Summary
Manganese (II) and manganese‐oxidizing bacteria were used as an efficient biological system for the degradation of the xenoestrogen 17α‐ethinylestradiol (EE2) at trace concentrations. Mn2+‐derived higher oxidation states of Mn (Mn3+, Mn4+) by Mn2+‐oxidizing bacteria mediate the oxidative cleavage of the polycyclic target compound EE2. The presence of manganese (II) was found to be essential for the degradation of EE2 by Leptothrix discophora, Pseudomonas putida MB1, P. putida MB6 and P. putida MB29. Mn2+‐dependent degradation of EE2 was found to be a slow process, which requires multi‐fold excess of Mn2+ and occurs in the late stationary phase of growth, implying a chemical process taking place. EE2‐derived degradation products were shown to no longer exhibit undesirable estrogenic activity.
Introduction
Manganese‐oxidizing bacteria oxidize Mn2+ to form insoluble Mn3+ and/or Mn4+ oxides, and this far best studied bacterial species have been isolated from manganese‐containing environments such as Mn‐oxide encrusted pipelines, manganese‐containing water pipes and aquifers, as well as from marine sediments (Schweisfurth, 1973; Nealson and Ford, 1980; Emerson and Ghiorse, 1992). However, the natural niche of manganese‐oxidizing bacteria appears not only to require the presence of manganese, but often also features a marked availability of usually recalcitrant organics in nature. Thus, an essential role of these bacteria in oxidation of organic material in organic‐rich environments has been postulated (Sunda and Kieber, 1994). Moreover, Mn2+‐oxidizing bacteria have been detected in environmental samples contaminated with recalcitrant xenobiotics, such as polycyclic aromatic hydrocarbons or methyl tert‐butyl ether (Bruns et al., 2001; Bodour et al., 2003; Raynal and Pruden, 2008).
To test the potential use of bacterial manganese oxidation to drive the degradation of recalcitrant polycyclic aromatic organic compounds, 17α‐ethinylestradiol (EE2) was chosen as target compound. EE2 is a persistent synthetic estrogen and is often present as a trace pollutant in waste and drinking water bearing the proven risk of undesirable hormonal effects on animals and humans (Thomas et al., 2001). The persistence of EE2 has prompted a number of attempts to achieve degradation of EE2 by bacteria, e.g. by use of Rhodococcus zopfii, Rhodococcus equi and Sphingobacterium sp. (Yoshimoto et al., 2004; Haiyan et al., 2007). All these bacteria require EE2 to be present at milligram levels. In situ, however, only trace concentrations of the pollutants are normally present in waste and drinking waters, which are unlikely to sustain bacterial growth, thus limiting the use of these bacteria for degradation of e.g. trace amounts of EE2. It has recently been demonstrated that commercially available manganese oxides as powder or as specifically produced nanoparticles can mediate removal of EE2 (de Rudder et al., 2004).
The main consideration for using biogenic Mn oxides produced by Mn‐oxidizing bacteria, instead of chemical Mn oxides as used before by de Rudder and colleagues (2004), was to explore the possibility of working at significantly lower (micromolar) concentrations of Mn. It was shown before that biogenic Mn oxides have different structure and surface areas compared with chemical oxides (Saratovsky et al., 2006), and this might bring about their increased activity towards EE2. Second, we are aiming at construction of a self‐regenerating system allowing cyclic oxidation and reduction of Mn oxides linked to continuous concomitant degradation of EE2.
In this study, we propose an efficient strategy for the removal of trace levels of xenoestrogens from environmental samples by biogenic generation of highly oxidized Mn oxides by manganese‐oxidizing bacteria, which in turn drive the indirect oxidation and cleavage of EE2. As degradation of substituted phenols, fluoroquinolone antibiotics, aniline and other aromatic amines by manganese oxides have also been reported (Stone, 1987; Laha and Luthy, 1990; Zhang and Huang, 2005), the use of highly oxidized biogenic Mn oxides can be expanded to the degradation of other organic pollutants. The use of manganese‐oxidizing bacteria can be a starting point for the development of a more general strategy for the purification of waste and drinking water from estrogens and other trace‐level organic contaminants.
