Summary
For over 40 years, natural products have served us well in combating cancer. The main sources of these successful compounds are microbes and plants from the terrestrial and marine environments. The microbes serve as a major source of natural products with anti‐tumour activity. A number of these products were first discovered as antibiotics. Another major contribution comes from plant alkaloids, taxoids and podophyllotoxins. A vast array of biological metabolites can be obtained from the marine world, which can be used for effective cancer treatment. The search for novel drugs is still a priority goal for cancer therapy, due to the rapid development of resistance to chemotherapeutic drugs. In addition, the high toxicity usually associated with some cancer chemotherapy drugs and their undesirable side‐effects increase the demand for novel anti‐tumour drugs active against untreatable tumours, with fewer side‐effects and/or with greater therapeutic efficiency. This review points out those technologies needed to produce the anti‐tumour compounds of the future.
Introduction
Natural products have been an overwhelming success in our society. The use of plant and microbial secondary metabolites has aided in doubling of our life span in the 20th century. They have reduced pain and suffering, and revolutionized medicine by enabling the transplantation of organs. Since their chemical diversity is based on biological and geographical diversity, the entire globe is explored for bioprospecting by researchers. Researchers have had easy access to terrestrial life from which most of the pharmaceutically successful natural products originate. However, the ocean hosts a vast repertoire of life forms brimming with natural products of potential pharmaceutical importance. Marine bioprospecting is a relatively new phenomenon; thus, marine life is a relatively unexplored area of opportunity. New methods are being developed to grow the so‐called ‘unculturable’ microbes from both the soil and the sea. Most biologically active natural products are secondary metabolites with complex structures. In some cases, the natural product itself can be used, but in others, derivatives made chemically or biologically are the molecules used in medicine. Biosynthetic pathways are often genetically manipulated to yield the desired product. With the advent of combinatorial biosynthesis, thousands of new derivatives can now be made by this biological technique, which is complementary to combinatorial chemistry.
Secondary metabolism evolved in nature in response to needs and challenges of the natural environment. Nature has been continually carrying out its own version of combinatorial chemistry (Verdine, 1996) for over the three billion years in which bacteria have inhabited the earth (Holland, 1998). During that time, there has been an evolutionary process going on in which producers of secondary metabolites evolved according to their local environments. If the metabolites were useful to the producing species, the biosynthetic genes were retained and genetic modifications further improved the process. Combinatorial chemistry practised by nature is much more sophisticated than combinatorial chemistry in the laboratory, yielding exotic structures rich in stereochemistry, concatenated rings and reactive functional groups (Verdine, 1996). As a result, an amazing variety and number of products have been found in nature. This natural wealth is tapped for drug discovery using high‐throughput screening and fermentation, mining genomes for cryptic pathways, and combinatorial biosynthesis to generate new secondary metabolites related to existing pharmacophores. The success of the pharmaceutical industry depends on the combination of complementary technologies such as natural product discovery, high‐throughput screening, genomics, proteomics, metabolomics and combinatorial biosynthesis.
About a million natural products are known. The number produced by plants has been estimated to be between 500 000 and 600 000 (Berdy, 1995; Mendelson and Balick, 1995). The structures of 160 000 natural products were already elucidated by the late 1990s, a value growing by 10 000 per year (Henkel et al., 1999). Of these, about 100 000 are from plants. There are more than 20 000 microbial secondary metabolites (Knight et al., 2003). With regard to biological activity, there are about 200 000 to 250 000 biologically active products (active and/or toxic). About 100 000 secondary metabolites of molecular weight less than 2500 have been characterized. About half are produced by microbes and the other half by plants (Fenical and Jensen, 1993; Berdy, 1995; Roessner and Scott, 1996a,b).
Many of the terrestrial natural products succeeded in clinical trials and have effectively served medicine, some for over 50 years. Over 60% of approved and pre‐NDA candidates are either natural products or related to them, not including biologicals such as vaccines and monoclonal antibodies (Cragg et al., 1997; Newman et al., 2003). Six per cent are natural products, 27% are derivatives of natural products, 5% are synthetic with natural product pharmacophores, and 23% are synthetic mimics of natural products. Almost half of the best selling pharmaceuticals are natural or are related to natural products. In early 2008, there were 225 natural product‐based drugs in various testing procedures such as preclinical, clinical phases I to III, and preregistration (Harvey, 2008). Of these, 108 were from plants, 61 were semisynthetic, 25 were from bacteria, 24 from animals and 7 were fungal in origin. Of the 18 000 known marine natural products, 22 of these or their chemical derivatives are in clinical trials.
With regard to the structures of natural products, the estimated number of known isoprenoids (including terpenoids and carotenoids) is 50 000 (McCaskill and Croteau, 1997). Terpenes number 30 500 and as pharmaceuticals had a market of $12 billion in 2002 (Verpoorte, 2000; Raskin et al., 2002). Other major categories include polyketides, including macrolides, non‐ribosomal peptides, etc. About 4000 of the known natural products are halogenated.
Cancer is a major group of diseases. There were 1.5 million new cases in the USA in 2008. The global market for anti‐tumour agents was $60 billion in 2007. Natural products are the most important anti‐cancer agents. Three quarters of anti‐tumour compounds used in medicine are natural products or related to them. Of the 140 anti‐cancer agents approved since 1940 and available for use, over 60% can be traced to a natural product. Of the 126 small molecules among them, 67% are natural in origin (Cragg and Newman, 2005). In 2000, 57% of all drugs in clinical trials for cancer were either natural products or their derivatives (Cragg and Newman, 2000). From 1981 to 2002, natural products were the basis of 74% of all new chemical entities for cancer. Of the 225 natural product‐based drugs in various stages of clinical testing in 2008 mentioned above (Harvey, 2008), the therapeutic categories targeted included 86 for cancer.
Compounds with anti‐tumour activity belong to several structural classes such as anthracyclines, enediynes, indolocarbazoles, isoprenoids, polyketide macrolides, non‐ribosomal peptides including glycopeptides, and others. Most of the polyketides are produced by bacteria and fungi (Rawls, 1998; Xue et al., 1999). They include a number of anti‐tumour drugs such as taxol, which is made by both plants and fungi. Halogenated anti‐tumour candidates include salinosporamide A and rebeccamycin (Neumann et al., 2008).
The anti‐tumour compounds act by several mechanisms such as inducing apoptosis (programmed cell death) through DNA cleavage mediated by topoisomerase I or II inhibition, mitochondrial permeabilization, inhibition of key enzymes involved in signal transduction (e.g. proteases), or cellular metabolism, and by inhibiting tumour‐induced angiogenesis (recruitment of new blood vessels).
Microbial anti‐tumour products
A great number of anti‐tumour compounds are natural products, mainly produced by microorganisms, or their derivatives. In particular, actinomycetes are the producers of a large number of natural products with anti‐tumour properties, many of which also have antimicrobial activity. Thus, a broad screening of antibiotically active molecules for antagonistic activity against organisms other than microorganisms was proposed in the 1980s in order to yield new and useful lives for ‘failed antibiotics’. This resulted in the development of a large number of simple in vitro laboratory tests, e.g. enzyme inhibition screens (Umezawa, 1972; 1982) to detect, isolate and purify useful compounds. As a result, we entered into a new era in which microbial metabolites were applied to diseases heretofore only treated with synthetic compounds, and much success was achieved. One such area was that of anti‐tumour agents.
In their review on the use of microbes to prescreen potential anti‐tumour compounds, Newman and Shapiro (2008) concluded that microorganisms have played an important role in identifying compounds with therapeutic benefit against cancer. Most of the important compounds used for chemotherapy of tumours are microbially produced antibiotics or their derivatives. Some of the microbial anti‐tumour agents are shown in Table 1. One of the earliest applications of a microbial product was actinomycin D for Wilm's tumour in children. Use of this compound against stage I or stage II Wilm's tumour has resulted in a 90% survival rate (Chung, 2009). The structures of some of the most useful anti‐tumour compounds are shown in Fig. 1 (Salas and Mendez, 1998). Also used for anti‐tumour therapy is the enzyme l‐asparaginase.
Table 1.
