
Figure 6.
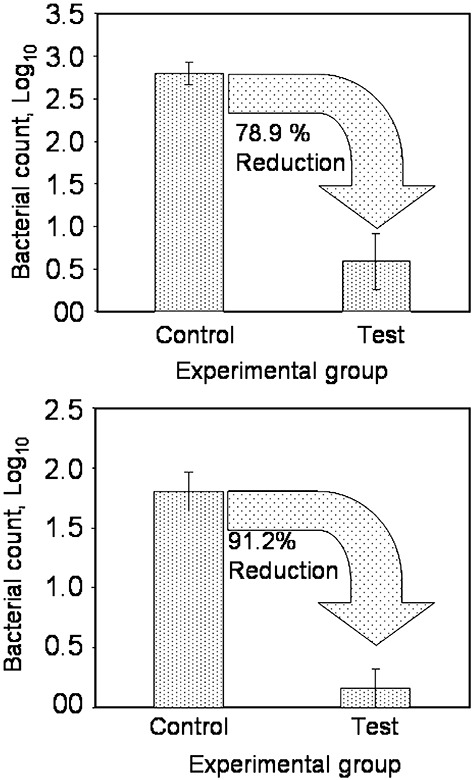
Total heterotrophic bacteria (A) and vibrio (B) population in Artemia nauplii, before (control) and after (test) photodynamic antimicrobial chemotherapy.
Discussion
Continuous evolution of bacterial pathogens demands a constant search for novel strategies to counter their threat to humans and animals alike. In this study we explored aPDT as an alternative strategy to counter pathogen infestations in shrimp larval rearing systems using a V. harveyi‐Artemia model. A commonly available inexpensive photosensitizer, RB was employed and its efficacy studied against a pathogenic, multiple antibiotic resistant V. harveyi strain. RB was selected as the photosensitizer based on its absorption in visible region, water solubility, high singlet oxygen quantum yield and inexpensiveness (Chang et al., 2008). Although, Gram‐negative bacteria are more susceptible to the cationic photosensitizer methylene blue, its high toxicity to Artemia salina even in the absence of light precludes its biological use (Peloi et al., 2008). Artemia nauplii are widely used as live feed in shrimp larval rearing systems, and reportedly an important route of pathogen entry (Triantaphyllidis et al., 1998; Lavens and Sorgeloos, 2000; Sorgeloos et al., 2001; Vaseeharan and Ramasamy, 2003). Therefore, producing pathogen free Artemia without antibiotic administration would plug not only a major pathogen entry point but also prevent antibiotic use in shrimp larval rearing systems. We observed that a photoexcitation for 30 min in the presence of 30 µM concentration of RB reduced the pathogen load without inducing toxicity to Artemia nauplii (Fig. 1) and the efficiency of aPDT increases with time of photoexcitation. Agreeably, an ε of 50% was achieved within the first 10 min of photosensitization of RB treated bacteria except, in those treated with 10 µM concentration. In contrast, both the highest concentrations of photosensitizer (RB) used or light alone independently could only induce a death rate of < 20%. This confirmed that the bacterial mortality obtained was indeed due to the photosensitizing effect of RB. Because total bacterial removal from the system may be detrimental to the remineralization capacities of a closed system (Riemann and Azam, 2002), the achievement of pathogen reduction at 30 µM concentration assumes significance. It may also be noted that RB at lower concentrations is non‐toxic, evidenced by its use in opthalmologic diagnosis (Paugh, 2008). In contrast, its higher concentrations can induce both apoptotic and non‐apoptotic cell deaths in different melanoma cells (Mousavi and Hersey, 2007) and inhibit P‐glycoprotein activities (Mizutani, 2009). Here in our observations, RB at 30 µM concentration resulted in > 80% death rate within 10 min. Such a reduction in bacterial load should be sufficient to prevent larval infections in hatcheries without overtly unbalancing the larval mesocosm.
To shed light on the targets of aPDT on V. harveyi, we used SDS and single cell gel electrophoresis assay (Comet assay). The continuous monitoring of incubation temperature (28 ± 1°C) during the 30 min photoexcitation ruled out possible thermal shock. In the SDS assay, the effect of aPDT on bacterial cell wall integrity is represented as a function of their sensitivity to detergent mediated cell lysis. The outer membrane if already damaged, when treated with a detergent such as sodium lauryl sulfate, will show a sharp decline in the optical density (OD600) due to leakage of cell content resulting in bacterial death (Lok et al., 2006). In the absence of any membrane damage, the optical density will not show sharp changes.
