Summary
The production of fuel ethanol from low‐cost lignocellulosic biomass currently suffers from several limitations. One of them is the presence of inhibitors in lignocellulosic hydrolysates that are released during pre‐treatment. These compounds inhibit growth and hamper the production of ethanol, thereby affecting process economics. To delineate the effects of such complex mixtures, we conducted a chemical analysis of four different real‐world lignocellulosic hydrolysates and determined their toxicological effect on yeast. By correlating the potential inhibitor abundance to the growth‐inhibiting properties of the corresponding hydrolysates, we identified furfural as an important contributor to hydrolysate toxicity for yeast. Subsequently, we conducted a targeted evolution experiment to improve growth behaviour of the half industrial Saccharomyces cerevisiae strain TMB3400 in the hydrolysates. After about 300 generations, representative clones from these evolved populations exhibited significantly reduced lag phases in medium containing the single inhibitor furfural, but also in hydrolysate‐supplemented medium. Furthermore, these strains were able to grow at concentrations of hydrolysates that effectively killed the parental strain and exhibited significantly improved bioconversion characteristics under industrially relevant conditions. The improved resistance of our evolved strains was based on their capacity to remain viable in a toxic environment during the prolonged, furfural induced lag phase.
Introduction
Lignocellulosic biomass such as straw or waste wood is an abundant low‐cost source for production of biofuels such as ethanol that does not compete for alimentational needs. Despite these advantages, turning plant biomass into ethanol remains primarily an economical challenge. For economic reasons, preferably all sugars present in the polymers hemicellulose and cellulose, should be converted to ethanol. To make the monomeric sugars contained in hemicellulose accessible for fermentation by microorganisms, biomass is pre‐treated under rough conditions. This pre‐treatment leaves the cellulose intact that is subsequently hydrolysed enzymatically. Pre‐treatment generates a wealth of compounds, many of which inhibit fermentation of Saccharomyces cerevisiae and other microorganisms (Palmqvist and Hahn‐Hagerdal, 2000a; Klinke et al., 2004). Usually, these compounds are classified into three groups based on their chemical structure: organic acids that originate from the breakdown of hemicellulose, phenolic compounds as breakdown products of the phenolic polymer lignin, and finally the furans furfural and 5‐hydroxy‐methylfurfural that are derived from elimination reactions of pentose and hexose sugars respectively. The inhibitory potential of these diverse compounds has typically been quantified in isolation and it is generally assumed that their combination poses the greatest challenge to the cell factories (van Maris et al., 2006; Almeida et al., 2007).
The inhibition mechanisms are as diverse as the compounds themselves and yeast have inherent mechanisms to counteract the negative impact of these compounds. Organic acids are believed to act mainly on membranes by disturbing the membrane potential and acidifying the cytoplasm (Russel, 1992). Yeasts can compensate for an acidic cytoplasm by energy‐dependent proton pumping (Eraso and Gancedo, 1987). The furan furfural has been shown to inhibit at least three enzymes in the central carbon metabolism (Modig et al., 2002). During fermentation, the aldehydes furfural and 5‐hydroxy‐methylfurfural are reduced to their corresponding and less toxic alcohols (Palmqvist and Hahn‐Hagerdal, 2000b; Mussatto and Roberto, 2004; Liu, 2006). Generally, the inhibition mechanisms and yeasts inherent strategy to cope with phenolic compounds has not been studied well yet.
To tackle the problem of toxicity, several strategies have been followed. One strategy is based on physical, chemical or biological detoxification prior to fermentation (Palmqvist and Hahn‐Hagerdal, 2000b). Another strategy focuses on optimizing fermentation control such that effects exerted by the toxic compounds can be minimized (Rudolf et al., 2004; 2005; Alkasrawi et al., 2006). A third strategy tackles the problem by improving the inherent resistance of the organism itself. To this end, several genes have been identified on a rational basis (Larsson et al., 2001) as well as with whole‐genome screening methods (Gorsich et al., 2005; Petersson et al., 2006) that convey tolerance against either single inhibitors or hydrolysates.
Here we aim to generate strains with a broadly improved tolerance against toxic hydrolysates made from different source materials. Therefore, we first identified the major toxicity factor in four different hydrolysates made from spruce, as well as barley and wheat straw by quantitative physiological assays. Based on the identified toxins, we devised a targeted evolutionary engineering approach with a single inhibitor. Finally, the physiological basis of the resistance was investigated in the successfully evolved strains.
Results
Hydrolysate composition and toxicity
In addition to the desired sugars, about 60 organic compounds are commonly found in lignocellulosic hydrolysates, many of which inhibit growth and reduce fermentation performance (Delgenes et al., 1996; Fenske et al., 1998; Klinke et al., 2001). To obtain first insight into the composition of four generally relevant hydrolysates (Table 1), we conducted a gas chromatography (GC) time‐of‐flight (TOF) mass spectrometry (MS) (GC‐TOF‐MS)‐based chemical analysis to identify the constituents (Fig. 1). Only compounds with a NIST‐library similarity of at least 800 were considered (Stein, 2005). The similarity score is a number between 0 and 999 and describes how well a collected spectrum matches with the reference spectrum in the library and values above 750 are considered to be indicative (LECO, 2004).
Table 1.
