Summary
The viscoelastic properties of mono‐microbial biofilms produced by ocular and reference staphylococcal strains were investigated. The microorganisms were characterized for their haemolytic activity and agr typing and the biofilms, grown on stainless steel surface under static conditions, were analysed by Confocal Laser Scanning Microscopy. Static and dynamic rheometric tests were carried out to determine the steady‐flow viscosity and the elastic and viscous moduli. The analysed biofilms showed the typical time‐dependent behaviour of viscoelastic materials with considerable elasticity and mechanical stability except for Staphylococcus aureus ATCC 29213 biofilm which showed a very fragile structure. In particular, S. aureus 6ME biofilm was more compact than other staphylococcal biofilms studied with a yield stress ranging between 2 and 3 Pa. The data obtained in this work could represent a starting point for developing new therapeutic strategies against biofilm‐associated infections, such as improving the drug effect by associating an antimicrobial agent with a biofilm viscoelasticity modifier.
Introduction
Biofilms may be considered as an ancestral selective event used by prokaryotes to adapt themselves in every environmental niche. In this way, microorganisms can survive to the external challenges including nutritional deprivation and environmental stress (Donlan and Costerton, 2002). The ‘free multicellularity’ can be considered, for the bacterial population, as the best programme of survival in stressed conditions (Branda and Kolter, 2004). Bacterial biofilms are able to display tolerance to host defences and antibiotic therapies. The clinical relevance of these microbial communities is related to their wide spread in the developed world together with the significant difficulties in their eradications (Mah and O'Toole, 2001; Lewis, 2005). Bacterial biofilms can be also defined as highly porous polymeric gels containing microorganisms, nucleic acids, proteins, extracellular polymeric substances (EPS) and water (Sutherland, 2001; Wloka et al., 2004). The typical components of EPS, such as alginate, gellan and xanthan, form a highly hydrated viscoelastic gel that provides strong mechanical stability and protects the microorganisms from desiccation and antimicrobial agents (Stoodley et al., 1999; Klapper et al., 2002). Indeed, recent studies have demonstrated the ability of biofilms to adapt themselves to changeable environmental shear stresses (Flemming et al., 2000). In addition, the dual nature of the viscoelastic properties of in vitro grown biofilms explains biofilm robustness: the elastic component absorbs stress energy through reversible deformation, while viscous flow overcomes internal stress by non‐reversible strain (Stoodley et al., 1999; Flemming et al., 2000; Korstgens et al., 2001; Shaw et al., 2004).
Staphylococci are important nosocomial pathogens: Staphylococcus aureus and Staphylococcus epidermidis have evolved to become highly adaptable human pathogens. Many staphylococcal infections result in acute disease; however, bacterial persistence and recurrent infections are also commonly observed among patients with indwelling medical devices, and the increasing use of such devices produced an increase in staphylococcal device‐related infection, in particular trough the biofilm formation (Fitzpatrick et al., 2005).
The ability to form biofilms contributes significantly to the pathogenesis of many S. aureus and S. epidermidis infections, including a variety of ocular diseases often associated with the biofilm formation on foreign materials such as contact lenses, scleral buckles and intraocular lenses which adhered to eye (Catalanotti et al., 2005; Okajima et al., 2006; Nostro et al., 2007).
The aim of this work was to evaluate the viscoelastic properties of the biofilms, grown under static conditions, of two ocular S. aureus (6ME and 815CT) derived from patients with community‐acquired ocular infections, and of two reference strains, S. aureus ATCC 29213 and S. epidermidis ATCC 35984. The examined bacteria, previously characterized for the icaA/icaD gene presence, the Congo red assay and the capability of forming biofilm on polystyrene surface (Blanco et al., 2005), were analysed for their haemolytic activity, the agr typing, and the formed biofilms, were observed by Confocal Laser Scanning Microscopy (CLSM). A cone/plate rheometer was used to measure the viscoelastic properties of these staphylococcal biofilms produced. This technique affords precise monitoring of shear stress and strain acting on the sample (Towler et al., 2003) and provide the opportunity of conducting both static and dynamic tests.
Results and discussion
The four strains investigated, evaluated for their haemolytic activity and agr system (Fig. 1A and B), correlated with a background of biofilm producer microorganisms (Cafiso et al., 2007; Yarwood et al., 2007). The biofilm developed by these bacteria was characterized by CLSM observations by using the COMSTAT image analysis software (Table 1). In particular, S. aureus ATCC 29213 biofilm (Fig. 1C, row 1) displayed a homogeneous structure (roughness coefficient = 0.63 ± 0.44) which reached an average thickness of 20.31 ± 8.26 µm. Staphylococcus aureus 6ME formed the most compact biofilm of the studied bacteria, with a biomass of 5.23 ± 1.97 (Fig. 1C, row 2). Staphylococcus aureus 815CT and S. epidermidis ATCC 35984 biofilms (Fig. 1C, rows 3 and 4 respectively) were characterized by more heterogeneous structures (roughness coefficient = 1.09 ± 0.25, 1.25 ± 0.12 respectively) than the biofilms formed by the other strains examined, reaching an average thickness of 22.35 ± 2.07 µm and 44.65 ± 12.4 respectively.
Figure 1.