We confirmed that the aPDT principally impacts the cell wall integrity of V. harveyi. The cell wall integrity of V. harveyi decreased by 50% in 30 min of aPDT. This effect was confirmed by extracting and quantifying the outer membrane proteins (Omps) of V. harveyi. Omps are located on the external surface of Gram‐negative bacteria in tight association with the peptidoglycan layer and play a key role in bacterial adaptation to environmental changes. The protein bands specific for Omps appeared in the SDS‐PAGE of untreated V. harveyi cells, whereas the aPDT‐treated cells did not yield any specific bands because of the drastic reduction in the concentration of Omps in these cells. The Omp concentration of control and aPDT‐treated samples were found to be 6.25 and 1 µg ml−1 respectively. Omps damage extends the access of the photosensitizer, Toludine blue, to inner membrane, making it more destructive (Sahu et al., 2009). Sahu and colleagues (2009) suggested the possibility of total destruction of the cell wall including the inner membrane of E. coli on aPDT (Sahu et al., 2009). They reported the appearance of deep grooves (∼ 200 nm) on the surface of E. coli at higher light doses leading to the leakage of the intracellular contents and concomitant cell flattening. This also correlates with the decreased absorbance in SDS assay. Studies by Tegos and Hamblin (2006) have demonstrated that RB and light probably kill bacteria by generating extracellular singlet oxygen that destroys the membrane from the outside in. Recently, Jin and colleagues (2010) demonstrated the photosensitized damage of cell wall of Gram‐negative and Gram‐positive bacteria using the photosensitizer haematoporphyrin monomethyl ether by atomic force microscopy.
Rose Bengal used in the present study is a standard photosensitizer, coming under the xanthene group of dyes (Wainwright, 1998). In standard photodynamic reactions, RB molecules absorb 450–580 nm wavelength light from a halogen lamp and are excited into a singlet state (1RB) with around 10−8 s lifetime. The excited molecules transfer energy to molecular oxygen (3O2) present in the proximity, leading to the generation of singlet oxygen (1O2) and initiate a cascade of energy transfer reactions and intermediates, which are collectively named as ROI (Banks et al., 1985). ROI induce a series of toxic reactions in living cells, including DNA damage and breakage, lipid and protein denaturation (Maisch et al., 2005). RB being an exogenous free dye enters the cell by diffusion‐controlled process rather than by active binding to the cell (Demidova and Hamblin, 2005). Therefore, the primary site of action of RB is the cell surface rather than the genetic material of V. harveyi. This was confirmed with comet assay result, where we observed neither medium nor heavy damage of DNA in aPDT‐treated cells. Reactive oxygen species, the main destructive force in aPDT, has a life‐time of ∼ 1–3 µs in water, ∼ 100 ns in lipid regions of cell membrane, and ∼ 250 ns in cytoplasm (Pandey and Zheng, 2000). Therefore, the diffusion range of singlet oxygen is predicted to be limited to approximately 45 nm in cellular media (Pandey and Zheng, 2000). This short lifetime and narrow diffusion range do not permit the ROI generated on the surface of bacteria to interact/destruct the genetic material of V. harveyi. Therefore, we attribute the photodynamic killing of V. harveyi to the cell wall disintegration by the reactive oxygen species produced upon photoexcitation of RB.
We evaluated the possibility of reducing bacterial pathogen entry into shrimp larval rearing systems by subjecting Artemia nauplii, a principle entry route of pathogens, to aPDT. Artemia, a crustacean, is a major live feed organism particularly in shrimp larval rearing. We exposed Artemia nauplii challenged with V. harveyi to aPDT and observed that the Vibrio population in aPDT‐treated animals reduced by ∼ 91% in 30 min. It may be noted that while Vibrio spp. are pathogenic to shrimp larvae, their absence too can negatively impacts larval survival because they perform vital role in the transformation of organic matter (Pruzzo et al., 2005). The photodynamic therapy described here reduces the total heterotrophic bacterial load of the system whereby making it a promising application in the closed shrimp larval rearing system. We believe that the rapid removal of pathogens is most important in larval rearing systems of shrimps as total mortality of these animals may set‐in within a short time of infection (Chen, 1992). Although many alternative strategies such as probiotics, immunostimulants and water quality management aim to restrict Vibrio population in larval rearing systems, all fail to respond to infections that have already set‐in forcing aquaculturists to depend on antibiotics for immediate resolution. However, the deleterious effects of residual antibiotics on humans and environment are more alarming and require immediate attention. Many countries have already banned/restricted the application of antibiotics in aquaculture and multilevel monitoring of the produce for antibiotic residues prevents antibiotic‐containing products from reaching consumers (Johnston and Santillo, 2002).
In this backdrop, the present study proposes the introduction of aPDT as an alternative strategy for negating/minimizing the use of antibiotics in shrimp larval rearing systems to control disease outbreaks. The advantages of aPDT, compared with the other alternative disease control strategies such as probiotics or immunostimulants, are its fast action and virtually no possibility of resistance development. Recurrence of bacterial diseases in high‐volume commercial tanks demands repetitive applications of excessive antibiotics. Maybe the high‐volume larval rearing tanks complicate the use of aPDT in its present capacity; nevertheless, the technique can easily be adapted when animals are being transferred from one tank to another, when volumes are considerably lower. During the transfer process, the larvae can be kept in small volumes of water for 30–60 min and exposed to aPDT. The RB containing water then becomes amenable to small scale biological or chemical treatments before discharge (Parham et al., 2011). aPDT could be very useful in the prevention of infections occurring through live feed such as Artemia, which can be treated in this manner before feeding. aPDT could also aid in the lowering of bacterial load of post larvae for transport to distant grow‐out farms.
