Summary
The position of high‐rate anaerobic technology (HR‐AnWT) in the wastewater treatment and bioenergy market can be enhanced if the range of suitable substrates is expanded. Analyzing existing technologies, applications and problems, it is clear that, until now, wastewaters with high lipids content are not effectively treated by HR‐AnWT. Nevertheless, waste lipids are ideal potential substrates for biogas production, since theoretically more methane can be produced, when compared with proteins or carbohydrates. In this minireview, the classical problems of lipids methanization in anaerobic processes are discussed and new concepts to enhance lipids degradation are presented. Reactors operation, feeding strategies and prospects of technological developments for wastewater treatment are discussed. Long‐chain fatty acids (LCFA) degradation is accomplished by syntrophic communities of anaerobic bacteria and methanogenic archaea. For optimal performance these syntrophic communities need to be clustered in compact aggregates, which is often difficult to achieve with wastewaters that contain fats and lipids. Driving the methane production from lipids/LCFA at industrial scale without risk of overloading and inhibition is still a challenge that has the potential for filling a gap in the existing processes and technologies for biological methane production associated to waste and wastewater treatment.
Introduction
The range of feedstock for biogas production spans from animal waste to municipal sludge, industrial wastewater and organic fractions of municipal solid waste as well as energy crops whenever available. The wide diversity of anaerobic technologies can be grouped in two main concepts: (i) facilities aiming at industrial wastewater treatment and (ii) facilities aiming at energy production. In general, the first group comprises more sophisticated technologies that operate with low solids content, high loading rates and sludge retention time much higher than hydraulic retention time. The second group includes anaerobic digestion (AD) plants dedicated to bioenergy production, which are usually completely mixed reactors, with a simple technological design. Since anaerobic processes possess multifunctional characteristics, energy is recovered from wastewater treatment facilities whenever it is economically feasible. On the other hand, organic waste stabilization and nutrient redistribution are, besides energy production, embedded objectives of any AD plant.
From the beginning of the eighties, high‐rate anaerobic wastewater treatment technology (HR‐AnWT) has become a standard for a certain range of industrial wastewaters. Thousands of full‐scale installations are in operation worldwide, treating mainly wastewater containing readily degradable organic pollutants such as volatile fatty acids and carbohydrates. Reliable technologies, such as the up‐flow anaerobic sludge blanket (UASB) reactor and the derived designs – expanded granular sludge bed (EGSB) and internal circulation (IC) reactors – promoted the confidence in AD technology. However, HR‐AnWT applications are still centred essentially in biodegradable effluents from distilleries, pulp and paper, breweries and beverage industries.
The position of HR‐AnWT in the wastewater treatment and bioenergy market can be further enhanced if the range of suitable substrates is expanded. Analysing the existing technologies, applications and problems, it is clear that wastewaters with high lipids content are not effectively treated yet by HR‐AnWT. Nevertheless, waste lipids are ideal substrates for methane production, since theoretically their degradation produces more biogas with higher methane content, when compared with proteins or carbohydrates (Table 1).
Table 1.
Potential biogas production from different classes of substrates.
| Component | Methanogenic reaction | Biogas (lg−1) | CH4 (%) |
|---|---|---|---|
| Lipids | C50H90O6 + 24.5H2O → 34.75CH4 + 15.25CO2 | 1.425 | 69.5 |
| Carbohydrates | C6H10O5 + H2O → 3CH4 + 3CO2 | 0.830 | 50.0 |
| Proteins | C16H24O5N4 + 14.5H2O → 8.25CH4 + 3.75CO2 + 4NH4+ + 4HCO3‐ | 0.921 | 68.8 |
The energy value of lipids makes them an ideal co‐substrate to increase the economical feasibility of any AD plant based on co‐digestion concepts. The net energy production increases significantly if a fraction of waste lipids is mixed in the feedstock. This has already been noticed by managers of AD plants, who are even willing to pay for lipids. However, without a proper feeding strategy, addition of waste lipids to an AD plant is risky, if accumulation of long‐chain fatty acids (LCFA) is not prevented. Understanding anaerobic degradation of lipids has therefore an immediate economical impact on AD plants.
The existing gap in HR‐AnWT for complex wastewaters with lipids and the importance of lipids as co‐substrates in AD plants make this issue of global interest in the environmental technology field. In this minireview, the classical problems of lipids degradation in anaerobic processes are discussed and new concepts to avoid inhibition by lipids and to enhance degradation are presented. Reactors operation, feeding strategies and prospects of technological developments for wastewater treatment are discussed. Finally, some hints on anaerobic bacteria and microbial communities that degrade LCFA are presented.
The role and drawbacks of LCFA in the anaerobic degradation of lipids
Lipids are LCFA bonded to glycerol, alcohols or other groups by an ester or ether linkage. Fats and oils are a subgroup of lipids that have the alcohol groups esterified with fatty acids, predominantly in the form of triglycerides (glycerol backbone with three LCFA). Fats contain saturated LCFA, and oils are normally composed of unsaturated fatty acids, which confer lower melting point.
Fats and oils are common contaminants of domestic sewage and industrial effluents from dairy industries (Perle et al., 1995), slaughterhouses (Sayed et al., 1988), livestock farms (Broughton et al., 1998), wool scouring facilities (Becker et al., 1999) and edible oil‐processing facilities (Beccari et al., 1996; Becker et al., 1999).
In general, hydrolysis of fats and oils to glycerol and LCFA proceeds rapidly in AD processes, resulting in the accumulation of LCFA in the wastewater (Hanaki et al., 1981; Angelidaki and Ahring, 1992). Over 90% of the chemical oxygen demand (COD) present in lipids is conserved, after hydrolysis, in the LCFA (Hanaki et al., 1981). The estimated biomass/substrate yield for the conversion of fat is 0.038 g VSS (g COD)−1, whereas for proteins and carbohydrates values of 0.2 and 0.35 g VSS (g COD)−1, respectively, are reported (Pavlostathis and Giraldo‐Gomez, 1991).
At neutral pH, LCFA are ionized and so it is appropriate to refer to them according to their carboxilate form: for instance, oleate and palmitate instead of oleic and palmitic acids. The concentration of lipids and LCFA in domestic and industrial wastewater is quite diverse. Wool scouring and olive mill processes can generate effluents with lipids concentrations in the range of 5–25 g l−1 (Beccari et al., 1998; Becker et al., 1999). Lower values were detected in a sunflower oil mill wastewater, with LCFA concentrations ranging from 0.2 to 1.3 g l−1 (Saatci et al., 2003). Total lipids in a dairy wastewater were reported to vary from 0.9 to 2.0 g l−1 (Kim et al., 2004a). In domestic sewage, lipids represent generally 20–25% of the total organic matter, with concentrations ranging from 40 to 100 mg l−1 (Quémeneur and Marty, 1994). Slaughterhouses can produce effluents with a total fat matter between 0.35 and 0.52 g l−1 (Sayed et al., 1988).
Table 2 presents the LCFA composition of lipid‐containing raw materials and wastewaters. It is very likely that the major constituents in raw materials are also present in the wastewaters generated during their processing. It is clear that palmitic acid and oleic acid are the most abundant saturated and unsaturated LCFA respectively.
Table 2.
LCFA commonly found in raw materials and wastewaters (showed as % of total LCFA) (adapted from Hwu, 1997).
| Raw materials/wastewaters | LCFA common name (structurea) | ||||||
|---|---|---|---|---|---|---|---|
| Lauric (C12:0) | Myristic (C14:0) | Palmitic (C16:0) | Palmitoleic (C16:1) | Stearic (C18:0) | Oleic (C18:1) | Linoleic (C18:2) | |
| Palm oil (1) | 1.4 | 42.9 | 0.7 | 4.8 | 39.0 | 10.0 | |
| Olive oil (1) | 14.3 | 1.4 | 2.4 | 71.4 | 5.5 | ||
| Soybean oil (1) | 1.0 | 11.0 | 4.8 | 21.9 | 49.0 | ||
| Cotton seed oil (1) | 1.4 | 25.7 | 1.0 | 2.9 | 15.2 | 51.9 | |
| Cocoa butter (1) | 26.7 | 0.5 | 32.9 | 33.8 | 4.3 | ||
| Whole milk (2) | 7.0 | 6.0 | 21.0 | 2.0 | 6.0 | 39.0 | 13.0 |
| Chicken fat (1) | 1.4 | 21.0 | 6.7 | 4.3 | 42.4 | 20.0 | |
| Beef tallow (1) | 1.0 | 2.6 | 28.1 | 3.8 | 20.0 | 37.6 | 2.9 |
| Domestic sewage (3) | 2.2 | 16.4 | 0.9 | 8.1 | 30.5 | 29.2 | |
| Dairy wastewater (4) | 27.0 | 7.0 | 37.0 | 13.0 | |||
Cn:d, where n is the number of carbon atoms and d the number of double bonds.
