Summary
The exploitation of microorganisms in natural or technological systems calls for monitoring tools that reflect their metabolic activity in real time and, if necessary, are flexible enough for field application. The Gibbs energy dissipation of assimilated substrates or photons often in the form of heat is a general feature of life processes and thus, in principle, available to monitor and control microbial dynamics. Furthermore, the combination of measured heat fluxes with material fluxes allows the application of Hess' law to either prove expected growth stoichiometries and kinetics or identify and estimate unexpected side reactions. The combination of calorimetry with respirometry is theoretically suited for the quantification of the degree of coupling between catabolic and anabolic reactions. New calorimeter developments overcome the weaknesses of conventional devices, which hitherto limited the full exploitation of this powerful analytical tool. Calorimetric systems can be integrated easily into natural and technological systems of interest. They are potentially suited for high‐throughput measurements and are robust enough for field deployment. This review explains what information calorimetric analyses provide; it introduces newly emerging calorimetric techniques and it exemplifies the application of calorimetry in different fields of microbial research.
Introduction
Multiplication and maintenance of biological matter is tied inherently to Gibbs energy dissipation in the forms of entropy (J K−1) and/or enthalpy (J) (von Stockar et al., 2006; 2007). In most of the studied microbiological processes the pressure is constant and the enthalpy is released or, in exceptional cases, consumed (Liu et al., 2001) as heat. This enthalpy/heat can be measured by a calorimeter. Because the Gibbs energy can be calculated from a known growth stoichiometry, calorimetry helps to identify the driving forces (enthalpic or entropic) of microbial growth (von Stockar and Liu, 1999). The more Gibbs energy is dissipated, the less will be available for biomass or product yield. In evolutionary terms, nature has had to find a compromise between high rates (high driving force) and high yields (low driving force). An important practical consequence of understanding the thermodynamics of microbial growth and product formation is that it allows the prediction of maximum possible yields (Heijnen, 1994; von Stockar et al., 2007), maintenance coefficients (Tijhuis et al., 1993), threshold concentrations and even maximum growth rates (Heijnen, 1999).
The heat itself depends on the stoichiometry of growth and product formation (it is a consequence of the first law of thermodynamics), whereas the heat production rate (J s−1, W) additionally depends on the process kinetics. The calorimetric measurement of this rate, combined with further analyses, thus provides insights into the stoichiometry and kinetics of a given bioprocess (bioprocess analysis).
The quantitative interpretation of calorimetric signals from complex systems involving species growing on complex substrate mixtures or even including food webs is difficult since it is almost impossible to balance the various enthalpy contributions. But even here, calorimetry may be extremely useful as it indicates metabolic changes in real time (von Stockar and Marison, 1989), thereby providing an ideal basis for bioprocess control. Besides the fast response times, the advantages of thermal sensors for bioprocess control include their robustness and the improved sensitivity achieved with any scale‐up because of the increasing ratio of heat‐producing volume to heat‐exchanging surface.
Measuring the heat production rate – requiring sophisticated measurement techniques at the bench scale – can develop into a significant problem at even larger scale when the heat exchange with the environment becomes negligible (i.e. adiabatic mode) (von Stockar and Marison, 1991). This means that most of the heat produced by the culture will raise the temperature and possibly deactivate the microbial catalyst, unless the bioreactor is equipped with appropriate cooling facilities. The quantitative knowledge of the microbial heat production rate is therefore crucial to successful bioreactor design.
The challenge of quantifying the sometimes relatively small biological heat production on the micro‐ or even nano‐watt scale spurred the development and optimization of the isothermal calorimeter (ITC). Due to its extraordinary sensitivity, this has contributed substantially to biochemical and biophysical research (Krell, 2008). Nevertheless, microcalorimetric research has hardly contributed to bioengineering progress in recent years. The reasons are: (i) difficult agitation and aeration in the microcalorimeter, (ii) complicated integration of additional microsensors like pH, redox, UV/VIS or pO2, and (iii) small sample volumes limited by the relatively small calorimetric vessel. These typically led to measurement conditions which often diverged in essence from those in the real process under consideration. Even so, the main weakness of conventional calorimeters is the low throughput which only allows relatively few bioprocesses to be followed in parallel whereas, for instance, optimizations of biotechnological strains are nowadays performed in plates with up to 1536 wells. Further restrictions are that (i) conventional calorimeters are not optimized for the specific requirements of bioprocesses and (ii) their integration into existing bioprocesses is difficult; whereas (iii) calorimeters are produced for a small market resulting in relatively high prices and (iv) they lack the robustness for application to field or dirty technical conditions. Therefore, calorimetry will never tap its full potential until at least some of these weaknesses are overcome.
Fortunately, recent trends in calorimetric developments such as:
megacalorimetry,
chip‐calorimetry (also called miniaturized, integrated circuit‐, nano‐calorimetry),
high‐throughput calorimetry (enthalpy arrays),
ultra‐sensitive calorimetry, and
photocalorimetry
promise a bright future for calorimetry as both scientific and technical sensor tools. The roots, the state of the art and the potential of calorimetry for microbial research and biotechnology will be discussed in the following sections.
Historical roots and modern trends in biocalorimetry
Heat has been a symbol of life and cold as a synonym for death since early historical times. This may explain why calorimetric measurements were made during the first studies that addressed the quantitative aspects of metabolic processes. So, ever since the first calorimetric experiments with guinea pigs more than 200 years ago by Crawford (1777) and Lavoisier (1780), it has been recognized that the heat produced from living matter is a real‐time measure of metabolic activity (Kleiber, 1961). The quantitative interpretation of the heat signal relies on the correlation between the growth stoichiometry and the heat signal via Hess' law. This quantitative view arose when Robert Mayer (1842) studied the physiology of horses and established the law of conservation of energy (The First Law of Thermodynamics). Max Rubner (1890), using a dog's metabolism, paved the way for the quantitative interpretation of biocalorimetric signals. He showed that in principle there were no thermodynamic differences between inanimate and living systems. Recently, quantitative calorimetry has become an even more important trend. Now it combines calorimetry with other measurements on/at‐line (Fourier transform infrared spectroscopy, dielectric spectroscopy, optical density, flow injection analysis, gas analysis) and/or off‐line (HPLC, enzymatic analysis of metabolites and products, flow cytometric analysis of cell states, etc.). It aims at verifying that a growth stoichiometry is complete or, if not, at calculating the missing components using enthalpy and elemental balances. Today the heat production rate can be quantitatively correlated to changes in metabolism and this holds true both for batch (Schumer et al., 2007) and for continuous cultivations (Maskow and Babel, 2001).
It is difficult to perform calorimetric experiments with microscopic living matter, but this is not due to their lower heat production rates per unit viable mass. In fact, exponentially growing methanogenic bacteria (900 W kg−1) (Schill et al., 1996) and yeasts (250 W kg−1) (von Stockar and Birou, 1989) for example are characterized by much higher specific heat production rates than for instance humans (1.3 W kg−1) (Jequier and Schutz, 1983), guinea pigs (3 W kg−1) (von Stockar and Marison, 1989) and lotus flowers (12 W kg−1) (Lamprecht et al., 1998). The difficulty rather results from the aqueous or heterogeneous environments for microbial life, media that have orders of magnitude higher heat storage capacity (water = 4200 kJ m−3 K−1; soil = 580–3100 kJ m−3 K−1) than air (1.2 kJ m−3 K−1) (T = 298 K, P = 101 325 Pa) together with the usually low volume fraction of heat‐producing microorganisms in the calorimetric vessel.
As early as 1856, Pierre Dubrunfaut had found an impressively simple solution to the problem and he is now by repute the father of the very topical megacalorimetry, i.e. calorimetry in reaction tanks of hundreds of litres or even m3 used for bioprocess control, which amounts to the second recent trend in calorimetry. Dubrunfaut performed an alcoholic fermentation of 2559 kg of sugar in an oak vessel of 21 400 l volume (Dubrunfaut, 1856). In this way he maximized the ratio of heat‐producing volume to heat‐exchanging surface. Closing the enthalpy balance, he calculated a fermentation heat of −94.9 kJ mol−1, a number surprisingly close to the now established value of −138.6 kJ mol−1 (von Stockar et al., 1993). New, more sensitive temperature probes, less temperature‐dependent electronic devices and progress in real‐time data acquisition facilitated the development of megacalorimetry. Megacalorimetry was already in 1991 suggested as a method for controlling large‐scale bioreactors (von Stockar and Marison, 1991). The present sensitivity of megacalorimetry at the pilot scale (0.3 m3) using PT 100 temperature probes (digital resolution 5 mK) (Anderson et al., 2002; Voisard et al., 2002) is approximately 50 mW l−1. A successful test of the practicability of the megacalorimetry measurement principle was performed in 2004 with baker's yeast at the 100 m3 scale (Türker, 2004). Newer research showed that the principle is not only applicable to bioreactors in the m3‐scale but also to bench‐scale bioreactors ‘off the peg’ (litre scale) (Schubert et al., 2007).
Whereas up‐scaling reduces the influence of environmental fluctuations, reduction in calorimeter size diminishes the amount of heat energy needed for heating the calorimetric device while speeding up the measurement. Developments towards short response and equilibration times, e.g. chip‐calorimetry (resulting in miniaturized, nano‐, and integrated circuit – IC calorimetry) are a third recent trend in calorimetry development. The principle of chip‐calorimetry is illustrated in Fig. 1. The first thin‐film chip‐calorimeter was developed during the early seventies (Greene et al., 1972) but recently new techniques of printed circuit design have boosted these developments, as testified by more than 70 publications in the last decade. As IC calorimeters nowadays measure a few nW, they are also called nano‐calorimeters. The expected progress in chip‐calorimeter development should lead to: (i) extremely small thermal detectors, (ii) minute sample mass requirements, (iii) increased sensitivity and (iv) much shorter response and equilibration times. Note that a small detector size is also crucial for the integration of calorimetric sensors into established bioreactors.
