Summary
Enterobacter sakazakii (Cronobacter spp.) is an opportunistic pathogen, which can cause rare, but life‐threatening infections in neonates and infants through feeding of a contaminated milk formula. We isolated 67 phages from environmental samples and tested their lytic host range on a representative collection of 40 E. sakazakii strains. A cocktail of five phages prevented the outgrowth of 35 out of 40 test strains in artificially contaminated infant formula. Two E. sakazakii phages represented prolate head Myoviridae. Molecular tests identified them as close relatives of Escherichia coli phage T4. The remaining three phages represented isometric head Myoviridae with large genome size of 140 and 200 kb, respectively, which belonged to two different DNA hybridization groups. A high dose of 108 pfu ml−1 of phage could effectively sterilize a broth contaminated with both high and low pathogen counts (106 and 102 cfu ml−1). In contrast, broth inoculated with 104 phage and 102 bacteria per ml first showed normal bacterial growth until reaching a cell titre of 105 cfu ml−1. Only when crossing this threshold, phage replication started, but it could not reduce the contamination level below 100 cfu ml−1. Phages could be produced with titres of 1010 pfu ml−1 in broth culture, but they were not stable upon freeze‐drying. Addition of trehalose or milk formula stabilized the phage preparation, which then showed excellent storage stability even at elevated temperature.
Introduction
Enterobacter sakazakii is an opportunistic pathogen, which can cause rare, but life‐threatening infections in neonates and infants. The original taxonomical name of E. sakazakii has been updated to Cronobacter spp. (Iversen et al., 2007a,b; 2008). In this communication we focus on the two main Cronobacter species, C. sakazakii and C. malonaticus, which collectively have been referred to as E. sakazakii group 1 strains (Iversen et al., 2007a). Infections caused by E. sakazakii have a high mortality rate and severe neurological sequelae are often observed in surviving patients (Muytjens et al., 1983; Mullane et al., 2007). Several studies have implicated rehydrated powdered infant formula and its preparation in hospital settings with disease transmission (Simmons et al., 1989; van Acker et al., 2001). Standard prevention strategies include dry‐cleaning and zoning in factories producing infant formula on one side and rigorous hygiene in preparing, storing and feeding of reconstituted powdered infant formula in the hospital. Proper handling of reconstituted infant formula is crucial as powdered formula is not prepared as a sterile pharmaceutical product, but as non‐sterile food product which may contain bacteria. The contamination level with Enterobacteriaceae is in general very low: less than one colony‐forming unit (cfu) per 100 gram of formula are commonly measured (Muytjens et al., 1988; Forsythe, 2005). However, outgrowth of even very low bacterial contamination may occur if the reconstituted product is kept at room temperature or in an incubator for some time. Risk assessment studies have not established an infectious dose for E. sakazakii, but it seems reasonable to equate an increased contamination level with an increased disease risk. Recommended E. sakazakii infection control measures include the refrigeration of the reconstituted formula and minimizing the time between preparation and consumption of the formula (World Health Organization, 2004). In view of the dramatic consequences, novel approaches to decrease the risk of E. sakazakii transmission from powdered milk to infants are clearly justified.
Bacteriophages, i.e. viruses that infect bacteria in a species‐specific way, are currently discussed as attractive alternatives to antibiotics for the treatment and prevention of bacterial infections. In addition, phages have been proposed for the control of food‐borne bacterial pathogens either by application to the factory (‘biosanitation’) or by addition to the food product as a supplementary hurdle to the outgrowth of the food pathogen (Fischetti, 2001; Merril et al., 2003). From the biotechnological viewpoint phages represent an attractive tool, as they can generally be isolated from the same environment where the food pathogen is encountered. Furthermore, phages are highly specific for their bacterial host, causing no damage to other bacterial species and thus lacking the undesired effects of antibiotics on the human commensal microbiota (Ashelford et al., 2003; Bruttin and Brüssow, 2005; Brüssow, 2005; Greer, 2005). Finally, phages do not infect human cells and adverse effects of phage ingestion are thus unlikely. In fact, phages are routinely consumed in significant numbers with fermented food (e.g. yoghurt, cheese, sauerkraut, pickles, salami) (Kennedy et al., 1986). Several recent studies have explored the potential of phages for controlling foodborne pathogens and spoilage organisms. Phage application lowered the number of Salmonella and Listeria monocytogenes on fresh‐cut fruit and soft cheese and the Food and Drug Administration has approved a Listeria phage cocktail for food applications (http://www.fda.gov/OHRMS/DOCKETS/98fr/cf0559.pdf) (Leverentz et al., 2001; 2003; 2004; Carlton et al., 2005). Phages have also been tested for the decontamination of chicken skin from Campylobacter and beef from Escherichia coli O157 (Atterbury et al., 2003; Goode et al., 2003; O'Flynn et al., 2004). A study exploring the use of two E. sakazakii‐specific phages for the decontamination of milk formula is following a similar line of thoughts (Kim et al., 2007).
Despite the fact that phage preparations are sold as a registered medicine in Russian pharmacies, microbiologists in the West are still split with respect to the prospect of phage use: phage enthusiasts and phage skeptics are met with equal frequency. It is therefore important to accumulate more experimental data to document impartially the potential and limitation of practical phage use. In the present communication we took E. sakazakii phages as a test case and explored their isolation and host range, their lytic potential and threshold phenomena, their purification and product stability with respect to their potential as a tool against a low level contamination of food with bacterial pathogen.
Results
The E. sakazakii strain collection
To represent a realistic option for a biotechnological application, phages must fulfil a number of requirements. As phages tend to be strain‐specific, the first crucial question is what coverage can be achieved with a phage cocktail on the target pathogen. To achieve an epidemiologically representative collection, twenty‐one E. sakazakii strains were selected from public sources and they were complemented with 39 isolates from our company culture collection to ensure a broad geographical coverage (Fig. 1C). Ribotyping identified 57 of the strains as belonging to E. sakazakii group 1 (Fig. 1A and B), which is clinically the most relevant group.
Figure 1.