Experimental procedures
Bacteria, Artemia and Photoexcitation conditions
Vibrio harveyi (MCCB 111) was obtained from the culture collection of National Centre for Aquatic Animal Health (NCAAH), Cochin University of Science and Technology, Cochin India. The strain was previously isolated from moribund Penaeus monodon larvae. V. harveyi was inoculated into ZoBell's marine broth prepared in 15 gl−1 seawater and grown overnight at 28 ± 1°C on a shaker at 120 r.p.m. Working cultures of V. harveyi was maintained on ZoBell's marine agar slants, subcultured every 2–3 weeks. The purity of the culture was confirmed by Gram staining and biochemical tests.
Gnotobiotic Artemia was prepared following the method of Sorgeloos and colleagues (1977). Artemia cysts (0.5 g) were hydrated in 45 ml tap water and kept in continuous suspension by sparging filter sterilized air from bottom. All materials used for rearing gnotobiotic artemia were previously sterilized. After 1 h, the suspension was diluted with an equal volume of sodium hypochlorite and incubated for 5–7 min. The decapsulated cysts were filtered through 100 µ sieve and washed thoroughly with sterile seawater to remove residual chlorine. Artemia nauplii thus reared were collected in a 120 µ sieve, washed thoroughly with sterile seawater, placed in a Petri dish with 10 ml seawater and treated with chloramphenicol (1 gl−1) for 30 min.
All samples for photoexcitation were placed under a 150 W halogen lamp with an output spectrum ranging from 450 to 600 nm. Samples (bacteria or artemia) were maintained in borosilicate glass vials with PTFE‐lined caps at a distance of 50 cm from the halogen lamp and shaken intermittently to negate any heating effect.
In vitro experiments
Bacterial killing assay. Borosilicate glass vials were filled with 106 cfu ml−1 of V. harveyi in 2 ml PBS (pH 7.3 ± 0.2) and 0–50 µM concentrations of RB in triplicate. Light controls were devoid of RB while dark controls were one set of all combinations aluminium foil double‐wrapped. The efficiency of photodynamic antimicrobial chemotherapy was expressed as percent death rate (Zheng and Zhu, 2003). One hundred microlitres of sample was retrieved from all vials at 10 min intervals and spread over the surface of ZoBell's marine agar plates in duplicates, incubated at 28 ± 1°C for 24 h before enumerating the total number of colonies. Death rate ε was calculated in comparison with light control using the following equation (Zheng and Zhu, 2003).
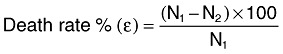 |
Where, N1 and N2 are the number of colonies developed on the control and experimental plate respectively.
Effect of aPDT on cell wall integrity. The effect of aPDT on cell wall integrity of V. harveyi was investigated by SDS assay following the method of Lok and colleagues (2006). The assay was repeated three times with duplicate tubes at each time. Briefly, overnight cultures of V. harveyi cells were washed copiously with sterile phosphate‐buffered saline (8 g NaCl, 0.2 g KCl and 1.44 g KH2PO4 prepared in distilled water 1 l, pH 7.4) and dispensed in sterile quartz cuvettes to a volume of 2 ml. After measuring the initial absorbance, the test solution was supplemented with RB (30 µM) and SDS (0.1%). A control cuvette without RB was maintained. The cuvettes were exposed to light for 60 min and the absorbance at 600 nm was recorded at every 15 min. The percentage decrease in absorbance compared with the initial reading was plotted against time.
The outer membrane protein profile of control and aPDT‐treated V. harveyi cells were evaluated following standard protocols (Laemmli, 1970; Sanderma and Stroming, 1972). The outer membrane protein was extracted according to Sabri and colleagues (2000) and Deb and colleagues (1995) with slight modifications. The control and aPDT‐treated cells of V. harveyi were washed copiously with sterile saline and resuspended in PBS containing 5 mM phenylmethylsulfonyl fluoride (PMSF). Cells were disrupted in an ultrasound sonicator by a number of 40 s pulses until the suspension became translucent. Unbroken cells and cellular debris were removed by centrifugation at 5000 g for 20 min. The pellet discarded, the supernatant collected was centrifuged at 100 000 g in an ultracentrifuge (Beckman Coulter) for 40 min at 4°C. The supernatant discarded, the pellet was resuspended in 2 vol of 2% (w/v) SDS, incubated at room temperature for 1 h and centrifuged at 100 000 g for 40 min at 4°C. This pellet was resuspended in saline and stored at −20°C until use. Outer membrane proteins (Omp) were analysed by SDS‐PAGE (Laemmli, 1970) with 15 % acrylamide in the separating gel and 5% acrylamide in the stacking gel. The proteins were visualized by staining with 0.2 % Coomassie Brilliant Blue G250. The Omp concentration was quantified against BSA as the standard using a spectrophotometer (Sanderma and Stroming, 1972).