Figure 1.
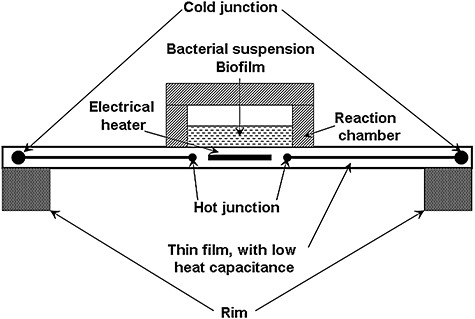
Measurement principle of a chip‐calorimeter.
In 2000 a micro‐machined calorimeter for the measurement of heat from as few as 106 eukaryotic cells was presented (Verhaegen et al., 2000). Two years later, Johannessen even measured calorimetrically the metabolism of individual cells in a volume of 0.72 nl (Johannessen et al., 2002). However, ultra‐low sample volume calorimeters for bioprocess control suffer from their low volume‐specific sensitivity. For example, the detection limit of the Johannessen calorimeter was only 18 W l−1. The newest developments of chip‐calorimeters achieve specific detection limits (Higuera‐Guisset et al., 2005) of 0.2 mW l−1 which are similar to that of the conventional microcalorimeter (0.3 mW l−1) (Lerchner et al., 2007).
The detection limit of 0.2 mW l−1 corresponds to an oxygen uptake rate of 1.2 × 10−8 g l−1s−1 (using the oxycaloric equivalent – see section The additional benefit of calorespirometric ratios below) or a substrate consumption rate of 1.1 × 10−8 g l−1s−1 (assuming aerobic combustion of glucose). For comparison, alternative monitoring technologies should be able to follow an hourly change in dissolved oxygen or glucose of less than 43 µg l−1 (approximately 0.5% h−1) or 40 µg l−1 respectively.
A weakness of the Higuera‐Guisset calorimeter is the relatively huge chamber volume of 600 µl. The chip‐calorimeter in one authors' lab fulfils the technical requirements for bioprocess control (2 mW l−1) with a 6 µl measurement chamber for most economic sample consumption (Lerchner et al., 2008a). Figure 2A shows substrate degradation and the formation characteristics of biomass and side‐products. The heat production rate can be calculated in advance from such data. The comparison of the calculated with the measured data (Fig. 2B) demonstrates clearly that such a nano‐calorimeter works well even at low biomass concentrations (> 1 mM = 0.025 g l−1) (Maskow et al., 2006a).
Figure 2.
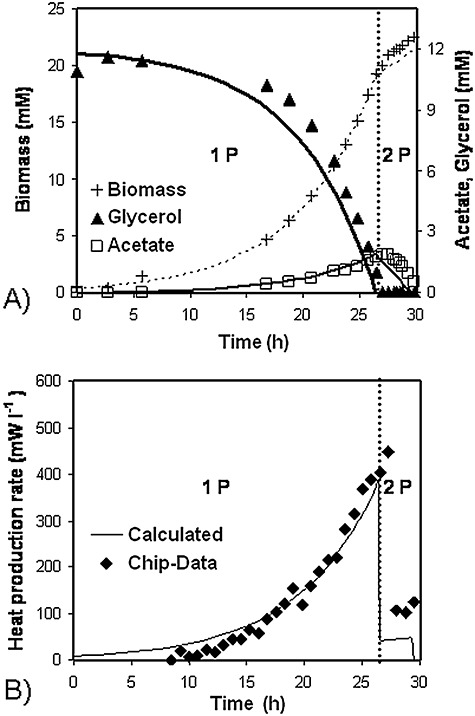
Testing a chip‐calorimeter with suspended E. coli DH5α cells aerobically growing on glycerol. Points and lines represent measurements and results of the thermokinetic modelling, respectively, in (A) and (B). During the first phase (1 P) the strain grows on glycerol and produces acetate whereas in the second growth phase (2 P) acetate is used as sole source of carbon and energy (Maskow et al., 2006a).
At present, even the most advanced calorimeters are too slow to compete with the robotic monitoring of metabolic activities in the 384‐ or 1536‐well microtitre plates that are used as diagnostic tools in the pharmaceutical industry and biotechnology. Multichannel calorimeters or so‐called enthalpy arrays would overcome this deadlock. In the early seventies, the identification of microorganisms using their characteristic heat production profiles was tried (Boling et al., 1973) and for that purpose a 50‐channel microcalorimeter was then developed by Instrumentation Laboratory (Lexington, MA, USA) (Russell et al., 1975). In the mid‐1990s Takahashi developed a 25‐channel instrument (Antoce et al., 1997) which was applied to studies on microbes (Koga et al., 2001). More recently, Thermometric (today TA Instruments) launched a 48‐channel instrument that was originally designed for stability/compatibility investigations of explosives or pharmaceutical agents. A Chinese group led by Jun Yao is extensively using this device for research into the toxic effects of foreign substances on microbes (for example see Yao et al., 2007). A fourth recent trend in biocalorimetry is thus the development of high‐throughput calorimeters. In 2004, Torres and colleagues (2004) introduced the term ‘Enthalpy Array’ and combined chip‐calorimeters in such a way that they were able to monitor 96 biochemical reactions in parallel. The company Vivactis (Vivactis NV, Leuven, Belgium) has developed a similar technology called microplate differential microcalorimetry (MIDICAL™) to follow 96 bioreactions in parallel. Both techniques have not been tested yet with microbial biocatalysts. It is the fact that microorganisms need to be surrounded by a suitable aqueous medium (of high heat capacitance and vaporization enthalpy) that influences measurement accuracy and requires further optimization. More than 10 years ago, an infrared imaging of 96‐well microtitre plates was used to measure the heat production of yeast and human adipocytes treated with thermogenic agents such as uncoupling protein‐2 (Paulik et al., 1998). The early results were promising but commercialization by Thermal Bioimaging, Cambridge, MA, USA – now Thermionic Imaging of the instrument failed because a way was not found to prevent vaporization in the plates. Another multiwall approach has been to microfabricate a thermoelectric device in which cells and related macromolecules are integrated with a thermopile (Page and Pizziconi, 1997;Pizziconi and Page, 1997). The model interactive system was the animal mast cell recognition of immunoglobulin A. In the bottom of microwells containing culture medium, the cells were stuck by fibronectin to only the active and not the reference junctions of thermopiles constructed of thin films of antimony and bismuth metals which gave the thermocouples. Activation by the calcium ionophore A23187 gave a heat production rate comparable to that obtained by conventional microcalorimetry. So far, reliability and reproducibility have proved to be insurmountable hurdles. However, it can be seen as a precursor to arrays of chip‐calorimeter. In the following, the information content of calorimetric signals and a few recent applications will be discussed in more detail.
Information content of calorimetric signals
Stoichiometry and kinetics of microbial growth and product formation
An engineer designing a biotechnological process or an environmental engineer assessing pollutant biodegradation requires knowledge of the bioconversion stoichiometry and kinetics. Both characteristics are tightly connected with the heat production rate. However, to analyse this correlation, the dynamic energy balance for the open technological or natural system of interest needs to be known. As early as 1993, von Stockar et al. described how to establish a dynamic energy balance for such a system (von Stockar et al., 1993).
Applying the dynamic energy balance, the reaction heat can be calculated. Under ideal conditions, the heat production rate, P, reflects only the progress of all the ongoing metabolic or chemical reactions (Eqn 1):
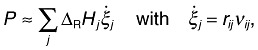 |
1 |
where rij and νij are the rate and the stoichiometric coefficient, respectively, of the component i participating in reaction j. The heat of non‐metabolic reactions, e.g. neutralization heats, can be obtained by the titrimetric consumption of alkali or acid using tabulated values for correction (von Stockar et al., 1993; Meier‐Schneiders et al., 1995b). The metabolic heat production rate can then be correlated with the stoichiometry of the microbial growth and the processes of product formation. The microbial growth reaction in its simplest aerobic case can be described stoichiometrically by Eqn (2):
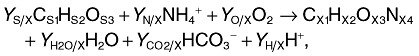 |
2 |
where CS1HS2OS3 is the applied carbon source, NH4+ and O2 stand for the nitrogen source and the terminal electron acceptor, respectively, and CX1HX2OX3NX4 is the elemental composition of the biomass. The elemental composition of bacteria has been averaged to C1H2O0.5N0.25 (Babel and Müller, 1985) or more recently to CH1.84O0.53N0.23 (Stephanopoulos et al., 1998). Both values are good proxies for stoichiometric considerations if no exact elemental composition is available. The six unknown yield coefficients (YS/X, YN/X, YO/X, YH2O/X, YCO2/X, YH/X) in Eqn (2) are not independent of each other because they have to satisfy five balances (four elemental balances and one charge balance). This means that adding the enthalpy balance and measuring the reaction enthalpy of the growth process ΔRHx allows the calculation of each of the unknown yield coefficients. Two reference states for the enthalpies are commonly used for this purpose, namely the constituent elements of all the involved species (enthalpies of formation) and the completely combusted state (enthalpies of combustion, ΔCH). Using the latter simplifies the enthalpy balance (Eqn 3):
| 3 |
The combustion enthalpies of each chemical species (Domalski, 1972) and also of the microbial biomass growing under different conditions (Cordier et al., 1987) are tabulated in the literature.
