Summary
Regulated promoters are useful tools for many aspects related to recombinant gene expression in bacteria, including for high‐level expression of heterologous proteins and for expression at physiological levels in metabolic engineering applications. In general, it is common to express the genes of interest from an inducible promoter controlled either by a positive regulator or by a repressor protein. In this review, we discuss established and potentially useful positively regulated bacterial promoter systems, with a particular emphasis on those that are controlled by the AraC‐XylS family of transcriptional activators. The systems function in a wide range of microorganisms, including enterobacteria, soil bacteria, lactic bacteria and streptomycetes. The available systems that have been applied to express heterologous genes are regulated either by sugars (l‐arabinose, l‐rhamnose, xylose and sucrose), substituted benzenes, cyclohexanone‐related compounds, ε‐caprolactam, propionate, thiostrepton, alkanes or peptides. It is of applied interest that some of the inducers require the presence of transport systems, some are more prone than others to become metabolized by the host and some have been applied mainly in one or a limited number of species. Based on bioinformatics analyses, the AraC‐XylS family of regulators contains a large number of different members (currently over 300), but only a small fraction of these, the XylS/Pm, AraC/PBAD, RhaR‐RhaS/rhaBAD, NitR/PnitA and ChnR/Pb regulator/promoter systems, have so far been explored for biotechnological applications.
Introduction
Expression of proteins in bacteria represents among the oldest techniques within recombinant DNA technology. The recombinant gene usually is placed under control of an inducible promoter on a multicopy vector and then transferred to a suitable bacterial host. For the most commonly used host organisms, a wide range of different expression systems are available, each with its specific characteristics. The goals when such tools are used may vary, ranging from high‐level protein production at industrial scales to low (physiological) levels of expression to control metabolic pathways. Ideally, expression systems should allow tight control of expression at all relevant levels. They should be inducible by agents or conditions that are cheap, should not require particular inducer uptake transport systems and the inducers should be metabolically inert relative to the host. It may also be preferable if the expression system works across species barriers, and for industrial applications, it is crucial that the entire system is stable during scale‐up under high‐cell‐density cultivation (HCDC). No such idealized system probably exists, and not all criteria are equally important for each specific application. On the other hand, knowledge of the characteristics of the many systems available may be very helpful for the researcher in order to be able to choose the system which is most likely best for each specific case.
Regulation of promoter activity is usually achieved by modulating environmental signals (pH, temperature, ligands) which are coupled to gene expression via transcriptional regulators that stimulate or repress transcription from specific promoters (Browning and Busby, 2004). The transcriptional regulators may function as activators (positive regulation), repressors (negative regulation) or both (positive and negative regulation). Inducer‐activator complexes stimulate transcription by binding to DNA at specific sites upstream of promoters and often proximal to the sites of binding for the host RNA polymerase (RNAP). They then interact with the polymerase or interfere in the transcription initiation process in such a way that transcription becomes facilitated (Adhya and Garges, 1990). In contrast, in the absence of inducer, repressors bind to operator sequences overlapping with or adjacent to the RNAP binding site, thereby blocking RNAP binding or inhibiting other steps of the transcription initiation process (Rojo, 1999). Upon activation, the inducer binds to the repressor, leading to a conformational change that makes the repressor unable to bind to the operator, allowing transcription by RNAP.
There is a continuous ongoing search for new expression systems that may either have more universally preferable properties, or are particularly useful for certain types of applications, often in specific hosts. In particular, there is a need for development of more gene expression systems that function in many different bacterial species and that can be used for fine‐tuning the expression of genes at low and physiologically relevant levels (Keasling, 1999; Terpe, 2006).
In the last decade due to the massive genome sequencing projects, identification of new inducible expression systems has become easier. By bioinformatics analyses, it is possible to rapidly identify numerous candidate systems, although it may require substantial amounts of experimental work to characterize the regulators. It is also often far from trivial to find out what particular inducer is activating the system. The regulatory proteins (transcription factors, TFs) controlling the expression may be classified according to sequence similarity of their DNA binding motifs or by alignments of their amino acid sequences. Currently, about 50 families of bacterial TFs have been reported (Rodionov, 2007), and the largest of these is LysR, followed by AraC‐XylS (see http://www.bactregulators.org). A common feature shared by many TFs is that they bind ligands with high specificity and this binding determines their activation state. The TFs may also be subjected to laboratory evolution strategies in order to change their ligand specificities (Calcagno Galvão and de Lorenzo, 2006). Interestingly, TFs have been depicted as molecular targets for drugs aiming at neutralizing or killing pathogenic bacteria (Bowser et al., 2007).
In this review, we will focus on TFs and their cognate promoters that are utilized to generate positively regulated expression tools, with a particular emphasis on regulators belonging to the AraC‐XylS family. Several positively regulated expression systems have already been developed for use in biotechnology, some are known but have not been developed into expression tools, and based on genome sequence analyses, numerous yet uncharacterized such systems certainly exist in nature. Some of the yet unexplored systems may potentially offer new and advantageous properties, and may be used in combination with existing systems to independently control the expression level of more than one gene in bacteria.
Overview of positively regulated expression systems that have been developed for biotechnological applications
The vast majority of all reported adjustable bacterial expression systems are negatively regulated. However, it has been proposed that positively regulated systems can confer certain advantages over those that are negatively regulated (Wilms et al., 2001 and references therein), particularly when very tight control (i.e. low background expression under non‐induced conditions) is needed (Altenbuchner and Mattes, 2005). Such properties are generally critical when expressing host‐toxic proteins, when running the production process under HCDC, and also when using the system as a tool for physiological studies. Positively regulated bacterial expression tools reported in the scientific literature are listed in Table 1.
Table 1.
Positively regulated bacterial expression systems and examples of biotechnological applications.
| Expression system | Regulator family | Representative characteristics and applications | Selected references |
|---|---|---|---|
| NisR/PnisA | Unknown | L. lactis expression vectors, food‐grade and antibacterial inducer nisin, tight control, limited host range. | Kuipers et al. (1993); De Ruyter et al. (1996); Bryan et al. (2000); Monnéet al. (2005) |
| SapR/PsapA and SapR/PsapIP | Unknown | pSIP vectors, induced by peptide sakacin A or P, tightly controlled and high‐level expression in L. sakei and L. plantarum. | Sørvig et al. (2003; 2005); Axelsson et al. (2003) |
| StbR/stbD | Unknown | pTRKH2 vector, induced by peptide‐pheromone (STP), regulated expression in S. thermophilus, relatively high background expression. | Blomqvist et al. (2006) |
| PrpR/PprpB | Unknown | Expression vector, propionate‐inducible, regulated and high‐level expression, S. enterica and other enteric bacteria. | Lee and Keasling (2005; 2006) |
| TipAL/PtipA | MerR‐SoxR | Expression vectors, thiostrepton‐inducible, regulated and high‐level expression in Streptomyces spp. | Kuhstoss and Rao (1991); Takanao et al. (1995); Ali et al. (2002); Dong et al. (2004) |
| PxylA | Unknown | Expression vectors, xylose‐inducible, function in low G + C gram‐positive bacteria. | Qasi et al. (2001); Radha and Gunasekaran (2008) |
| PsacB | Unknown | Expression vectors, sucrose‐inducible, adjustable and high‐level expression in Bacillus spp | Ye et al. (1999); Biedendieck et al. (2007) |
| SnpR/PsnpA | LysR | E. coli‐Streptomyces shuttle vectors, high level expression in Streptomyces spp, no inducer reported. | DeSanti and Strohl (2003); Nikodinovic and Priestly (2006) |
| AlkS/PalkB | LysR | E. coli expression vectors, alkane and DCPK inducible, high‐level expression, HCDC. | Panke et al. (1999); Makart et al. (2007) |
| XylR/Pu | XylR‐NtrC | Broad‐host‐range expression vectors, inducers are substituted benzenes, catabolite repressed, growth phase regulated. | Blatny et al. (1997a,b) |
| AraC/PBAD | AraC‐XylS | Expression vectors, l‐arabinose‐inducible, high‐level expression phage display, HCDC, metabolic engineering, broad‐host range. | Guzman et al. (1995); DeLisa et al. (1999); Sukchawalita et al. (1999); Huang et al. (2000); Lim et al. (2000); Loessner et al. (2007) |
| RhaR‐RhaS/rhaBAD | AraC‐XylS | E. coli expression vectors, l‐rhamnose‐inducible, tigh tregulation, high‐level expression. | Haldimann et al. (1998); Wilms et al. (2001) |
| XylS/Pm | AraC‐XylS | Broad host‐range expression vectors, multiple inducers (substituted benzenes), tight control, high‐level expression, metabolic engineering, HCDC. | HCDC.Mermod et al. (1986); Blatny et al. (1997a,b); Brautaset et al., 1998; 2000); Winther‐Larsen et al., 2000a,b; Gimmestad et al. (2003); Sletta et al. (2004; 2007); Bakkevig et al. (2005) |
| NitR/PnitA | AraC‐XylS | Expression vector, hyper‐inducible (ε‐caprolactam) high‐level expression, functions in Streptomyces strains. | Komeda et al. (1996); Herai et al. (2004) |
| ChnR/Pb | AraC‐XylS | Broad‐host‐range vectors, cyclohexanone‐inducible, high‐level and tightly controlled expression, metabolic engineering. | Steigedal and Valla (2008) |
Peptide induced expression systems
The popular nisin‐based system originating from Lactococcus lactis is somewhat atypical in that nisin (the inducer) is an antibacterial peptide (for a review, see Mierau and Kleerebezem, 2005) which can inhibit growth of gram‐positive bacteria. Nisin is ribosomally synthesized as a 57‐residue precursor peptide and then subjected to various modifications, eventually resulting in a secreted 34‐residue mature peptide. This peptide is a food grade inducer that can be extracted and purified from milk and it is useful for production of heterologous proteins in L. lactis. The nisin biosynthetic gene cluster in L. lactis was found to be under positive control of the NisR regulator (Kuipers et al., 1993). NisR, together with NisK, constitute a two‐component signal transduction system that responds to nisin. By placing the nisin gene promoter, PnisA, together with the nisR gene, a set of shuttle vectors for nisin‐inducible and tightly controlled heterologous protein expression in L. lactis bacteria have been constructed (De Ruyter et al., 1996; Bryan et al., 2000; Mierau and Kleerebezem, 2005; Monnéet al., 2005; Wu et al., 2006). One important limitation by using lactic acid bacteria as hosts for heterologous protein production is their low metabolic capacity typically resulting in relative poor volumetric yields (Mierau and Kleerebezem, 2005). The NisR/PnisA system, including NisK, has been demonstrated to function also in other gram‐positive bacteria, including streptococcal and enterococcal species (Eichenbaum et al., 1998; Bryan et al., 2000). Nisin acts as an inducer on the outside of the cells and is sensed by NisK. However, due to the antibacterial properties of nisin for a number of gram‐positive bacterial species, the host range of this expression system may be limited.