Effect of aPDT on genetic stability. The effect of aPDT on the stability of genetic material was investigated by comet assay following the method of Singh and colleagues (1999). Briefly, 106 bacterial cells were mixed with 500 µl of 0.5 % low melting point agarose prepared in TAE buffer containing RNase (5 µg ml−1), SDS (10 %) and lysozyme (0.5 mg ml−1). Bacterial cells impregnated in agarose solution were layered over a microscopic slide pre‐coated with a thin film of 0.5% agarose solidified by incubating at 4°C for 30 min. Slides were then incubated at 37°C for 30 min and cells lysed by immersing in lysis solution containing NaCl (0.15 M), Tris (10 mM, pH 10), EDTA (100 mM), Triton × 100 (1 %) for 1 h at 37°C. The slides were again immersed in a solution containing NaCl (0.15 M), Tris (10 mM, pH 7.4), and EDTA (10 mM) and proteinase K (1 mg ml−1) for 2 h at 37°C to digest cellular proteins. The slides were equilibrated with 300 mM sodium acetate and electrophoresed at 50 V for 15–20 min. Following electrophoresis, the slides were immersed in 1 M ammonium acetate in ethanol for 30 min, then in absolute ethanol for 1 h, air dried at 25°C, immersed in 70% ethanol for 30 min and air dried. Slides were stained with 1 ml freshly prepared solution of 1 µl SyBr green in TE buffer. The comets were observed under a fluorescent microscope (Olympus BX 51) equipped with dichroic filter pairs (Excitation filter: 470–490 nm, Emission filter 520 nm, Dichroic 500 nm) and a CCD camera. The comet length was measured and processed using the software Image Pro. The nucleic acids were classified based on comet length as low or no damage (0–10 mm length), medium (10–20 mm) and heavy (> 20 mm) damaged groups and expressed as % number of incidence in the histogram. The assay was repeated three times with duplicate tubes at each time.
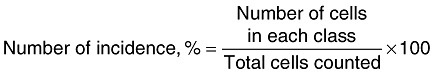 |
In vivo experiments
Evaluation of the toxicity of RB to Artemia nauplii. Gnotobiotic Artemia prepared as mentioned earlier were dispensed in the wells of a 24‐well microplate containing 2 ml sterile seawater at a density of 10 animals per well. RB in 0–250 µM concentrations range was added to the wells and the plate exposed to light for 180 min. A set of each was maintained without exposure to light as dark control. Following the incubation, differential counts of live and dead Artemia nauplii were recorded. All the combinations were in quadruplicate.
Efficiency of aPDT in eliminating V. harveyi from Artemia nauplii rearing system. Ten individuals of gnotobiotic Artemia were deposited in Petri dishes filled with 10 ml seawater containing 105 cfu ml−1V. harveyi and incubated for 3 h following which the animals were washed with sterile seawater and divided into two groups designated as control and test. The test groups were subjected for aPDT by exposing to light for 30 min in the presence of RB (30 µM). Both the groups were transferred into microcentrifuge tubes and homogenized with a micropestle for evaluating the total heterotrophic bacteria and Vibrio populations. One hundred microlitres of the homogenate was plated on ZoBell's marine and TCBS agar plates respectively and colonies counted after 24 h incubation at 28°C. The experiments were repeated thrice. Smooth, opaque and thin‐edged fermentative or non‐fermentative colonies on TCBS agar were picked and confirmed as Vibrio by assessing cytochrome c oxidase activity, glucose dissimilation and sensitivity to the vibriostatic agent 2,2‐diamine‐6,7‐diisopropylpteridine phosphate (O/129). Pseudomonas gives oxidation reaction to glucose while Aeromonas and Vibrio are fermentative. Aeromonas spp. are generally resistant to O/129 while Vibrio are sensitive.
Statistical analysis
Differences in the death rate of V. harveyi on photoexcitation with different concentrations of RB were analysed using one way anova. A significance level P < 0.05 was considered (Bailey, 1995).