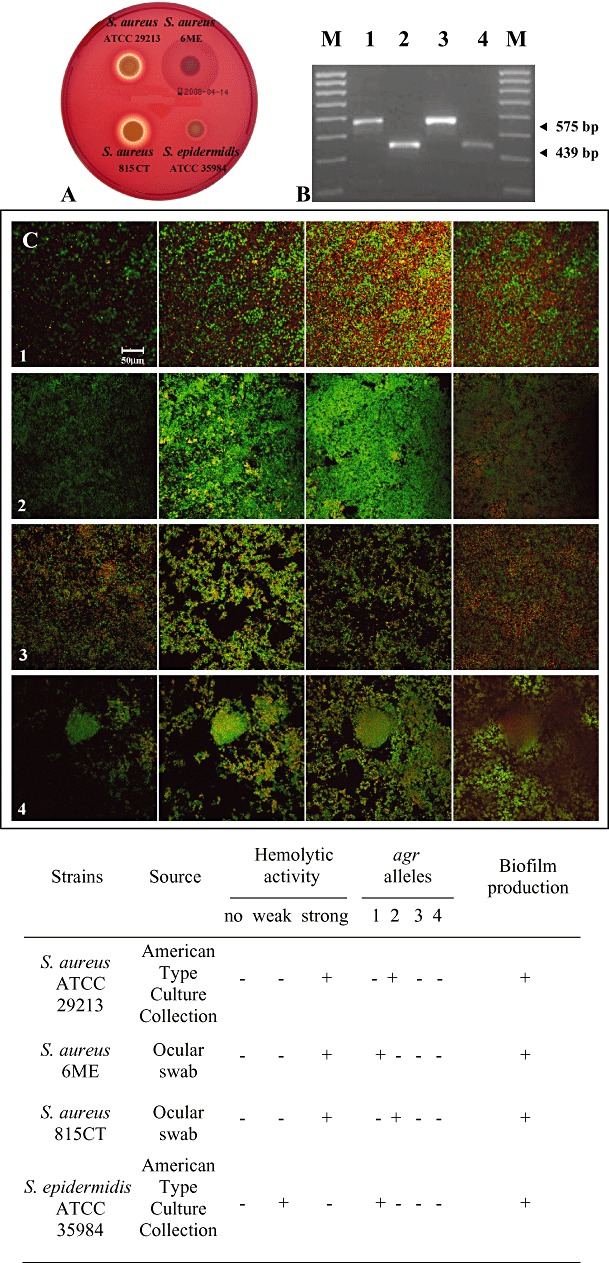
Characterization of staphylococcal strains. A. Tryptic Soy Blood Agar plates showing strong β‐haemolytic activity in S. aureus ATCC 29213 and S. aureus 815CT (top and down on left); strong and weak α‐haemolytic activity in S. aureus 6ME and S. epidermidis ATCC 35984 respectively (top and down on right). B. Multiplex‐PCR of agr genotype products of S. aureus ATCC 29213 (lane 1), S. aureus 6ME (lane 2), S. aureus 815CT (lane 3), S. epidermidis ATCC 35984 (lane 4); M indicates the DNA molecular weight marker (0.1 kb). For multiplex‐PCR, two primer sets were prepared: one to amplify agr S. aureus alleles (agr 1–4sa) and another to amplify S. epidermidis alleles (agr 1–3se). The sizes of the identified allele products were 439 bp for agr‐1 and 575 bp for agr‐2. C. CLSM images of stacks, from left to right, derived from one representative field taken from biofilm of (1) S. aureus ATCC 29213, (2) S. aureus 6ME, (3) S. aureus 815CT, (4) S. epidermidis ATCC 35984 respectively. Each sample was stained by using Live/Dead kit. The biofilms were sectioned from the top to the bottom. The two stain stock solutions (SYTO 9 and propidium iodide) were added the steel surface and the samples were observed using a ZEISS LSM510 META (Jena) confocal microscope, using the 488 nm line from an argon ion laser and 535 nm band pass emission filter. A Zeiss 40×/1.3 oil and 10×/0.3 numerical aperture objective was used to collect all image stacks. Original magnification, 400×. Scale bar = 50 µm. The table at the bottom of the figure summarizes all the detected characteristics.
Table 1.
Biomass per unit area, roughness coefficient and average thickness of biofilms from Staphylococcus aureus ATCC 29213, S. aureus 6ME, S. aureus 815CT, Staphylococcus epidermidis ATCC 35984 (COMSTAT analysis).
| Strain | Biomass per unit area [µm3/(µm2)−1] | Roughness coefficient | Average thickness (µm) |
|---|---|---|---|
| S. aureus ATCC 29213 | 4.04 ± 1.29a | 0.63 ± 0.44 | 20.31 ± 8.26 |
| S. aureus 6ME | 5.23 ± 1.97a | 0.86 ± 0.32 | 27.40 ± 7.63 |
| S. aureus 815CT | 2.54 ± 0.85 | 1.09 ± 0.25 | 22.35 ± 2.07 |
| S. epidermidis ATCC 35984 | 2.49 ± 0.87 | 1.25 ± 0.12 | 44.65 ± 12.4b |
P < 0.05 compared with S. epidermidis ATCC 35984 and S. aureus 815CT.
P < 0.01 compared with S. aureus ATCC 29213, S. aureus 6ME and S. aureus 815CT.