Results and discussion
In order to test if manganese oxides derived from oxidation of Mn2+ by manganese‐oxidizing bacteria may mediate degradation of EE2, four different microbial strains namely Leptothrix discophora, Pseudomonas putida strains LMG 2321, 2322 and 2323, which are known to catalyse oxidation of Mn2+, were tested for EE2 degradation. These strains were grown on a complete medium with glucose as a carbon source and with a small amount of EE2 (0.5 µM) in presence or absence of different concentrations of Mn2+ (25, 50, 75, 100 µM). These concentrations are known to induce oxidation of manganese by these manganese‐oxidizing bacterial species. All tested strains have shown the ability to oxidize Mn2+, as observed by the decrease of Mn2+ content (Table 1) and also by accumulation of Mn oxides, visible as insoluble brown or orange particulate matter. The amount of EE2 degraded by L. discophora and LMG 2322 strains was proportional with the oxidized Mn2+. Without manganese only a slight decrease of EE2 (around 10%) was detected (Table 1), which was independent from the culture growth and may be attributed to adsorption of EE2 to biomass. All the remaining levels of EE2 were found to be very stable in the Mn‐free bacterial cultures even after 4 weeks of incubation (data not shown). Elevated concentrations of Mn2+ (higher than 100 µM) negatively affected oxidation of Mn2+ and the degradation of EE2 (data not shown), which again points at Mn2+ oxidation to be a prerequisite to the degradation of EE2.
Table 1.
Degradation of EE2 by different manganese‐oxidizing strains at different initial concentrations of MnCl2.
| Strains | Initial Mn2+ (µM) | EE2 degraded (µM) | Mn2+ oxidized (µM) | EE2 adsorbed to Mn oxides, % |
|---|---|---|---|---|
| L. discophora | 0 | 0.074 | ||
| 25 | 0.477 | 25 | 0 | |
| 50 | 0.5 | 50 | 0 | |
| 75 | 0.5 | 75 | 0 | |
| 100 | 0.5 | 100 | 0 | |
| P. putida 2321 | 0 | 0.013 | ||
| 25 | 0.466 | 25 | 2.5 | |
| 50 | 0.479 | 50 | 0 | |
| 75 | 0.5 | 75 | 0 | |
| 100 | 0.481 | 100 | 0 | |
| P. putida 2322 | 0 | 0.042 | ||
| 25 | 0.5 | 25 | 0 | |
| 50 | 0.5 | 50 | 0 | |
| 75 | 0.5 | 75 | 0 | |
| 100 | 0.5 | 100 | 0 | |
| P. putida 2323 | 0 | 0.049 | ||
| 25 | 0.443 | 25 | 0 | |
| 50 | 0.433 | 50 | 5.6 | |
| 75 | 0.144 | 59.1 | 8.2 | |
| 100 | 0 | 100 | 0 |
EE2 degradation was measured by HPLC analysis, and adsorption of EE2 to biogenic manganese oxides were determined as described in Experimental procedures section. All parameters were taken after 48 h of growth. For each measurement, data represent a mean value obtained from three identical cultures.
