Summary
Campylobacter jejuni is a major foodborne pathogen of animal origin and a leading cause of bacterial gastroenteritis in humans. During the past decade, especially since the publication of the first C. jejuni genome sequence, major advances have been made in understanding the pathobiology and physiology of this organism. It is apparent that C. jejuni utilizes sophisticated mechanisms for effective colonization of the intestinal tracts in various animal species. Although Campylobacter is fragile in the environment and requires fastidious growth conditions, it exhibits great flexibility in the adaptation to various habitats including the gastrointestinal tract. This high adaptability is attributable to its genetically, metabolically and phenotypically diverse population structure and its ability to change in response to various challenges. Unlike other enteric pathogens, such as Escherichia coli and Salmonella, Campylobacter is unable to utilize exogenous glucose and mainly depends on the catabolism of amino acids as a carbon source. Campylobacter proves highly mutable in response to antibiotic treatments and possesses eukaryote‐like dual protein glycosylation systems, which modify flagella and other surface proteins with specific sugar structures. In this review we will summarize the distinct biological traits of Campylobacter and discuss the potential biotechnological approaches that can be developed to control this enteric pathogen.
Introduction
Campylobacter is a microaerophilic Gram‐negative bacterium and is among the leading causes of human gastroenteritis (Allos, 2001; Olson et al., 2008), responsible for 400–500 million cases of infection each year worldwide (Ruiz‐Palacios, 2007). Campylobacteriosis in humans presents as diarrhoea, fever and abdominal cramps and is recognized as a major risk factor for the onset of Guillain‐Barré syndrome, which is a serious post‐infection complication characterized by acute and progressive neuromuscular paralysis (Allos, 2001). Among Campylobacter species, Campylobacter jejuni accounts for more than 92% of human infections (Friedman et al., 2000; Gillespie et al., 2008) with an infectious dose as low as 500 bacteria (Robinson, 1981). The organism is transmitted to humans through a variety of sources, such as contact with infected pets or consumption of contaminated water or milk. However, the most frequent source of C. jejuni infection is contaminated, undercooked poultry meat (Olson et al., 2008). Campylobacter‐induced enteritis is usually self‐limiting and does not require antimicrobial treatment, but clinical therapy, often using erythromycin or fluoroquinolone antibiotics, is warranted for cases involving high fever, bloody diarrhoea or immunocompromised patients (Blaser and Engberg, 2008). The increasing prevalence of drug‐resistant Campylobacter has compromised the effectiveness of these antibiotics and poses a significant threat to public health in many countries (Blaser and Engberg, 2008).
Facilitated by the decoding of genome sequences, our understanding of Campylobacter as a zoonotic pathogen significantly advanced in the last decade. The progress is especially prominent in the areas of metabolic pathways, genetic variability, adaptation mechanisms, protein glycosylation systems, colonization factors and molecular basis of antibiotic resistance. These recent advances reveal distinct features of this pathogenic organism, provide new insights into Campylobacter physiology and pathobiology, and open potential avenues for the development of intervention strategies to prevent and control Campylobacter colonization in animal reservoirs and infection of the human host. This review will summarize these advances with a particular emphasis on the unique aspects of Campylobacter biology. The implications of these findings for biotechnological applications will also be discussed. Detailed discussion on Campylobacter pathogenesis and host immune responses has been presented in several recent review papers (Young et al., 2007; Janssen et al., 2008; Poly and Guerry, 2008; Zilbauer et al., 2008) and will not be a focus of this review.
Central metabolism and carbon flux surrounding pyruvate
With the exception of 6‐phosphofructokinase (Pfk), C. jejuni maintains a complete Embden‐Meyerhof‐Parnas (EMP) pathway and citric acid cycle (CAC) (Parkhill et al., 2000). Although C. jejuni possesses a fructose‐1,6‐bisphosphatase orthologue (Fbp) (Velayudhan and Kelly, 2002), the lack of Pfk results in the inability to catalyse the conversion of fructose‐6‐phosphate to fructose‐1,6‐bisphosphate (Fig. 1) and consequently renders this organism unable to utilize exogenous glucose (Vandamme et al., 2005). Pyruvate is a central metabolite connecting the EMP pathway and CAC, but is not formed from glucose in C. jejuni; yet acetyl‐CoA is still formed in the cell (Mendz et al., 1997; St Maurice et al., 2007). Many anaerobes produce acetyl‐CoA from the oxidative decarboxylation of pyruvate catalysed by pyruvate : ferredoxin oxidoreductase (PFOR) and reduce ferredoxin (White, 2007). Different from these anaerobes, members of the ε‐proteobacteria including C. jejuni use flavodoxin as the preferred electron carrier (Cremades et al., 2005). Campylobacter jejuni homologues of flavoprotein (FldA; Cj1382c) and flavodoxin : quinole reductase (Fqr; Cj0559) are likely involved in this process.
Figure 1.
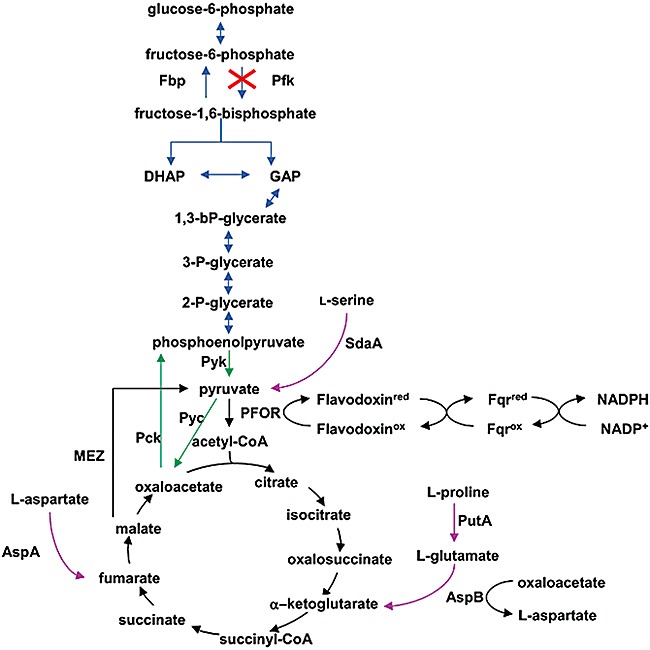
Central metabolism of C. jejuni and carbon flux surrounding pyruvate. Pathways and reactions are distinguished by the colour of arrows connecting intermediates; blue, possible anabolic role of EMP pathway; magenta, preferred amino acid catabolism; green, potential futile cycle; black, oxidative citric acid cycle and pyruvate carboxylation/decarboxylation. This composite figure is adapted from previously published information (Velayudhan and Kelly, 2002; St Maurice et al., 2007; Guccione et al., 2008).
An endogenous source of pyruvate is the catabolism of glucogenic amino acids producing α‐keto or α‐oxoacids (Fig. 1). Thus, amino acids likely play an important role in Campylobacter metabolism (Guccione et al., 2008). Several amino acids, particularly l‐aspartate, l‐glutamate, l‐serine and l‐proline, are significantly depleted from the culture media by C. jejuni strain NCTC 11168 (Guccione et al., 2008), and the ability to utilize l‐asparagine and glutathione is highly strain‐specific (Hofreuter et al., 2008). Transamination of alanine, possibly by putative aminotransferases (Cj0762c or Cj0150c), and dehydration of serine by SdaA (Velayudhan et al., 2004) will each produce pyruvate. Proline is converted to glutamate through a glutamate‐5‐semialdehyde intermediate (Cj1503c, PutA), and subsequent transamination of glutamate and oxaloacetate by AspB will produce α‐ketoglutarate and aspartate (Guccione et al., 2008). The deamination of aspartate by AspA yields fumarate (Guccione et al., 2008). Fumarate and α‐ketoglutarate, formed from these amino acids, can enter the CAC. Malate is converted to pyruvate by the malic enzyme (MEZ), and oxaloacetate is first converted to phosphoenolpyruvate (PEP) by phosphoenolpyruvate carboxykinase (PckA) and then to pyruvate by pyruvate kinase (Pyk) (Velayudhan and Kelly, 2002; Sauer and Eikmanns, 2005). The lack of a PEP synthase in C. jejuni suggests that decarboxylation of oxaloacetate by PckA is the main mechanism of PEP production (Velayudhan and Kelly, 2002). Consistent with this notion, a pckA mutant could not be constructed, thus pckA is likely an essential gene in C. jejuni (Velayudhan and Kelly, 2002). Although the lack of Pfk results in the inability to utilize exogenous glucose, the presence of an Fbp orthologue (Cj0840c) strongly suggests an anabolic role for the EMP pathway, requiring the synthesis of PEP. Anabolically formed glucose may have several metabolic fates, including incorporation into lipooligosaccharides (LOS), capsular polysaccharides (CPS), or protein glycans or entry into the pentose phosphate pathway. Interestingly, the C. jejuni genome encodes a functional pyruvate kinase (Pyk), posing a potential futile cycle between PEP, pyruvate and oxaloacetate and resulting in the net loss of one ATP (Velayudhan and Kelly, 2002). The role of Pyk in Campylobacter metabolism remains unclear, but may be involved in the catabolism of PEP derived from an unknown substrate that enters the EMP pathway subsequent to the formation of fructose‐1,6‐bisphosphate (Velayudhan and Kelly, 2002).
Aerobic and anaerobic respiration
The highly branched electron transport system of C. jejuni is capable of both anaerobic and aerobic respiration (Fig. 2), affording this organism the flexibility to respire under varying levels of available electron donors and terminal acceptors. Additionally, branches that use terminal oxidases with high oxygen affinity remove trace oxygen, thereby creating a low intracellular oxygen state that is conducive to the activity of oxygen sensitive enzymes and the use of alternative terminal electron acceptors. Early reports on the respiratory system of C. jejuni reported high activities when membrane vesicles were incubated with hydrogen and formate, moderate rates with malate, succinate and lactate, and poor activity with NADH as electron donors (Hoffman and Goodman, 1982). Sulfite, which is normally an inhibitory substance added to food as a preservative, is a respiratory substrate for C. jejuni (Myers and Kelly, 2005). A gluconate dehydrogenase (GADH) was recently reported to enable C. jejuni to respire using gluconate as an electron donor at elevated temperatures. Mutation of GADH led to a marked impairment in the colonization of poultry intestines (Pajaniappan et al., 2008). Campylobacter jejuni possesses a cluster of genes (nuoA‐N) encoding homologues of the NADH : ubiquinone oxidoreductase complex (also known as NADH dehydrogenase or Complex I in other bacteria) (Parkhill et al., 2000). Most of the genes that encode Complex I are intact except for homologues of the NADH dehydrogenase subunits (nuoE and nuoF), accounting for the poor respiratory ability of C. jejuni using NADH as an electron donor (Hoffman and Goodman, 1982). In their place within the operon are two genes (Cj1574c and Cj1575c) of unknown function (Weerakoon and Olson, 2008), and it is hypothesized that Cj1574c and Cj1575c accept electrons from reduced flavoprotein (FldA), rather than NADH (Weerakoon and Olson, 2008). Analysis of the C. jejuni NCTC 11168 genome revealed no α‐ketoglutarate dehydrogenase homologue; however, OOR (encoded by oorDABC) is a functionally equivalent redox complex that catalyses the conversion of α‐ketoglutarate to succinyl‐CoA (Hughes et al., 1998) and transfers electrons to FldA (Weerakoon and Olson, 2008), providing a source of electrons for Complex I.
Figure 2.
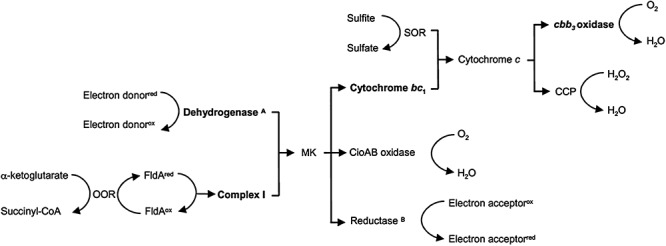
Schematic diagram of the highly branched respiratory pathway in C. jejuni. APrimary dehydrogenases that oxidize formate, hydrogen, lactate and succinate. Electrons from gluconate likely enter at the level of cytochrome c.BReductases that transfer electrons onto fumarate, nitrate, nitrite and S‐and N‐oxides. Bold type: potential coupling sites associated with proton translocation and energy conservation. MK, Menaquinone pool.
