Summary
Indole is an extracellular biofilm signal for Escherichia coli, and many bacterial oxygenases readily convert indole to various oxidized compounds including 7‐hydroxyindole (7HI). Here we investigate the impact of indole and 7HI on Pseudomonas aeruginosa PAO1 virulence and quorum sensing (QS)‐regulated phenotypes; this strain does not synthesize these compounds but degrades them rapidly. Indole and 7HI both altered extensively gene expression in a manner opposite that of acylhomoserine lactones; the most repressed genes encode the mexGHI‐opmD multidrug efflux pump and genes involved in the synthesis of QS‐regulated virulence factors including pyocyanin (phz operon), 2‐heptyl‐3‐hydroxy‐4(1H)‐quinolone (PQS) signal (pqs operon), pyochelin (pch operon) and pyoverdine (pvd operon). Corroborating these microarray results, indole and 7HI decreased production of pyocyanin, rhamnolipid, PQS and pyoverdine and enhanced antibiotic resistance. In addition, indole affected the utilization of carbon, nitrogen and phosphorus, and 7HI abolished swarming motility. Furthermore, 7HI reduced pulmonary colonization of P. aeruginosa in guinea pigs and increased clearance in lungs. Hence, indole‐related compounds have potential as a novel antivirulence approach for the recalcitrant pathogen P. aeruginosa.
Introduction
Cell signals can be promiscuous. For example, Escherichia coli senses the quorum‐sensing (QS) signal autoinducer‐2 (AI‐2) that is produced by Vibrio harveyi to assess changes in its cell population (Xavier and Bassler, 2005), and Pseudomonas aeruginosa responds to AI‐2 and modulates its gene expression pattern including pathogenicity, although it does not itself produce AI‐2 (Duan et al., 2003). In addition, Salmonella enterica and E. coli detect acylhomoserine lactones (AHLs) via SdiA, although they do not synthesize AHLs (Michael et al., 2001), and E. coli decreases biofilm formation in the presence of AHLs through SdiA (Lee et al., 2007a). Salmonella enterica enhances drug resistance in response to indole, although it does not produce indole (Nikaido et al., 2008), and we found that biofilm formation of P. aeruginosa and P. fluorescens increases in the presence of indole, even though these pseudomonads do not produce indole (Lee et al., 2007a). Furthermore, the large quantities of indole secreted by E. coli[up to 0.6 mM in Luria–Bertani (LB) medium] (Domka et al., 2006) may be hydroxylated by other bacteria such as Burkholderia cepacia G4 (Rui et al., 2005) to form hydroxyindoles that both increase and decrease biofilm formation in E. coli O157:H7 (Lee et al., 2007b).
A variety of bacteria produce indole from l‐tryptophan such as E. coli (Crawford and Yanofsky, 1958), Vibrio vulnificus (Dalsgaard et al., 1999), Haemophilus influenzae (Stull et al., 1995), Pasteurella multocida (Clemons and Gadberry, 1982), Klebsiella oxytoca (Liu et al., 1997) and Proteus vulgaris (DeMoss and Moser, 1969); a NCBI blast search shows more than 27 genera utilize tryptophanase (tnaA) to convert tryptophan into indole, pyruvate and ammonia (Stewart and Yanofsky, 1985). Indole is an extracellular E. coli signal (Wang et al., 2001) that inhibits biofilms (Lee et al., 2007a), and it works in a QS fashion (Lee et al., 2007b) primarily at temperatures less than 37oC in E. coli (Lee et al., 2008). Indole with E. coli also influences motility, acid resistance, chemotaxis and attachment to epithelial cells (Domka et al., 2006; Bansal et al., 2007; Lee et al., 2007a,b). It also controls plasmid stability by delaying cell division in E. coli (Chant and Summers, 2007), induces the expression of multidrug exporter genes and increases drug resistance (Hirakawa et al., 2005). Hence, indole is widespread in the environment, controls many phenotypes and may be encountered by other bacteria.
Pseudomonas aeruginosa is virulent to a variety of hosts including man, plants and invertebrates (Lewenza et al., 2005). Pseudomonas aeruginosa captures iron with two endogenous siderophores, pyoverdine and pyochelin (Michel et al., 2005), and both contribute to its virulence (Cox, 1982; Meyer et al., 1996). RhlR‐regulated rhamnolipids are glycolipids with biosurfactant properties that are involved in bacterial virulence (Zulianello et al., 2006). Pyocyanin from P. aeruginosa has antibiotic activity towards competing bacteria including indole‐producing E. coli (Hassett et al., 1992), and 2‐heptyl‐3‐hydroxy‐4(1H)‐quinolone (PQS) is structurally similar to pyocyanin and possesses antimicrobial activity (Mashburn and Whiteley, 2005). Therefore, P. aeruginosa utilizes several virulence factors.
