Summary
Laccase efficiently catalyses polymerization of phenolic compounds. However, knowledge on applications of polymers synthesized in this manner remains scarce. Here, the potential of laccase‐catalysed polymerization of natural phenols to form products useful in hair dyeing was investigated. All 15 tested phenols yielded coloured products after laccase treatment and colour diversity was attained by using mixtures of two phenolic monomers. After exploring colour differentiation pattern of 120 different reactions with statistical regression analysis, three monomer combinations, namely gallic acid and syringic acid, catechin and catechol, and ferulic acid and syringic acid, giving rise to brown, black, and red materials, respectively, were further characterized because such colours are commercially important for grey hair dyeing. Selected polymers could strongly absorb visible light and their hydrodynamic sizes ranged from 100 to 400 nm. Analyses of enzyme kinetic constants, liquid chromatography and electrospray ionization‐mass spectrometry (ESI‐MS) coupled with collision‐induced dissociation MS/MS indicate that both monomers in reactions involving catechin and catechol, and ferulic acid and syringic acid, are coloured by heteropolymer synthesis, but the gallic acid/syringic acid combination is based on homopolymer mixture formation. Comparison of colour parameters from these three reactions with those of corresponding artificial homopolymer mixtures also supported the idea that laccase may catalyse either hetero‐ or homo‐polymer synthesis. We finally used selected materials to dye grey hair. Each material coloured hair appropriately and the dyeing showed excellent resistance to conventional shampooing. Our study indicates that laccase‐catalysed polymerization of natural phenols is applicable to the development of new cosmetic pigments.
Introduction
Laccases are copper‐containing enzymes catalysing the monoelectronic oxidation of substrates (e.g. phenols and aromatic or aliphatic amines) to their corresponding radicals, using molecular oxygen. The enzymes are particularly widespread in ligninolytic basidiomycetes, but also occur in certain prokaryotes, insects and plants (Claus, 2003; Baldrian, 2006), indicating that the laccase redox process is ubiquitous in nature. Laccase oxidative activities have been extensively characterized because of the potential for industrial applications. The most interesting features of oxidative reactions catalysed by laccases are broad substrate specificity and involvement of molecular oxygen as the final electron acceptor (Riva, 2006). Based on both of these properties, several investigators have shown that laccase use is effective and eco‐friendly in specific applications including paper pulp bleaching, organic syntheses and degradation of recalcitrant xenobiotics (Reid and Paice, 1994; Riva, 2006; Jeon et al., 2008; Kunamneni et al., 2008; Mikolasch and Schauer, 2009; Murugesan et al., 2009).
As phenol oxidases, laccases can efficiently catalyse polymerization of phenolic moieties. Polymerization is initiated by formation of radical cations followed by intermolecular attack to produce dimers (Mikolasch and Schauer, 2009). Oligomers and polymers can be synthesized from the dimers, but over much longer incubation times. Laccase‐based polymerization of phenolic compounds is known to play an important role in several biosynthetic steps of plants and microorganisms (O'Malley et al., 1993; Tasi et al., 1999). Apart from biological roles in polymerization, laccase application in functional polymer synthesis is also of interest. Moreover, laccase‐catalysed grafting of functional molecules on wood (Fackler et al., 2008; Kudanga et al., 2008) and cellulose (Elegir et al., 2008) confers new properties to fibres in an eco‐friendly manner. In particular, use of naturally occurring phenols such as flavonoids for polymer synthesis (Uyama and Kobayashi, 2002; Desentis‐Mendoza et al., 2006) is attractive because such reactions fulfill the basic requirements of ‘green’ chemistry, in that (i) little toxic waste is produced as the monomers are eco‐friendly and an enzyme is a ‘green’ catalyst; and (ii) polymer synthesis mimics a natural phenomenon responsible for synthesis of flavonoid polymers such as tannins and proanthocyanidins. The attributes of polymers synthesized by laccase action on natural phenols have been explored for potential applications in bioactive material syntheses (Uyama and Kobayashi, 2002; 2006) and textile colouration (Covington et al., 2005; Kim et al., 2007; 2008). In the latter field, natural flavonoids (e.g. morin, rutin and quercetin) have been employed to dye fabrics such as flax and cellulose with laccase‐mediated polymerization (Kim et al., 2007; 2008). Such polymerized flavonoids are generally dark brown in colour, and the synthesized polymers can be coupled to fabric surfaces by manipulation of pH, temperature and mechanical agitation.
It has been customary for many years to dye human hair to conceal the grey that signifies advancing age. Conventional hair dyeing methodology, which has been successfully commercialized, is based on the combined action of three reactive species: dye precursors, colour modifiers and oxidizing agents. The latter compounds induce polymerization of dye precursors and colour modifiers, resulting in formation of coloured dyes. The extent of dye diffusion into hair and the polymeric dye molecular sizes determine colour permanence of dyed hair to shampooing. The most widely used oxidizing agent and dye precursors are hydrogen peroxide (H2O2) and phenylenediamines respectively (Wall, 1972). Although H2O2 efficiently polymerizes phenylenediamines concomitant with adsorption of polymers to hair (Wall, 1972; Chandrashekara and Ranganathaiah, 2009), some health concerns and hair damage have been recognized. First, the oxidation power of H2O2 is very strong, and severe hair damage can result from H2O2 use (Takada et al., 2003). Second, toxicological studies show that phenylenediamines, used as dye precursors, are potential carcinogens and allergic sensitizers in humans (Chen et al., 2006; Huang et al., 2007). Some patents claim that oxidases such as peroxidase and laccase can be used to generate useful hair dyes, but the procedures continue to employ phenylenediamines and other synthetic dye precursors, which may be toxic to humans, and experimental approaches for evaluating enzymatically synthesized polymeric dyes are also scarce (Onuki et al., 2004; Lang and Cotteret, 2007). Hence, it was of interest to explore laccase‐based polymerization of naturally occurring phenols for potential product application to hair dyeing, because such an approach might overcome the current problems with H2O2‐ and phenylenediamine‐based dyeing systems.
Although some investigators showed that laccase‐catalysed polymerization of single phenolic compounds gives rise to coloured compounds for textile dyeing (Shin et al., 2001; Kim et al., 2007; 2008), colour diversity was too limited for applications in cosmetic pigments. To overcome this limitation, we employed novel natural phenols derived from lignin, tannin and plant hormone, and based colouration reactions with laccase catalysis on use of dual‐monomer cocktails leading to heteropolymer or homopolymer mixture formation. Colour parameters of dual monomer reactions were compared with those of single monomer reactions to evaluate whether dual monomer use is effective to achieve polymer colour diversity. In addition, relations between colour parameters of each monomer and tendency of each monomer to generate different colours with other monomers were evaluated by linear regression analysis. From the colour diversity, we could select three kinds of two‐monomer phenol cocktails generating black, brown or red colourations that might be commercially valuable for dyeing of grey hair. Most Asians have naturally black hair, and brown and red hair colours are also cosmetically popular. Selected colouration reactions were further characterized by colourimetry, UV‐visible spectrophotometry, electrophoretic light scattering spectrophotometry, liquid chromatography, electrospray ionization‐mass spectrometry (ESI‐MS) coupled with collision‐induced dissociation (CID) MS/MS, and enzyme kinetic constants. We finally employed our novel products to dye grey hair and tested colour permanence to conventional shampooing.