(1) Taylor (1965); (2) Hanaki and colleagues (1981); (3) Quémeneur and Marty (1994); (4) Kim and colleagues (2004a).
Research on the application of anaerobic technology to treat wastewaters containing lipids/LCFA has been emerging in the past 25 years. As discussed below, two main problems specifically related to the treatment of these effluents were identified and characterized: (i) sludge flotation and biomass washout due to the adsorption of lipids/LCFA onto the biomass, and (ii) inhibition of acetogenic bacteria and methanogenic archaea by LCFA.
LCFA adsorption onto the sludge – sludge flotation and sludge washout
Sludge flotation and sludge washout due to the adsorption of fatty matter onto the biomass are widely reported in the literature. Samson and colleagues (1985) referred the failure of an industrial scale UASB reactor treating milk fat, due to sludge flotation. Hawkes and colleagues (1995) observed poor biomass retention in four different reactors treating ice‐cream wastewater with high fat content. Rinzema and colleagues (1989; 1993) tested the treatability of LCFA‐containing wastewaters in UASB reactors. When the reactors were overloaded a severe washout caused by flotation was observed. Sam‐Soon and colleagues (1991) used a UASB reactor to study oleic acid degradation and reported that the original inoculated granules suffered from disintegration and encapsulation by a gelatinous and whitish mass.
LCFA have an amphiphilic structure; they are composed of a hydrophobic aliphatic tail and a hydrophilic carboxylic head. From a thermodynamic viewpoint disintegration of granules can be expected because at neutral pH LCFA act as surfactants, lowering the surface tension. According to Thaveesri and colleagues (1995) the adhesion of hydrophilic cells appeared to be enhanced at a low liquid surface tension, while the adhesion of hydrophobic cells was favoured at a high surface tension. Acetogens were characterized as mostly hydrophobic and therefore the low‐surface‐tension environments imposed by LCFA may cause a sloughing‐off from granular sludge and the selective washout of these microorganisms (Daffonchio et al., 1995). Hwu et al. (1997a) concluded that typical operating parameters of EGSB reactors (up‐flow velocity > 4 m h−1, hydraulic retention time < 10 h) resulted in a poor treatment of LCFA containing wastewaters. The recirculation of the washed out biomass was beneficial to enhance the overall performance (Hwu et al., 1997b).
Hwu and colleagues (1998a) showed that the specific LCFA organic load necessary to induce complete sludge flotation [0.203 g COD (g VSS)−1 day−1] corresponded to a LCFA concentration of 263 mg LCFA l−1, which was far below the minimum inhibitory concentration (401 mg LCFA l−1) of methanogenesis. This suggested that deterioration of the UASB process by LCFA adsorption and consequent sludge washout are likely to occur prior to inhibition of the methanogenic archaea by the LCFA.
Jeganathan and colleagues (2006), using UASB reactors to treat a complex oily wastewater from a food industry, reported that although approximately 75% of COD was degraded to methane at an organic loading rate (OLR) of about 2.5 g COD l−1 day−1, the system performance declined sharply at higher loading rates. An increase in loading to 5 g COD l−1 day−1 caused fat, oil and grease (FOG) accumulation in the sludge and increased foam production. This reduced the degradation to 40–50%. These authors also reported that accumulation of FOG in the biomass was the critical parameter governing the high‐rate anaerobic reactor performance and further suggested the need for periodic reseeding of anaerobic reactor systems treating oily wastes, since the loss of sludge in the bed, due to washout, increased the FOG accumulation onto the biomass and consequent reactor failure.
Inhibition of anaerobic microbial communities by LCFA
Although derived essentially from the interpretation of batch experiments, the effect of LCFA on the methanogenic and acetogenic microorganisms is documented (Hanaki et al., 1981; Koster and Cramer, 1987; Angelidaki and Ahring, 1992; Rinzema et al., 1994; Lalman and Bagley, 2000; 2001; 2002). Both acetoclastic and hydrogenotrophic methanogens are affected by LCFA, although acetoclastic methanogens are apparently more affected by the presence of these compounds (Hanaki et al., 1981; Hwu and Lettinga, 1997; Alves et al., 2001; Lalman and Bagley, 2001). Inhibitory effects of unsaturated LCFA are reported to be more severe than those of saturated LCFA (Lalman and Bagley, 2002).
In the early 1980s, Hanaki and colleagues (1981) performed several batch experiments where they found that glucose fermentation was not affected by the presence of LCFA, the addition of acetate and butyrate intensified the toxic effect of LCFA, and oleate was less inhibitory than a LCFA mixture. Angelidaki and Ahring (1992) suggested that the response to the addition of neutral lipids may depend on the degree of biomass adaptation, whereas the addition of free LCFA above a certain concentration may directly result in process failure, due to a permanent toxic effect of these compounds towards acetogenic bacteria and methanogenic archaea. Rinzema and colleagues (1994) validated this concept and found that LCFA exerted a bactericidal effect on methanogenic archaea. This conclusion was based on the observation that acetoclastic methanogens did not adapt to LCFA neither upon repeated exposure to toxic concentrations, nor after prolonged exposure to non‐toxic concentrations. The recovery after a lag phase usually observed in batch assays was ascribed to growth of a few survivors. For many years it was believed that high‐rate treatment of lipid‐rich effluents was not possible. Hwu (1997) tried to enhance the anaerobic treatment of wastewater containing oleic acid and found a higher susceptibility of suspended sludge than granular sludge to LCFA toxicity. This observation in batch assays, though interesting, was of little practical relevance, since granular sludge was not structurally stable when LCFA were present.
Lalman and Bagley (2002) reported only a small inhibition of hydrogenotrophic methanogens in the presence of linoleate (C18:2), oleate (C18:1) and stearate (C18:0), individually or in mixture. The mechanism of inhibition by LCFA was related to their adsorption onto the cell surface, affecting the transport and/or protective functions of the cell (Demeyer and Henderickx, 1967; Galbrait and Miller, 1973; Rinzema, 1988). This led to the hypothesis that the inhibitory effect of LCFA is determined by the LCFA : biomass surface ratio, although in several other studies the LCFA concentration was found to determine inhibition by LCFA (Rinzema et al., 1994; Kim et al., 2004a). However, other authors showed that inhibition by LCFA is not permanent and that adaptation of biomass to lipids/LCFA can occur (Broughton et al., 1998; Alves et al., 2001; Kim et al., 2004a; Pereira et al., 2004).
New concepts on LCFA inhibition and degradation
As a consequence of LCFA accumulation onto the sludge, treatment of lipids/LCFA‐containing wastewaters in conventional up‐flow anaerobic reactors can lead to complete sludge washout and process failure, which seems to be more due to particular problems of fluid dynamics than to microbial activity inhibition. In fact, microbial injure due to intensive (high concentration) and extended (long time) contact between anaerobic sludge and LCFA was found to be less severe than could be expected (Alves et al., 2001; Pereira et al., 2002a; 2004; 2005). When performing a routine assessment of the specific methanogenic activity of sludge collected from a continuous reactor fed with oleic acid, a surprisingly high methane production was observed in the blank vials, where no external substrate was added (Alves et al., 2001). The observed methane production resulted from the degradation of substrate that accumulated onto the sludge during the reactor's operation, contradicting the accepted theories of permanent LCFA toxicity and inhibition. This finding led to the development of new concepts and encouraged the study of the microbiology of LCFA degradation in anaerobic bioreactors. The scheme presented in Fig. 1 illustrates the sequence of continuous LCFA feeding, LCFA accumulation and consequent sludge flotation and washout. Subsequent batch incubation of the sludge taken from the reactor, containing biomass‐associated LCFA, results in methane production. The proposed mechanisms of LCFA accumulation onto the biomass are adsorption, entrapment within the flocks structure and precipitation with divalent ions (Pereira et al., 2005). Fig. 2 shows a microbial aggregate collected from a UASB reactor fed with oleic acid. The whitish matter embedding the cells consisted, in that case, of more than 80% palmitic acid (Pereira et al., 2005).