In C. jejuni, the electron flow from donor to acceptor, through dehydrogenases to reductases, and oxidases, is carried by lipid‐soluble menaquinone and two types of cytochromes (b‐ and c‐type). Although menaquinone can be detected from members of the Campylobacter genus (Moss et al., 1984), the C. jejuni genome reveals no obvious homologues to the Escherichia coli MenZ‐G enzymes catalysing the conversion of chorismate to menaquinone (Parkhill et al., 2000). An alternative pathway for menaquinone synthesis, termed the futalosine pathway, was recently described in Streptomyces coelicolor (Hiratsuka et al., 2008). This study suggested a role for this alternative pathway in menaquinone synthesis in C. jejuni.
Campylobacter is able to respire both aerobically and anaerobically, utilizing oxygen or alternative electron acceptors to terminate the electron transport chain. Campylobacter jejuni possesses two terminal oxidases, including a high‐affinity cbb3‐type cytochrome c oxidase and a cyanide insensitive oxidase (Smith et al., 2000). In terms of aerobic respiration through the high‐affinity oxidase, electrons fed into the menaquinone pool are transferred to cytochrome bc1 (Complex III) then to a periplasmic cytochrome c (Fig. 2). The cbb3‐type oxidase (CcoNOPQ; Cj1487c‐Cj1490c) has very high affinity for oxygen (Km = 40 nM) (Jackson et al., 2007), which allows C. jejuni to aerobically respire under oxygen‐limiting conditions such as those encountered in a host's intestine. The second branch at the terminal oxidase level of aerobic respiration is mediated by CioAB (cyanide insensitive oxidase, formerly CydAB) and has a low affinity to oxygen (Jackson et al., 2007). The CioA mutants colonized the poultry intestine as efficiently as the wild‐type, but CcoN mutants were completely impaired in colonization indicating a significant role for oxygen during in vivo colonization (Weingarten et al., 2008). Although the conditions encountered by C. jejuni within the animal host may be considered anaerobic, it is plausible that oxygen perfusion from actively respiring host epithelial cells provide an oxygen gradient in the mucosal layer approaching anoxic conditions near the intestinal lumen; thus, the role of terminal oxidases, especially the high‐affinity cbb3‐type, in the animal host may be energy conservation through scavenging host‐provided oxygen (Weingarten et al., 2008).
In addition to aerobic respiration, C. jejuni is capable of anaerobic respiration. The electron transfer from cytochrome c to H2O2 is facilitated by two different periplasmic cytochrome cperoxidases (CCP; Cj0020c and Cj0358). Although Cj0358 plays only a minor role in colonization (Bingham‐Ramos and Hendrixson, 2008), Cj0020c plays an important role in chicken colonization and was named docA (determinant of colonization) (Hendrixson and Dirita, 2004). The contribution of periplasmic CCP to colonization remains unclear, but may be involved in the removal of endogenously produced H2O2 during formate respiration (Atack and Kelly, 2007). Anaerobic respiration in C. jejuni is also accomplished by a variety of reductases that transfer electrons from the menaquinone pool onto fumarate (FrdABC; Cj0408‐Cj0410), nitrate (NapAB; Cj0780 and Cj0781), nitrite (NrfA; Cj1357c) and N‐ and S‐oxides (TorAC; Cj0264c and Cj0265c) (Sellars et al., 2002; Pittman et al., 2007). While the high‐affinity cbb3‐type terminal oxidase plays a significant role in the colonization of poultry intestines, the role of terminal reductases is less clear. TorA plays a minimal role in poultry colonization (Weingarten et al., 2008) with conflicting evidence presented for respiration using nitrate (Pittman et al., 2007; Weingarten et al., 2008). These discrepancies may be attributed to genetic differences among strains, inoculum dose, or diet fed to the chickens and microbiota of the chicken intestine affecting the production or availability of terminal electron acceptors. The versatile respiratory system of Campylobacter provides for the cell's energy demands and detoxifies potentially damaging compounds, such as nitrite, nitric oxide and sulfite.
Dual protein glycosylation systems in C. jejuni
Campylobacter jejuni possesses both O‐linked and N‐linked protein glycosylation systems (Szymanski et al., 2005; Guerry and Szymanski, 2008). The N‐linked protein glycosylation occurs at the carboxamide side‐chain of asparagine, while the O‐linked protein glycosylation system mediates the attachment of glycans to the hydroxyl group of serine or threonine residues on a protein. Flagellin is the only O‐glycoprotein in C. jejuni and is extensively modified with pseudaminic acid (Thibault et al., 2001). In C. jejuni NCTC 11168, the DNA locus responsible for O‐linked protein glycosylation consisting of about 50 genes, and the glycosylation locus is highly variable among different strains of C. jejuni (Karlyshev et al., 2005). Mutations eliminating glycans from flagella reduced Campylobacter adherence to and invasion of INT407 cells, decreased auto‐agglutination and attenuated diarrhoea in a ferret animal model (Guerry et al., 2006). Thus, the Campylobacter flagella require glycosylation to carry out its virulence‐associated functions (Guerry et al., 2006). It was believed that N‐linked glycoproteins were exclusive to eukaryotes; however, recent evidence of this post‐translational modification has been documented in archaea and eubacteria (Schaffer et al., 2001). The first bacterial N‐linked glycosylation pathway was reported in C. jejuni (Szymanski et al., 1999), and Campylobacter is the only bacterium known to possess both O‐ and N‐linked protein glycosylation systems to date (Szymanski et al., 2005). N‐linked protein glycosylation in C. jejuni is carried out by the pgl locus consisting of 11 genes (pglA‐F, pglH‐K and gne) (Szymanski et al., 1999; Linton et al., 2002; Alaimo et al., 2006; Kelly et al., 2006). Analogous to dolichyl‐phosphate in eukaryotes, undecaprenyl‐phosphate serves as a carrier for the cytosolic addition of UDP‐activated oligosaccharides (Feldman et al., 2005). PglD, PglE and PglF convert UDP‐N‐acetyl glucosamine (GlcNAc) to UDP‐bacillosamine that is attached to the undecaprenyl pyrophosphate by PglC. The gne gene encodes an epimerase that interconverts glucose and GlcNAc to galactose and N‐acetyl galactosamine (GalNAc), respectively. Sequential addition of GalNAc residues are achieved by the transferase activities of PglA, PglJ and PglH, with PglI forming the branching β1,3 bond to glucose (Glover et al., 2005; Linton et al., 2005). The entire heptasaccharide is flipped across the cytoplasmic membrane into the periplasm by PglK (formerly WlaB) (Alaimo et al., 2006; Kelly et al., 2006), and transferred to the Asn residue of the D/E‐X1‐N‐X2‐S/T (X1 and X2 are any amino acids other than proline) glycan accepting sequon by PglB, an oligosaccharyl transferase (Kowarik et al., 2006). The bacterial glycan acceptor sequon requires the negatively charged side‐chain of aspartate or glutamate at the −2 position, which is not required by eukaryotes, suggesting that C. jejuni has a more specific attachment site of the N‐glycan than eukaryotes (Kowarik et al., 2006). Interestingly, the C. jejuni pgl gene cluster also functionally mediates N‐linked protein glycosylation in E. coli and can modify proteins with diverse glycan structures (Feldman et al., 2005; Wacker et al., 2006).
Genetic and phenotypic instability
A distinct feature of Campylobacter is its genetic and phenotypic instability resulting from high‐frequency mutation and horizontal gene transfer. The instability results in remarkable strain‐to‐strain variations, creating a significant obstacle for deciphering gene functions in Campylobacter. The high mutation rate is attributable to an incomplete mismatch DNA repair system. The mutH, mutL, mutS and uvrD genes are key components of the mismatch repair system in bacteria, and mutants defective in any of these genes show a hypermutable phenotype with a 100‐ to 1000‐fold increase in mutation frequency (Miller, 1996). Campylobacter jejuni does not have mutH, mutL and uvrD homologues, but has a variant copy of mutS (mutS2) (Parkhill et al., 2000). MutS2, however, is not involved in mismatch repair and was shown to suppress intergenomic recombination in Helicobacter pylori (Kang et al., 2005). The inability of C. jejuni to recognize and repair mismatch errors through the MutHLS complex may lead to the observed high frequency of spontaneous point mutations and phase variation, contributing to genetic diversity in Campylobacter.
Horizontal gene transfer is also involved in generating genetic variation in Campylobacter. Comparative genomic studies revealed that C. jejuni has hypervariable gene clusters, which are located on chromosome and functionally associated with biosynthesis of surface structures (LOS, CPS and flagella) and restriction–modification systems (Parkhill et al., 2000; Taboada et al., 2004). These hypervariable clusters have a different G+C composition from that of the rest of the chromosome (Parkhill et al., 2000; Hofreuter et al., 2006), suggesting that horizontal gene transfer may be responsible for their acquisition. Natural transformation, conjugation and transduction are the mechanisms of horizontal gene transfer in bacteria, and C. jejuni can use all three mechanisms. Natural transformation significantly contributes to genetic diversity and the spread of antibiotic resistance determinants in this bacterium. For example, co‐culture or co‐colonization of C. jejuni strains harbouring different antibiotic resistance markers generated C. jejuni populations possessing resistance to multiple antibiotics, and this process was prevented in the Cj1211 mutant that was deficient in natural transformation (de Boer et al., 2002; Jeon et al., 2008). Although a complete understanding of the overall mechanism of natural transformation is still lacking and the recognition sequence for natural transformation has not been identified in Campylobacter, multiple factors contributing to this process have been reported (Guerry et al., 1994; Wiesner et al., 2003; Larsen et al., 2004; Jeon and Zhang, 2007; Jeon et al., 2008; 2009).
Compared with natural transformation, limited information is available for conjugation and transduction in Campylobacter. Campylobacter jejuni strain RM1221 contains a Mu‐like prophage located in its chromosome (Fouts et al., 2005), which was not found in the genome sequence of C. jejuni NCTC 11168 (Parkhill et al., 2000). The Mu‐like bacteriophage present in strain RM1221 appears to be widely prevalent in many C. jejuni and C. coli strains and contributes to the genetic diversity of this genus (Parker et al., 2006; Clark and Ng, 2008). Recently, evidence of intragenomic and interstrain recombination, mediated by bacteriophages, was reported in C. jejuni (Scott et al., 2007a,b). Conjugative plasmids have been described in Campylobacter (Taylor et al., 1981; Bacon et al., 2000), which were transferrable between different strains in culture media and in the avian intestine (Avrain et al., 2004). The exact contribution of conjugation and transduction to genetic diversity in Campylobacter awaits further investigation.
Flagellum‐mediated motility and secretion of virulence factors
The motility of Campylobacter is imparted by polar flagella and is recognized as an important virulence factor. Challenge of human volunteers with a mixture of motile and non‐motile C. jejuni resulted in the isolation of only motile C. jejuni (Black et al., 1988), implying a significant role of motility in colonization. Flagella are involved in auto‐agglutination (Misawa and Blaser, 2000) and biofilm formation (Joshua et al., 2006; Kalmokoff et al., 2006). Structurally, the flagellar filament consists of flagellin subunits encoded by two tandem genes, flaA and flaB (Nuijten et al., 1990; Guerry et al., 1991). While the expression of flaA, encoding the major flagellin (FlaA) subunit, is σ28‐dependent (Nuijten et al., 1990; Guerry et al., 1991), flaB is expressed by a σ54‐dependent promoter and produces the minor flagellin (FlaB) subunit (Guerry et al., 1991). FlaA and FlaB are highly immunogenic (Shoaf‐Sweeney et al., 2008), although immune recognition is independent of toll‐like receptor 5 (Andersen‐Nissen et al., 2005). Mutation of flaA, but not flaB, reduced motility significantly, rendered Campylobacter less invasive to human INT407 cells and decreased the level of chicken colonization (Guerry et al., 1991; Wassenaar et al., 1991; 1993). Although the flaA mutation affected both auto‐agglutination and biofilm formation, flaB is only involved in biofilm formation but not auto‐agglutination (Golden and Acheson, 2002; Kalmokoff et al., 2006). Analysis of the genome sequence of C. jejuni identified two additional flagellin paralogues, flaC (Cj0720c) and flaD (Cj0887c) (Parkhill et al., 2000). Inactivation of flaC did not affect motility, but reduced invasion of HEp‐2 cells (Song et al., 2004) and pellicle formation (Kalmokoff et al., 2006). Similar to the flaA mutation, the flaD mutation caused defects in motility and auto‐agglutination; however, FlaD is not detected in the flagellar filament of C. jejuni (Golden and Acheson, 2002).