Results and discussion
Monomer blending strategy to achieve diverse colours
We used 15 plant‐derived phenolic monomers for colouration reactions. Most phenolic compounds are lignin‐derived monophenols (Camarero et al., 2005; Ruiz‐Duenas and Martinez, 2009), but gallic acid, catechin and catechol are natural polyphenols, and salicylic acid (a plant hormone) (Raskin, 1992) and tyramine (occurring naturally in vegetables) (Ly et al., 2008) were also assessed in the present work. In principle, polymerization of natural phenolic monomers derived from plant fibres such as lignin and tannin results in artificial fibre synthesis structurally mimicking plant‐derived fibres. Thus, application of plant fibre‐like polymer synthesis in hair dyeing would be eco‐friendly and safe compared with conventional phenylenediamines‐based hair dyeing methodology that has proven to be toxic to humans (Chen et al., 2006; Huang et al., 2007). Kim et al. showed that laccase‐catalysed polymerization of single natural polyphenols (e.g. morin, rutin and quercetin) can generate coloured compounds to dye fabrics such as flax and cellulose (Kim et al., 2007; 2008), but colour diversity was too limited for applying the products in cosmetic or food processing industry. Beyond natural polyphenols, we additionally employed lignin‐derived monophenols and other kinds of plant‐derived phenols, and then evaluated whether monomer blending is effective to diversify colour.
Colour values (a*, b* and L*) of natural phenolic compounds in aqueous solution were very similar to those of distilled water, indicating that phenolic compounds are poor absorbers of visible light. However, addition of laccase to samples containing single monomers or pairs of monomers resulted in visible colour generation (see Fig. S1). In general, L* (luminosity) values were evenly distributed ranging from 0 (black) to 100 (white), but a* and b* values were usually positive, suggesting that blue or green polymers are hardly achieved by laccase‐based polymerization of the naturally occurring phenols tested in the present work (Fig. 1 and Fig. S1). We also plotted L* values against a*/b* values to evaluate colour diversity, as shown in Fig. 1. The plot clearly shows that polymer colours after two‐monomer polymerizations were more diverse than seen when single‐monomer solutions were polymerized. We next evaluated the extent of colour parameter differences by monomer blending with respect to colour parameters of single monomer‐based colourations to confirm which monomers are prone to generating diverse colours with other monomers. Figure 2A shows that the extent of distribution of E* value {a representative colour value calculated from the three colour parameters using the formula: [(L*)2 + (a*)2 + (b*)2]1/2} differences by the presence of other kinds of natural phenols is narrow in cases of gallic acid and catechol although the E* value differences with the single monomers (gallic acid and catechol) were observed. These results indicate that visible colours generated from gallic acid or catechol can be changed with little colour diversity by other monomers. On the other hand, tyramine and salicylic acid show a much more broad distribution of E* value differences with other monomers (Fig. 2A), suggesting that the natural phenols can act as effective ingredients that induce colour diversity during polymeric dye synthesis. This biased tendency of each monomer for colour differentiation with other monomers raises the question whether the tendency is predictable with respect to three colour parameters of each monomer. To answer the question, first a representative value of each monomer describing the extent of colour differentiation was obtained by the standard deviation calculation from the values shown in Fig. 2A (Fig. 2B). Linear regression analysis was then performed using six variables, i.e. L*, a*, b* (L*)2 (a*)2 and (b*)2 of each monomer. The best fit is a model equation:
Figure 1.
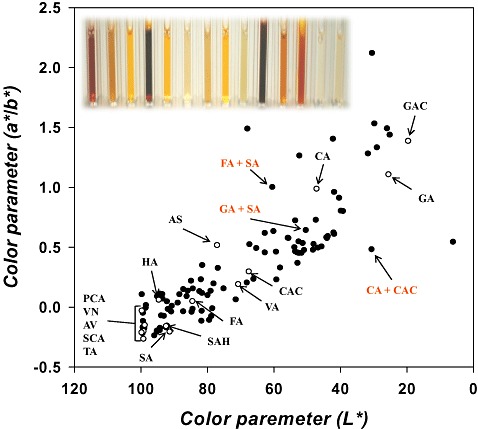
Distribution of colour values obtained from 120 different kinds of laccase‐catalysed colouration reactions containing single (empty circles) or dual monomers (filled circles). The inset is a representative photograph of colouration reactions (colours were shown in cases of single monomers). L* values from 0 (black) to 100 (white) represent luminosity and a*/b* values indicate the ratios of green‐red to blue‐yellow chromatic factors. AS, acetosyringone; VA, vanillic acid; SA, syringic acid; GA, gallic acid; HA, homovanillyl alcohol; PCA, p‐coumaric acid; VN, vanillin; SAH, syringaldehyde; AV, acetovanillone; GAC, guaiacol; FA, ferulic acid; CA, catechin; SCA, salicylic acid; TA tyramine; CAC, catechol.
Figure 2.
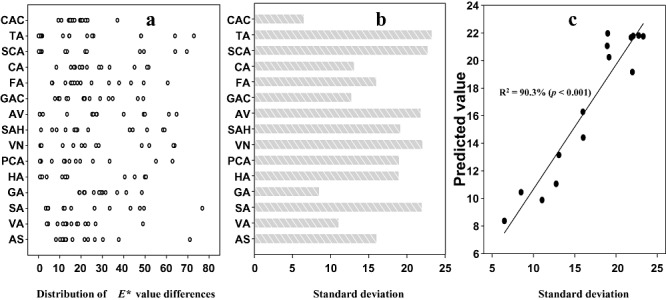
(A) Distribution of E* value differences by the presence of additional monomers with respect to E* values of single monomers. (B) Standard deviation of the E* value differences. (C) Linear regression analysis to fit the standard deviation of the E* value differences with six variables obtained from each monomer colouration (L*, P < 0.05; a*, P > 0.05; b*, P > 0.05; (L*)2, P < 0.05; (a*)2, P > 0.05; (b*)2, P > 0.05). E* value integrating all three colour parameters, L*, a* and b*, was calculated using the formula: [(L*)2 + (a*)2 + (b*)2]1/2. AS, acetosyringone; VA, vanillic acid; SA, syringic acid; GA, gallic acid; HA, homovanillyl alcohol; PCA, p‐coumaric acid; VN, vanillin; SAH, syringaldehyde; AV, acetovanillone; GAC, guaiacol; FA, ferulic acid; CA, catechin; SCA, salicylic acid; TA, tyramine; CAC, catechol.
 |
The R‐squared statistic was 90.3% (P < 0.001), i.e. the fitted model explained 90.3% of the variability in the extent of colour differentiation and the P‐values of different variables obtained by comparing the model with a simpler model omitting one variable indicate that L* value among three colour parameters of individual monomers is a main contributor to this model (Fig. 2C), suggesting that achromatic factors affecting colour luminosity could be a significant indicator of colour differentiation with other monomers under our dual‐monomer blending system. This new approach to evaluate the tendency of colour generation would be useful for the characterization of polymeric dye synthesis because, to date, little has been known about the methodology determining important factors to diversify colours.