The so‐called pSIP vectors have been developed based on regulatory genes and promoters involved in the production of the bacteriocins sakacin A and sakacin P in Lactobacillus sakei (Axelsson et al., 2003). The vectors contain a sakacin A or P promoter, PsapA and PsapIP respectively together with a two‐component regulatory system (Sørvig et al., 2003; 2005), encoded by the sapRK operon, analogous to the nisin‐based system (see above). Recombinant expression can be induced by adding sakacin induction peptides to the growth medium, and the vectors were demonstrated useful for tightly regulated and high‐level expression of heterologous proteins in L. sakei and Lactobacillus plantarum (Sørvig et al., 2005).
Blomqvist and colleagues (2006) recently reported the construction of the vector pTRKH2, which carries the peptide pheromone‐inducible StbR/stbD regulator/promoter elements from Streptococcus thermophilus. The vector was used for regulated expression of the gusA reporter gene in S. thermophilus, and one drawback observed was a relative high background expression level. To our knowledge, this expression system has not been tested in other bacterial species.
The PrpR/PprpB regulator/promoter system
Lee and Keasling (2005; 2006) described the construction of propionate‐inducible expression vectors carrying the PrpR/PprpB regulator/promoter system. The PprpB promoter is responsible for transcription of the propionate catabolic genes and transcription is under positive control by the PrpR regulator. The authors demonstrated dose‐dependent heterologous expression with up to 1500‐fold induction ratios of various reporter genes in Escherichia coli and in Salmonella enterica. Propionate is cheap, it enters the host cell by passive diffusion (i.e. no particular transport system is required) and the background expression level in the absence of the inducer is very low. This system should be useful both for high‐level protein expression and for metabolic engineering studies. It should however be noticed that PprpB has CAP‐dependent activation and accordingly the carbon source used in the growth medium can severely affect background expression. Moreover, the inducer propionate can likely be metabolised and modified by many bacterial species.
The TipAL/PtipA regulator/promoter system
The thiostrepton‐inducible promoter PtipA from Streptomyces lividans was placed on a high‐copy‐number plasmid and used for adjustable expression of recombinant proteins in Streptomyces species (Kuhstoss and Rao, 1991; Takanao et al., 1995). Later, the MerR‐SoxR family type positive PtipA regulator TipAL was identified and characterized, and placed together with PtipA on plasmid vectors useful for thiostrepton‐induced recombinant expression in Streptomyces and other gram‐positive bacteria (Ali et al., 2002; Dong et al., 2004). Interestingly, expression from PtipA is under osmotic regulation and adjustment of growth medium osmolarity led to increased and prolonged TipAL‐dependent expression of heterologous genes in S. lividans (Ali et al., 2002). One important observation was that under conditions of high osmolarity, basal expression of this system increased considerably. It should also be pointed out that the inducer molecule, thiostrepton, is an antibiotic, and this may limit the host‐range of this expression system.
The PxylA and the Psac promoters
Both the xylose‐inducible PxylA promoter (Qasi et al., 2001; Radha and Gunasekaran, 2008) and the sucrose‐inducible Psac promoter (Ye et al., 1999; Biedendieck et al., 2007) have proven to be useful for regulated and high‐level recombinant gene expression of prokaryotic and eukaryotic proteins in Bacillus megaterium and other AT‐rich gram‐positive bacteria. The Psac promoter was shown to display an induction ratio of up to 350‐fold in B. megaterium. The PxylA promoter was demonstrated to be useful for regulated high‐level production of the protease keratinase in this organism. A common limitation with both these promoters is that the inducer molecules sucrose and xylose are both typically metabolized by host cells, and accordingly the availability of mutant host strains deficient in their utilization can be preferred when using these expression systems. To our knowledge, no TFs for any of these two promoters have to date been identified.
The SnpR/PsnpA regulator/promoter system
The LysR family represents the largest family of positively regulated TFs (for a review see Schell, 1993), yet few of its members have been used as tools for recombinant expression. However, the SnpR activated PsnpA promoter originating from Streptomyces sp. strain C5 was characterized (DeSanti and Strohl, 2003) and later applied for high‐level heterologous expression in Streptomyces species (Nikodinovic and Priestley, 2006). To our knowledge, no inducer has been reported for this expression system, and it has been shown that SnpR‐mediated expression from PsnpA is modulated by the host physiology and activated in the stationary phase. Accordingly, this system has certain limitations when controlled recombinant expression is needed (DeSanti and Strohl, 2003).
The AlkS/PalkB regulator/promoter system
The OCT plasmid of Pseudomonas oleovorans GPo1 carries two alk gene clusters encoding a set of enzymes for the degradation of alkanes (van Beilen et al., 1994). Expression of one of these clusters is controlled by the alkane‐responsive AlkS/PalkB regulator/promoter system. AlkS is a LysR family positive regulator that, in addition to alkanes, can be activated by the inducer molecule dicyclopropylketone (DCPK) (Sticher et al., 1997). The activated AlkS then binds to its cognate promoter PalkB and stimulates transcription. Expression vectors carrying the AlkS/PalkB genetic elements have been constructed and demonstrated to posses several favourable properties. For example, the DCPK inducer is water‐soluble and metabolically inert (Makart et al., 2007). Moreover, this inducer presumably requires no active uptake system to enter the cells (Sven Panke and Bernard Witholt, pers. comm.). The AlkS/PalkB expression system is not subject to catabolite repression in E. coli (Staijen et al., 1999). AlkS/PalkB has been used for tightly controlled and high‐level expression of recombinant xylene oxidase for biotransformation of styrene into (S)‐styrene oxide (Panke et al., 1999), and it has also been shown to function well for induced production of recombinant proteins under HCDC of E. coli (Makart et al., 2007).
The XylR/Pu regulator/promoter system
XylR/Pu controls expression of the upper‐pathway genes of the Pseudomonas putida TOL plasmid pWW0, and this operon is positioned physically together with the meta‐operon which is controlled by XylS/Pm (see The XylS/Pm regulator/promoter system). XylR belongs to the XylR‐NtrC family of TFs (see Table 1) and it binds to toluene‐based inducers and positively activates transcription from the Pu promoter. Interestingly, XylR also exerts positive control on the expression of the important TF XylS, which will be described in more detail below. The XylR/Pu genetic elements were fused to the RK2 broad‐host‐range minimal replication elements, and the resulting expression vectors were found to be useful particularly for fine‐tuning recombinant expression of heterologous genes at physiological levels (Blatny et al., 1997a,b). It should be noticed that Pu is under catabolite repression control (Duetz et al., 1996) and this expression system becomes leakier as the cells enter stationary phase (Blatny et al., 1997a).
The remaining expression systems listed in Table 1 are all members of the AraC‐XylS family of positive transcription regulators, and we will in the following pay particular attention to this important family.
The AraC‐XylS family of positively regulated TFs
Members of the AraC‐XylS family are among the most commonly applied positive regulators in experiments involving recombinant gene expression control in bacteria. Prior to the applied use, this family of regulators have attracted scientific interest for decades as novel model systems for basic research on bacterial gene regulation. The origin and biology of the AraC‐XylS family, and in particular the ‘mother’ protein AraC, have been extensively reviewed previously (Gallegos et al., 1997; Martin and Rosner, 2001; Egan, 2002; Tobes and Ramos, 2002; Schleif, 2003). Members of the group all share primary amino acid sequence homology to AraC, which was the first bacterial transcriptional activator described (Englesberg et al., 1965; Greenblatt and Schleif, 1971). Some years after the discovery of AraC, the RhaR and RhaS proteins were reported (Tobin and Schleif, 1987; 1990). Since then the family has grown considerably and was recently reported to include above 300 different members (Tobes and Ramos, 2002; Ruiz et al., 2003; Tropel and van der Meer, 2004), represented in a wide range of both gram‐positive and gram‐negative bacterial species. The vast majority of the family members are positive regulators, but some of them can also act as negative regulators. As a direct consequence of the massive ongoing bacterial genome sequencing ( http://www.genomesonline.org/), the total number of putative AraC‐XylS TFs identified is several thousands (Rodionov, 2007), and this number is likely to increase even more in the future. The members of the AraC‐XylS family regulate diverse cellular functions, such as carbon metabolism, various stress responses including antibiotic biosynthesis, and also pathogenesis (Gallegos et al., 1997). Interestingly, one member was recently demonstrated to be associated with cell survival following DNA damage in Bacteroides fragilis (Casanueva et al., 2008). The AraC‐XylS family sub‐group that regulates carbon metabolism is characterized by being of similar molecular sizes (about 300 amino acids), and they stimulate transcription from cognate promoters in response to the presence of effectors. Below we discuss the well‐characterized family members AraC and XylS as representative models to describe biology and structure/function characteristics of the AraC‐XylS family regulators.
The AraC/PBAD regulator/promoter system
AraC is the best‐characterized member of the AraC‐XylS family and it regulates transcription from promoters involved in catabolism of l‐arabinose, which is also the inducer of the system (Englesberg et al., 1965). When l‐arabinose is present, it positively activates transcription from the ara promoters. Interestingly, when l‐arabinose is not present, this protein also actively represses transcription from at least one of these promoters, PBAD (Englesberg et al., 1969). This promoter is catabolite‐repressed, so uninduced levels of transcription is further reduced by cell growth in the presence of glucose (Miyada et al., 1984). The AraC protein is a homodimeric protein, and each of its monomers contains a conserved C‐terminal DNA binding domain made up of about 100 amino acids and an N‐terminal non‐conserved dimerization domain. The latter domain also contains an l‐arabinose binding pocket (Wilcox and Meuris, 1976; Steffen and Schleif, 1977; Soisson et al., 1997a,b). In the absence of l‐arabinose, the AraC dimer binds to two DNA sites O2 and I1 separated by 210 base pairs (Fig. 1). This generates a characteristic DNA loop (Schleif, 1988) which in turn negatively interferes with the access of RNAP to the promoter in the looping region. When l‐arabinose is present and bound to AraC, instead of looping the regulator binds to the I1 and I2 sites on the DNA, leading to stimulation of transcription from the PBAD promoter via direct interaction with the host RNAP (Zhang et al., 1996). AraC can also downregulate its own expression by binding to an operator close to the araC promoter PC (see Schleif, 2003).
Figure 1.