Flagellar expression is subject to phase variation and contributes to the unstable motile phenotype in Campylobacter. Phase variation between motile and non‐motile phenotypes occurs frequently in vitro and in vivo (Caldwell et al., 1985; Karlyshev et al., 2002; Hendrixson, 2006; 2008), and is mediated by slipped‐strand mispairing primarily in homopolymeric or occasionally heteropolymeric tracts, resulting in frameshift mutations in the genes associated with flagellar biosynthesis and regulation (flhA, flgS, flgR and maf) (Park et al., 2000; Karlyshev et al., 2002; Hendrixson, 2006; 2008). The high‐frequency phase variation was attributed to the A/T‐rich genetic content of the C. jejuni chromosome and the absence of an intact mismatch repair system (Hendrixson, 2006).
The flagellar apparatus in C. jejuni is involved in secretion of virulence‐associated proteins, such as Cia, FlaC and FspA. Cia (C ampylobacterinvasion antigen) proteins are associated with C. jejuni invasion of INT407 cells (Konkel et al., 1999), and their secretion requires the function of the flagellar basal body, hook and filament (Konkel et al., 2004). Mutation of the basal rod (flgF) and hook (flgE) elements prevented the secretion of FlaC, suggesting that FlaC is secreted via the flagellar apparatus. Secreted FlaC binds to HEp‐2 cells and the FlaC mutant demonstrated reduced invasion compared with the wild‐type strain (Song et al., 2004). FspA (flagellar secreted protein), a small acidic protein, is secreted by the flagellar system in C. jejuni (Poly et al., 2007). Although the exact function of FspA is unknown, a variant of FspA (FspA2) induced apoptosis in INT407 cells. Given the fact that C. jejuni lacks a typical type III secretion system, the flagellar secretion apparatus is likely an alternative mechanism for secretion of virulence factors in this pathogen.
Adaption to environmental stresses
As a fastidious, asaccharolytic and microaerobic bacterium, C. jejuni utilizes elaborate adaptation mechanisms to survive environmental stresses (Murphy et al., 2006), such as temperature shift, oxygen tension and nutrient depletion, which occur during transmission between the environment and animal hosts and within the host's intestine. The minimal temperature at which C. jejuni can grow is between 31°C and 32°C, with an optimal growth temperature of 42°C (Hazeleger et al., 1998). Unlike E. coli, C. jejuni lacks the genes encoding the key components of cold shock response such as CspA (Parkhill et al., 2000), which facilitates protein synthesis by blocking the formation of unfavorable secondary structures in mRNAs during cold shock (Jiang et al., 1997). Temperature downshift to below the minimal growth temperature resulted in massive downregulation of gene expression, including those involved in biosynthesis of macromolecules; however, some genes encoding heat shock proteins, chaperones and energy metabolism were upregulated, suggesting that C. jejuni utilizes active mechanisms for adaptation to low temperatures (Moen et al., 2005). Temperature upshift (from 37°C to 42°C) led to upregulation of genes encoding heat shock proteins, chaperones and chaperonins, and downregulation of genes encoding ribosomal proteins (Stintzi, 2003). These findings suggest that with both temperature upshift and downshift C. jejuni reduces energy consumption and invokes the heat shock protection mechanism.
RpoS, a sigma factor associated with stationary phase response, is absent in C. jejuni (Parkhill et al., 2000). Despite this fact, active transcriptomic, metabolic and phenotypic changes occur in Campylobacter during the transition from exponential to stationary phase, including downregulation of genes associated with electron transport and protein synthesis, induction of protection mechanisms (heat shock response and oxidative stress resistance), shift in nutrient utilization and increase in motility (Wright et al., 2009). For effective colonization and infection, C. jejuni must be able to survive acid stresses in the stomach. Exposure to acid stress in vitro and in vivo (piglet stomach) induced the expression of genes involved in heat shock response and oxidative stress resistance (Reid et al., 2008). DNA microarray profiles of Campylobacter transcripts recovered from rabbit ileal loops, into which C. jejuni was directly inoculated without passing through the stomach, showed marked upregulation of the genes involved in oxidative stress response, heat shock response, iron metabolism, bile resistance (cmeABC), flagellar biosynthesis and the stringent response (Stintzi et al., 2005), suggesting that C. jejuni utilizes sophisticated mechanisms for adaptation in the intestinal environment. SpoT, a key regulator for stringent response, modulates Campylobacter survival in stationary phase and the resistance to oxidative stress and rifampicin (Gaynor et al., 2005). Expression of spoT was upregulated upon contact with INT407 cells and inactivation of spoT in Campylobacter reduced adherence, invasion and intracellular survival (Gaynor et al., 2005). Together, these findings suggest that modulating the expression of genes involved in heat shock response and oxidative stress may be a common mechanism utilized by Campylobacter to adapt to various stresses.
Mechanisms for in vivo colonization
As a zoonotic pathogen, Campylobacter colonizes the gastrointestinal tracts of a variety of animal hosts, either as a commensal or as a pathogen. Understanding the mechanisms Campylobacter uses for colonization may yield control strategies. In particular, C. jejuni colonization of the chicken intestinal tract has received considerable attention because faecal contamination of chicken carcasses during the slaughter process poses a significant risk for human exposure. To date, flagella and motility are the most well‐defined colonization factors, and it is clear that non‐motile Campylobacter cannot establish colonization in animal intestinal tracts. In addition, recent work also identified other factors that are required for effective colonization.
Adherence to epithelial cells of the intestine may be required for C. jejuni to resist intestinal peristalsis and expulsion. Several proteins have been identified in Campylobacter that mediate adherence to cultured cells including flagella (McSweegan and Walker, 1986), CadF (C ampylobacteradhesion to fibronectin) (Konkel et al., 1997), PEB1a (Pei et al., 1998), PEB4 (Asakura et al., 2007) and JlpA (jejunilipoprotein A) (Jin et al., 2001). Proteomic analysis demonstrated that FlaA, CadF, PEB1a and PEB4 are associated with the membrane fraction and are recognized by convalescent human sera (Cordwell et al., 2008). Consistent with their function in cellular adherence, most of these proteins are associated with the colonization of animal hosts. For example, mutations of peb1a and peb4 decreased murine colonization (Pei et al., 1998; Asakura et al., 2007), and a CadF mutant demonstrated impaired colonization in chickens (Ziprin et al., 1999).
Bile is normally present in intestinal tracts and is a natural detergent with antimicrobial activities. Thus, resistance to bile is an essential mechanism for enteric bacteria to colonize animal intestines. In Campylobacter, bile resistance is primarily mediated by the CmeABC multidrug efflux pump (Lin et al., 2002; 2003). The CmeABC mutant was completely impaired for colonization of the chicken intestine due to the drastically increased sensitivity to bile compounds (Lin et al., 2003). Consistent with its important role in bile resistance and in vivo colonization, cmeABC is inducible by bile salts in culture media (Lin et al., 2005b). The induced expression of cmeABC in the intestine was also demonstrated in a rabbit ileal loop model using DNA microarray (Stintzi et al., 2005). These findings indicate that CmeABC is an important factor for Campylobacter adaptation in bile‐containing environments including the intestinal tract.
Another important determinant for successful colonization is the ability to acquire adequate nutrients in vivo. Amino acids are the primary carbon source for Campylobacter with the preferred use of aspartate, glutamate, serine and proline (Guccione et al., 2008). Mutation of genes involved in the catabolism of aspartate (aspA) (Guccione et al., 2008) and serine (sdaA) (Velayudhan et al., 2004) reduced chicken colonization. Additionally, sdaA expression is upregulated during chicken colonization (Woodall et al., 2005), further suggesting an important role for serine catabolism during intestinal colonization. Recently, strain‐specific utilization of asparagine, glutamine and glutathione was described in C. jejuni strain 81‐176, and the ability to utilize these substrates was correlated with enhanced colonization of specific tissues in mice (Hofreuter et al., 2008). The strain‐dependent substrate utilization is due to the presence of γ‐glutamyl transpeptidase (GGT) and an exported form of asparaginase (AnsB) in C. jejuni 81‐176. The ggt gene is also present in C. jejuni strain 81116 and mutation of this gene significantly reduced the duration of C. jejuni colonization in murine and avian hosts (Hofreuter et al., 2006; Barnes et al., 2007). In addition, signature‐tagged transposon mutagenesis identified multiple genes contributing to C. jejuni colonization in chickens and some of the identified genes encode amino acid transporters (Hendrixson and Dirita, 2004).
The C. jejuni glycome also contributes to colonization of animal intestines. Mutation of genes necessary for N‐linked protein glycosylation reduced Campylobacter colonization in chickens (Hendrixson and Dirita, 2004; Jones et al., 2004; Karlyshev et al., 2004; Kelly et al., 2006). Mutagenesis of all putative N‐glycosylated proteins in C. jejuni identified Cj1496c as a colonization determinant, but glycosylation of this protein did not seem to be required for its function in colonization (Kakuda and Dirita, 2006). Capsular polysaccharides influence Campylobacter colonization and virulence. A kpsM mutant of C. jejuni strain NCTC 11168 demonstrated a complete lack of colonization in chickens (Jones et al., 2004), and a kpsM mutation in a different strain (81‐176) reduced the invasion of INT 407 cells and decreased the development of diarrhoea in the ferret model (Bacon et al., 2001).
Multiple two‐component regulatory systems (12 response regulators and 7 sensor kinases in strain NCTC 11168) have been identified in C. jejuni (Parkhill et al., 2000; Fouts et al., 2005), and several of them were shown to play a role in intestinal colonization. RacRS (reduced ability to colonize) modulates Campylobacter gene expression in a temperature‐dependent manner. The racR mutation reduced bacterial growth at 42°C and impaired C. jejuni colonization in chickens (Bras et al., 1999). Inactivation of DccRS (diminished capacity to colonize) did not impair the growth of Campylobacter in culture media, but resulted in a deficiency in the colonization of mice and chickens (MacKichan et al., 2004). Inactivation of CbrR (C ampylobacterbile resistance regulator) rendered Campylobacter more sensitive to bile salts than the wild‐type strain and significantly reduced chicken colonization, presumably due to the increased sensitivity to bile (Raphael et al., 2005). The cognate sensor kinase for CbrR and how it regulates bile resistance are still unknown. FlgRS is a two‐component regulatory system associated with the expression of flagellin subunit genes (Hendrixson and Dirita, 2003; Wösten et al., 2004), and mutation of FlgRS reduced the colonization level in chickens (Hendrixson and Dirita, 2004; Wösten et al., 2004). These findings suggest that two‐component regulatory systems are important for Campylobacter adaptation in the intestinal tract, but in most cases the exact environmental stimuli recognized by the two‐component systems are unknown.
In addition to two‐component regulatory systems, C. jejuni also utilizes other transcriptional regulators for adaptation and colonization. Some examples include Fur and CmeR. As a transcriptional repressor, Fur controls iron homeostasis and modulates the expression of multiple genes in C. jejuni (van Vliet et al., 1998; Palyada et al., 2004). Mutation of Fur significantly reduced the colonization of Campylobacter in chickens (Palyada et al., 2004). CmeR belongs to the TetR family of transcriptional regulators (Ramos et al., 2005). In Campylobacter, CmeR functions as a repressor for CmeABC and modulates the expression level of this efflux pump (Lin et al., 2005a). A recent study using DNA microarray also revealed that CmeR is a pleiotropic regulator affecting the expression of multiple genes and is required for optimal colonization of Campylobacter in chickens (Guo et al., 2008). The regulation of cmeABC by CmeR is through direct binding of CmeR to the promoter region of cmeABC (Lin et al., 2005a). Bile compounds inhibit the binding of CmeR to the promoter DNA of cmeABC (Lin et al., 2005b), which explains why bile salts induce the expression of cmeABC. The recent crystallization work revealed a two‐domain structure of CmeR, including a DNA‐binding motif formed by the N‐termini of the CmeR dimer and a large tunnel‐like ligand‐binding cavity in the C‐terminal domain of each monomer (Gu et al., 2007; Routh et al., 2009). The tunnel is surrounded by mostly hydrophobic residues and is predicted to be able to accommodate large, negatively charged bile acid molecules. Based on the structural data and the fact that bile compounds induced the expression of CmeR‐regulated genes (Guo et al., 2008), it is speculated that bile acids and potentially other unidentified signals in the gut interact with the ligand‐binding pocket of CmeR, which triggers a conformational change in the DNA‐binding domain, leading to the release of CmeR from its target promoters. A model illustrating the regulatory mechanism of CmeR is shown in Fig. 3.