With respect to chemistry, the colourful polymer synthesis under our dual‐monomer system involves mainly two different pathways, in that (i) homopolymer mixtures are formed by sequential formation of homopolymers consisting of individual monomers; (ii) heteropolymer synthesis proceeds via oxidative coupling of both monomers catalysed by laccase or cross‐linking of the preformed homopolymers. Such two pathways may be kinetically accelerated when relatively inactive monomers become reactive radicals by a laccase‐mediator system. In fact, natural phenols can act as laccase mediators (Camarero et al., 2005) and co‐polymerization of different phenols can occur in a laccase‐mediator system (Jeon et al., 2008). Each natural phenol has different reactivity with laccase due to substrate specificity of the enzyme and also shows different behaviours when it is transformed to its radical due to its chemical structure affording radical stability (Camarero et al., 2005; 2008). Hence, factors leading to homo‐ or hetero‐polymer synthesis under our dual‐monomer system can be both intrinsic properties of phenoxyl radicals formed by laccase and laccase catalytic bias on its substrates. When the phenoxyl radicals formed from one monomer preferentially attack themselves rather than the other monomer, homopolymer would be mainly synthesized. Similarly, the distinct catalytic specificity of laccase between both monomers would also result in homopolymer synthesis because one monomer showing low reactivity with the enzyme remains little reactive, thus little involved in the polymer synthesis until the other reactive monomer is completely consumed. In fact, we observed that sequential or simultaneous removal of two monomers supporting our hypotheses can happen under our dual‐monomer system when selected polymeric reactions were further characterized (see below).
Characterization of selected coloured polymers
From the colour library we made in this study, three reactions containing gallic acid and syringic acid, catechin and catechol, and ferulic acid and syringic acid, which gave rise to brown, black and red in colour, respectively, were selected for further characterization because such colours are of commercial interest for dyeing of grey hair (Figs 1 and 4). Unlike conventional dye compounds such as azo and sulfur dyes (Towns, 1999; Fu and Wang, 2008), polymeric dye colours change with monomer polymerization extent which, in turn, depends on polymerization time. Generation of desired colours within a few hours is critical for commercial application of in situ reactions to grey hair dyeing (Lang and Cotteret, 2007). Therefore, colour changes of the selected reactions were measured over time (Fig. 3). In general, the coordinate values (L*, a*, b*) of all three reactions approached the (0, 0, 0) coordinate value with increasing incubation time, indicating that initial chromatic factors causing development of bright red and yellow polymers were attenuated and, at the same time, activities of achromatic factors dimming colours were enhanced. Interestingly, a combination of catechin and catechol achieved the (0, 0, 0) coordinate value within 12 h, thus achieving a complete black colour. Dyeing of grey hair blackish, for cosmetic purposes, has become increasingly popular in many countries due to the similarity of the colour with that of natural human hair. The most widely used dye precursor affording a dark black colour is p‐phenylenediamine, but this is both allergenic and carcinogenic (Marcoux et al., 2002; Chen et al., 2006; Huang et al., 2007). The catechin/catechol mixture could be especially useful as an alternative to p‐phenylenediamine for hair blackening, as both materials are natural polyphenols. Brown and red colourations were also attained by polymerization of gallic acid with syringic acid and ferulic acid with syringic acid respectively (Fig. 4).
Figure 4.
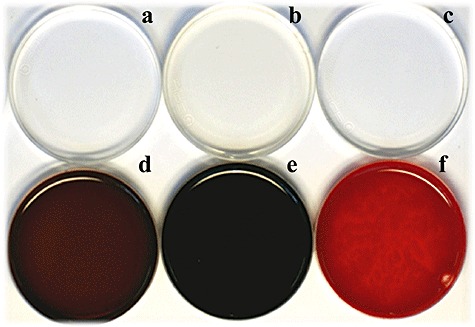
Photographs of selected colouration solutions in Petri dishes with or without laccase. The photograph was taken after 22 h incubation. (A) Gallic acid plus syringic acid without laccase. (B) Catechin plus catechol without laccase. (C) Ferulic acid plus syringic acid without laccase. (D) Gallic acid plus syringic acid with laccase. (E) Catechin plus catechol with laccase. (F) Ferulic acid plus syringic acid with laccase.
Figure 3.
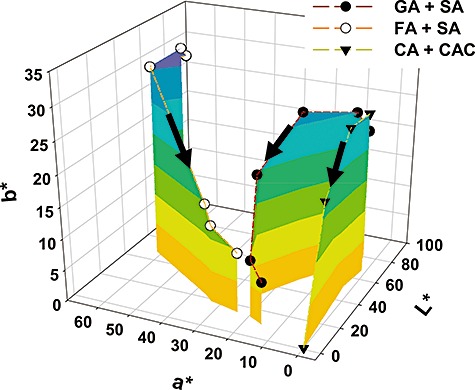
Colour value change of selected laccase‐catalysed colouration reactions with different incubation times. The colour values were measured after 0.5, 1, 2, 12, 15 and 22 h by a liquid colourimetry. GA, gallic acid; SA, syringic acid; FA, ferulic acid; CA, catechin; CAC, catechol.
To further characterize the valuable polymeric reactions, we evaluated the extent of monomer removal over time using RP‐HPLC (Fig. 5). In colour generation reactions using either catechin and catechol or ferulic acid and syringic acid, both monomer concentrations decreased simultaneously upon laccase catalytic action. However, in polymerization of gallic acid with syringic acid, the two monomers were sequentially removed by laccase. Thus, most gallic acid was removed within 1 h during which time the concentration of syringic acid fell only slightly. Such simultaneous or sequential monomer removal pattern suggests that colouration of gallic acid and syringic acid is based on homopolymer synthesis, whereas in the cases of ferulic acid and syringic acid, and catechin and catechol, heteropolymer is formed by laccase action. To prove experimentally homo‐ or hetero‐polymer synthesis in the selected colourations, we employed ESI‐MS coupled with CID MS/MS. As shown in Table 1, the presence of only gallic acid homo‐dimer at initial incubation step was revealed by comparison of CID MS/MS of [M − H]‐ ion of the dimer with CID MS/MS of standard gallic acid. On the other hand, hetero‐dimer and hetero‐trimer consisting of either ferulic acid and syringic acid or catechin and catechol were observed in the colouration reactions, indicating that hetero‐polymers were easily formed at oligomer levels. Together with HPLC profiles with incubation times, this CID MS/MS data strongly suggest that colouration with gallic acid and syringic acid proceeds with homo‐polymer synthesis, whereas colouration with either ferulic acid and syringic acid or catechin and catechol results in hetero‐polymer synthesis.
Figure 5.