Acknowledgments
I.S.B. and A.A. acknowledge Department of Biotechnology (DBT), Govt of India for financial assistance (BT/HRD/01/01/2004). A.A. is thankful to Director, NIO and Scientist‐in‐Charge NIO‐RC Cochin. Aparna and Esha are thankful to DBT for their M. Tech. fellowship. C.J. is a recipient of Dr D.S. Kothari Postdoctoral Fellowship, University Grants Commission. We thank three anonymous reviewers for their constructive criticisms that improved the manuscript. This is also an NIO contribution, No. 5050.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Death rate of V. harveyi at increasing concentrations of photosensitizer alone at different time intervals.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Abdallah F., Kallel H., Bakhrouf A. Enzymatic, outer membrane proteins and plasmid alterations of starved Vibrio parahaemolyticus and Vibrio alginolyticus cells in seawater. Arch Microbiol. 2009;191:493–500. doi: 10.1007/s00203-009-0477-8. [DOI] [PubMed] [Google Scholar]
- Abraham T.J., Manley R., Palaniappan R., Dhevendaran K. Pathogenicity and antibiotic sensitivity of luminous Vibrio harveyi isolated from diseased penaeid shrimp. J Aquacult Trop. 1997;12:1–8. [Google Scholar]
- Almeida A., Cunha A., Gomes N.C.M., Alves E., Costa L., Faustino M.A.F. Phage Therapy and Photodynamic Therapy: Low Environmental Impact Approaches to Inactivate Microorganisms in Fish Farming Plants. Mar Drugs. 2009;7:268–313. doi: 10.3390/md7030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alminov R.I. The role of antibiotics and antibiotic resistance in nature. Environ Microbiol. 2009;11:2970–2988. doi: 10.1111/j.1462-2920.2009.01972.x. [DOI] [PubMed] [Google Scholar]
- Anas A., Lowman D.W., Williams D.L., Millen S., Pai S.S., Sajeevan T.P. Alkali insoluble glucan extracted from Acremonium diospyri is a more potent immunostimulant in the Indian White Shrimp, Fenneropenaeus indicus than alkali soluble glucan. Aquac Res. 2009;40:1320–1327. et al. [Google Scholar]
- Angulo F.J., Nargund V.N., Chiller T.C. Evidence of an association between use of anti‐microbial agents in food animals and anti‐microbial resistance among bacteria isolated from humans and the human health consequences of such resistance. J Vet Med B Infect Dis Vet Public Health. 2004;51:374–379. doi: 10.1111/j.1439-0450.2004.00789.x. [DOI] [PubMed] [Google Scholar]
- Bailey N. Cambridge University Press; 1995. [Google Scholar]
- Banks J.G., Board R.G., Carter J., Dodge A.D. The cytotoxic and photodynamic inactivation of microorganisms by RoseBengal. J Appl Bacteriol. 1985;58:391–400. doi: 10.1111/j.1365-2672.1985.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Cabello F.C. Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ Microbiol. 2006;8:1137–1144. doi: 10.1111/j.1462-2920.2006.01054.x. [DOI] [PubMed] [Google Scholar]
- Cabiscol E., Tamarit J., Ros J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int Microbiol. 2000;3:3–8. [PubMed] [Google Scholar]
- Caminos D.A., Durantini E.N. Photodynamic inactivation of Escherichia coli immobilized on agar surfaces by a tricationic porphyrin. Bioorg Med Chem. 2006;14:4253–4259. doi: 10.1016/j.bmc.2006.01.058. [DOI] [PubMed] [Google Scholar]
- Casteel M.J., Jayaraj K., Gold A., Ball L.M., Sobsey M.D. Photoinactivation of hepatitis A virus by synthetic porphyrins. Photochem Photobiol. 2004;80:294–300. doi: 10.1562/2004-04-05-RA-134. [DOI] [PubMed] [Google Scholar]
- Chang C.‐C., Yang Y.‐T., Yang J.‐C., Wu H.‐D., Tsai T. Absorption and emission spectral shifts of rose bengal associated with DMPC liposomes. Dyes Pigments. 2008;79:170–175. [Google Scholar]
- Chen D. An overview of the disease situation, diagnostic techniques, treatments and preventives used on shrimp farms in China. In: Fulks W., Main K.L., editors. The Oceanic Institute; 1992. pp. 47–56. [Google Scholar]
- Costa L., Alves E., Carvalho C.M.B., Tome J.P.C., Faustino M.A.F., Neves M. Sewage bacteriophage photoinactivation by cationic porphyrins: a study of charge effect. Photochem Photobiol Sci. 2008;7:415–422. doi: 10.1039/b712749a. et al. [DOI] [PubMed] [Google Scholar]
- Deb A., Bhattacharyya D., Das J. A 25‐Kda Beta‐Lactam‐Induced Outer‐Membrane Protein of Vibrio‐Cholerae – Purification and Characterization. J Biol Chem. 1995;270:2914–2920. doi: 10.1074/jbc.270.7.2914. [DOI] [PubMed] [Google Scholar]