For each strain studied, 24 z‐stacks (six image stacks from two channels in two independent experiments) were studied. All the CLSM experiments were performed in triplicate; standard deviation is shown.
The rheological properties measured are shown in Table 2. The S. aureus ATCC 29213 biofilm structure was dispersed in PBS when the lowest shear stress was imposed. In fact, both creep and oscillatory tests carried out on this sample displayed water‐like rheological properties. Probably the interaction forces between the biofilm EPS polymeric constituents were very weak, so that the biofilm structure was easily dispersed in the medium when mechanically stressed. The biofilm disruption was also confirmed by direct observation. CLSM images of S. aureus ATCC 29213 biofilm showed a homogeneous structure characterized by a great number of dead cells at the bottom of the biofilm, suggesting that the water‐like rheological properties might be associated to an easier biofilm detachment due to the low number of live adhered cells. On the contrary, S. aureus 6ME formed a compact biofilm constituted by a significant amount of live cells also at the bottom of the structure supporting the hypothesis of both a strong cellular adhesion to the surface and an organized structure correlated to a strong viscoelastic nature. Similar viscoelastic properties were shown by S. aureus 815CT and S. epidermidis ATCC 35984 although the biofilms formed were more heterogeneous than the one of S. aureus 6ME.
Table 2.
Rheological properties of Staphylococcus aureus and Staphylococcus epidermidis biofilms.
| Strains | η0 (Pa·s) | Je0 (Pa−1) | G′ (Pa) | G″ (Pa) | Yield stress | Elastic relaxation time (λ) (min) |
|---|---|---|---|---|---|---|
| S. aureus ATCC 29213 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| S. aureus 6ME | (2.1 ± 1.7) × 106a | (5.9 ± 4.1) × 10−3a | (2.1 ± 0.9) × 103a | 72 ± 57a | 2.0–3.0 Pa | 18.2 ± 3.3 |
| S. aureus 815CT | (1.9 ± 1.2) × 105 | (3.5 ± 3.2) × 10−2 | (1.5 ± 1.1) × 102 | 11 ± 9.9 | 0.1–0.5 Pa | 17.5 ± 3.1 |
| S. epidermidis ATCC 35984 | (46 ± 5.0) × 103 | (5.9 ± 3.8) × 10−2 | (4.5 ± 4.4) × 102 | 25 ± 23 | 0.5–1.0 Pa | 19.2 ± 4.3 |
P < 0.1 compared with S. epidermidis ATCC 35984.
Values (±SD) are means of five experiments; each experiment was performed in triplicate.
n.d., not determined.
Staphylococcus aureus 6ME, S. aureus 815CT and S. epidermidis ATCC 35984 biofilms displayed a viscoelastic nature, as reported in the literature for biofilms of other bacterial species (Stoodley et al., 1999; 2002; Korstgens et al., 2001; Klapper et al., 2002; Shaw et al., 2004; Vinogradov et al., 2004; Rupp et al., 2005; Towler et al., 2007; Yarwood et al., 2007). The creep and recovery curves of these three staphylococcal biofilms showed the typical time‐dependent behaviour of viscoelastic materials. As an example, the creep and recovery curves of S. aureus 6ME are reported in Fig. 2A. The curves of the strain plotted against time displayed five characteristic regions: (i) an instantaneous elastic deformation, (ii) a retarded, time‐dependent deformation, (iii) a constant rate viscous flow region, the linear portion of the graph (constant slope), (iv) an instantaneous elastic recovery on removal of the shear stress, and (v) a residual strain due to viscous flow (Vinogradov et al., 2004). The steady‐flow viscosities 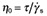 (where τ is the applied stress and
(where τ is the applied stress and 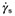 is the constant shear rate reached at the steady state) were obtained from the constant rate viscous flow region of the creep curves. The constant slope of the linear portion of the curve of strain versus time was extrapolated back to zero time, and the intercept γe0 was divided by the applied stress τ to get the value of the so‐called equilibrium compliance Je0, a measure of the elasticity of viscoelastic materials at short times (Barnes et al., 1989): high values of Je0 indicate a high compliance of the sample to flow. It was observed that the S. aureus 6ME biofilm linear viscoelastic range (LVR) reached 2.0 Pa; indeed, its creep compliances [J(t) = y(t)/τ] at 0.5, 1.0 and 2.0 Pa overlapped. Instead, for τ = 3.0 Pa, this biofilm showed a very high deformation without overlapping the J(t) (Fig. 2B); this is a non‐linear viscoelastic behaviour and then the LVR of the sample does not go over 2 Pa. At the same time the creep deformation observed for a shear stress of 3 Pa was totally irreversible. This is synonymous of extreme modification of the original structure of the sample, which does not show any recovery and behaves like a viscous liquid. The oscillation tests confirmed that the LVR of this biofilm reached 2.0 Pa and that a stress of 3.0 Pa determined non‐linear viscoelastic behaviour and also a solid‐like to liquid‐like transition. In fact, a perfect overlapping of the oscillation‐frequency‐sweep test results could be observed at very low deformation (0.1–0.25%), and at 0.5, 1.0 and 2.0 Pa (Fig. 2C), whereas the result at 3.0 Pa of the oscillation‐frequency‐sweep tests did not overlap the others and displayed a non‐linear relationship between the elastic (G′) and the viscous (G″) moduli and the frequency (Fig. 2D). Moreover, for τ = 3.0 Pa, the elastic modulus G′ had a smaller value than that of the viscous modulus G″, while up to 2.0 Pa, there was the typical behaviour of stable elastic gels, with