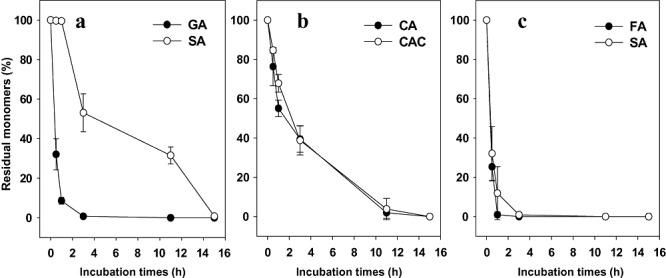
Time‐courses of monomer removal in selected laccase‐catalysed colouration reactions. Means and standard deviations for triplicate samples shown. GA, gallic acid; SA, syringic acid; FA, ferulic acid; CA, catechin; CAC, catechol.
Table 1.
CID MS/MS of homo‐ or hetero‐oligomers formed in dual‐monomer colourations and comparison with CID MS/MS of standard monomers.a
| Selected colouration | Theoretical [M − H]‐ | Experimental [M − H]‐ | CID MS/MS of [M − H]‐ (%, relative abundance) | Standard monomers | Theoretical [M − H]‐ | Experimental [M − H]‐ | CID MS/MS of [M − H]‐ (%, relative abundance) | |
|---|---|---|---|---|---|---|---|---|
| GA + SA | [GA + GA − H]‐ | 337.0 | 337.7 | 337.7 (5.1), 169.0 (100), 125.2 (17.9) | [GA − H]‐ | 169.0 | 169.0 | 169.0 (17.6), 125.0 (100), 81.1 (11.3), 78.9 (12.5) |
| SA + FA | [FA + FA − H]‐ | 385.1 | 385.3 | 385.3 (20.8), 267.3 (100), 193.1 (54.3), 149.4 (11.9), 134.2 (50.8) | [FA − H]‐ | 193.1 | 193.1 | 193.1 (4.6), 178.2 (22.3), 149.1 (6.9), 134.0 (100) |
| [FA + SA − H]‐ | 389.1 | 390.5 | 390.5 (3.8), 197.2 (28.9), 193.3 (100), 149.0 (10.2), 134.0 (21.9), 123.0 (11.1), 95.0 (3.9) | |||||
| [SA + SA + FA − H]‐ or [SA + FA + SA − H]‐ | 585.1 | 584.5 | 584.5 (6.2), 197.1 (95.5), 193.1 (50.0), 182.0 (100), 178.2 (15.6), 166.9 (15.6), 138.0 (21.9), 134.0 (34.4), 122.8 (9.3), 94.6 (15.6) | [SA − H]‐ | 197.1 | 197.1 | 197.1 (13.5), 182.0 (59.4), 166.9 (43.1), 138.0 (20.8), 122.8 (100), 95.0 (27.1) | |
| CA + CAC | [CA + CAC − H]‐ | 397.1 | 397.1 | 397.1 (60.0), 289.1 (46.6), 125.1 (100), 108.6 (13.3) | [CA − H]‐ | 289.1 | 289.1 | 289.1 (21.4), 125.2 (14.3), 108.8 (100) |
| [CAC + CAC − H]‐ | 217.1 | 271.1 | 189.2 (35.0), 161.1 (37.7), 108.7 (100), 65.2 (12.8), 41.0 (16.7) | |||||
| [CA + CA − H]‐ | 577.1 | 578.4 | 289.2 (100), 125.0 (13.4), 109.2 (15.3) | [CAC − H]‐ | 109.0 | 108.5 | 108.5 (6.8), 65.1 (72.8), 41.2 (100) | |
| [CA + CA + CAC − H]‐ or [CA + CAC + CA − H]‐ | 685.2 | 685.2 | 282.4 (100), 108.9 (59.2) | |||||
Colourations for 30 min incubation were analysed. The m/z values of all other possible dimers and trimers were negligible compared with noise signals.
GA, gallic acid; SA, syringic acid; FA, ferulic acid; CA, catechin; CAC, catechol.
We next evaluated kinetic constants of Trametes versicolor laccase to confirm whether the simultaneous or sequential removal is attributed to distinct laccase catalytic activity on both monomers. Trametes versicolor laccase oxidizes more efficiently gallic acid than syringic acid, according to the higher turnover number (Vmax) under the condition that substrate concentration is much higher than its Km value (Table 2). However, in spite of the higher turnover number of ferulic acid and catechin than syringic acid and catechol, respectively, the simultaneous removal pattern under the dual‐monomer systems was observed (Fig. 5). The higher Vmax values indicate that phenoxyl radicals from ferulic acid and catechin are dominant in the initial colouration. Therefore, the simultaneous removal pattern could be explained by tendency of preformed radicals to attack other phenols. It is not unreasonable that phenoxyl radicals generated from gallic acid are scavenged with gallic acid itself, but in the case of ferulic acid and catechin, the radicals are scavenged concomitant with intermolecular attack to syringic acid and catechol, resulting in the initiation of heteropolymer synthesis. The hypothesis is also supported by the ESI‐MS analysis (Table 1).
Table 2.
Kinetic constants of T. versicolor laccase for oxidation of selected natural phenols.
| Plant‐derived phenols | λ (nm) | ε (M−1 cm−1) | Km (µM) | Vmax (U mg−1) | [Substrate]a/Km |
|---|---|---|---|---|---|
| Gallic acid | 306 | 3 200 | 521 ± 75.3 | 1112.8 ± 137.2 | 56.5 |
| Syringic acid | 300 | 8 500 | 11.8 ± 3.3 | 309.1 ± 25.6 | 2144.1 |
| Ferulic acid | 320 | 12 500 | 19.9 ± 4.3 | 719.6 ± 23.9 | 1291.5 |
| Catechin | 450 | 2 200 | 173.0 ± 54.2 | 1843.9 ± 156.1 | 99.6 |
| Catechol | 435 | 2 600 | 362.0 ± 59.5 | 196.4 ± 25.5 | 125.6 |
[Substrate] indicates monomer concentration (µM) used for colouration reactions of hair dyeing.
Means and standard deviations for triplicate samples are shown.
Such sequential removal of two monomers also suggests that polymerization of the two‐monomer system might simply result in homopolymer mixtures. To answer this question as another evidence of homo‐ or hetero‐polymer synthesis, we compared the colour values of the three selected colouration reaction products with those of artificial homopolymer mixtures (Table 3). The colour values of artificial mixtures of polymers derived from catechin (alone) and catechol (alone), and from ferulic acid (alone) and syringic acid (alone), made by mechanical homopolymer blending, were significantly different from those of the colouration polymerizations using two‐monomer mixtures. Consistent with HPLC profile, ESI‐MS analysis and kinetic constants (Tables 1 and 2, and Fig. 5), this also indicates that both reactions after monomer blending resulted in heteropolymer synthesis by laccase. On the other hand, when polymers made from gallic acid (alone) and syringic acid (alone) were blended, all colour values overlapped (within an error range) with those of the polymer from the two‐monomer mixture, indicating that co‐incubation of gallic acid and syringic acid with laccase did not induce heteropolymer formation. In this experiment, we assumed that half of the added laccase reacted with each monomer, but laccase shows catalytic bias (caused by different affinities for distinct monomers). Thus, varying oxidation rates of different phenolic substrates (Camarero et al., 2005) can yield homopolymer mixtures in which the products have different molar ratios. It will thus be necessary to further investigate laccase‐catalysed polymer synthesis, particularly by nuclear magnetic resonance spectrometry (Manda et al., 2005), to fully understand heteropolymer formation processes.