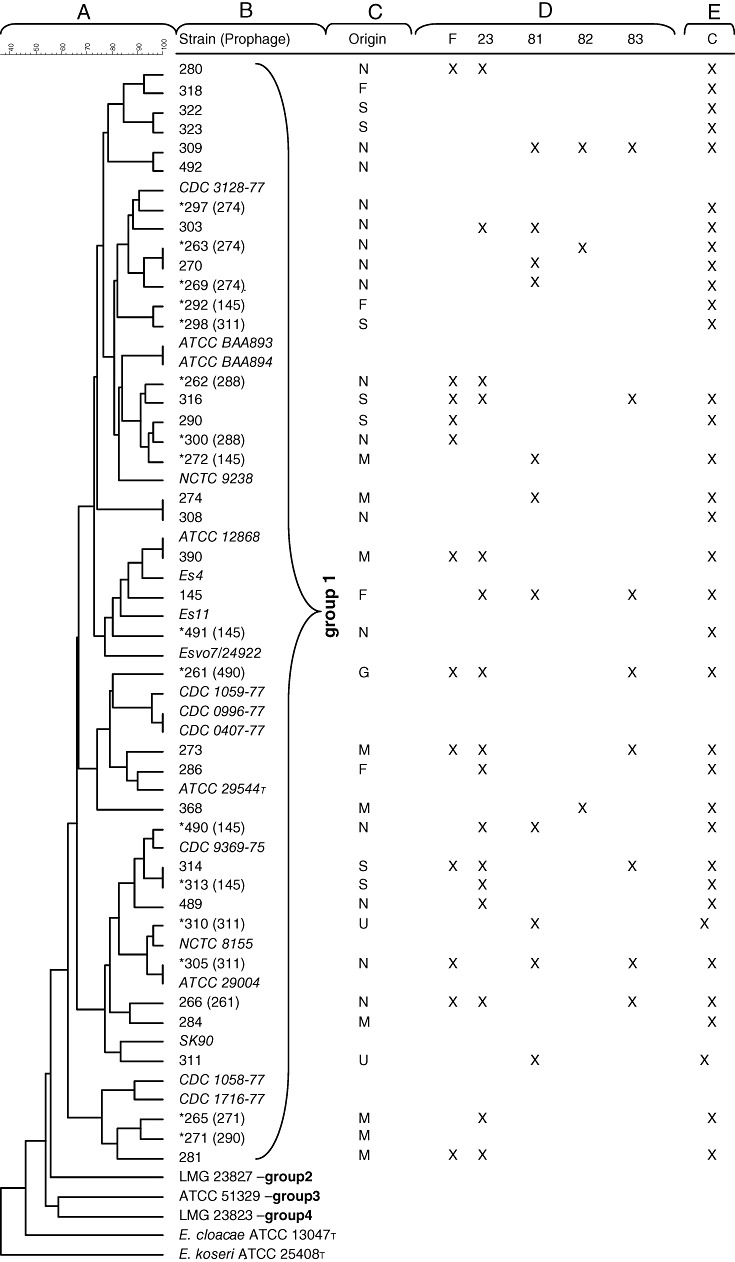
E. sakazakii host strain differentiation. From left to right: Dendrogram of 39 strains of E. sakazakii (A) (the scale bar shows the percentage of similarity), obtained after restriction with EcoRI. Groups 1–4 indicate assignment of the strains to 16S rRNA groups (Iversen et al., 2007a). The FSM strain designation (with reference strains in italics) is shown (B). The star symbol (*) indicates the presence of a prophage and the strain designation in parenthesis corresponds to the indicator strain used to reveal it. Origins (N, the Netherlands; F, France; S, Switzerland; M, Malaysia; G, Germany; U, USA) of the selected strains (C). Susceptibility of the E. sakazakii strain from the corresponding row to infection with E. sakazakii phages F, 23, 81, 82 and 83 as determined by the plaque assay using 105 pfu ml−1 on BHI‐agar; ‘X’ indicates observation of phage plaques (D). Enterobacter sakazakii challenge test in infant formula using a cocktail of the five phages showing the broadest host range on E. sakazakii; ‘X’ indicates prevention of outgrowth of 102 cfu ml−1E. sakazakii when phage was added at 108 pfu ml−1 (E).
Prophage induction
The easiest source of phages is prophages induced from the lysogenic bacterium. Seventeen from our 39 collection strains (44%) released phages when induced with mitomycin C (Fig. 1B). We were able to define at least three groups of prophages by host range, as five induced prophages could be propagated on the non‐lysogenic E. sakazakii strain 145, three prophages on strain 311 and three prophages on strain 274 respectively. As some induced prophages could also be propagated on lysogenic strains, different immunity groups must exist in E. sakazakii phages. We observed sometimes complicated relationships, e.g. the prophage from strain 266 could be propagated on lysogen 261, while the prophage from 261 grew on lysogenic strain 490 and the prophage from 490 could infect the non‐lysogenic strain 145 (Fig. 1B).
Isolation of virulent phages
As temperate phages from Enterobacteriaceae are known to carry bacterial pathogenicity genes, induced prophages present a safety risk for practical phage application. To enrich for obligate virulent (‘lytic’) phages, we screened cell‐free environmental samples from milk powder factories, waste water and sewage treatment plants for E. sakazakii phages. Overall, 25 out of 83 environmental samples (30%) yielded a phage. Samples from sewage treatment plants provided more frequently E. sakazakii‐specific phages (16 positive out of 18 samples analysed) than samples from factory environments (8 positive out of 50 samples analysed). A total of 67 phages were isolated.
Host range
The host range of the individual phage isolates was determined on the group 1 E. sakazakii strains with the help of the plaque assay. Some environmental phage isolates were strain‐specific, others infected a few strains (e.g. phage 82), while a few environmental phage isolates infected up to 16 of the 39 strains of our collection (e.g. phage 23 infected 41% of the pathogenic strains) (Fig. 1D). With a combination of five phages, namely isolates F, 23, 81, 82 and 83, we achieved 72% coverage on our 39 test strains (Fig. 1E). These five phages were then investigated in more detail. In the spot assay on a large number of food pathogens, phages 81 and 83 showed lytic activity also on Citrobacter freundii. Phage 23, which showed the broadest host range on E. sakazakii strains, even infected three other genera (Hafnia, Serratia, Klebsiella) (Table 1). However, a panel of 11 commensal E. coli strains from the ECOR collection were not infected.
Table 1.
Specificity test.
| Strains | Phages | ||||
|---|---|---|---|---|---|
| F | 23 | 81 | 82 | 83 | |
| Enterobacter agglomerans (FSM 415) | X | X | |||
| Enterobacter cloacae (FSM 90) | X | X | X | ||
| Hafnia alvei (FSM 42) | X | ||||
| Shigella dysenteriae (FSM 1) | X | ||||
| Serratia rubidea (FSM 248) | X | ||||
| Klebsiella pneumoniae (FSM 36) | X | ||||
| Citrobacter freundii (FSM 34) | X | X | |||
| Salmonella Typhimurium (FSM 496) | X | ||||
X, lysis. Strains which were not inhibited by the E. sakazakii phages were the following: Enterobacter aerogenes (FSM 149), Enterobacter gergoviae (FSM 268), E. coli (FSM 219) and ECOR strains 1, 2, 4, 5, 6, 8, 9, 11, 12, 13 and 14 from the standard E. coli reference strain collection (Ochman and Selander, 1984), Escherichia hermanii (FSM 39), Serratia liquefaciens (FSM 142), Klebsiella oxytoca (FSM 38), Salmonella enterica (FSM 495), S. Enteritidis (FSM 331), S. seftenberg (FSM 473), Yersinia enterolitica (FSM 67), Proteus mirabilis (FSM 69), P. vulgaris (FSM 63), Providencia rettgeri (FSM 65), Pseudomonas aeruginosa (FSM 36, FSM 37), P. fluorescens (FSM 30), Aeromonas hydrophila (FSM 32), Acinetobacter baumanii (FSM 406), Listeria monocytogenes (FSM 39) and Staphylococcus aureus (FSM 115).