Some microbial anti‐tumour compounds.
| Group | Example(s) |
|---|---|
| Aromatic polyketides (anthracyclines) | Daunorubicin, doxorubicin (adriamycin), epirubicin, pirirubicin, idarubicin, valrubicin, amrubicin |
| Glycopeptides | Bleomycin, phleomycin |
| Non‐ribosomal peptides | Actinomycin D (dactinomycin) |
| Anthracenones | Mithramycin, streptozotecin, pentostatin |
| Quinones | Mitosanes mitomycin C |
| Polyketides | Enediynes calicheamycin |
| Indolocarbazoles | Glycosides rebeccamycin |
| Polyketides | Macrolide lactones epotihilones, ixebepilone |
| Nucleosides | 2″‐deoxycoformycin (pentostatin) |
| Halogenated compounds | Salinosporamide A |
Figure 1.
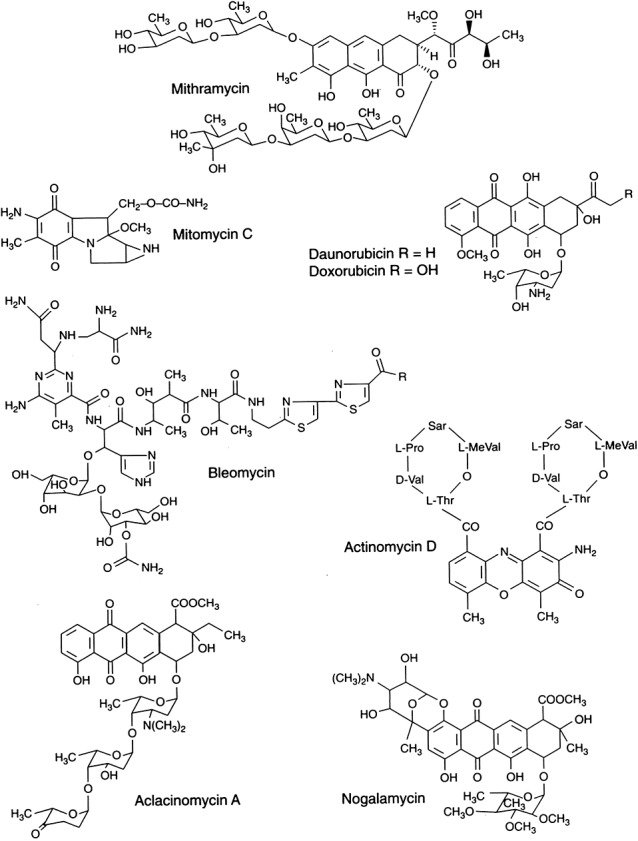
Structures of some anti‐tumour agents with clinical application. Reprinted from Salas and Mendez (1998) with permission.
Bleomycin is a glycopeptide produced by Streptoalloteichus hindustanus. It is used for squamous cell carcinomas, Hodgkin's lymphomas and testis tumours. A derivative of the bleomycin family, pingyangmycin, has been used in cancer therapy in China since 1978 (Zhen and Li, 2009). Another bleomycin derivative, Blenoxane, is used clinically with other compounds against lymphomas, skin carcinomas and tumours of the head, neck and testicles (Tao et al., 2010).
Derivatives (analogues) are usually made chemically or by manipulation of fermentation conditions. For example, addition of KBr to the rebeccamycin producer, Saccharothrix aerocolonigenes, yielded a brominated rebeccamycin (Lam et al., 1991). Addition of dl‐fluorotryptopan yielded two new fluorinated rebeccamycins and addition of dl‐fluorotryptophan led to a third (Lam et al., 2001). In clinical testing are the rebeccamycin derivative edotecarin and the geldanomycin derivative 17‐allylaminogeldanomycin. Interestingly, a fourth‐generation tetracycline known as SF 2575, produced by Streptomyces sp., has low antibiotic activity but a high level of activity against P388 leukaemia cells in vitro and many other types of cancer cells (Pickens et al., 2009). It appears to act against DNA topoisomerases I and II, the target of camptothecins and doxorubicin respectively.
A dramatic example of the power of natural products in the cure of cancer is that of metastatic testicular cancer. Although this type of cancer is rather uncommon, i.e. it was responsible for only 1% of male malignancies in the USA, it did cause 80 000 cases in the year 2000. Indeed, it is the most common carcinoma in men aged 15–35. The cure rate for disseminated testicular cancer was 5% in 1974; today it is 90% mainly due to the use of a triple combination of the microbial product bleomycin, the plant compound etoposide and the synthetic agent cisplatin (Einhorn and Donohue, 2002).
Anthracyclines
Anthracyclines are among the most well‐known clinically used anti‐tumour agents, especially daunorubicin (daunomycin), doxorubicin (14‐hydroxydaunorubicin); adriamycin, carminemycin and aclarubicin. The biosynthesis of daunorubicin and doxorubicin are depicted in Fig. 2 (Strohl et al., 1998).
Figure 2.
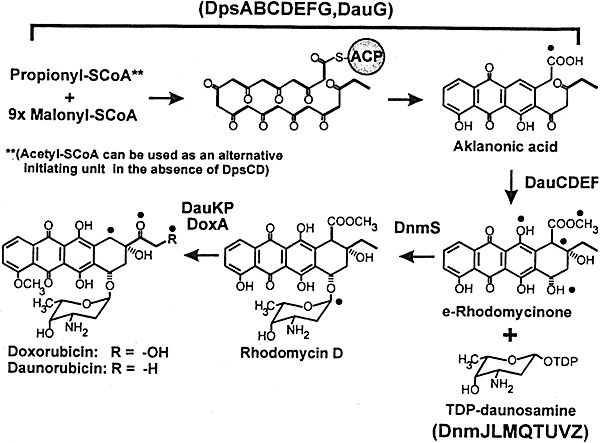
Abbreviated pathway for biosynthesis of daunorubicin and doxorubicin. Reprinted from Strohl and colleagues (1998) with permission.
A novel anthracycline, 11‐hydroxyaclacinomycin A, was produced by cloning the doxorubicin resistance gene and the aklavinone 11‐hydroxylase gene dnrF from the doxorubicin producer, Streptomyces peucetius ssp. caesius, into the aclacinomycin A producer (Hwang et al., 1995). The hybrid molecule showed greater activity against leukaemia and melanoma than aclacinomycin A. Another hybrid molecule produced was 2″‐amino‐11‐hydroxyaclacinomycin Y, which is highly active against tumours (Kim et al., 1996). Additional anthracyclines have been made by introducing DNA from Streptomyces purpurascens into Streptomyces galilaeus, both of which normally produce known anthracyclines (Niemi and Mäntsälä, 1995). Other novel anthracyclines were produced by cloning DNA from the nogalomycin producer, Streptomyces nogalater, into Streptomyces lividans and into an aclacinomycin‐negative mutant of S. galilaeus (Ylihonko et al., 1996). Cloning of the actI, actIV and actVII genes from Streptomyces coelicolor into the 2‐hydroxyaklavinone producer, S. galilaeus 31671, yielded the novel hybrid metabolites, desoxyerythrolaccin and 1‐O‐methyl‐desoxyerythrolaccin (Strohl et al., 1991). Similar studies yielded the novel metabolite aloesaponarin II (Bartel et al., 1990). Epirubicin (4′‐epidoxorubicin) is a semisynthetic anthracycline with less cardiotoxicity than doxorubicin (Arcamone et al., 1975). Genetic engineering of a blocked S. peucetius strain provided a new method to produce it (Madduri et al., 1998). The gene introduced was avrE of the avermectin‐producing Streptomyces avermitilis or the eryBIV genes of the erythromycin producer, Saccharopolyspora erythrea. These genes and the blocked gene in the recipient are involved in deoxysugar biosynthesis.
Enediynes
Enediynes are among the strongest naturally produced anti‐tumour compounds but are extremely toxic due to their action of causing apoptosis in normal cells as well as in tumour cells. They include calicheamicin, dynemicin A, esparamicin, kerdarcidin and neocarzinostatin. In recent years, scientists are trying to design nontoxic enediyne‐based anti‐tumour drugs (Kraka and Cremer, 2000). Progress towards this goal is proceeding by merging amidines with the natural enediyne dynemicin A (Kraka et al., 2008).