Figure 1.
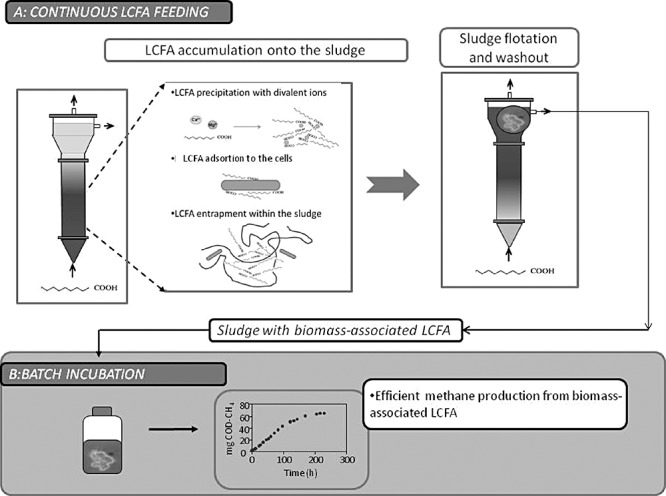
Schematic representation of the phenomena of LCFA accumulation onto the sludge during the continuous operation of a reactor fed with LCFA, sludge flotation, sludge washout and methane production in batch vials from the degradation of the biomass‐associated LCFA (adapted from Sousa et al., 2009).
Figure 2.
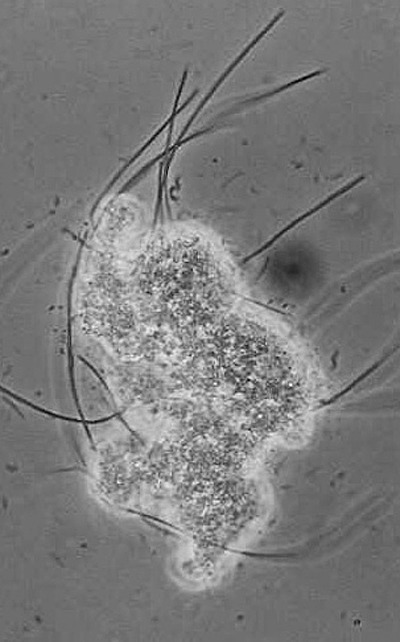
Example of a microbial aggregate collected from a lab‐scale UASB reactor fed with oleic acid.
The difference in properties of granular and suspended sludge in terms of their capacity to accumulate and to degrade the accumulated (or biomass associated) LCFA was studied by Pereira and colleagues (2002a). Two EGSB reactors were fed with oleic acid‐based synthetic effluents. One EGSB was inoculated with suspended sludge and the other one with granular sludge. Sludge samples were collected at different operating times and incubated in batch vials, where the methane production from the degradation of the accumulated LCFA was monitored. Suspended sludge turned out to be more efficient than granular sludge to accumulate and to degrade LCFA. A maximum specific LCFA accumulation of 3271 ± 877 mg COD‐LCFA (g VSS)−1, and a maximum methane production rate of 434 ± 60 mg COD‐CH4 (g VSS)−1 day−1 was exhibited by the suspended sludge, which was about 1.3 and 3 times, respectively, the maximum values exhibited by the granular sludge (Pereira et al., 2002a). Additionally, for the suspended sludge, the methane production rate in batch was enhanced by stirring and was inhibited by the addition of oleic acid. Extraction and GC analysis revealed that the main adsorbed substrate was palmitate, and not oleate. Apparently, the conversion of oleate to palmitate occurred easily in the reactor, but further degradation of palmitate did not occur.
Pursuing the investigation, the specific methanogenic activity (SMA) of sludge samples, containing biomass‐associated LCFA content between 1000 and 5000 mg COD‐LCFA (g VSS)−1, was determined before and after the conversion to methane of that embedded LCFA (Pereira et al., 2004). Acetate and H2/CO2 were used as individual substrates for SMA experiments. In general, the loaded sludge had no quantifiable activity except with H2 as substrate (Table 3). After the degradation of the biomass‐associated LCFA, a significant increase on the SMA was observed for the selected substrates.
Table 3.
Specific methanogenic activities exhibited by three different sludges, before and after the conversion to methane of the biomass‐associated LCFA (adapted from Pereira et al., 2004).
| Sludge‐specific LCFA content [mg COD‐LCFA (g VSS)−1] | Specific methanogenic activity [mg COD‐CH4 (g VSS)−1 day−1] | |||
|---|---|---|---|---|
| Acetate | H2/CO2 | |||
| Before | After | Before | After | |
| 1221 ± 144 | 143 ± 29 | 326 ± 13 | 1462 ± 94 | 1670 ± 81 |
| 2838 ± 63 | 0 | 579 ± 4 | 1218 ± 1 | 2817 ± 146 |
| 4571 ± 257 | 0 | 533 ± 95 | 401 ± 21 | 2709 ± 38 |
This result demonstrated that the inhibition by LCFA is reversible in the range of LCFA content between 1000 and 5000 mg COD‐LCFA (g VSS)−1, contradicting the previously reported bactericidal or permanent toxic effects of LCFA (Rinzema, 1988; Angelidaki and Ahring, 1992). This opened new scenarios for the anaerobic treatment of wastewater with high lipid content. The low SMA measured before the depletion of the biomass‐associated LCFA may result from a strong effect of transport (diffusion) limitations imposed by the LCFA layer surrounding the cells, which could hamper the access of the added substrates, as well as the subsequent biogas release. This is reinforced by the fact that with H2, the smallest molecule used as electron donor, methane production was directly observed, suggesting a fast transport of this small compound through the LCFA layer. Transport limitations phenomena may also be responsible for the observed lag phases that previously have been ascribed to mechanisms of cell wall damage and bactericidal effects. However, although the hypothesis of transport limitations seems to be reasonable, a reversible metabolic inhibitory effect would result in similar observations. Probably both phenomena are involved, their relative importance being dependent on the specific LCFA content of the sludge (Pereira et al., 2004).
The evidence of physical inhibition due to transport limitation effects was further clarified by Pereira and colleagues (2005). The important conclusion was reinforced; the inhibitory effect on the methanogenic activity, after the contact of the biomass with LCFA, is reversible.
From this ensemble of new results it became clear that LCFA‐rich wastewater treatment can be feasible. Sequencing phases of continuous feeding and batch degradation of the accumulated substrate was then postulated as a possible practical solution for the treatment of this kind of effluents.
New reactor concept for HR‐AnWT of complex wastewater with high lipid content
Since the success of conventional anaerobic treatment systems is based on optimization of biomass sedimentation, flotation leads to washout and subsequent process disruption. Table 4 gives an overview of studies in which LCFA‐containing wastewater was treated in anaerobic bioreactor studies. Problems of sludge flotation or unsuccessful granulation were ascribed to treatment failure of industrial and pilot scale UASB reactors treating lipids/LCFA‐containing wastewater (Samson et al., 1985; Rinzema, 1988; Sam‐Soon et al., 1991; Hawkes et al., 1995; Hwu, 1997). Reasonable solutions to overcome flotation problems were searched: sieve drums, biomass recirculation or partial phase separation were tentatively applied for that purpose (Rinzema, 1988; Hamdi et al., 1992; Hwu, 1997; Beccari et al., 1998). To prevent washout induced by LCFA adsorption, a sequential process with a feeding and a reaction phase was suggested as the preferred technology for anaerobic LCFA removal from wastewater (Pereira et al., 2005). It was further postulated that the specific contact area between bacteria and LCFA should be maximized to minimize mass transfer limitations. The sequential process was applied at lab scale by Cavaleiro and colleagues (2009) and the results obtained showed that this operating mode was the best for sludge acclimation. After the third cycle a specialized anaerobic microbial community was developed, able to efficiently convert the LCFA to methane. Continuous treatment was then applied, achieving volumetric loading rates up to 21 kg COD m−3 day−1 with 72% conversion efficiency to methane. From the current problems encountered at industrial scale with LCFA and the research results from Pereira and colleagues (2002a,b,c; 2004; 2005) and Cavaleiro and colleagues (2009), two main principles may be postulated for the design of a reactor capable of high‐rate anaerobic treatment of LCFA‐containing wastewater. These constitute the base of a new reactor concept, designated as Inverted Anaerobic Sludge Bed (IASB) reactor (Alves et al., 2007): (i) maximize the contact area between biomass and LCFA to optimize LCFA adsorption, and (ii) use flotation as the primary biomass retention technique. These two principles imply that conventional primary biomass retention techniques such as granulation or biomass fixation cannot be applied. However, a settling step is still needed, because sludge settles well again after effective LCFA conversion. This settled sludge can subsequently be intimately contacted with LCFA‐containing wastewater to maximize adsorption. Thus, a sludge recycle loop should be present over the reactor. This loop could further provide the mild shear stress needed to maximize the sludge surface area. Additionally, it provides the means to control mixing intensity inside the reactor and limit possible mass transfer limitations even further. A pilot scale proof of concept is running presently in a slaughterhouse located in the Nord of Portugal.