Table 3.
Colour value difference between homopolymer blending and monomer blending.a
| Plant‐derived phenols | L* | a* | b* | |
|---|---|---|---|---|
| Homopolymer blending (separated reactions and mixed later) | GA (reacted) + SA (reacted) | 14.4 ± 4.5 | 17.7 ± 5.1 | 9.5 ± 3.0 |
| FA (reacted) + SA (reacted) | 29.3 ± 4.0 | 16.9 ± 2.7 | 18.7 ± 4.3 | |
| CA (reacted) + CAC (reacted) | 14.0 ± 2.5 | 17.8 ± 3.2 | 9.87 ± 2.9 | |
| Monomer blending (mixed already before laccase addition) | GA + SA | 11.2 ± 3.9 | 15.2 ± 4.5 | 7.7 ± 3.8 |
| FA + SA | 12.9 ± 2.7 | 27.0 ± 3.8 | 9.5 ± 1.7 | |
| CA + CAC | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
Homopolymer blending indicates mechanical mixtures of two half volume colouration reactions containing 50% laccase and single monomers. Monomer blending indicates colouration reactions containing laccase and dual monomers. Colour values were measured after 22 h incubation. Means and standard deviations for triplicate samples shown.
We next divided the selected colouration polymers into soluble and insoluble components, to characterize visible light absorbance capacities of soluble polymers and the hydrodynamic size distributions of insoluble polymers. Hair dyeing is based on diffusion of dye molecules, particularly into hair cuticle cavities of the cortex layers, resulting in sorption of colourful materials (Chandrashekara and Ranganathaiah, 2009). Polymeric dye size affects sorption and the visible light absorbance capacities of dyes determine hair colour after sorption. Therefore, it was important to evaluate light absorbance capacities and size distributions of the new polymeric dyes. After 24 h laccase incubation with catechin and catechol, or ferulic acid and syringic acid, insoluble polymers of hydrodynamic sizes 90–220 nm were found, but polymers obtained from the gallic acid and syringic acid mixture were slightly larger, and had a broader size distribution, from 130–400 nm (Fig. 6A). Visible light absorbance spectra showed that the absorbance capacities of soluble polymers obtained from gallic acid and syringic acid, or catechin and catechol, increased with decreasing wavelengths. However, the red polymers obtained in the reaction of ferulic acid and syringic acid (Fig. 4F) absorbed visible light maximally at the specific wavelength of 483 nm (Fig. 6B).
Figure 6.
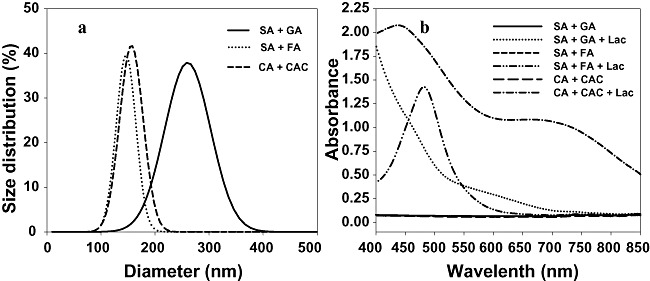
Hydrodynamic size distribution of insoluble polymers (A) and visible light absorbance capacity of soluble polymers (B) obtained from selected laccase‐catalysed colouration reactions. GA, gallic acid; SA, syringic acid; FA, ferulic acid; CA, catechin; CAC, catechol.
Grey keratin hair dyeing by selected polymerizations
Organic materials synthesized by laccase‐catalysed polymerization of naturally occurring phenols could be particularly useful as cosmetics because such materials can scavenge reactive radicals, thus also serving as anti‐oxidants (Uyama and Kobayashi, 2002; 2006), and can absorb light in the visible range, making them suitable for cosmetic colouring applications. Such applications include both the final polymeric product and the in situ reaction per se. In situ polymeric reactions generating visible colours are based on the same chemical principle used in conventional hair dyeing employing H2O2 and dye precursors (Takada et al., 2003). Hence, in situ enzymatic polymerization of natural phenols could be used as an alternative to the H2O2‐based hair dyeing system.
To explore this possibility, we employed selected colouration reactions for grey keratin hair dyeing. After 22 h reaction time, the grey hair became dyed (Fig. 7). In situ polymerizations using gallic acid and syringic acid, and catechin and catechol, provided the hair with deep colours that are relatively similar to colours under aqueous solutions, but when the ferulic acid/syringic acid mixture was employed, the colour of dyed hair was rather light, indicating that diffusion of the polymeric dyes into the hair was inefficient (Figs 4 and 7). We next evaluated the colour permanence to conventional shampooing of dyed hair, because colour staying power on shampooing determines hair colouring type (temporary, semi‐permanent or permanent) (Lang and Cotteret, 2007). Even after repeated shampooing with excess detergent, colour changes in all dyed hairs tested were negligible (Fig. 8), indicating that laccase polymerization of natural phenols can provide stable coloured polymers resistant to shampooing. Apart from colour permanence to shampooing, the time required to dye grey hair (less than 1 h) is also critical for practical application of this methodology. In general, efficient fibre swelling promoting dye sorption and sorption speed can be achieved by high pH and temperature (Kim et al., 2008), but such reaction conditions are obviously inappropriate on a human scalp as well as a laccase activity. Alternatively, chemical swelling agents (Burkinshaw et al., 2002) that are both non‐toxic and compatible with laccase catalytic action may accelerate diffusion of coloured dyes into hair that is a natural keratin protein fibre. Currently, we incorporate a chemical swelling agent into enzymatic colouration processes to accelerate diffusion of dye into keratin hair.
Figure 7.
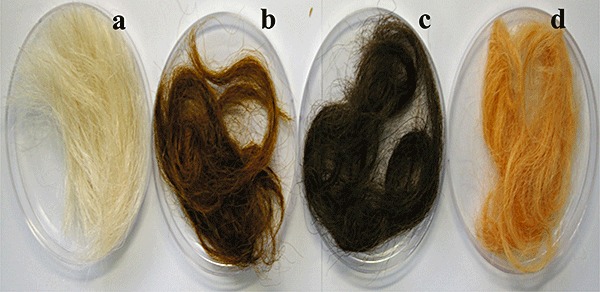
Grey keratin hair dyeing by selected laccase‐catalysed colouration reactions. (A) Virgin keratin hair. (B) Keratin hair dyed with gallic acid plus syringic acid. (C) Keratin hair dyed with catechin plus catechol. (D) Keratin hair dyed with ferulic acid plus syringic acid.
Figure 8.