Overview of the used hydrolysates. Furfural and 5‐hydroxy‐methylfurfural were determined with HPLC, vanillin with GC‐TOF‐MS.
| Source material | Pre‐treatment | Furfural (mM) | Vanillin (mM) | 5‐hydroxy‐methylfurfural (mM) | Provider/reference |
|---|---|---|---|---|---|
| Own analysis: | |||||
| Wheat straw I | Acid STEX | 5.0 | 0.6 | 1.4 | Institut Français du Pétrole, SAF‐ISIS |
| Wheat straw II | Acid STEX | 7.1 | 0.8 | 2.2 | Institut Français du Pétrole, SAF‐ISIS |
| Spruce | Acid steam | 11.5 | 1.0 | 17.0 | Guido Zacchi |
| Barley straw | Acid steam | 30.0 | 0.7 | 7.9 | Guido Zacchi |
| Literature: | |||||
| Spruce | Dilute acid (one/two step) | 5.2/10.4 | 0.8 | 15/46 | Larsson et al. (1999); Nilvebrant et al. (2003) |
| Corn stover | Steam | 115 | N/I | 0.5 | Öhgren et al. (2006) |
| Wheat straw | Wet oxidation | N/I | 0.2 | N/I | Klinke et al. (2003) |
| Willow | Acid steam | N/I | 2.7 | N/I | Jönsson et al. (1998) |
| Sugar cane | Steam | 20.0 | N/I | 4.8 | Martin et al. (2001) |
| Poplar | Dilute acid | N/I | 0.10 | N/I | Fenske et al. (1998) |
| Switchgrass | Dilute acid | N/I | 0.10 | N/I | Fenske et al. (1998) |
| Corn stover | Dilute acid | N/I | 0.12 | N/I | Fenske et al. (1998) |
N/I, not identified; STEX, steam explosion.
Figure 1.
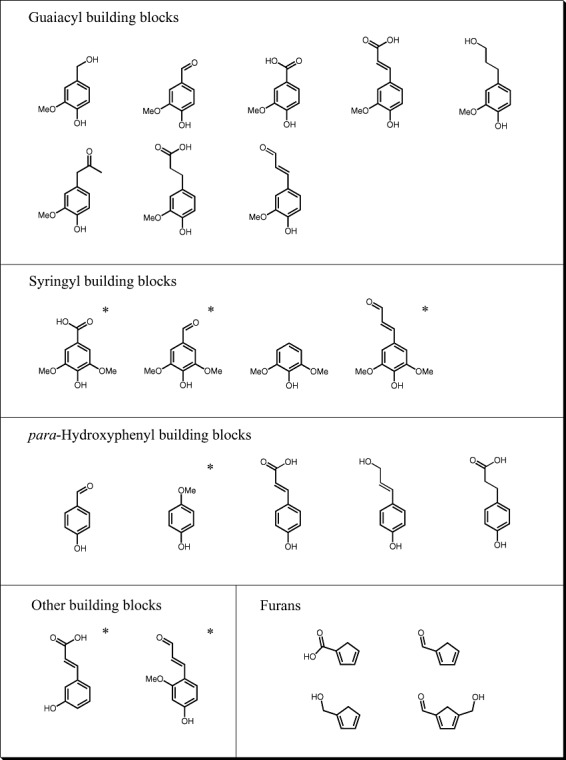
GC‐TOF‐MS identified compounds in the four hydrolysates. *Compounds only found in wheat and barley straw hydrolysate.
Besides the well‐known furans, the identified compounds are mostly lignin‐building blocks, which are commonly categorized into para‐hydroxyphenyl, guaiacyl or syringyl residues harbouring compounds. Only two phenolic compounds did not fall into these three categories. Almost all compounds were found in all four hydrolysates with the exception of syringyl residue phenols, which we only detected in wheat and barley straw hydrolysate. Not surprisingly, the syringyl residue phenols are known to be building blocks of herbaceous lignin but not wood lignin (Campbell and Sederoff, 1996; Lawther et al., 1996). We then compared the list of constituents before and after fermentation of hydrolysates with TMB3400 under anaerobic conditions. Most strikingly, all aldehyde functional groups were reduced to their corresponding alcohols, whereas compounds with alcohol or carboxyl functional groups remained untouched in the medium without any apparent metabolization.
Based on our chemical analysis of the four hydrolysates, as well as previous toxicological investigations (Delgenes et al., 1996; Klinke et al., 2004), we chose vanillin as a representative from the phenol group and the furans furfural and 5‐hydroxy‐methylfurfural to assess toxicity in more detail. Dose–response curves of increasing inhibitor concentration on the growth rate of the yeast TMB3400 identify vanillin as the most and 5‐hydroxy‐methylfurfural as the least toxic compound (Fig. 2). The question then was whether the actual hydrolysates contained growth‐affecting concentrations of these three inhibitors. In addition to our own measurements, we also searched for reported inhibitor concentrations in published hydrolysate compositions (Table 1). The actual concentration of 5‐hydroxy‐methylfurfural in the hydrolysates was below a relevant level. Furfural and vanillin in contrast were found in concentration ranges that affect growth significantly.
Figure 2.
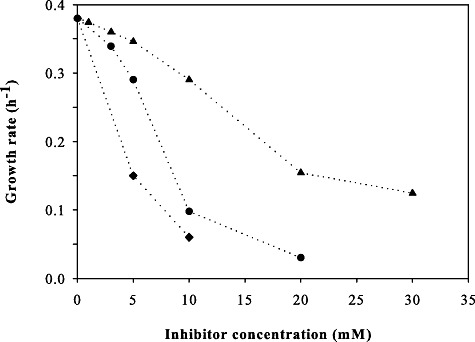
Inhibitory effect of 5‐hydroxy‐methylfurfural (▴), vanillin (♦) and furfural (●) on the growth rate of TMB3400 in aerobic glucose minimal medium.
To further characterize the relationship between composition and toxicity, we determined EC50 values on growth rates in the presence of different hydrolysates and correlated them to the concentrations of individual inhibitors present in the hydrolysates. A clear dependency of EC50 to the concentration of toxins was found for furfural; i.e. the more furfural the hydrolysate contains, the lower the EC50 value (Fig. 3). Thus, we conclude that furfural is the main contributor to the toxicity of the tested hydrolysates. We cannot fully exclude, however, that at least vanillin is also important, as it displays a correlation for three of the four tested hydrolysates. Only barley hydrolysate constitutes an exception. As this hydrolysate contains high concentrations of furfural, the correlation of vanillin and EC50 might be masked.
Figure 3.