The reaction heat of the growth process varies between +90 kJ C‐mol−1 biomass (endothermic methanogenesis from acetate by Methanobacterium soehngenii (von Stockar and Liu, 1999) and −3730 kJ C‐mol−1 biomass [extreme exothermic methanogenesis from H2 and CO2 by Methanobacterium thermoautotrophicum (Schill et al., 1999)]. A typical value for the oxidative metabolism of Saccharomyces cerevisiae is −190 kJ C‐mol−1 (von Stockar and Birou, 1989). In the case of chemostatic growth, Eqn (1) reads:
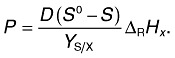 |
4 |
The dilution rate D and the substrate concentration of the reactor input S0 are predefined by the operator. The residual substrate concentration S is usually low in comparison with S0 in chemostatic bacterial or yeast cultures and can thus be neglected in enthalpic and stoichiometric balances. Therefore, the measured P reflects ΔRHx and, using the balances of the enthalpy and the elements, provides the real‐time stoichiometry.
Any conventional bioreactor can easily be used as a calorimeter simply by equipping it with a highly sensitive temperature probe (ΔT < 1 mK) and applying dynamic energy balances (Schubert et al., 2007). Such a solution is much cheaper and more flexible than conventional reaction calorimeters or the tailor‐made fermentation calorimeters described by Meier‐Schneiders et al. (Meier‐Schneiders et al., 1995a; Meier‐Schneiders and Schäfer, 1996). Such an improvised calorimeter works with a sensitivity of approximately 50 mW l−1 (Schubert et al., 2007).
The additional benefit of calorespirometric ratios
An old, simple and well‐established approach to characterize metabolic activities of microorganisms in the environment or in instruments is the measurement of the oxygen uptake rate (OUR, rO2 in mol‐O2 l−1 h−1) and carbon dioxide evolution rate (CER, rCO2 in mol‐CO2 l−1 h−1). Additional information can be gained by combining calorimetric measurements with respirometric data (i.e. OUR and CER). In 1917, Thornton (1917) plotted the combustion enthalpy of multiple organic compounds versus the degree of reductance γi (Eqn 7) and found a good linear correlation [the slope depending on the applied data base 107–120 kJ e‐mol−1 (Cordier et al., 1987)].
| 5 |
γi in Eqn 5 is related to 1 C‐mol of the compound i containing eHi hydrogen atoms, eOi oxygen atoms and eNi nitrogen atoms. In this concept all carbon compounds are arranged from γi = 0 (CO2, the most oxidized compound with the lowest energy) until γi = 8 (CH4, the most reduced compound with the highest energy).
This means that there is a tight correlation between the heat evolved (rq in W) and the OUR (O2 accepts four electrons). This ratio (rq/rO2) is called the oxycaloric equivalent (Gnaiger and Kemp, 1990) and usually attains values between −430 and −480 kJ mol‐O2−1 with an averaged value of −455 ± 15 kJ mol‐O2−1 (Gnaiger and Kemp, 1990). The oxycaloric equivalent is widely used in connection with indirect calorimetry (i.e. calculation of the enthalpy from OUR measurements). Because in aerobic metabolism, oxygen is predominately consumed during the electron transport phosphorylation, the OUR and thus the calorimetric signal are considered to reflect the catabolic side of the metabolism. The monitoring of both heat production rate and OUR has the potential to reveal product formations because the oxycaloric equivalent depends on the class of biologically converted compounds (Kemp, 2000). Additionally, any strong deviation in the oxycaloric equivalent indicates clearly either partially anaerobic metabolism or anaerobic zones in the considered bioreactor or ecosystem. Note that any oxidation (in the electron transport phosphorylation or via oxydoreductases) has also to fulfil approximately the oxycaloric equivalent except when peroxides are the oxidation products.
Contrary to the oxycaloric equivalent, the ratio of the heat released to CER (rq/rCO2) depends on the degree of reductance γS of the organic compound S undergoing combustion (Eqn 6) (Hansen et al., 2004):
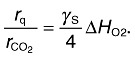 |
6 |
In the case that the energy from the catabolic process is used to drive anabolic processes, then the ratio of heat production rate to the CER provides information on the yield coefficient YX/S (Hansen et al., 2004) (Eqn 7).
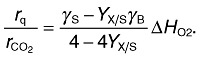 |
7 |
For that purpose, it is necessary to estimate the relative degree of reductance for biomass γX. This is facilitated by the fact that the elementary composition of biomass and thus the degree of reductance are nearly independent of the bacterial strain or of the growth condition (Stephanopoulos et al., 1998).
In summary, the calorespirometric ratios measured for live microorganisms, tissues or complex environmental samples (e.g. soil) typically deviate from predicted oxycaloric equivalent and contain information about the coupling between catabolic and anabolic processes. In combination with biochemical models it is possible even to obtain information about the metabolic pathways. Thus, interpretations of calorespirometric ratios require a biochemical model. For instance, there are reports of calorespirometric values as large as nearly −1100 kJ mol‐O2−1 (Schön and Wadsö, 1988) which have been ascribed to anaerobic metabolism leading to the production of lactate under aerobic conditions.
Modern application fields
Real‐time analysis of whole‐cell biotransformations
In addition to the above, the ratio of the heat production rate rq to the substrate consumption rate rS contains information about the yield coefficient and, hence, about the growth stoichiometry (Eqn 8).
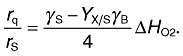 |
8 |
For instance, the switch in S. cerevisiae from oxidative (−190 kJ C‐mol−1) to fermentative (−11.6 kJ C‐mol−1) metabolism (von Stockar and Birou, 1989) or even the energetically much smaller switch in bacteria from phenol assimilation via the ortho pathway (−361 kJ C‐mol−1) to the meta pathway (−313 kJ C‐mol−1) (Maskow and Babel, 1998) can be detected calorimetrically in real time. The metabolic main fluxes can be estimated by connecting all the fluxes leaving and entering the cell calculated from the yield coefficients with a metabolic map. The maps can be taken from books (Michal, 1999) or internet resources such as http://www.biocyc.org or http://www.genome.jp/kegg/. The potential of calorimetry for online stoichiometry and online analysis of the main metabolic fluxes will be illustrated with the following example.
Halomonas elongata maintains an osmotic balance by synthesis and accumulation of 5,6‐tetrahydro‐2‐methyl‐4‐pyrimidinecarboxylic acid (ectoine). It was continuously cultured in a fermenter calorimeter in which the salt concentration was increased linearly. All other factors influencing growth including D and S0 were kept constant. Equation 4 predicts there will be a constant heat production rate, because it does not account for the energy expenditure of osmoadaptation. However, Fig. 3 shows that heat production rate in fact decreased in two phases, a linear first one followed by a more drastic drop. Whilst the sharp reduction in heat production rate during the second phase reflected the increasing residual substrate, the initial linear decrease could only be explained assuming a changed growth stoichiometry and consequently changed metabolic fluxes.
Figure 3.
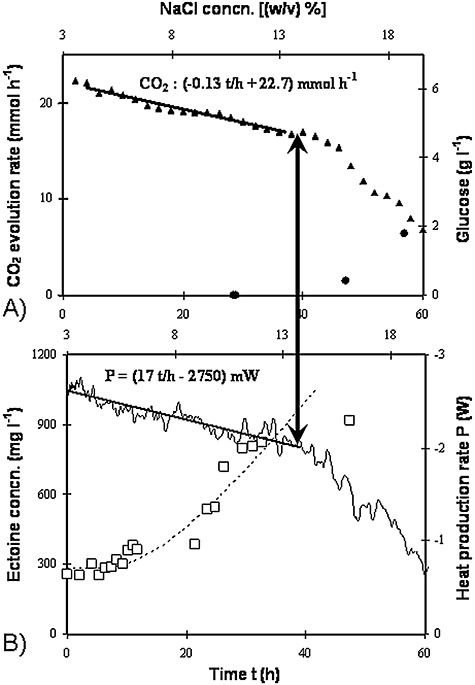
The transient growth pattern of H. elongata growing on glucose with increasing sodium chloride concentration, as functions of time (primary abscissa) and NaCl concentration (secondary abscissa). The gradient started from chemostatic growth [dilution rate, 0.071 h−1; input concentration, 10 g l−1 glucose; salt concentration, 3 (w/v)%]. A. Closed triangles, CO2 evolution rate; bold line, interpolation of CO2 release; closed circles, residual glucose concentration. B. Thin line, heat production rate; bold line, interpolation of heat production rate; dotted line, course of ectoine content assuming a linear increase in its rate of formation; open squares, measured ectoine concentration (Maskow and Babel, 2001).
Applying balances of substances (substrate, nitrogen source, ectoine‐free biomass, ectoine) and elements (carbon and nitrogen), the best description of the haloadaptation was obtained assuming constant changes in ectoine formation (Eqn 9) and growth rate (Eqn 10) vs. time t:
| 9 |
| 10 |
The closed enthalpy balance achieved by applying the calorimetrically determined heat production rate shows that the ectoine production rate is indeed the main haloadaptation process in the survival of H. elongata. Other generally known adaptation processes, e.g. synthesis of other compatible solutes, enzymes, increased energy‐driven transport processes, especially for Na+, or changes in the cell wall, seem less important for H. elongata, at least from the energetic point of view.