Table 4.
Treatment of wastewater containing lipids and LCFA in different anaerobic reactors (adapted from Sousa, 2007).
| Type of wastewater | Type of reactor | Temperature (°C) | HRT (days) | OLR (g COD l−1 day−1) | Specific OLR [g COD (g VSS)−1 day−1] | COD removal (%) | CH4 yield [lCH4 (g COD)−1] | Reference |
|---|---|---|---|---|---|---|---|---|
| LCFA mixture | EGSB | 30 | 0.25–0.13 | 4–8 | 0.20–0.41 | 44–69 | ND | Hwu et al. (1998b) |
| LCFA mixture | EGSB | 55 | 0.25–0.13 | 4–8 | 0.23–0.47 | 66–73 | ND | |
| LCFA mixture | UASB | 35 | 0.7–1.2 | 3.2–9.4 | 0.09–0.25 | 82–93 | ND | Hwu et al. (1998a) |
| LCFA mixturea | CSTR + UASB | 35 | 2.9 | 0.2–2.7 | ND | 60–95 | ND | Kim et al. (2004b) |
| Oleate (+milk)b | EGSB | 37 | 1 | 4–8 | 1.39–2.78 | 69–97 | 0.03–0.28 | Pereira et al. (2002b) |
| Oleate (+milk)b | AF | 37 | 3.3–0.64 | 0.7–12.5 | 0.09–1.67 | 80–95 | 0.09–0.36 | |
| Oleate | EGSB | 37 | 1.18 | 3.3 | 0.85 | 75–86 | 0.03 | Pereira et al. (2005) |
| Palmitate | EGSB | 37 | 1.14 | 3.2 | 0.83 | 90–95 | 0.03 | |
| Saccharose + oleate | UASB | 35 | 1 | 4.2–6.3 | 0.25–0.38 | 76–90 | ND | Miranda et al. (2006) |
| Saccharose + oleate | DAEB | 35 | 1 | 4.2–6.3 | NA | 77–93 | ND | |
| Saccharose + stearate | UASB | 35 | 1 | 4.2–6.4 | 0.25–0.38 | 77–90 | ND | |
| Saccharose + stearate | DAEB | 35 | 1 | 4.2–6.4 | NA | 76–90 | ND | |
| Dairy wastewater | UASB | 35 | 6–40 | 2.0–4.5 | ND | 79–99 | ND | Gavala et al. (1999) |
| Dairy wastewater | IFB | 35 | 63.6–3 | 0.5–10 | ND | 75–98 | ND | Arnaiz et al. (2003) |
| ITB | 35 | 66.6–3 | 0.5–12 | ND | 75–98 | ND | ||
| Dairy wastewater | BFBR | – | 0.3–0.5 | 10 (up to) | ND | 85–90 | Approximately 0.37 | Haridas et al. (2005) |
| Ice‐cream factory wastewater | AF | 35 | 0.9 | 6.4 | ND | 67 | 0.36 | Hawkes et al. (1995) |
| Contact process | 35 | 5.5 | 1.1 | ND | 82 | 0.39 | ||
| Fluidized bed reactor | 35 | 1.5 | 4.2 | ND | 56 | 0.37 | ||
| UASB | 35 | 1.6 | 2.2 | ND | 49 | 0.19 | ||
| Food‐processing wastewater | Multi‐stage UASB | 55 | 0.14 | 50 | 2.29 | 60–70 | ND | Tagawa et al. (2002) |
| Food‐processing wastewater | UASB | 35 | 5 | 2.7–5.2 | 0.19–0.37 | 94–98 | 0.24–0.32 | Jeganathan et al. (2006) |
| UASB | 35 | 2.5–1.25 | 1.3–8.0 | 0.07–0.42 | 84–89 | 0.24–0.48 | ||
| (PBR+) UASB | 35 | 2.5–1.25 | 1.3–4.2 | 0.06–0.18 | 86–90 | 0.18–0.42 | ||
| Slaughterhouse wastewater | EGSB | 35 | 0.2 | 15 (up to) | ND | 67 | ND | Núnez and Martínez (1999) |
| Slaughterhouse wastewater | ASBR | 25 | 2.9 | 31.3c | ND | 94 | ND | Masse et al. (2002) |
| Slaughterhouse wastewater | UASB | 33 | 0.3–0.1 | 13–30 | ND | 60–93 | 0.20–0.28 | Torkian et al. (2003) |
| Slaughterhouse wastewater | Draw‐and‐fill reactor | 35 | 20 | 0.20 | – | 48 | 0.30d | Fountoulakis et al. (2008) |
| Slaughterhouse + olive mill wastewater (1:1) | 35 | 20 | 2.1 | – | 85 | 0.17d | ||
| Olive mill wastewater | 35 | 20 | 4 | – | 75 | 0.11d | ||
| Palm oil mill wastewater | MABR | – | 3–10 | 1.6–5.3 | ND | 87–95 | 0.32–0.42 | Faisal and Unno (2001) |
| Palm oil mill wastewater | UASFF | 38 | 3–1.5 | 1.8–23.2 | 0.18–1.23 | 89–97 | 0.31–0.35 | Najafpour et al. (2006) |
| Sunflower oil factory wastewater | UASB | 37 | 2–2.8 | 1.6–7.8 | ND | 87 | 0.16–0.35 | Saatci et al. (2003) |
Glucose was used as a co‐substrate during reactor operation.
Skim milk was used as co‐substrate during the start‐up period.
Calculated with basis on the 1 h time feeding of the reactor (subsequent reaction and settling phases lasted for 69 h).
lCH4 (g COD added)−1.
HRT, hydraulic retention time; OLR, organic loading rate; COD, chemical oxygen demand; UASB, up‐flow anaerobic sludge bed reactor; CSTR, continuously stirred tank reactor; EGSB, expanded granular sludge bed reactor; DAEB, down‐flow anaerobic expanded bed reactor; IFB, inverse fluidized bed reactor; ITB, inverse turbulent bed reactor; BFBR, buoyant filter bioreactor; AF, anaerobic filter; PBR, packed bed reactor (containing immobilized lipase beads); ASBR, anaerobic sequencing batch reactor; UASFF, up‐flow anaerobic sludge‐fixed film reactor; MABR, modified anaerobic baffled bioreactor; ND, not determined; NA, not applicable; –, information not available.
Kinetics of LCFA degradation
Existing information about the kinetics of LCFA degradation under anaerobic conditions is not vast (Table 5). The complex nature of LCFA–cells interaction, and the potential of biomass acclimation, make it difficult to obtain objective data about kinetics of LCFA degradation and inhibition by LCFA. In the Anaerobic Digestion Model (ADM) No. 1 inhibition under transient high LCFA concentrations is not described (Batstone et al., 2002).
Table 5.
Summary of kinetics data on anaerobic LCFA degradation.
| Substrate | KS kg COD m−3 | Y COD/CODa | µmax day−1 | Reference |
|---|---|---|---|---|
| Oleate/manure/oil | 0.058 | 0.05 | 0.55 | Angelidaki et al. (1999) |
| Stearate | 0.295 | 0.055 | 0.1 | Novak and Carlson (1970) |
| Palmitate | 0.41 | 0.054 | 0.11 | Novak and Carlson (1970) |
| Myristate | 1.23 | 0.053 | 0.08 | Novak and Carlson (1970) |
| Oleate | 9.21 | 0.054 | 0.44 | Novak and Carlson (1970) |
| Linoleate | 5.19 | 0.055 | 0.55 | Novak and Carlson (1970) |
| Slaughterhouse (stearate) | 0.1 | 0.021 | 7.7 | Salminen et al. (2000) |
| Slaughterhouse (palmitate) | 0.1 | 0.004 | 0.89 | Salminen et al. (2000) |
| Slaughterhouse | 0.102 | – | – | Masse et al. (2002) |
| Oleate + skim milk | – | 0.11–0.20 | 0.15–0.25 | Alves (1998) |
| LCFA oxidation | 0.105–3.18 | 0.06–0.16 | 0.085–0.55 | Pavlostathis and Giraldo‐Gomez (1991) |
KS, half‐saturation constant; Y, biomass/substrate yield; μmax, maximum specific growth rate.
produced biomass COD/consumed substrate COD.