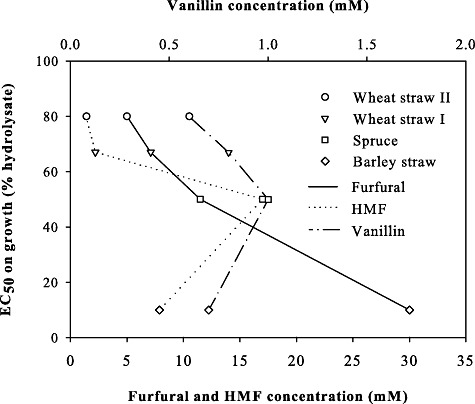
Correlation between EC50 and single inhibitor concentration in different lignocellulosic hydrolysates. HMF, 5‐hydroxy‐methylfurfural.
Evolution of improved furfural tolerance
As furfural was identified as the most relevant inhibitor in all four hydrolysates, we initiated a long‐term evolutionary adaptation experiment where S. cerevisiae TMB3400 was challenged with increasing furfural concentrations. For this purpose, aerobic batch cultures were serially transferred in minimal media with increasing furfural concentrations (Liu et al., 2005). To monitor the adaptation progress, we determined population growth phenotypes after every 5th transfer in minimal medium containing 17 mM furfural (Fig. 4). After an extended lag phase of about 90 h, even the un‐adapted parental strain began to grow, but adapted populations exhibited increasingly shorter lag phases. The most rapid shortening of lag phases occurred between transfers 5 and 20. As the following transfers yielded only minor improvements, the evolutionary adaptation experiment reached apparently a plateau.
Figure 4.
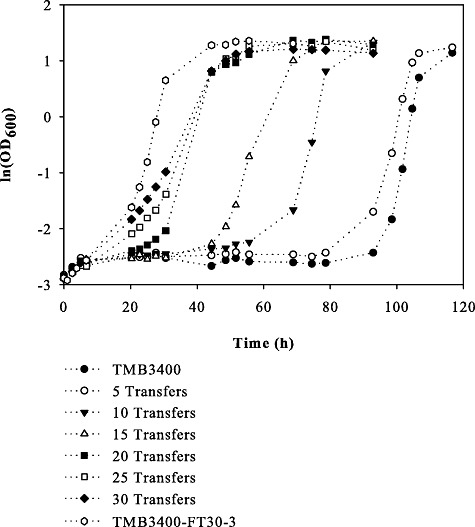
Growth of evolved populations in aerobic minimal medium containing 17 mM furfural.
From the population that was transferred 30 times, corresponding to approximately 300 generations, we isolated 15 clones and analysed their physiology in more detail. Based on the growth behaviour on minimal medium containing 17 mM furfural and an inoculum size of 0.025 gcdw l−1, the isolated clones can be grouped in three types based on their lag phases (Table 2): type A with 90 h and parent‐like lag phase, type B with 16 h lag phase and type C with 7 h lag phase. We then verified the genetic stability of type B and type C strains by serial transfer for five times on YPG/agar plates in the absence of furfural. While the type B strains retained their growth phenotype in the presence of furfural, type C strains had lag phases indistinguishable from type B strains after the transfers. Thus, the general inhibitor tolerance is genetically stable even in the absence of selection.
Table 2.
Physiological parameters of isolated clones from the evolved population.
| Yeast strain | Type | Lag phase (h) | Maximal specific growth rate (h−1) |
|---|---|---|---|
| TMB3400 | – | 90 ± 5 | 0.38 ± 0.01 |
| TMB3400‐FT30‐1, 4, 8, 9, 10, 11, 12, 14 and 15 | A | 90 ± 11 | N/D |
| TMB3400‐FT30‐2, 3, 5 and 7 | B | 16 ± 2 | 0.39 ± 0.01 |
| TMB3400FT30‐6 and 13 | C | 7 | 0.35 ± 0.01 |
Maximal specific growth rates were determined in aerobic glucose minimal medium.
Growth behaviour of the evolved strains on hydrolysate
To verify our initial hypothesis that strains adapted to the major toxicity factor furfural have also an advantage in actual hydrolysates, we tested the evolved strain TMB3400‐FT30‐3 as a representative clone of type B strains and the parent in glucose minimal medium containing 40% v/v of the medium toxic spruce hydrolysate. The experiment was performed under anaerobic conditions to resemble typical industrial ethanol production conditions. Akin to the experiments with the pure inhibitor, the lag phase of TMB3400‐FT30‐3 was significantly shorter compared with the parent strain (Fig. 5). Higher hydrolysate concentrations of 50% v/v prevents growth of the parent strains for at least 300 h, whereas the evolved strains managed to grow after 24 h. This finding underpins our hypothesis that furfural is indeed the major toxicity component of the tested hydroysates.
Figure 5.
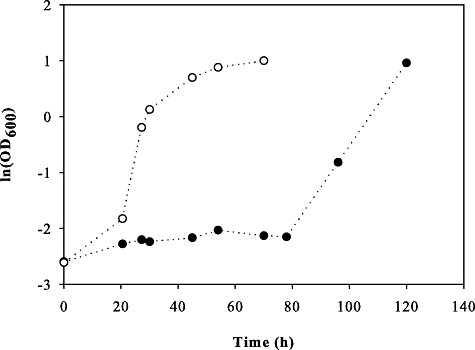
Growth of TMB3400 (●) and the evolved clone TMB3400‐FT30‐3 (○) in anaerobic glucose minimal medium containing 40% (v/v) spruce hydrolysate.
Growth behaviour of the evolved strains on furfural‐containing medium
We then wondered what, on a physiological level, would allow the evolved strains to reduce their lag phase so strongly. In accordance with previous investigations (Palmqvist and Hahn‐Hagerdal, 2000b; Liu et al., 2004; Mussatto and Roberto, 2004), we found furfural to be almost quantitatively reduced to furfuryl alcohol. To resolve whether this reduction was growth‐associated, we tracked the fate of furfural by high‐performance liquid chromatography (HPLC) measurement during an aerobic batch experiment. As can be seen from Fig. 6B and C, furfural reduction occurred mainly during lag phase. This conversion was almost quantitative and the furfuryl alcohol was not further metabolized to a significant extent. Surprisingly, the same furfural reduction rates of 12.2 ± 2.7 mM h−1 gcdw−1 were determined for the parent and the evolved strains during the first 10 h of the experiment. The same rate was also determined for other evolved strains (data not shown).