Figure 3.
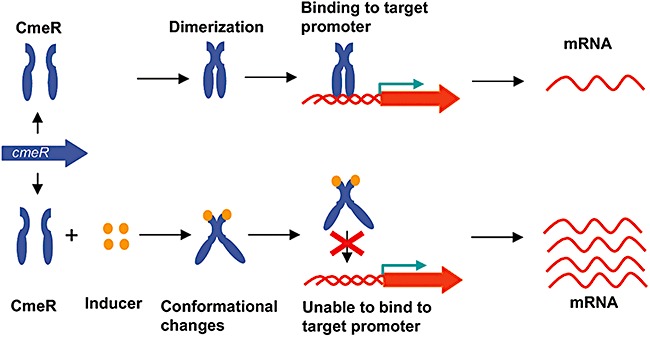
CmeR regulation of gene expression in Campylobacter. In the absence of an inducer, dimeric CmeR binds to target promoters and inhibit the expression of target genes (e.g. cmeABC). In the presence of an inducer (e.g. bile salts), the interaction of an inducer with the C‐terminal ligand‐binding pocket of CmeR triggers a conformational change in the N‐terminal DNA binding domain, preventing the binding of CmeR to target promoters and leading to enhanced expression of target genes.
Mechanisms of antibiotic resistance
Campylobacter has developed resistance to multiple antibiotics and the general resistance mechanisms to clinically important antibiotics are illustrated in Fig. 4 and reviewed elsewhere (Zhang and Plummer, 2008). Depending on their chemical and structural properties, antibiotics enter bacterial cells through different routes. Small hydrophilic antibiotics can pass through membrane porins, while hydrophobic antibiotics diffuse through the lipid membrane (Nikaido, 2003). The first obstacle for antibiotics is cell permeability, which is affected by various membrane and surface structures. Campylobacter jejuni has both LOS and CPS, which contribute to the hydrophilic nature of the bacterial surface. A recent study demonstrated that mutagenesis of LOS, but not CPS, significantly sensitized C. jejuni (strain NCTC 11168) to erythromycin, a hydrophobic macrolide antibiotic, suggesting that LOS reduces the permeability to hydrophobic antibiotics (Jeon et al., 2009). Once antimicrobials traverse the cytoplasmic membrane, they are extruded by multidrug efflux transporters, which reduce the intracellular drug concentration and constitute a second defence mechanism against antibiotics. Although the genome sequences of C. jejuni revealed the presence of multiple putative drug efflux transporters of different families, most of them have not been functionally characterized. CmeABC, an RND‐type efflux transporter, appears to be the primary drug efflux pump in Campylobacter and confers resistance to structurally diverse antibiotics as well as bile compounds (Lin et al., 2002; Pumbwe and Piddock, 2002). As discussed earlier, CmeABC is also required for Campylobacter colonization in the chicken intestine (Lin et al., 2003). Thus, this efflux pump has an important natural function in addition to conferring antibiotic resistance.
Figure 4.
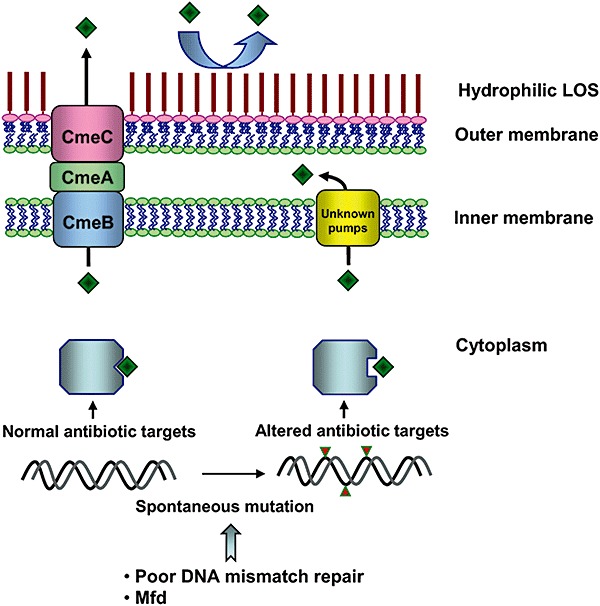
Mechanisms associated with Campylobacter resistance to macrolide and fluoroquinolone antibiotics. LOS reduces the uptake of hydrophobic antibiotics (e.g. macrolide); efflux pumps (such as CmeABC and other uncharacterized efflux transporters) decrease the intracellular concentration of antibiotics; and chromosomal mutations reduce the affinity of antibiotics to their targets. Mfd and the lack of an intact mismatch repair system enhance the spontaneous mutation rate in Campylobacter.
Another mechanism utilized by Campylobacter for antibiotic resistance is target modification. Spontaneous point mutations in the quinolone resistance determining region of GyrA reduce the affinity of fluoroquinolones (FQs) to DNA gyrase and result in resistance to this class of antibiotics (Engberg et al., 2001). Likewise, certain mutations in the 23S rRNA subunit mediate resistance to macrolide antibiotics (Gibreel et al., 2005). Due to the clinical importance of FQs and macrolides in treating campylobacteriosis, the rising prevalence of resistance to these two classes of antibiotics, especially to FQs, has become a concern for public health. Campylobacter has a high mutation frequency (up to 10−6) for FQ resistance, which is affected by the function of Mfd (Yan et al., 2006; Han et al., 2008). Mfd is a transcription–repair coupling factor involved in strand‐specific DNA repair (Selby and Sancar, 1994). In contrast to its mutation‐frequency‐decline function in E. coli, the Mfd orthologue in C. jejuni actually promotes the emergence of spontaneous FQ‐resistant mutants in Campylobacter (Han et al., 2008). Knocking out the mfd gene in Campylobacter resulted in a 100‐fold reduction in the number of spontaneous mutants resistant to ciprofloxacin, while overexpression of mfd increased the mutant numbers, suggesting that Mfd modulates the spontaneous mutation rates in Campylobacter. Due to the high spontaneous mutation rate and adaptive response in gene expression, FQ‐resistant Campylobacter rapidly emerge from a FQ‐susceptible population when exposed to FQ antibiotics. This has been shown by both in vitro and in vivo treatment studies (McDermott et al., 2002; Luo et al., 2003; Han et al., 2008) and represents a distinct feature of FQ‐resistance development in Campylobacter. The resistance‐conferring mutation in GyrA not only affects the susceptibility of Campylobacter to FQ antibiotics, but also modulates the fitness of this organism because FQ‐resistant Campylobacter can out‐compete FQ‐susceptible Campylobacter in the absence of antibiotic selection pressure (Luo et al., 2005). In contrast to FQ resistance, the spontaneous mutation rate to macrolide resistance is low (< 10−9), and the development of stable macrolide‐resistant mutants appears to require long‐term exposure to the antibiotics (Lin et al., 2007; Luangtongkum et al., 2009), which explains, at least partly, why the overall prevalence of macrolide‐resistant Campylobacter is lower than that of FQ‐resistant Campylobacter.
Biotechnological approaches for control of Campylobacter
Control of Campylobacter represents a major goal for improving food safety and public health. As Campylobacter is a foodborne pathogen of animal origin and is transmitted to humans through the food chain, intervention strategies should consider the ecological aspects of the organism and can be designed to target the pathogen in both animal and human hosts, as well as the different segments of food production systems. Given the fact that contaminated poultry meat is a major source of human infections with Campylobacter, reduction of this pathogen in commercial chickens both at the pre‐slaughter and post‐slaughter stages has been a focus of investigation. A quantitative risk assessment estimated that a two‐log reduction in the number of Campylobacter on chicken carcasses could lead to a 30‐fold reduction in the incidence of human campylobacteriosis (Rosenquist et al., 2003).
Bacteriophage therapy has received considerable attention lately. Administering lytic bacteriophages to artificially contaminated chicken carcasses or Campylobacter‐colonized chickens reduced the level of C. jejuni contamination or colonization with varying success (Atterbury et al., 2003; Loc et al., 2005; Wagenaar et al., 2005). The efficacy of phage therapy is affected by phage types and treatment doses (Loc et al., 2005). In addition, treatment of Campylobacter colonization with bacteriophages was shown to be effective only for a short period, presumably due to development of phage resistance (Loc et al., 2005; Wagenaar et al., 2005). One potential way to avoid this limitation is to apply this treatment to chickens in the final days preceding slaughter, which may effectively reduce the quantity of Campylobacter that are introduced to the abattoir and consequently decrease chicken carcass contamination.
Recent advances in the field of metagenomics present a novel approach to the rational design of competitive exclusion products to potentially reduce Campylobacter colonization. Previously, competitive exclusions were attempted using culture‐based, mucous‐associated bacteria and yielded insufficient protection and inconsistent results (Schoeni and Doyle, 1992; Schoeni and Wong, 1994). The culture‐based method potentially misses key constituents, such as fungi, anaerobes or fastidious microbes, resulting in non‐reproducible effects of this treatment approach. The recently developed molecular techniques, such as oligonucleotide fingerprinting of rDNA genes (OFRG) (Patton et al., 2008) and high‐throughput pyrosequencing of rDNA genes (Qu et al., 2008), provide powerful tools to assess the microbial ecology of the gut. Application of OFRG, in conjunction with antibiotic dissection of the microbial community in the turkey ceca, revealed a possible association between the presence of a subspecies of Megamonas hypermegale and Campylobacter suppression (A. Scupham, pers. comm.). As technology and data analysis methods advance, it is possible that more effective competitive exclusion products can be developed to control Campylobacter in animal reservoirs.
Bacteriocins are ribosomally synthesized peptides produced by bacteria that inhibit the growth of other bacteria (Cotter et al., 2005). Several bacteriocins, notably bacteriocin OR‐7 from Lactobacillus salivarius (Stern et al., 2006) and E‐760 from Enterococcus (Line et al., 2008), demonstrated significant antagonistic effects on Campylobacter. Administration of either bacteriocin to chickens reduced the colonization level of Campylobacter by more than 106 colony‐forming units per gram of cecal contents (Stern et al., 2006; Line et al., 2008). These results suggest that bacteriocin treatment is a promising approach for the control of Campylobacter. When used prior to slaughter, this strategy may be effective in reducing chicken carcass contamination by Campylobacter.
Vaccines may be designed to prevent Campylobacter infection in humans or in the chicken host. Various approaches for immunization against Campylobacter colonization in chickens have been discussed in a recent review (de Zoete et al., 2007). Whole‐cell vaccines appear to confer marginal protection in chickens (Noor et al., 1995; Rice et al., 1997). Heterologous expression of Campylobacter antigens in an attenuated Salmonella strain has been used as a potential vector vaccine for poultry immunization, and this approach was met with varying success (Wyszynska et al., 2004; Sizemore et al., 2006). Surface‐exposed proteins, such as flagellin, MOMP (the major outer membrane protein) and adhesins (e.g. PEB1), may serve as potential subunit vaccine candidates. For example, vaccination of mice with recombinant FlaA or PEB1 demonstrated partial protection against subsequent C. jejuni challenge (Lee et al., 1999; Du et al., 2008). A CPS conjugate vaccine of C. jejuni 81‐176 provided complete protection against diarrhoea from homologous challenge, but did not prevent colonization in the New World monkey (Monteiro et al., 2008). Alternatively, secreted proteins can also be exploited as vaccine candidates. The flagella apparatus is known to secrete virulence factors (e.g. FlaC, Cia, FspA1 and FspA2), and vaccination of mice with recombinant FlaC, FspA1 and FspA2 elicited a humoral response and yielded partial protection against homologous challenge (Baqar et al., 2008). Despite these studies, an effective vaccine against Campylobacter has yet to be developed. As Campylobacter strains are genetically and antigenically diverse, an ideal vaccine should have the ability to elicit a protective immunity against a broad range of Campylobacter subtypes. Proteomics provides a promising tool for global profiling of Campylobacter antigens (Scott and Cordwell, 2009). Combination of proteomics with immunoblotting may identify protective antigens for vaccine development.