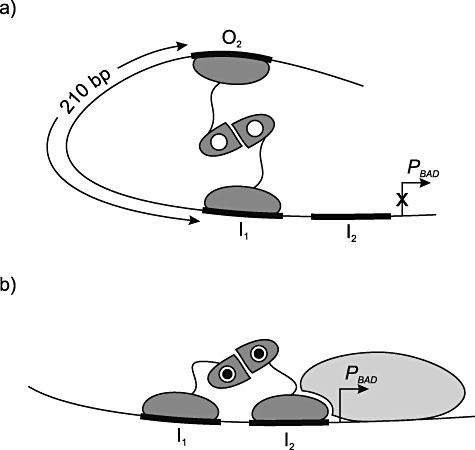
Binding of AraC to PBAD in the absence (A) and presence (B) of the inducer molecule l‐arabinose (closed circle) (modified with permission from Schleif, 2003). O2, I1 and I2 are DNA binding sites for AraC. The RNAP is depicted in light colour. For details see text section The AraC/PBADregulator/promoter system.
Experimental work involving functional expression, purification and biochemical characterization of AraC is technically very difficult, as reviewed by Schleif (2003). This property also holds for a large number of the AraC‐XylS family members; they are extremely insoluble. The major reason is predicted to be due to the unusually long contact area between the protein DNA binding domain and its target DNA (almost 40 base pairs). Each monomer contacts two major groove regions of the DNA (see Fig. 1) using two helix–turn–helix motifs (HTH) (Ogden et al., 1980; Hendricksen and Schleif, 1985; Brunelle and Schleif, 1989; Carra and Schleif, 1993). It has been proposed that the AraC protein, in particular the DNA binding domain, does not complete folding in the absence of DNA (Schleif, 2003). A partially folded DNA binding domain is sensitive to proteases and with an excessive number of hydrophobic residues exposed, this may also lead to aggregation. Due to all these problems, the AraC protein crystal structure was determined more than 20 years after its discovery (Soisson et al., 1997a,b). The technical difficulties experienced with AraC are most likely one major reason why few of the large number of TFs of this family have not yet been characterized biochemically (Martin and Rosner, 2001). Currently, 3D structures have been experimentally solved for only three members; AraC, and the monomeric regulators RobA, and MarA (Rhee et al., 1998; Kwon et al., 2000). The structure of the MarA protein is shown in Fig. 2. The conserved C‐terminal domain of the TF contains the characteristic HTH motif critical for DNA binding. In contrast, the N‐terminal domains among AraC‐XylS family TFs are structurally highly divergent, supporting the assumption that the insolubility properties of these proteins can be mainly assigned to the DNA binding domain.
Figure 2.
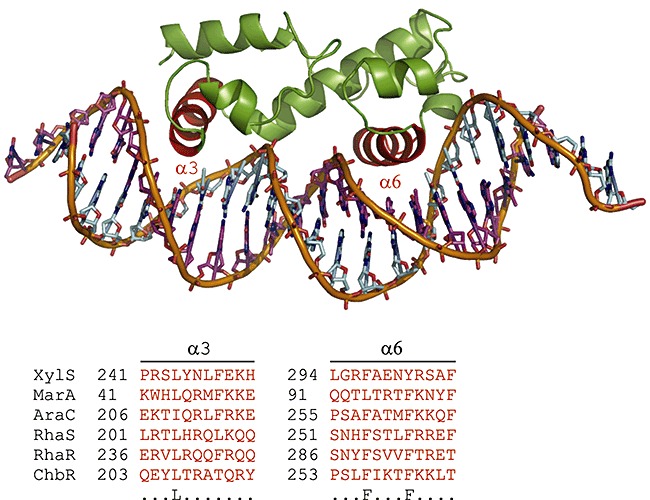
AraC‐XylS family regulators' characteristic HTH DNA binding motif is shown by using the member MarA as a model (α‐helixes 3 and 6 in red colour). Conserved amino acid residues are depicted in the bottom row. The alignment was derived from the full‐length primary sequences of the given TFs by using the PROMALS3D web server (Pei et al., 2008). Parameters were left at default values. The figure was prepared by using PyMOL (DeLano, 2003). Note that MarA binds DNA as a monomer.
The XylS/Pm regulator/promoter system
A handful of the known AraC‐XylS family TFs, including XylS, control catabolic operons and they generally act as activators in the presence of a chemical effector molecule (for a review, see Tropel and van der Meer, 2004). The P. putida TOL plasmid encodes a pathway for catabolism of toluene and xylenes (Worsey and Williams, 1975), and the genes involved are grouped into the upper‐ and meta‐pathway operons, positively regulated by TFs XylR and XylS respectively. The xylS gene can be transcribed from two alternative and individually regulated promoters, Ps1 and Ps2; XylR can activate transcription from Ps1 while transcription from Ps2 is constitutive and low. Interestingly, translation of xylS mRNA generated from the Ps1 promoter is 10 times more efficient than that generated from Ps2 (González‐Pérez et al., 2004). Together, this ensures a tightly controlled and balanced expression level of XylS, and it gives rise to two modes of Pm activation according to the XylS concentrations in the cells. When overexpressed, XylS can bind to the operator sequence Om in the absence of inducer and activate transcription from the meta‐pathway Pm promoter (Marqués et al., 1999). In the presence of a benzoate‐derived inducer, on the other hand, XylS activates transcription from Pm at low protein concentrations (for a review, see Ramos et al., 1997). Pm is special in that the in vivo transcription from this promoter can be mediated by RNAP with different alternative σ factors, σ32 or σ38, depending on the growth phase (Marqués et al., 1999). Regardless of the growth phase, expression from Pm remains dependent on XylS (see above), and the transcription start point is the same (Marqués et al., 1995; Domínguez‐Cuevas et al., 2006). A number of σ38‐dependent promoters have also been reported to be transcribed in vivo by RNAP with an alternative bound σ factor depending on the physiological conditions; however, in all cases so far reported (except for Pm), σ70 was responsible for the alternative transcription (Busby and Ebright, 1997).
As described for AraC (see above), XylS is also a modular protein with a conserved C‐terminal domain for DNA binding and interactions with RNAP, and with a non‐conserved N‐terminal domain responsible for effector binding and protein dimerization. Interestingly, AraC and XylS show some degree of primary sequence homology also in the N‐terminal region, and it has been proposed that these two proteins use similar mechanisms by which the effector controls the activity of the TF (Kaldalu et al., 2000). The DNA binding motif and the effector binding pocket in XylS are not independent domains, as shown by intramolecular dominance of C‐terminal mutations over N‐terminal mutations and by the reversal of this dominance in double mutants tested in vitro (Mìchan et al., 1992). Ruiz and Ramos (2001 and 2002) constructed XylS double mutants with substitutions in positions 137 and 153 of the C‐terminal domain, and the mutant proteins displayed altered effector profile suggesting that these two residues are critical for effector recognition and regulator activation to stimulate transcription from Pm.
In the C‐terminal end of XylS, a conserved stretch of about 100 amino acids is proposed to contain totally seven α‐helices, including two HTH binding motifs (α2‐T‐α3 and α5‐T‐α6), connected by a linker α‐helix (α4) and two flanking α‐helices (α1 and α7). This structural organization has also been experimentally confirmed for the monomeric MarA protein (Rhee et al., 1998) (see Fig. 2). The second HTH motif (α5‐T‐α6) is the most conserved in the proteins of the AraC‐XylS family proteins (Gallegos et al., 1997), suggesting a common role for this motif in all these regulators. Besides, mutational analyses have shown that the α5‐T‐α6 motif takes part in DNA binding by AraC and similar experiments have confirmed the role of this motif for XylS activity. In addition, conserved residues located outside of the HTH motifs were found to play important structural roles for XylS function (Manzanera et al., 2000).
XylS (like AraC) binds to DNA as a dimer, to a 35‐bp‐long region of the promoter (Hendricksen and Schleif, 1985; Kaldalu et al., 2000), and genetic analyses established that this TF recognizes two 15‐bp direct repeats (TGCA‐N6‐GGNTA) (Kessler et al., 1993; Gallegos et al., 1997; González‐Pérez et al., 1999; 2002). It was demonstrated that XylS interacts with the C‐terminal domain of the RNAP α subunit during activation of transcription from the Pm promoter, analogous to what has been found for the AraC‐XylS family members AraC, MarA, SoxS, Rob, AlkA and RhaS (Ruiz et al., 2001 and references therein). As reported for AraC, Rob and RhaS, the binding sites for XylS and RNAP overlap (Busby and Ebright, 1997; Dhiman and Schleif, 2000; González‐Pérez et al., 2002). It was suggested that this overlap may facilitate interactions between XylS and the α subunit of RNAP (González‐Péres et al., 2002). This may be different in other members of this family, e.g. for Ada (Landini et al., 1998) and MarA (Martin et al., 2000).
Recombinant expression systems based on AraC‐XylS family TFs and their cognate promoters
To date, only a handful of AraC‐XylS family regulator genes with their cognate promoters have been applied to construct expression vectors for various biotechnological applications (Table 1). A common trait observed with those that have been tested and used for such purposes is that they are tightly controlled and they are also demonstrated to generally be functioning in different bacterial species. Each of them is described below in some detail.
AraC/PBAD can be used to achieve high‐level and tightly controlled gene expression in different bacterial species
In general, genes cloned under the control of the PBAD promoter can be efficiently expressed, and this system also allows tight regulation and inexpensive induction with l‐arabinose (see above). In the presence of this sugar, the PBAD promoter is rapidly turned on, while in the absence of l‐arabinose and in the presence of glucose, very low or undetectable levels of transcription occurs (Lee et al., 1991; Johnson and Schleif, 1995). Guzman and colleagues (1995) took advantage of the many favourable properties of AraC/PBAD to construct the so‐called pBAD expression vectors, carrying the ColE1 origin for replication in E. coli. In host strains deficient in ara genes (that cannot metabolize the effector molecule l‐arabinose which in turn remains at a constant concentration in the cells), expression of cloned genes reaches maximal induction upon adding as little as 0.001% l‐arabinose, while in l‐arabinose fermenting strains, addition of about 1% inducer is necessary to achieve full induction (Mayer, 1995). The system has also been demonstrated to function for recombinant protein production under HCDC (DeLisa et al., 1999). Huang and colleagues (2000) constructed phage display vectors using AraC together with PBAD, and the vectors turned out to be useful to exhibit controlled expression of proteins on the surface of the bacteriophage M13. A typical challenge faced when using phage display is toxicity problems of the recombinant protein in the host bacterium, eventually leading to libraries dominated by deletion mutants (Fischer et al., 1994; Huang et al., 2000). One strategy to avoid such problems is to ensure tightly controlled expression of the heterologous protein, and the amount of protein displayed on the phage by using the AraC/PBAD system could be modulated by the amount of l‐arabinose inducer present in the growth medium during phage propagation. The active transport needed for l‐arabinose to enter the cells may confer some application limitations with this system (see the next section).