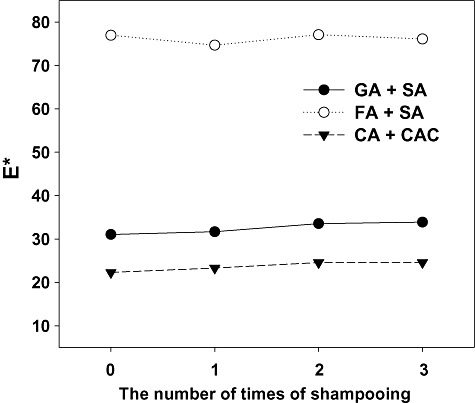
Colour permanence of dyed keratin hairs to shampooing. E* value was calculated using the formula: [(L*)2 + (a*)2 + (b*)2]1/2. GA, gallic acid; SA, syringic acid; FA, ferulic acid; CA, catechin; CAC, catechol.
Overall, our findings indicate that laccase‐based polymerization of natural phenols based on monomer blending strategy can result in colourful polymer synthesis leading to the discovery of desired colours that may be useful in cosmetic or food processing industry as eco‐friendly organic pigments. The most natural phenols we tested are derived from edible plant fibres such as lignin and tannin. Therefore, our novel strategy to make polymeric dyes by structurally mimicking plant fibre synthesis would be a promising ‘green’ technology to overcome current limitation of conventional polymeric or tar dyes that are widely used in cosmetic or food processing industry. In fact, in this study we found novel combinations of natural phenols leading to polymeric dyes synthesis whose colours are very useful in hair dyeing and also characterized natural phenols capable of generating diverse colours under the dual‐monomer system.
Experimental procedures
Chemicals
The phenolic compounds mentioned in Fig. 1 were purchased from Sigma‐Aldrich except for ferulic acid. Ferulic acid, 2,2′‐azino‐bis‐(3‐ethylbenzothiazoline‐6‐sulfonate) (ABTS), and T. versicolor laccase were supplied by Fluka. The laccase was used without further purification. However, we confirmed that laccase is 41 kDa and that there are no other laccase isozymes, via polyacrylamide gel electrophoresis on native PAGE and subsequent incubation with guaiacol (Murugesan et al., 2007). Peroxidase activities such as lignin and Mn2 ± dependent peroxidases were also negligible compared with the laccase activity. Grey yak hairs were provided by PheonixKorea (Hwaseong, Korea).
Enzyme assays
Laccase activity was measured at 30°C using 1 mM ABTS as the substrate (Wolfenden and Wilson, 1982). The assay mixture (1 ml) contained 880 µl of 100 mM sodium acetate buffer pH 5.0, 100 µl of ABTS stock (final concentration 1 mM), and 20 µl of appropriately diluted enzyme. The absorbance increase of the assay mixture was monitored at 420 nm (E420 = 36 000 M−1 cm−1) using a UV‐visible spectrophotometer (Cary 3‐Bio, Varian, France). The enzyme activities were expressed in international units (U), defined as the amount of enzyme needed to produce 1 µmol of oxidized ABTS min−1. Commercial T. versicolor laccase showed 6.62 × 105 U mg−1.
Screening for colour generation
Colouration reactions by laccase and phenolic monomers were performed at room temperature in 100 mM sodium‐acetate buffer pH 5.0, containing 25% (v/v) absolute ethanol, under complete darkness to exclude possible involvement of light‐induced phenol oxidation. Trametes versicolor laccase (4.3 mg ml−1) was dissolved in 100 mM sodium‐acetate buffer pH 5.0 for catalysis stock. Each phenolic compound (5 mg ml−1) was dissolved in 100 mM sodium‐acetate buffer pH 5.0, containing 50% (v/v) absolute ethanol. Reaction mixtures (20 ml) were adjusted to contain 25% (v/v) absolute ethanol after adding laccase (6 µg ml−1) and single or dual phenolic compounds (25 µg ml−1): in the case of dual monomers, we mixed two kinds of monomers (1:1 mass ratio) and the concentration sum of two monomers was 25 µg ml−1. We assessed colour differences of 120 kinds of colouration reactions using the standard commission international de L'Eclairage (CIE L*a*b*) colour system that is utilized extensively in examining various materials with regard to colour (Abu‐bakr et al., 2000; Ruiz et al., 2008). L* values from 0 (black) to 100 (white) represent luminosity, a* values range from −60 (green) to 60 (red), and b* values range from −60 (blue) to 60 (yellow). Colour values of 35 h incubated reaction mixtures were measured by a chromameter (CM‐3500d, Minolta, Japan) tristimulus colour analyser after calibrated with distilled water and black porcelain reference plate. Statistical analysis for linear regression was performed by using GraphPad Instat software (Ver. 3.05).
Characterization of selected colouration reactions
Based on the screening study, three different dual‐monomer cocktails, namely gallic acid and syringic acid, catechin and catechol, and ferulic acid and syringic acid, were selected for further characterization. We employed colourimetry, liquid chromatography, UV‐visible spectrophotometry, light scattering spectrophotometry, and ESI‐MS coupled with CID MS/MS to characterize polymer synthesis in the selected colourations. Unlike the screening experiments, we used much higher concentrations of monomers (10 mg ml−1) and laccase enzyme (0.25 mg ml−1) to maximize the efficiency of colouration reactions under same buffer condition (20 ml): dual monomers were mixed with 1:1 mass ratio. Change of each colour value with incubation times was measured by a chromameter (CM‐3500d, Minolta, Japan) tristimulus colour analyser. The extent of each monomer removal in the colouration reactions was analysed by reverse phase‐high performance liquid chromatograpy (RP‐HPLC) after adding 50% of acetonitrile to inactivate laccase enzyme and filtering through 0.45 µm syringe filter (Millipore PTFE type). Residual monomers were calculated from peak areas using calibration curve of standards. RP‐HPLC was performed in an Agilent 1100 series equipped with a diode array detector (Agilent G1315A, Agilent, Germany) and a ZORBAX SB C‐18 column at 25°C, with an aqueous solvent system (flow rate, 1.0 ml min−1) containing 0.1% (w/v) ortho‐phosphoric acid and 10% acetonitrile: in the case of ferulic acid, the aqueous solvent system containing 30% acetonitrile was used due to its hydrophobicity. Absorbance during RP‐HPLC was monitored at 267 nm for gallic acid and syringic acid, 276 nm for catechin and catechol, and 286 nm for ferulic acid.
ESI‐MS coupled with CID MS/MS (API 2000, Applied Biosystem, USA) was performed with negative mode (−4000 V). We used deionized water as reaction solution to exclude salts inhibiting measuring accurate m/z values. After 30 min reactions, the reactions were extracted with ethyl acetate and the extracts were then dried by nitrogen purging. Finally, the extracts were dissolved in 50% acetonitrile aqueous solutions. Before analysing the reaction samples, we first obtained CID MS/MS data of each monomer (e.g. gallic acid, ferulic acid, syringic acid, catechin and catechol). In this case, we used pure standard chemicals purchased from Sigma‐Aldrich to obtain reliable CID MS/MS data. We then compared CID MS/MS fragmentation patterns of hetero‐ or homo‐dimer and timer ions with those of standard monomers to elucidate the monomer composition of the dimer and trimer ions.