G′ > G″. This viscoelastic behaviour corresponded to a compact biofilm characterized by a higher biomass value than the other analysed biofilms. Creep analysis made it possible to estimate an η0 mean value of 2.1 × 106 (±1.7 × 106) Pa·s, and a Je0 mean value of 5.9 × 10−3 (±4.1 × 10−3) Pa−1, while the oscillatory tests yielded a storage modulus mean value of 2.1 × 103 (±0.9 × 103) Pa and a loss modulus value of 72 (±57) Pa. Also the other two biofilms analysed show a solid‐like to liquid‐like transition for a certain value of shear stress. We indicate this sample‐characteristic value of shear stress as yield stress τy. Staphylococcus aureus 815CT biofilm showed a yield stress between 0.1 and 0.5 Pa (just above the LVR of the sample), an η0 mean value of 1.9 × 105 (±1.2 × 105) Pa·s, a G′ of 1.5 × 102 (±1.1 × 102) Pa and a G″ of 11 (±9.9) Pa. The wide range of these values is not surprising, given the high grade of variability in density and structures previously reported in the literature on biofilms (Towler et al., 2003; Laspidou and Aravas, 2007). Finally, for the S. epidermidis ATCC 35984 biofilm, a linear viscoelastic behaviour was observed only for small τ values, not much beyond 0.5 Pa. The creep tests conducted at a τ of 1.0 Pa showed a strain response dissimilar to that of a viscoelastic material and characteristic for a viscous fluid, suggesting that 1.0 Pa exceeded the yield point of the S. epidermidis ATCC 35984 biofilm. The dynamic tests confirmed a transition phase between 0.5 and 1.0 Pa. The creep tests determined an η0 value of 46 × 103 (±5.0 × 103) Pa·s and a Je0 value of 5.9 × 10−2 (±3.8 × 10−2) Pa−1 while the oscillatory tests gave a G′ mean value of 4.5 × 102 (±4.4 × 102) Pa and a G″ mean value of 25 (±23) Pa.
is the constant shear rate reached at the steady state) were obtained from the constant rate viscous flow region of the creep curves. The constant slope of the linear portion of the curve of strain versus time was extrapolated back to zero time, and the intercept γe0 was divided by the applied stress τ to get the value of the so‐called equilibrium compliance Je0, a measure of the elasticity of viscoelastic materials at short times (Barnes et al., 1989): high values of Je0 indicate a high compliance of the sample to flow. It was observed that the S. aureus 6ME biofilm linear viscoelastic range (LVR) reached 2.0 Pa; indeed, its creep compliances [J(t) = y(t)/τ] at 0.5, 1.0 and 2.0 Pa overlapped. Instead, for τ = 3.0 Pa, this biofilm showed a very high deformation without overlapping the J(t) (Fig. 2B); this is a non‐linear viscoelastic behaviour and then the LVR of the sample does not go over 2 Pa. At the same time the creep deformation observed for a shear stress of 3 Pa was totally irreversible. This is synonymous of extreme modification of the original structure of the sample, which does not show any recovery and behaves like a viscous liquid. The oscillation tests confirmed that the LVR of this biofilm reached 2.0 Pa and that a stress of 3.0 Pa determined non‐linear viscoelastic behaviour and also a solid‐like to liquid‐like transition. In fact, a perfect overlapping of the oscillation‐frequency‐sweep test results could be observed at very low deformation (0.1–0.25%), and at 0.5, 1.0 and 2.0 Pa (Fig. 2C), whereas the result at 3.0 Pa of the oscillation‐frequency‐sweep tests did not overlap the others and displayed a non‐linear relationship between the elastic (G′) and the viscous (G″) moduli and the frequency (Fig. 2D). Moreover, for τ = 3.0 Pa, the elastic modulus G′ had a smaller value than that of the viscous modulus G″, while up to 2.0 Pa, there was the typical behaviour of stable elastic gels, with G′ > G″. This viscoelastic behaviour corresponded to a compact biofilm characterized by a higher biomass value than the other analysed biofilms. Creep analysis made it possible to estimate an η0 mean value of 2.1 × 106 (±1.7 × 106) Pa·s, and a Je0 mean value of 5.9 × 10−3 (±4.1 × 10−3) Pa−1, while the oscillatory tests yielded a storage modulus mean value of 2.1 × 103 (±0.9 × 103) Pa and a loss modulus value of 72 (±57) Pa. Also the other two biofilms analysed show a solid‐like to liquid‐like transition for a certain value of shear stress. We indicate this sample‐characteristic value of shear stress as yield stress τy. Staphylococcus aureus 815CT biofilm showed a yield stress between 0.1 and 0.5 Pa (just above the LVR of the sample), an η0 mean value of 1.9 × 105 (±1.2 × 105) Pa·s, a G′ of 1.5 × 102 (±1.1 × 102) Pa and a G″ of 11 (±9.9) Pa. The wide range of these values is not surprising, given the high grade of variability in density and structures previously reported in the literature on biofilms (Towler et al., 2003; Laspidou and Aravas, 2007). Finally, for the S. epidermidis ATCC 35984 biofilm, a linear viscoelastic behaviour was observed only for small τ values, not much beyond 0.5 Pa. The creep tests conducted at a τ of 1.0 Pa showed a strain response dissimilar to that of a viscoelastic material and characteristic for a viscous fluid, suggesting that 1.0 Pa exceeded the yield point of the S. epidermidis ATCC 35984 biofilm. The dynamic tests confirmed a transition phase between 0.5 and 1.0 Pa. The creep tests determined an η0 value of 46 × 103 (±5.0 × 103) Pa·s and a Je0 value of 5.9 × 10−2 (±3.8 × 10−2) Pa−1 while the oscillatory tests gave a G′ mean value of 4.5 × 102 (±4.4 × 102) Pa and a G″ mean value of 25 (±23) Pa.