T4 Myoviridae of E. sakazakii
Two of the phages, namely phages 81 and 82, both sewage isolates from Switzerland, showed in negative stain electron microscopy the morphology of typical T4‐like Myoviridae, the prototype of the obligate virulent (‘lytic’) phages. These phages are characterized by elongated heads, collars, contractile tails and typical baseplates with tail spikes (Fig. 2C and D). Tail fibres were not observed, but might have been lost during the preparation of the phages for electron microscopy. Genome analysis supported the morphological diagnosis of T4‐like phages: in pulsed‐field gel electrophoresis the DNA from phages 81 and 82 showed a molecular size slightly below 200 kbp, the typical genome size range for T4‐like phages (Fig. 3A). A PCR test based on the T4 gene g32, which is diagnostic for close relatives of E. coli phage T4 was negative for them (data not shown) (Monod et al., 1997). However, a PCR test based on the better conserved T4 gene g23, encoding the major head protein (Tétart et al., 2001), yielded a clear amplification product for phages 81 and 82, which co‐migrated with the amplification product of the T4 control (Fig. 3B). The PCR result was confirmed by Southern hybridization: a labelled T4 probe cross‐hybridized at moderate intensity with phage 81 and 82 DNA (Fig. 4B), characterizing them as relatives of E. coli phage T4.
Figure 2.

Negative‐staining electron microscopy of CsCl‐purified E. sakazakii phages constituting the 5‐phage cocktail. (A) phage F; (B) phage 23; (C) phages 81; (D) phage 82 and (E) phage 83. The negative stain was ammonium molybdate/bacitracine. Scale bars, 100 nm.
Figure 3.

Molecular characterization of the phage genomes from the 5‐phage cocktail.
A. Pulsed‐field gel electrophoresis of E. sakazakii phages. (1), phage F; (2), phage 23; (3), phage 81; (4), phage 82; (5), phage 83; and (M), size marker (phage  concatemers, 50 kb DNA ladder, Promega).
B. PCR analysis on phage DNA with primers Mzia1 and CAP8 amplifying the major head gene g23 (Tétart et al., 2001). (M), Smartladder (Eurogentec); (+), T4 positive control; (1), phage F; (2), phage 23; (3), phage 81; (4), phage 82; (5), phage 83; and (−), negative control without DNA.
concatemers, 50 kb DNA ladder, Promega).
B. PCR analysis on phage DNA with primers Mzia1 and CAP8 amplifying the major head gene g23 (Tétart et al., 2001). (M), Smartladder (Eurogentec); (+), T4 positive control; (1), phage F; (2), phage 23; (3), phage 81; (4), phage 82; (5), phage 83; and (−), negative control without DNA.
Figure 4.

Restriction analysis and Southern hybridization of E. sakazakii phages. (A) DraI restriction digests of E. sakazakii phage DNA. (M), Smartladder (Eurogentec); (T4), T4 phage; (1), phage F; (2), phage 23; (3), phage 81; (4), phage 82; and (5), phage 83. (B, C) Corresponding Southern blot hybridization using DIG‐labelled phage T4 DNA (B) or phage F DNA (C) as a probe respectively.
Isometric head Myoviridae of E. sakazakii
Phages F and 23, both isolated from a milk powder factory, and phage 83, a sewage isolate from Switzerland, represent Myoviruses with isometric heads (Fig. 2A, B and E). The DNA from phages F and 83 migrated with a molecular weight slightly below 150 kb (Fig. 3A). Phage 23, although presenting a similar virion morphology, (Fig. 2) showed a genome size in excess of 200 kbp (Fig. 3A). We confirmed by Southern hybridization that phage 23 on one side and phages F and 83 on the other side represent two distinct groups. A labelled DNA probe from phage F cross‐hybridized with phage 83 DNA, but not with phage 23 DNA (Fig. 4C).
Challenge tests: high contamination level
Next we investigated the effect of the isolated phages on E. sakazakii grown in brain–heart infusion (BHI) broth. The medium was inoculated with a high bacterial titre of 106 cfu ml−1. The culture was then split in two halves. One half received a high phage dose of 108 plaque‐forming units (pfu) ml−1[multiplicity of infection (moi) = 100] from either the isometric head Myophage F (Fig. 5A) or the prolate head T4‐like Myophage 82 (Fig. 5B), while the other halves remained uninfected. Until 3 h post inoculation the optical density reading showed a parallel OD increase in uninfected and infected cultures. This phase was followed by an OD decrease in the infected culture while the control culture demonstrated an ongoing growth (Fig. 5A and B). The 100‐fold phage titre increase, which we observed 3 h post infection, indicated that the OD decrease in the infected culture was the result of a lytic phage infection. Essentially identical results were obtained for E. sakazakii strain 316 infected with phage F (Fig. 5A) and E. sakazakii strain 368 infected with T4 like Myophage 82 (Fig. 5B). After 12 h, no outgrowth was observed for phage 82‐infected strain 368, while outgrowth was starting for phage F (data not shown).
Figure 5.

Challenge tests. A. Prevention of the outgrowth of 106 cfu ml−1E. sakazakii strain 316 in the presence of 108 pfu ml−1 phage F (filled squares). The growth of the strain 316 in the absence of phage is documented by empty squares. The squares represent OD600 readings. The replication of phage F on the infected strain 316 is documented as pfu ml−1 readings (filled circles). B. A parallel experiment demonstrates the effect of phage 82 on E. sakazakii strain 368. C. Prevention of the outgrowth of 102 cfu ml−1E. sakazakii strain 316 in the presence of 108 pfu ml−1 phage F (filled squares). The growth of the strain 316 in the absence of phage is documented by empty squares. Bacterial and phage growth are documented as cfu ml−1 and pfu ml−1 (filled circles) respectively. D. Prevention of the outgrowth of 102 cfu ml−1E. sakazakii strain 316 in the presence of 104 pfu ml−1 phage F (filled squares). The growth of the strain 316 in the absence of phage is documented by empty squares. Bacterial and phage growth are documented as cfu ml−1 and pfu ml−1 (filled circles) respectively.
Challenge tests: low contamination level
As 106 cfu ml−1 is an unrealistic high initial contamination level for E. sakazakii, we repeated the experiment with a lower bacterial dose as inoculum. When the broth was seeded with 102 cfu ml−1, strain 316 grew to a final titre of 108 cfu ml−1 over the next 10 h (Fig. 5C). In the presence of a high dose of 108 pfu ml−1 of phage F (moi = 106), no outgrowth of the bacterial culture was observed. Actually, at 7 out of 10 time points the broth culture was sterile and the highest measured cell count was 10 cfu ml−1 (Fig. 5C). Note that at time zero, no viable cells were recovered. This phenomenon can be explained either enzymatically (‘lysis from without’) or through the rapid adsorption of the phages to the cells, preventing outgrowth after plating. The initially measured phage titre in the culture of 108 pfu ml−1, representing the inoculated phage, remained unchanged over the experiment.