Epothilones
An unusual source of secondary metabolites are the myxobacteria, relatively large Gram‐negative rods which move by gliding or creeping. They form fruiting bodies and have a very diverse morphology. Over 400 compounds had been isolated from these organisms by 2005, but the first in clinical trials were the epothilones, potential anti‐tumour agents, which act like taxol (see Plant anti‐tumour agents below) but are active versus taxol‐resistant tumours (Kowalski et al., 1997). Epothilones are 16‐member ring polyketide macrolide lactones produced by the myxobacterium Sorangium cellulosum, which were originally developed as antifungal agents against rust fungi (Gerth et al., 1996; 2000), but have found their use as anti‐tumour compounds. Their structures are shown in Fig. 3 (Goodin et al., 2004). They contain a methylthiazole group attached by an olefinic bond. They are active against breast cancer and other forms of cancer (Chou et al., 1998). They bind to and stabilize microtubules essential for DNA replication and cell division, even more so than taxol. One epothilone, ixebepilone (Ixempra), produced chemically from epothilone B and which targets microtubules, has been recently approved by FDA. By preventing the disassembly of microtubules, epothilones cause arrest of the tumour cell cycle at the GM2/M phase and induce apoptosis. The mechanism is similar to that of taxol but epothilones bind to tubulin at different binding sites and induce microtubule polymerization. Production of epothilone B by S. cellulosum is accompanied by the undesirable epothilone A. Production of epothilone B over A is favoured by adding sodium propionate to the medium. Epothilone polyketides are more water‐soluble than taxol. The epothilone gene cluster has been cloned, sequenced, characterized and expressed in the faster growing S. coelicolor, resulting in the production of epothilones A and B (Julien et al., 2000; Tang et al., 2000).
Figure 3.
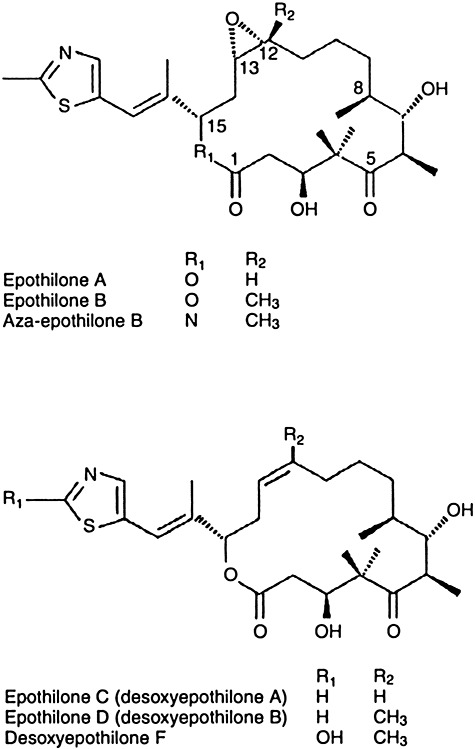
Structures of epothilones. Reprinted from Goodin and colleagues (2004) with permission.
Anti‐angiogenic agents
Angiogenesis is necessary for tumours to obtain oxygen and nutrients. Tumours actively secrete growth factors, which trigger angiogenesis. The concept of angiogenesis was established by Professor Judah Folkman (Cao and Langer, 2008). He proposed that tumour growth depends on angiogenesis and proposed the use of angiogenesis inhibitors as anti‐tumour agents, i.e. to target activated endothelial cells. He further proposed that the vascular endothelial growth factor (VEGF) is involved in angiogenesis and that it could be a target for anti‐angiogenic drugs. Fumagillin, produced by Aspergillus fumigatis, was one of the first agents found to act as an anti‐angiogenesis compound. Next to come along were its oxidation product ovalacin and the fumagillin analogue TNP470 (= AGM‐1470) (Ingber et al., 1990; Corey et al., 1994). TNP470 binds to and inhibits type 2 methionine aminopeptidase (MetAP2) (Griffith et al., 1997; Sin et al., 1997). This interferes with aminoterminal processing of methionine, which may lead to inactivation of enzymes essential for growth and migration of endothelial cells (Antoine et al., 1994; Turk et al., 1999). In animal models, TNP470 effectively treated many types of tumour and metastases (Yanase et al., 1993; Tanaka et al., 1995; Sasaki et al., 1998).
The monoclonal antibody Avastin (bevacizumab) is a first‐line remedy for metastatic coleorectal cancer and is an angiogenesis inhibitor. Antiangiogenic agents Pegaptanib (Macugen) and ranibizumab (Lucentis) were approved by FDA. Macugen is an aptomer of the VEGF and Lucentis is an anti‐VEGF antibody. By the end of 2007, 23 anti‐angiogenic drugs were in Phase III clinical trials and more than 30 were in Phase II. By 2008, 10 anti‐angiogenesis drugs had been approved. Eight are used against cancer and two are employed for treatment of age‐related macular degeneration. Anti‐angiogenesis therapy is now known as one of the four major types of cancer treatment.
Rapamycin (sirolimus) derivatives
This narrow spectrum polyketide antifungal agent (Vezina et al., 1975) attained stature not as an antibiotic but as a potent immunosuppressive agent used widely for organ transplantation. Rapamycin's mode of action is that of inhibiting the mTOR (mammalian target of rapamycin) phosphatidylinositol lipid kinase. Interestingly, it was also found to have anti‐tumour activity (Brown et al., 2003) by interfering with angiogenesis (Guba et al., 2002; Pray, 2002) and inducing apoptosis. Rapamycin was the basis of chemical modification and these efforts yielded important products such as temsirolimus (CCI‐779; (Torisel), everolimus and deforolimus (A23573). Temsirolimus, an mTOR protein kinase inhibitor, has been approved by FDA for renal cell carcinoma (Rini et al., 2007), while everolimus and deferolimus, are in clinical trials against cancer (Bailly, 2009).
Statins
The statins inhibit de novo production of cholesterol in the liver, the major source of blood cholesterol, thus lowering cholesterol levels in humans. They are microbially produced enzyme inhibitors, inhibiting 3‐hydroxy‐3‐methyl‐coenzyme A reductase, the regulatory and rate‐limiting enzyme of cholesterol biosynthesis in liver. As a result, they are the leading group of pharmaceuticals in the world. One of the most useful statins in lovastatin (monocolin K, mevinolin) produced by Monascus ruber (Endo, 1979) and Aspergillus terreus (Alberts et al., 1980). Statins also have anti‐cancer activity, i.e. they inhibit in vitro and in vivo growth of pancreatic tumours. They also sensitize tumours to cytostatic drugs such as gemcitabrine which is used for pancreatic cancer (Mistafa and Stenius, 2009). It has been shown that there is over a 50% reduction in risk of metastatic or fatal prostate cancer among people taking statins and an 80% reduction in pancreatic cancer among people using statins for 4 years. It has been found that lovastatin has anti‐tumour activity against Lewis Lung Carcinoma cells (Ho and Pan, 2009).
Searching for new agents
High‐throughput screening of 16 000 compounds for selective inhibition of cancer stem cells surprisingly revealed salinomycin to be the best (Gupta et al., 2009). In fact, it was 100 times more active than taxol. Salinomycin is used as a coccidiostat in poultry and other livestock and as an agent increasing feed efficiency in ruminant animals. Also active were etoposide, abamectin and nigericin. Both salinomycin and nigericin are structurally related polyether potassium ionophores.
Genetic manipulation is one way to obtain novel anti‐tumour agents. This is carried out by expressing biosynthetic gene clusters from anti‐tumour pathways in other organiasms. This has resulted in the formation of some novel hydroxylated and glycosylated anti‐tumour agents (Salas and Mendez, 1998).
The concept of genome mining was developed after it was found that the S. coelicolor genome contained 22 gene clusters encoding production of secondary metabolites but only four known products. The technique is useful for identifying genetic units with potential for synthesizing new drugs and has yielded many new antibiotics and anti‐tumour agents (Wilkinson and Micklefield, 2007; Gross, 2009; Zerikly and Challis, 2009). One anti‐cancer compound developed by Thallion Pharmaceuticals is ECO‐4601. It inhibits the Ras‐mitogen‐activated phosphokinase (MAPK) pathway and binds selectively to PBR (peripheral benzodiazapine receptor), which is overexpressed in many types of tumour. It is currently in clinical trials. As a result of genome mining with Micromonospora sp., a new anti‐tumour drug was discovered (Zazopoulos et al., 2003). The compound, ECO‐04601, is a farnesylated dibenzodiazapene, which induces apoptosis.
A new antibiotic showing anti‐tumour activity was isolated from a co‐culture present in the drainage of an abandoned mine, where the prevalent nutrient conditions created an extreme environment (Park et al., 2009). Such an environment results in defensive and offensive microbial interactions for survival of microbes. The two members of the co‐culture were the bacterium Sphingomonas sp. strain KMK‐001, and the fungus Aspergillus fumigatus strain KMC‐90. A new diketopiperazine disulfide, glionitrin A, was isolated from the co‐culture but was not detected in monoculture broths of KMK‐001 or KMC‐901. Glionitrin A is a (3S, 10aS) diketopiperazine disulfide containing a nitro aromatic ring. It displayed significant antibiotic activity against a series of microbes including methicillin‐resistant Staphylococcus aureus. An in vitro MTT cytotoxicity assay revealed that it has potent submicromolar cytotoxic activity against four human cancer cell lines: HCT‐116, A549, AGS and DU145.