Figure 2.
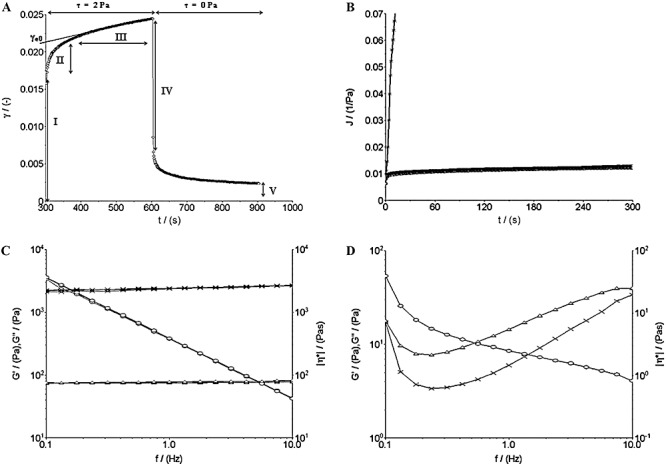
Representative rheological data of a S. aureus 6ME biofilm. A. Creep and recovery. The five characteristic regions of the strain (γ) versus time curve of a viscoelastic material are shown: (I) instantaneous elastic strain, (II) delayed deformation, (III) steady‐state viscous response, (IV) instantaneous elastic recovery and (V) residual strain. B. Creep compliance curves at different τ0 values. Up to shear stresses of 2 Pa (□, 0.5 Pa; Δ, 1.0 Pa; ◊, 2.0 Pa), the curves overlap (linear response to shear); for 3 Pa (×) there is a drastic increase of the creep compliance (yield stress exceeded). C. Dynamic tests in the linear viscoelastic range (LVR) (×, storage modulus G′; Δ, loss modulus G″; ○, dynamic viscosity). D. Oscillation‐frequency‐sweep test at 3.0 Pa.
On the basis of our results, the biofilms of S. aureus 6ME, S. aureus 815CT and S. epidermidis ATCC 35984 can be considered gel systems with considerable elasticity and mechanical stability. Their slime matrix might behave as a physical barrier that blocks xenobiotic access to the cells (de Carvalho, 2007). The knowledge of the biofilm mechanical properties is of relevance, especially in prosthetic devices, such as contact lenses, catheters, and vascular and orthopaedic implants, in which the bacterial biofilm can be subjected to shear stresses that vary in magnitude and frequency (Stoodley et al., 1999; Rupp et al., 2005). In particular, the staphylococcal biofilm plays role in a variety of ocular infections associated with materials used for the eye (Catalanotti et al., 2005; Zegans et al., 2005; Okajima et al., 2006; Duggirala et al., 2007), and an understanding of the characteristics of these biofilms could help in the development of new drug strategies in ophthalmology (Duggirala et al., 2007). Indeed, we tested intact biofilms using dynamic methods in which shear stresses of various moduli were oscillated at various frequencies. To our knowledge, few experimental studies were carried out for the determination of the physical properties of attached biofilms and, in the present study, the viscoelasticity of biofilms, evaluated both by creep experiments and by dynamic tests, provided more precise measurements of the material characteristics. Rheometry is a well‐established technique for measuring properties of viscoelastic samples and it has the advantage that shear stresses and strain acting on the sample are precisely monitored (Towler et al., 2003; Vinogradov et al., 2004; Aravas and Laspidou, 2008). As previously reported the biofilms produced in different laboratories are greatly heterogeneous (Houari et al., 2008; Rogers et al., 2008), but a commonality of the elastic relaxation times (λ, the time scale separating solid and fluid behaviour) of different biofilms can be observed (Shaw et al., 2004). Indeed our measurements indicate that biofilms elastic relaxation times are very similar, and a value of about 18 min was observed for all the samples (Table 2).
Recent years have seen an increase in ocular diseases provoked by S. aureus and S. epidermidis biofilms and the biofilm viscoelasticity analysed in this study emphasizes their important role in the ocular infection spreading and in the resistance to detachment as previously demonstrated by Rupp and colleagues (2005). These authors showed that the viscoelastic behaviour of S. aureus biofilm promotes the rolling migration and the dissemination of non‐motile bacteria in a protected biofilm state favouring the persistence of the infection.
Taken together, the observations collected in the present study could represent a starting point for developing new therapeutic strategies against biofilm‐associated infections, such as improving the drug effect by associating an antimicrobial agent with a biofilm viscoelasticity modifier.