In the next experiment we investigated the effect of moderate amount of phage on a low number of cells in the BHI broth. The medium was again inoculated with 102 cfu ml−1E. sakazakii strain 316, but this time with a 10 000‐fold lower phage dose of 104 pfu ml−1 of phage F (note, however, that the moi is still high: 100). The cell counts of the phage‐containing culture increased in parallel with the uninfected control culture until reaching a titre of 105 cfu ml−1 at 6 h after inoculation (Fig. 5D). At 7 h post inoculation, the cell count decreased in the infected culture while the cell numbers continued to increase in the uninfected control culture. We could observe a phage titre increase starting at 7 h post infection, demonstrating that the cell titre decrease is the result of phage infection. However, the phage infection did not sterilize the culture – about 100 cfu ml−1E. sakazakii was maintained in the medium. Twenty surviving colonies were picked from the plates and tested for phage susceptibility: 18 turned out to be resistant to phage F.
Lysis test
As an industrial reality check, reconstituted infant formula was inoculated with individual E. sakazakii strains from our collection using 102 cfu ml−1 as bacterial titre and 108 pfu ml−1 for the 5‐phage cocktail. At this moi of 106, the outgrowth of 36 of the 40 tested E. sakazakii strains (90%) was prevented when tested over a 4‐h incubation period at 30°C (Fig. 1E). Two of the four E. sakazakii strains, which were not lysed by the phage cocktail in reconstituted infant formula, belonged to the same branch of the ribotype cluster (strains 262, 300). However, at an moi of 104, the phage cocktail failed to prevent outgrowth of the test strains (data not shown).
Production and stability of phages
In 1 l batches of BHI broth culture we achieved yields of 109–1010 pfu ml−1 for the E. sakazakii phages. We used a simple phage isolation procedure: the phage lysate was cleared by centrifugation, the supernatant was sterile filtered and the phages were collected by centrifugation. The pellet was suspended into buffered physiological saline supplemented with magnesium ions for stabilization of the phages and subsequently freeze‐dried. All five phages constituting the cocktail showed substantial if not dramatic loss in infectivity upon freeze‐drying. In the best case phage F showed a 99% loss of infectivity, while the infectivity of phages 23 and 83 was totally lost (Fig. 6). Addition of the sugar trehalose prevented the loss of infectivity in phages F and 82, dramatically ameliorated the survival of phages 81 and 82, but had only moderate effects on the survival of phage 83 (Fig. 6). A number of other sugars (sucrose, sorbitol, maltodextrine) had comparable effects on the survival of phage infectivity (data not shown). When phage F was grown in a reconstituted infant formula to test its survival when freeze‐dried directly in the finished product, the phage survived the freeze‐drying without the need of trehalose (data not shown). Freeze‐dried phage F with or without the addition of trehalose underwent stability tests for long‐term storage at 4, 25 and 37°C. Over an observation period of more than 5 months, the trehalose‐containing phage preparation showed only a small titre reduction (Table 2). In contrast, phage F, which was freeze‐dried in physiological saline or infant formula, showed an important titre loss over time that was accelerated by increasing storage temperature (Table 2). Notably, phage F freeze‐dried in the presence of trehalose (but not with other sugars) survived as a powder a heat‐treatment of 70°C at least for 3 days (data not shown).
Figure 6.

Stability of E. sakazakii phages towards freeze‐drying. The bars represent data from phages F, 23, 81, 82 and 83 before (first bars) and after (second bars) freeze‐drying in phage buffer and before (third bars) and after (fourth bars) freeze‐drying in phage buffer plus 5% trehalose. Values are the average of three replicates and standard deviations are indicated.
Table 2.
Storage stability of freeze‐dried phage F.
| 4°C | 25°C | 37°C | |
|---|---|---|---|
| Buffer | 16.10 | 9.59 | 6.56a |
| Buffer + trehalose 5% | 115.78 | 53.76 | 37.63 |
| Infant formula | 36.27 | 14.20 | 7.17 |
| Infant formula + trehalose 5% | 48.55 | 19.80 | 11.67 |
Regression line only over 28 days, as afterwards phage numbers had fallen below detection level.
Phage F was stored after freeze‐drying in the presence of various cryoprotective agents and at various temperatures for a total of 175 days. Phage titres were determined after 7, 14, 21, 28, 56, 98, 133 and 175 days. Half‐lives are given in days.
Discussion
Bacteriophages have been proposed as novel tools against food contamination with bacterial pathogens. As FDA approval has already been received for a Listeria phage cocktail in food production (http://www.fda.gov/OHRMS/DOCKETS/98fr/cf0559.pdf), one might expect further food applications of phages in the near future. However, before phage application becomes a reality, a number of practical problems have to be solved.
The first challenge for any phage application is the isolation of specific phages. Knowing the niche of the target bacterium is frequently crucial for the successful isolation of phages. The natural niche of E. sakazakii has not been definitively identified – it might well represent the gut of insects (Hamilton et al., 2003). As for many other Enterobacteriaceae, E. sakazakii phages are easily isolated from sewage. Kim and colleagues isolated five phages when working with six E. sakazakii strains (Kim et al., 2007); we isolated 67 phages when using 39 E. sakazakii strains as test strains. The isolation of E. sakazakii phages is therefore not problematic. An alternative source of phages is lysogenic cells. In fact, we could propagate induced prophages from 17 out of 39 test strains, raising the number of isolated phages to 84.
The next question is whether the isolated set of phages is safe for a food application. As temperate phages from Enterobacteriaceae are known to carry important bacterial virulence determinants (Brüssow et al., 2004), induced prophages are a doubtful source of phages and should a priori be excluded from phage application in food. Virulent phages (in the phage therapy context perhaps better called ‘obligately lytic’ phages) were classically distinguished from temperate phages by plaque characteristics, namely a clear versus a turbid appearance. Only two out of the six phages isolated by the Zürich lab showed clear plaques, suggesting a majority of temperate phages in their isolates. In addition, one of their two phages with clear plaques showed the morphology of a B1 Siphovirus (Kim et al., 2007). The systematic analysis of bacterial genomes has demonstrated that this morphological group of phages contributes the majority of integrated prophage genomes associated with virulence genes (Canchaya et al., 2003). Lambdoid Siphoviridae from Enterobacteriaceae should therefore be excluded from practical application. As a result, only one phage from Kim and colleagues (2007) represented a safe class of phages, namely an A2 Myovirus resembling a T4 phage.
When we screened our E. sakazakii phages for broad‐host range, we apparently selected for clear plaque Myoviridae as previously observed with E. coli phages (Chibani‐Chennoufi et al., 2004; Brüssow, 2005). Within the five selected phages, two represented A2 group (prolate head) Myoviridae. Their morphology, large genome size, resistance to restriction enzymes, a diagnostic PCR and Southern hybridization characterized them as relatives of the E. coli phage T4, the prototype of the professional ‘lytic’ phage and the safest candidate for phage application. The three other broad‐host range E. sakazakii phages, which we isolated, belong to the A1 (isometric head) Myoviridae. Genome size (140 versus 200 kb) and Southern hybridization attributed them to two distinct phage families. Such phages might be quite common in this bacterial species, as another A1 E. sakazakii Myovirus was reported previously (Loessner et al., 1993). Related phages have been reported for E. coli and Klebsiella, but none of these has been sequenced (H. Ackermann, pers. comm.). One thus cannot make a positive statement about their suitability for practical phage application from a safety point of view. Therefore, it is advisable to sequence a representative of both A1 Myoviridae from E. sakazakii and screen them for undesired genes (Zuber et al., 2007), before including them into a phage cocktail.