Plant anti‐tumour products
Plants have been a useful source of approved anti‐cancer agents (Dholwani et al., 2008). Since 1961, nine plant‐derived compounds have been approved for use as anti‐cancer drugs in the USA (Table 2). Various groups are described below.
Table 2.
Some approved plant‐derived anti‐tumour compounds.
| Vinblastine (Velban) |
| Vincristine (Oncovin) |
| Etoposide |
| Teniposide |
| Taxol (paclitaxel) |
| Navelbine (Vinorelbine) |
| Taxotere (Docetaxel) |
| Camptothecin (Camptosar, Campto) |
| Topotecan (Hycamtin) |
| Irinotecan |
Alkaloids
Vinca monoterpene indole alkaloids, such as vinblastine and vincristine, orginate from the Madagascar periwinkle plant (Catharanthus roseus). Vinblastine is commonly used to treat cancers such as Hodgkin's lymphoma. Vinblastine and vincristine have important pharmacological activities but are synthetically challenging. Metabolic engineering of alkaloid biosynthesis can provide an efficient and environmentally friendly route to analogues of these pharmaceutically valuable natural products. The enzyme at the entry point of the pathway, strictosidine synthase, has a narrow substrate range and thus limits a pathway engineering approach. However, it has been demonstrated by Bernhardt and colleagues (2007) that with a different expression system and screening method, it is possible to rapidly identify strictosidine synthase variants that accept tryptamine analogues not utilized by the wild‐type enzyme. The variants are used in a stereoselective synthesis of β‐carboline analogues and are assessed for biosynthetic competence within the terpene indole alkaloid pathway. These results present an opportunity to explore metabolic engineering of ‘unnatural’ product production in the plant periwinkle.
Madagascar periwinkle also produces serpentines, which have shown promise as anti‐cancer agents. Other plant‐produced compounds have shown pharmacological activities including anti‐cancer activity but are too toxic for use in humans. However, researchers have been able to produce a range of halogenated alkaloids (Duflos et al., 2000). This strategy could help expand the range of available drug candidates for cancer.
Camptothecin is a modified monoterpene indole alkaloid produced by certain plants (angiosperms) (Wall and Wani, 1996). It also is produced by the endophytic fungus, Entrophospora infrequens, from the plant Nathapodytes foetida. In view of the low concentration of camptothecin in tree roots and poor yield from chemical synthesis, the fungal fermentation is very promising for industrial production. Camptothecin is used for recurrent colon cancer and has unusual activity against lung, ovarian, and uterine cancer (Amna et al., 2006). Colon cancer is the second leading cause of cancer fatalities in the USA and the third most common cancer among US citizens. Camptothecin is known commercially as Camptosar and Campto and achieved sales of $1 billion in 2003 (Lorence and Nessler, 2004). Camptothecin's water‐soluble derivatives irinotecan and topotecan are also used clinically.
The cellular target of camptothecin is type I DNA topoisomerase. When patients become resistant to irenotecan, its use can be prolonged by combining it with the monoclonal antibody Erbitux (Cetuximab). Erbitux blocks a protein that stimulates tumour growth and the combination helps metastatic colorectal cancer patients expressing epidermal growth factor receptor (EGFR). This protein is expressed in 80% of advanced metastatic colorectal cancers. The drug combination reduces invasion of normal tissues by tumour cells and the spread of tumours to new areas.
Taxol (paclitaxel), a diterpene alkaloid, has been a very successful anti‐tumour molecule. An outline of taxol biosynthesis is shown in Fig. 4 (Dejong et al., 2005). It was originally discovered in plants but has also been found to be a fungal metabolite (Stierle et al., 1993). Fungi, such as Taxomyces adreanae, Pestalotiopsis microspora, Tubercularia sp. and Phyllosticta citricarpa, produce taxol (Stierle et al., 1993; Li et al., 1996; Wang et al., 2000). Production by P. citricarpa is rather low, i.e. 265 µg l−1 (Kumaran et al., 2008). However, it is claimed that another fungus, Alternaria alternate var monosporus from the bark of Taxus yunanensis, after ultraviolet and nitrosoguanidine mutagenesis, can produce taxol at the high level of 227 mg l−1 (Duan et al., 2008). Originally isolated from the bark of the Pacific yew tree (Taxus brevifolia), taxol showed anti‐tumour activity but it took six trees of 100 years of age to treat one cancer patient (Horwitz, 1994). Today, it is produced by plant cell culture or by semisynthesis from taxoids made by Taxus species. These species make more than 350 known taxoid compounds (Baloglu and Kingston, 1999).
Figure 4.
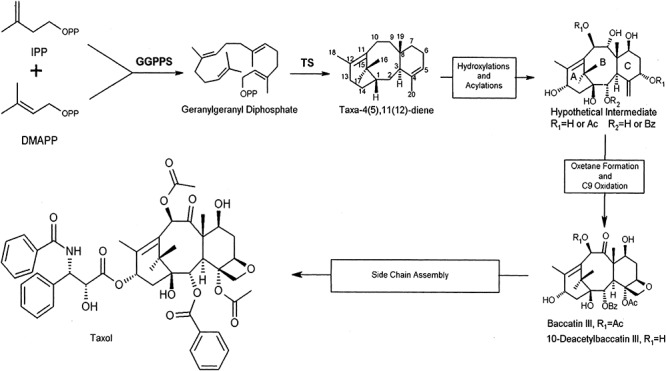
Outline of taxol biosynthesis. Reprinted from DeJong and colleagues (2005) with permission.
Early genetic engineering of Saccharomyces cerevisiae yielded no taxadiene (the taxol precursor) because too little of the intermediate, geranylgeranyl diphosphate, was formed. When the Taxus canadensis geranylgeranyl diphosphate synthase gene was introduced into S. cerevisiae, 1 mg l−1 of taxadiene was obtained (Dejong et al., 2005). More recent metabolic engineering has yielded a S. cerevisiae strain producing over 8 mg l−1 taxadiene and 33 mg l−1 of geranyl geraniol.
The use of cells of the plant Taxus chinensis to produce taxol became the industrial means to make the compound. The addition of methyl jasmonate, a plant signal transducer, increased production from 28 to 110 mg l−1 (Yukimune et al., 1996). The optimum temperature for growth of T. chinensis is 24°C and that for taxol synthesis is 29°C. Shifting from 24°C to 29°C at 14 days gave 137 mg l−1 at 21 days (Choi et al., 2000). There is a 6‐week process yielding 153 mg l−1 with T. chinensis (Bringi and Kadkade, 1993). Mixed cultures of Taxus plant cells and taxol‐producing endohytic fungi are under investigation (Li et al., 2009).
Taxol had sales of $1.6 billion in 2005. It is approved for breast and ovarian cancer and acts by blocking depolymerization of microtubules. In addition, taxol promotes tubulin polymerization and inhibits rapidly dividing mammalian cancer cells (Manfredi and Horowitz, 1984). Taxanes and camptothecins alone accounted for approximately one‐third of the global anti‐cancer market in 2002, with a market value of over $2.75 billion. An analogue of taxol, docetaxel (Taxotere), had sales of $3 billion in 2009 (Thayer, 2010).
Taxol also has antifungal activity by the same microtubule mechanism especially against oomycetes (Strobel and Long, 1998; Strobel, 2002). Oomycetes are water moulds exemplified by plant pathogens, such as Phytophthora, Pythium and Aphanomyces.
Etoposide and teniposide
These two compounds were derived as semisynthetic derivatives of podophyllotoxin, an antimitotic metabolite of the roots of the mayapple plant, Podophyllum peltatam (Deorukhkar, 2007; Nobili et al., 2009). The mayapple plant is an old herbal remedy. Etoposide is a topoisomerase II inhibitor. This essential enzyme is involved in eukaryotic cell growth by regulating levels of DNA supercoiling (Bender et al., 2008). Etoposide was approved for lung cancer, choriocarcinoma, ovarian and testicular cancer, lymphoma, and acute myeloid leukaemia. Teniposide was approved for tumours of the central nervous system, malignant lymphoma and bladder cancer.