Experimental procedures
Bacteria and biofilm preparation
Staphylococcus aureus ATCC 29213 (LGC), S. aureus 6ME, S. aureus 815CT and S. epidermidis ATCC 35984 (LCG) were the microorganisms used for the experiments. All bacteria were previously characterized for some biofilm‐related properties (Blanco et al., 2005). Strains were cultured on Mannitol Salt Agar (MSA, Oxoid) at 37°C for 24–48 h, identified by Api‐Staph System (Biomerieux) and stored at −20°C until use.
For the biofilm preparation, a bacterial colony of each examined microorganism, grown on MSA, was cultured overnight at 37°C in Tryptic Soy Broth (TSB, Oxoid) plus 0.5% glucose, then diluted 1:10 (v/v) in TSB plus 0.5% glucose and incubated for 2 h at 37°C in an orbital shaker at 160 r.p.m. Refreshed broth cultures were adjusted to 0.04–0.05 optical density (OD600), corresponding to 0.9–1.3 × 107 colony‐forming units (cfu) ml−1, and the total count was also confirmed using a Burker chamber (Fortuna). Broth cultures diluted 1:100 (v/v) were used for preparing samples for CLSM and rheometric analysis.
All the experiments were performed in triplicate.
Haemolytic activity
For the evaluation of the haemolytic activity of S. aureus and S. epidermidis strains, 5 µl of refreshed broth cultures grown in TSB, for 2 h at 37°C in an orbital shaker at 160 r.p.m. and adjusted to 0.04–0.05 OD600, were spotted onto Tryptic Soy Agar (TSA) plus 5% sheep sterile blood (BioMerieux) and incubated for 48 h at 37°C, then chilled at 4°C for 1 h. Subsequently, the no‐haemolytic activity and the weak, normal or strong haemolytic activities were evaluated (Goerke et al., 2007).
agr genotyping by multiplex polymerase chain reaction
Bacterial DNA was extracted using QIAamp Tissue DNA isolation Minikit and used as template for the specific detection of agr alleles in S. aureus and S. epidermidis according to Lina and colleagues (2003).
Polymerase chain reactions (PCRs) were performed in a 2700 Thermocycler (PE‐Applied Biosystems) and the amplification reaction was performed in a total volume of 25 µl containing 2.5 ml of 10× PCR buffer, 1.5 mM MgCl2, 200 mM (each) deoxinucleotide triphosphates (dNTPs), 2 U of Amplitaq DNA polymerase, 20 mM of each primer and 50 ng of bacterial DNA. After 5 min of denaturation at 95°C, each reaction was amplified for 25 cycles as follows: 1 min at 94°C, 1 min of annealing at 58°C and 1 min at 72°C. After the last cycle, the extension was continued for 10 min at 72°C. Each oligonucleotide sequence used in this work was synthesized by Primm. The PCR products were examined by electrophoresis in 2% (w/v) agarose gel at 100 V for 40 min. Gels were stained with ethidium bromide and photographed.
All the experiments were performed in triplicate.
CLSM analysis
The CLSM images of 48 h biofilm grown on stainless steel 1.5 × 2 × 0.08 cm surfaces were evaluated by using the COMSTAT image analysis software package (MathWorks, Natick, MA, USA) (Heydorn et al., 2000). For each strain studied, 24 z‐stacks (six image stacks from two channels in two independent experiments) were studied. The evaluation of the differences statistically significant (P < 0.05) was performed by means of anova followed by Dunnett's t‐test.
All the CLSM experiments were performed three times with similar results.
Rheometry methods
The viscoelastic properties of the samples were evaluated using a Haake M.A.R.S. II Thermo Scientific modular rheometer equipped with a cone and plate sensor system (Fig. 3). The low non‐rotating element that received the sample was a stainless steel plate (Din No. 1.4841). The biofilms were grown directly on the surface of the rheometer plate, in order to preserve their structure intact. A stainless steel ring was fixed around the edge of the device with 4% bacteriological agar (Fig. 3A); the diluted broth culture (40 ml) was added to this reactor and incubated at 37°C for 48 h. Then, the biofilm was washed with sterile PBS to remove the planctonic cells. Before placing the plate on the rheometer, the stainless steel ring was substituted with a glass ring sealed with 2% bacteriological agar around the edge of the plate to allow visual inspection during the measurements; the sample sandwiched between the cone and the plate was surrounded by 2 ml of sterile PBS at 37°C in order to prevent dehydration of the biofilm (Fig. 3B, insert). In order to minimize the ‘squeezin G’ of water out of the macropores and channels of the biofilm, the rate by which the upper sensor reached the measurement position was controlled so that the normal force acting on the sample did not exceed the value of 0.1 N. The samples were allowed to undergo an ‘adjustment’ period of 5 min after loading (Vinogradov et al., 2004). All tests were carried out at the controlled temperature of 37 ± 0.3°C, with the aid of a Haake Phoenix Thermo Electron Corporation thermo‐controller system. The RheoWin software 3.61 (Thermo Fisher Scientific) was used for data evaluation. Creep and recovery tests were performed, applying a constant shear stress (τ) for 300 s followed by a 300 s recovery period (τ = 0); the resulting shear strain (γ) was measured over time (t). These tests were performed at several different τ (allowing the samples to undergo an adjustment period of 300 s between each test), in order to identify the LVR of the samples, where γ was directly proportional to τ. Dynamic tests were carried out in order to estimate the elastic (G′) and the viscous (G″) moduli of the biofilms, and for the determination of their LVR, the region characterized by stress‐strain linear proportionality and by constant values of the G′ and G″ moduli. A set of three oscillation frequency sweep tests at strains of 0.100%, 0.125% and 0.250%, respectively, across a frequency range from 0.1 to 10 Hz (previously identified as the frequency range satisfying the linear viscoelasticity for all the evaluated biofilms), was performed for each sample, and the complex shear viscosity (η*), the elastic and the viscous moduli were measured as a function of frequency. By sweeping across the same frequency range, another series of oscillation frequency sweep tests was performed in controlled stress mode, for different values of stress. In our studies, the dynamic approach was useful for confirming the LVR determined by the creep tests series. The measurements were carried out in the LVR, as otherwise the results would be dependent on the experimental details and not unique to the material (Barnes et al., 1989).