The next practical question is whether the isolated phages infect a sufficient percentage of the target strains. With a combination of the five phages, we achieved 72% coverage on our 39 test strains (Fig. 1D). A relatively broad host range seems to be a general property of Enterobacter phages. Loessner and colleagues isolated phages with a very broad host range that infected in addition to their host Enterobacter cloacae also Enterobacter agglomerans, E. sakazakii, E. coli and Serratia marcescens (Loessner et al., 1993). Enterobacter phages are thus clearly versatile and even cross boundaries between bacterial genera. We observed the same broad host range with our E. sakazakii phage isolates. We have not met this property with other phages which we handled in our laboratory. Therefore, E. sakazakii might be quite exceptional regarding this property, but part of this observation might also reflect unusually closely related genera within the Enterobacteriaceae family. Notably, our E. sakazakii phages did not infect E. coli. As E. coli is also a common gut commensal in newborns, lack of activity of the phage cocktail on gut commensals should also be a criterion of phage selection.
The next point on a checklist for selection of appropriate phages is their lytic potential on the target species. With both morphotypes of Myoviridae we observed not only a strong growth inhibition, but even loss of viable cells when high cell numbers were inoculated with a high phage dose at 37°C. Kim and colleagues reported very similar results when using the T4‐like Myovirus at 37°C, while at 24°C and 12°C their T4 phage was bacteriostatic, but not bacteriolytic. The Siphovirus they used showed a temperature‐ and moi‐dependent lytic activity (Kim et al., 2007).
These researchers argue that their massive contamination with pathogen represents a worst case scenario. However, high bacterial counts represent a good cellular substrate as many phages can only replicate above a given threshold level of target cells. For E. coli phages this threshold has been determined to 104 cfu ml−1 (Wiggins and Alexander, 1985). The fundamentals of phage therapy have been explored by mathematical models. Some researchers dealt with pharmacokinetic aspects of phage use, modelling the temporal change of the phage concentration in the infected patient and at the site of infection during time. The self‐amplifying property of phages implicates indeed novel kinetic phenomena not known from antibiotics (Payne and Jansen, 2003). Others studied the interaction of combining two antimicrobial principles namely antibiotics and phages, which could lead both to interference and synergy between different antimicrobials (Comeau et al., 2007). Still others addressed problems of pharmacodynamics describing the changes in phages concentration with the growth and death rates of the bacterial population based on ecological models of predator–prey relationships (Levin and Bull, 2004). Evolutionary biologists concentrated on the biological aspects of different phage mutation rates and their impact on the arms race between bacteria and phages and the development of resistances (Kysela and Turner, 2007). Much discussion went into phage therapy as a density‐dependent kinetic process (Payne and Jansen, 2001). Ecologists demonstrated threshold phenomena by combining theoretical calculations with in vitro and in vivo experiments using T4 phage and E. coli. Notably, they confirmed the 104 cfu ml−1 threshold with in vitro experiments (Weld et al., 2004). Microbiologists denied that there is a metabolic basis for a phage replication threshold. They argued that the concept of the moi is wrong as the infection process does not only depend on the relative ratio of phage to target bacteria, but also on the absolute numbers of both partners (Kasman et al., 2002). Based on the mathematics governing the movements and binding of inert colloid particles under the influence of Brownian motion, they developed a model that distinguishes between initial moi (which is a pure phage/cell ratio) and actual moi (a ratio depending on absolute densities). They predicted that a very high actual moi is needed to achieve the infection of low numbers of target cells. This leads to the paradoxical prediction that almost the same number of phages is needed to infect 99.99% of 106 cells (what is expected for an moi of 10 under high cell counts) as to infect 102 cells.
Our experiments concur with this prediction: when we infected a low pathogen number with low phage titres, the cell grew out – apparently undisturbed by the phage – until they reached a titre of 104 to 105 cfu ml−1. Only after crossing this threshold, we observed phage replication and the lysis of E. sakazakii cells (Fig. 5D). Threshold phenomena might be a particularly relevant problem for phage use in biosanitation because food products tend to be contaminated with very low pathogen titres that frequently do not exceed 100 cfu g−1. In fact, we achieved with replicating phages only a suppression of the E. sakazakii cell count to about 100 cfu ml−1, but no sterilization of the broth. Part of the survival of the target cells might, however, be due to the selection of a phage‐resistance phenotype and not threshold phenomena. After longer incubation time Kim and colleagues also observed an outgrowth of T4 phage‐resistant bacteria (Kim et al., 2007). The dual problem of inefficient replication of phage on small bacterial populations and the selection of phage‐resistant bacterial derivatives might limit the use of E. sakazakii phages. Indeed, when we and Kim and colleagues used 102 cfu ml−1 as E. sakazakii inoculum, we could maintain the cell titre ≤ 10 cfu ml−1 only when using a high Myophage dose of 108 pfu ml−1. Even slightly decreasing the Myophage titre by 100‐fold allowed in both studies the survival of E. sakazakii. High phage titres were also necessary to reduce the Campylobacter or Salmonella contamination from retail poultry products (Atterbury et al., 2003; Goode et al., 2003) or E. coli O157:H7 from beef meat surfaces (O'Flynn et al., 2004), suggesting a general effect. A two‐way dialogue between biologists developing mathematical models of phage–bacterium interaction on one side and applied microbiologists studying this interaction with experiments in a complex food matrix or an experimental animal on the other side is necessary to develop phage therapy and prophylaxis. The experimentalists will constrain the mathematical parameters by experimental observations, while the model builder will help the experimentalist to understand otherwise paradoxical observations.
If one wants to assure product safety of infant formulae from E. sakazakii contamination, high amounts of phages have therefore to be added to the product. Can these phage titres be produced at reasonable costs? Without extensive optimization E. sakazakii phages grew in our hands to a titre of up to 1010 pfu ml−1 in 1 l containers. From a biotechnological viewpoint large‐scale phage production on a pathogenic strain is not desirable. Containment measures during phage growth and diligent purification of the phage from the lysed pathogen culture will increase the phage production cost. In view of its polyvalent character, production of E. sakazakii phages on non‐pathogenic Enterobacter species like E. cloacae might be an option which could substantially reduce phage production costs.
Are E. sakazakii phages stable enough to survive their preparation by freeze drying needed for the production of a powdered milk formula? Four of the five E. sakazakii phages which we investigated suffered substantial losses. However, the disaccharide trehalose and a few other sugars increased the stability of all but one of the tested phages. These results show that, for a practical future application such as preparing a cocktail of freeze‐dried phages, phages would need to be freeze‐dried individually, in order to compensate for different losses during freeze‐drying.