The increase in ectoine formation rate of 2.6 mg l−1 h−2 at the expense of only 1.6 mg l−1 h−2 lesser biomass formation rate is only possible assuming that the carbon substrate glucose was converted into ectoine with approximately 100% carbon conversion efficiency (CCE) (Maskow and Babel, 2001). The maximum theoretically possible CCE can be calculated by combining the known pathway for the synthesis of ectoine from the metabolites aspartic β‐semialdehyde and acetylCoA (Peters et al., 1990) with the different known glucose assimilation pathways, i.e. those of Emden–Meyerhof–Parnas (EMP) and Entner Doudoroff (ED). The precursor oxalacetate (leading to aspartic β‐semialdehyde) can be synthesized: (i) through heterotrophic CO2 fixation or (ii) via the glyoxylate shunt. The respective balance equations are:
(i) pyruvate carboxylation
EMP:
| 11 |
ED:
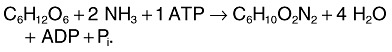 |
12 |
(ii) glyoxylate shunt
EMP:
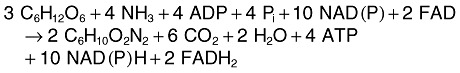 |
13 |
ED:
 |
14 |
Comparison of the balance equations (i) and (ii) shows that Eqn (11) and Eqn (12) can be understood as linear combinations of two times Eqn (13) and Eqn (14), respectively, plus the respective biological combustion of a third glucose molecule, in total to provide the energy required for the biomass synthesis. Therefore, growth‐associated ectoine formation occurs without net carboxylation and comparison of Eqn (11) and Eqn (12) shows that the CCE (approximately 100%) is nearly independent of the glucose assimilation pathways that differ only slightly in the energy expenditure. Thus, the selected examples appear to confirm that calorimetry provides not only information on growth stoichiometry and kinetics but furthermore it allows some conclusions to be drawn about the metabolic fluxes in combination with metabolic maps.
Real‐time control of whole‐cell biotransformations
A further scrutiny of Fig. 3 shows that the boundary between the first growth phase (haloadaptation) and the second one (increase in the residual substrate) coincided with the maximum ectoine production rate. The boundary was indicated by a change in the slope of the heat production rate. If this phenomenon is a rule rather than an exception, it can be used principally to control bioprocesses in such a way that the maximum quantity of the assimilated carbon flows into the desired product and the residue is just sufficient to compensate for the dilution of the microbial catalyst. This was successfully tested for the continuous conversion of phenol into ectoine with Halomonas sp. EF11 (Maskow and Kleinsteuber, 2004). As with glucose as the substrate, the maximum ectoine formation rate coincided with a change in the heat production rate. This principle can also be applied to control the continuous biosynthesis of other stress‐induced products. For instance, it was shown for the synthesis of a product of an overflow metabolism (i.e. PHA) (Maskow and Babel, 2000). This confirmed that calorimetry can be used to control, stabilize and maximize the microbial conversion of toxic substrates into target products. The application of the described solutions for bioprocess control requires the envelopment of the bioreactor by a calorimetric system to form a complete heat‐balanced bioreactor – CHB.
An alternative would be to continuously sample and calorimetrically monitor the culture in a measuring loop (ML), which is an especially interesting way to monitor small‐ and medium‐sized bioreactors (Fig. 4). It is also highly flexible because it allows the connection of a flow‐through calorimeter or chip‐calorimeter with any type of bioreactor. Two possible disadvantages of employing a calorimetric sensor in a ML are clogging and microbial settlement on the inner surfaces of the tubing. The most serious drawbacks, however, result from metabolic processes that continue during transport in the transmission tubing, but become rate‐limiting and thereby influence the calorimetric signal. As an example, during batch or fed batch processes the depletion of oxygen in the flow line is a major problem. This could be solved though for a low‐density culture of S. cerevisiae growing on glucose by the introduction of air bubbles into the flow in the ratio of 1:1 (Larsson et al., 1991). In a bacterial chemostat culture, however, it is not the oxygen but rather the residual carbon substrate that is consumed in the transmission tube and thus biases the calorimetric signal. This is difficult to counteract but it can be described mathematically to interpret the signal which is then employed for bioprocesses control (Maskow et al., 2006b). The results of this simulation show that: (i) the flow‐through calorimeter measures just a part of the real heat production rate, and (ii) the discrepancy between the heat production rate measured in the CHB and the value measured in the ML diminishes with the decreasing length of the transmission tubing and when D (dilution rate of the chemostat) approaches the maximum specific growth rates. The increased channelling of substrate carbon into a target product (examples are overflow metabolites formed at increasing C/N ratios or compatible solutes formed at high salinity) leads to the decreasing availability of carbon and energy for growth and reduces the maximum specific growth rate. It is predicted that as soon as the specific growth rate equals the dilution rate, there is a rapid increase in the measured heat production rate, because the residual substrate is metabolized in the thermal sensor and not previously consumed in the transmission tubing leading to it. This behaviour is independent of the toxicity of the carbon substrate. It is perhaps the most important result of the simulation, because this behaviour opens the opportunity to keep bioprocesses at the point of maximum channelling of the carbon substrate into the target product. Figure 5 shows that these predictions are correct. Software maintained the dilution rate at 0.091 h−1 and the phenol supply rate was enhanced until there was a jump in the heat production rate. At this point, the phenol supply rate was reduced for a 15 min period. Afterwards, the process was continued with the phenol supply rate returning to its pre‐intoxication level (see Fig. 5). Finally, after the defined time interval, the programme increased the phenol supply rate slightly until the next sudden rise in the heat production rate. In this way the process was maintained for several weeks at approximately 78% of the phenol assimilation rate, 100% of the biomass formation rate and 72% of the product formation rate (i.e. poly‐3‐hydroxybutyrate – PHB) compared with the independently determined optimum rates (Fig. 5 shows only the first 4 days).
Figure 4.
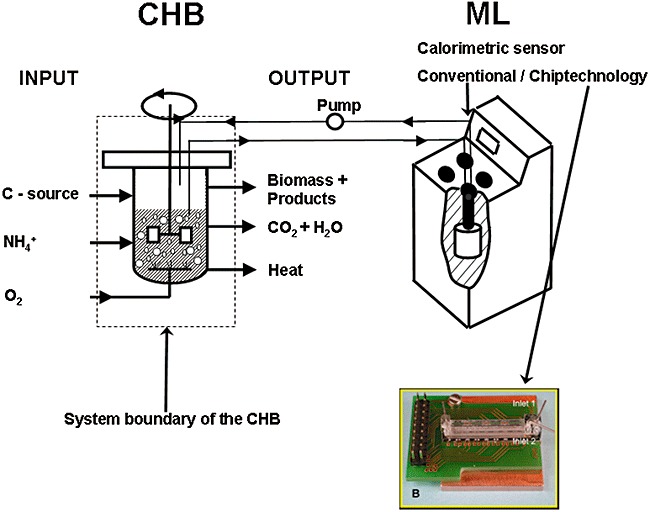
Experimental set‐up combining a complete heat‐balanced bioreactor (CHB) with a flow‐through calorimeter as part of a measuring loop (ML) (Maskow et al., 2004; 2006b).
Figure 5.
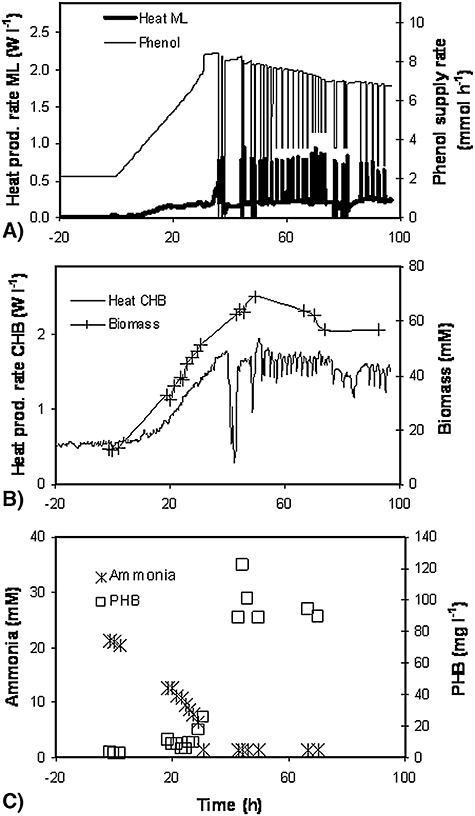
Development of various parameters during heat production rate controlled continuous conversion (D = 0.091 h−1, VReactor = 2.2 l) of phenol into biomass and PHB by Variovorax paradoxus. The phenol concentration gradient was started at zero time (Maskow et al., 2006b).
Another way to keep the process at the maximum productivity is to apply small pulses of the carbon substrate and the chip‐calorimetric monitoring of the chemostat response (T. Schubert, F. Lerchner, H. Harms, T. Mashow, unpublished). In a chemostat operating under carbon‐limited conditions at dilution rates far from the maximum specific growth rate, the carbon substrate concentration is usually low. A substrate pulse then will lead to a rapid increase in substrate concentration, in an enhanced assimilation rate and finally in an elevated heat production rate. However, chemostats operating at their maximum capacity (i.e. the dilution rate equals to the maximum specific growth rate) cannot respond to the substrate pulse. Figure 6 shows the respective modelling results and chip‐calorimetric experiments, which confirm the potential of such a calorimetric technique to control continuous bioprocesses. The substrate pulse can be added to the bacterial suspension on the calorimeter chip to prevent any disturbance of the controlled bioprocess.
Figure 6.