It is likely that P. aeruginosa encounters indole because (i) P. aeruginosa is ubiquitous (Stover et al., 2000), (ii) P. aeruginosa is found in the gut where E. coli is dominant (Wang et al., 2006) and produces gut‐derived generalized sepsis (Matsumoto et al., 1999), and (iii) P. aeruginosa may also encounter E. coli outside the human body as E. coli exists outside the human host as well. Hence, it is possible Pseudomonas encounters indole and 7‐hydroxyindole (7HI).
In this study, we used both DNA microarrays and phenotype arrays to investigate the impact of the E. coli signal indole and hydroxylated indole (7HI) on global gene expression and QS‐regulated phenotypes of P. aeruginosa PAO1. Indole and 7HI altered extensively gene expression, decreased the production of various virulence factors, decreased swarming motility and increased antibiotic resistance in P. aeruginosa. Furthermore, 7HI reduced colonization of P. aeruginosa in guinea pig lungs and increased clearance in the gastrointestinal region.
Results
Indole and 7HI are non‐toxic
To test the toxicity with P. aeruginosa, the specific growth rate was measured with indole, 7HI and plant hormone indole‐3‐acetic acid (IAA, structurally similar plant hormone which served as a negative control). In the absence of indole compounds, the specific growth rate was 1.5 ± 0.1 h−1 whereas the growth rate was 1.51 ± 0.06 h−1 with 1.0 mM indole, 1.89 ± 0.01 h−1 with 0.5 mM 7HI and 1.64 ± 0.04 h−1 with 1.0 mM IAA. Hence, indole, 7HI and IAA do not decrease the specific growth rate of P. aeruginosa at these concentrations, and the effects of indole and 7HI on P. aeruginosa are not due to toxicity. However, higher concentrations (2 mM) decreased the specific growth by 14% for indole (1.31 ± 0.01 h−1) and by 47% for 7HI (0.8 ± 0.2 h−1).
Indole and 7HI stimulate biofilm formation
As indole and 7HI increased P. aeruginosa biofilm formation at 30°C (Lee et al., 2007b), we tested different concentrations of indole, 7HI and IAA for their ability to affect P. aeruginosa biofilm formation in 96‐well plates after 24 h at 37°C (Fig. S1). Indole increased P. aeruginosa biofilm formation up to 1.8 ± 0.6‐fold (0–1 mM) compared with no indole addition and did not affect cell growth. 7HI also increased biofilm formation 2.5 ± 0.8‐fold in a dose–response manner (0–0.75 mM) compared with no 7HI addition without affecting cell growth. Notably, 7HI (0.5 mM) increased the liquid/solid (bottom) biofilm formation sixfold (0.60 ± 0.21 versus 0.10 ± 0.07). Therefore, indole and 7HI increase P. aeruginosa biofilm formation although P. aeruginosa does not synthesize these compounds. However, IAA did not influence the biofilm formation of P. aeruginosa (Fig. S1).
Indole and 7HI are global regulators
To investigate the genetic mechanism of biofilm enhancement by indole and 7HI on a global basis, we performed three sets of microarray experiments with 7 h P. aeruginosa biofilm cells: (i) a comparison of cells with and without 1.0 mM indole, (ii) a comparison of cells with and without 0.5 mM 7HI, and (iii) a comparison of cells with and without 1.0 mM IAA (negative control). The expression data for the biofilm samples are summarized in Table 1.
Table 1.
Indole and 7HI regulate quorum‐sensing genes in P. aeruginosa PAO1.