Figure 6.
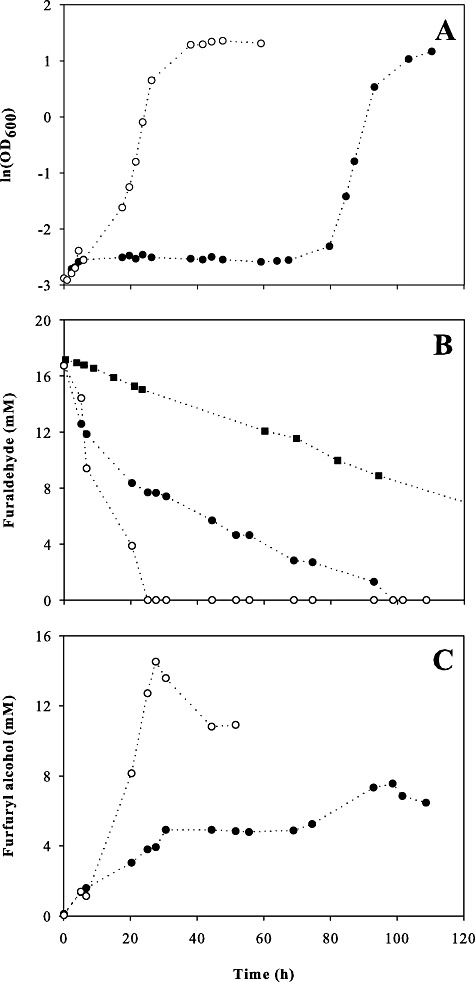
Time‐courses of cell growth (A), furfural (B) and furfuryl alcohol concentrations in cultures of TMB3400 (●), TMB3400‐FT30‐3 (○) and without the addition of cells (▪) (evaporation) in aerobic minimal medium containing 17 mM furfural.
After 10 h, the furfural reduction rate of TMB3400 slowed down significantly and the production of furfuryl alcohol ceased concomitantly. The parental strain only resumed to grow when furfural had evaporated from the shake flask down to a concentration of about 3 mM. The evolved mutant TMB3400‐FT30‐3 in contrast, while still in lag phase, continued to reduce furfural at the same rate as during the first 10 h. The furfural concentration thus decreased much faster to an apparent threshold concentration of 2–3 mM upon which growth resumed.
Thus, we conclude that the evolved resistance of our mutants is caused by their ability to maintain furfural reduction activity during extended lag phases. A possible reason for the decreasing reduction rate in the parent strain would be a cytotoxic effect of furfural. To test this hypothesis, we performed viability platings during the lag phase that showed a clear drop of viability of TMB3400 after the cells were inoculated in medium containing furfural (Fig. 7). Upon some recovery, the parent strain started to die with continuously decreasing colony‐forming units after about 10 h. While the evolved strain remained viable and thus continued to reduce furfural at a high rate, the surviving parental cells managed to grow after the furfural has passively evaporated from the shake flask (data not shown). Hence, the reduced reduction rate in case of the parent strain TMB3400 is mainly caused by a drop in viability that was apparently absent in the evolved strains.
Figure 7.
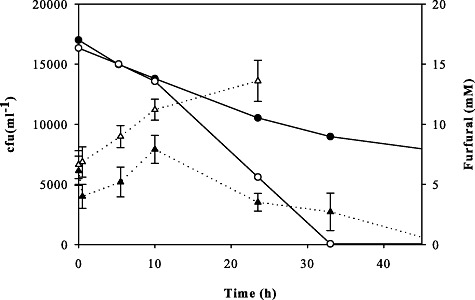
Colony‐forming unit counts (triangles) and furfural concentration (bullets) of TMB3400 (closed symbols) and TMB3400‐FT30‐3 (open symbols) in aerobic glucose minimal medium with 17 mM furfural.
Performance of the evolved strains under process‐relevant conditions
For reliable toxicity quantification and consistency reasons, we had used very low inocula in all above experiments. To finally demonstrate that our strains are potentially useful under industrial process conditions, we conducted an anaerobic bioconversion at high concentrations (80% v/v) of toxic barley straw hydrolysate with TMB3400 and TMB3400‐FT30‐3 and high inoculum sizes of 5 and 10 gcdw l−1 (Nilsson et al., 2005; Rudolf et al., 2007) (Fig. 8). Under these conditions, little cell growth occurs (an increase of about 2 gcdw l−1 over the whole process), but the high biomass concentration can effectively catalyse also conversion of sugars like xylose that do not support growth efficiently (Wahlbom et al., 2003; Sonderegger et al., 2004). While the final ethanol titres were identical for both strains and conditions, the evolved strain produced the maximum ethanol concentration much faster (Fig. 8). At a density of 5 gcdw l−1, the process time gain was 30 h and at 10 gcdw l−1 still 8 h (data not shown). We thus conclude that the evolved tolerance is also relevant under more realistic conditions, by reducing process times from 50 to 20 h.
Figure 8.
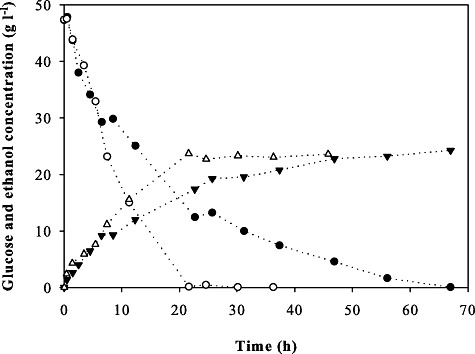
Time‐courses of glucose (bullets) and ethanol (triangles) concentrations during an anaerobic bioconversion in 80% v/v barley straw hydrolysate with TMB3400 (closed symbol) and TMB3400‐FT30‐3 (open symbols) with 5 gcdw l−1 initial biomass.