Interestingly, by using alternative broad‐host‐range replicons, it has been demonstrated that AraC/PBAD can function well in several bacterial species, including Corynebacterium glutamicum and Xanthomonas sp. (Ben–Saumoun et al., 1999; Newman and Fuqua, 1999; Sukchawalita et al., 1999). Lee and colleagues (2007) recently constructed novel araC mutants by directed enzyme evolution that displayed altered effector affinities, including 10 times higher sensitivity to l‐arabinose, and improved tolerance to IPTG. The IPTG is a commonly used inducer molecule for the popular Plac promoter; however, this compound is also an inhibitor of the AraC/PBAD system. This mutant AraC regulator can be useful in cases where Plac and PBAD are applied simultaneously for controlled recombinant expression in the same host cell.
The RhaR‐RhaS/rhaBAD system shares many expression properties with AraC/PBAD
The l‐rhamnose regulons of E. coli consist of the rhaT and the operons rhaSR and rhaBAD. The E. coli rhaSR operon encodes the l‐rhamnose‐responsive AraC‐XylS type TFs RhaS and RhaR, while rhaT and rhaBAD encode l‐rhamnose catabolic and transport enzymes respectively (Gallegos et al., 1997). Both RhaS and RhaR are activated by l‐rhamnose. l‐rhamnose acts as an inducer which binds to the regulator RhaR which then activates transcription of the rhaSR operon leading to induced expression of RhaR and RhaS. The latter protein in turn acts as an activator in the positive control of rhaT and the rhaBAD operon (Fig. 3). The l‐rhamnose regulons are, as described for the ara genes (see above), regulated by catabolite repression and full activation also requires the cyclic AMP receptor protein CRP in addition to RhaS and RhaR (Gallegos et al., 1997; Wickstrum et al., 2005). The rhaBAD promoter is, as shown for PBAD, an interesting tool for regulated recombinant expression in E. coli (Haldimann et al., 1998). This system has been stated to be even more tightly regulated than AraC/PBAD and recombinant production of l‐N‐carbamoylase using rhaBAD has been demonstrated to function well under HCDC (Wilms et al., 2001). As mentioned for PBAD, it may be advantageous also for the rhaBAD promoter to use genetically engineered host strains that cannot metabolize the l‐rhamnose inducer.
Figure 3.
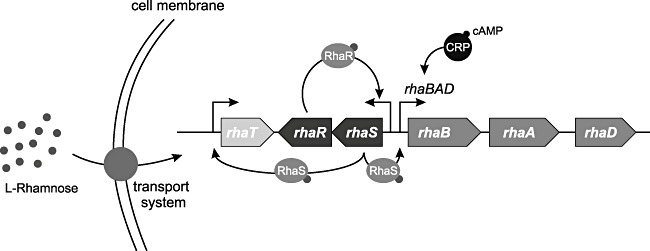
Mechanism of induction of the RhaR‐RhaS/rhaBAD system (modified with permission from Altenbuchner and Mattes, 2005). l‐rhamnose is actively transported into the cells and it binds to RhaS which then becomes activated and stimulates transcription of rhaT (encoding l‐rhamnose transporter protein) and the rhaBAD operon (encoding l‐rhamnose catabolic enzymes). RhaR is also activated by l‐rhamnose and stimulates transcription of the rhaSR operon (encoding RhaR and RhaS). The catabolite repression is indicated. For further details see text section The RhaR‐RhaS/rhaBAD system shares many expression properties with AraC/PBAD.
At this point, it should be mentioned that both l‐rhamnose and l‐arabinose require transport systems to enter the cells, and it has been stated that expression systems induced by such compounds display limitations with respect to dose‐dependent induction (Keasling 1999). Typically, the genes encoding the respective transport systems are also controlled by the inducer, leading to an ‘all‐or‐nothing’ induction response; i.e. in a cell culture some cells are fully induced while some are not. Khlebnikov and colleagues (2002) demonstrated how this could be circumvented by using engineered E. coli host cells that overexpressed the l‐arabinose transporter AraE.
Broad‐host‐range expression systems based on XylS/Pm
The XylS/Pm regulator/promoter system has been used for the construction of multiple different expression systems which together represent a wide range of properties and application ranges. The first example of using this cassette to construct expression tools was described more than 20 years ago (Mermod et al., 1986). The vectors were based on the broad‐host‐range RSF1010 replicon, and they were shown to be useful for various levels of regulated expression of the XylE reporter protein in a relatively large number of different gram‐negative species. The vectors were later modified by using a mutant XylS protein, designated XylStr6, with altered effector affinity and which mediated up to eightfold higher expression levels from Pm compared with the wild‐type XylS (Ramos et al., 1988). These studies demonstrated that XylS/Pm can function well for regulated expression of heterologous proteins in a wide range of different gram‐negative bacterial species.
The xylS/Pm expression cassette was later fused also to the broad‐host‐range minimal replication elements oriV and trfA from the RK2 plasmid (Blatny et al., 1997a). The trfA gene encodes a replication initiation protein also exerting negative control of replication from oriV. Different versions of the plasmids were constructed with various antibiotic markers, multiple cloning sites and oriT for conjugal transfer, resulting in a set of broad‐host‐range cloning and expression vectors (Blatny et al., 1997b). In all the vectors the xylS gene is constitutively transcribed from its native Ps2 promoter, and the gene of interest is placed under transcriptional control by Pm (Fig. 4). The application potential of these vectors for both high‐ and low‐level regulated gene expression in E. coli, Pseudomonas aeruginosa and Xanthomonas campestris was indicated (Blatny et al., 1997a,b). In particular, the plasmid pJB658 which is a combined cloning and expression vector (Blatny et al., 1997b) has proven to be very useful for many different aspects of recombinant expression. Relevant characteristics of this vector includes adjustable vector copy number (between 5 and 100 per chromosome), several cheap benzoic acid derivatives such as m‐toluic acid can be used, they act in a dose‐dependent manner and inducer uptake is presumably passive (no transport system required) (Lambert and Stratford, 1999 and references therein) which further simplifies the use of the system across species barriers. pJB658 was used to control sugar metabolism in E. coli by fine‐tuning the expression levels of a number of phosphoglucomutase mutant enzymes genes with different kinetic properties (Brautaset et al., 1998; 2000). The same plasmid was used to control xanthan biosynthesis in a xanA‐deficient X. campestris mutant strain by regulated low‐level expression of the xanA gene, encoding a bifunctional phosphomannomutase/phosphoglucomutase (Winther‐Larsen et al., 2000a). The unique ability of fine‐tuning recombinant expression levels at low levels by using Pm/xylS has also been demonstrated in alginate producing Pseudomonas fluorescens (Gimmestad et al., 2003; Bakkevig et al., 2005). The phosphomannomutase gene algC and the mannuronan C‐5‐epimerase gene algG were individually put under transcriptional control of Pm and introduced into the bacterial chromosome by using transposons. The phosphomannomutase is involved in alginate biosynthesis while the mannuronan C‐5‐epimerase is involved in the concomitant alginate polymer modification. In this way, the alginate production level and the alginate composition in the resulting recombinant strains could be modulated by adding inducer at various concentrations.
Figure 4.
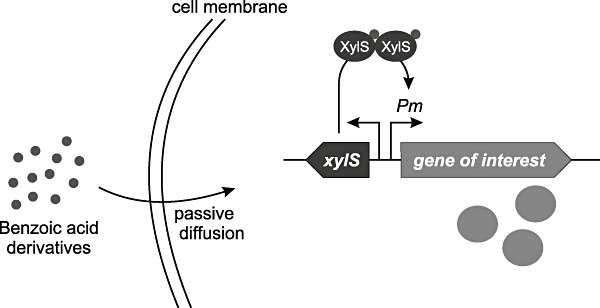
The XylS/Pm expression system. Inducer molecules enter cells passively and bind to XylS which then becomes activated. The activated XylS stimulates transcription from Pm. For details see text section Broad‐host‐range expression systems based on XylS/Pm.
By using firefly luciferase as a reporter protein, it was shown that the induced expression levels both in E. coli and P. aeruginosa could be varied over a wide range by using different types and different concentrations of inducers (Winther‐Larsen et al., 2000a). By integrating the Pm/xylS expression cassette into the E. coli chromosome, recombinant expression levels of the reporter protein could be controlled at very low levels. The −10 region of the Pm promoter sequence was also randomly mutagenized by so‐called doped oligonucleotides. Pm promoter mutants with enhanced induced expression levels were identified, as well as mutants with lower uninduced levels compared with the wild‐type Pm (Winther‐Larsen et al., 2000b). One interesting finding was that expression from Pm can be modulated by the growth conditions of the recombinant cells, and in particular the pH of the growth medium was found to be critical. At a relatively high pH expression from Pm decreases drastically, and it was postulated to be related to the dissociation state of the inducer. By combining the effects of environmental and genetic parameters expression from Pm could be varied over an almost hundred‐thousand‐fold continuous range (Winther‐Larsen et al., 2000a,b).
Plasmid pJB658 has also been demonstrated to be highly useful for industrial‐level production of three secreted human medically relevant proteins; single‐chain antibody fragment (scFv‐phOx), interferon α2b and granulocyte macrophage colony‐stimulating factor, under HCDC in E. coli (Sletta et al., 2007). The advantage of optimizing, and not maximizing, expression levels was demonstrated to be crucial to achieve high‐level functional expression of antibody fragment scFv‐phOx in E. coli (Sletta et al., 2004; 2007). Protein scFv‐phOx is host toxic and must be translocated to the periplasm to fold into soluble and functional form, and this translocation was guided by using a signal sequence as fusion partner. A very low background expression prior to induction, together with the strong induced expression from Pm, was demonstrated to be crucial to maintain plasmid stability under HCDC and to achieve very high volumetric production yields (Fig. 5).
Figure 5.
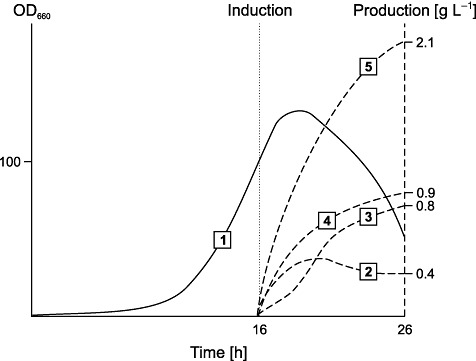
Fermentation course of recombinant E. coli cells expressing totally 2.1 g L−1 of single‐cell antibody fragment scFv‐phOx (P) by using the XylS/Pm expression system. Lines: 1, growth curve; 2, soluble P in periplasm; 3, soluble P in growth medium; 4, insoluble P in cytoplasm; 5, total soluble + insoluble P (data imported from Sletta et al., 2004; 2007).