- Defoirdt T., Boon N., Sorgeloos P., Verstraete W., Bossier P. Alternatives to antibiotics to control bacterial infections: luminescent vibriosis in aquaculture as an example. Trends Biotechnol. 2007;25:472–479. doi: 10.1016/j.tibtech.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Demidova T.N., Hamblin M.R. Effect of cell‐photo sensitizer binding and cell density on microbial photoinactivation. Antimicrob Agents Chemother. 2005;49:2329–2335. doi: 10.1128/AAC.49.6.2329-2335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly R.F., McCarron P.A., Tunney M.M. Antifungal photodynamic therapy. Microbiol Res. 2008;163:1–12. doi: 10.1016/j.micres.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Ergaieg K., Seux R. A comparative study of the photoinactivation of bacteria by meso‐substituted cationic porphyrin, rose Bengal and methylene blue. Desalination. 2009;248:32–41. [Google Scholar]
- Garland M.J., Cassidy C.M., Woolfson D., Donnelly R.F. Designing photosensitizers for photodynamic therapy: strategies, challenges and promising developments. Future Med Chem. 2009;1:667–691. doi: 10.4155/fmc.09.55. [DOI] [PubMed] [Google Scholar]
- Hameed A.S.S., Balasubramanian G. Antibiotic resistance in bacteria isolated from Artemia nauplii and efficacy of formaldehyde to control bacterial load. Aquaculture. 2000;183:195–205. [Google Scholar]
- Humpries K.M., Sweda L.I. Selective inactivation of α‐ketoglutarate dehydrogenase: reaction of lipoic acid with 4‐hydroxy‐2‐nonenal. Biochemistry. 1998;37:15835–15841. doi: 10.1021/bi981512h. [DOI] [PubMed] [Google Scholar]
- Jacoby G.A. Mechanisms of resistance to quinolones. Clin Infect Dis. 2005;41:S120–S126. doi: 10.1086/428052. [DOI] [PubMed] [Google Scholar]
- Jemli M., Alouini Z., Sabbahi S., Gueddari M. Destruction of fecal bacteria in wastewater by three photosensitizers. J Environ Monit. 2002;4:511–516. doi: 10.1039/b204637g. [DOI] [PubMed] [Google Scholar]
- Jin H., Huang X., Chen Y., Zhao H., Ye H., Huang F. Photoinactivation effects of hematoporphyrin monomethyl ether on Gram‐positive and ‐negative bacteria detected by atomic force microscopy. Appl Microbiol Biotechnol. 2010;88:761–770. doi: 10.1007/s00253-010-2747-4. et al. [DOI] [PubMed] [Google Scholar]
- Johnston P., Santillo D. 2002.
- Jori G., Brown S.B. Photosensitized inactivation of microorganisms. Photochem Photobiol Sci. 2004;3:403–405. doi: 10.1039/b311904c. [DOI] [PubMed] [Google Scholar]
- Karunasagar I., Pai R., Malathi G.R., Karunasagar I. Mass Mortality of Penaeus‐Monodon Larvae Due to Antibiotic‐Resistant Vibrio‐Harveyi Infection. Aquaculture. 1994;128:203–209. [Google Scholar]
- Komerik N., Nakanishi H., MacRobert A.J., Henderson B., Speight P., Wilson M. In vivo killing of Porphyromonas gingivalis by toluidine blue‐mediated photosensitization in an animal model. Antimicrob Agents Chemother. 2003;47:932–940. doi: 10.1128/AAC.47.3.932-940.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka K., Goslinski T. Photodynamic therapy in dentistry. J Dent Res. 2007;86:694–707. doi: 10.1177/154405910708600803. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of Structural Proteins During Assembly of Head of Bacteriophage‐T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lavens P., Sorgeloos P. The history, present status and prospects of the availability of Artemia cysts for aquaculture. Aquaculture. 2000;181:397–403. [Google Scholar]
- Lee C.F., Lee C.J., Chen C.T., Huang C.T. delta‐Aminolaevulinic acid mediated photodynamic antimicrobial chemotherapy on Pseudomonas aeruginosa planktonic and biofilm cultures. J Photochem Photobiol B. 2004;75:21–25. doi: 10.1016/j.jphotobiol.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Lok C.‐N., Ho C.‐M., Chen R., He Q.‐Y., Yu W.‐Y., Sun H. Proteomic Analysis of the Mode of Antibacte rial Action of Silver Nanoparticles. J Proteome Res. 2006;5:916–924. doi: 10.1021/pr0504079. et al. [DOI] [PubMed] [Google Scholar]
- Magaraggia M., Faccenda F., Gandolfi A., Jori G. Treatment of microbiologically polluted aquaculture waters by a novel photochemical technique of potentially low environmental impact. J Environ Monit. 2006;8:923–931. doi: 10.1039/b606975d. [DOI] [PubMed] [Google Scholar]
- Maisch T., Bosl C., Szeimies R.M., Lehn N., Abels C. Photodynamic effects of novel XF porphyrin derivatives on prokaryotic and eukaryotic cells. Antimicrob Agents Chemother. 2005;49:1542–1552. doi: 10.1128/AAC.49.4.1542-1552.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchie G., Lavens P., Sorgeloos P. Optimization of dietary vitamin C in fish and crustacean larvae: a review. Aquaculture. 1997;155:165–181. [Google Scholar]