Other compounds
The naphthoquinone pigment shikonin, a herbal medicine remedy, is produced by cell culture of the plant Lithospermum erythrorhizon, mainly for cosmetic use. Unexpectedly, shikonin and two derivatives were found to inhibit tumour growth in mice bearing Lewis Lung Carcinoma (Lee et al., 2008). Other plant natural products such as the isoflavone genistine, indole‐3‐carbinol (I3C), 3,3′‐diindolemethane, curcumin (−)‐epigallocatechin‐3‐gallate, resveratrol and lycopene are known to inhibit the growth of cancer cells (Sarkar et al., 2009). These natural compounds appear to act by interference in multiple cellular signalling pathways, activating cell death signals, and bringing on apoptosis of cancer cells without negatively affecting normal cells.
Marine anti‐tumour products
Although the ocean represents the centre of biological diversity with 34 of 37 phyla of life represented (compared with only 17 on land), prospecting marine resources for biotechnological use, particularly in drug discovery, is a relatively recent activity. Marine microorganisms encompass a complex and diverse assemblage of microscopic life forms, of which it is estimated that only 1% have been cultured or identified. Coral reefs and other highly diverse ecosystems, such as mangroves and sea grass, have been targeted for bioprospecting because they host a high level of biodiversity and are often characterized by intense competition for space, leading to chemical warfare among sessile organisms. Marine sponges produce numerous bioactive compounds with promising pharmaceutical properties. Cytarabine (Cytostar) used for non‐Hodgkin's lymphoma was originally derived from a sponge (Rayl, 1999). Sponges have been frequently hypothesized to contain compounds of bacterial origin and the bacterial symbionts have long been suspected to be the true producers of many drug candidates. Marine products with anti‐tumour activity are listed in Table 3.
Table 3.
Marine products with anti‐tumour activity.
| Cytarabine (Cyto star) |
| Pederin |
| Theopederins |
| Annamides |
| Trabectedin (Yondelis) |
| Aplidine |
| Ecteinascidin 743 (ET743) |
Scientists have recently identified an uncultured Pseudomonas sp. symbiont as the most likely producer of the defensive anti‐tumour polyketide pederin in Paederus fuscipes beetles by cloning the putative biosynthesis genes. Closely related genes have also been isolated from the highly complex metagenome of the marine sponge Theonella swinhoei, which is the source of the onnamides and theopederins, a group of polyketides that structurally resemble pederin. Sequence features of the isolated genes clearly indicate that they may belong to a prokaryotic genome and are responsible for the biosynthesis of almost the entire portion of the polyketide structure that is correlated with anti‐tumour activity. Besides providing further proof for the role of the related beetle symbiont‐derived genes, these findings raise intriguing ecological and evolutionary questions and have important general implications for the sustainable production of otherwise inaccessible marine drugs by using biotechnological strategies (Piel et al., 2004).
Sixty‐eight per cent of the pharmaceutically useful marine natural products employed are for cancer and the rest are used for inflammation, pain, asthma and Alzheimer's disease. Global sales of marine biotechnology products including anti‐cancer compounds exceeded $3.2 billion in 2007. Since 2007, the marine alkaloid trabectedin (Yondelis) was approved by FDA (Bailly, 2009). Another anti‐tumour compound, aplidine, a cyclic peptide, has Orphan Drug Status in Europe for acute lymphocytic leukaemia and is in Phase II clinical trials in the USA. Ecteinascidin 743 (ET 743) is in Phase III trials against sarcoma. A major problem in this field is that less than 1% of the commensal microbiotic consorta of marine invertebrates are culturable at this time.
Curacin A, obtained from a marine cyanobacterium Lyngbya majuscula isolated in Curacao, showed potent anti‐tumour activity (Blokhin et al., 1995). Other anti‐tumour agents derived from marine sources include eleutherobin, discodermolide, bryostatins, dolastatins and cephalostatins.
The symbionts of marine invertebrate animals continue to reveal interesting natural products (Dunlap et al., 2007). Variants of the toxic dolastin from the sea hare Dolabella auricalaria seem promising against cancer. These include soblidofin (T2F 1027), which completed Phase II against soft tissue sarcoma, and synthadotin (tasidotin, 1LX 651), which is at the same clinical stage against melanoma, prostate and non‐small cell lung cancers. These are thought to be produced by cyanobacteria sequestered by the marine invertebrates in their diet.
The marine environment continues to offer new opportunities for the discovery of anti‐tumour agents (Jensen et al., 2005). The new actinomycete genus Salinispora and its two species, S. tropica and S. arenicola, have been isolated around the world. These require seawater for growth. Salinispora tropica makes a new bicyclic γ‐lactone β‐lactam called salinosporamide A, which is a proteasome inhibitor (Macherla et al., 2005) in Phase II clinical trials against multiple myeloma and mantle cell lymphoma. Also the genus Marinophilus contains species that produce novel polyenes, which have no antifungal activity but display potent anti‐tumour activity.
Final comments
How can we come up with the anti‐tumour compounds of the future? High‐throughput screening and combinatorial chemistry have not provided the numbers of high‐quality leads that were anticipated. Combinatorial chemistry mainly yields minor modifications of present day drugs and absolutely requires new scaffolds, such as natural products, on which to build. Although comparative genomics is capable of disclosing new targets for drugs, the number of targets is so large that it requires tremendous investments of time and money to set up all the screens necessary to exploit this resource. This can only be handled by high‐throughput screening methodology which demands libraries of millions of chemical entities. It is clear that the future success of the pharmaceutical industry depends not only on high‐throughput screening and combinatorial chemistry, but on the combining of complementary technologies, such as natural product discovery, genomics, proteomics, metabolomics, metagenomics, structure‐function drug design, semi‐synthesis, recombinant DNA methodology, genome mining and combinatorial biosynthesis. In addition, ‘intelligent screening’ methods, robotic separation with structural analysis, metabolic engineering and synthetic biology offer exciting technologies for new natural product drug discovery and the future development of anti‐tumour compounds.
References
- Alberts A.W., Chen J., Kuron G., Hunt V., Huff J., Hoffman C. Mevinolin. A highly potent competitive inhibitor of hydroxymethylglutaryl‐coenzyme A reductase and a cholesterol‐lowering agent. Proc Natl Acad Sci USA. 1980;77:3957–3961. doi: 10.1073/pnas.77.7.3957. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amna T., Puri S.C., Verma V., Sharma J.P., Khajuria R.K., Spiteller M., Qasi G.N. Bioreactor studies on the endophytic fungus Entrophospora for the production of an anticancer alkaloid camptothecin. Can J Microbiol. 2006;52:189–196. doi: 10.1139/w05-122. [DOI] [PubMed] [Google Scholar]
- Antoine N., Greimers R., De Roanne C., Kusaka M., Heinen E., Simar L.J., Castronovo V. AGM‐1470, a potent angiogenesis inhibitor, prevents the entry of normal but not transformed endothelial cells into the G1 phase of the cell cycle. Cancer Res. 1994;54:2073–2076. [PubMed] [Google Scholar]
- Arcamone F., Penco S., Vigevani A., Redaelli S., Franchi G., Di Marco A. Synthesis and antitumor properties of new glycosides of daunomycinone and adriamycinone. J Med Chem. 1975;18:703–707. doi: 10.1021/jm00241a013. et al. [DOI] [PubMed] [Google Scholar]
- Bailly C. Ready for a comeback of natural products in oncology. Biochem Pharmacol. 2009;77:1447–1457. doi: 10.1016/j.bcp.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Baloglu E., Kingston D.G.I. The taxane diterpenoids. J Nat Prod. 1999;62:1448–1472. doi: 10.1021/np990176i. [DOI] [PubMed] [Google Scholar]
- Bartel P.L., Zhu C.B., Lampel J.S., Dosch D.C., Connors N.C., Strohl W.R. Biosynthesis of anthraquinones by interspecies cloning of actinorhodin genes in streptomycetes: clarification of actinorhodin gene functions. J Bacteriol. 1990;172:4816–4826. doi: 10.1128/jb.172.9.4816-4826.1990. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R.P., Jablonksy M.J., Shadid M., Romaine I., Dunlap N., Anklin C. Substituents on etoposide that interact with human topoisomerase IIα in the binary enzyme‐drug complex: contributions to etoposide binding and activity. Biochemistry. 2008;46:4501–4509. doi: 10.1021/bi702019z. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdy J. Are actinomycetes exhausted as a source of secondary metabolites? Biotechnologia. 1995;7–8:13–34. [Google Scholar]
- Bernhardt P., McCoy E., O'Connor S.E. Rapid identification of enzyme variants for reengineered alkaloid biosynthesis in periwinkle. Chem Biol. 2007;14:888–897. doi: 10.1016/j.chembiol.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokhin A.V., Yoo H.D., Geralds R.S., Nagle D.G., Gerwick W.H., Hamel E. Characterization of the interaction of the marine cyanobacterial natural product curacin A with the colchicine site of tubulin and initial structure‐activity studies with analogues. Mol Pharmacol. 1995;48:523–531. [PubMed] [Google Scholar]
- Bringi V., Kadkade P.G. 1993. , and ) Enhanced production of taxol and taxanes by cell cultures of Taxus species. Patent WO93/17121.