For both inoculum sizes and strains, glucose consumption and ethanol production were similar during the first 7 h. But after this period, the parental strain slowed down consuming glucose and producing ethanol. This finding is similar to what we observed in the experiments with low inoculi: our evolved strains appear to be more fit after a certain period of time under stressful conditions than their parent.
Discussion
A chemical and toxicological analysis of four different lignocellulosic hydrolysates clearly identified furfural as the main toxic agent for yeast. Based on this knowledge, we designed a long‐term evolution experiment, where significantly increased tolerance of a semi‐industrial yeast strains to toxic hydrolysates was achieved by selection for growth in furfural‐containing media. This increased tolerance of the evolved strains had three main characteristics: (i) growth at higher hydrolysate concentrations, (ii) significantly reduced lag phases and (iii) reduced process times in a process‐relevant fermentation set‐up.
Previous adaptation studies reported inhibitor‐tolerant strains with increased furfural reduction rates (Liu et al., 2005; Martin et al., 2007). In contrast, our adaptation experiment gave rise to strains with indistinguishable furfural reduction rates but increased viabilities under furfural stress. The most probable explanation is the different selection regimes: Martin employed a chemostat with increasing hydrolysate concentrations for selection and Liu added furfural only after growth was logarithmic. In contrast, our cells came from a late exponential phase and were challenged at a low inoculum size to relatively high amounts of furfural. Such a regime is quite rigorous and hence selection is mainly targeted at survival. One result from the increased viability is the ability of our evolved strains to withstand and grow at high hydrolysate concentration with low inoculum concentration where the parent does not grow at all. The previously evolved strains have only been shown to grow in hydrolysates with relatively high initial cell densities.
The inherent strategy of yeast to counteract the negative impact of furfural and other aldehydes is to reduce them to the less toxic alcohols. Prominent candidates for catalysing such reactions are alcohol dehydrogenases and aldo‐keto reductases, and overexpression of alcohol dehydrogenases has been demonstrated to convey tolerance against furfural (Larroy et al., 2002a,b; Petersson et al., 2006). While aldo‐keto reductases generally display a broad substrate specificity and preference for catalysing the carbonyl reduction (Kuhn et al., 1995; Nakamura et al., 1997; Hur and Wilson, 2000; Chang et al., 2007), little is known about their potential role in inhibitor detoxification. As our evolved strains did not exhibit increased furfural reduction rates in any of the investigated mutants, however, it is unlikely that increased expression levels of these enzymes contributed significantly to the evolved tolerance.
The primary advantage of our evolved strain is their capacity to withstand and remain active at higher concentrations of furfural or hydrolysates than their already quite tolerant parent (Sonderegger et al., 2004). This is most apparent during the lag phase upon inoculation in high furfural‐containing media. Lag phase cells are more sensitive to stress, as they cannot rapidly repair damage by synthesising new macromolecules. The detrimental effect of furfural is believed to result from its accumulation in the cell, thereby inhibiting enzymes of the central carbon metabolism and disturbing the cells energy balance (Modig et al., 2002; Sarvari Horvath et al., 2003). Furthermore, furfural induces damage in vacuolar and mitochondrial membranes and reactive oxygen species accumulation, which in turn can cause cell death (Almeida et al., 2007). Survival during lag phases depends on many factors that include the state of the inoculum, regulation of gene expression, translation, post‐translational protein modification and autophagy (Gray et al., 2004), any of which could potentially contribute to the evolved phenotype.
Taken together, we hypothesise that our evolved strains have a general fitness gain compared with the parental strain upon furfural exposition, which allows them to survive the adverse conditions better than the parental strain. Hence, our strains have the potential to reduce inoculum sizes and process times, thereby contributing to the economical feasibility of an ethanol production process.
Experimental procedures
Yeast strain, media and cultivation conditions
All experiments were conducted with a recombinant xylose consuming S. cerevisiae strain TMB3400 (Wahlbom et al., 2003). TMB3400 originates from the industrial strain USM21 (van der Westhuizen and Pretorius, 1992), which was metabolically engineered for anaerobic growth on xylose by the addition of three genes from Pichia stipitis followed by EMS mutagenesis and selection to increase performance. USM21 itself originates from a cross between strains used for wine making. Due to its origin, TMB3400 is at least diploid and generally referred to as a robust half‐industrial strain.
Yeast was cultivated in complex YPG medium containing 10 g l−1 yeast extract, 20 g l−1peptone and 20 g l−1d‐glucose or in minimal medium with 20 g l−1d‐glucose described elsewhere (Verduyn et al., 1992), buffered with 50 mM potassium‐hydrogen‐phthalate at pH 5. Media for anaerobic cultivations additionally contained 0.01 g l−1 ergosterol and 0.4 g l−1 Tween 80. Hydrolysate was supplemented after sterile filtration, 100% hydrolysate thereby corresponding to the slurry obtained. As the pH of the hydrolysates was acidic, the pH of all media containing hydrolysates was adjusted to 5 prior to sterile filtration by addition of KOH. Aerobic cultivation was conducted in baffled 500 ml shake flasks. Anaerobic cultivations were performed in either 10 ml Hungate tubes filled with 7 ml medium or 125 ml septum flasks, filled with 50 ml medium. Anaerobic conditions were attained by flushing the media with nitrogen prior to incubation. All growth experiments were conducted at 30°C.
Evolution experiment
TMB3400 was grown aerobically in baffled shake flasks with minimal medium containing 3 mM furfural. From cultures in late exponential phase, at least 107 cells [amount calculated from the optical density at 600 nm (OD600)] were transferred to the next shake flask. Upon shortening of lag phases, the furfural concentration was increased in the subsequent shake flask (up to 20 mM after reaching transfer number 30). Populations were tested for growth every 5th transfer. After 30 transfers, single clones were obtained from populations by several serial streak‐outs on YPG/agar plates.