| PA# | Gene | Fold changea | Description | ||
|---|---|---|---|---|---|
| Indole versus no indole | 7HI versus no 7HI | IAA versus no IAA | |||
| Multidrug efflux transporter | |||||
| PA4205 | mexG | −13.0 | −7.0 | −1.1 | Hypothetical protein, part of the mexGHI‐opmD cluster |
| PA4206 | mexH | −11.3 | −5.7 | −1.3 | Efflux membrane fusion protein precursor |
| PA4207 | mexI | −6.1 | −4.0 | −1.3 | Efflux transporter |
| PA4208 | opmD | −7.5 | −4.6 | −1.1 | Probable outer membrane efflux protein |
| Phenazine (pyocyanin) synthesis | |||||
| PA1901 | phzC2 | −2.5 | −2.1 | −1.2 | Phenazine biosynthesis protein PhzC |
| PA1902 | phzD2 | −3.2 | −3.0 | −1.1 | Phenazine biosynthesis protein PhzD |
| PA1903 | phzE2 | −3.5 | −2.6 | −1.2 | Phenazine biosynthesis protein PhzE |
| PA1904 | phzF2 | −4.0 | −4.3 | −1.1 | Phenazine biosynthesis protein |
| PA1905 | phzG2 | −3.2 | −3.2 | −1.2 | Pyridoxamine 5′‐phosphate oxidase |
| PA2274 | −4.9 | −3.2 | −1.1 | Possible monooxygenase, involved in phenazine biosynthesis | |
| PA4210 | phzA1 | −2.5 | −3.7 | −1.1 | Phenazine biosynthesis protein |
| PA4211 | phzB1 | −2.3 | −2.3 | −1.3 | Phenazine biosynthesis protein |
| PA4217 | phzS | −2.8 | −2.8 | −1.1 | Flavin‐containing monooxygenase |
| PQS synthesis | |||||
| PA0996 | pqsA | −1.9 | −2.0 | −1.1 | PqsA, probable coenzyme A ligase |
| PA0997 | pqsB | −2.3 | −2.5 | −1.1 | PqsB, homologous to beta‐keto‐acyl‐acyl‐carrier protein synthase |
| PA0998 | pqsC | −1.7 | −1.9 | −1.1 | PqsC, homologous to beta‐keto‐acyl‐acyl‐carrier protein synthase |
| PA0999 | pqsD | −1.3 | −1.9 | −1.1 | PqsD, 3‐oxoacyl‐[acyl‐carrier‐protein] synthase III |
| PA1000 | pqsE | −2.0 | −2.1 | −1.1 | PqsE, quinolone signal response protein |
| PA1003 | mvfR | 1.3 | −1.1 | −1.1 | MvfR (PqsR), PQS transcriptional regulator |
| Pyochelin synthesis | |||||
| PA4225 | pchF | −2.0 | −1.1 | 1.3 | Pyochelin synthetase |
| PA4226 | pchE | −2.8 | −1.3 | 1.2 | Dihydroaeruginoic acid synthetase |
| PA4227 | pchR | −1.5 | −1.2 | 1.1 | Transcriptional regulator PchR |
| PA4228 | pchD | −3.2 | −1.3 | 1.2 | Pyochelin biosynthesis protein PchD |
| PA4229 | pchC | −2.3 | −1.1 | 1.2 | Pyochelin biosynthetic protein PchC |
| PA4230 | pchB | −2.0 | −1.2 | 1.2 | Salicylate biosynthesis protein PchB |
| PA4231 | pchA | −2.1 | −1.3 | 1.2 | Salicylate biosynthesis isochorismate synthase |
| Pyoverdine synthesis | |||||
| PA2385 | pvdQ | −2.1 | −1.6 | 1.1 | pyoverdine biosynthesis protein PvdQ |
| PA2393 | pvdM | −2.5 | −1.1 | 2.1 | pyoverdine biosynthesis protein PvdM |
| PA2394 | pvdN | −2.5 | −1.2 | 1.3 | pyoverdine biosynthesis protein PvdN |
| PA2395 | pvdO | −2.0 | −1.1 | 1.5 | pyoverdine biosynthesis protein PvdO |
| PA2397 | pvdE | −2.0 | −1.4 | 1.1 | pyoverdine biosynthesis protein PvdE |
| PA2424 | pvdL | −2.3 | −1.3 | 1.9 | pyoverdine biosynthesis protein PvdV |
| PA2426 | pvdS | −6.1 | −2.6 | 2.0 | Sigma factor PvdS, transcriptional regulator |
| Metabolism | |||||
| PA0283 | sbp | −8.6 | −1.7 | 1.3 | Sulfate‐binding protein precursor |
| PA1838 | cysI | −6.1 | −2.1 | 1.2 | Sulfite reductase |