The specific methane production rate from LCFA has been reported by several authors. Hwu and colleagues (1997b) reported a value of 600 mg COD (g VSS)−1 day−1 in the washed‐out biomass from an EGSB reactor fed with oleic acid. Pereira and colleagues (2004) reported kinetic values of degradation of biomass‐associated LCFA; a maximum of 434 mg COD‐CH4 (g VSS)−1 day−1 was obtained for a sludge containing a specific LCFA content of 743 mg COD‐LCFA (g VSS)−1, while a value of 241 mg COD‐CH4 (g VSS)−1 day−1 was obtained for a sludge containing a specific LCFA content of 3272 mg COD‐LCFA (g VSS)−1. A simple Haldane substrate inhibition model was not adequate to describe the kinetics of methane production from biomass‐associated LCFA. Cavaleiro and colleagues (2009) reported a maximum value of 1170 mg COD‐CH4 (g VSS)−1 day−1 in a continuous reactor fed with high loads of skim milk and oleate.
Microbiology of anaerobic LCFA degradation
LCFA degradation in anaerobic bioreactors is accomplished by syntrophic communities of acetogenic bacteria and methanogenic archaea. LCFA are converted to acetate and hydrogen by obligate hydrogen‐producing acetogens – OHPA (Schink, 1997). To date, only 14 acetogenic microorganisms have been described that degrade butyrate or higher fatty acids in syntrophy with hydrogen‐consuming microorganisms (McInerney et al., 2008). They all belong to the families Syntrophomonadaceae (McInerney, 1992; Zhao et al., 1993; Wu et al., 2006; Sousa et al., 2007a) and Syntrophaceae (Jackson et al., 1999), and live together with hydrogen‐consuming methanogenic archaea or sulfate‐reducing bacteria. However, only seven species are described that can use straight‐chain LCFA with more than 12 carbon atoms: Syntrophomonas sapovorans (Roy et al., 1986), Syntrophomonas saponavida (Lorowitz et al., 1989), Syntrophomonas curvata (Zhang et al., 2004), Syntrophomonas zehnderi (Sousa et al., 2007a), Syntrophomonas palmitatica (Hatamoto et al., 2007a), Thermosyntropha lipolytica (Svetlitshnyi et al., 1996) and Syntrophus aciditrophicus (Jackson et al., 1999).
Among these microorganisms only four species have the capability of utilizing mono‐ and/or polyunsaturated LCFA (with more than 12 carbon atoms): S. sapovorans (Roy et al., 1986), S. curvata (Zhang et al., 2004), T. lipolytica (Svetlitshnyi et al., 1996) and the recently isolated S. zehnderi (Sousa et al., 2007a) (Table 6). The latter bacterium was obtained from an anaerobic bioreactor treating an oleate‐based effluent.
Table 6.
Characteristics of some syntrophic LCFA‐degrading bacteria (adapted from Sousa, 2007).
| LCFA‐degrading bacteria | Morphological characteristics | LCFA utilization range |
|---|---|---|
Syntrophomonas sapovoransa
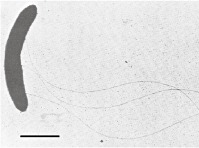
|
Short curved rods (0.5 × 2.5 µm) Slightly motile Gram‐negative Two to four flagella Non‐spore forming | Degrades linear saturated fatty acids with 4–18 carbon atoms in co‐culture with Methanospirillum hungatei. Mono‐ and di‐unsaturated LCFA, such as oleate (C18:1) and linoleate (C18:2), are also oxidized by the co‐culture |
Syntrophomonas curvatab
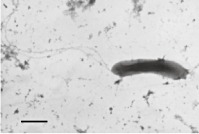
|
Slightly curved rods (0.5–0.7 × 2.3–4.0 µm) Motile Gram‐negative One or three flagella inserted in both poles Non‐spore forming | Degrades linear saturated fatty acids with 4–18 carbon atoms in co‐culture with M. hungatei. Oleate (C18:1) is also oxidized by the co‐culture |
Syntrophus aciditrophicusc
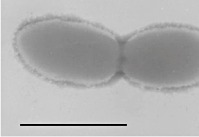
|
Rod‐shaped cells (0.5–0.7 × 1.0–1.6 µm) Non‐motile Gram‐negative Non‐spore forming | Degrades linear saturated fatty acids with more than four carbon atoms (C4:0 to C8:0, C16:0, C18:0) in co‐culture with H2‐utilizing Desulfovibrio sp. or Methanospirillum hungatei |
Syntrophomonas zehnderid
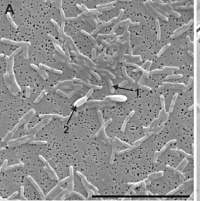
|
Curved rods (approximately 0.4–0.7 × 2.0–4.0 mm) Variable response to Gram staining Slight twitching Motility Spore formation during growth on oleate in co‐culture with a methanogen that utilizes hydrogen and formate | Degrades oleate, a mono‐unsaturated fatty acid, and straight‐chain fatty acids C4:0–C18:0 in syntrophic association with Methanobacterium formicicum |
Bars equal 1 µm, except for picture d where the bar represents 10 µm.
To date there are few studies on the microbial diversity of anaerobic communities that degrade LCFA. Microbial diversity of granular and suspended sludge was assessed during a long‐term operation of two anaerobic up‐flow bioreactors fed with increasing concentrations of oleate. Due to partial flotation caused by LCFA accumulation, a physical segregation of the sludge in two fractions was observed: a whitish floating fraction, at the top, and a settled fraction at the bottom (Pereira et al., 2002c).
Differences in the community structure between these two fractions were assessed by comparison of denaturating gradient gel electrophoresis (DGGE) profiles. Similarity indices between bottom and top sludge fractions attained values as low as 56.7% and 29.4% for the granular and suspended sludge respectively. Additionally, a shift in the community structure was observed in both sludges during the operation. Similarity indices between the original granular and suspended sludges and the respective top sludge fractions at the end of the operation were 17.3% and 15.2%.
Shigematsu and colleagues (2006) used a 16S rRNA gene approach to study the microbial communities present in a chemostat fed with a mixture of oleic and palmitic acids. Members belonging to Syntrophomonadaceae were detected in the chemostat, although the most predominant microorganisms belonged to the Bacteroidetes and Spirochaetes phyla. Based on this fact, the authors suggested that members of those phyla could play a role in LCFA degradation. The diversity and dynamics of biomass in reactors that treat saturated (palmitate) and unsaturated (oleate) LCFA were studied by 16S ribosomal RNA gene‐targeted molecular techniques (Sousa et al., 2007b,c). These studies revealed that the bacterial community of the sludges is quite complex. Besides bacteria known to be able to degrade LCFA, bacteria were detected that are likely involved in the degradation of short‐chain fatty acids, suggesting that during LCFA degradation short‐chain fatty acids are formed from LCFA. Bacterial communities were dominated by members of the Clostridiaceae and Syntrophomonadaceae families (Sousa et al., 2007b). However, a significant part of the retrieved bacterial 16S rRNA gene sequences (53%) were most similar to those of yet uncultured microorganisms, with the majority assigned to the phylum Firmicutes. Members of Proteobacteria and Bacteroidetes were also found.
A further interesting observation was the difference in the dominant population obtained with oleate and palmitate. The oleate‐degrading community is able to rapidly degrade palmitate, which is obvious as palmitate is a key intermediate in oleate degradation. However, the consortium enriched with palmitate degraded oleate only poorly. This seems to reflect the above‐mentioned characteristics of LCFA‐degrading bacteria. All bacteria that degrade unsaturated fatty acids also degrade saturated fatty acids, but the opposite is not the case. The likely biochemical mechanism to degrade unsaturated fatty acids seems to be a coupled hydrogenation and β‐oxidation. Besides OHPA that degrade unsaturated LCFA, bacteria exist that have the ability to hydrogenate unsaturated LCFA to saturated LCFA (Maia et al., 2007; Paillard et al., 2007).