The distinct features of Campylobacter biology provide potential targets for the development of new antimicrobials or alternatives to antibiotics. Campylobacter preferentially uses flavodoxins as electron carriers and is predicted to synthesize menaquinone through the futalosine pathway (Cremades et al., 2005; Hiratsuka et al., 2008). The lack of flavodoxins and the futalosine pathway in humans and their important role in C. jejuni metabolism make them attractive therapeutic targets. As CmeABC is a key player in the resistance to antibiotics and in intestinal colonization, inhibition of CmeABC represents a plausible approach for controlling Campylobacter. A few studies have explored this possibility using efflux pump inhibitors, such as phenyl‐arginine‐β‐naphthylamide (PAβN) and 1‐(1‐naphthylmethyl)‐piperazine (NMP), which reduced Campylobacter resistance to antibiotics and bile salts in culture media (Lin and Martinez, 2006; Gibreel et al., 2007; Hannula and Hanninen, 2008). However, the tested inhibitors including PAβN had a limited effect on Campylobacter colonization in chickens (Lin and Martinez, 2006). To date, CmeABC‐specific inhibitors have not been identified. Elucidation of the three‐dimensional structure of the efflux proteins may facilitate the design of small molecular blockers that can be used to inhibit the function of CmeABC. Recently, Jeon and Zhang (2009) examined the feasibility of using antisense technology to silence the function of CmeABC and found that a CmeA‐specific peptide nucleic acid sensitized C. jejuni to antibiotics. Whether this method can be optimized for in vivo use in preventing Campylobacter colonization awaits further investigation. To be effective in vivo, efflux pump inhibitors must be stable in the gastrointestinal tract, have a low toxicity to animal hosts and specifically target Campylobacter without inhibiting the normal gut flora.
In summary, the recent advances in understanding Campylobacter biology have provided us with new opportunities to develop anti‐Campylobacter strategies. As research efforts expand from the genome to the transcriptome, proteome, glycome, metabolome and metagenome, we will be better equipped with biotechnological tools to control Campylobacter infection in both animal reservoirs and humans. In addition, the biological systems in Campylobacter may be exploited for broad biotechnological applications. For example, the N‐glycosylation system in C. jejuni may be used to produce glycosylated recombinant proteins because this system is functional in E. coli (Wacker et al., 2002). The PglB enzyme of the C. jejuni glycosylation pathway was also shown to be able to transfer the O‐polysaccharide from a lipid carrier to an acceptor protein in E. coli and Salmonella (Feldman et al., 2005; Wacker et al., 2006). The functionality of the Campylobacter glycosylation system in heterologous bacterial hosts and its relaxed substrate specificity provide a suitable system to engineer glycoproteins for various biotechnological applications, such as production of glycoconjugate vaccines.
Acknowledgments
The work conducted in Zhang's laboratory is supported by National Institute Health Grant RO1DK063008 and National Research Initiative Competitive grants 2005‐51110‐03273 and 2006‐34211‐17310 from the US Department of Agriculture Cooperative State Research, Education, and Extension Services.
References
- Alaimo C., Catrein I., Morf L., Marolda C.L., Callewaert N., Valvano M.A. Two distinct but interchangeable mechanisms for flipping of lipid‐linked oligosaccharides. EMBO J. 2006;25:967–976. doi: 10.1038/sj.emboj.7601024. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allos B.M. Campylobacter jejuni infections: update on emerging issues and trends. Clin Infect Dis. 2001;32:1201–1206. doi: 10.1086/319760. [DOI] [PubMed] [Google Scholar]
- Andersen‐Nissen E., Smith K.D., Strobe K.L., Barrett S.L., Cookson B.T., Logan S.M., Aderem A. Evasion of Toll‐like receptor 5 by flagellated bacteria. Proc Natl Acad Sci USA. 2005;102:9247–9252. doi: 10.1073/pnas.0502040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura H., Yamasaki M., Yamamoto S., Igimi S. Deletion of peb4 gene impairs cell adhesion and biofilm formation in Campylobacter jejuni. FEMS Microbiol Lett. 2007;275:278–285. doi: 10.1111/j.1574-6968.2007.00893.x. [DOI] [PubMed] [Google Scholar]
- Atack J.M., Kelly D.J. Structure, mechanism and physiological roles of bacterial cytochrome c peroxidases. Adv Microb Physiol. 2007;52:73–106. doi: 10.1016/S0065-2911(06)52002-8. [DOI] [PubMed] [Google Scholar]
- Atterbury R.J., Connerton P.L., Dodd C.E., Rees C.E., Connerton I.F. Application of host‐specific bacteriophages to the surface of chicken skin leads to a reduction in recovery of Campylobacter jejuni. Appl Environ Microbiol. 2003;69:6302–6306. doi: 10.1128/AEM.69.10.6302-6306.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrain L., Vernozy‐Rozand C., Kempf I. Evidence for natural horizontal transfer of tetO gene between Campylobacter jejuni strains in chickens. J Appl Microbiol. 2004;97:134–140. doi: 10.1111/j.1365-2672.2004.02306.x. [DOI] [PubMed] [Google Scholar]
- Bacon D.J., Alm R.A., Burr D.H., Hu L., Kopecko D.J., Ewing C.P. Involvement of a plasmid in virulence of Campylobacter jejuni 81‐176. Infect Immun. 2000;68:4384–4390. doi: 10.1128/iai.68.8.4384-4390.2000. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon D.J., Szymanski C.M., Burr D.H., Silver R.P., Alm R.A., Guerry P. A phase‐variable capsule is involved in virulence of Campylobacter jejuni 81‐176. Mol Microbiol. 2001;40:769–777. doi: 10.1046/j.1365-2958.2001.02431.x. [DOI] [PubMed] [Google Scholar]
- Baqar S., Applebee L.A., Gilliland T.C., Jr, Lee L.H., Porter C.K., Guerry P. Immunogenicity and protective efficacy of recombinant Campylobacter jejuni flagellum‐secreted proteins in mice. Infect Immun. 2008;76:3170–3175. doi: 10.1128/IAI.00076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes I.H., Bagnall M.C., Browning D.D., Thompson S.A., Manning G., Newell D.G. Gamma‐glutamyl transpeptidase has a role in the persistent colonization of the avian gut by Campylobacter jejuni. Microb Pathog. 2007;43:198–207. doi: 10.1016/j.micpath.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham‐Ramos L.K., Hendrixson D.R. Characterization of two putative cytochrome c peroxidases of Campylobacter jejuni involved in promoting commensal colonization of poultry. Infect Immun. 2008;76:1105–1114. doi: 10.1128/IAI.01430-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R.E., Levine M.M., Clements M.L., Hughes T.P., Blaser M.J. Experimental Campylobacter jejuni infection in humans. J Infect Dis. 1988;157:472–479. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- Blaser M.J., Engberg J. Clinical aspects of Campylobacter jejuni and Campylobacter coli infections. In: Nachamkin I., Szymanski C., Blaser M.J., editors. ASM Press; 2008. pp. 99–121. [Google Scholar]
- De Boer P., Wagenaar J.A., Achterberg R.P., Van Putten J.P., Schouls L.M., Duim B. Generation of Campylobacter jejuni genetic diversity in vivo. Mol Microbiol. 2002;44:351–359. doi: 10.1046/j.1365-2958.2002.02930.x. [DOI] [PubMed] [Google Scholar]
- Bras A.M., Chatterjee S., Wren B.W., Newell D.G., Ketley J.M. A novel Campylobacter jejuni two‐component regulatory system important for temperature‐dependent growth and colonization. J Bacteriol. 1999;181:3298–3302. doi: 10.1128/jb.181.10.3298-3302.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell M.B., Guerry P., Lee E.C., Burans J.P., Walker R.I. Reversible expression of flagella in Campylobacter jejuni. Infect Immun. 1985;50:941–943. doi: 10.1128/iai.50.3.941-943.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C.G., Ng L.K. Sequence variability of Campylobacter temperate bacteriophages. BMC Microbiol. 2008;8:49. doi: 10.1186/1471-2180-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordwell S.J., Len A.C., Touma R.G., Scott N.E., Falconer L., Jones D. Identification of membrane‐associated proteins from Campylobacter jejuni strains using complementary proteomics technologies. Proteomics. 2008;8:122–139. doi: 10.1002/pmic.200700561. et al. [DOI] [PubMed] [Google Scholar]
- Cotter P.D., Hill C., Ross R.P. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol. 2005;3:777–788. doi: 10.1038/nrmicro1273. [DOI] [PubMed] [Google Scholar]
- Cremades N., Bueno M., Toja M., Sancho J. Towards a new therapeutic target: Helicobacter pylori flavodoxin. Biophys Chem. 2005;115:267–276. doi: 10.1016/j.bpc.2004.12.045. [DOI] [PubMed] [Google Scholar]
- Du L.F., Li Z.J., Tang X.Y., Huang J.Q., Sun W.B. Immunogenicity and immunoprotection of recombinant PEB1 in Campylobacter jejuni‐infected mice. World J Gastroenterol. 2008;14:6244–6248. doi: 10.3748/wjg.14.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg J., Aarestrup F.M., Taylor D.E., Gerner‐Smidt P., Nachamkin I. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg Infect Dis. 2001;7:24–34. doi: 10.3201/eid0701.010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M.F., Wacker M., Hernandez M., Hitchen P.G., Marolda C.L., Kowarik M. Engineering N‐linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichia coli. Proc Natl Acad Sci USA. 2005;102:3016–3021. doi: 10.1073/pnas.0500044102. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouts D.E., Mongodin E.F., Mandrell R.E., Miller W.G., Rasko D.A., Ravel J. Major structural differences and novel potential virulence mechanisms from the genomes of multiple Campylobacter species. PLoS Biol. 2005;3:e15. doi: 10.1371/journal.pbio.0030015. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman C.R., Neimann J., Wegener H.C., Tauxe R.V. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. In: Nachamkin I., Blaser M.J., editors. ASM Press; 2000. pp. 121–138. [Google Scholar]
- Gaynor E.C., Wells D.H., MacKichan J.K., Falkow S. The Campylobacter jejuni stringent response controls specific stress survival and virulence‐associated phenotypes. Mol Microbiol. 2005;56:8–27. doi: 10.1111/j.1365-2958.2005.04525.x. [DOI] [PubMed] [Google Scholar]
- Gibreel A., Kos V.N., Keelan M., Trieber C.A., Levesque S., Michaud S., Taylor D.E. Macrolide resistance in Campylobacter jejuni and Campylobacter coli: molecular mechanism and stability of the resistance phenotype. Antimicrob Agents Chemother. 2005;49:2753–2759. doi: 10.1128/AAC.49.7.2753-2759.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibreel A., Wetsch N.M., Taylor D.E. Contribution of the CmeABC efflux pump to macrolide and tetracycline resistance in Campylobacter jejuni. Antimicrob Agents Chemother. 2007;51:3212–3216. doi: 10.1128/AAC.01592-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie I.A., O'Brien S.J., Penman C., Tompkins D., Cowden J., Humphrey T.J. Demographic determinants for Campylobacter infection in England and Wales: implications for future epidemiological studies. Epidemiol Infect. 2008;136:1717–1725. doi: 10.1017/S0950268808000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover K.J., Weerapana E., Imperiali B. In vitro assembly of the undecaprenylpyrophosphate‐linked heptasaccharide for prokaryotic N‐linked glycosylation. Proc Natl Acad Sci USA. 2005;102:14255–14259. doi: 10.1073/pnas.0507311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden N.J., Acheson D.W. Identification of motility and autoagglutination Campylobacter jejuni mutants by random transposon mutagenesis. Infect Immun. 2002;70:1761–1771. doi: 10.1128/IAI.70.4.1761-1771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu R., Su C.C., Shi F., Li M., McDermott G., Zhang Q., Yu E.W. Crystal structure of the transcriptional regulator CmeR from Campylobacter jejuni. J Mol Biol. 2007;372:583–593. doi: 10.1016/j.jmb.2007.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guccione E., Leon‐Kempis M.R., Pearson B.M., Hitchin E., Mulholland F., Van Diemen P.M. Amino acid‐dependent growth of Campylobacter jejuni: key roles for aspartase (AspA) under microaerobic and oxygen‐limited conditions and identification of AspB (Cj0762), essential for growth on glutamate. Mol Microbiol. 2008;69:77–93. doi: 10.1111/j.1365-2958.2008.06263.x. et al. [DOI] [PubMed] [Google Scholar]