On the basis of the presented results, what is the prospect for E. sakazakii phage application? Product safety can only be reached with high phage concentrations added to the finished product, which raises the question of phage safety for infants, product availability and phage production cost. Part of these issues might be solved when producing the phages on a non‐pathogenic propagation strain with easy growth properties making an industrial up‐scaling possible. Phage safety issues could be addressed with genome sequencing and volunteer tests. As very high phage concentrations are needed to assure product safety, one might wonder whether biotechnologically produced phage lysins might not be an easier solution. However, it might well turn out that replication‐competent phages could protect the consumer even after consumption of the milk with low E. sakazakii counts. Like in a medical phage therapy application, E. sakazakii phages could keep the in vivo intestinal outgrowth of the pathogen in check before it reaches concentrations allowing a systemic spread. Two animal models have been proposed for E. sakazakii infection, namely an oral and intraperitoneal infection in suckling mice (Pagotto et al., 2003) and intracranial infection in neonatal rats (Townsend et al., 2007) where the in vivo efficacy of phage addition could be tested.
Experimental procedures
Bacterial strains, media and growth conditions
Enterobacter sakazakii strains (listed in Fig. 1 with name, origin and description) and strains from other species used throughout this study are part of the Nestlé Food Safety Microbiology (FSM) culture collection and were isolated from various factories and food samples worldwide. Enterobacter sakazakii strains were grown at 30°C and 37°C in BHI broth (Oxoid) and in reconstituted powdered infant formula (Nestlé).
Isolation of bacteriophages
Environmental samples were collected from sewage treatment plants (entry water, primary and secondary mud, exit water) and milk powder factory environments (dust and dirt collected during cleaning). The samples were homogenized in BHI broth, inoculated with exponentially growing bacterial cultures at an OD600 of 0.1 (corresponding to approximately 5 × 106 cfu ml−1) and incubated at 30°C for 5 h with gentle agitation (100 r.p.m.), left overnight at room temperature, centrifuged at 1000 g for 10 min and filtered through a 0.2‐µm‐pore‐size Minisart filter (polyethersulfone membrane). The presence of phage was tested with the plaque assay.
Purification and propagation of bacteriophages
One well‐separated phage plaque was chosen for amplification from each positive environmental sample. The plaque was transferred with a toothpick into 4 ml BHI broth, inoculated with exponentially growing bacterial culture and incubated at 30°C for 5 h with gentle agitation (100 r.p.m.). After centrifugation, the lysate was filtered through a 0.2‐µm‐pore‐size Minisart filter. Plaque purification was repeated twice. Enterobacter sakazakii strain 316 was used for the propagation of phage F, strain 390 for phage 23, strain 309 for phage 81and phage 83 and strain 368 for phage 82.
Host range determination
In the spot test 105 pfu in 10 µl were applied on BHI top agar inoculated with 100 µl of exponentially growing bacterial culture. The plates were incubated overnight at 37°C. Positive tests were verified by the plaque assay.
Induction of temperate phages by mitomycin C
Mitomycin C was added to exponentially growing cells at an OD of 0.1 to a final concentration of 0.3 µg ml−1 followed by overnight incubation at 37°C. The filtrate of all mitomycin C‐treated cultures was tested in the plaque assay against all 40 E. sakazakii strains.
E. sakazakii challenge test
Tubes containing 9 ml BHI broth or reconstituted infant formula were inoculated with E. sakazakii to the final concentration of 102 or 106 cfu ml−1 and phages (single phage or phage cocktail) to a final concentration of 104 or 108 pfu ml−1 as specified in the results section. For each experiment a control tube without phages was included. The tubes were incubated at 30°C. The cell and phage counts were followed every hour. The colony‐forming units were determined on BHI agar plates incubated overnight at 30°C.
Stability experiments
Phages F, 23, 81, 82 and 83 from infected broth cultures were filtrated and collected by centrifugation (16 000 g for 30 min). Phages were re‐suspended in phage buffer (20 mM Tris‐HCl pH 7.4, 100 mM NaCl, 10 mM MgSO4), or in phage buffer plus 5% trehalose (Merck), infant formula or infant formula plus 5% trehalose. From these samples 1 ml aliquots containing approximately 1010 pfu ml−1 were freeze‐dried in glass vials in a Telstar freeze‐dryer (freezing, 10 min, −50°C; chamber vacuum 0.8 mbar; primary drying 1 h, −50°C, 0.05 mbar and 8 h, −15°C, 0.05 mbar; secondary drying 8 h, 0°C and 6.5 h, 28°C). Aliquots were rehydrated with 1 ml TS (Tryptone 1 g l−1, NaCl 8.5 g l−1) directly after freeze‐drying and after 7, 14, 21, 28, 56, 98, 133 and 175 days storage at 4°C, 25°C, 37°C and 70°C, as indicated. Infectious phages were counted as triplicates in the plaque assay. Statistical analysis and calculations of phage half‐lives were performed using Microsoft Excel. Regression lines were calculated on the log10 phage titre plotted against time and half‐lives were determined as follows: half‐life = log10 2/‐gradient of the regression line (Jepson and March, 2004).
Electron microscopy
Phages were purified on a discontinuous CsCl gradient (CsCl at 1.35, 1.53 and 1.65 g ml−1) as described previously (Chibani‐Chennoufi et al., 2004). A drop of phage suspension was applied to a Formvar carbon‐coated copper grid. After 5 min the suspension was removed and immediately replaced by a mixture 1:1 of solution A (2% ammonium molybdate at pH 7.0) and solution B (11% bacitracin in distilled water). The liquid was removed after 1 min. The grids were examined in a Philips CM12 transmission electron microscope at 80 kV at magnifications of 176 000 or 224 000 times. Images were processed using Adobe Photoshop software.
Pulsed‐field gel electrophoresis
Blocks of 1% agarose containing 109 pfu of the indicated phage were prepared using the CHEF Mammalian Genomic DNA Plug Kit (Bio‐Rad) according to the manufacturer's instructions. Analysis of the blocks was performed by electrophoresis in a CHEF‐DRII apparatus (Bio‐Rad) in 1% agarose and 0.5% TBE (45 mM Tris‐borate, 1 mM EDTA) for 20 h at 6 V cm−1 and 14°C with a pulse time of 1–20 s. The gel was stained with ethidium bromide.
Southern blot
Phage DNA was isolated using the Lambda Midi Kit (Qiagen). Restriction enzymes were used according to the manufacturer's instructions. DraI‐digested phage DNA was separated on a 1% agarose gel, according to standard procedures (Sambrook et al., 1989). Southern blotting was performed with Perkin Elmer Gene Screen Hybridization Transfer membranes. Probes consisting of total DNA of phages T4 and F, respectively, were randomly labelled using the DIG High Prime DNA Labeling and Detection Kit (Roche Diagnostics). Prehybridization and hybridization were carried out at 37°C. The membranes were washed at 65°C in 0.5 × SSC and 0.1% SDS and exposed to Hyperfilm autoradiography film (Amersham).
PCR
Diagnostic PCR specific for T4‐like phages was performed on 100 ng phage DNA according to published protocols. For the amplification of gene 23 the primers Mzia1 (5′‐TGTTATIGGTATGGTICGICGTGCTAT‐3′) and CAP8 (5′‐TGAAGTTACCTTCACCACGACCGG‐3′) were used (Monod et al., 1997; Tétart et al., 2001). PCR conditions consisted of an initial denaturation step at 94°C for 10 min; DNA was amplified for 28 and 35 cycles, respectively, in a Gene Amp PCR system (Applied Biosystems). The cycling parameters were as follows: 30 s at 96°C, 2 min at 62°C and 54°C, respectively, 3 and 5 min at 72°C, respectively, with a final extension of 5 min at 72°C. The PCR products were separated on a 1% agarose gel.