Hyper‐inducible expression in streptomycetes using NitR/PnitA
Streptomycetes are popular production hosts for a wide range of useful compounds, such as antibiotics, pesticides, immunosuppressive compounds and also enzymes. However, tools for regulated recombinant gene expression in these bacteria are in general poorly developed. In a report by Herai and colleagues (2004), plasmid vectors carrying the Rhodococcus rhodochrous J1 NitR/PnitA system were described. The AraC‐family activator NitR controls transcription from the PnitA promoter of the nitrilase gene nitA in R. rhodochrous (Komeda et al., 1996). Nitriles are generally cell‐toxic compounds; however, some microorganisms can use such compounds as carbon and nitrogen sources. Nitrilases are enzymes that catalyse the cleavage of nitriles to the corresponding acids and ammonia, and in R. rhodochrous, nitrilase can constitute up to 35% of all soluble proteins under induced conditions, e.g. in the presence of isovaleronitrile (Komeda et al., 1996). However, this compound is both cell‐toxic and expensive and accordingly not well suited as an inducer molecule for practical applications. It was recently demonstrated that NitR can be activated by ε‐caprolactam which is an alternative inexpensive, water‐soluble and safe inducer that has no obvious influence of the physiological conditions of streptomycetes (Herai et al., 2004). By using NitR/PnitA and ε‐caprolactam, tightly controlled and high‐level expression of reporter genes in several different streptomycete strains was demonstrated (Herai et al., 2004). Another important trait observed with this system was that ε‐caprolactam induction is dose‐dependent, a highly useful property when fine‐tuning of expression levels are needed, e.g. to optimize conditions for host‐toxic proteins and also for physiological studies. Considering the molecular size and structure, it is likely that ε‐caprolactam does not need any transport system to enter the bacterial cells.
Regulated heterologous expression using ChnR/Pb
Steigedal and Valla (2008) recently searched the AraC‐XylS database in order to identify new expression cassette candidates with similar properties as those of XylS/Pm (see sections the XylS/Pm regulator/promoter system and broad‐host‐range expression systems based on XylS/Pm), and the ChnR/Pb regulator/promoter system in Acinetobacter sp. strain NCIMB 9871 was further investigated. The chnR gene is part of a gene cluster responsible for cyclohexanol oxidation, suggesting that the system could be activated by several different inducers, in addition to cyclohexanone (Iwaki et al., 1999), which does not need any particular transport system, and also that the inducers are not metabolized by most heterologous hosts strains. The genetic elements were cloned into a RK2‐based plasmid vector and tested for recombinant expression using various induction conditions in both E. coli and P. fluorescens. Cyclohexanone was found to be the most efficient inducer of this system in E. coli using firefly luciferase as a reporter protein. The maximum expression level obtained with the ChnR/Pb system was lower compared with that of XylS/Pm (see above); however, both systems shared the ability to display an inducer concentration‐dependent response, and they both also function well in different gram‐negative species. ChnR/Pb, in combination with Pm/xylS, was used to control the monomer composition of alginate in P. fluorescens (Steigedal and Valla, 2008), and this system should therefore represent a valuable new broad‐host‐range expression tool.
The AraC‐XylS family as a source of TFs for identification of novel expression systems
Along with the growing exploitation of not yet characterized bacterial species for various biotechnological applications, there is a need for new and flexible expression systems. The identification and application of the ChnR/Pb expression system from Acinetobacter sp. strain NCIMB 9871 (see above) successfully demonstrated an approach in which the AraC‐XylS family was screened in silico to identify promoter/regulator elements useful to develop a new bacterial expression system. In this particular case, some biological knowledge of the system in question was available (Iwaki et al., 1999). Considering the broad diversity of biological functions likely to be controlled by AraC‐XylS type of TFs, we propose that additional candidates with a potential for development of novel expression tools for both gram‐positive and gram‐negative bacteria may be identified from this family in the future.
As we see it, two major challenges may be met in this approach: the first challenge is the identification of correct cognate promoter(s) that are under transcriptional control of the identified AraC‐XylS regulator. It is well known that several AraC‐XylS regulators have been found to control expression of multiple different promoters (Gallegos et al., 1997; Rodionov, 2007). This is a common feature shared by many bacterial TFs; although they bind to their TF binding sites (TFBSs) in a sequence specific manner, the sequences of the binding sites of a particular TF located upstream of different genes in the same genome may vary significantly, allowing for a more flexible transcriptional control. Potentially, this may lead to unpredicted binding to chromosomal host sites when using such expression systems in a heterologous host, and the biological effects of such events cannot be easily foreseen. The size of a bacterial TFBS is typically 16–20 nucleotides, and for TFs that recognize and bind to DNA as homodimers, it follows that the TFBSs usually possess an intrinsic symmetry. Accordingly, inverted and direct repeats are the most common structures of TFBSs, while some homomultimeric TFs cooperatively bind more complex TFBSs composed of both inverted and direct repeats, e.g. AraC in E. coli. Consensus sequences for important types of TFs have been reported, including for AraC‐XylS family regulators, and computational algorithms have been developed for searching genomes for TFBSs based on such consensus signatures (for review see Zhou and Yang, 2006; Rodionov, 2007). As such in silico methods constantly improve, they will most likely become powerful tools to rapidly identify and map new AraC‐XylS TF‐controlled promoters. It should also be mentioned that many AraC‐XylS family TF genes are located in close vicinity to the genes or operons they control, which further simplifies the identification of the desired promoters and their cognate regulators.
The second major challenge in selecting new AraC‐XylS family members for construction of expression vectors will likely be to identify the appropriate inducer compound(s) that modulates regulator activity. Interestingly, it was recently shown that the AraC‐XylS family Rns regulator in E. coli presumably does not respond to any ligand (Basturea et al., 2008), demonstrating that this information may not be straightforward to obtain. One could argue that the ongoing basic research on gene regulation in bacteria will eventually also generate new functional knowledge on so far poorly characterized AraC‐XylS family members, useful for easy selection of promising candidates for constructing new expression tools. According to Ibarra and colleagues (2008), about 60 different AraC‐XylS regulators have to date been experimentally characterized, and this number is likely to increase in the future. For example, the first example of a cellobiose‐induced AraC‐XylS regulator was recently reported (Joshi et al., 2007). However, as we see it, a deep basic insight regarding regulator/promoter/RNAP interactions should be no prerequisite for using newly identified regulators as expression cassettes. Ibarra and colleagues (2008) defined a consensus DNA binding domain based on alignment of all experimentally characterized AraC‐XylS proteins, and by using this domain, almost 2000 putative AraC‐XylS protein sequences were discovered in 212 bacterial genomes. The domain was then used as a template to generate a phylogenetic tree and as a tool to predict the putative regulatory role of new members of this family based on their sequence proximity to a particular functional cluster in the tree. By this approach, functional regulatory roles of about 75% of all identified AraC‐XylS regulators were assigned (Ibarra et al., 2008). It should however be stated that the accuracy of these predictions remains, to our knowledge, yet to be experimentally verified.
An interesting structure‐based drug design strategy was described to identify chemical ligands that could bind to and inactivate AraC‐XylS regulators (Bowser et al., 2007). In principle, a similar approach could be used to identify inducers for a given AraC‐XylS type of activator as long as a candidate cognate promoter is available. By placing a suitable reporter under control of this promoter, high‐throughput screening of thousands of relevant chemical compounds can be done in a laboratory, eventually leading to the identification of useful effector compounds.
Acknowledgments
This work was funded by a grant from the Research Council of Norway.