Growth tests
To assess growth performance, media containing either a single inhibitor or hydrolysate were inoculated with a mid‐exponential pre‐culture at a cell dry weight of 0.025 g l−1. Growth was monitored by measuring OD600. Due to changing absorbance properties of the hydrolysate during the course of fermentation, OD600 was measured as follows: the absorbance of the cell broth was measured, the sample was centrifuged and the absorbance of the supernatant measured and used as reference. For external metabolites, cell broth was withdrawn, centrifuged and the supernatant frozen for subsequent analysis by HPLC.
For fermentations with high inoculum sizes, yeast was first grown in aerobic shake flasks in glucose minimal medium with 40 g l−1 glucose as carbon source. A calculated volume of cell broth that would yield a desired final biomass concentration in the fermentation was then harvested by centrifugation as the culture was still exponentially growing. The cells were dumped into hydrolysate supplemented with the double amount of salts and vitamins and buffered with 20 mM potassium‐hydrogen‐phthalate at a pH of 5 and glucose concentration adjusted to 50 g l−1.
To quantify toxicity, EC50 is defined as the concentration of a compound at which the growth rate is reduced to 50% compared with a control without inhibitors. These values were obtained from dose–response curves of hydrolysate concentration versus growth rate, determined in minimal medium under anaerobic conditions. They indicate how toxic a hydrolysate is. Lag phases are determined by the x‐axis intercept of the intersection from the linearized exponential growth curve with the baseline made up in the absence of growth and is expressed in hours.
Analytical procedures
Extracellular metabolites such as sugars, alcohols and organic acids were determined with an HPLC system (Agilent HP1100), equipped with a polymer column (Aminex HPX‐87H from Bio‐Rad). The eluent was 5 mM H2SO4 and the column was heated to 60°C. The compounds were detected and quantified with a refractive index detector and an UV/Vis‐detector. Concentrations of 5‐hydroxy‐methylfurfural, furfural and furfuryl alcohol were determined with the same HPLC system but with an end‐capped C18 column (Synergi Hydro‐RP from Phenomenex) and a UV‐detector at 280 nm (furfural) and 210 nm (furfuryl alcohol). Elution was carried out at a flow rate of 0.7 ml min−1 with nanopure water and the column was heated to 35°C. Quantification of all substances was achieved by external standards with the corresponding pure substance obtained from Sigma.
For hydrolysate composition, samples were extracted twice with ethylacetate. The solvent was evaporated in a vacuum centrifuge (UniVapo 150 ECH with a UniJet II vacuum pump from UniEquip) and the pellet re‐suspended in ethylacetate. To achieve good GC separation, the alcohol functional groups were derivatized with N‐tert‐butyl‐dimethylsilyl‐acetamid to yield the corresponding silyl‐ether by the addition of an approximately 10‐fold excess of N‐tert‐butyl‐dimethylsilyl‐acetamid. The GC system was an Agilent 6890N equipped with an HP‐5 column (Agilent). The GC protocol was as follows: 1 µl split‐injection 1:20, 2 min at 100°C, then continuous increase to 180°C at 20°C min−1, followed by an increase to 300°C at 20°C min−1 and a final minute at 300°C. The MS spectra were collected with a LECO Pegasus III time of flight MS system, masses were collected from 35 to 800 amu. Compounds were identified by comparison against the NIST‐library (Stein, 2005) based on their fragmentation profile. Quantification was achieved by measuring an external standard consisting of the pure substance obtained from Sigma.
Hydrolysates
Both wheat straw hydrolysates were prepared by soaking the source material (20% dry matter) in 0.08 N H2SO4 for 16 h followed by steam explosion at 19.5 bars and a pH of 4.7 for 150 s, followed by enzymatic hydrolysis. Both batches were prepared by saf‐isis (Souston, France) together with the Institut Français du Pétrole (Lyon, France). Barley straw hydrolysate was prepared according to Linde and colleagues (2006) and produced by the lab of Guido Zacchi (Lund, Sweden). This hydrolysate was prepared without enzymatic hydrolysis, so 20 g l−1 glucose was added separately for growth experiments. Spruce hydrolysate was prepared by impregnating the wood chips with 2.5% SO2 followed by pre‐treatment at 215°C for 5 min.
Acknowledgments
This work was supported by the NILE project within European Commission Framework VI. We thank the providers of the hydrolysates: Institut Français du Pétrole, Lyon (France); SAF‐ISIS, Soustons (France) and Guido Zacchi, Lund (Sweden).