The initial concentrations of Mn2+ used in this study are known from the earlier studies to induce Mn oxidation, but were by far represented in excess compared with 0.5 µM EE2. In order to establish a lower limit of Mn2+ which starts to catalyse degradation of EE2, LMG 2322 was grown with 0.5 µM of EE2 and 1, 10 and 50 µM Mn2+. Degradation of EE2 is dependent on the amount of Mn2+ present in the medium and that multi‐fold excess of Mn2+ was required (Fig. 1). Most of EE2 was degraded in the late stationary phase and thus was not linked to the growth of the bacteria (Fig. 1). Moreover, the degradation of EE2 occurred in time after Mn oxidation: for the culture with 50 µM of Mn2+ most of EE2 was degraded in the period between 24 and 36 h, whereas most of Mn2+ was oxidized earlier, in the period between 18 and 24 h (Fig. 1). This delay is likely to be linked to the primary accumulation of significant amounts of biogenic Mn oxides, which then start to react with EE2. Thus, degradation of EE2 is a slow chemical process that is not dependent on the presence of viable bacterial cells, but rather on the amount of biogenic Mn oxides produced. Influence of microbial activity on Mn‐dependent degradation of EE2 was tested by addition of sodium azide, a known inhibitor of electron transport‐linked oxidation. Pseudomonas putida was grown in presence of 100 µM of MnCl2 until orange coloured Mn oxides were visible and EE2 and sodium azide at a concentration of 1 mM were added. A culture without sodium azide was used as a control. Addition of sodium azide still allowed 80% degradation of EE2, whereas without azide complete degradation of EE2 occured. We suspect that the 20% inhibition caused by azide could be linked to inability of the azide‐treated cultures to regenerate Mn oxides.
Figure 1.
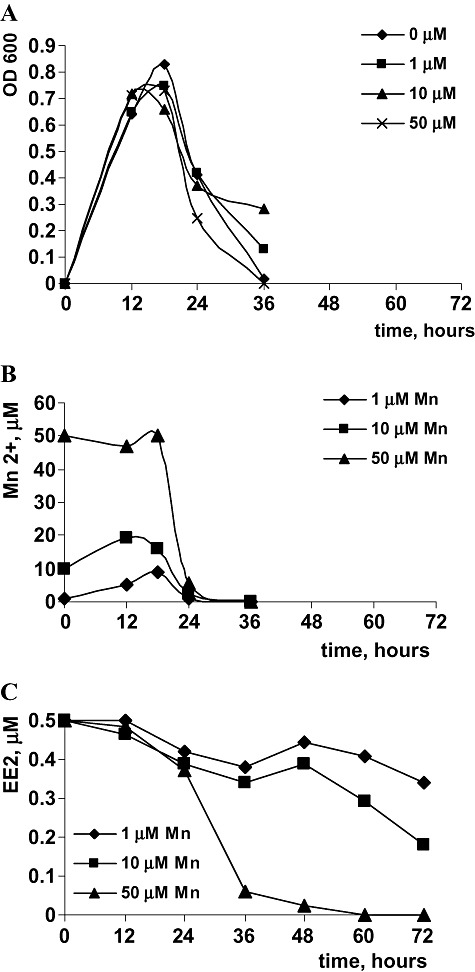
A. Growth of P. putida in the presence of 0.5 µM of EE2 and 1, 10 and 50 µM of Mn2+. B. Dynamics of Mn2+ oxidation. C. Dynamics of degradation of 0.5 µM of EE2 with different initial concentrations of Mn2+. For each measurement, data represent a mean value obtained from three identical cultures.
Earlier it was suggested that EE2 may be adsorbed in significant amounts to chemically synthesized manganese oxides (de Rudder et al., 2004). In order to rule out the adsorption of EE2 to insoluble Mn oxides generated by the Mn2+‐oxidizing bacteria and thus to distinguish it from oxidation of EE2, cultures were treated with ascorbic acid, which is known to reduce higher oxidation states of Mn3+/4+ back to readily soluble Mn2+ ions (Stone and Morgan, 1984), thereby releasing potentially adsorbed EE2. With few exceptions (e.g. LMG 2321 at 25 mM Mn2+, LMG 2323 at 50 µM and 100 µM Mn2+), where small amounts of apparently adsorbed EE2 were recovered after ascorbic acid treatment, in all other Mn2+‐supplied samples no additional EE2 was released after treatment with ascorbic acid (Table 1). Ascorbic acid by itself did not affect the concentration of EE2, as shown by EE2 concentrations of Mn‐free control cultures before and after the treatment with ascorbic acid (data not shown). Moreover, upon careful examination of HPLC spectra an intermediary product of EE2 was detected at low levels and with a retention time of 9.8. Because this product disappeared in a manganese‐dependent fashion and had no measurable estrogenic effect, its structure was not further examined. Therefore, decreased levels of EE2 in the respective Mn‐containing cultures are accomplished by true degradation, rather than by adsorption to the insoluble Mn oxides that are formed.