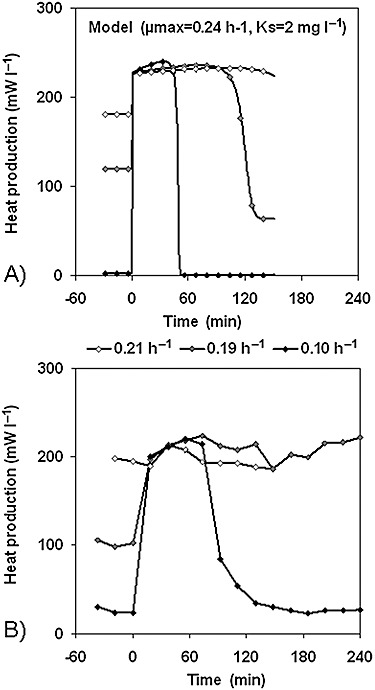
The influence of substrate pulses (1 mM glucose) on the heat production rate of a continuous culture of Halomonas elongata[S0 = 6.67 mM; NaCl = 5 (w/v)%] growing at different dilution rates on glycerol. A. Modelling results. B. Experimental results.
Analytical potential for biodegradation processes with potentially low heat production rate
Many environmental scientists currently are interested in heterotrophic denitrifying and autotrophic nitrifying activities or processes of biogas generation. Such processes often generate extreme low amounts of heat. For analysing such processes, ultra‐sensitive bench‐scale calorimeters were developed (Garci'a‐Payo et al., 2002) and successfully applied to analyse endothermic processes during biogas generation (Liu et al., 2001) as well as extremely low energy‐generating waste water treatment processes (Aulenta et al., 2002). A further field requiring extremely sensitive calorimeters is the degradation of micropollutants. The interest in the latter is caused by their ubiquity in the environment, their apparent persistence and their potentially deleterious effect on human health. The problems in measuring degradation kinetics arise not only from the low water solubility of many of these compounds but also from their trend to interact with the surfaces of bioreactors or sampling devices. This makes classical, conventional analytics difficult and may lead to substantial errors. A way out could be to monitor the process by calorimetry. Unfortunately, the biodegradation of these compounds often proceeds so slowly (< 0.5 µmol l−1 h−1) or the concentration changes are so small (< 0.3 µmol l−1) that the resulting specific reaction heat or specific heat production rate is on the order of few J l−1 or mW l−1, respectively, which thus makes measurements difficult. However, by combining the advantages of isothermal titration calorimetry (ITC) with a newly developed thermokinetic model, Buchholz and colleagues (2007) were able to derive parameters from the thermal signal of trace pollutants (180 ng or 60 µg l−1 anthracene) (Fig. 7). The reproducibility of the kinetic parameters was better than of those obtained by conventional HPLC analysis (Table 1). Furthermore, the biodegradation progress was recorded online without sampling or sample preparation.
Figure 7.
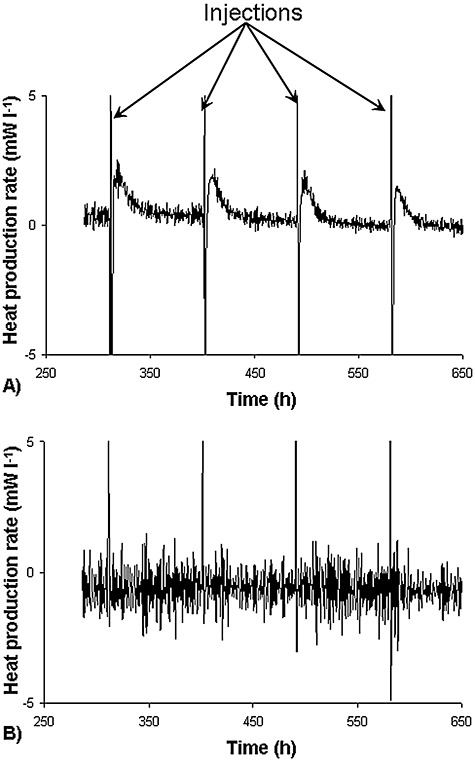
Heat production rate (P) response by Mycobacteriumfrederiksbergense LB501T (A) and by a sterile reference solution (B) on addition of 180 ng anthracene (60 µg l−1) (Buchholz et al., 2007).
Table 1.
Comparison of the kinetic parameter of anthracene biodegradation by Mycobacterium frederiksbergense LB501T determined calorimetrically and by conventional analytics for different salt contents (Buchholz et al., 2007).
| Salt content (g l−1) | Calorimetry | Conventional analytics (HPLC) | ||
|---|---|---|---|---|
| qmax (mol mg‐Protein−1 h−1) | ks (mol l−1) | qmax (mol mg‐Protein−1 h−1) | ks (mol l−1) | |
| 0 | (9.0 ± 0.3) × 10−8 | (3.2 ± 0.3) × 10−7 | (12.2 ± 3.7) × 10−8 | (4.6 ± 0.7) × 10−7 |
| 20 | (5.3 ± 0.6) × 10−8 | (3.5 ± 0.3) × 10−7 | (8.4 ± 1.0) × 10−8 | (3.3 ± 0.8) × 10−7 |
| 40 | (4.3 ± 0.4) × 10−8 | (3.2 ± 0.1) × 10−7 | (3.6 ± 0.6) × 10−8 | (3.1 ± 0.6) × 10−7 |
Mariana and co‐workers showed recently (F. Mariana, F. Bucholz, H. Harms, U. Szewzyk, T. Mashow, unpublished) that this principle also can be applied to different bacterial strains that degrade other pollutants. Even the degradation of substances that are not totally mineralized can be quantified in such a way and the thermokinetic evaluation can be applied to estimate the achieved degree of mineralization.
Ecological calorimetry
The available energy and the energy transfer from one trophic level to the next are important factors shaping the structure and biodiversity of ecosystems. The fraction of energy at one trophic level that passes on to the next higher level (ecological efficiency) is usually low – in the order of 5–20% (Lindeman, 1942) and decreases with higher trophic levels (Burns, 1989). Calorimetry may not only measure the energy loss during passage between trophic levels and the energy contents of predator and prey. It may also reveal the energy conversion in real time. These advantages were recognized for macrobiotic organisms many years ago and indirect calorimetry was applied to measure it. However, such investigations on microorganisms are rare these days. In a pioneering study therefore, Guosheng and colleagues (2003) measured the heat production rate of an Escherichia coli strain infected by the virulent T4 phage. Such phages kill the infected bacteria via the lytic cycle and produce 50 and more new phages per infected cell. The authors did not take the next step to interpret the data quantitatively with respect to changes in the growth stoichiometry and kinetics. From an ecological point of view, bacteria and their phages are extremely important because they represent the most abundant, widespread and diverse group of biological entities on the planet (5 × 1030 bacteria on the planet and 10 times more phages). Metabolically active bacteria are the main target for phage attacks and are killed by phage infections, thus maintaining the balance in the ecosystems. Temperate phages following the lysogenic cycle are also highly important. In these cases, the viral genome is integrated into the host DNA as a so‐called prophage and normally multiplied together with the host DNA. The host strain produces phages only after exposure to stress. The lysogenic infection cycle was investigated calorimetrically with the intention of relating the measured heat production rate with changes in the growth stoichiometry and kinetics. Figure 8 compares the growth pattern of E. coli growing on glucose and treated with methotrexate as the inducer of phage production. The investigated E. coli strain carries the genome of a temperate phage (i.e. λ phage).
Figure 8.
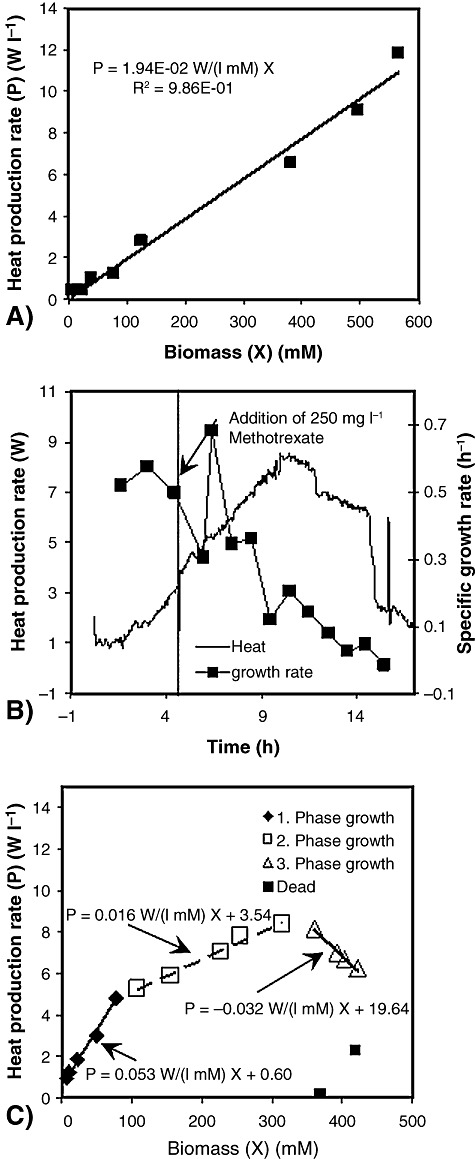
The growth pattern of E. coli carrying the genome of a λ phage. A. Specific heat production rate without methotrexate. B. The heat production rate and the specific growth rate upon addition of 250 mg l−1 methotrexate. C. The specific heat production rate on the addition of 1100 mg l−1 methotrexate (T. Maskow, B. Kiesel, T. Schubert, Z. Yong, C. Rerat, H. Harms, J. Yao, unpublished).