| PA3441 | ssuF | −7.5 | −5.7 | 1.2 | SsuF, part of the ssu locus |
| PA3442 | ssuB | −4.6 | −2.6 | 1.3 | SsuB, part of the ssu locus |
| PA3443 | ssuC | −3.2 | −2.6 | 1.3 | SsuC, part of the ssu locus |
| PA3444 | ssuD | −6.1 | −2.1 | 1.1 | SsuD, part of the ssu locus |
| PA3445 | ssuA | −6.5 | −2.8 | 2.8 | SsuA, part of the ssu locus |
| PA3446 | ssuE | −6.5 | −1.6 | 1.5 | SsuE, part of the ssu locus |
| PA4442 | cysN | −4.6 | −2.0 | −1.1 | ATP sulfurylase GTP‐binding subunit |
| PA4443 | cysD | −4.9 | −1.7 | 1.2 | ATP sulfurylase small subunit |
| PA5025 | metY | −2.6 | −1.3 | −1.1 | Homocysteine synthase |
| PA5427 | adhA | 2.1 | 2.6 | −1.1 | Alcohol dehydrogenase |
| Fimbriae and pili | |||||
| PA2131 | −1.9 | −2.6 | 1.0 | Probable pili assembly chaperone | |
| PA2570 | pa1L | 1.6 | −2.8 | 1.1 | PA‐I galactophilic lectin |
| PA4084 | cupB3 | −2.8 | −4.0 | 1.5 | Probable fimbrial biogenesis usher protein |
| PA4085 | cupB2 | −3.0 | −2.1 | 1.1 | Probable pili assembly chaperone |
| PA4297 | tadG | 2.3 | 3.5 | 1.7 | TadG |
| PA4298 | 1.6 | −3.2 | 1.2 | Hypothetical protein | |
| PA4299 | tadD | 1.7 | −2.6 | 1.1 | Flp pilus assembly protein TadD |
| PA4300 | tadC | 1.7 | −2.3 | 1.3 | Flp pilus assembly protein TadC |
| PA4301 | tadB | 1.6 | −3.0 | 1.5 | Flp pilus assembly protein TadB |
| PA4302 | tadA | 1.9 | −2.5 | 1.2 | Flp pilus assembly protein, ATPase CpaF |
| PA4303 | tadZ | 1.7 | −2.3 | 1.2 | Flp pilus assembly protein, ATPase CpaE |
| PA4304 | rcpA | 2.0 | −1.9 | 1.0 | Flp pilus assembly protein, secretin CpaC |
| PA4305 | rcpC | 1.6 | −2.5 | 1.1 | Flp pilus assembly protein CpaB |
| PA4306 | flp | 1.5 | −2.8 | 1.1 | Flp pilus assembly protein, pilin Flp |
| PA4651 | 2.0 | −3.5 | 1.3 | Probable pili assembly chaperone | |
| Transport of small molecules | |||||
| PA1108 | −1.9 | −5.7 | 1.5 | Probable MFS transporter | |
| PA2092 | −2.6 | −4.0 | 1.4 | Probable MFS transporter | |
| PA2204 | −9.8 | −1.9 | 1.1 | Binding protein component of ABC transporter | |
| PA2328 | −6.1 | −5.3 | 1.1 | Hypothetical protein in the cluster of ABC transporter | |
| PA2329 | −5.7 | −5.7 | 1.0 | Probable ATP‐binding component of ABC transporter | |
| PA2330 | −4.9 | −4.3 | 1.1 | Hypothetical protein in the cluster of ABC transporter | |
| PA2331 | −5.7 | −4.0 | −1.1 | Hypothetical protein in the cluster of ABC transporter | |
| PA3531 | bfrB | 3.2 | 2.3 | −1.2 | Bacterioferritin, transport of small molecules |
| PA3926 | −1.7 | −2.0 | 1.3 | Probable MFS transporter | |
| PA4514 | piuA | −4.3 | −2.5 | 1.1 | Probable outer membrane receptor for iron transport |
| Oxygenases | |||||
| PA2512 | antA | 5.3 | −2.0 | 9.2 | Anthranilate dioxygenase large subunit |
| PA2513 | antB | 6.1 | −2.6 | 1.6 | Anthranilate dioxygenase small subunit |
| PA2514 | antC | 3.0 | −2.8 | 1.6 | Anthranilate dioxygenase reductase |
| PA0106 | coxA | 3.2 | 1.4 | 1.0 | Cytochrome c oxidase, subunit I |
| PA0107 | 2.8 | 1.3 | −1.1 | Predicted cytochrome oxidase assembly factor | |
| PA0108 | coIII | 3.2 | 1.2 | −1.1 | Cytochrome c oxidase, subunit III |
| PA0109 | 2.0 | −1.1 | 1.1 | Hypothetical protein in the cluster of oxidase | |