- Guerry P., Szymanski C.M. Campylobacter sugars sticking out. Trends Microbiol. 2008;16:428–435. doi: 10.1016/j.tim.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Guerry P., Alm R.A., Power M.E., Logan S.M., Trust T.J. Role of two flagellin genes in Campylobacter motility. J Bacteriol. 1991;173:4757–4764. doi: 10.1128/jb.173.15.4757-4764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., Pope P.M., Burr D.H., Leifer J., Joseph S.W., Bourgeois A.L. Development and characterization of recA mutants of Campylobacter jejuni for inclusion in attenuated vaccines. Infect Immun. 1994;62:426–432. doi: 10.1128/iai.62.2.426-432.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., Ewing C.P., Schirm M., Lorenzo M., Kelly J., Pattarini D. Changes in flagellin glycosylation affect Campylobacter autoagglutination and virulence. Mol Microbiol. 2006;60:299–311. doi: 10.1111/j.1365-2958.2006.05100.x. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B., Wang Y., Shi F., Barton Y.W., Plummer P., Reynolds D.L. CmeR functions as a pleiotropic regulator and is required for optimal colonization of Campylobacter jejuni in vivo. J Bacteriol. 2008;190:1879–1890. doi: 10.1128/JB.01796-07. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Sahin O., Barton Y.W., Zhang Q. Key role of Mfd in the development of fluoroquinolone resistance in Campylobacter jejuni. PLoS Pathog. 2008;4:e1000083. doi: 10.1371/journal.ppat.1000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula M., Hanninen M.L. Effect of putative efflux pump inhibitors and inducers on the antimicrobial susceptibility of Campylobacter jejuni and Campylobacter coli. J Med Microbiol. 2008;57:851–855. doi: 10.1099/jmm.0.47823-0. [DOI] [PubMed] [Google Scholar]
- Hazeleger W.C., Wouters J.A., Rombouts F.M., Abee T. Physiological activity of Campylobacter jejuni far below the minimal growth temperature. Appl Environ Microbiol. 1998;64:3917–3922. doi: 10.1128/aem.64.10.3917-3922.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrixson D.R. A phase‐variable mechanism controlling the Campylobacter jejuni FlgR response regulator influences commensalism. Mol Microbiol. 2006;61:1646–1659. doi: 10.1111/j.1365-2958.2006.05336.x. [DOI] [PubMed] [Google Scholar]
- Hendrixson D.R. Restoration of flagellar biosynthesis by varied mutational events in Campylobacter jejuni. Mol Microbiol. 2008;70:519–536. doi: 10.1111/j.1365-2958.2008.06428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrixson D.R., Dirita V.J. Transcription of sigma54‐dependent but not sigma28‐dependent flagellar genes in Campylobacter jejuni is associated with formation of the flagellar secretory apparatus. Mol Microbiol. 2003;50:687–702. doi: 10.1046/j.1365-2958.2003.03731.x. [DOI] [PubMed] [Google Scholar]
- Hendrixson D.R., Dirita V.J. Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Mol Microbiol. 2004;52:471–484. doi: 10.1111/j.1365-2958.2004.03988.x. [DOI] [PubMed] [Google Scholar]
- Hiratsuka T., Furihata K., Ishikawa J., Yamashita H., Itoh N., Seto H., Dairi T. An alternative menaquinone biosynthetic pathway operating in microorganisms. Science. 2008;321:1670–1673. doi: 10.1126/science.1160446. [DOI] [PubMed] [Google Scholar]
- Hoffman P.S., Goodman T.G. Respiratory physiology and energy conservation efficiency of Campylobacter jejuni. J Bacteriol. 1982;150:319–326. doi: 10.1128/jb.150.1.319-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofreuter D., Tsai J., Watson R.O., Novik V., Altman B., Benitez M. Unique features of a highly pathogenic Campylobacter jejuni strain. Infect Immun. 2006;74:4694–4707. doi: 10.1128/IAI.00210-06. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofreuter D., Novik V., Galan J.E. Metabolic diversity in Campylobacter jejuni enhances specific tissue colonization. Cell Host Microbe. 2008;4:425–433. doi: 10.1016/j.chom.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Hughes N.J., Clayton C.L., Chalk P.A., Kelly D.J. Helicobacter pylori porCDAB and oorDABC genes encode distinct pyruvate:flavodoxin and 2‐oxoglutarate:acceptor oxidoreductases which mediate electron transport to NADP. J Bacteriol. 1998;180:1119–1128. doi: 10.1128/jb.180.5.1119-1128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R.J., Elvers K.T., Lee L.J., Gidley M.D., Wainwright L.M., Lightfoot J. Oxygen reactivity of both respiratory oxidases in Campylobacter jejuni: the cydAB genes encode a cyanide‐resistant, low‐affinity oxidase that is not of the cytochrome bd type. J Bacteriol. 2007;189:1604–1615. doi: 10.1128/JB.00897-06. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen R., Krogfelt K.A., Cawthraw S.A., Van Pelt W., Wagenaar J.A., Owen R.J. Host‐pathogen interactions in Campylobacter infections: the host perspective. Clin Microbiol Rev. 2008;21:505–518. doi: 10.1128/CMR.00055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon B., Zhang Q. Cj0011c, a periplasmic single‐ and double‐stranded DNA‐binding protein, contributes to natural transformation in Campylobacter jejuni. J Bacteriol. 2007;189:7399–7407. doi: 10.1128/JB.01012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon B., Zhang Q. Sensitization of Campylobacter jejuni to fluoroquinolone and macrolide antibiotics by antisense inhibition of the CmeABC multidrug efflux transporter. J Antimicrob Chemother. 2009;63:946–948. doi: 10.1093/jac/dkp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon B., Muraoka W., Sahin O., Zhang Q. Role of Cj1211 in natural transformation and transfer of antibiotic resistance determinants in Campylobacter jejuni. Antimicrob Agents Chemother. 2008;52:2699–2708. doi: 10.1128/AAC.01607-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon B., Muraoka W., Scupham A., Zhang Q. Roles of lipooligosaccharide and capsular polysaccharide in antimicrobial resistance and natural transformation of Campylobacter jejuni. J Antimicrob Chemother. 2009;63:462–468. doi: 10.1093/jac/dkn529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Hou Y., Inouye M. CspA, the major cold‐shock protein of Escherichia coli, is an RNA chaperone. J Biol Chem. 1997;272:196–202. doi: 10.1074/jbc.272.1.196. [DOI] [PubMed] [Google Scholar]
- Jin S., Joe A., Lynett J., Hani E.K., Sherman P., Chan V.L. JlpA, a novel surface‐exposed lipoprotein specific to Campylobacter jejuni, mediates adherence to host epithelial cells. Mol Microbiol. 2001;39:1225–1236. doi: 10.1111/j.1365-2958.2001.02294.x. [DOI] [PubMed] [Google Scholar]
- Jones M.A., Marston K.L., Woodall C.A., Maskell D.J., Linton D., Karlyshev A.V. Adaptation of Campylobacter jejuni NCTC11168 to high‐level colonization of the avian gastrointestinal tract. Infect Immun. 2004;72:3769–3776. doi: 10.1128/IAI.72.7.3769-3776.2004. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshua G.W., Guthrie‐Irons C., Karlyshev A.V., Wren B.W. Biofilm formation in Campylobacter jejuni. Microbiology. 2006;152:387–396. doi: 10.1099/mic.0.28358-0. [DOI] [PubMed] [Google Scholar]
- Kakuda T., Dirita V.J. Cj1496c encodes a Campylobacter jejuni glycoprotein that influences invasion of human epithelial cells and colonization of the chick gastrointestinal tract. Infect Immun. 2006;74:4715–4723. doi: 10.1128/IAI.00033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmokoff M., Lanthier P., Tremblay T.L., Foss M., Lau P.C., Sanders G. Proteomic analysis of Campylobacter jejuni 11168 biofilms reveals a role for the motility complex in biofilm formation. J Bacteriol. 2006;188:4312–4320. doi: 10.1128/JB.01975-05. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Huang S., Blaser M.J. Structural and functional divergence of MutS2 from bacterial MutS1 and eukaryotic MSH4‐MSH5 homologs. J Bacteriol. 2005;187:3528–3537. doi: 10.1128/JB.187.10.3528-3537.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlyshev A.V., Linton D., Gregson N.A., Wren B.W. A novel paralogous gene family involved in phase‐variable flagella‐mediated motility in Campylobacter jejuni. Microbiology. 2002;148:473–480. doi: 10.1099/00221287-148-2-473. [DOI] [PubMed] [Google Scholar]
- Karlyshev A.V., Everest P., Linton D., Cawthraw S., Newell D.G., Wren B.W. The Campylobacter jejuni general glycosylation system is important for attachment to human epithelial cells and in the colonization of chicks. Microbiology. 2004;150:1957–1964. doi: 10.1099/mic.0.26721-0. [DOI] [PubMed] [Google Scholar]
- Karlyshev A.V., Ketley J.M., Wren B.W. The Campylobacter jejuni glycome. FEMS Microbiol Rev. 2005;29:377–390. doi: 10.1016/j.femsre.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Kelly J., Jarrell H., Millar L., Tessier L., Fiori L.M., Lau P.C. Biosynthesis of the N‐linked glycan in Campylobacter jejuni and addition onto protein through block transfer. J Bacteriol. 2006;188:2427–2434. doi: 10.1128/JB.188.7.2427-2434.2006. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel M.E., Garvis S.G., Tipton S.L., Anderson D.E., Jr, Cieplak W., Jr Identification and molecular cloning of a gene encoding a fibronectin‐binding protein (CadF) from Campylobacter jejuni. Mol Microbiol. 1997;24:953–963. doi: 10.1046/j.1365-2958.1997.4031771.x. [DOI] [PubMed] [Google Scholar]
- Konkel M.E., Kim B.J., Rivera‐Amill V., Garvis S.G. Bacterial secreted proteins are required for the internaliztion of Campylobacter jejuni into cultured mammalian cells. Mol Microbiol. 1999;32:691–701. doi: 10.1046/j.1365-2958.1999.01376.x. [DOI] [PubMed] [Google Scholar]
- Konkel M.E., Klena J.D., Rivera‐Amill V., Monteville M.R., Biswas D., Raphael B., Mickelson J. Secretion of virulence proteins from Campylobacter jejuni is dependent on a functional flagellar export apparatus. J Bacteriol. 2004;186:3296–3303. doi: 10.1128/JB.186.11.3296-3303.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowarik M., Young N.M., Numao S., Schulz B.L., Hug I., Callewaert N. Definition of the bacterial N‐glycosylation site consensus sequence. EMBO J. 2006;25:1957–1966. doi: 10.1038/sj.emboj.7601087. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J.C., Szymanski C., Guerry P. N‐linked protein glycosylation is required for full competence in Campylobacter jejuni 81‐176. J Bacteriol. 2004;186:6508–6514. doi: 10.1128/JB.186.19.6508-6514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L.H., Burg E., III, Baqar S., Bourgeois A.L., Burr D.H., Ewing C.P. Evaluation of a truncated recombinant flagellin subunit vaccine against Campylobacter jejuni. Infect Immun. 1999;67:5799–5805. doi: 10.1128/iai.67.11.5799-5805.1999. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Martinez A. Effect of efflux pump inhibitors on bile resistance and in vivo colonization of Campylobacter jejuni. J Antimicrob Chemother. 2006;58:966–972. doi: 10.1093/jac/dkl374. [DOI] [PubMed] [Google Scholar]