References
- Alkasrawi M., Rudolf A., Liden G., Zacchi G. Influence of strain and cultivation procedure on the performance of simultaneous saccharification and fermentation of steam pretreated spruce. Enzyme Microb Technol. 2006;38:279–286. [Google Scholar]
- Almeida J.R.M., Modig T., Petersson A., Hahn‐Hagerdal B., Liden G., Gorwa‐Grauslund M.F. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J Chem Technol Biotechnol. 2007;82:340–349. [Google Scholar]
- Campbell M.M., Sederoff R.R. Variation in lignin content and composition – mechanism of control and implications for the genetic improvement of plants. Plant Physiol. 1996;110:3–13. doi: 10.1104/pp.110.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q., Griest T.A., Harter T.M., Petrash J.M. Functional studies of aldo‐keto reductases in Saccharomyces cerevisiaeBiochimica Et Biophysica Acta‐Molecular. Cell Res. 2007;1773:321–329. doi: 10.1016/j.bbamcr.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgenes J.P., Moletta R., Navarro J.M. Effects of lignocellulose degradation products on ethanol fermentations of glucose and xylose by Saccharomyces cerevisiae, Zymomonas mobilisPichia stipitis, and Candida shehatae. Enzyme Microb Technol. 1996;19:220–225. [Google Scholar]
- Eraso P., Gancedo C. Activation of yeast plasma membrane ATPase by acid pH during growth. FEBS Lett. 1987;224:187–192. doi: 10.1016/0014-5793(87)80445-3. [DOI] [PubMed] [Google Scholar]
- Fenske J.J., Griffin D.A., Penner M.H. Comparison of aromatic monomers in lignocellulosic biomass prehydrolysates. J Ind Microbiol Biotechnol. 1998;20:364–368. [Google Scholar]
- Gorsich S.W., Dien B.S., Nichols N.N., Slininger P.J., Liu Z.L., Skory C.D. Tolerance to furfural‐induced stress is associated with pentose phosphate pathway genes ZWF1, GND1, RPE1, and TKL1 in Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2005;71:1–11. doi: 10.1007/s00253-005-0142-3. [DOI] [PubMed] [Google Scholar]
- Gray J.V., Petsko G.A., Johnston G.C., Ringe D., Singer R.A., Werner‐Washburne M. Sleeping beauty’: quiescence in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2004;68:187–206. doi: 10.1128/MMBR.68.2.187-206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur E., Wilson D.K. Crystallization and aldo‐keto reductase activity of Gcy1p from Saccharomyces cerevisiae. Acta Crystallogr D Biol Crystallogr. 2000;56:763–765. doi: 10.1107/s0907444900004704. [DOI] [PubMed] [Google Scholar]
- Jönsson L.J., Palmqvist E., Nilvebrant N.O., Hahn‐Hagerdal B. Detoxification of wood hydrolysates with laccase and peroxidase from the white‐rot fungus Trametes versicolor. Appl Microbiol Biotechnol. 1998;49:91–104. [Google Scholar]
- Klinke H.B., Thomsen A.B., Ahring B.K. Potential inhibitors from wet oxidation of wheat straw and their effect on growth and ethanol production by Thermoanaerobacter mathranii. Appl Microbiol Biotechnol. 2001;57:631–638. doi: 10.1007/s002530100825. [DOI] [PubMed] [Google Scholar]
- Klinke H.B., Olsson L., Thomsen A.B., Ahring B.K. Potential inhibitors from wet oxidation of wheat straw and their effect on ethanol production of Saccharomyces cerevisiae: wet oxidation and fermentation by yeast. Biotechnol Bioeng. 2003;81:738–747. doi: 10.1002/bit.10523. [DOI] [PubMed] [Google Scholar]
- Klinke H.B., Thomsen A.B., Ahring B.K. Inhibition of ethanol‐producing yeast and bacteria by degradation products produced during pre‐treatment of biomass. Appl Microbiol Biotechnol. 2004;66:10–26. doi: 10.1007/s00253-004-1642-2. [DOI] [PubMed] [Google Scholar]
- Kuhn A., Vanzyl C., Vantonder A., Prior B.A. Purification and Partial Characterization of an Aldo‐Keto Reductase from Saccharomyces cerevisiae. Appl Environ Microbiol. 1995;61:1580–1585. doi: 10.1128/aem.61.4.1580-1585.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larroy C., Fernandez M.R., Gonzalez E., Pares X., Biosca J.A. Characterization of the Saccharomyces cerevisiae YMR318C (ADH6) gene product as a broad specificity NADPH‐dependent alcohol dehydrogenase: relevance in aldehyde reduction. Biochem J. 2002a;361:163–172. doi: 10.1042/0264-6021:3610163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larroy C., Pares X., Biosca J.A. Characterization of a Saccharomyces cerevisiae NADP(H)‐dependent alcohol dehydrogenase (ADHVII), a member of the cinnamyl alcohol dehydrogenase family. Eur J Biochem. 2002b;269:5738–5745. doi: 10.1046/j.1432-1033.2002.03296.x. [DOI] [PubMed] [Google Scholar]
- Larsson S., Reiman A., Nilverbrant N., Jönsson L.J. Comparison of different methods for the detoxification of lignocellulose hydrolysate of spruce. Appl Biochem Biotechnol. 1999;77:91–104. [Google Scholar]
- Larsson S., Cassland P., Jonsson L.J. Development of a Saccharomyces cerevisiae strain with enhanced resistance to phenolic fermentation inhibitors in lignocellulose hydrolysates by heterologous expression of laccase. Appl Environ Microbiol. 2001;67:1163–1170. doi: 10.1128/AEM.67.3.1163-1170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther J.M., Sun R.C., Banks W.B. Fractional characterization of alkali‐labile lignin and alkali‐insoluble lignin from wheat straw. Ind Crops Prod. 1996;5:291–300. [Google Scholar]
- LECO. LECO Corporation; 2004. [Google Scholar]
- Linde M., Galbe M., Zacchi G. Steam pretreatment of acid‐sprayed and acid‐soaked barley straw for production of ethanol. Appl Biochem Biotechnol. 2006;130:546–562. doi: 10.1385/abab:130:1:546. [DOI] [PubMed] [Google Scholar]
- Liu L. Genomic adaptation of ethanologenic yeast to biomass conversion inhibitors. Appl Microbiol Biotechnol. 2006;73:27–36. doi: 10.1007/s00253-006-0567-3. [DOI] [PubMed] [Google Scholar]