| PA0110 | 2.6 | 1.4 | 1.1 | Hypothetical protein in the cluster of oxidase | |
| PA0111 | 3.0 | 1.3 | 1.1 | Hypothetical protein in the cluster of oxidase | |
| PA0112 | 3.0 | 1.1 | 1.1 | Hypothetical protein in the cluster of oxidase | |
| PA0113 | 3.0 | 1.4 | −1.3 | Probable cytochrome c oxidase assembly | |
| Others | |||||
| PA0715 | 3.7 | 1.4 | −2.0 | Probable bacteriophage protein | |
| PA0716 | 5.7 | 7.0 | −1.3 | Probable bacteriophage protein | |
| PA0740 | sdsA1 | −8.6 | −3.0 | 1.1 | Probable beta‐lactamase |
| PA1109 | −2.6 | −5.7 | 2.5 | Transcriptional regulator | |
| PA2258 | ptxR | −1.6 | −3.2 | 1.9 | Transcriptional regulator PtxR |
| PA3153 | wzx | 2.6 | 1.0 | −1.4 | O‐antigen translocase |
| PA3154 | wzy | 3.5 | 1.0 | −1.3 | B‐band O‐antigen polymerase |
| PA3155 | wbpE | 2.8 | 1.5 | −1.2 | Probable aminotransferase WbpE |
| PA3156 | wbpD | 2.3 | 1.4 | −1.1 | Probable acetyltransferase |
| PA3234 | yjcG | 4.3 | 2.0 | −1.1 | Probable sodium:solute symporter |
| PA3337 | rfaD | 2.0 | 2.5 | 1.1 | ADP‐l‐glycero‐d‐mannoheptose 6‐epimerase |
| PA3450 | lsfA | −6.1 | −1.4 | 1.1 | Probable antioxidant protein |
| PA3721 | nalC | 3.0 | −1.1 | 1.6 | Transcriptional regulator NalC, induced by MvfR |
| PA4563 | rpsT | 2.8 | 2.8 | −1.1 | 30S ribosomal protein S20 |
| Hypothetical proteins | |||||
| PA0284 | −13.9 | −1.6 | 1.2 | Hypothetical protein, induced by MvfR | |
| PA0492 | −4.3 | −3.5 | 1.9 | Hypothetical protein | |
| PA0696 | −4.3 | −2.5 | 1.3 | Hypothetical protein | |
| PA0939 | −1.1 | −5.3 | 1.6 | Hypothetical protein | |
| PA1190 | 5.7 | 1.6 | 1.2 | Hypothetical protein | |
| PA1837 | −4.6 | −2.1 | 1.1 | Hypothetical protein | |
| PA1914 | 1.1 | −6.1 | 1.1 | Hypothetical protein | |
| PA1953 | −3.2 | −6.1 | 1.5 | Hypothetical protein | |
| PA2036 | −2.0 | −7.0 | 1.1 | Hypothetical protein | |
| PA2078 | −3.0 | −5.7 | 1.6 | Hypothetical protein | |
| PA2419 | −1.6 | 6.1 | 1.5 | Hypothetical protein | |
| PA2805 | 2.3 | 2.1 | 1.1 | Hypothetical protein | |
| PA3080 | 3.2 | 1.1 | −1.1 | Hypothetical protein | |
| PA3235 | 4.0 | 2.5 | 1.1 | Hypothetical protein | |
| PA3237 | −1.9 | −6.1 | 1.9 | Hypothetical protein | |
| PA3572 | 2.0 | 2.5 | 1.1 | Hypothetical protein | |
| PA3719 | 4.6 | 1.3 | 1.3 | Hypothetical protein | |
| PA3720 | 2.6 | 1.2 | 1.6 | Hypothetical protein | |
| PA3931 | −5.3 | −1.7 | 1.0 | Hypothetical protein | |
| PA4087 | −2.5 | −3.5 | 1.0 | Hypothetical protein | |
| PA4359 | −4.0 | −1.1 | 1.3 | Hypothetical protein | |
| PA4377 | 3.7 | 1.7 | 1.4 | Hypothetical protein | |
| PA4614 | 3.5 | 1.4 | 1.1 | Hypothetical protein | |
| PA4638 | 6.5 | −1.6 | −1.2 | Hypothetical protein | |
| PA4683 | −2.8 | −1.3 | 1.1 | Hypothetical protein | |
Partial list of differentially expressed genes in LB medium after 7 h for (i) biofilm cells grown with 1.0 mM indole versus no indole, (ii) biofilm cells grown with 0.5 mM 7‐hydroxyindole (7HI) versus no 7HI and (iii) biofilm cells grown with 1.0 mM indole‐3‐acetic acid (IAA, control compound) versus no IAA. Most significant changes are shown in bold.