To measure enzyme kinetic constants, initial oxidation rates were estimated by decreases in substrate absorbance in the case of ferulic acid or by increase in product absorbance in the cases of gallic acid, syringic acid, catechin and catechol. Different concentrations of substrates with a constant amount of laccase (25 mg l−1) in 100 mM sodium citrate buffer pH 5.0 were used to calculate kinetic constants (Km and Vmax) by the Lineweaver–Burk equation. The molar absorbances of the substrates were estimated under reaction conditions. The molar absorbances of the reaction products were obtained after complete substrate oxidation with laccase.
Insoluble polymers were harvested by centrifugation (15 000 rpm for 10 min) from the 22 h incubated colouration reactions. The polymers were washed three times with distilled water by repeated centrifugation and gentle pipetting, and the washed polymers were suspended in distilled water to measure hydrodynamic size distribution using an electrophoretic light scattering spectrophotometer (ELS 8000, Otsuka, Japan). Soluble polymers were also obtained by filtering through 0.45 µm syringe filter (Millipore PTFE type) from the colouration reactions. Visible light absorbance characteristics of soluble polymers were evaluated using a UV‐visible spectrophotometer (Cary 3‐Bio, Varian, France).
Grey keratin hair dyeing and colour permanence test to shampooing
Each tress of grey yak hair (7 cm length, 3 g) was completely soaked in sodium acetate buffer pH 5.0 (30 ml), containing 25% (v/v) absolute ethanol and dye precursors (10 mg ml−1): dual monomers were mixed with 1:1 mass ratio. Dyeing process was initiated with the addition of laccase enzyme (0.25 mg ml−1). After 22 h incubation in a shaking incubator (160 rpm at 28°C), the tresses were washed with excessive water for 5 min. Colour permanence of dyed keratin hairs to shampooing was evaluated by E* value change with repeated shampooing. Detergents used for shampooing are a mixture of ammonium lauryl sulfate and ammonium laureth sulfate (3:1 mass ratio) that are widely used for conventional shampoos (Bushman and Wis, 1982). Washing solution containing the detergent mixture and each tress of dyed keratin hair (50:1 mass ratio) was incubated in a shaking incubator (160 rpm at 28°C) for 10 min. After finishing detergent treatment, tresses were washed with excessive water for 5 min and then colour values (L*, a* and b*) of the tresses were measured using a chromameter (LabScan XE, Hunter lab, USA) tristimulus colour analyser calibrated with a white porcelain reference plate. This process was repeated three times. E* value was calculated using the formula: [(L*)2 + (a*)2 + (b*)2]1/2 (Abu‐bakr et al., 2000).
Acknowledgments
We thank Gye‐Hyen Kim (POSTECH) and Dr Jin‐Sung Kim (Chonbuk national university hospital) for advice in statistical analysis and ESI‐CID MS/MS analysis respectively. J.‐R. Jeon thanks Dr Petr Baldrian (Academy of Science of the Czech Republic) for helpful discussion. This work was financially supported by Amorepacific Corporation.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Colour values of laccase-catalysed polymeric reactions containing single or dual monomers. The values were measured after 35 h incubation by a liquid colourimetry. AS, acetosyringone; VA, vanillic acid; SA, syringic acid; GA, gallic acid; HA, homovanillyl alcohol; PCA, p-coumaric acid; VN, vanillin; SAH, syringaldehyde; AV, acetovanillone; GAC, guaiacol; FA, ferulic acid; CA, catechin; SCA, salicylic acid; TA, tyramine; CAC, catechol.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Abu‐bakr N., Han L., Okamoto A., Iwaku M. Color stability of compomer after immersion in various media. J Esthet Dent. 2000;12:258–263. doi: 10.1111/j.1708-8240.2000.tb00232.x. [DOI] [PubMed] [Google Scholar]
- Baldrian P. Fungal laccases‐occurrence and properties. FEMS Microbiol Rev. 2006;30:215–242. doi: 10.1111/j.1574-4976.2005.00010.x. [DOI] [PubMed] [Google Scholar]
- Burkinshaw S.M., Froehling P.E., Mignanelli M. The effect of hyperbranched polymers on the dyeing of polypropylene fibres. Dyes Pigments. 2002;53:229–235. [Google Scholar]
- Bushman D.W., Wis R. 1982.
- Camarero S., Ibarra D., Jesus M., Martinez M.J., Martinez A.T. Lignin‐derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes. Appl Environ Microbiol. 2005;71:1775–1784. doi: 10.1128/AEM.71.4.1775-1784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarero S., Canas A.I., Nousiainen P., Record E., Lomancolo A., Martinez M.J., Martinez A.T. p‐Hydroxycinnamic acids as natural mediators for laccase oxidation of recalcitrant compounds. Environ Sci Technol. 2008;42:6703–6709. doi: 10.1021/es8008979. [DOI] [PubMed] [Google Scholar]
- Chandrashekara M.N., Ranganathaiah C. Diffusion of permanent liquid dye molecules in human hair investigated by positron lifetime spectroscopy. Colloids Surf B: Biointerf. 2009;40:129–134. doi: 10.1016/j.colsurfb.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Chen S.C., Chen C.H., Chen C.L., Hsu L.S., Huang Y.C., Chung K.T., Chye S.M. P‐Phenylenediamine induces p53‐mediated apoptosis in Mardin‐Darby canine kidney cells. Toxicol In Vitro. 2006;20:801–807. doi: 10.1016/j.tiv.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Claus H. Laccases and their occurrence in prokaryotes. Arch Microbiol. 2003;179:145–150. doi: 10.1007/s00203-002-0510-7. [DOI] [PubMed] [Google Scholar]
- Covington A.D., Evans C.S., Lilley T.H., Suparno O. Collagen and polyphenols: new relationships and new outcomes. Part 2. Phenolic reactions for simultaneous tanning and coloring. J Am Leather Chem As. 2005;100:336–343. [Google Scholar]
- Desentis‐Mendoza R.M., Hernandez‐Sanchez H., Moreno A., Rojas del C.E., Chel‐Guerrero L., Tamariz J., Jaramillo‐Flores M.E. Enzymatic polymerization of phenolic compounds using laccase and tyrosinase from Ustilago maydis. Biomacromolecules. 2006;7:1845–1854. doi: 10.1021/bm060159p. [DOI] [PubMed] [Google Scholar]
- Elegir G., Kindl A., Sadocco P., Orlandi M. Development of antimicrobial cellulose packaging through laccase‐mediated grafting of phenolic compounds. Enzyme Microb Technol. 2008;43:84–92. [Google Scholar]
- Fackler K., Kuncinger T., Ters T., Srebotnik E. Laccase‐catalyzed functionalization with 4‐hydroxy‐3‐methoxybenzylurea significantly improves internal bond of particle boards. Holzforschung. 2008;62:223–229. [Google Scholar]
- Fu T.L., Wang I.J. Synthesis and characterization of some novel polyfunctionally substituted indeno[2,1‐b]thiophene compounds derived from indanones. Dyes Pigments. 2008;76:590–595. [Google Scholar]
- Huang Y.C., Hung W.C., Kang W.Y., Chen W.T., Chai C.Y. Phenylenediamine induced DNA damage in SV‐40 immortalized human uroepithelial cells and expression of mutant p53 and COX‐2 proteins. Toxicol Lett. 2007;170:116–123. doi: 10.1016/j.toxlet.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Jeon J.R., Murugesan K., Kim Y.M., Kim E.J., Chang Y.S. Synergistic effect of laccase mediators on pentachlorophenol removal by Genoderma lucidum laccase. Appl Microbiol Biotechnol. 2008;81:783–790. doi: 10.1007/s00253-008-1753-2. [DOI] [PubMed] [Google Scholar]
- Kim S., Moldes D., Cavaco‐Paulo A. Laccases for enzymatic colouration of unbleached cotton. Enzyme Microb Technol. 2007;40:1788–1793. [Google Scholar]
- Kim S., Lopez C., Guebitz G., Cavaco‐Paulo A. Biological coloration of flax fabrics with flavonoids using laccase from Trametes hirsula. Eng Life Sci. 2008;8:324–330. [Google Scholar]
- Kudanga T., Prasetyo E.N., Sipila J., Nousiainen P., Widsten P., Kandelbauer A. Laccase‐mediated wood surface functionalization. Eng Life Sci. 2008;3:297–302. et al. [Google Scholar]
- Kunamneni A., Camarero S., Garcia‐Burgos C., Plou F.J., Ballesteros A., Alcalde M. Engineering and applications of fungal laccases for organic synthesis. Microb Cell Factories. 2008;7:32–48. doi: 10.1186/1475-2859-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang G., Cotteret J. 2007.