As oxidation of EE2 by Mn oxides may result in production of metabolites with undesirable estrogenic activity, we performed yeast estrogen screen (YES) bioassays. The YES assay is based on the use of a recombinant yeast strain that harbours a human estrogen receptor gene and is used to measure the estrogenic activity of environmental samples (Pierrat et al., 1992; Baker, 2001). Estrogenic response of the culture of the strain LMG 2322 grown with 0.5 µM EE2 and 100 µM of Mn2+ was comparable with the one of negative control, indicating that no residual estrogenic activity in this Mn‐containing sample was present where EE2 was found to be eliminated (Fig. 2). By contrast, in the corresponding culture grown in the absence of Mn2+ where EE2 was still present, strong estrogenic response was found as expected (Fig. 2).
Figure 2.
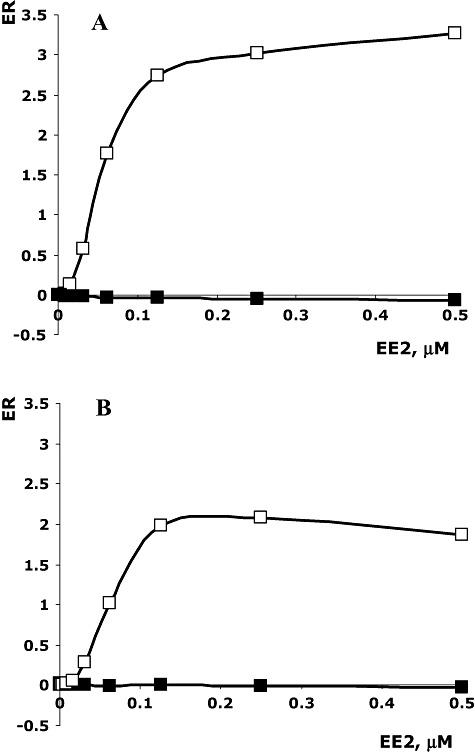
Estrogenic responses of cultures of P. putida 2322 grown with 0.5 µM EE2 and with/or without 100 µM of MnCl2 (black or white squares, correspondingly) (B). Estrogenic responses of distilled water (black squares) and a solution of 0.5 µM EE2 (white squares) were used as corresponding controls (A).
It was shown that degradation of EE2 may be linked to the extracellular enzymatic activity in other organisms, such as to the manganese peroxidase and multicopper oxidase activities of white rot fungi (Suzuki et al., 2003). This is unlikely to be the case for the manganese‐oxidizing bacteria tested here for the following reasons: (i) the rate of EE2 degradation is strongly correlated with the amount of Mn2+ oxidized and such correlation is normally unlikely to be linked to the enzymatic activity; (ii) oxidation of EE2 does not coincide in time with the oxidation of Mn2+, when the Mn2+‐oxidizing enzyme is produced, but it is delayed and rather linked with the period when most of Mn2+ has been oxidized and the bulk of Mn oxides have been accumulated; (iii) enzymatic treatment of the Mn2+‐ and EE2‐containing culture with pronase at two different concentrations of the enzyme (100 µM, 1 mM) with the aim to abolish the extracellular enzymatic activity has not resulted in any noticeable inhibition of the EE2 degradation (data not shown).