- Brown V.I., Fang J., Alcorn K., Barr R., Kim J.M., Wasserman R., Grupp S.A. Rapamycin is active against B‐precursor leukemia in vitro and in vivo, an effect that is modulated by IL‐7‐mediated signaling. Proc Natl Acad Sci USA. 2003;100:15113–15118. doi: 10.1073/pnas.2436348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Langer R. A review of Judah Folkman's remarkable achievements in biomedicine. Proc Natl Acad Sci USA. 2008;105:13203–13205. doi: 10.1073/pnas.0806582105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.‐K., Kim S.‐I., Son J.‐S., Hong S.‐S., Lee H.‐S., Chung I.‐S., Lee H.‐J. Intermittent maltose feeding enhances paclitaxel production in suspension culture of Taxus chinensis cells. Biotechnol Lett. 2000;22:1793–1796. [Google Scholar]
- Chou T.C., Zhang X.G., Harris C.R., Kuduk S.D., Balog A., Savin K.A. Desoxyepothilone B is curative against human tumor xenografts that are refractive to paclitaxel. Proc Natl Acad Sci USA. 1998;95:9642–9647. doi: 10.1073/pnas.95.26.15798. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K.‐T. H. Boyd Woodruff (b. 1917): antibiotics hunter and distinguished soil microbiologist. SIM News. 2009;59:178–185. [Google Scholar]
- Corey E.J., Guzman‐Perez A., Noe M.C. Short enantioselective synthesis of (−)‐ovalicin, a potent inhibitor of angiogenesis, using substrate‐enhanced catalytic asymmetric dihydroxylation. J Am Chem Soc. 1994;116:12109–12110. [Google Scholar]
- Cragg G.M., Newman D.J. Antineoplastic agents from natural sources: achievements and future directions. Expert Opin Investig Drugs. 2000;9:2783–2797. doi: 10.1517/13543784.9.12.2783. [DOI] [PubMed] [Google Scholar]
- Cragg G.M., Newman D.J. Plants as a source of anti‐cancer agents. J Ethnopharmacol. 2005;100:72–79. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Cragg G.M., Newman D.J., Snader K.M. Natural products in drug discovery and development. J Nat Prod. 1997;60:52–60. doi: 10.1021/np9604893. [DOI] [PubMed] [Google Scholar]
- Dejong J.M., Liu Y., Bollon A.P., Long R.M., Jennewein S., Williams D., Croteau R.B. Genetic engineering of taxol biosynthetic genes in Saccharomyces cerevisiae. Biotechnol Bioeng. 2005;93:212–224. doi: 10.1002/bit.20694. [DOI] [PubMed] [Google Scholar]
- Deorukhkar A. Back to basics: how natural products can provide the basis for new therapeutics. Expert Opin Investig Drugs. 2007;16:1753–1773. doi: 10.1517/13543784.16.11.1753. [DOI] [PubMed] [Google Scholar]
- Dholwani K.K., Saluja A.K., Gupta A.R., Shah D.R. A review on plant‐derived natural products and their analogs with anti‐tumor activity. Indian J Pharmacol. 2008;40:49–58. doi: 10.4103/0253-7613.41038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L.‐L., Chen H.‐R., Chen J.‐P., Li W.‐P., Hong L. Screening the high‐yield paclitaxel producing strain Alternaria alternate var monosporus. Chin J Antibiot. 2008;33:650–652. [Google Scholar]
- Duflos A., Fahy J., Thillaye du Boullay V., Barret J.‐M., Hill B. 2000.
- Dunlap W.C., Battershill C.N., Liptrot C.H., Cobb R.E., Bourne D.G., Jaspars M. Biomedicinals from the phytosymbionts of marine invertebrates: a molecular approach. Methods. 2007;42:358–376. doi: 10.1016/j.ymeth.2007.03.001. et al. [DOI] [PubMed] [Google Scholar]
- Einhorn L., Donohue J. Cis‐diamminedichloroplatinum, vinblastine, and bleomycin combination chemotherapy in disseminated testicular cancer. J Urol. 2002;167:928–932. [PubMed] [Google Scholar]
- Endo A. Monacolin K, a new hypocholsterolemic agent produced by a Monascus species. J Antibiot. 1979;32:852–854. doi: 10.7164/antibiotics.32.852. [DOI] [PubMed] [Google Scholar]
- Fenical W., Jensen P.R. Marine microorganisms: a new biomedical resource. In: Attaway D., Zaborsky O., editors. Plenum Press; 1993. pp. 419–457. [Google Scholar]
- Gerth K., Bedorf N., Hoefle G., Irschik H., Reichenbach H. Epothilones A and B: antifungal and cytotoxic compounds from Sorangium cellulosum (myxobacteria); production, physico‐chemical and biological properties. J Antibiot. 1996;49:560–563. doi: 10.7164/antibiotics.49.560. [DOI] [PubMed] [Google Scholar]
- Gerth K., Steinmetz H., Hofle G., Reichenbach H. Studies on biosynthesis of epothilones: the biosynthetic origin of the carbon skeleton. J Antibiot. 2000;53:1373–1377. doi: 10.7164/antibiotics.53.1373. [DOI] [PubMed] [Google Scholar]
- Goodin S., Kane M.P., Rubin E.H. Epothilones: mechanism of action and biologic activity. J Clin Oncol. 2004;22:2015–2025. doi: 10.1200/JCO.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Griffith E.C., Su Z., Turk B.E., Chen S., Chang Y.H., Wu Z. Methionine aminopeptidase (type 2) is the common target for angiogenesis inhibitors AGM‐1470 and ovalicin. Chem Biol. 1997;4:461–471. doi: 10.1016/s1074-5521(97)90198-8. [DOI] [PubMed] [Google Scholar]
- Gross H. Genomic mining – a concept for the discovery of new bioactive natural products. Curr Opin Drug Discov Devel. 2009;12:207–219. [PubMed] [Google Scholar]
- Guba M., von Breitenbuch P., Steinbauer M., Koehl G., Flegel S., Hornung M. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. et al. [DOI] [PubMed] [Google Scholar]
- Gupta P., Onder T., Jiang G., Tao K., Kuperwasser C., Weinberg R., Lander E. Identification of selective inhibitors of cancer stem cells by high‐throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A.L. Natural products in drug discovery. Drug Discov Today. 2008;13:894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Henkel T., Brunne R.M., Mueeller H., Reichel H. F. Statistical investigation into structural complementarity of natural products and synthetic compounds. Angew Chem Int Ed Engl. 1999;38:643–647. doi: 10.1002/(SICI)1521-3773(19990301)38:5<643::AID-ANIE643>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Ho B.‐Y., Pan T.‐M. The Monascus metabolite monacolin K reduces tumor progression and metastasis of Lewis lung carcinoma cells. J Agric Food Chem. 2009;57:8258–8265. doi: 10.1021/jf901619w. [DOI] [PubMed] [Google Scholar]
- Holland H.D. Evidence for life on earth more than 3850 million years ago. Science. 1998;275:38–39. doi: 10.1126/science.275.5296.38. [DOI] [PubMed] [Google Scholar]
- Horwitz S.B. Taxol (paclitaxel): mechanisms of action. Ann Oncol. 1994;5(6):S3–S6. [PubMed] [Google Scholar]
- Hwang C.K., Kim H.S., Hong Y.S., Kim Y.H., Hong S.K., Kim S.J., Lee J.J. Expression of Streptomyces peucetius genes for doxorubicin resistance and aklavinone 11‐hydroxylase in Streptomyces galilaeus ATCC 31133 and production of a hybrid aclacinomycin. Antimicrob Agents Chemother. 1995;39:1616–1620. doi: 10.1128/aac.39.7.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D., Fujita T., Kishimoto S., Sudo K., Kanamaru T., Brem H., Folkman J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature. 1990;348:555–557. doi: 10.1038/348555a0. [DOI] [PubMed] [Google Scholar]