Figure 3.

Rheometry equipment. A. The rheometer measuring plate with a removable stainless steel ring containing a bacterial biofilm. B. Thermo Scientific modular rheometer with the plate (insert) colonized with biofilm in testing mode.
Nomenclature
Dimensions in terms of mass (M), length (L) and time (T):
| f | frequency (1/T) |
| G′ | storage modulus (M/LT2) |
| G″ | loss modulus (M/LT2) |
| γ | deformation (L/L) |
| γe0 | elastic deformation (L/L) |
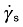 |
steady‐flow shear rate (1/T) |
| η0 | steady‐flow viscosity (M/LT) |
| |η*| | complex viscosity (M/LT) |
| J | creep compliance (LT2/M) |
| Je0 | equilibrium compliance (LT2/M) |
| λ | elastic relaxation time (T) |
| τ | shear stress (M/LT2). |
References
- Aravas N., Laspidou C.S. On the calculation of the elastic modulus of a biofilm streamer. Biotechnol Bioeng. 2008;101:196–200. doi: 10.1002/bit.21865. [DOI] [PubMed] [Google Scholar]
- Barnes H.A., Hutton J.F., Walters K. Linear viscoelasticity. In: Barnes H.A., Hutton J.F., Walters K., editors. Elsevier Science Publishers B. V.; 1989. pp. 37–54. [Google Scholar]
- Blanco A.R., Sudano‐Roccaro A., Spoto G.C., Nostro A., Rusciano D. Epigallocatechin gallate inhibits biofilm formation by ocular staphylococcal isolates. Antimicrob Agents Chemother. 2005;49:4339–4343. doi: 10.1128/AAC.49.10.4339-4343.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda S.S., Kolter R. Multicellularity and biofilms. In: Ghannoum M., O'Toole G.A., editors. ASM Press; 2004. pp. 20–27. [Google Scholar]
- Cafiso V., Bertuccio T., Santagati M., Demelio V., Spina D., Nicoletti G., Stefani S. agr‐genotyping and transcriptional analysis of biofilm‐producing Staphylococcus aureus. FEMS Immunol Med Microbiol. 2007;51:220–227. doi: 10.1111/j.1574-695X.2007.00298.x. [DOI] [PubMed] [Google Scholar]
- De Carvalho C.C.C.R. Biofilms: recent developments on an old battle. Recent Patents Biotechnol. 2007;1:49–57. doi: 10.2174/187220807779813965. [DOI] [PubMed] [Google Scholar]
- Catalanotti P., Lanza M., Del Prete A., Lucido M., Catania M.R., Gallè F. Slime‐producing Staphylococcus epidermidis and S. aureus in acute bacterial conjunctivitis in soft contact lens wearers. New Microbiol. 2005;28:345–354. et al. [PubMed] [Google Scholar]
- Donlan R.M., Costerton J.W. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggirala A., Kenchappa P., Sharma S., Peeters J.K., Ahmed N., Garg P. High‐resolution genome profiling differentiated Staphylococcus epidermidis isolated from patients with ocular infections and normal individuals. Invest Ophthalmol Vis Sci. 2007;48:3239–3245. doi: 10.1167/iovs.06-1365. et al. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick F., Humphreys H., O'Gara J.P. The genetics of staphylococcal biofilm formation – will a greater understanding of pathogenesis lead to better management of device‐related infection? Clin Microbiol Infect. 2005;11:967–973. doi: 10.1111/j.1469-0691.2005.01274.x. [DOI] [PubMed] [Google Scholar]
- Flemming H.C., Wingender J., Mayer C., Koerstgens V., Borchard W. Cohesiveness in biofilm matrix polymers. In: Allison D., Gilbert P., Lappin‐Scott H.M., Wilson M., editors. University Press; 2000. pp. 87–105. [Google Scholar]
- Goerke C., Gressinger M., Endler K., Breitkopf C., Wardecki K., Stern M. High phenotypic diversity in infecting but not in colonizing Staphylococcus aureus populations. Environ Microbiol. 2007;9:3134–3142. doi: 10.1111/j.1462-2920.2007.01423.x. et al. [DOI] [PubMed] [Google Scholar]
- Heydorn A., Nielsen A.T., Hentzer M., Sternberg C., Givskov M., Ersbøll B.K., Molin S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000;146:2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- Houari A., Picard J., Habarou H., Galas L., Vaudry H., Heim V., Di Martino P. Rheology of biofilms formed at the surface of NF membranes in a drinking water production unit. Biofouling. 2008;24:235–240. doi: 10.1080/08927010802023764. [DOI] [PubMed] [Google Scholar]
- Klapper I., Rupp C.J., Cargo B., Purvedorj R., Stoodley P. Viscoelastic fluid description of bacterial biofilm material properties. Biotechnol Bioeng. 2002;80:289–296. doi: 10.1002/bit.10376. [DOI] [PubMed] [Google Scholar]