- Mizutani T. Toxicity of xanthene food dyes by inhibition of human drug‐metabolizing enzymes in a noncompetitive manner. J Environ Public Health. 2009;2009:1–9. doi: 10.1155/2009/953952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi S.H., Hersey P. Role of caspases and reactive oxygen species in rose Bengal‐induced toxicity in melanoma cells. Iran J Basic Med Sci. 2007;10:118–123. [Google Scholar]
- Nakonechny F., Firer M.A., Nitzan Y., Nisnevitch M. Intracellular antimicrobial photodynamic therapy: a novel technique for efficient eradication of pathogenic bacteria. Photochem Photobiol. 2010;86:1350–1355. doi: 10.1111/j.1751-1097.2010.00804.x. [DOI] [PubMed] [Google Scholar]
- O'Riordan K., Akilov O.E., Hasan T. The potential for photodynamic therapy in the treatment of localized infections. Photodiagn Photodyn Ther. 2005;2:247–262. doi: 10.1016/S1572-1000(05)00099-2. [DOI] [PubMed] [Google Scholar]
- Pai S.S., Anas A., Jayaprakash N.S., Priyaja P., Sreelakshmi B., Preetha R. Penaeus monodon larvae can be protected from Vibrio harveyi infection by pre‐emptive treatment of a rearing system with antagonistic or non‐antagonistic bacterial probiotics. Aquac Res. 2010;41:847–860. et al. [Google Scholar]
- Pandey R.K., Zheng G. Pophyrins as photosensitizers in photodynamic therapy. In: Kadish K.M., Smith K.M., Guilarf R., editors. Academic Press; 2000. p. 341. [Google Scholar]
- Parham H., Zargar B., Heidari Z., Hatamie A. Magnetic solid‐phase extraction or rose bengal using iron oxide nanoparticles modified with cetyltrimethylammonium bromide. J Iran Chem Soc. 2011;8:S9–S16. [Google Scholar]
- Paugh J.R. Dyes. In: Bartlett J.D., Jaanus S.D., editors. Elsevier Health Sciences; 2008. pp. 283–295. [Google Scholar]
- Peloi L.S., Soares R.R.S., Biondo C.E.G., Souza V.R., Hioka N., Kimura E. Photodynamic effect of light‐emitting diode light on cell growth inhibition induced by methylene blue. J Biosci. 2008;33:231–237. doi: 10.1007/s12038-008-0040-9. [DOI] [PubMed] [Google Scholar]
- Poirel L., Liard A., Rodriguez‐Martinez J.M., Nordmann P. Vibrionaceae as a possible source of Qnr‐like quinolone resistance determinants. J Antimicrob Chemother. 2005;56:1118–1121. doi: 10.1093/jac/dki371. [DOI] [PubMed] [Google Scholar]
- Pruzzo C., Huq A., Colwell R.R., Donelli G. Pathogenic Vibrio species in the marine and estuarine environment. In: Belkin S.S., Colwell R.R., editors. Springer; 2005. pp. 217–252. [Google Scholar]
- Rhodes G., Huys G., Swings J., McGann P., Hiney M., Smith P., Pickup R.W. Distribution of oxytetracycline resistance plasmids between aeromonads in hospital and aquaculture environments: Implication of Tn1721 in dissemination of the tetracycline resistance determinant Tet A. Appl Environ Microbiol. 2000a;66:3883–3890. doi: 10.1128/aem.66.9.3883-3890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes L.D., Grayson T.H., Alexander S.M., Strom M.S. Description and characterization of IS994, a putative IS3 family insertion sequence from the salmon pathogen, Renibacterium salmoninarum. Gene. 2000b;244:97–107. doi: 10.1016/s0378-1119(99)00573-9. [DOI] [PubMed] [Google Scholar]
- Riemann L., Azam F. Widespread N‐acetyl‐D‐glucosamine uptake among pelagic marine bacteria and its ecological implications. Appl Environ Microbiol. 2002;68:5554–5562. doi: 10.1128/AEM.68.11.5554-5562.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robicsek A., Sahm D.F., Strahilevitz J., Jacoby G.A., Hooper D.C. Broader distribution of plasmid‐mediated quinolone resistance in the United States. Antimicrob Agents Chemother. 2005;49:3001–3003. doi: 10.1128/AAC.49.7.3001-3003.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robicsek A., Strahilevitz J., Jacoby G.A., Macielag M., Abbanat D., Park C.H. Fluoroquinolone‐modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nat Med. 2006;12:83–88. doi: 10.1038/nm1347. et al. [DOI] [PubMed] [Google Scholar]
- Roque A., Molina‐Aja A., Bolan‐Mejia C., Gomez Gil B. In vitro susceptiblity to 15 antibiotics of vibrios isolated from peaneid shrimps in Northwestern Mexico. Int J Antimicrob Agents. 2001;17:383–387. doi: 10.1016/s0924-8579(01)00308-9. [DOI] [PubMed] [Google Scholar]
- Sabri M.Y., Zamri‐Saad M., Mutalib A.R., Israf D.A., Muniandy N. Efficacy of an outer membrane protein of Pasteurella haemolytica A2, A7 or A9‐enriched vaccine against intratracheal challenge exposure in sheep. Vet Microbiol. 2000;73:13–23. doi: 10.1016/s0378-1135(99)00205-9. [DOI] [PubMed] [Google Scholar]