To conclude, manganese‐oxidizing bacteria are of particular biotechnological interest, not only with respect to their use in the well‐established biological treatment of manganese‐containing waters, but also for bioremediation of environments contaminated with trace concentrations of recalcitrant organic pollutants. We presented the indirect oxidation of EE2 by biogenic manganese oxides as a concept of a novel biological process aiming to eliminate trace concentrations of organic pollutants from environmental samples. The initial attack of highly oxidized manganese oxides on EE2 is likely to address chemical features of the phenolic groups also found in other polycyclic aromatic compounds, which could thus likewise be degraded by highly oxidized manganese. The breakdown of other compounds like amines, aromatic amines and xenobiotics with sulphydryl groups were also reported to be chemically degraded by Mn oxides, and are thus also likely to be degraded in the presence of manganese‐oxidizing bacteria and Mn2+. Our future studies will be focused on designing a bioprocess aiming at the degradation of EE2 by Mn‐oxidizing bacteria. A serious step to consider is that the lowest concentration which still allows efficient degradation of EE2 found in this study was 50 µM, which is still five times higher than the allowed concentrations of manganese in drinking water. However, designing a bioreactor with alternating conditions would result in both complete degradation of EE2 and complete oxidation of Mn. The oxidized insoluble manganese oxides would then precipitate and could be this way removed from the water. Generally, these results draw attention to the potential value of microbial extracellular oxidative processes (like bacterial manganese oxidation) for biotechnological processes aiming at degradation of trace pollutants.
Experimental procedures
Bacterial strains and culture conditions
Leptothrix discophora (LMG 8142, Belgian coordinated collections of microorganisms) and P. putida strains MnB1 (LMG 2321), MnB6 (LMG 2322) and MnB29 (LMG 2323) were cultivated on a medium described by Boogerd and de Vrind (1987). To assess degradation of EE2 and to determine manganese oxidation by the manganese‐oxidizing bacteria, two types of overnight pre‐cultures were set without and with filter‐sterilized manganese chloride at a concentration of 25 µM. On the following day, 50 ml of the medium with EE2 (0.5 µM) and MnCl2 at different concentrations (0, 25, 50, 75, 100 µM) were inoculated, bacterial growth (by measuring OD600) was monitored and concentrations of EE2 and Mn2+ were measured.
Measurement of Mn oxidation
Mn2+ oxidation was monitored in 2 ml aliquots from the cultures, which were centrifuged at 13 400 g for 30 min. After Mn2+ concentrations were determined by using the formaldoxime assay described by Bartley and colleagues (1957). Briefly, 3 ml of the supernatant was mixed with 1 ml of formaldoxime hydrochloride solution (Sigma‐Aldrich, 0.5 g dissolved in 10 ml of distilled water). After 10 min reaction time, absorption was measured at 455 nm. The sample solutions were read against a manganese‐free solution treated in the same way.
HPLC analysis
Culture aliquots were filtered through a 0.22 µm filter prior to HPLC analysis. In order to test for potential adsorption of EE2 to particulate Mn oxides generated by bacterial oxidation of Mn2+, ascorbic acid (0.1–1 M) was added to 5 ml culture aliquots, and samples were shaken for 15 min at 30°C. The samples were analysed for EE2 before and after the treatment with ascorbic acid, which, being a strong reductant, was expected to release adsorbed EE2 by regenerating soluble Mn2+ from insoluble Mn oxides. EE2 and its potential metabolites were analysed by reversed phase HPLC with an ASI‐100 autosampler, P580 pump and STH585 column oven (Dionex, Sunnyvale, CA, USA) on an Agilent Zorbax SB‐C18 column (150 mm × 4.6 mm, 5 µm). Elution was performed at 25°C and a flow rate of 1 ml/min with solvent A (100% acetonitrile) and solvent B (MilliQ + 0.1% formic acid). The injection volume was 50 µl and the elution program was as follows: 2 min isocratic at 85% B, linear gradient to 60% B in 5 min, linear to 35% B in 15 min, linear to 0% B (or 100% A) in 3 min follow by 5 min isocratic 100% A, linear gradient to 85% B and isocratic at 85% for 5 min. Detection of EE2, E2 and E3 was performed at an excitation‐emission wavelength of 280–310 nm (Yoon et al., 2003) by RF2000 Fluorescence detector (Dionex) and E1 was detected with UV‐VIS at 200 nm (UVD340S, Dionex). Standards ranging from 2 to 1000 µg l−1 for EE2 for calibration were prepared, starting from individual ethanol stock solution containing 1 g l−1 of EE2.