Figure 8 demonstrates clearly the ability of calorimetry to reflect changes in the growth stoichiometry (specific heat production per mol biomass) and kinetics due to phage infection. Different growth phases can be clearly distinguished (i.e. 1. growth with prophages; 2. growth during the hijack by the phage of the replication engine; 3. growth after release of the phages; 4. death). It was shown that calorimetry is superior to the other monitoring tools because it can follow infections in real time.
Analysis of immobilized cells and biofilms
In the past, nearly all calorimetric measurements have addressed planktonic microorganisms, whereas an estimated 90% of all microorganisms live in biofilms. This surprising disproportion is caused by the demands of treating the thermodynamic data from immobilized biomass. Comparatively recent data from pioneering calorimetric measurements have not been completely exploited in quantitative terms (Wentzien et al., 1994; von Rege and Sand, 1998). Traditional biofilm experiments are usually carried out in flow lines. Therefore the transfer of this technique to calorimetry requires biofilm cultivation in flow‐through calorimeters. It has allowed the detection of biofilm poisoning (Wentzien et al., 1994; von Rege and Sand, 1998) and detachment (Peitzsch et al., 2007) within a few minutes. The weaknesses of biofilm investigations with conventional calorimeters have been (i) the costs of the calorimeters, (ii) their inadequacy for high‐throughput measurements, (iii) the occupation of the instruments for weeks of biofilm cultivation, and (iv) the restrictions regarding the substratum for biofilm growth due to the required compatibility with calorimetric measurements. The recently emerging chip‐calorimetric technique overcomes these weaknesses by employing exchangeable flow‐chambers. Today, using a chip with four thermopiles, heat production rates can be determined with spatial and temporal resolutions of 2.5 cm−1 and 2 s−1 (Lerchner et al., 2008b). With the achieved detection limit of 20 nW, already it is possible to analyse early‐stage biofilms (approximately 3 × 105 cells cm−2).
Inclusion of light energy in calorimetric measurements
Considering the importance of photosynthesis in nature, it is surprising that its energetics rarely has been probed by direct calorimetry. Probably this is because of the unintentional introduction of heat with the light required to drive the photosynthesis. In microcalorimetry, it is difficult to balance the incident light from a single source split prior to entering the reference and measuring vessels by light guides. It may be that carefully matched light‐emitting diodes (LEDs) will solve the problem. Banks of them were used in bench‐scale calorimetry, but difficulties arise from light escaping from the bioreactor at low biomass early in a batch culture as well as from the challenge to deliver enough light to the biological material later in the growth process. However, the largest problem is keeping the photobioreactor thermally isolated from its surroundings. All potential future photocalorimetrists should be aware that only a minor fraction of the sun's energy absorbed by phototrophs is stored in photosynthate, while most of it is dissipated as heat.
As reviewed in 2006 by Mukhanov and one of the authors (Mukhanov and Kemp, 2006), microcalorimetry with a light source has only made a minimal contribution thus far to our knowledge of photosynthesis. These authors used a microcalorimeter with a single, external argon light source split to light guides which entered the reference and test vessels. Measurements were performed simultaneously with those by a polarographic oxygen sensor, the two to measure the energy flows in Dunaliella maritima at saturating light intensities. By constructing energy balances they identified an extra source of heat that may have been due to non‐photochemical quenching to protect the vital photosystem 11 D protein. These authors have recently replaced the argon light source with independently controlled LEDs in each vessel in order to achieve better balance (Mukhanov and Kemp, 2009).
Using small‐scale photocalorimetry, Magee and colleagues (1939) made the most telling contribution to our knowledge of photosynthesis by showing that the quantum efficiency of the process in Chlorella vulgaris was 0.077, which means that ∼13 photons were needed to produce one molecule glucose from CO2. This is remarkably close to values recently obtained by other means (Govindjee, 1999) and in concordance with the accepted photosynthesis Z‐scheme (Walker, 2002; Kramer et al., 2004). The photosynthetic efficiency (PE) can thus be studied in lab‐scale calorimeters acting as bioreactors as shown by von Stockar and Marison. In the same lab, Janssen and colleagues (2004) illuminated the most recent version of the bioreactor containing a C. vulgaris culture with banks of 1452 red‐light (660 nm) LEDs mounted just outside a plexiglass insulating box and giving a light input of 0.88 W l−1.
After a dark baseline heat flux, presumably due to respiration, was achieved (see Fig. 9), the lights were switched on (B) to initiate photoautotrophic growth. Immediately there was a large increase in net heat flux (mW l−1) which was the measure of the light energy absorption and subsequent dissipation as heat of much of it. Because the biomass was low at this stage, energy absorption by the calorimeter hardware was the source of some of the produced heat. Of course, heat absorption in the process of endothermic photosynthesis was swamped by the heat dissipation. As the cells grew in number, the net heat flux increased because they were intercepting more of the incident light. At the point when the cells had absorbed all the available light at constant intensity (Fig. 9), meaning that light was the limiting substrate, the net heat flux reached a plateau with the cells in linear growth, as measured off‐line. Then, photosynthesis was stopped at its maximal activity (see Fig. 9C) with its electron transport chain inhibitors, 3‐(3,4‐dichlorophenyl)‐1,1‐dimethylurea (DCMU) and 2,5‐dibromo‐3‐methyl‐6‐isopropyl‐p‐benzoquinone (DBMIB). The rate of photosynthesis could be measured only at this point in the batch culture but the use of respiratory poisons caused cell death so truly inactive, ‘live’ biomass was not achieved in this experiment. When the light was switched off (see Fig. 9D), the extent of the reduction in net heat flux gave the total light energy stored as biomass. The ratio between it and the entire light input during the batch culture gave PE, the fraction of light energy stored as biomass, as 7.1%. Improvements to the lighting set‐up increased the light input to 1.35 W l−1 and raised the PE to 10.5%.
Figure 9.
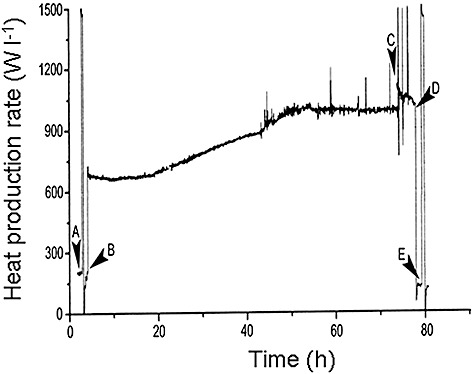
The net heat flux (W l−1) during the batch cultivation of C. vulgaris in a bioreactor. The light energy was provided by a bank of red LEDs at 0.88 W l−1. Events: (A) heat calibration; (B) lights on; (C) addition of DCMU and DBMIB; (D) lights off; (E) heat calibration.
Clearly, the photocalorimeter could measure the light energy required for photoautotrophic growth but there is the need for the continuous measurement of light energy accumulation in the biomass rather than only a snapshot. This requires increased light input and better distribution of the light energy across the calorimeter surface as achieved in a later study by Janssen and colleagues (2007). Although at a higher level than previously, the overall form of the net heat flow rate (W) curve was similar to that in Fig. 9. The metabolic activity was stopped by replacing the normal gas flow with that of pure nitrogen so that all the light energy was dissipated as heat to give the biochemical heat flow rate (qbio) after online correction for baseline variations. Providing all the light had been absorbed within the calorimeter, the heat flow rate was only the qbio flow rate and thus the photosynthetic rate. The corresponding PE was 14%. As biomass increased, PE decreased gradually to 5.4% at the end of the culture because of an increasing zone of no light penetration, i.e. the light path of the bioreactor is too long. It was concluded that, although the calorimeter measured photoautotrophic growth online, a short light‐path photocalorimeter is required fully to realize the potential of the technique.
Future prospect of biocalorimetry
The costs of bioprocess optimization contribute significantly to the market prices of biotechnological products. Thus, there is a strong economic incentive for improved process monitoring. Also in ecosystems there is a strong requirement for easily manageable, robust monitoring tools providing information about the catabolic processes and coupling to biomass formation. Calorimetry is a promising monitoring tool for this purpose, because it provides real‐time information about the growth rates and the stoichiometry. Megacalorimetry and chip‐calorimetry overcome the most serious weaknesses of conventional calorimetric devices such as high costs, slow response times and difficult integration in current bioprocesses. Furthermore, arrays of chip‐calorimeters emerging since 2004 have the potential to monitor different bioprocesses simultaneously. Further miniaturization may permit calorimetric measurement at various locations inside large bioreactors or ecosystems. Undesired process heterogeneities that are caused by incomplete mixing or anaerobic zones in ecosystems can be revealed in this way. The small size of chip modules in combination with the low costs that would result from its production in large series will allow its use as off‐line biofilm support to be integrated in various technical systems followed by the emplacement in a calorimeter unit for activity investigations. Finally, it is envisaged that this technology will be applied as an environmental monitor. For instance, the reactions of appropriate immobilized cell cultures associated with calorimeter chips could be used for online monitoring of the chemical quality of drinking and waste water. The real‐time recognition of the passage of toxicants could be used automatically to shut down affected lines.