- Liu Z.L., Slininger P.J., Dien B.S., Berhow M.A., Kurtzman C.P., Gorsich S.W. Adaptive response of yeasts to furfural and 5‐hydroxymethylfurfural and new chemical evidence for HMF conversion to 2,5‐bis‐hydroxymethylfuran. J Ind Microbiol Biotechnol. 2004;31:345–352. doi: 10.1007/s10295-004-0148-3. [DOI] [PubMed] [Google Scholar]
- Liu Z.L., Slininger P.J., Gorsich S.W. Enhanced biotransformation of furfural and hydroxymethylfurfural by newly developed ethanologenic yeast strains. Appl Biochem Biotechnol. 2005;121:451–460. [PubMed] [Google Scholar]
- Van Maris A.J., Abbott D.A., Bellissimi E., Brink J., Kuyper M., Luttik M.A. Alcoholic fermentation of carbon sources in biomass hydrolysates by Saccharomyces cerevisiae: current status. Antonie Van Leeuwenhoek. 2006;90:391–418. doi: 10.1007/s10482-006-9085-7. et al. [DOI] [PubMed] [Google Scholar]
- Martin C., Wahlbom C.F., Galbe M., Jönsson L., Hahn‐Hagerdal B. 2001;7:361–367. [Google Scholar]
- Martin C., Marcet M., Almazan O., Jonsson L.J. Adaptation of a recombinant xylose‐utilizing Saccharomyces cerevisiae strain to a sugarcane bagasse hydrolysate with high content of fermentation inhibitors. Bioresour Technol. 2007;98:1767–1773. doi: 10.1016/j.biortech.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Modig T., Liden G., Taherzadeh M.J. Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem J. 2002;363:769–776. doi: 10.1042/0264-6021:3630769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussatto S.I., Roberto I.C. Alternatives for detoxification of diluted‐acid lignocellulosic hydrolyzatesfor use in fermentative processes: a review. Bioresour Technol. 2004;93:1–10. doi: 10.1016/j.biortech.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Kondo S., Kawai Y., Nakajima N., Ohno A. Amino acid sequence and characterization of aldo‐keto reductase from bakers' yeast. Biosci Biotechnol Biochem. 1997;61:375–377. doi: 10.1271/bbb.61.375. [DOI] [PubMed] [Google Scholar]
- Nilsson A., Gorwa‐Grauslund M.F., Hahn‐Hagerdal B., Liden G. Cofactor dependence in furan reduction by Saccharomyces cerevisiae in fermentation of acid‐hydrolyzed lignocellulose. Appl Environ Microbiol. 2005;71:7866–7871. doi: 10.1128/AEM.71.12.7866-7871.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilvebrant N.O., Persson P., Reimann A., De Sousa F., Gorton L., Jonsson L.J. Limits for alkaline detoxification of dilute‐acid lignocellulose hydrolysates. Appl Biochem Biotechnol. 2003;105:615–628. doi: 10.1385/abab:107:1-3:615. [DOI] [PubMed] [Google Scholar]
- Öhgren K., Rudolf A., Galbe M., Zacchi G. Fuel ethanol production from steam‐pretreated corn stover using SSF at higher dry matter content. Biomass Bioenergy. 2006;30:863–869. [Google Scholar]
- Palmqvist E., Hahn‐Hagerdal B. Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour Technol. 2000a;74:25–33. [Google Scholar]
- Palmqvist E., Hahn‐Hagerdal B. Fermentation of lignocellulosic hydrolysates. I: inhibition and detoxification. Bioresour Technol. 2000b;74:17–24. [Google Scholar]
- Petersson A., Almeida J.R., Modig T., Karhumaa K., Hahn‐Hagerdal B., Gorwa‐Grauslund M.F., Liden G. A 5‐hydroxymethyl furfural reducing enzyme encoded by the Saccharomyces cerevisiae ADH6 gene conveys HMF tolerance. Yeast. 2006;23:455–464. doi: 10.1002/yea.1370. [DOI] [PubMed] [Google Scholar]
- Rudolf A., Galbe M., Liden G. Controlled fed‐batch fermentations of dilute‐acid hydrolysate in pilot development unit scale. Appl Biochem Biotechnol. 2004;114:601–617. doi: 10.1385/abab:114:1-3:601. [DOI] [PubMed] [Google Scholar]
- Rudolf A., Alkasrawi M., Zacchi G., Liden G. A comparison between batch and fed‐batch simultaneous saccharification and fermentation of steam pretreated spruce. Enzyme and Microbial Technology. 2005;37:195–204. [Google Scholar]
- Rudolf A., Lequeux G., Liden G. Controlled pilot development unit‐scale fed‐batch cultivation of yeast on spruce hydrolysates. Biotechnol Prog. 2007;23:351–358. doi: 10.1021/bp060256u. [DOI] [PubMed] [Google Scholar]
- Russel J.B. Another explanation for the toxicity of fermentation acids at low pH: anion accumulation versus uncoupling. J Appl Bacteriol. 1992;73:363–370. [Google Scholar]
- Sarvari Horvath I., Franzen C.J., Taherzadeh M.J., Niklasson C., Liden G. Effects of furfural on the respiratory metabolism of Saccharomyces cerevisiae in glucose‐limited chemostats. Appl Environ Microbiol. 2003;69:4076–4086. doi: 10.1128/AEM.69.7.4076-4086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderegger M., Jeppsson M., Larsson C., Gorwa‐Grauslund M.F., Boles E., Olsson L. Fermentation performance of engineered and evolved xylose‐fermenting Saccharomyces cerevisiae strains. Biotechnol Bioeng. 2004;87:90–98. doi: 10.1002/bit.20094. et al. [DOI] [PubMed] [Google Scholar]
- Stein S.E. 2005.
- Verduyn C., Postma E., Scheffers W.A., Van Dijken J.P. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous‐culture study on the regulation of respiration and alcoholic fermentation. Yeast. 1992;8:501–517. doi: 10.1002/yea.320080703. [DOI] [PubMed] [Google Scholar]
- Wahlbom C.F., Van Zyl W.H., Jonsson L.J., Hahn‐Hagerdal B., Otero R.R. Generation of the improved recombinant xylose‐utilizing Saccharomyces cerevisiae TMB 3400 by random mutagenesis and physiological comparison with Pichia stipitis CBS 6054. FEMS Yeast Res. 2003;3:319–326. doi: 10.1016/S1567-1356(02)00206-4. [DOI] [PubMed] [Google Scholar]
- Van Der Westhuizen T.J., Pretorius I.S. The value of electrophoretic fingerprinting and karyotyping in wine yeast breeding programmes. Antonie Van Leeuwenhoek. 1992;61:249–257. doi: 10.1007/BF00713932. [DOI] [PubMed] [Google Scholar]