References
- Adhya S., Garges S. Positive control. J Biol Chem. 1990;265:10797–10800. [PubMed] [Google Scholar]
- Ali N., Herron P.R., Evans M.C., Dyson P.J. Osmotic regulation of the Streptomyces lividans thiostrepton‐inducible promoter PtipA. Microbiology. 2002;148:381–390. doi: 10.1099/00221287-148-2-381. [DOI] [PubMed] [Google Scholar]
- Altenbuchner J., Mattes R. Escherichia coli. In: Gellissen G., editor. WILEY‐VCH Verlag GmbH and Co. KGaA; 2005. pp. 7–43. [Google Scholar]
- Axelsson L., Lindstad G., Naterstad K. Development of an inducible gene expression system for Lactobacillus sakei. Lett Appl Microbiol. 2003;37:1–6. doi: 10.1046/j.1472-765x.2003.01360.x. [DOI] [PubMed] [Google Scholar]
- Bakkevig K., Sletta H., Gimmestad M., Aune R., Ertesvåg H., Degnes K. Role of the Pseudomonas fluorescens alginate lyase (AlgL) in clearing the periplasm of alginates not exported to the extracellular environment. J Bacteriol. 2005;187:8375–8384. doi: 10.1128/JB.187.24.8375-8384.2005. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basturea G.N., Bodero M.D., Moreno M.E., Munson G.P. Residues near the amino‐terminus of Rns are essential for positive autoregulation and DNA binding. J Bacteriol. 2008;190:2279–2285. doi: 10.1128/JB.01705-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben–Saumoun K., Leblon G., Reyes O. Positively regulated expression of the Escherichia coli araBAD promoter in Corynebacterium glutamicum. FEMS Microbiol Lett. 1999;174:125–130. doi: 10.1111/j.1574-6968.1999.tb13558.x. [DOI] [PubMed] [Google Scholar]
- Biedendieck R., Gamer M., Jaensch L., Meyer S., Rohde M., Deckwer W.D., Jahn D. A sucrose‐inducible promoter system for the intra‐ and extracellular protein production in Bacillus megaterium. J Biotechnol. 2007;132:426–430. doi: 10.1016/j.jbiotec.2007.07.494. [DOI] [PubMed] [Google Scholar]
- Blatny J.B., Brautaset T., Winther‐Larsen H.C., Haugan K., Valla S. Construction of a versatile set of broad‐host‐range cloning and expression vectors based on the RK2 replicon. Appl Environ Microbiol. 1997a;63:370–379. doi: 10.1128/aem.63.2.370-379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatny J.B., Brautaset T., Winther‐Larsen H.C., Karunakaran P., Valla S. Improved broad‐host‐range RK2 vectors useful for high and low regulated gene expression levels in gram‐negative bacteria. Plasmid. 1997b;38:35–51. doi: 10.1006/plas.1997.1294. [DOI] [PubMed] [Google Scholar]
- Blomqvist T., Steinmoen H., Håvarstein L.S. Pheromone‐induced expression of recombinant proteins in Streptococcus thermophilus. Arch Microbiol. 2006;186:465–473. doi: 10.1007/s00203-006-0162-0. [DOI] [PubMed] [Google Scholar]
- Bowser T.E., Bartlett V.J., Grier M.C., Verma A.K., Warchol T., Levy S.B., Alekshun M.N. Novel anti‐infection agents: small‐molecule inhibitors of bacterial transcription factors. Bioorg Med Chem Lett. 2007;17:5652–5655. doi: 10.1016/j.bmcl.2007.07.072. [DOI] [PubMed] [Google Scholar]
- Brautaset T., Petersen S., Valla S. An experimental study on carbon flow in Escherichia coli as a function of kinetic properties and expression levels of the enzyme phosphoglucomutase. Biotechnol Bioeng. 1998;58:299–302. [PubMed] [Google Scholar]
- Brautaset T., Petersen S.B., Valla S. In vitro determined kinetic properties of mutant phosphoglucomutases and their effects on sugar metabolism in Escherichia coli. Metab Eng. 2000;2:104–114. doi: 10.1006/mben.1999.0145. [DOI] [PubMed] [Google Scholar]
- Browning D.F., Busby S.J. The regulation of bacterial transcription factors. Nat Rev Microbiol. 2004;2:57–65. doi: 10.1038/nrmicro787. [DOI] [PubMed] [Google Scholar]
- Brunelle A., Schleif R. Determining residue‐base interactions between AraC protein and aral DNA. J Mol Biol. 1989;209:607–622. doi: 10.1016/0022-2836(89)90598-6. [DOI] [PubMed] [Google Scholar]
- Bryan E.M., Bae T., Kleerebezem M., Dunny G.M. Improved vectors for nisin‐controlled expression in gram‐negative bacteria. Plasmid. 2000;44:183–190. doi: 10.1006/plas.2000.1484. [DOI] [PubMed] [Google Scholar]
- Busby S., Ebright R.H. Transcription activation at class II CAP‐dependent promoters. Mol Microbiol. 1997;23:853–859. doi: 10.1046/j.1365-2958.1997.2771641.x. [DOI] [PubMed] [Google Scholar]
- Calcagno Galvão T., De Lorenzo V. Transcriptional regulators á la carte: engineering new effector specificities in bacterial regulatory proteins. Curr Opin biotechnol. 2006;17:34–42. doi: 10.1016/j.copbio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Carra J., Schleif R. Variation of half‐site organization and DNA looping by AraC protein. EMBO. 1993;12:35–44. doi: 10.1002/j.1460-2075.1993.tb05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanueva A.I., Paul L., Patrick S., Abratt V.R. An AraC/XylS family transcriptional regulator homologue from Bacteroides fragilis is associated with cell survival following DNA damage. FEMS Microbiol Lett. 2008;278:249–256. doi: 10.1111/j.1574-6968.2007.01004.x. [DOI] [PubMed] [Google Scholar]
- De Ruyter P.G., Kuipers O.P., De Vos W.M. Controlled gene expression systems for Lactococcus lactis with the food‐grade inducer nisin. Appl Environ Microbiol. 1996;62:3662–3667. doi: 10.1128/aem.62.10.3662-3667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLano W.L. DeLano Scientific; 2003. [Google Scholar]
- DeLisa M.P., Li J., Rao R., Weigand W.A., Beentley W.E. Monitoring GFP‐operon fusion protein expression during high cell density cultivation of Escherichia coli using an on‐line optical sensor. Biotechnol Bioeng. 1999;65:54–64. [PubMed] [Google Scholar]
- DeSanti C.L., Strohl W.R. Characterization of the Streptomyces sp. strain C5 locus and development of snp‐derived expression vectors. Appl Environ Microbiol. 2003;69:1647–1654. doi: 10.1128/AEM.69.3.1647-1654.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman A., Schleif R. Recognition of overlapping nucleotides by AraC and the sigma subunit of RNA polymerase. J Bacteriol. 2000;182:5076–5081. doi: 10.1128/jb.182.18.5076-5081.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez‐Cuevas P., Marín P., Ramos J.L., Marqués S. RNA polymerase holoenzymes can share a single transcription start site from the Pm promoter: critical nucleotides in the −7 to −18 region are needed toselect between RNA polymerase with σ32 or σ38. J Biol Chem. 2006;280:41315–41323. doi: 10.1074/jbc.M505415200. [DOI] [PubMed] [Google Scholar]
- Dong L., Nakashima N., Tamura N., Tamura T. Isolation and characterization of the Rhodococcus opacus thiostrepton‐inducible genes tipAL and tipAS: application for recombinant protein expression in Rhodococcus. FEMS Microbiol Lett. 2004;237:35–40. doi: 10.1016/j.femsle.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Duetz W.A., Marqués S., Wind B., Ramos J.L., Van Andel J.G. Catabolite repression of the toluene degradation pathway in Pseudomonas putida harboring pWW0 under various conditions of nutrient limitation in chemostat culture. Appl Environ Microbiol. 1996;62:601–606. doi: 10.1128/aem.62.2.601-606.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan S.M. Growing repertoire of AraC/XylS activators. J Bacteriol. 2002;184:5529–5532. doi: 10.1128/JB.184.20.5529-5532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum Z., Federle M.J., Marra D., De Vos W.M., Kuipers O.P., Kleerebezem M., Scott J.R. Use of the Lactococcal nisA promoter to regulate gene expression in gram‐positive bacteria: comparison of induction level and promoter strength. Appl Environ Microbiol. 1998;64:2763–2769. doi: 10.1128/aem.64.8.2763-2769.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englesberg E., Irr J., Power J., Lee N. Positive control of enzyme synthesis by gene C in the L‐arabinose system. J Bacteriol. 1965;90:946–957. doi: 10.1128/jb.90.4.946-957.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englesberg E., Squires C., Meronk F. The L‐arabinose operon in Escherichia coli B/r: a genetic demonstration of two functional states of the product of a regulator gene. Proc Natl Acad Sci USA. 1969;62:1100–1107. doi: 10.1073/pnas.62.4.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer P., Leu S.‐J.C., Yang Y.‐Y., Chen P.P. Rapid simultaneous screening for DNA integrity and antigen specificity of clones selected by phage display. Biotechniques. 1994;16:828–830. [PubMed] [Google Scholar]
- Gallegos M.‐T., Schleif R., Bairoch A., Hofmann K., Ramos J.L. AraC/XylS family of transcriptional regulators. Microbiol Mol Biol Rev. 1997;61:393–410. doi: 10.1128/mmbr.61.4.393-410.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimmestad M., Sletta H., Ertesvaag H., Bakkevig K., Jain S., Skjåk‐Bræk G. The Pseudomonas fluorescens AlgG protein, but not its mannuronan C‐5‐epimerase activity, is needed for alginate polymer function. J Bacteriol. 2003;185:3515–3523. doi: 10.1128/JB.185.12.3515-3523.2003. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González‐Pérez M.M., Ramos J.L., Gallegos M.T., Marqués S. Critical nucleotides in the upstream region of the XylS‐dependent TOL meta‐cleavage pathway operon promoter as deduced from analysis of mutants. J Biol Chem. 1999;274:2286–2290. doi: 10.1074/jbc.274.4.2286. [DOI] [PubMed] [Google Scholar]
- González‐Pérez M.M., Marqués S., Domínguez‐Cuevas P., Ramos J.L. XylS activator and RNA polymerase binding sites at the Pm promoter overlap. FEBS Lett. 2002;519:117–122. doi: 10.1016/s0014-5793(02)02730-8. [DOI] [PubMed] [Google Scholar]
- González‐Pérez M.M., Ramos J.L., Marqués S. Cellular XylS levels are a function of transcription of xylS from two independent promoters and the differential efficiency of translation of the two mRNAs. J Bacteriol. 2004;186:1898–1901. doi: 10.1128/JB.186.6.1898-1901.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J., Schleif R. Arabinose C protein: regulation of the arabinose operon in vitro. Nature (London) New Biol. 1971;233:166–170. doi: 10.1038/newbio233166a0. [DOI] [PubMed] [Google Scholar]