Hatamoto and colleagues (2007b,c) used stable isotope probing with 13C palmitate as a substrate to identify the microorganisms directly involved in palmitate degradation. Members of the phyla Bacteroidetes and Spirochaetes, the family Syntrophaceae within the Deltaproteobacteria, and members of the family Syntrophomonadaceae and genus Clostridium within the Firmicutes were found in clone libraries from heavy rRNA fractions. These results confirm that phylogenetically diverse bacterial groups were active in situ in the degradation of LCFA under methanogenic conditions.
The effect of sulfate addition to methanogenic LCFA‐degrading sludges was studied (Sousa et al., 2009). When sludges were exposed to sulfate, sulfide was produced and methane formation decreased. Nevertheless, although many sulfate‐reducing bacteria are known that can degrade LCFA, OHPA remained dominantly present in the reactors even after a long exposure to high sulfate concentrations (Sousa et al., 2009). The results suggest that hydrogen consumption by methanogens is taken over by hydrogen‐consuming sulfate reducers, which are known to have a higher affinity for hydrogen than methanogens.
Conclusions and future perspectives
Lipids are suitable substrates for high‐rate anaerobic wastewater treatment and are also ideal co‐substrates for AD plants. Provided the appropriate technology is utilized and the right feeding strategy is followed, lipids can be effectively converted to methane by syntrophic consortia of acetogenic bacteria and methanogenic archaea. Driving the methane production from lipids/LCFA at industrial scale, without risk of inhibition, is still a challenge that has the potential for filling a gap in the existing processes and technologies for biomethane production associated to waste and wastewater treatment. A new reactor concept is proposed that provide primary biomass retention through flotation and secondary biomass retention through settling. The potential of this reactor to treat effluents with high concentrations of lipids/LCFA is being explored at pilot scale.
The types of bacteria involved in the methanogenic conversion of LCFA are known and the biochemical mechanism of LCFA degradation by β‐oxidation is rather well understood. However, the initial steps in the anaerobic conversion of unsaturated LCFA remain unclear. Besides OHPA that degrade unsaturated LCFA, bacteria exist that have the ability to hydrogenate unsaturated LCFA to saturated LCFA. The position of the latter bacteria in LCFA degradation in bioreactors requires further study. In principle this conversion can be coupled to growth and these bacteria may compete with hydrogenotrophic methanogens for hydrogen.
LCFA require the syntrophic cooperation of OHPA and methanogens. These syntrophic communities perform optimally when they are organized in micro‐colonies; at short intermicrobial distances the rate of interspecies hydrogen transfer is enhanced. Presently, it is unclear how the fatty structure of the substrate interferes with these communities. It is not clear how the micro‐colonies develop in a fatty matrix and what is the effect on hydrogen transfer. As hydrogen is poorly soluble in water, it cannot be excluded that interspecies hydrogen transfer is enhanced when the matrix is LCFA rather than water.
Acknowledgments
The authors gratefully acknowledge the financial support provided by the Portuguese Science Foundation (FCT) through the project FAT‐METHANE (POCTI/CTA/46328/2002) and the Grants PRAXIS XXI/BD/20326/99, SFRH/BPD/14591/2003 and SFRH/BD/24256/2005. The financial support of the Netherlands Science Foundation through the divisions STW, ALW and CW, and of the Portuguese National Institute of Industrial property – INPI, are gratefully acknowledged. A special thank to the Lettinga Associates Foundation for the financial support and recognition through the Lettinga Award 2004, attributed to M. Alves.
References
- Alves M.M. 1998.
- Alves M.M., Vieira J.A., Pereira R.M., Pereira M.A., Mota M. Effects of lipids and oleic acid on biomass development in anaerobic fixed‐bed reactors. Part II: oleic acid toxicity and biodegradability. Water Res. 2001;35:264–270. doi: 10.1016/s0043-1354(00)00242-6. [DOI] [PubMed] [Google Scholar]
- Alves M.M., Picavet M.A., Pereira M.A., Cavaleiro A.J., Sousa D.Z. 2007.
- Angelidaki I., Ahring B.K. Effects of free long‐chain fatty acids on thermophilic anaerobic digestion. Appl Microbiol Biotechnol. 1992;37:808–812. doi: 10.1007/BF00176668. [DOI] [PubMed] [Google Scholar]
- Angelidaki I., Ellegaard L., Ahring B.K. A comprehensive model of anaerobic bioconversion of complex substrates to biogas. Biotechnol Bioeng. 1999;63:363–372. doi: 10.1002/(sici)1097-0290(19990505)63:3<363::aid-bit13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Arnaiz C., Buffiere P., Elmaleh S., Lebrato J., Moletta R. Anaerobic digestion of dairy wastewater by inverse fluidization: the inverse fluidized bed and the inverse turbulent bed reactors. Environ Technol. 2003;24:1431–1443. doi: 10.1080/09593330309385687. [DOI] [PubMed] [Google Scholar]
- Batstone D.J., Keller J., Angelidaki I., Kalyuzhnyi S.V., Pavlostathis S.G., Rozzi A. The IWA Anaerobic Digestion Model No 1 (ADM1) Water Sci Technol. 2002;45:65–73. et al. [PubMed] [Google Scholar]
- Beccari M., Bonemazzi F., Majone M., Riccardi C. Interaction between acidogenesis and methanogenesis in the anaerobic treatment of olive oil mill effluents. Water Res. 1996;30:183–189. [Google Scholar]
- Beccari M., Majone M., Torrisi L. Two‐reactor system with partial phase separation for anaerobic treatment of olive oil mill effluents. Water Sci Technol. 1998;38:53–60. [Google Scholar]
- Becker P., Koster D., Popov M.N., Markossian S., Antranikian G., Markl H. The biodegradation of olive oil and the treatment of lipid‐rich wool scouring wastewater under aerobic thermophilic conditions. Water Res. 1999;33:653–660. [Google Scholar]
- Broughton M.J., Thiele J.H., Birch E.J., Cohen A. Anaerobic batch digestion of sheep tallow. Water Res. 1998;32:1423–1428. [Google Scholar]
- Cavaleiro A.J., Salvador A.F., Alves J.I., Alves M.M. Continuous high rate anaerobic treatment of oleic acid based wastewater is possible after a step feeding start‐up. Environ Sci Technol. 2009 doi: 10.1021/es8031264. [DOI] [PubMed] [Google Scholar]
- Daffonchio D., Thaveesri J., Verstraete W. Contact angle measurement and cell hydrophobicity of granular sludge from upflow anaerobic sludge bed reactors. Appl Environ Microbiol. 1995;61:3676–3680. doi: 10.1128/aem.61.10.3676-3680.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeyer D.I., Henderickx H.K. Effect of C18 unsaturated fatty acids on methane production in vitro by mixed rumen bacteria. Biochim Biophys Acta. 1967;137:484–497. doi: 10.1016/0005-2760(67)90130-0. [DOI] [PubMed] [Google Scholar]
- Faisal M., Unno H. Kinetic analysis of palm oil mill wastewater treatment by a modified anaerobic baffled reactor. Biochem Eng J. 2001;9:25–31. [Google Scholar]
- Fountoulakis M.S., Drakopoulou S., Terzakis S., Georgaki E., Manios T. Potential for methane production from typical Mediterranean agro‐industrial by‐products. Biomass Bioenergy. 2008;32:155–161. [Google Scholar]
- Galbrait H., Miller T.B. Physicochemical effects of long‐chain fatty‐acids on bacterial‐cells and their protoplasts. J Appl Bacteriol. 1973;36:647–658. doi: 10.1111/j.1365-2672.1973.tb04150.x. [DOI] [PubMed] [Google Scholar]
- Gavala H.N., Kopsinis H., Skiadas I.V., Stamatelatou K., Lyberatos G. Treatment of dairy wastewater using an Upflow Anaerobic Sludge Blanket reactor. J Agric Engng Res. 1999;73:59–63. [Google Scholar]
- Hamdi M., Festino C., Aubart C. Anaerobic digestion of olive mill wastewaters in fully mixed reactors and in fixed film reactors. Process Biochem. 1992;27:37–42. [Google Scholar]
- Hanaki K., Nagase M., Matsuo T. Mechanism of inhibition caused by long‐chain fatty acids in anaerobic digestion process. Biotechnol Bioeng. 1981;23:1591–1610. [Google Scholar]
- Haridas A., Suresh S., Chitra K.R., Manilal V.B. The Buoyant Filter Bioreactor: a high‐rate anaerobic reactor for complex wastewater – process dynamics with dairy effluent. Water Res. 2005;39:993–1004. doi: 10.1016/j.watres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Hatamoto M., Imachi H., Yashiro Y., Fukayo S., Ohashi A., Harada H. Syntrophomonas palmitatica sp nov., an anaerobic, syntrophic, long‐chain fatty‐acid‐oxidizing bacterium isolated from methanogenic sludge. Int J Syst Evol Microbiol. 2007a;57:2137–2142. doi: 10.1099/ijs.0.64981-0. [DOI] [PubMed] [Google Scholar]
- Hatamoto M., Imachi H., Yashiro Y., Ohashi A., Harada H. Diversity of anaerobic microorganisms involved in long‐chain fatty acid degradation in methanogenic sludges as revealed by RNA‐based stable isotope probing. Appl Environ Microbiol. 2007b;73:4119–4127. doi: 10.1128/AEM.00362-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatamoto M., Imachi H., Ohashi A., Harada H. Identification and cultivation of anaerobic, syntrophic long‐chain fatty acid‐degrading microbes from mesophilic and thermophilic methanogenic sludges. Appl Environ Microbiol. 2007c;73:1332–1340. doi: 10.1128/AEM.02053-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes F.R., Donnelly T., Anderson G.K. Comparative performance of anaerobic digesters operating on ice‐cream wastewater. Water Res. 1995;29:525–533. [Google Scholar]
- Hwu C.‐S. 1997.