- Jensen P.R., Mincer T.J., Williams P.G., Fenical W. Marine actinomycete diversity and natural product discovery. Antonie Van Leeuwenhoek. 2005;87:43–48. doi: 10.1007/s10482-004-6540-1. [DOI] [PubMed] [Google Scholar]
- Julien B., Shah S., Ziermann R., Goldman R., Katz L., Khosla C. Isolation and characterization of the epothilone biosynthetic gene cluster from Sorangium cellulosum. Gene. 2000;16:153–160. doi: 10.1016/s0378-1119(00)00149-9. [DOI] [PubMed] [Google Scholar]
- Kim H.‐S., Hong Y.‐S., Kim Y.‐H., Yoo O.‐J., Lee J.‐J. New anthracycline metabolites produced by the aklavinone 11‐hydroxylase gene in Streptomyces galilaeus ATCC 3113. J Antibiot. 1996;49:355–360. doi: 10.7164/antibiotics.49.355. [DOI] [PubMed] [Google Scholar]
- Knight V., Sanglier J.J., DiTullio D., Braccili S., Bonner P., Waters J. Diversifying microbial natural products for drug discovery. Appl Microbiol Biotechnol. 2003;62:446–458. doi: 10.1007/s00253-003-1381-9. et al. [DOI] [PubMed] [Google Scholar]
- Kowalski R.J., Giannakakou P., Gunasekera S.P., Longley R.E., Day B.W., Hamel E. The microtubule‐stabilizing agent discodermolide competitively inhibits the binding of paclitaxel (Taxol) to tubulin polymers, enhances tubulin nucleation reactions more potently than paclitaxel, and inhibits the growth of paclitaxel‐resistant cells. Mol Pharmacol. 1997;52:4613–4622. [PubMed] [Google Scholar]
- Kraka E., Cremer D. Computer design of anticancer drugs. A new enediyne warhead. J Am Chem Soc. 2000;122:8245–8264. [Google Scholar]
- Kraka E., Tuttle T., Cremer D. Design of a new warhead for the natural enediyne dynemicin A. An increase of biological activity. J Phys Chem B. 2008;112:2661–2670. doi: 10.1021/jp0773536. [DOI] [PubMed] [Google Scholar]
- Kumaran R.S., Muthumary J.P., Hur B.‐K. Taxol from Phyllosticta citricarpa, a leaf spot fungus of the angiosperm Citrus medica. J Biosci Bioeng. 2008;106:103–106. doi: 10.1263/jbb.106.103. [DOI] [PubMed] [Google Scholar]
- Lam K.S., Schroeder D.R., Veitch J.K., Matson J.A., Forenza S. Isolation of a bromo analog of rebeccamycin from Saccharothrix aerocolonigenes. J Antibiot. 1991;44:934–939. doi: 10.7164/antibiotics.44.934. [DOI] [PubMed] [Google Scholar]
- Lam K.S., Schroeder D.R., Veitch J.M., Colson K.L., Matson J.A., Rose W.C. Production, isolation and structure determination of novel fluoroindolocarbazoles from Saccharothrix aerocolonigenes ATCC 39243. J Antibiot. 2001;54:1–9. doi: 10.7164/antibiotics.54.1. et al. [DOI] [PubMed] [Google Scholar]
- Lee H.J., Lee H.J., Magesh V., Nam D., Lee E.O., Ahn K.S. Shikonin, acetylshikonin, and isobutyroylshikonin inhibit VEGF‐induced angiogenesis and suppress tumor growth in Lewis lung carcinoma‐bearing mice. Yakugaku Zasshi. 2008;128:1681–1688. doi: 10.1248/yakushi.128.1681. et al. [DOI] [PubMed] [Google Scholar]
- Li J.‐Y., Strobel G., Sidhu R., Hess W.M., Ford E.J. Endophytic taxol‐producing fungi from bald cypress, Taxodium distichum. Microbiology. 1996;142:2223–2226. doi: 10.1099/13500872-142-8-2223. [DOI] [PubMed] [Google Scholar]
- Li Y.‐C., Tao W.‐Y., Cheng L. Paclitaxel production using co‐culture of Taxus suspension cells and paclitaxel‐producing endophytic fungi in a co‐bioreactor. Appl Microbiol Biotechnol. 2009;83:233–239. doi: 10.1007/s00253-009-1856-4. [DOI] [PubMed] [Google Scholar]
- Lorence A., Nessler C.L. Camptothecin, over four decades of surprising findings. Phytochemistry. 2004;65:2735–2749. doi: 10.1016/j.phytochem.2004.09.001. [DOI] [PubMed] [Google Scholar]
- McCaskill D., Croteau R. Prospects for the bioengineering of isoprenoid biosynthesis. Adv Biochem Eng Biotechnol. 1997;55:107–146. doi: 10.1007/BFb0102064. [DOI] [PubMed] [Google Scholar]
- Macherla V.R., Mitchell S.S., Manam R.R., Reed K.A., Chao T.H., Nicholson B. Structure‐activity relationship studies of salinosporamide A (NPI‐0052), a novel marine derived proteasome inhibitor. J Med Chem. 2005;48:3684–3687. doi: 10.1021/jm048995+. et al. [DOI] [PubMed] [Google Scholar]
- Madduri K., Kennedy J., Rivola G., Inventi‐Solari A., Filippini S., Zanuso G. Production of the antitumor drug epirubicin (4′‐epidoxorubicin) and its precursor by a genetically engineered strain of Streptomyces peucetius. Nat Biotechnol. 1998;16:69–74. doi: 10.1038/nbt0198-69. et al. [DOI] [PubMed] [Google Scholar]
- Manfredi J., Horowitz S. Taxol: an antimitotic agent with a new mechanism of action. Pharmacol Ther. 1984;25:83–125. doi: 10.1016/0163-7258(84)90025-1. [DOI] [PubMed] [Google Scholar]
- Mendelson R., Balick M.J. The value of undiscovered pharmaceuticals in tropical forests. Econ Bot. 1995;49:223–227. [Google Scholar]
- Mistafa O., Stenius U. Statins inhibit Akt/PKB signaling via P2X7 receptor in pancreatic cancer cells. Biochem Pharmacol. 2009;78:1115–1126. doi: 10.1016/j.bcp.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Neumann C.S., Fujimori D.G., Walsh C.T. Halogenation strategies in natural product biosynthesis. Chem Biol. 2008;15:99–109. doi: 10.1016/j.chembiol.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Newman D.J., Shapiro S. Microbial prescreens for anticancer activity. SIM News. 2008;58:132–150. [Google Scholar]
- Newman D.J., Cragg G.M., Snader K.M. Natural products as sources of new drugs over the period 1981–2002. J Nat Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- Niemi J., Mäntsälä P. Nucleotide sequences and expression of genes from Streptomyces purpurascens that cause the production of new anthracyclines in Streptomyces galilaeus. J Bacteriol. 1995;177:2942–2945. doi: 10.1128/jb.177.10.2942-2945.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobili S., Lippi D., Witort E., Donnini M., Bausi L., Mini E., Capaccioli S. Natural compounds for cancer treatment and prevention. Pharmacol Res. 2009;59:365–378. doi: 10.1016/j.phrs.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Park H.B.B., Kwon H.C., Lee C.‐H., Yang H.O. Glionitrin A, an antibiotic‐antitumor metabolite derived from competitive interaction between abandoned mine microbes. J Nat Prod. 2009;72:248–252. doi: 10.1021/np800606e. [DOI] [PubMed] [Google Scholar]
- Pickens L.B., Kim W., Wang P., Zhou H., Watanabe K., Gomi S., Tay Y. Biochemical analysis of the biosynthetic pathway of an anticancer tetracycline SF2575. J Am Chem Soc. 2009;131:17677–17689. doi: 10.1021/ja907852c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel J., Hui D., Wen G., Butzke D., Platzer M., Fusetani N., Matsunaga S. Antitumor polyketide biosynthesis by an uncultivated bacterial symbiont of the marine sponge Theonella swinhoei. Proc Natl Acad Sci USA. 2004;101:16222–16227. doi: 10.1073/pnas.0405976101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pray L. Strange bedfellows in transplant drug therapy. Scientist. 2002;16:36. [Google Scholar]