- Guzman L.‐M., Belin D., Carson M.J., Beckwith J. Tight regulation, modulation, and high‐level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldimann A., Daniels L.D., Wanner B.L. Use of new methods for construction of tightly regulated arabinose and rhamnose promoter fusions in studies of the Escherichia coli phosphate regulons. J Bacteriol. 1998;180:1277–1286. doi: 10.1128/jb.180.5.1277-1286.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricksen W., Schleif R. A dimer of AraC protein contacts three adjacent major groove regions of the aral DNA site. Proc Natl Acad Sci USA. 1985;82:3129–3133. doi: 10.1073/pnas.82.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herai S., Hashimoto Y., Higashibata H., Maseda H., Ikeda H., Omura S., Kobayashi M. Hyper‐inducible expression system for streptomycetes. Proc Natl Acad Sci USA. 2004;28:14031–14035. doi: 10.1073/pnas.0406058101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., McKevitt M., Palzkill T. Use of the arabinose PBAD promoter for tightly regulated display of proteins in bacteriophage. Gene. 2000;251:187–197. doi: 10.1016/s0378-1119(00)00210-9. [DOI] [PubMed] [Google Scholar]
- Ibarra J.A., Pérez‐Rueda E., Segovia L., Puente J.L. The DNA‐binding domain as a functional indicator: the case of the AraC‐XylS family transcription factors. Genetica. 2008;133:65–76. doi: 10.1007/s10709-007-9185-y. [DOI] [PubMed] [Google Scholar]
- Iwaki H., Haseguwa Y., Teraoka M., Tokuyama T., Bergeron H., Lau P.C. Identification of a transcriptional activator (ChnR) and a 6‐oxohexanoate dehydrogenase (ChnE) in the cyclohexanol catabolic pathway in Acinetobacter sp. strain NCIMB 9871 and localization of the genes that encode them. Appl Environ Microbiol. 1999;65:5158–5162. doi: 10.1128/aem.65.11.5158-5162.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.M., Schleif R.F. In vivo kinetics of the arabinose promoters in Escherichia coli. J Bacteriol. 1995;177:3438–3442. doi: 10.1128/jb.177.12.3438-3442.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi M.V., Bignell D.R., Johnsen E.G., Sparks J.P., Gibson D.M., Loria R. The AraC/XylS regulator TxtR modulates thaxtomin biosynthesis and virulence in Streptomyces scabies. Mol Microbiol. 2007;66:633–642. doi: 10.1111/j.1365-2958.2007.05942.x. [DOI] [PubMed] [Google Scholar]
- Kaldalu N., Toots U., Lorenzo D.E.V., Ustav M. Functional domains of the TOL plasmid transcription factor XylX. J Bacteriol. 2000;182:1118–1126. doi: 10.1128/jb.182.4.1118-1126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keasling J.D. Gene‐expression tools for the metabolic engineering of bacteria. Trends Biotechnol. 1999;17:452–460. doi: 10.1016/s0167-7799(99)01376-1. [DOI] [PubMed] [Google Scholar]
- Kessler B., De Lorenzo V., Timmis K.N. Identification of a cis‐acting sequence within the Pm promoter of the TOL plasmid which confers XylS‐mediated responsiveness to substituted benzoates. J Mol Biol. 1993;230:699–703. doi: 10.1006/jmbi.1993.1189. [DOI] [PubMed] [Google Scholar]
- Khlebnikov A., Skaug T., Keasling J.D. Modulation of gene expression from the arabinose‐inducible araBAD promoter. J Ind Microbiol Biotechnol. 2002;29:34–37. doi: 10.1038/sj.jim.7000259. [DOI] [PubMed] [Google Scholar]
- Komeda H., Hori Y., Kobayashi M., Shimizu S. Transcriptional regulation of the Rhodococcus rhodochrous J1 nitA gene encoding nitrilase. Proc Natl Acad Sci USA. 1996;93:10572–10577. doi: 10.1073/pnas.93.20.10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhstoss S., Rao R.N. A thiostrepton‐inducible expression vector for use in Streptomyces spp. Gene. 1991;103:97–99. doi: 10.1016/0378-1119(91)90398-u. [DOI] [PubMed] [Google Scholar]
- Kuipers O.P., Beerthuyzen M.M., Siezen R.J., De Vos W.M. Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis: requirement of expression of the nisA and nisI genes for development of immunity. Eur J Biochem. 1993;216:281–291. doi: 10.1111/j.1432-1033.1993.tb18143.x. [DOI] [PubMed] [Google Scholar]
- Kwon H.J., Bennik M.H., Demple B., Ellenberger T. Crystal structure of the Escherichia coli Rob transcription factor in complex with DNA. Nat Struct Biol. 2000;7:424–430. doi: 10.1038/75213. [DOI] [PubMed] [Google Scholar]
- Lambert R.J., Stratford M. Weak‐acid preservatives: modelling microbial inhibition and response. J Appl Microbiol. 1999;86:157–164. doi: 10.1046/j.1365-2672.1999.00646.x. [DOI] [PubMed] [Google Scholar]
- Landini P., Bown J.A., Volkert M.R., Busby S.J. Ada protein‐RNA polymerase sigma subunit interaction and alpha subunit‐promoter DNA interaction are necessary at different steps in transcription initiation at the Escherichia coli ada and aidB promoters. J Biol Chem. 1998;273:13307–13312. doi: 10.1074/jbc.273.21.13307. [DOI] [PubMed] [Google Scholar]
- Lee K.Y., Hopkins J.D., O'Brien T.F., Syvanen M. Gly‐238‐Ser substitution changes the substrate specificity of the SHV class A β‐lactamases. Proteins Struct Funct Genet. 1991;11:45–51. doi: 10.1002/prot.340110106. [DOI] [PubMed] [Google Scholar]
- Lee S.K., Keasling J.D. A propionate‐inducible expression system for enteric bacteria. Appl Environ Microbiol. 2005;71:6856–6862. doi: 10.1128/AEM.71.11.6856-6862.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.K., Keasling J.D. Effect of glucose or glycerol as the sole carbon source on gene expression from the Salmonella prpBCDE promoter in Escherichia coli. Biotechnol Prog. 2006;22:1547–1551. doi: 10.1021/bp060193f. [DOI] [PubMed] [Google Scholar]
- Lee S.K., Chou H.H., Pfleger B.F., Newman J.D., Yoshikuni Y., Keasling J.D. Directed evolution of AraC for improved compatibility of arabinose‐ and lactose‐inducible promoters. Appl Environ Microbiol. 2007;73:5711–5715. doi: 10.1128/AEM.00791-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H.K., Jung K.H., Park D.H., Chung S.I. Production characteristics of interferon‐a using an L‐arabinose promoter system in a high‐cell‐density culture. Appl Microbiol Biotechnol. 2000;53:201–208. doi: 10.1007/s002530050009. [DOI] [PubMed] [Google Scholar]
- Loessner H., Endmann A., Leschner S., Westphal K., Rohde M., Miloud T. Remote control of tumor‐targeted Salmonella enterica serovar typhimurium by the use of L‐arabinose as inducer of bacterial gene expression in vivo. Cell Microbiol. 2007;9:1529–1537. doi: 10.1111/j.1462-5822.2007.00890.x. et al. [DOI] [PubMed] [Google Scholar]
- Makart S., Heinemann M., Panke S. Characterization of the AlkS/P(alkB)‐expression system as an efficient tool for the production of recombinant proteins in Escherichia coli fed‐batch fermentations. Biotechnol Bioeng. 2007;96:326–336. doi: 10.1002/bit.21117. [DOI] [PubMed] [Google Scholar]
- Manzanera M., Marqués S., Ramos J.L. Mutational analysis of the highly conserved C‐terminal residues of the XylS protein, a member of the AraC family of transcriptional regulators. FEBS Lett. 2000;476:312–317. doi: 10.1016/s0014-5793(00)01749-x. [DOI] [PubMed] [Google Scholar]
- Marqués S., Gallegos M.T., Ramos J.L. Role of sigma S in transcription from the positively controlled Pm promoter of the TOL plasmid of Pseudomonas putida. Mol Microbiol. 1995;18:851–857. doi: 10.1111/j.1365-2958.1995.18050851.x. [DOI] [PubMed] [Google Scholar]
- Marqués S., Manzanera M., González‐Pérez M.M., Ramos J.L. The XylS‐dependent Pm promoter is transcribed in vivo by RNA polymerase with sigma 32 or sigma 38 depending on the growth phase. Mol Microbiol. 1999;31:1105–1113. doi: 10.1046/j.1365-2958.1999.01249.x. [DOI] [PubMed] [Google Scholar]
- Martin R.G., Gillette W.K., Rosner J.L. Promoter discrimination by the related transcriptional activators MarA and SoxS: differential regulation by differential binding. Mol Microbiol. 2000;35:623–634. doi: 10.1046/j.1365-2958.2000.01732.x. [DOI] [PubMed] [Google Scholar]
- Martin R.G., Rosner J.L. The AraC transcriptional activators. Curr Opin Microbiol. 2001;4:132–137. doi: 10.1016/s1369-5274(00)00178-8. [DOI] [PubMed] [Google Scholar]
- Mayer M. A new set of useful cloning and expression vectors derived from pBlueScript. Gene. 1995;16:41–46. doi: 10.1016/0378-1119(95)00389-n. [DOI] [PubMed] [Google Scholar]
- Mermod N., Ramos J.L., Lehrbach P.R., Timmis K.N. Vector for regulated expression of cloned genes in a wide range of gram‐negative bacteria. J Bacteriol. 1986;167:447–454. doi: 10.1128/jb.167.2.447-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierau I., Kleerebezem M. 10 years of the nisin‐controlled gene expression system (NICE) in Lactococcus lactis. Appl Microbiol Biotechnol. 2005;68:705–717. doi: 10.1007/s00253-005-0107-6. [DOI] [PubMed] [Google Scholar]
- Miyada C.G., Stotzfus L., Wilcox G. Regulation of the araC gene of Escherichia coli, catabolite repression, autoregulation and effect on araBAD expression. Proc Natl Acad Sci USA. 1984;81:4120–4124. doi: 10.1073/pnas.81.13.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mìchan C., Kessler B., De Lorenzo V., Timmis K.N., Ramos J.L. XylS domain interactions can be deduced from the intra allelic dominance in double mutants. Mol Gen Genet. 1992;235:406–412. doi: 10.1007/BF00279387. [DOI] [PubMed] [Google Scholar]
- Monné M., Chan K.W., Slotboom D.J., Kunji E.R. Functional expression of eukaryotic proteins in Lactococcus lactis. Protein Sci. 2005;14:3048–3056. doi: 10.1110/ps.051689905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J.R., Fuqua C. Broad‐host‐range expression vectors that carry the L‐arabinose‐inducible Escherichia coli araBAD promoter and the AraC regulator. Gene. 1999;227:197–203. doi: 10.1016/s0378-1119(98)00601-5. [DOI] [PubMed] [Google Scholar]
- Nikodinovic J., Priestley N.D. A second generation gnd‐derived Escherichia coli‐Streptomyces shuttle expression vector that is generally transferable by conjugation. Plasmid. 2006;56:223–227. doi: 10.1016/j.plasmid.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Ogden S., Haggerty D., Stoner C.M., Kolodrubetz S., Schleif R. The Escherichia coli L‐arabinose operon: binding sites of the regulatory proteins and a mechanism of positive and negative regulation. Proc Natl Acad Sci USA. 1980;77:3346–3350. doi: 10.1073/pnas.77.6.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panke S., Meyer A., Huber C.M., Witholt B., Wubbolts M.G. An alkane‐responsive expression system for the production of fine chemicals. Appl Environ Microbiol. 1999;65:2324–2332. doi: 10.1128/aem.65.6.2324-2332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei J., Kim B., Grishin N.V. PROMALS3D: a tool for multiple protein sequence and structure alignments. Nucleic Acids Res. 2008;36:2295–2300. doi: 10.1093/nar/gkn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qasi S.N., Rees C.E., Mellits K.H., Hill P.J. Development of gfp vectors for expression in Listeria monocytogenes and other low G + C gram positive bacteria. Microb Ecol. 2001;41:301–309. doi: 10.1007/s002480000091. [DOI] [PubMed] [Google Scholar]