- Lin J., Michel L.O., Zhang Q. CmeABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrob Agents Chemother. 2002;46:2124–2131. doi: 10.1128/AAC.46.7.2124-2131.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Sahin O., Michel L.O., Zhang Q. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni. Infect Immun. 2003;71:4250–4259. doi: 10.1128/IAI.71.8.4250-4259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Akiba M., Sahin O., Zhang Q. CmeR functions as a transcriptional repressor for the multidrug efflux pump CmeABC in Campylobacter jejuni. Antimicrob Agents Chemother. 2005a;49:1067–1075. doi: 10.1128/AAC.49.3.1067-1075.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Cagliero C., Guo B., Barton Y.W., Maurel M.C., Payot S., Zhang Q. Bile salts modulate expression of the CmeABC multidrug efflux pump in Campylobacter jejuni. J Bacteriol. 2005b;187:7417–7424. doi: 10.1128/JB.187.21.7417-7424.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Yan M., Sahin O., Pereira S., Chang Y.J., Zhang Q. Effect of macrolide usage on emergence of erythromycin‐resistant Campylobacter isolates in chickens. Antimicrob Agents Chemother. 2007;51:1678–1686. doi: 10.1128/AAC.01411-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Line J.E., Svetoch E.A., Eruslanov B.V., Perelygin V.V., Mitsevich E.V., Mitsevich I.P. Isolation and purification of enterocin E‐760 with broad antimicrobial activity against gram‐positive and gram‐negative bacteria. Antimicrob Agents Chemother. 2008;52:1094–1100. doi: 10.1128/AAC.01569-06. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton D., Allan E., Karlyshev A.V., Cronshaw A.D., Wren B.W. Identification of N‐acetylgalactosamine‐containing glycoproteins PEB3 and CgpA in Campylobacter jejuni. Mol Microbiol. 2002;43:497–508. doi: 10.1046/j.1365-2958.2002.02762.x. [DOI] [PubMed] [Google Scholar]
- Linton D., Dorrell N., Hitchen P.G., Amber S., Karlyshev A.V., Morris H.R. Functional analysis of the Campylobacter jejuni N‐linked protein glycosylation pathway. Mol Microbiol. 2005;55:1695–1703. doi: 10.1111/j.1365-2958.2005.04519.x. et al. [DOI] [PubMed] [Google Scholar]
- Loc C.C., Atterbury R.J., El Shibiny A., Connerton P.L., Dillon E., Scott A., Connerton I.F. Bacteriophage therapy to reduce Campylobacter jejuni colonization of broiler chickens. Appl Environ Microbiol. 2005;71:6554–6563. doi: 10.1128/AEM.71.11.6554-6563.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luangtongkum T., Jeon B., Han J., Plummer P.J., Logue C.M., Zhang Q. Antibiotic resistance in Campylobacter: molecular mechanisms and ecology of emergence, transmission and persistence. Future Microbiol. 2009;4:189–200. doi: 10.2217/17460913.4.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo N., Sahin O., Lin J., Michel L.O., Zhang Q. In vivo selection of Campylobacter isolates with high levels of fluoroquinolone resistance associated with gyrA mutations and the function of the CmeABC efflux pump. Antimicrob Agents Chemother. 2003;47:390–394. doi: 10.1128/AAC.47.1.390-394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo N., Pereira S., Sahin O., Lin J., Huang S., Michel L., Zhang Q. Enhanced in vivo fitness of fluoroquinolone‐resistant Campylobacter jejuni in the absence of antibiotic selection pressure. Proc Natl Acad Sci USA. 2005;102:541–546. doi: 10.1073/pnas.0408966102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott P.F., Bodeis S.M., English L.L., White D.G., Walker R.D., Zhao S. Ciprofloxacin resistance in Campylobacter jejuni evolves rapidly in chickens treated with fluoroquinolones. J Infect Dis. 2002;185:837–840. doi: 10.1086/339195. et al. [DOI] [PubMed] [Google Scholar]
- MacKichan J.K., Gaynor E.C., Chang C., Cawthraw S., Newell D.G., Miller J.F., Falkow S. The Campylobacter jejuni dccRS two‐component system is required for optimal in vivo colonization but is dispensable for in vitro growth. Mol Microbiol. 2004;54:1269–1286. doi: 10.1111/j.1365-2958.2004.04371.x. [DOI] [PubMed] [Google Scholar]
- McSweegan E., Walker R.I. Identification and characterization of two Campylobacter jejuni adhesins for cellular and mucous substrates. Infect Immun. 1986;53:141–148. doi: 10.1128/iai.53.1.141-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendz G.L., Ball G.E., Meek D.J. Pyruvate metabolism in Campylobacter spp. Biochim Biophys Acta. 1997;1334:291–302. doi: 10.1016/s0304-4165(96)00107-9. [DOI] [PubMed] [Google Scholar]
- Miller J.H. Spontaneous mutators in bacteria: insights into pathways of mutagenesis and repair. Annu Rev Microbiol. 1996;50:625–643. doi: 10.1146/annurev.micro.50.1.625. [DOI] [PubMed] [Google Scholar]
- Misawa N., Blaser M.J. Detection and characterization of autoagglutination activity by Campylobacter jejuni. Infect Immun. 2000;68:6168–6175. doi: 10.1128/iai.68.11.6168-6175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen B., Oust A., Langsrud O., Dorrell N., Marsden G.L., Hinds J. Explorative multifactor approach for investigating global survival mechanisms of Campylobacter jejuni under environmental conditions. Appl Environ Microbiol. 2005;71:2086–2094. doi: 10.1128/AEM.71.4.2086-2094.2005. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro M.A., Baqar S., Hall E.R., Chen Y.H., Porter C.K., Bentzel D.E. A capsule polysaccharide conjugate vaccine against diarrheal disease caused by Campylobacter jejuni. Infect Immun. 2008;77:1128–1136. doi: 10.1128/IAI.01056-08. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C.W., Kai A., Lambert M.A., Patton C. Isoprenoid quinone content and cellular fatty acid composition of Campylobacter species. J Clin Microbiol. 1984;19:772–776. doi: 10.1128/jcm.19.6.772-776.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C., Carroll C., Jordan K.N. Environmental survival mechanisms of the foodborne pathogen Campylobacter jejuni. J Appl Microbiol. 2006;100:623–632. doi: 10.1111/j.1365-2672.2006.02903.x. [DOI] [PubMed] [Google Scholar]
- Myers J.D., Kelly D.J. A sulphite respiration system in the chemoheterotrophic human pathogen Campylobacter jejuni. Microbiology. 2005;151:233–242. doi: 10.1099/mic.0.27573-0. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor S.M., Husband A.J., Widders P.R. In ovo oral vaccination with Campylobacter jejuni establishes early development of intestinal immunity in chickens. Br Poult Sci. 1995;36:563–573. doi: 10.1080/00071669508417802. [DOI] [PubMed] [Google Scholar]
- Nuijten P.J., Van Asten F.J., Gaastra W., Van Der Zeijst B.A. Structural and functional analysis of two Campylobacter jejuni flagellin genes. J Biol Chem. 1990;265:17798–17804. [PubMed] [Google Scholar]
- Olson C.K., Ethelberg S., Van Pelt W., Tauxe R.V. Epidemiology of Campylobacter jejuni infections in industrialized nations. In: Nachamkin I., Szymanski C., Blaser M.J., editors. ASM Press; 2008. pp. 163–189. [Google Scholar]
- Pajaniappan M., Hall J.E., Cawthraw S.A., Newell D.G., Gaynor E.C., Fields J.A. A temperature‐regulated Campylobacter jejuni gluconate dehydrogenase is involved in respiration‐dependent energy conservation and chicken colonization. Mol Microbiol. 2008;68:474–491. doi: 10.1111/j.1365-2958.2008.06161.x. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palyada K., Threadgill D., Stintzi A. Iron acquisition and regulation in Campylobacter jejuni. J Bacteriol. 2004;186:4714–4729. doi: 10.1128/JB.186.14.4714-4729.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.F., Purdy D., Leach S. Localized reversible frameshift mutation in the flhA gene confers phase variability to flagellin gene expression in Campylobacter coli. J Bacteriol. 2000;182:207–210. doi: 10.1128/jb.182.1.207-210.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C.T., Quinones B., Miller W.G., Horn S.T., Mandrell R.E. Comparative genomic analysis of Campylobacter jejuni strains reveals diversity due to genomic elements similar to those present in C. jejuni strain RM1221. J Clin Microbiol. 2006;44:4125–4135. doi: 10.1128/JCM.01231-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhill J., Wren B.W., Mungall K., Ketley J.M., Churcher C., Basham D. The genome sequence of the food‐borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. et al. [DOI] [PubMed] [Google Scholar]
- Patton T.G., Scupham A.J., Bearson S.M., Carlson S.A. Characterization of fecal microbiota from a Salmonella endemic cattle herd as determined by oligonucleotide fingerprinting of rDNA genes. Vet Microbiol. 2008;136:285–292. doi: 10.1016/j.vetmic.2008.10.032. [DOI] [PubMed] [Google Scholar]
- Pei Z., Burucoa C., Grignon B., Baqar S., Huang X.Z., Kopecko D.J. Mutation in the peb1A locus of Campylobacter jejuni reduces interactions with epithelial cells and intestinal colonization of mice. Infect Immun. 1998;66:938–943. doi: 10.1128/iai.66.3.938-943.1998. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman M.S., Elvers K.T., Lee L., Jones M.A., Poole R.K., Park S.F., Kelly D.J. Growth of Campylobacter jejuni on nitrate and nitrite: electron transport to NapA and NrfA via NrfH and distinct roles for NrfA and the globin Cgb in protection against nitrosative stress. Mol Microbiol. 2007;63:575–590. doi: 10.1111/j.1365-2958.2006.05532.x. [DOI] [PubMed] [Google Scholar]
- Poly F., Guerry P. Pathogenesis of Campylobacter. Curr Opin Gastroenterol. 2008;24:27–31. doi: 10.1097/MOG.0b013e3282f1dcb1. [DOI] [PubMed] [Google Scholar]
- Poly F., Ewing C., Goon S., Hickey T.E., Rockabrand D., Majam G. Heterogeneity of a Campylobacter jejuni protein that is secreted through the flagellar filament. Infect Immun. 2007;75:3859–3867. doi: 10.1128/IAI.00159-07. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumbwe L., Piddock L.J. Identification and molecular characterisation of CmeB, a Campylobacter jejuni multidrug efflux pump. FEMS Microbiol Lett. 2002;206:185–189. doi: 10.1111/j.1574-6968.2002.tb11007.x. [DOI] [PubMed] [Google Scholar]
- Qu A., Brulc J.M., Wilson M.K., Law B.F., Theoret J.R., Joens L.A. Comparative metagenomics reveals host specific metavirulomes and horizontal gene transfer elements in the chicken cecum microbiome. PLoS ONE. 2008;3:e2945. doi: 10.1371/journal.pone.0002945. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J.L., Martinez‐Bueno M., Molina‐Henares A.J., Teran W., Watanabe K., Zhang X. The TetR family of transcriptional repressors. Microbiol Mol Biol Rev. 2005;69:326–356. doi: 10.1128/MMBR.69.2.326-356.2005. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael B.H., Pereira S., Flom G.A., Zhang Q., Ketley J.M., Konkel M.E. The Campylobacter jejuni response regulator, CbrR, modulates sodium deoxycholate resistance and chicken colonization. J Bacteriol. 2005;187:3662–3670. doi: 10.1128/JB.187.11.3662-3670.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid A.N., Pandey R., Palyada K., Naikare H., Stintzi A. Identification of Campylobacter jejuni genes involved in the response to acidic pH and stomach transit. Appl Environ Microbiol. 2008;74:1583–1597. doi: 10.1128/AEM.01507-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice B.E., Rollins D.M., Mallinson E.T., Carr L., Joseph S.W. Campylobacter jejuni in broiler chickens: colonization and humoral immunity following oral vaccination and experimental infection. Vaccine. 1997;15:1922–1932. doi: 10.1016/s0264-410x(97)00126-6. [DOI] [PubMed] [Google Scholar]
- Robinson D.A. Infective dose of Campylobacter jejuni in milk. Br Med J (Clin Res Ed) 1981;282:1584. doi: 10.1136/bmj.282.6276.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist H., Nielsen N.L., Sommer H.M., Norrung B., Christensen B.B. Quantitative risk assessment of human campylobacteriosis associated with thermophilic Campylobacter species in chickens. Int J Food Microbiol. 2003;83:87–103. doi: 10.1016/s0168-1605(02)00317-3. [DOI] [PubMed] [Google Scholar]
- Routh M.D., Su C.C., Zhang Q., Yu E.W. Structures of AcrR and CmeR: insight into the mechanisms of transcriptional repression and multi‐drug recognition in the TetR family of regulators. Biochim Biophys Acta. 2009;1794:844–851. doi: 10.1016/j.bbapap.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐Palacios G.M. The health burden of Campylobacter infection and the impact of antimicrobial resistance: playing chicken. Clin Infect Dis. 2007;44:701–703. doi: 10.1086/509936. [DOI] [PubMed] [Google Scholar]