- Raskin I., Ribnicky D.M., Komarnytsky S., Nebojsa I., Poulev A., Borisjuk N. Plants and human health in the twenty‐first century. Trends Biotechnol. 2002;20:522–531. doi: 10.1016/s0167-7799(02)02080-2. et al. [DOI] [PubMed] [Google Scholar]
- Rawls R.L. Modular enzymes. Chem Eng News. 1998;76:29–32. [Google Scholar]
- Rayl A.J.S. Oceans: medicine chests of the future? Scientist. 1999;13:1. [Google Scholar]
- Rini B., Kar S., Kirkpatrick P. Temsirolimus. Nat Rev Drug Discov. 2007;6:599–600. [Google Scholar]
- Roessner C.A., Scott A.I. Achieving natural product synthesis and diversity via catalytic networking ex vivo. Chem Biol. 1996a;3:325–330. doi: 10.1016/s1074-5521(96)90114-3. [DOI] [PubMed] [Google Scholar]
- Roessner C.A., Scott A.I. Genetically engineered synthesis of natural products: from alkaloids to corrins. Annu Rev Microbiol. 1996b;50:467–490. doi: 10.1146/annurev.micro.50.1.467. [DOI] [PubMed] [Google Scholar]
- Salas J.A., Mendez C. Genetic manipulation of antitumor‐agent biosynthesis to produce novel drugs. Trends Biotechnol. 1998;16:475–482. doi: 10.1016/s0167-7799(98)01198-6. [DOI] [PubMed] [Google Scholar]
- Sarkar F.H., Li Y., Wang Z., Kong D. Cellular signalling perturbation by natural products. Cell Signal. 2009;21:1541–1547. doi: 10.1016/j.cellsig.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A., Alcalde R.E., Nishiyama A., Lim D.D., Mese H., Akedo H., Matsumura T. Angiogenesis inhibitor TNP‐470 inhibits human breast cancer osteolytic bone metastasis in nude mice through the reduction of bone resorption. Cancer Res. 1998;58:462–467. [PubMed] [Google Scholar]
- Sin N., Meng L., Wang M.Q.W., Wen J.J., Bornmann W.G., Crews C.M. The anti‐angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP‐2. Proc Natl Acad Sci USA. 1997;94:6099–6103. doi: 10.1073/pnas.94.12.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stierle A., Strobel G., Stierle D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science. 1993;260:214–216. doi: 10.1126/science.8097061. [DOI] [PubMed] [Google Scholar]
- Strobel G.A. Rainforest endophytes and bioactive products. Crit Rev Biotechnol. 2002;22:315–333. doi: 10.1080/07388550290789531. [DOI] [PubMed] [Google Scholar]
- Strobel G.A., Long D.M. Endophytic microbes embody pharmaceutical potential. ASM News. 1998;64:263–268. [Google Scholar]
- Strohl W.R., Bartel P.L., Li Y., Connors N.C., Woodman R.H. Expression of polyketide biosynthesis and regulatory genes in heterologous streptomycetes. J Ind Microbiol. 1991;7:163–174. doi: 10.1007/BF01575879. [DOI] [PubMed] [Google Scholar]
- Strohl W.R., Dickens M.L., Rajgarhia V.B., Walczak R., Woo A., Priestley N.D. Biochemistry, molecular biology and protein‐protein interactions in daunorubicin/doxorubicin biosynthesis. Dev Ind Microbiol – BMP '97. 1998;97:15–22. [Google Scholar]
- Tanaka T., Konno H., Matsuda I., Nakamura S., Baba S. Prevention of hepatic metastasis of human colon cancer by angiogenesis inhibitor TNP‐470. Cancer Res. 1995;55:836–839. [PubMed] [Google Scholar]
- Tang L., Shah S., Chung L., Carney J., Katz L., Khosla C., Julien B. Cloning and heterologous expression of the epothilone gene cluster. Science. 2000;287:640–642. doi: 10.1126/science.287.5453.640. [DOI] [PubMed] [Google Scholar]
- Tao M., Wang L., Wendt‐Pienkowski E., Zhang N., Yang D., Galm U. Functional characterization of tlmH in Streptoalloteichus hindustanus E465‐94 ATCC 31158 unveiling new insight into tallysomycin biosynthesis and affording a novel bleomycin analog. Mol Biosyst. 2010;6:349–356. doi: 10.1039/b918106g. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer A. More than a supplier. Chem Eng News. 2010;88:25–27. [Google Scholar]
- Turk B.E., Griffith E.C., Wolf S., Biemann K., Chang Y.H., Liu J.O. Selective inhibition of amino‐terminal methionine processing by TNP‐470 and ovalicin in endothelial cells. Chem Biol. 1999;6:823–833. doi: 10.1016/s1074-5521(99)80129-x. [DOI] [PubMed] [Google Scholar]
- Umezawa H. University Park Press; 1972. [Google Scholar]
- Umezawa H. Low‐molecular‐weight enzyme inhibitors of microbial origin. Annu Rev Microbiol. 1982;36:75–99. doi: 10.1146/annurev.mi.36.100182.000451. [DOI] [PubMed] [Google Scholar]
- Verdine G.L. The combinatorial chemistry of nature. Nature. 1996;384:11–13. doi: 10.1038/384011a0. [DOI] [PubMed] [Google Scholar]
- Verpoorte R. Pharmacognosy in the new millennium: leadfinding and biotechnology. J Pharm Pharmacol. 2000;52:253–262. doi: 10.1211/0022357001773931. [DOI] [PubMed] [Google Scholar]
- Vezina C., Kudelski A., Sehgal S.N. Rapamycin (AY‐22, 989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot. 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- Wall M.E., Wani M.C. Camptothecin and taxol: from discovery to clinic. J Ethnopharmacol. 1996;51:239–254. doi: 10.1016/0378-8741(95)01367-9. [DOI] [PubMed] [Google Scholar]
- Wang J.F., Li G.L., Lu H.Y., Zhang Z.H., Huang Y.J., Su W.J. Taxol from Tubercularia sp. Strain TF5, an endophytic fungus of Taxus mairei. FEMS Microbiol Lett. 2000;193:249–253. doi: 10.1111/j.1574-6968.2000.tb09432.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson B., Micklefield J. Mining and engineering natural‐product biosynthetic pathways. Nat Chem Biol. 2007;3:379–386. doi: 10.1038/nchembio.2007.7. [DOI] [PubMed] [Google Scholar]
- Xue J., Duda L.‐C., Smith K.E., Fedorov A.V., Johnson P.D., Hulbert S.L. Electronic structure near the Fermi surface in the quasi‐one‐dimensional conductor Li0.9Mo6O17. Phys Rev Lett. 1999;83:1235–1238. et al. [Google Scholar]
- Yanase T., Tamura M., Fujita K., Kodama S., Tanaka K. Inhibitory effect of angiogenesis inhibitor TNP‐470 on tumor growth and metastasis of human cell lines in vitro and in vivo. Cancer Res. 1993;53:2566–2670. [PubMed] [Google Scholar]
- Ylihonko K., Hakala J., Kunnari T., Mäntsälälä P. Production of hybrid anthracycline antibiotics by heterologous expression of Streptomyces nogalater nogalamycin biosynthesis genes. Microbiology. 1996;142:1965–1972. doi: 10.1099/13500872-142-8-1965. [DOI] [PubMed] [Google Scholar]
- Yukimune Y., Tabata H., Higashi Y., Hara Y. Methyl jasmonate‐induced overproduction of paclitaxel and baccatin III in Taxus cell suspension cultures. Nat Biotechnol. 1996;14:1129–1132. doi: 10.1038/nbt0996-1129. [DOI] [PubMed] [Google Scholar]
- Zazopoulos E., Huang K., Staffa A., Liu W., Bachmann B.O., Nonaka K. A genomics‐guided approach for discovering and expressing cryptic metabolic pathways. Nat Biotechnol. 2003;21:187–190. doi: 10.1038/nbt784. et al. [DOI] [PubMed] [Google Scholar]
- Zerikly M., Challis G.L. Strategies for the discovery of new natural products by genome mining. Chembiochem. 2009;10:625–633. doi: 10.1002/cbic.200800389. [DOI] [PubMed] [Google Scholar]
- Zhen Y.‐S., Li D.‐D. Antitumor antibiotic pingyangmycin: research and clinical use for 40 years. Chin J Antibiot. 2009;34:577–580. [Google Scholar]