- Sahu K., Bansal H., Mukherjee C., Sharma M., Gupta P.K. Atomic force microscopic study on morphological alterations induced by photodynamic action of Toluidine Blue O in Staphylococcus aureus and Escherichia coli. J Photochem Photobiol B. 2009;96:9–16. doi: 10.1016/j.jphotobiol.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Sanderma H., Stroming J. Biosynthesis of peptidoglycan of bacterial cell‐wall .27. Purification and properties of C55‐isoprenoid alcohol phosphokinase from Staphylococcus aureus. J Biol Chem. 1972;247:5123–5131. [PubMed] [Google Scholar]
- Sies H. Damage to plasmid DNA by singlet oxygen and its protection. Mutat Res. 1993;299:183–191. doi: 10.1016/0165-1218(93)90095-u. [DOI] [PubMed] [Google Scholar]
- Sies H., Menck C.F. Singlet oxygen induced DNA damage. Mutat Res. 1992;275:367–375. doi: 10.1016/0921-8734(92)90039-r. [DOI] [PubMed] [Google Scholar]
- Singh N.P., Stephens R.E., Singh H., Lai H. Visual quantification of DNA double‐strand breaks in bacteria. Mutat Res. 1999;429:159–168. doi: 10.1016/s0027-5107(99)00124-4. [DOI] [PubMed] [Google Scholar]
- Sorgeloos P., Bossuyt E., Laviña E., Baeza‐Mesa M., Persoone G. Decapsulation of Artemia cysts: a simple technique for the improvement of the use of brine shrimp in aquaculture. Aquaculture. 1977;12:311–315. [Google Scholar]
- Sorgeloos P., Dhert P., Candreva P. Use of the brine shrimp, Artemia spp., in marine fish larviculture. Aquaculture. 2001;200:147–159. [Google Scholar]
- Sorum H. Antimicrobial drug resistance in fish pathogens. In: Aarestrup F.M., editor. American Society for Microbiology; 2006. pp. 213–238. [Google Scholar]
- Spanggaard B., Huber I., Nielsen J., Sick E.B., Pipper C.B., Martinussen T. The probiotic potential against vibriosis of the indigenous microflora of rainbow trout. Environ Microbiol. 2001;3:755–765. doi: 10.1046/j.1462-2920.2001.00240.x. et al. [DOI] [PubMed] [Google Scholar]
- Spesia M.B., Lazzeri D., Pascual L., Rovera M., Durantini E.N. Photoinactivation of Escherichia coli using porphyrin derivatives with different number of cationic charges. FEMS Immunol Med Microbiol. 2005;44:289–295. doi: 10.1016/j.femsim.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Tegos G.P., Hamblin M.R. 2006.
- Triantaphyllidis G.V., Abatzopoulos T.J., Sorgeloos P. Review of the biogeography of the genus Artemia (Crustacea, Anostraca) J Biogeogr. 1998;25:213–226. [Google Scholar]
- Vaseeharan B., Ramasamy P. Control of pathogenic Vibrio spp. by Bacillus subtilis BT23, a possible probiotic treatment for black tiger shrimp Penaeus monodon. Lett Appl Microbiol. 2003;36:83–87. doi: 10.1046/j.1472-765x.2003.01255.x. [DOI] [PubMed] [Google Scholar]
- Verschuere L., Rombaut G., Sorgeloos P., Verstraete W. 2000a. [DOI] [PMC free article] [PubMed]
- Verschuere L., Rombaut G., Sorgeloos P., Verstraete W. Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev. 2000b;64:655–671. doi: 10.1128/mmbr.64.4.655-671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright M. Photodynamic antimicrobial chemotherapy (PACT) J Antimicrob Chemother. 1998;42:13–28. doi: 10.1093/jac/42.1.13. [DOI] [PubMed] [Google Scholar]
- Wainwright M. Photoantimicrobials‐So what's stopping us? Photodiagn Photodyn Ther. 2009;6:167–169. doi: 10.1016/j.pdpdt.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Wainwright M. ‘Safe’ photoantimicrobials for skin and soft‐tissue infections. Int J Antimicrob Agents. 2010;36:14–18. doi: 10.1016/j.ijantimicag.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Wainwright M., Phoenix D.A., Laycock S.L., Wareing D.R.A., Wright P.A. Photobactericidal activity of phenothiazinium dyes against methicillin‐resistant strains of Staphylococcus aureus. FEMS Microbiol Lett. 1998;160:177–181. doi: 10.1111/j.1574-6968.1998.tb12908.x. [DOI] [PubMed] [Google Scholar]
- Weber J.T., Mintz E.D., Canizares R., Semiglia A., Gomez I., Sempertegui R. Epidemic Cholera in Ecuador – Multidrug‐Resistance and Transmission by Water and Seafood. Epidemiol Infect. 1994;112:1–11. doi: 10.1017/s0950268800057368. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P.N., Mak S.K., Lo M.W., Lo K.Y., Tong G.M.W., Wong Y., Wong A.K.M. Vibrio vulnificus peritonitis after handling of seafood in a patient receiving CAPD. Am J Kidney Dis. 2005;46:E87–E90. doi: 10.1053/j.ajkd.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Zheng L.Y., Zhu J.F. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr Polym. 2003;54:527–530. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Death rate of V. harveyi at increasing concentrations of photosensitizer alone at different time intervals.