- Hwu C.‐S., Lettinga G. Acute toxicity of oleate to acetate‐utilizing methanogens in mesophilic and thermophilic anaerobic sludges. Enzyme Microb Technol. 1997;21:297–301. [Google Scholar]
- Hwu C.‐S., Molenaar G., Garthoff J., Van Lier J.B., Lettinga G. Thermophilic high‐rate anaerobic treatment of wastewater containing long‐chain fatty acids: impact of reactor hydrodynamics. Biotechnol Lett. 1997a;19:447–451. [Google Scholar]
- Hwu C.‐S., Van Beek B., Van Lier J.B., Lettinga G. Thermophilic high‐rate anaerobic treatment of wastewater containing long‐chain fatty acids: effect of washed out biomass recirculation. Biotechnol Lett. 1997b;19:453–456. [Google Scholar]
- Hwu C.‐S., Tseng S.‐K., Yuan C.‐Y., Kulik Z., Lettinga G. Biosorption of long‐chain fatty acids in UASB treatment process. Water Res. 1998a;32:1571–1579. [Google Scholar]
- Hwu C.‐S., Van Lier J.B., Lettinga G. Physicochemical and biological performance of expanded granular sludge bed reactors treating long‐chain fatty acids. Process Biochem. 1998b;33:75–81. [Google Scholar]
- Jackson B.E., Bhupathiraju V.K., Tanner R.S., Woese C.R., McInerney M.J. Syntrophus aciditrophicus sp. nov., a new anaerobic bacterium that degrades fatty acids and benzoate in syntrophic association with hydrogen‐using microorganisms. Arch Microbiol. 1999;171:107–114. doi: 10.1007/s002030050685. [DOI] [PubMed] [Google Scholar]
- Jeganathan J., Nakhla G., Bassi A. Long‐term performance of high‐rate anaerobic reactors for the treatment of oily wastewater. Environ Sci Technol. 2006;40:6466–6472. doi: 10.1021/es061071m. [DOI] [PubMed] [Google Scholar]
- Kim S.‐H., Han S.‐K., Shin H.‐S. Kinetics of LCFA inhibition on acetoclastic methanogenesis, propionate degradation and β‐oxidation. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2004a;39:1025–1037. doi: 10.1081/ese-120028411. [DOI] [PubMed] [Google Scholar]
- Kim S.‐H., Han S.‐K., Shin H.‐S. Two‐phase anaerobic treatment system for fat‐containing wastewater. J Chem Technol Biotechnol. 2004b;79:63–71. [Google Scholar]
- Koster I.W., Cramer A. Inhibition of methanogenesis from acetate in granular sludge by long‐chain fatty acids. Appl Environ Microbiol. 1987;53:403–409. doi: 10.1128/aem.53.2.403-409.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalman J.A., Bagley D.M. Anaerobic degradation and inhibitory effects of linoleic acid. Water Res. 2000;34:4220–4228. doi: 10.1016/s0043-1354(00)00593-5. [DOI] [PubMed] [Google Scholar]
- Lalman J.A., Bagley D.M. Anaerobic degradation and methanogenic inhibitory effects of oleic and stearic acids. Water Res. 2001;35:2975–2983. doi: 10.1016/s0043-1354(00)00593-5. [DOI] [PubMed] [Google Scholar]
- Lalman J.A., Bagley D.M. Effects of C18 long chain fatty acids on glucose, butyrate and hydrogen degradation. Water Res. 2002;36:3307–3313. doi: 10.1016/s0043-1354(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Lorowitz W.H., Zhao H., Bryant M.P. Syntrophomonas wolfei subsp saponavida subsp. nov., a long chain fatty‐acid degrading, anaerobic, syntrophic bacterium –Syntrophomonas wolfei subsp. wolfei subsp. nov. – and emended descriptions of the genus and species. Int J Syst Bacteriol. 1989;39:122–126. [Google Scholar]
- McInerney M.J. The genus Syntrophomonas, and other syntrophic bacteria. In: Balows A., Trüper H.G., Dworkin M., Harder W., Schleifer K.H., editors. Springer; 1992. pp. 2048–2057. [Google Scholar]
- McInerney M.J., Struchtemeyer C.G., Sieber J., Mouttaki H., Stams A.J., Schink B. Physiology, ecology, phylogeny, and genomics of microorganisms capable of syntrophic metabolism. Ann N Y Acad Sci. 2008;1125:58–72. doi: 10.1196/annals.1419.005. et al. [DOI] [PubMed] [Google Scholar]
- Maia M.R.G., Chaudhary L.C., Figueres L., Wallace R.J. Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie Van Leeuwenhoek. 2007;91:303–314. doi: 10.1007/s10482-006-9118-2. [DOI] [PubMed] [Google Scholar]
- Masse L., Massé D.I., Kennedy K.J., Chou S.P. Neutral fat hydrolysis and long‐chain fatty acid oxidation during anaerobic digestion of slaughterhouse wastewater. Biotechnol Bioeng. 2002;79:43–52. doi: 10.1002/bit.10284. [DOI] [PubMed] [Google Scholar]
- Miranda L.A.S., Henriques J.A.P., Monteggia L.O. Performance of UASB and DAEB reactors in the anaerobic digestion of synthetic wastewater containing sodium oleate and sodium stearate. Water Sci Technol. 2006;54:127–133. doi: 10.2166/wst.2006.495. [DOI] [PubMed] [Google Scholar]
- Najafpour G.D., Zinatizadeh A.A.L., Mohamed A.R., Hasnain Isa M., Nasrollahzadeh H. High‐rate anaerobic digestion of palm oil mill effluents in an upflow anaerobic sludge‐fixed film bioreactor. Process Biochem. 2006;41:370–379. [Google Scholar]
- Novak J.T., Carlson D.A. The kinetics of anaerobic long‐chain fatty‐acid degradation. J Water Pollut Control Fed. 1970;42:1932–1943. [Google Scholar]
- Núnez L.A., Martínez B. Anaerobic treatment of slaughterhouse wastewater in an Expanded Granular Sludge Bed (EGSB) reactor. Water Sci Technol. 1999;40:99–106. [Google Scholar]
- Paillard D., McKain N., Chaudhary L.C., Walker N.D., Pizette F., Koppova I. Relation between phylogenetic position, lipid metabolism and butyrate production by different Butyrivibrio‐like bacteria from the rumen. Antonie Van Leeuwenhoek. 2007;91:417–422. doi: 10.1007/s10482-006-9121-7. et al. [DOI] [PubMed] [Google Scholar]
- Pavlostathis S.G., Giraldo‐Gomez E. Kinetics of anaerobic treatment: a critical review. Crit Rev Environ Control. 1991;21:411–490. [Google Scholar]
- Pereira M.A., Pires O.C., Mota M., Alves M.M. Anaerobic degradation of oleic acid by suspended and granular sludge: identification of palmitic acid as a key intermediate. Water Sci Technol. 2002a;45:139–144. [PubMed] [Google Scholar]
- Pereira M.A., Mota M., Alves M.M. Operation of an anaerobic filter and an EGSB reactor for the treatment of an oleic acid‐based effluent: influence of inoculum quality. Process Biochem. 2002b;37:1025–1031. [Google Scholar]
- Pereira M.A., Roest K., Stams A.J.M., Mota M., Alves M.M., Akkermans A. Molecular monitoring of microbial diversity in expanded granular sludge bed (EGSB) reactors treating oleic acid. FEMS Microbiol Ecol. 2002c;41:95–103. doi: 10.1111/j.1574-6941.2002.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Pereira M.A., Sousa D.Z., Mota M., Alves M.M. Mineralization of LCFA associated with anaerobic sludge: kinetics, enhancement of methanogenic activity, and effect of VFA. Biotechnol Bioeng. 2004;88:502–511. doi: 10.1002/bit.20278. [DOI] [PubMed] [Google Scholar]
- Pereira M.A., Pires O.C., Mota M., Alves M.M. Anaerobic biodegradation of oleic and palmitic acids: evidence of mass transfer limitations caused by long chain fatty acid accumulation onto the anaerobic sludge. Biotechnol Bioeng. 2005;92:15–23. doi: 10.1002/bit.20548. [DOI] [PubMed] [Google Scholar]
- Perle M., Kimchie S., Shelef G. Some biochemical aspects of the anaerobic degradation of dairy wastewater. Water Res. 1995;29:1549–1554. [Google Scholar]
- Quémeneur M., Marty Y. Fatty‐acids and sterols in domestic wastewaters. Water Res. 1994;28:1217–1226. [Google Scholar]
- Rinzema A. 1988.