Overall, we found the addition of indole regulates significantly 532 genes more than twofold in biofilm cells at 7 h; 88 genes were induced and 444 genes were repressed. Similarly, the addition of 7HI regulates significantly 733 genes more than twofold in biofilm cells at 7 h; 25 genes were induced and 708 genes were repressed. In contrast, the addition of IAA altered expression of 10‐fold fewer genes (only 71 genes more than twofold); 57 genes were induced and 14 genes were repressed. Therefore, indole and 7HI primarily repress genes in P. aeruginosa. To confirm the presence of indole and 7HI in the microarray samples, the extracellular indole and 7HI were measured using HPLC. The level of indole decreased from 1.0 mM to 0.68 mM in 7 h (the negative control without indole addition showed zero indole). Also, the level of extracellular 7HI decreased from 0.5 mM to 0.154 mM after 7 h.
Indole represses the mexGHI‐opmD multidrug efflux genes and QS‐regulated genes
The most noticeable change of gene expression with indole was that the mexGHI‐opmD multidrug efflux genes were highly repressed (6‐ to 13‐fold), and many genes involved in the synthesis of QS‐regulated virulence factors were repressed (Table 1). Specifically, the phenazine synthesis operon (phz) which is involved in the pyocyanin biosynthesis, the PQS synthesis operon (pqs), the pyochelin synthesis operon (pch) and the pyoverdine synthesis operon (pvd) were repressed (two‐ to sixfold) by indole. Also, several genes involved in the transport of small molecules were repressed by indole. Among genes involved in metabolism, genes involved in sulfur metabolism, such as sbp, the whole ssuFBCDAE locus that plays a key role in organosulfur uptake (Kahnert et al., 2000), and cysI and cysND, were significantly repressed (three‐ to eightfold) by indole (Table 1). This suggests that indole downregulates sulfur uptake of P. aeruginosa. In contrast, several oxidase genes and antABC‐encoding anthranilate dioxygenase genes were induced (three‐ to sixfold) by indole, while 7HI repressed the antABC operon. However, the transcriptional level of the QS signal regulators, such as RhlRI, LasRI and MvfR (PqsR), was not altered by indole or 7HI.
7HI represses the mexGHI‐opmD multidrug efflux genes and QS‐regulated genes
Like indole, 7HI repressed (four‐ to sevenfold) the four mexGHI‐opmD multidrug efflux genes and genes involved in the synthesis of QS‐regulated virulence factors (e.g. phz operon, pqs operon and pvdS) (Table 1). Unlike indole, 7HI did not change the expression of pyochelin synthesis genes.
Note, the control IAA did not change the transcription of nearly all the genes involved in virulence (Table 1); therefore, the impact on virulence is specific for indole and 7HI. The most induced gene (9.2‐fold) in the presence of IAA was antA (encodes the anthranilate dioxygenase large subunit). Also induced were six genes involved in metabolism (two‐ to threefold) and 12 genes involved in the transport of small molecules (two‐ to eightfold).
Verification of DNA microarray results
Real‐time reverse transcription polymerase chain reaction (RT‐PCR) was used to verify gene expression for several of the highly differentially expressed loci in the two sets of the DNA microarray experiments (response to indole and 7HI). Using independent cultures, RT‐PCR showed differential changes in expression comparable to the DNA microarrays. For the indole experiments, both techniques (RT‐PCR versus microarray) showed the genes were repressed in a similar manner: 14.1‐ versus 13.0‐fold for mexG, 26.4‐ versus 11.3‐fold for mexH, 1.9‐ versus 6.1‐fold for mexI, 5.6‐ versus 6.1‐fold for pvdS, 10.0‐ versus 7.5‐fold for ssuF and 12.7‐ versus 13.9‐fold for PA0284. Similarly, for the 7HI experiments, both techniques (RT‐PCR versus microarray) showed the genes were repressed in a similar manner: 54.7‐ versus 7.0‐fold for mexG, 4.0‐ versus 5.7‐fold for mexH, 2.8‐ versus 4.0‐fold for mexI, 3.8‐ versus 2.6‐fold for pvdS, 3.0‐ versus 5.7‐fold for ssuF and 2.6‐ versus 1.6‐fold for PA0284.