- Ly D., Kang K., Choi J.Y., Ishihara A., Back K., Lee S.G. HPLC analysis of serotonin, tryptamine, tyramine, and the hydroxycinnamic acid amides of serotonin and tyramine in food vegetables. J Med Food. 2008;11:385–389. doi: 10.1089/jmf.2007.514. [DOI] [PubMed] [Google Scholar]
- Manda K., Hammer E., Mikolasch A., Niedermeyer T., Dec J., Daniel A.J. Laccase‐induced cross‐coupling of 4‐aminobenzoic acid with para‐dihydroxylated compounds 2,5‐dihydroxy‐N‐(2‐hydroxyethyl)‐benzamide and 2,5‐dihydroxylbenzoic acid methyl ester. J Mol Catal B Enzym. 2005;35:86–92. et al. [Google Scholar]
- Marcoux D., Couture‐Tridel P.M., Riboulet‐Delmas G., Sasseville D. Sensitization to para‐phenylenediamine from a streetside temporary tattoo. Pediatr Dermatol. 2002;19:498–502. doi: 10.1046/j.1525-1470.2002.00218.x. [DOI] [PubMed] [Google Scholar]
- Mikolasch A., Schauer F. Fungal laccases as tools for the synthesis of new hybrid molecules and biomaterials. Appl Microbiol Biotechnol. 2009;82:605–624. doi: 10.1007/s00253-009-1869-z. [DOI] [PubMed] [Google Scholar]
- Murugesan K., Nam I.H., Kim Y.M., Chang Y.S. Decolorization of reactive dyes by a thermostable laccase produced by Ganoderma lucidum in solid state culture. Enzyme Microb Technol. 2007;40:1662–1672. [Google Scholar]
- Murugesan K., Yang I.H., Kim Y.M., Jeon J.R., Chang Y.S. Enhanced transformation of malachite green by laccase of Genoderma lucidum in the presence of natural phenolic compounds. Appl Microbiol Biotechnol. 2009;82:341–350. doi: 10.1007/s00253-008-1819-1. [DOI] [PubMed] [Google Scholar]
- O'Malley D.M., Whetten R., Bao W., Chen C.L., Sederoff R.R. The role of laccase in lignification. Plant J. 1993;4:751–757. [Google Scholar]
- Onuki T., Noguchi M., Mitamura J. 2004.
- Raskin I. Role of salicylic acid in plants. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:439–463. [Google Scholar]
- Reid I.D., Paice M.G. Biological bleaching of kraft pulps by white‐rot fungi and their enzymes. FEMS Microbial Rev. 1994;13:369–376. [Google Scholar]
- Riva S. Laccases: blue enzymes for green chemistry. Trends Biotechnol. 2006;24:219–226. doi: 10.1016/j.tibtech.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Ruiz D., Reich M., Bureau S., Renard C.M.G.C., Audergon J.M. Application of reflectance colorimeter measurements and infrared spectroscopy methods to rapid and nondestructive evaluation of carotenoids content in Apricot (Prunus armeniaca L.) J Agric Food Chem. 2008;56:4916–4922. doi: 10.1021/jf7036032. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Duenas F.J., Martinez A.T. Microbial degradation of lignin: how a bulky recalcitrant polymer is efficiently recycled in nature and how we can take advantage of this. Microb Biotechnol. 2009;2:164–177. doi: 10.1111/j.1751-7915.2008.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H‐.S., Guebitz G., Cavaco‐Paulo A. In Situ’ enzymatically prepared polymers for wool coloration. Macromol Mater Eng. 2001;286:691–694. [Google Scholar]
- Takada K., Nakamura A., Matsuo N., Inoue A., Someya K., Shimogaki H. Influence of oxidative and/or reductive treatment on human hair (I): Analysis of hair‐damage after oxidative and/or reductive treatment. J Oleo Sci. 2003;52:541–548. [Google Scholar]
- Tasi H.F., Wheeler M.H., Chang Y.C., Kwon‐Chung K.J. A developmentally regulated gene cluster involved in condial pigment biosynthesis in Aspergillus fumigates. J Bacteriol. 1999;181:6469–6477. doi: 10.1128/jb.181.20.6469-6477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towns A.D. Developments in azo disperse dyes derived from heterocyclic diazo components. Dyes Pigments. 1999;42:3–28. [Google Scholar]
- Uyama H., Kobayashi S. Enzyme‐catalyzed polymerization to functional polymers. J Mol Catal B Enzym. 2002;19:117–127. [Google Scholar]
- Uyama H., Kobayashi S. Enzymatic synthesis and properties of polymers from polyphenols. Adv Polym Sci. 2006;194:51–67. [Google Scholar]
- Wall F.E. Bleaches, hair colorings, and dye removers. In: Balsam M.S., Sagarin E., editors. Academic press; 1972. pp. 279–243. [Google Scholar]
- Wolfenden B.S., Wilson R.L. Radical cations as reference chromogens in kinetic studies of one‐electron transfer reactions: pulse radio analysis studies of 2,2′‐azinobis‐(3‐ethlbenzthiazoline‐bisulfonate) J Chem Soc Perkin Trans. 1982;II:805–812. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Colour values of laccase-catalysed polymeric reactions containing single or dual monomers. The values were measured after 35 h incubation by a liquid colourimetry. AS, acetosyringone; VA, vanillic acid; SA, syringic acid; GA, gallic acid; HA, homovanillyl alcohol; PCA, p-coumaric acid; VN, vanillin; SAH, syringaldehyde; AV, acetovanillone; GAC, guaiacol; FA, ferulic acid; CA, catechin; SCA, salicylic acid; TA, tyramine; CAC, catechol.