- Korstgens V., Flemming H.C., Wingender J., Borchard W. Uniaxial compression measurement device for investigation of the mechanical stability of biofilms. J Microbiol Methods. 2001;46:9–17. doi: 10.1016/s0167-7012(01)00248-2. [DOI] [PubMed] [Google Scholar]
- Laspidou C.S., Aravas N. Variation in the mechanical properties of a porous multi‐phase biofilm under compression due to void closure. Water Sci Technol. 2007;55:447–453. doi: 10.2166/wst.2007.289. [DOI] [PubMed] [Google Scholar]
- Lewis K. Persister cells and the riddle of biofilm survival. Biochemistry. 2005;70:267–274. doi: 10.1007/s10541-005-0111-6. [DOI] [PubMed] [Google Scholar]
- Lina G., Boutite F., Tristan A., Bes M., Etienne J., Vandenesch F. Bacterial competition for human nasal cavity colonization: role of Staphylococcal agr alleles. Appl Environ Microbiol. 2003;69:18–23. doi: 10.1128/AEM.69.1.18-23.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah T.F., O'Toole G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- Nostro A., Roccaro A.S., Bisignano G., Marino A., Cannatelli M.A., Pizzimenti F.C. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Med Microbiol. 2007;56:519–523. doi: 10.1099/jmm.0.46804-0. et al. [DOI] [PubMed] [Google Scholar]
- Okajima Y., Kobayakawa S., Tsuji A., Tochikubo T. Biofilm formation by Staphylococcus epidermidis on intraocular lens material. Invest Ophthalmol Vis Sci. 2006;47:2971–2975. doi: 10.1167/iovs.05-1172. [DOI] [PubMed] [Google Scholar]
- Rogers S.S., Van Der Walle C., Waigh T.A. Microrheology of bacterial biofilms in vitroStaphylococcus aureus and Pseudomonas aeruginosa. Langmuir. 2008;24:13549–13555. doi: 10.1021/la802442d. [DOI] [PubMed] [Google Scholar]
- Rupp C.J., Fux C.A., Stoodley P. Viscoelasticity of Staphylococcus aureus biofilms in response to fluid shear allows resistance to detachment and facilitates rolling migration. Appl Environ Microbiol. 2005;71:2175–2178. doi: 10.1128/AEM.71.4.2175-2178.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw T., Winston M., Rupp C.J., Klapper I., Stoodley P. Commonality of elastic relaxation times in biofilms. Phys Rev Lett. 2004;93:1–4. doi: 10.1103/PhysRevLett.93.098102. [DOI] [PubMed] [Google Scholar]
- Stoodley P., Lewandowski Z., Boyle J.D., Lappin‐Scott H.M. Structural deformation of bacterial biofilms caused by short‐term fluctuations in fluid shear: an in situ investigation of biofilm rheology. Biotechnol Bioeng. 1999;65:83–92. [PubMed] [Google Scholar]
- Stoodley P., Cargo R., Rupp C.J., Wilson S., Klapper I. Biofilm material properties as related to shear‐induced deformation and detachment phenomena. J Ind Microbiol Biotechnol. 2002;29:361–367. doi: 10.1038/sj.jim.7000282. [DOI] [PubMed] [Google Scholar]
- Sutherland I.W. Exopolysaccharides in biofilms, flocs and related structures. Water Sci Technol. 2001;43:77–86. [PubMed] [Google Scholar]
- Towler B.W., Rupp C.J., Cunningham A.B., Stoodley P. Viscoelastic properties of a mixed culture biofilm from rheometer creep analysis. Biofouling. 2003;19:279–285. doi: 10.1080/0892701031000152470. [DOI] [PubMed] [Google Scholar]
- Towler B.W., Cunningham A., Stoodley P., McKittrick L. A model of fluid–biofilm interaction using a Burger material law. Biotechnol Bioeng. 2007;96:259–271. doi: 10.1002/bit.21098. [DOI] [PubMed] [Google Scholar]
- Vinogradov A.M., Winston M., Rupp C.J., Stoodley P. Rheology of biofilms formed from the dental plaque pathogen Streptococcus mutans. Biofilms. 2004;1:49–56. [Google Scholar]
- Wloka M., Rehage H., Flemming H.C., Wingender J. Rheological properties of viscoelastic biofilm extracellular polymeric substances and comparison to the behavior of calcium alginate gels. Colloid Polym Sci. 2004;282:1067–1076. [Google Scholar]
- Yarwood J.M., Paquette K.M., Tikh I.B., Volper E.M., Greenberg E.P. Generation of virulence factor variants in Staphylococcus aureus biofilms. J Bacteriol. 2007;189:7961–7967. doi: 10.1128/JB.00789-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegans M.E., Shanks R.M., O'Toole G.A. Bacterial biofilms and ocular infections. Ocul Surf. 2005;3:73–80. doi: 10.1016/s1542-0124(12)70155-6. [DOI] [PubMed] [Google Scholar]