Ribotyping and data analysis
Ribotyping was carried out in a RiboPrinter system (Qualicon) using the restriction enzyme EcoRI, (Qualicon), as described elsewhere (Bruce, 1996; Guillaume‐Gentil et al., 2002). Ribopattern with a similarity coefficient higher than 0.93 were grouped into a ribogroup. Normalized images of the ribopattern were exported from the Riboprinter into the BioNumerics software (version 4.1; Applied Maths) and the patterns analysed by upgma using a DICE coefficient with bandmatching of 1.5% and optimization of 1%.
Acknowledgments
We thank Annick Lardeau and Eva Bidlas for technical assistance and Marie‐Lise Dillmann for providing the electron microscopy pictures. We are grateful to Sebastien Populaire for statistical advice.
References
- Van Acker J., De Smet F., Muyldermans G., Bougatef A., Naessens A., Lauwers S. Outbreak of necrotizing enterocolitis associated with Enterobacter sakazakii in powdered milk formula. J Clin Microbiol. 2001;39:293–297. doi: 10.1128/JCM.39.1.293-297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashelford K.E., Day M.J., Fry J.C. Elevated abundance of bacteriophage infecting bacteria in soil. Appl Environ Microbiol. 2003;69:285–289. doi: 10.1128/AEM.69.1.285-289.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atterbury R.J., Connerton P.L., Dodd C.E., Rees C.E., Connerton I.F. Application of host‐specific bacteriophages to the surface of chicken skin leads to a reduction in recovery of Campylobacter jejuni. Appl Environ Microbiol. 2003;69:6302–6306. doi: 10.1128/AEM.69.10.6302-6306.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J. Automated system rapidly identifies and characterizes microorganisms in food. Food Technol. 1996;50:77–81. [Google Scholar]
- Bruttin A., Brüssow H. Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrob Agents Chemother. 2005;49:2874–2878. doi: 10.1128/AAC.49.7.2874-2878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüssow H. Phage therapy: the Escherichia coli experience. Microbiology. 2005;151:2133–2140. doi: 10.1099/mic.0.27849-0. [DOI] [PubMed] [Google Scholar]
- Brüssow H., Canchaya C., Hardt W.D. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol Mol Biol Rev. 2004;68:560–602. doi: 10.1128/MMBR.68.3.560-602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canchaya C., Proux C., Fournous G., Bruttin A., Brüssow H. Prophage genomics. Microbiol Mol Biol Rev. 2003;67:238–276. doi: 10.1128/MMBR.67.2.238-276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton R.M., Noordman W.H., Biswas B., De Meester E.D., Loessner M.J. Bacteriophage P100 for control of Listeria monocytogenes in foods: genome sequence, bioinformatic analyses, oral toxicity study, and application. Regul Toxicol Pharmacol. 2005;43:301–312. doi: 10.1016/j.yrtph.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Chibani‐Chennoufi S., Sidoti J., Bruttin A., Kutter E., Sarker S., Brüssow H. In vitro and in vivo bacteriolytic activities of Escherichia coli phages: implications for phage therapy. Antimicrob Agents Chemother. 2004;48:2558–2569. doi: 10.1128/AAC.48.7.2558-2569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeau A.M., Tétart F., Trojet S.N., Prère M.F., Krisch H.M. Phage‐Antibiotic Synergy (PAS): beta‐lactam and quinolone antibiotic stimulate virulent phage growth. PLoS ONE. 2007;2:e799. doi: 10.1371/journal.pone.0000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V.A. Phage antibacterials make a comeback. Nat Biotechnol. 2001;19:734–735. doi: 10.1038/90777. [DOI] [PubMed] [Google Scholar]
- Forsythe S.J. Enterobacter sakazakii and other bacteria in powdeerd infant milk formula. Matern Child Nutr. 2005;1:44–50. doi: 10.1111/j.1740-8709.2004.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode D., Allen V.M., Barrow P.A. Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl Environ Microbiol. 2003;69:5032–5036. doi: 10.1128/AEM.69.8.5032-5036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer G.G. Bacteriophage control of foodborne bacteriat. J Food Prot. 2005;68:1102–1111. doi: 10.4315/0362-028x-68.5.1102. [DOI] [PubMed] [Google Scholar]
- Guillaume‐Gentil O., Scheldeman P., Marugg J., Herman L., Joosten H., Heyndrickx M. Genetic heterogeneity in Bacillus sporothermodurans as demonstrated by ribotyping and repetitive extragenic palindromic‐PCR fingerprinting. Appl Environ Microbiol. 2002;68:4216–4224. doi: 10.1128/AEM.68.9.4216-4224.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.V., Lehane M.J., Braig H.R. Isolation of Enterobacter sakazakii from midgut of Stomoxys calcitrans. Emerg Infect Dis. 2003;9:1355–1356. doi: 10.3201/eid0910.030218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen C., Lehner A., Mullane N., Bidlas E., Cleenwerck I., Marugg J. The taxonomy of Enterobacter sakazakii: proposal of a new genus Cronobacter gen. nov. and descriptions of Cronobacter sakazakii comb. nov. Cronobacter sakazakii subsp. sakazakii, comb. nov., Cronobacter sakazakii subsp. malonaticus subsp. nov., Cronobacter turicensis sp. nov., Cronobacter muytjensii sp. nov., Cronobacter dublinensis sp. nov. and Cronobacter genomospecies 1. BMC Evol Biol. 2007a;7:64. doi: 10.1186/1471-2148-7-64. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen C., Lehner A., Mullane N., Marugg J., Fanning S., Stephan R., Joosten H. Identification of ‘Cronobacter’ spp. (Enterobacter sakazakii. J Clin Microbiol. 2007b;45:3814–3816. doi: 10.1128/JCM.01026-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen C., Mullane N., McCardell B., Tall B.D., Lehner A., Fanning S. Cronobacter gen. nov., a new genus to accommodate the biogroups of Enterobacter sakazakii, and proposal of Cronobacter sakazakii gen. nov. comb. nov., C. malonaticus sp. nov., C. turicensis sp. nov., C. muytjensii sp. nov., C. dublinensis sp. nov., Cronobacter genomospecies 1, and of three subspecies, C. dublinensis sp. nov. subsp. dublinensis subsp. nov., C. dublinensis sp. nov. subsp. lausannensis subsp. nov., and C. dublinensis sp. nov. subsp. lactaridi subsp. nov. Int J Syst Evol Microbiol. 2008;58:1442–1447. doi: 10.1099/ijs.0.65577-0. et al. [DOI] [PubMed] [Google Scholar]
- Jepson C.D., March J.B. Bacteriophage lambda is a highly stable DNA vaccine delivery vehicle. Vaccine. 2004;22:2413–2419. doi: 10.1016/j.vaccine.2003.11.065. [DOI] [PubMed] [Google Scholar]