Indole and 7HI decrease QS‐regulated virulence phenotypes
As the microarray data showed that indole and 7HI repressed genes involved in the synthesis of QS‐regulated virulence factors (Table 1), we assayed phenotypes related to the three QS systems of P. aeruginosa including the RhlR‐regulated phenotypes [pyocyanin (Gallagher et al., 2002), rhamnolipid production (Ochsner and Reiser, 1995) and swarming motility (Déziel et al., 2003)], LasR‐ and RhlR‐regulated PQS production (Diggle et al., 2006) and LasR‐regulated elastase production (Gambello and Iglewski, 1991). Production of the siderophore pyoverdine, a virulence factor in P. aeruginosa (Lamont and Martin, 2003), was also measured as indole and 7HI repressed the pyoverdine synthesis genes and a transcriptional regulator pvdS (Table 1).
As expected, indole and 7HI decreased PQS production by 5.0 ± 2‐fold and 10 ± 5‐fold, decreased pyocyanin production by 7.1 ± 0.6‐fold and 11 ± 3‐fold, decreased rhamnolipid production by 2.2 ± 0.7‐fold and 2.0 ± 0.5‐fold, and decreased pyoverdine production by 1.7 ± 0.3‐fold and 3.2 ± 0.3‐fold respectively (Figs 1A and S2 for PQS production); however, indole and 7HI did not alter elastase production. Therefore, the decreased production of these QS‐regulated virulence factors upon addition of indole and 7HI corroborated the microarray data (Table 1). Notably, the impact of 7HI on the production of PQS and pyoverdine was more significant than that with indole. In contrast, IAA did not significantly change the production of the four virulence factors; hence the changes in the regulated phenotypes are specific for indole and 7HI.
Figure 1.
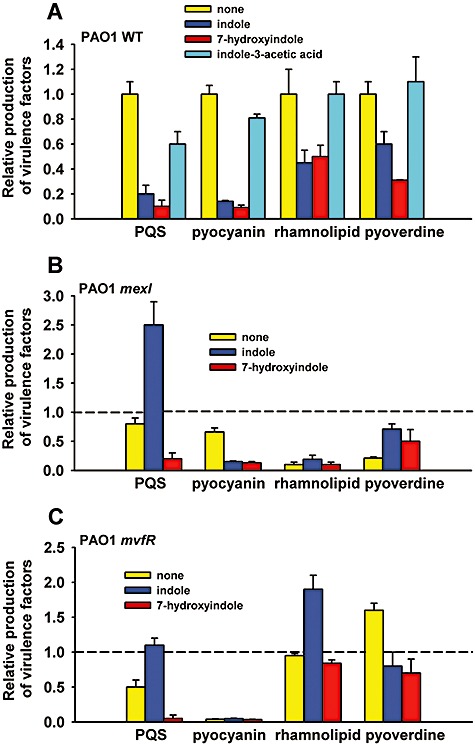
Reduction of virulence factors by indole and 7HI. Production of virulence factors with 1.0 mM indole, 0.5 mM 7HI and 1.0 mM IAA (negative control) with P. aeruginosa PAO1 (A), with P. aeruginosa PAO1 mexI (B) and P. aeruginosa PAO1 mvfR (C). For clarity, wild‐type values are not shown in (B) and (C) so bars indicate the relative amount of each compound made compared with the wild‐type strain without indole or 7HI added. Each experiment was performed with at least two independent cultures. Data show the average of the replicates, and one standard deviation is shown.
7HI abolishes swarming
The impact of indole and 7HI on the swimming, swarming and twitching motility of P. aeruginosa was tested as swarming motility plays an important role in P. aeruginosa biofilm formation (Overhage et al., 2007), swarming is related to QS (Köhler et al., 2000), and our microarray data showed that QS genes (e.g. phz operon, pqs operon and pvdS) were repressed by 7HI (Table 1). Indole and 7HI both decreased slightly swimming motility; the swimming halo diameters at 24 h were 3.24 ± 0.08 cm (no addition), 2.75 ± 0.01 cm (indole) and 2.28 ± 0.01 cm (7HI). Twitching motility was not significantly changed with indole and 7HI. However, indole significantly decreased swarming motility, and 7HI abolished swarming motility (Fig. 2).
Figure 2.