- Sauer U., Eikmanns B.J. The PEP‐pyruvate‐oxaloacetate node as the switch point for carbon flux distribution in bacteria. FEMS Microbiol Rev. 2005;29:765–794. doi: 10.1016/j.femsre.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Schaffer C., Graninger M., Messner P. Prokaryotic glycosylation. Proteomics. 2001;1:248–261. doi: 10.1002/1615-9861(200102)1:2<248::AID-PROT248>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Schoeni J.L., Doyle M.P. Reduction of Campylobacter jejuni colonization of chicks by cecum‐colonizing bacteria producing anti‐C. jejuni metabolites. Appl Environ Microbiol. 1992;58:664–670. doi: 10.1128/aem.58.2.664-670.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeni J.L., Wong A.C. Inhibition of Campylobacter jejuni colonization in chicks by defined competitive exclusion bacteria. Appl Environ Microbiol. 1994;60:1191–1197. doi: 10.1128/aem.60.4.1191-1197.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A.E., Timms A.R., Connerton P.L., El Shibiny A., Connerton I.F. Bacteriophage influence Campylobacter jejuni types populating broiler chickens. Environ Microbiol. 2007a;9:2341–2353. doi: 10.1111/j.1462-2920.2007.01351.x. [DOI] [PubMed] [Google Scholar]
- Scott A.E., Timms A.R., Connerton P.L., Loc C.C., Adzfa R.K., Connerton I.F. Genome dynamics of Campylobacter jejuni in response to bacteriophage predation. PLoS Pathog. 2007b;3:e119. doi: 10.1371/journal.ppat.0030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott N.E., Cordwell S.J. Campylobacter proteomics: guidelines, challenges and future perspectives. Expert Rev Proteomics. 2009;6:61–74. doi: 10.1586/14789450.6.1.61. [DOI] [PubMed] [Google Scholar]
- Selby C.P., Sancar A. Mechanisms of transcription‐repair coupling and mutation frequency decline. Microbiol Rev. 1994;58:317–329. doi: 10.1128/mr.58.3.317-329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellars M.J., Hall S.J., Kelly D.J. Growth of Campylobacter jejuni supported by respiration of fumarate, nitrate, nitrite, trimethylamine‐N‐oxide, or dimethyl sulfoxide requires oxygen. J Bacteriol. 2002;184:4187–4196. doi: 10.1128/JB.184.15.4187-4196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaf‐Sweeney K.D., Larson C.L., Tang X., Konkel M.E. Identification of Campylobacter jejuni proteins recognized by chicken maternal antibodies. Appl Environ Microbiol. 2008;74:6867–6875. doi: 10.1128/AEM.01097-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore D.R., Warner B., Lawrence J., Jones A., Killeen K.P. Live, attenuated Salmonella typhimurium vectoring Campylobacter antigens. Vaccine. 2006;24:3793–3803. doi: 10.1016/j.vaccine.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Smith M.A., Finel M., Korolik V., Mendz G.L. Characteristics of the aerobic respiratory chains of the microaerophiles Campylobacter jejuni and Helicobacter pylori. Arch Microbiol. 2000;174:1–10. doi: 10.1007/s002030000174. [DOI] [PubMed] [Google Scholar]
- Song Y.C., Jin S., Louie H., Ng D., Lau R., Zhang Y. FlaC, a protein of Campylobacter jejuni TGH9011 (ATCC43431) secreted through the flagellar apparatus, binds epithelial cells and influences cell invasion. Mol Microbiol. 2004;53:541–553. doi: 10.1111/j.1365-2958.2004.04175.x. et al. [DOI] [PubMed] [Google Scholar]
- St Maurice M., Cremades N., Croxen M.A., Sisson G., Sancho J., Hoffman P.S. Flavodoxin:quinone reductase (FqrB): a redox partner of pyruvate:ferredoxin oxidoreductase that reversibly couples pyruvate oxidation to NADPH production in Helicobacter pylori and Campylobacter jejuni. J Bacteriol. 2007;189:4764–4773. doi: 10.1128/JB.00287-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern N.J., Svetoch E.A., Eruslanov B.V., Perelygin V.V., Mitsevich E.V., Mitsevich I.P. Isolation of a Lactobacillus salivarius strain and purification of its bacteriocin, which is inhibitory to Campylobacter jejuni in the chicken gastrointestinal system. Antimicrob Agents Chemother. 2006;50:3111–3116. doi: 10.1128/AAC.00259-06. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzi A. Gene expression profile of Campylobacter jejuni in response to growth temperature variation. J Bacteriol. 2003;185:2009–2016. doi: 10.1128/JB.185.6.2009-2016.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzi A., Marlow D., Palyada K., Naikare H., Panciera R., Whitworth L., Clarke C. Use of genome‐wide expression profiling and mutagenesis to study the intestinal lifestyle of Campylobacter jejuni. Infect Immun. 2005;73:1797–1810. doi: 10.1128/IAI.73.3.1797-1810.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski C.M., Yao R., Ewing C.P., Trust T.J., Guerry P. Evidence for a system of general protein glycosylation in Campylobacter jejuni. Mol Microbiol. 1999;32:1022–1030. doi: 10.1046/j.1365-2958.1999.01415.x. [DOI] [PubMed] [Google Scholar]
- Szymanski C.M., Goon S., Allan B., Guerry P. Protein glycosylation in Campylobacter. In: Ketley J.M., Konkel M.E., editors. Horizon Bioscience; 2005. pp. 259–274. [Google Scholar]
- Taboada E.N., Acedillo R.R., Carrillo C.D., Findlay W.A., Medeiros D.T., Mykytczuk O.L. Large‐scale comparative genomics meta‐analysis of Campylobacter jejuni isolates reveals low level of genome plasticity. J Clin Microbiol. 2004;42:4566–4576. doi: 10.1128/JCM.42.10.4566-4576.2004. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D.E., De Grandis S.A., Karmali M.A., Fleming P.C. Transmissible plasmids from Campylobacter jejuni. Antimicrob Agents Chemother. 1981;19:831–835. doi: 10.1128/aac.19.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault P., Logan S.M., Kelly J.F., Brisson J.R., Ewing C.P., Trust T.J., Guerry P. Identification of the carbohydrate moieties and glycosylation motifs in Campylobacter jejuni flagellin. J Biol Chem. 2001;276:34862–34870. doi: 10.1074/jbc.M104529200. [DOI] [PubMed] [Google Scholar]
- Vandamme P., Dewhirst F.E., Paster B.J., On S.L. Genus I. Campylobacter. In: Garrity G., Brenner D.J., Krieg N.R., Staley J.T., editors. Springer; 2005. pp. 1147–1161. [Google Scholar]
- Velayudhan J., Kelly D.J. Analysis of gluconeogenic and anaplerotic enzymes in Campylobacter jejuni: an essential role for phosphoenolpyruvate carboxykinase. Microbiology. 2002;148:685–694. doi: 10.1099/00221287-148-3-685. [DOI] [PubMed] [Google Scholar]
- Velayudhan J., Jones M.A., Barrow P.A., Kelly D.J. L‐serine catabolism via an oxygen‐labile L‐serine dehydratase is essential for colonization of the avian gut by Campylobacter jejuni. Infect Immun. 2004;72:260–268. doi: 10.1128/IAI.72.1.260-268.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vliet A.H., Wooldridge K.G., Ketley J.M. Iron‐responsive gene regulation in a Campylobacter jejuni fur mutant. J Bacteriol. 1998;180:5291–5298. doi: 10.1128/jb.180.20.5291-5298.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker M., Linton D., Hitchen P.G., Nita‐Lazar M., Haslam S.M., North S.J. N‐linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science. 2002;298:1790–1793. doi: 10.1126/science.298.5599.1790. et al. [DOI] [PubMed] [Google Scholar]
- Wacker M., Feldman M.F., Callewaert N., Kowarik M., Clarke B.R., Pohl N.L. Substrate specificity of bacterial oligosaccharyltransferase suggests a common transfer mechanism for the bacterial and eukaryotic systems. Proc Natl Acad Sci USA. 2006;103:7088–7093. doi: 10.1073/pnas.0509207103. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar J.A., Van Bergen M.A., Mueller M.A., Wassenaar T.M., Carlton R.M. Phage therapy reduces Campylobacter jejuni colonization in broilers. Vet Microbiol. 2005;109:275–283. doi: 10.1016/j.vetmic.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Wassenaar T.M., Bleumink‐Pluym N.M., Van Der Zeijst B.A. Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion. EMBO J. 1991;10:2055–2061. doi: 10.1002/j.1460-2075.1991.tb07736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenaar T.M., Van Der Zeijst B.A., Ayling R., Newell D.G. Colonization of chicks by motility mutants of Campylobacter jejuni demonstrates the importance of flagellin A expression. J Gen Microbiol. 1993;139:1171–1175. doi: 10.1099/00221287-139-6-1171. [DOI] [PubMed] [Google Scholar]
- Weerakoon D.R., Olson J.W. The Campylobacter jejuni NADH: ubiquinone oxidoreductase (complex I) utilizes flavodoxin rather than NADH. J Bacteriol. 2008;190:915–925. doi: 10.1128/JB.01647-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten R.A., Grimes J.L., Olson J.W. Role of Campylobacter jejuni respiratory oxidases and reductases in host colonization. Appl Environ Microbiol. 2008;74:1367–1375. doi: 10.1128/AEM.02261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. Bioenergetics in the cytosol. In: White D., editor. Oxford University Press; 2007. pp. 181–195. [Google Scholar]
- Wiesner R.S., Hendrixson D.R., Dirita V.J. Natural transformation of Campylobacter jejuni requires components of a type II secretion system. J Bacteriol. 2003;185:5408–5418. doi: 10.1128/JB.185.18.5408-5418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodall C.A., Jones M.A., Barrow P.A., Hinds J., Marsden G.L., Kelly D.J. Campylobacter jejuni gene expression in the chick cecum: evidence for adaptation to a low‐oxygen environment. Infect Immun. 2005;73:5278–5285. doi: 10.1128/IAI.73.8.5278-5285.2005. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wösten M.M., Wagenaar J.A., Van Putten J.P. The FlgS/FlgR two‐component signal transduction system regulates the fla regulon in Campylobacter jejuni. J Biol Chem. 2004;279:16214–16222. doi: 10.1074/jbc.M400357200. [DOI] [PubMed] [Google Scholar]
- Wright J.A., Grant A.J., Hurd D., Harrison M., Guccione E.J., Kelly D.J., Maskell D.J. Metabolite and transcriptome analysis of Campylobacter jejuni in vitro growth reveals a stationary‐phase physiological switch. Microbiology. 2009;155:80–94. doi: 10.1099/mic.0.021790-0. [DOI] [PubMed] [Google Scholar]
- Wyszynska A., Raczko A., Lis M., Jagusztyn‐Krynicka E.K. Oral immunization of chickens with avirulent Salmonella vaccine strain carrying C. jejuni 72Dz/92 cjaA gene elicits specific humoral immune response associated with protection against challenge with wild‐type Campylobacter. Vaccine. 2004;22:1379–1389. doi: 10.1016/j.vaccine.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Yan M., Sahin O., Lin J., Zhang Q. Role of the CmeABC efflux pump in the emergence of fluoroquinolone‐resistant Campylobacter under selection pressure. J Antimicrob Chemother. 2006;58:1154–1159. doi: 10.1093/jac/dkl412. [DOI] [PubMed] [Google Scholar]
- Young K.T., Davis L.M., Dirita V.J. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol. 2007;5:665–679. doi: 10.1038/nrmicro1718. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Plummer P.J. Mechanisms of antibiotic resistance in Campylobacter jejuni. In: Nachamkin I., Szymanski C., Blaser M.J., editors. ASM Press; 2008. pp. 263–276. [Google Scholar]
- Zilbauer M., Dorrell N., Wren B.W., Bajaj‐Elliott M. Campylobacter jejuni‐mediated disease pathogenesis: an update. Trans R Soc Trop Med Hyg. 2008;102:123–129. doi: 10.1016/j.trstmh.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Ziprin R.L., Young C.R., Stanker L.H., Hume M.E., Konkel M.E. The absence of cecal colonization of chicks by a mutant of Campylobacter jejuni not expressing bacterial fibronectin‐binding protein. Avian Dis. 1999;43:586–589. [PubMed] [Google Scholar]
- De Zoete M.R., Van Putten J.P., Wagenaar J.A. Vaccination of chickens against Campylobacter. Vaccine. 2007;25:5548–5557. doi: 10.1016/j.vaccine.2006.12.002. [DOI] [PubMed] [Google Scholar]