Nomenclature
| D | dilution rate (h−1) |
| KS | half saturation constant of the growth kinetics (mol l−1) |
| P | specific heat production rate (W l−1) |
| ri,j | rate of the species i participation on reaction j (mol l−1 s−1) |
| S | substrate concentration (mol l−1) |
| T | temperature (K) |
| t | time (h) |
| Y | stoichiometric/yield coefficient (mol mol−1) |
| ΔRH | enthalpy of reaction (kJ mol−1) |
| νI,j | stoichiometry coefficient of the ith species participating on the jth reaction |
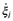 |
progress of the reaction j (mol l−1 s−1) |
Superscripts
| 0 | in the bioreactor input |
Subscripts
| C | combustion |
| N | nitrogen |
| O | oxygen |
| R | reaction |
| S | substrate |
| S1, S2, S3 | number of the carbon, hydrogen or oxygen atoms in one molecule of the carbon substrate |
| X1, X2, X3 | number of the carbon, hydrogen or oxygen atoms in a hypothetical molecule of biomass |
| X | biomass |
References
- Anderson R.K.I., Jayaraman K., Voisard D., Marison I.W., Von Stockar U. Heat flux as an on‐line indicator of metabolic activity in pilot scale bioreactor during the production of bacillus thuringiensis var. galleriae‐based biopesticides. Thermochim Acta. 2002;386:127–138. [Google Scholar]
- Antoce O.A., Antoce V., Takahashi K., Pomohaci N., Namolosanu I. A calorimetric method applied to the study of yeast growth inhibition by alcohols and organic acids. Am J Enol Viticult. 1997;48:413–422. [Google Scholar]
- Aulenta F., Bassani C., Ligthart J., Majone M., Tilche A. Calorimetry: a tool for assessing microbial activity under aerobic and anoxic conditions. Water Res. 2002;36:1297–1305. doi: 10.1016/s0043-1354(01)00337-2. [DOI] [PubMed] [Google Scholar]
- Babel W., Müller R.H. Correlation between cell composition and carbon conversion efficiency in microbial growth: a theoretical study. Appl Microbiol Biotechnol. 1985;22:201–207. [Google Scholar]
- Boling E.A., Blanchard G.C., Russell W.J. Bacterial identification by microcalorimetry. Nature. 1973;241:472–473. doi: 10.1038/241472a0. [DOI] [PubMed] [Google Scholar]
- Buchholz F., Wick L.Y., Harms H., Maskow T. The kinetics of polycyclic aromatic hydrocarbon (PAH) biodegradation assessed by isothermal titration calorimetry (ITC) Thermochim Acta. 2007;458:47–53. [Google Scholar]
- Burns T.P. Lindeman's contradiction and the trophic structure of ecosystems. Ecology. 1989;70:1355–1362. [Google Scholar]
- Cordier J.L., Butsch B.M., Birou B., Von Stockar U. The relationship between elemental composition and heat of combustion of microbial biomass. Appl Microbiol Biotechnol. 1987;25:305–312. [Google Scholar]
- Domalski E.S. Selected values of heats of combustion and heats of formation of organic compounds containing the elements C, H, N, O, P and S. J Phys Chem Ref Data. 1972;1:221–276. [Google Scholar]
- Dubrunfaut M. Note sur al chaleur et le travail mecanique produits lar la fermentation vineuse. C R Acad Sci. 1856;42:945–948. [Google Scholar]
- Garci'a‐Payo M.C., Ampuero S., Liu J.S., Marison I.W., Von Stockar U. The development and characterization of a high resolution bio‐reaction calorimeter for weakly exothermic cultures. Thermochimica Acta. 2002;391:25–39. [Google Scholar]
- Gnaiger E., Kemp R.B. Anaerobic metabolism in aerobic mammalian cells: information from the ratio of calorimetric heat flux and respirometric oxygen flux. Biochim Biophys Acta. 1990;1016:328–332. doi: 10.1016/0005-2728(90)90164-y. [DOI] [PubMed] [Google Scholar]
- Govindjee On the requirement of a minimum number of four versus eight quanta of light for the evolution of oxygen in photosynthesis: a historical note. Photosynth Res. 1999;59:249–254. [Google Scholar]
- Greene R.L., King C.N., Zubeck R.B., Hauser J.J. Specific heat of granular aluminum films. Phys Rev B. 1972;6:3297–3305. [Google Scholar]
- Guosheng L., Yi L., Xiangdong C., Peng L., Ping S., Songsheng Q. Study on interaction between T 4 phage and Escherichia coli B by microcalorimetric method. J Virol Methods. 2003;112:137–143. doi: 10.1016/s0166-0934(03)00214-3. [DOI] [PubMed] [Google Scholar]
- Hansen L.D., Macfarlane C., McKinnon N., Smith B.N., Criddle R.S. Use of calorespirometric ratios, heat per CO2 and heat per O2, to quantify metabolic paths and energetics of growing cells. Thermochim Acta. 2004;422:55–61. [Google Scholar]
- Heijnen J.J. Bioenergetics of microbial growth. In: Flickinger M.C., Drew S.W., editors. John Wiley & Sons; 1999. pp. 267–291. [Google Scholar]
- Heijnen S.J. Thermodynamics of microbial growth and its implications for process design. Trends Biotechnol. 1994;12:483–492. [Google Scholar]
- Higuera‐Guisset J., Rodriguez‐Viejo J., Chacon M., Munoz F.J., Vigues N., Mas J. Calorimetry of microbial growth using a thermopile based microreactor. Thermochim. Acta. 2005;427:187–191. et al. [Google Scholar]
- Janssen M., Patino R., Von Stockar U. Application of bench‐scale biocalorimetry to photoautotrophic cultures. Thermochim Acta. 2004;435:18–27. [Google Scholar]
- Janssen M., Wijffels R., Von Stockar U. Biocalorimetric monitoring of photoautotrophic batch cultures. Thermochim Acta. 2007;458:54–64. [Google Scholar]
- Jequier E., Schutz Y. Long‐term measurements of energy expenditure in humans using a respiration chamber. Am J Clin Nutr. 1983;38:989. doi: 10.1093/ajcn/38.6.989. [DOI] [PubMed] [Google Scholar]
- Johannessen E.A., Weaver J.M.R., Bourova L., Svoboda P., Cobbold P.H., Cooper J.M. Micromachined nanocalorimetric sensor for ultra‐low‐volume cell‐based assays. Anal Chem. 2002;74:2190–2197. doi: 10.1021/ac011028b. [DOI] [PubMed] [Google Scholar]
- Kemp R.B. Fire burn and cauldron bubble (W. Shakespeare): what the calorimetric–respirometric (CR) ratio does for our understanding of cells? Thermochim Acta. 2000;355:115–124. [Google Scholar]
- Kleiber M. Wiley; 1961. [Google Scholar]
- Koga K., Hiraoka S.I., Kim Y.S., Hagiwara D., Suehiro Y., Sakamoto Y., Takahashi K. Calorimetric studies on the ability of organic matter decomposition by microbes in different kinds of soils. Cal Therm Anal. 2001;28:54–61. [Google Scholar]
- Kramer D.M., Avenson T.J., Edwards G.E. Dynamic flexibility in the light reactions of photosynthesis governed by both electron and proton transfer reactions. Trends Plant Sci. 2004;9:349–357. doi: 10.1016/j.tplants.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Krell T. Microcalorimetry: a response to challenges in modern biotechnology. Microbial Biotech. 2008;1:126–136. doi: 10.1111/j.1751-7915.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht I., Seymour R.S., Schultze‐Motel P. Direct and indirect calorimetry of thermogenic flowers of the sacred lotus, Nelumbo nucifera. Thermochim Acta. 1998;309:5–16. [Google Scholar]
- Larsson C., Lidén G., Niklasson C., Gustafsson L. Calorimetric control of fed‐batch cultures of Saccharomyces cerevisiae. Bioprocess Biosyst Eng. 1991;7:151–155. [Google Scholar]
- Lerchner J., Maskow T., Wolf G. Chip calorimetry and its use for biochemical and cell biological investigations. Chem Eng Process. 2007;47:991–999. [Google Scholar]
- Lerchner J., Wolf A., Schneider H.J., Mertens F., Kessler E., Baier V. Nano‐calorimetry of small‐sized biological samples. Thermochim Acta. 2008a;477:48–53. et al. [Google Scholar]
- Lerchner J., Wolf A., Buchholz F., Mertens F., Neu T.R., Harms H., Maskow T. Miniaturized calorimetry – a new method for real‐time biofilm activity analysis. J Microbiol Methods. 2008b;74:74–81. doi: 10.1016/j.mimet.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Liu J.S., Marison I.W., Von Stockar U. Microbial growth by a net heat up‐take: a calorimetric and thermodynamic study on acetotrophic methanogenesis by Methanosarcina barkeri. Biotechnol Bioeng. 2001;75:170–180. doi: 10.1002/bit.1176. [DOI] [PubMed] [Google Scholar]
- Lindeman R.L. The trophic–dynamic aspect of ecology. Ecology. 1942;23:399–414. [Google Scholar]
- Magee J.L., DeWitt T.W., Smith E.C., Daniels F. A photocalorimeter: the quantum efficiency of photosynthesis in algae. J Am Chem Soc. 1939;61:3529–3533. [Google Scholar]
- Maskow T., Babel W. Calorimetric investigations of bacterial growth on phenol‐efficiency and velocity of growth as a function of the assimilation pathways. Thermochim Acta. 1998;309:97–103. [Google Scholar]
- Maskow T., Babel W. Calorimetrically recognized maximum yield of poly‐3‐hydroxybutyrate (PHB) continuously synthesized from toxic substrates. J Biotechnol. 2000;77:247–253. doi: 10.1016/s0168-1656(99)00220-5. [DOI] [PubMed] [Google Scholar]