Yeast estrogen screen
The YES assay is based on the use of a recombinant yeast strain that harbours a human estrogen receptor gene (Pierrat et al., 1992) and is used to measure the estrogenic activity of environmental samples (Baker, 2001). The YES assay was performed on stationary‐grown cultures of manganese‐oxidizing bacteria. The bacteria were grown either with manganese and EE2 or with manganese alone (control conditions) for 72 h (until late‐stationary stage of growth) as described above. One millilitre of each culture was then filtered through a 0.22 µm‐filter and filtrates were used for the YES assay. The YES assay was performed as described (De Boever et al., 2001) in 96‐well plates in which 10 µl of filtrates diluted twice with dimethyl sulfoxide were incubated with 240 µl of the genetically modified Saccharomyces cerevisiae at an optical density of 0.25 at 610 nm. Additional plates with serial dilutions of either the medium alone (negative control), or of medium supplemented with EE2 in DMSO (positive control) were made and equally tested. Estrogenic activity responses were recorded as the absorbance at 540 nm divided by the optical density at 630 nm [(A540/A630)net] (SunriseTM, plate reader, Tecan, Grödig, Austria). Based on the data obtained, dose–response curves for doses versus activity were generated. The data were fitted by a four‐parametric logistic model using the Marquardt‐Levenberg algorithm (Sigmaplot 4.0; SPSS Inc., Chicago, IL, USA; De Boever et al., 2001).
Acknowledgments
The authors would like to acknowledge EC for financial support within the frame of the Neptune project (036845, SUST DEV‐2005‐3.II.3.2). J.S. would like to acknowledge Massimo Marzorati for critical reading of the manuscript.
References
- Baker V.A. Endocrine disrupters – testing strategies to assess human hazard. Toxicol In Vitro. 2001;15:413–419. doi: 10.1016/s0887-2333(01)00045-5. [DOI] [PubMed] [Google Scholar]
- Bartley W., Notton B.M., Werkheiser W.C. Determination of manganese in tissue extracts by means of formaldoxime. Biochem J. 1957;67:291–295. doi: 10.1042/bj0670291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodour A.A., Wang J.M., Brusseau M.L., Maier R.M. Temporal change in culturable phenanthrene degraders in response to long‐term exposure to phenanthrene in a soil column system. Environ Microbiol. 2003;5:888–895. doi: 10.1046/j.1462-2920.2003.00481.x. [DOI] [PubMed] [Google Scholar]
- De Boever P., Demare W., Vanderperren E., Cooreman K., Bossier P., Verstraete W. Optimization of a yeast estrogen screen and its applicability to study the release of estrogenic isoflavones from a soygerm powder. Environ Health Perspect. 2001;109:691–697. doi: 10.1289/ehp.01109691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boogerd F.C., De Vrind J.P. Manganese oxidation by Leptothrix discophora. J Bacteriol. 1987;169:489–494. doi: 10.1128/jb.169.2.489-494.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns M.A., Hanson J.R., Mefford J., Scow K.M. Isolate PM1 populations are dominant and novel methyl tert‐butyl ether‐degrading in compost biofilter enrichments. Environ Microbiol. 2001;3:220–225. doi: 10.1046/j.1462-2920.2001.00184.x. [DOI] [PubMed] [Google Scholar]