- Kasman L.M., Kasman A., Westwater C., Dolan J., Schmidt M.G., Norris J.S. Overcoming the phage replication threshold:a mathematical model with implications for phage therapy. J Virol. 2002;76:5557–5564. doi: 10.1128/JVI.76.11.5557-5564.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy J.E., Jr, Wei C.I., Oblinger J.L. Methodology for enumeration of coliphages in foods. Appl Environ Microbiol. 1986;51:956–962. doi: 10.1128/aem.51.5.956-962.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.P., Klumpp J., Loessner M.J. Enterobacter sakazakii bacteriophages can prevent bacterial growth in reconstituted infant formula. Int J Food Microbiol. 2007;115:195–203. doi: 10.1016/j.ijfoodmicro.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Kysela D.T., Turner P.E. Optimal bacteriophage mutation rates for phage therapy. J Theor Biol. 2007;249:411–421. doi: 10.1016/j.jtbi.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Leverentz B., Conway W.S., Alavidze Z., Janisiewicz W.J., Fuchs Y., Camp M.J. Examination of bacteriophage as a biocontrol method for Salmonella on fresh‐cut fruit: a model study. J Food Prot. 2001;64:1116–1121. doi: 10.4315/0362-028x-64.8.1116. et al. [DOI] [PubMed] [Google Scholar]
- Leverentz B., Conway W.S., Camp M.J., Janisiewicz W.J., Abuladze T., Yang M. Biocontrol of Listeria monocytogenes on fresh‐cut produce by treatment with lytic bacteriophages and a bacteriocin. Appl Environ Microbiol. 2003;69:4519–4526. doi: 10.1128/AEM.69.8.4519-4526.2003. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverentz B., Conway W.S., Janisiewicz W., Camp M.J. Optimizing concentration and timing of a phage spray application to reduce Listeria monocytogenes on honeydew melon tissue. J Food Prot. 2004;67:1682–1686. doi: 10.4315/0362-028x-67.8.1682. [DOI] [PubMed] [Google Scholar]
- Levin B.R., Bull J.J. Population and evolutionary dynamics of phage therapy. Nat Rev Microbiol. 2004;2:166–173. doi: 10.1038/nrmicro822. [DOI] [PubMed] [Google Scholar]
- Loessner M.J., Neugirg E., Zink R., Scherer S. Isolation, classification and molecular characterization of bacteriophages for Enterobacter species. J Gen Microbiol. 1993;139:2627–2633. doi: 10.1099/00221287-139-11-2627. [DOI] [PubMed] [Google Scholar]
- Merril C.R., Scholl D., Adhya S.L. The prospect for bacteriophage therapy in Western medicine. Nat Rev Drug Discov. 2003;2:489–497. doi: 10.1038/nrd1111. [DOI] [PubMed] [Google Scholar]
- Monod C., Repoila F., Kutateladze M., Tétart F., Krisch H.M. The genome of the pseudo T‐even bacteriophages, a diverse group that resembles T4. J Mol Biol. 1997;267:237–249. doi: 10.1006/jmbi.1996.0867. [DOI] [PubMed] [Google Scholar]
- Mullane N.R., Iversen C., Healy B., Walsh C., Whyte P., Wall P.G. Enterobacter sakazakii an emerging bacterial pathogen with implications for infant health. Minerva Pediatrica. 2007;59:137–148. et al. [PubMed] [Google Scholar]
- Muytjens H.L., Zanen H.C., Sonderkamp H.J., Kollee L.A., Wachsmuth I.K., Farmer J.J., III Analysis of eight cases of neonatal meningitis and sepsis due to Enterobacter sakazakii. J Clin Microbiol. 1983;18:115–120. doi: 10.1128/jcm.18.1.115-120.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muytjens H.L., Roelofs‐Willemse H., Jaspar G.H. Quality of powdered substitutes for breast milk with regard to members of the family Enterobacteriaceae. J Clin Microbiol. 1988;26:743–746. doi: 10.1128/jcm.26.4.743-746.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flynn G., Ross R.P., Fitzgerald G.F., Coffey A. Evaluation of a cocktail of three bacteriophages for biocontrol of Escherichia coli O157:H7. Appl Environ Microbiol. 2004;70:3417–3424. doi: 10.1128/AEM.70.6.3417-3424.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H., Selander R.K. Standard reference strains of Escherichia coli from natural populations. J Bacteriol. 1984;157:690–693. doi: 10.1128/jb.157.2.690-693.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagotto F.J., Nazarowec‐White M., Bidawid S., Farber J.M. Enterobacter sakazakii: infectivity and enterotoxin production in vitro and in vivo. J Food Prot. 2003;66:370–375. doi: 10.4315/0362-028x-66.3.370. [DOI] [PubMed] [Google Scholar]
- Payne R.J., Jansen V.A. Understanding bacteriophage therapy as a density‐dependent kinetic process. J Theor Biol. 2001;208:37–48. doi: 10.1006/jtbi.2000.2198. [DOI] [PubMed] [Google Scholar]
- Payne R.J., Jansen V.A. Pharmacokinetic principles of bacteriophage therapy. Clin Pharmacokinet. 2003;42:315–325. doi: 10.2165/00003088-200342040-00002. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Simmons B.P., Gelfand M.S., Haas M., Metts L., Ferguson J. Enterobacter sakazakii infections in neonates associated with intrinsic contamination of a powdered infant formula. Infect Control Hosp Epidemiol. 1989;10:398–401. doi: 10.1086/646060. [DOI] [PubMed] [Google Scholar]
- Townsend S.M., Hurrell E., Gonzalez‐Gomez I., Lowe J., Frye J.G., Forsythe S., Badger J.L. Enterobacter sakazakii invades brain capillary endothelial cells, persists in human macrophages influencing cytokine secretion and induces severe brain pathology in the neonatal rat. Microbiology. 2007;153:3538–3547. doi: 10.1099/mic.0.2007/009316-0. [DOI] [PubMed] [Google Scholar]
- Tétart F., Desplats C., Kutateladze M., Monod C., Ackermann H.W., Krisch H.M. Phylogeny of the major head and tail genes of the wide‐ranging T4‐type bacteriophages. J Bacteriol. 2001;183:358–366. doi: 10.1128/JB.183.1.358-366.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weld R.J., Butts C., Heinemann J.A. Models of phage growth and their applicability to phage therapy. J Theor Biol. 2004;227:1–11. doi: 10.1016/S0022-5193(03)00262-5. [DOI] [PubMed] [Google Scholar]
- Wiggins B.A., Alexander M. Minimum bacterial density for bacteriophage replication: implications for significance of bacteriophages in natural ecosystems. Appl Environ Microbiol. 1985;49:19–23. doi: 10.1128/aem.49.1.19-23.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. World Health Organization; 2004. Enterobacter sakazakii and other microorgansims in powdered infant formula. Geneva, Switzerland: [Google Scholar]
- Zuber S., Ngom‐Bru C., Barretto C., Bruttin A., Brussow H., Denou E. Genome analysis of phage JS98 defines a fourth major subgroup of T4‐like phages in Escherichia coli. J Bacteriol. 2007;189:8206–8214. doi: 10.1128/JB.00838-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