- Maskow T., Babel W. Calorimetrically obtained information about the efficiency of ectoine synthesis from glucose in Halomonas elongata. Biochim Biophys Acta. 2001;1527:4–10. doi: 10.1016/s0304-4165(01)00115-5. [DOI] [PubMed] [Google Scholar]
- Maskow T., Kleinsteuber S. Carbon and energy fluxes during haloadaptation of Halomonas sp. EF11 growing on phenol. Extremophiles. 2004;8:133–141. doi: 10.1007/s00792-003-0372-1. [DOI] [PubMed] [Google Scholar]
- Maskow T., Olomolaiye D., Breuer U., Kemp R. Flow calorimetry and dielectric spectroscopy to control the bacterial conversion of toxic substrates into polyhydroxyalkanoates. Biotechnol Bioeng. 2004;85:547–552. doi: 10.1002/bit.10903. [DOI] [PubMed] [Google Scholar]
- Maskow T., Lerchner J., Peitzsch M., Harms H., Wolf G. Chip calorimetry for the monitoring of whole cell biotransformation. J Biotechnol. 2006a;122:431–442. doi: 10.1016/j.jbiotec.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Maskow T., Müller S., Losche A., Harms H., Kemp R. Control of continuous polyhydroxybutyrate synthesis using calorimetry and flow cytometry. Biotechnol Bioeng. 2006b;93:541. doi: 10.1002/bit.20743. [DOI] [PubMed] [Google Scholar]
- Meier‐Schneiders M., Schäfer F. Quantification of small enthalpic differences in anaerobic microbial metabolism – a calorimetry‐supported approach. Thermochim Acta. 1996;275:1–16. [Google Scholar]
- Meier‐Schneiders M., Grosshans U., Busch C., Eigenberger G. Biocalorimetry‐supported analysis of fermentation processes. Appl Microbiol Biotechnol. 1995a;43:431–439. [Google Scholar]
- Meier‐Schneiders M., Schäfer F., Grosshans U., Busch C. Impact of carbon dioxide evolution on the calorimetric monitoring of fermentations. Thermochim Acta. 1995b;251:85–97. [Google Scholar]
- Michal G. Wiley; 1999. [Google Scholar]
- Mukhanov V.S., Kemp R.B. Simultaneous photocalorimetric and oxygen polarographic measurements on Dunaliella maritima cells reveal a thermal discrepancy that could be due to nonphotochemical quenching. Thermochim Acta. 2006;446:11–19. [Google Scholar]
- Mukhanov V.S., Kemp R.B. Design and experience of using light‐emitting diodes (LEDs) as the inbuilt light source for a customised differential photomicrocalorimeter. J Therm Anal Calorimetry. 2009;95:731–736. [Google Scholar]
- Page D.L., Pizziconi V.B. A cell‐based immunobiosensor with engineered molecular recognition – Part II: enzyme amplification systems. Biosens Bioelectron. 1997;12:457–466. doi: 10.1016/s0956-5663(96)00069-3. [DOI] [PubMed] [Google Scholar]
- Paulik M.A., Buckholz R.G., Lancaster M.E., Dallas W.S., Hull‐Ryde E.A., Weiel J.E., Lenhard J.M. Development of infrared imaging to measure thermogenesis in cell culture: thermogenic effects of uncoupling protein‐2, troglitazone, and β‐adrenoceptor agonists. Pharm Res. 1998;15:944–949. doi: 10.1023/a:1011993019385. [DOI] [PubMed] [Google Scholar]
- Peitzsch M., Kiesel K., Harms H., Maskow T. Real time analysis of Escherichia coli biofilms using calorimetry. Chem Eng Proc. 2007;47:1000–1006. [Google Scholar]
- Peters P., Galinski E.A., Truper H.G. The biosynthesis of ectoine. FEMS Microbiol Lett. 1990;71:157–162. [Google Scholar]
- Pizziconi V.B., Page D.L. A cell‐based immunobiosensor with engineered molecular recognition – Part I: design feasibility. Biosens Bioelectron. 1997;12:287–299. doi: 10.1016/s0956-5663(96)00068-1. [DOI] [PubMed] [Google Scholar]
- Von Rege H., Sand W. Evaluation of biocide efficacy by microcalorimetric determination of microbial activity in biofilms. J Microbiol Methods. 1998;33:227–235. [Google Scholar]
- Russell W.J., Zettler J.F., Blanchard G.C., Boling E.A. Wiley; 1975. [Google Scholar]
- Schill N., Van Gulik W.M., Voisard D., Von Stockar U. Continuous cultures limited by a gaseous substrate: development of a simple, unstructured mathematical model and experimental verification with Methanobacterium thermoautotrophicum. Biotechnol Bioeng. 1996;51:645–658. doi: 10.1002/(SICI)1097-0290(19960920)51:6<645::AID-BIT4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Schill N.A., Liu J.S., Von Stockar U. Thermodynamic analysis of growth of Methanobacterium thermoautotrophicum. Biotechnol Bioeng. 1999;64:74–81. doi: 10.1002/(sici)1097-0290(19990705)64:1<74::aid-bit8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Schön A., Wadsö I. The use of microcalorimetry in studies of mammalian cells. J Therm Anal Calorim. 1988;33:47–54. [Google Scholar]
- Schubert T., Breuer U., Harms H., Maskow T. Calorimetric bioprocess monitoring by small modifications to a standard bench‐scale bioreactor. J Biotechnol. 2007;130:24–31. doi: 10.1016/j.jbiotec.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Schumer D., Breuer U., Harms H., Maskow T. Thermokinetic analysis reveals the complex growth and haloadaptation pattern of the non‐conventional yeast Debaryomyces hansenii. Eng Life Sci. 2007;7:1–10. [Google Scholar]
- Stephanopoulos G.N., Aristidou A.A., Nielsen J. Academic Press; 1998. [Google Scholar]
- Von Stockar U., Birou B. The heat generated by yeast cultures with a mixed metabolism in the transition between respiration and fermentation. Biotechnol Bioeng. 1989;34:86–101. doi: 10.1002/bit.260340112. [DOI] [PubMed] [Google Scholar]
- Von Stockar U., Liu J.S. Does microbial life always feed on negative entropy? Thermodynamic analysis of microbial growth. Biochem Biophys Acta. 1999;1412:191–211. doi: 10.1016/s0005-2728(99)00065-1. [DOI] [PubMed] [Google Scholar]
- Von Stockar U., Marison I.W. The use of calorimetry in biotechnology. Adv Biochem Eng Biot. 1989;40:93–136. [Google Scholar]
- Von Stockar U., Marison I.W. Large‐scale calorimetry and biotechnology. Thermochim Acta. 1991;193:215–242. [Google Scholar]
- Von Stockar U., Gustafsson L., Larsson C., Marison I., Tissot P., Gnaiger E. Thermodynamic considerations in constructing energy balances for cellular growth. Biochim Biophys Acta. 1993;1183:221–240. [Google Scholar]
- Von Stockar U., Maskow T., Liu J., Marison I.W., Patino R. Thermodynamics of microbial growth and metabolism: an analysis of the current situation. J Biotechnol. 2006;121:517–533. doi: 10.1016/j.jbiotec.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Von Stockar U., Vojinovića V., Maskow T., Liu J.‐S. Can microbial growth yield be estimated using simple thermodynamic analogies to technical processes. Chem Eng Proc. 2007;47:980–990. [Google Scholar]
- Thornton W. The relation of oxygen to the heat of combustion of organic compounds. Philos Mag. 1917;33:196–203. [Google Scholar]
- Tijhuis L., Van Loosdrecht M.C.M., Heijnen J.J. A thermodynamically based correlation for maintenance Gibbs energy requirements in aerobic and anaerobic chemotrophic growth. Biotechnol Bioeng. 1993;42:509–519. doi: 10.1002/bit.260420415. [DOI] [PubMed] [Google Scholar]
- Torres F.E., Kuhn P., De Bruyker D., Bell A.G., Wolkin M.V., Peeters E. Enthalpy arrays. Proc Natl Acad Sci USA. 2004;101:9517–9522. doi: 10.1073/pnas.0403573101. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türker M. Development of biocalorimetry as a technique for process monitoring and control in technical scale fermentations. Thermochim Acta. 2004;419:73–81. [Google Scholar]
- Verhaegen K., Baert K., Simaels J., Van Driessche W. A high‐throughput silicon microphysiometer. Sens Actuators B. 2000;82:186–190. [Google Scholar]
- Voisard D., Pugeaud P., Kumar A.R., Jenny K., Jayaraman K., Marison I.W., Von Stockar U. Development of a large‐scale biocalorimeter to monitor and control bioprocesses. Biotechnol Bioeng. 2002;80:125–138. doi: 10.1002/bit.10351. [DOI] [PubMed] [Google Scholar]
- Walker D.A. The Z‐scheme – down hill all the way. Trends Plant Sci. 2002;7:183–185. doi: 10.1016/s1360-1385(02)02242-2. [DOI] [PubMed] [Google Scholar]
- Wentzien S., Sand W., Albertsen A., Steudel R. Thiosulfate and tetrathionate degradation as well as biofilm generation by Thiobacillus intermedius and Thiobacillus versutus studied by microcalorimetry, HPLC, and ion‐pair chromatography. Arch Microbiol. 1994;161:116–125. [Google Scholar]
- Yao J., Wang C., Xu F., Tian L., Wang Y., Chen H. An in vitro microcalorimetric method for studying the toxic effect of cadmium on microbial activity of an agricultural soil. Ecotoxicology. 2007;16:503–509. doi: 10.1007/s10646-007-0157-x. et al. [DOI] [PubMed] [Google Scholar]