- De Rudder J., Van de Wiele T.V., Dhooge W., Comhaire F., Verstraete W. Advanced water treatment with manganese oxide for the removal of 17alpha‐ethinylestradiol (EE2) Water Res. 2004;38:184–192. doi: 10.1016/j.watres.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Emerson D., Ghiorse W.C. Isolation, cultural maintenance, and taxonomy of a sheath‐forming strain of Leptothrix discophora and characterization of manganese‐oxidizing activity associated with the sheath. Appl Environ Microbiol. 1992;58:4001–4010. doi: 10.1128/aem.58.12.4001-4010.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiyan R., Shulan J., Ahmad N., Dao W., Chengwu C. Degradation characteristics and metabolic pathway of 17alpha‐ethinylestradiol by Sphingobacterium sp. JCR5. Chemosphere. 2007;66:340–346. doi: 10.1016/j.chemosphere.2006.04.064. [DOI] [PubMed] [Google Scholar]
- Laha S., Luthy R.G. Oxidation of aniline and other primary aromatic amines by manganese dioxide. Environ Sci Technol. 1990;24:363–373. [Google Scholar]
- Nealson K.H., Ford J. Surface enhancement of bacterial manganese oxidation: implications for aquatic environments. Geomicrobiol J. 1980;2:21–37. [Google Scholar]
- Pierrat B., Heery D.M., Lemoine Y., Losson R. Functional analysis of the human estrogen receptor using a phenotypic transactivation assay in yeast. Gene. 1992;119:237–245. doi: 10.1016/0378-1119(92)90277-v. [DOI] [PubMed] [Google Scholar]
- Raynal M., Pruden A. Aerobic MTBE biodegradation in the presence of BTEX by two consortia under batch and semi‐batch conditions. Biodegradation. 2008;19:269–282. doi: 10.1007/s10532-007-9133-7. [DOI] [PubMed] [Google Scholar]
- Saratovsky I., Wightman P.G., Pasten P.A., Gaillard J.F., Poeppelmeier K.R. Manganese‐oxides: parallels between abiotic and biotic structures. J Am Chem Soc. 2006;128:11188–11198. doi: 10.1021/ja062097g. [DOI] [PubMed] [Google Scholar]
- Schweisfurth R. Manganese‐oxidizing bacteria. Isolation and identification of various manganese‐oxidizing bacteria. Z Allg Mikrobiol. 1973;13:341–347. [PubMed] [Google Scholar]
- Stone A.T. Reductive dissolution of manganese (III/IV) oxides by substituted phenols. Environ Sci Technol. 1987;21:979–988. doi: 10.1021/es50001a011. [DOI] [PubMed] [Google Scholar]
- Stone A.T., Morgan J.J. Reduction and dissolution of manganese (III) and manganese (IV) oxides by organics. Survey of the reactivity of organics. Environ Sci Technol. 1984;18:617–624. doi: 10.1021/es00126a010. [DOI] [PubMed] [Google Scholar]
- Sunda W.G., Kieber D.J. Oxidation of humic substances by manganese oxides yields low‐molecular‐weight organic substrates. Nature. 1994;367:62–64. [Google Scholar]
- Suzuki K., Hirai H., Murata H., Nishida T. Removal of estrogenic activities of 17ß‐estradiol and ethinylestradiol by ligninolytic enzymes from white rot fungi. Water Research. 2003;37:1972–1975. doi: 10.1016/S0043-1354(02)00533-X. [DOI] [PubMed] [Google Scholar]
- Thomas K.V., Hurst M.R., Matthiessen P., Waldock M.J. Characterization of estrogenic compounds in water samples collected from United Kingdom estuaries. Environ Toxicol Chem. 2001;20:2165–2170. doi: 10.1897/1551-5028(2001)020<2165:coeciw>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Yoon Y., Westerhoff P., Snyder S.A., Esparza M. HPLC‐fluorescence detection and adsorption of bisphenol A, 17beta‐estradiol, and 17alpha‐ethynyl estradiol on powdered activated carbon. Water Res. 2003;37:3530–3537. doi: 10.1016/S0043-1354(03)00239-2. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Nagai F., Fujimoto J., Watanabe K., Mizukoshi H., Makino T. Degradation of estrogens by Rhodococcus zopfii and Rhodococcus equi isolates from activated sludge in wastewater treatment plants. Appl Environ Microbiol. 2004;70:5283–5289. doi: 10.1128/AEM.70.9.5283-5289.2004. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Huang C.H. Oxidative transformation of fluoroquinolone antibacterial agents and structurally related amines by manganese oxide. Environ Sci Technol. 2005;15:4474–4483. doi: 10.1021/es048166d. [DOI] [PubMed] [Google Scholar]