- Radha S., Gunasekaran P. Sustained expression of keratinase gene under PxylA and PamyL promoters in the recombinant Bacillus megaterium MS941. Bioresour Technol. 2008;99:5528–5537. doi: 10.1016/j.biortech.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Ramos J.L., González‐Carrero M., Timmis K.N. Broad‐host range expression vectors containing manipulated meta‐cleavage pathway regulatory elements of the TOL plasmid. FEBS Lett. 1988;226:241–246. doi: 10.1016/0014-5793(88)81431-5. [DOI] [PubMed] [Google Scholar]
- Ramos J.L., Marqués S., Timmis K.N. Transcriptional control of the Pseudomonas TOL plasmid catabolite operons is achieved through an interplay of host factors and plasmid‐encoded regulators. Annu Rev Microbiol. 1997;51:341–373. doi: 10.1146/annurev.micro.51.1.341. [DOI] [PubMed] [Google Scholar]
- Rhee S., Martin R.G., Rosner J.L., Davies D.R. A novel DNA‐binding motif in MarA: the first structure for an AraC family transcriptional activator. Proc Natl Acad Sci USA. 1998;95:10413–10418. doi: 10.1073/pnas.95.18.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov D.A. Comparative genomic reconstruction of transcriptional regulatory networks in bacteria. Chem Rev. 2007;107:3467–3497. doi: 10.1021/cr068309+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo F. Repression of transcription initiation in bacteria. J Bacteriol. 1999;181:1987–2991. doi: 10.1128/jb.181.10.2987-2991.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz R., Ramos J.L. Residues 137 and 153 of XylS influence contacts with the C‐terminal end of the RNA polymerase alpha subunit. Biochem Biophys Res Commun. 2001;287:519–521. doi: 10.1006/bbrc.2001.5615. [DOI] [PubMed] [Google Scholar]
- Ruiz R., Ramos J.L. Residues 137 and 153 at the N terminus of the XylS protein influence the effector profile of this transcriptional regulator and the s factor used by RNA polymerase to stimulate transcription from its cognate promoter. J Biol Chem. 2002;277:7282–7286. doi: 10.1074/jbc.M110226200. [DOI] [PubMed] [Google Scholar]
- Ruiz R., Ramos J.L., Egan S.M. Interactions of the XylS regulators with the C‐terminal domain of the RNA polymerase a subunit influence the expression level from the cognate Pm promoter. FEBS Lett. 2001;491:207–211. doi: 10.1016/s0014-5793(01)02192-5. [DOI] [PubMed] [Google Scholar]
- Ruiz R., Marqués S., Ramos J.L. Leucines 193 and 194 at the N‐terminal domain of the XylS protein, the positive regulator of the TOL meta‐cleavage pathway, are involved in dimerization. J Bacteriol. 2003;185:3036–3041. doi: 10.1128/JB.185.10.3036-3041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell M.A. Molecular biology of the LysR family of transcriptional regulators. Annu Rev Microbiol. 1993;47:597–626. doi: 10.1146/annurev.mi.47.100193.003121. [DOI] [PubMed] [Google Scholar]
- Schleif R. DNA looping. Science. 1988;240:127–128. doi: 10.1126/science.3353710. [DOI] [PubMed] [Google Scholar]
- Schleif R. AraC protein: a love‐hate relationship. BioEssays. 2003;25:274–282. doi: 10.1002/bies.10237. [DOI] [PubMed] [Google Scholar]
- Sletta H., Nedal A., Aune T.E.V., Hellebust H., Hakvåg S., Aune R. Broad‐host‐range plasmid pJB658 can be used for industrial‐level production of a secreted host‐toxc single‐chain antibody fragment in Escherichia coli. Appl Environ Microbiol. 2004;70:7033–7039. doi: 10.1128/AEM.70.12.7033-7039.2004. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletta H., Tøndervik A., Hakvåg S., Vee Aune T.E., Nedal A., Aune R. The presence of N‐terminal secretion signal sequences leads to strong stimulation of the total expression levels of three tested medically important proteins during high‐cell‐density cultivations of Escherichia coli. Appl Environ Microbiol. 2007;73:906–912. doi: 10.1128/AEM.01804-06. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soisson S., MacDougall‐Shackleton B., Schleif R., Wolberger C. Structural basis for ligand‐regulated oligomerization of AraC. Science. 1997a;276:421–425. doi: 10.1126/science.276.5311.421. [DOI] [PubMed] [Google Scholar]
- Soisson S., MacDougall‐Shackleton B., Schleif R., Wolberger C. The 1.6 Å crystal structure of the AraC sugar‐binding and dimerization domain complexed with D‐fucose. J Mol Biol. 1997b;273:226–237. doi: 10.1006/jmbi.1997.1314. [DOI] [PubMed] [Google Scholar]
- Staijen I.E., Marcionelli R., Witholt B. The PalkBFGHJKL promoter is under carbon catabolite repression control in Pseudomonas oleovorans but not in Escherichia coli alk+ recombinants. J Bacteriol. 1999;181:1610–1617. doi: 10.1128/jb.181.5.1610-1616.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen D., Schleif R. Overproducing AraC protein with lambda‐arabinose transducing phage. Molec Gen. 1977;157:133–339. doi: 10.1007/BF00268671. [DOI] [PubMed] [Google Scholar]
- Steigedal M., Valla S. The Acinetobacter sp. chnB promoter together with its cognate positive regulator ChnR is an attractive new candidate for metabolic engineering applications in bacteria. Metab Eng. 2008;10:121–129. doi: 10.1016/j.ymben.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Sticher P., Jaspers M.C., Stemmler K., Harms H., Zehnder A.J., Van Der Meer J.R. Development and characterization of a whole‐cell bioluminescent sensor for bioavailable middle‐chain alkanes in contaminated groundwater samples. Appl Environ Microbiol. 1997;63:4053–4060. doi: 10.1128/aem.63.10.4053-4060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukchawalita R., Vattanaviboona P., Sallabhana R., Mongkolsuk S. Construction and characterization of regulated L‐arabinose inducible broad host range expression vectors in Xanthomonas. FEMS Microbiol Lett. 1999;181:217–223. doi: 10.1111/j.1574-6968.1999.tb08847.x. [DOI] [PubMed] [Google Scholar]
- Sørvig E., Grönqvist S., Naterstad K., Mathiesen G., Eijsink V.G.H., Axelsson L. Construction of vectors for inducible gene expression in Lactococcus sakei and L. plantarum. FEMS Microbiol Lett. 2003;229:119–126. doi: 10.1016/S0378-1097(03)00798-5. [DOI] [PubMed] [Google Scholar]
- Sørvig E., Mathiesen G., Naterstad K., Eijsink V.G.H., Axelsson L. High‐level, inducible gene expression in Lactobacillus sakei and Lactobacillus plantarum using versatile expression vectors. Microbiology. 2005;151:2439–2449. doi: 10.1099/mic.0.28084-0. [DOI] [PubMed] [Google Scholar]
- Takanao E., White J., Thompson C.J., Bibb M.J. Construction of Thiostrepton‐inducible, high‐copy‐number expression vectors for the use in Streptomyces spp. Gene. 1995;166:133–137. doi: 10.1016/0378-1119(95)00545-2. [DOI] [PubMed] [Google Scholar]
- Terpe K. Overview of bacterial expression systems for heterologous protein production: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol. 2006;72:211–222. doi: 10.1007/s00253-006-0465-8. [DOI] [PubMed] [Google Scholar]
- Tobes R., Ramos J.L. AraC‐XylS database: a family of positive transcriptional factors in bacteria. Nucl Acids Res. 2002;30:318–321. doi: 10.1093/nar/30.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin J.F., Schleif R. Positive regulation of the Escherichia coli L‐rhamnose operon is mediated by the products of tandemly repeated regulatory genes. J Mol Biol. 1987;196:789–799. doi: 10.1016/0022-2836(87)90405-0. [DOI] [PubMed] [Google Scholar]
- Tobin J.F., Schleif R. Purification and properties of RhaR, the positive regulator of the L‐rhamnose operons of Escherichia coli. J Mol Biol. 1990;211:75–89. doi: 10.1016/0022-2836(90)90012-B. [DOI] [PubMed] [Google Scholar]
- Tropel D., Van Der Meer J.R. Bacterial transcriptional regulators for degradation of pathways of aromatic compounds. Microbiol Mol Biol Rev. 2004;68:474–500. doi: 10.1128/MMBR.68.3.474-500.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrum J.R., Santangelo T.J., Egan S.M. Cyclic AMP receptor protein and RhaR synergistically activate transcription from the L‐rhamnose‐responsive rhaSR promoter in Escherichia coli. J Bacteriol. 2005;187:6708–6719. doi: 10.1128/JB.187.19.6708-6718.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox G., Meuris P. Stabilization and size of AraC protein. Mol Gen Genet. 1976;145:97–100. doi: 10.1007/BF00331563. [DOI] [PubMed] [Google Scholar]
- Wilms B., Hauck A., Reuss M., Syldatk C., Mattes R., Siemann M., Altenbuchner J. High‐cell‐density fermentation for production of L‐N‐carbamoylase using an expression system base on the Escherichia coli rhaBAD promoter. Biotechnol Bioeng. 2001;73:95–103. doi: 10.1002/bit.1041. [DOI] [PubMed] [Google Scholar]
- Winther‐Larsen H.C., Josefsen K.D., Brautaset T., Valla S. Parameters affecting gene expression from the Pm promoter in gram‐negative bacteria. Metab Eng. 2000a;2:79–91. doi: 10.1006/mben.1999.0142. [DOI] [PubMed] [Google Scholar]
- Winther‐Larsen H.C., Blatny J.M., Valand B., Brautaset T., Valla S. Pm promoter expression mutants and their use in broad‐host‐range RK2 plasmid vectors. Metab Eng. 2000b;2:92–103. doi: 10.1006/mben.1999.0143. [DOI] [PubMed] [Google Scholar]
- Worsey M.J., Williams P.A. Metabolism of toluene and xylenes by Pseudomonas putida (arvilla) mt‐2: evidence for a new function of the TOL plasmid. J Bacteriol. 1975;124:7–13. doi: 10.1128/jb.124.1.7-13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.M., Lin C.F., Chang Y.C., Chung T.C. Construction and characterization of nisin‐controlled expression vectors for use in Lactococcus reuteri. Biosci Biotechnol Biochem. 2006;70:757–767. doi: 10.1271/bbb.70.757. [DOI] [PubMed] [Google Scholar]
- Ye R., Kim J.H., Kim B.G., Szarka S., Sihota E., Wong S.L. High‐level secretory production of intact, biologically active staphylokinase from Bacillus subtilis. Biotechnol Bioeng. 1999;62:87–96. [PubMed] [Google Scholar]
- Zhang X., Reeder T., Schleif R. Transcription activation parameters at araPBAD. J Mol Biol. 1996;258:14–24. doi: 10.1006/jmbi.1996.0230. [DOI] [PubMed] [Google Scholar]
- Zhou D., Yang R. Global analysis of transcription regulation in prokaryotes. Cell Mol Life Sci. 2006;63:2260–2290. doi: 10.1007/s00018-006-6184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beilen J.B., Wubbolts M.G., Witholt B. Genetics of alkane oxidation by Pseudomonas oleovorans. Biodegradation. 1994;5:161–174. doi: 10.1007/BF00696457. [DOI] [PubMed] [Google Scholar]