- Rinzema A., Alphenaar A., Lettinga G. The effect of lauric acid shock loads on the biological and physical performance of granular sludge in UASB reactors digesting acetate. J Chem Technol Biotechnol. 1989;46:257–266. [Google Scholar]
- Rinzema A., Alphenaar A., Lettinga G. Anaerobic digestion of long‐chain fatty‐acids in UASB and expanded granular sludge bed reactors. Process Biochem. 1993;28:527–537. [Google Scholar]
- Rinzema A., Boone M., Van Knippenberg K., Lettinga G. Bactericidal effect of long chain fatty acids in anaerobic digestion. Water Environ Res. 1994;66:40–49. [Google Scholar]
- Roy F., Samain E., Dubourguier H.C., Albagnac G. Syntrophomonas sapovorans sp. nov., a new obligately proton reducing anaerobe oxidizing saturated andunsaturated long‐chain fatty acids. Arch Microbiol. 1986;145:142–147. [Google Scholar]
- Saatci Y., Arslan E.I., Konar V. Removal of total lipids and fatty acids from sunflower oil factory effluent by UASB reactor. Bioresour Technol. 2003;87:269–272. doi: 10.1016/s0960-8524(02)00255-9. [DOI] [PubMed] [Google Scholar]
- Salminen E., Rintala J., Lokshina L.Y., Vavilin V.A. Anaerobic batch degradation of solid poultry slaughterhouse waste. Water Sci Technol. 2000;41:33–41. [PubMed] [Google Scholar]
- Sam‐Soon P., Loewenthal R.E., Wentzel M.C., Marais G.V.R. A long‐chain fatty acid, oleate, as sole substrate in upflow anaerobic sludge bed (UASB) reactor systems. Water SA. 1991;17:31–36. [Google Scholar]
- Samson R., Van den Berg B., Peters R., Hade C. Dairy waste treatment using industrial‐scale fixed‐film and upflow sludge bed anaerobic digesters: design and start‐up experience. In: Bell J.M., editor. Butterworth Publ; 1985. pp. 235–241. [Google Scholar]
- Sayed S., Van Der Zanden J., Wijffels R., Lettinga G. Anaerobic degradation of the various fractions of slaughterhouse wastewater. Biol Waste. 1988;323:117–142. [Google Scholar]
- Schink B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev. 1997;61:262–280. doi: 10.1128/mmbr.61.2.262-280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigematsu T., Tang Y., Mizuno Y., Kawaguchi H., Norimvra S., Kida K. Microbial diversity of mesophilic methanogenic consortium that degrade long‐chain fatty acids in chemostat cultivation. J Biosci Bioeng. 2006;102:535–544. doi: 10.1263/jbb.102.535. [DOI] [PubMed] [Google Scholar]
- Sousa D.Z. University of Minho; 1997. [Google Scholar]
- Sousa D.Z., Smidt H., Alves M.M., Stams A.J.M. Syntrophomonas zehnderi sp. nov., an anaerobe that degrades long chain fatty acids in co‐culture with Methanobacterium formicicum. Int J Syst Evol Microbiol. 2007a;57:609–615. doi: 10.1099/ijs.0.64734-0. [DOI] [PubMed] [Google Scholar]
- Sousa D.Z., Pereira M.A., Smidt H., Stams A.J.M., Alves M.M. Molecular assessment of complex microbial communities degrading long chain fatty acids in methanogenic bioreactors. FEMS Microbiol Ecol. 2007b;60:252–265. doi: 10.1111/j.1574-6941.2007.00291.x. [DOI] [PubMed] [Google Scholar]
- Sousa D.Z., Pereira M.A., Stams A.J.M., Alves M.M., Smidt H. Microbial communities involved in anaerobic degradation of unsaturated or saturated long chain fatty acids. Appl Environ Microbiol. 2007c;73:1054–1064. doi: 10.1128/AEM.01723-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa D.Z., Alves J.I., Alves M.M., Smidt H., Stams A.J.M. Effect of sulfate on methanogenic communities that degrade unsaturated and saturated long‐chain fatty acids (LCFA) Environ Microbiol. 2009;11:68–80. doi: 10.1111/j.1462-2920.2008.01740.x. [DOI] [PubMed] [Google Scholar]
- Svetlitshnyi V., Rainey F., Wiegel J. Thermosyntropha lipolytica gen. nov., sp. nov., a lipolytic, anaerobic, alkalitolerant, thermophilic bacterium utilizing short‐ and long‐chain fatty acids in syntrophic coculture with a methanogenic archaeum. Int J Syst Bacteriol. 1996;46:1131–1137. doi: 10.1099/00207713-46-4-1131. [DOI] [PubMed] [Google Scholar]
- Tagawa T., Takahashi H., Sekiguchi Y., Ohashi A., Harada H. Pilot‐plant study on anaerobic treatment of a lipid‐ and protein‐rich food industrial wastewater by a thermophilic multi‐staged UASB reactor. Water Sci Technol. 2002;45:225–230. [PubMed] [Google Scholar]
- Taylor R.J. Unilever Ltd; 1965. [Google Scholar]
- Thaveesri J., Daffonchio D., Liessens B., Vandermeren P., Verstraete W. Granulation and sludge bed stability in upflow anaerobic sludge bed reactors in relation to surface thermodynamics. Appl Environ Microbiol. 1995;61:3681–3686. doi: 10.1128/aem.61.10.3681-3686.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkian A., Eqbali A., Hashemian S.J. The effect of organic loading rate on the performance of UASB reactor treating slaughterhouse effluent. Resour Conserv Recycling. 2003;40:1–11. [Google Scholar]
- Wu C., Liu X., Dong X. Syntrophomonas erecta subsp. sporosyntropha subsp. nov., a spore‐forming bacterium that degrades short chain fatty acids in co‐culture with methanogens. Syst Appl Microbiol. 2006;29:457–462. doi: 10.1016/j.syapm.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Zhang C., Liu X., Dong X. Syntrophomonas curvata sp. nov., an anaerobe that degrades fatty acids in co‐culture with methanogens. Int J Syst Evol Microbiol. 2004;54:969–973. doi: 10.1099/ijs.0.02903-0. [DOI] [PubMed] [Google Scholar]
- Zhao H., Yang D., Woese C.R., Bryant M.P. Assignment of fatty acid‐β‐oxidizing syntrophic bacteria to Syntrophomonadaceae fam. nov. on the basis of 16S rRNA sequence analysis. Int J Syst Bacteriol. 1993;43:278–286. doi: 10.1099/00207713-43-2-278. [DOI] [PubMed] [Google Scholar]