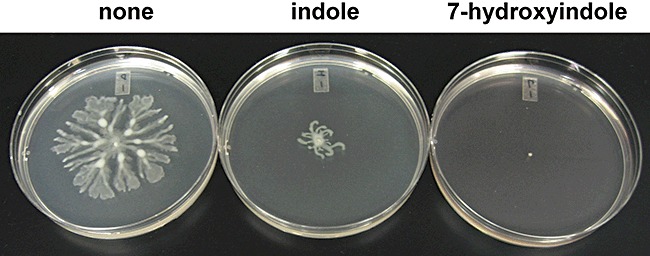
Inhibition of swarming motility by indole and 7HI. Swarming motility of P. aeruginosa on BM2 medium with 0.5% agar with 1.0 mM indole and 0.5 mM 7HI after 28 h. Each experiment was performed with at least two independent cultures and one representative data set is shown.
Indole and 7HI increase antibiotic resistance
As MexGHI‐OpmD efflux pump mutants of P. aeruginosa show enhanced antibiotic resistance (Aendekerk et al., 2005) and as our microarray data showed that these efflux genes were the most repressed genes by indole and 7HI (Table 1), we assayed the antibiotic resistance of P. aeruginosa upon addition of indole and 7HI with five antibiotics. Four antibiotics (tetracycline, gentamicin, kanamycin and vanadyl sulfate) were investigated by measuring the survival rate, and ampicillin was investigated by using a minimal inhibition concentration (MIC) method. Indole and 7HI increased tetracycline resistance by 5 ± 3‐fold and 4 ± 3‐fold, respectively, and increased gentamicin resistance by 2 ± 2‐fold and 3 ± 2‐fold, respectively, and 7HI increased kanamycin resistance by 2.5 ± 0.5‐fold (Fig. S3). Hence, our results agreed well with previous results using mexI and opmD mutants with kanamycin and tetracycline (Aendekerk et al., 2005) in that repression of MexGHI‐OpmD by indole and 7HI enhanced antibiotic resistance consistently. However, indole and 7HI did not change the survival rates (∼55%) in the presence of 5 mM vanadyl sulfate (Aendekerk et al., 2002). Similarly, the MIC of ampicillin was increased in the presence of indole and 7HI (300 µg ml−1 for no indole or 7HI, 500 µg ml−1 with indole and 600 µg ml−1 with 7HI). Therefore, indole and 7HI enhance P. aeruginosa antibiotic resistance.
Indole affects many cell phenotypes
As the regulation of 532 genes were altered by adding indole as shown by the DNA microarrays (Table 1), we explored the impact of adding indole to P. aeruginosa on 756 phenotypes using phenotype arrays (Table S1). The phenotype arrays showed that the addition of indole severely suppressed growth with many carbon sources (e.g. d‐saccharic acid, l‐alanine, glucuronamide, m‐tartaric acid, d‐aspartic acid and α‐methyl‐d‐galactoside), many nitrogen sources (e.g. xanthosine, tyramine, cytidine, cytosine, guanine and d‐lysine), many phosphorus sources (e.g. d‐mannose‐1‐phosphate, d‐2‐phospho‐glyceric acid and cytidine‐2‐monophosphate) and three sulfur sources (lanthionine, glutathione, and l‐cysteine). The reduced cell growth in the presence of indole with three sulfur sources corroborates our microarray data in that indole repressed genes involved in sulfur uptake (sbp, ssuFBCDAE, cysI and cysND) (Table 1), which suggests that indole decreases sulfur uptake in P. aeruginosa. Overall, indole alters the carbon, nitrogen, sulfur and phosphorus metabolism of P. aeruginosa.
P. aeruginosa degrades indole and 7HI
In the microarray samples of P. aeruginosa, the concentration of indole and 7HI decreased significantly after 7 h (1.0 mM indole decreased to 0.68 mM and 0.5 mM 7HI decreased to 0.154 mM); hence, the degradation of indole and 7HI was quantified by measuring the concentration of extracellular indole and 7HI using HPLC. It was found that P. aeruginosa degrades indole in LB medium at 1.04 ± 0.04 nmol min−1 (mg protein)−1 and degrades 7HI in LB medium at 2.6 ± 0.1 nmol min−1 (mg protein)−1 while there was no significant change of indole and 7HI concentrations with the negative control (dead cells) (Fig. 3A and B). Furthermore, P. aeruginosa PAO1 does not utilize either indole (1 or 2 mM) or 7HI (0.5 or 2 mM) as a sole source of carbon and energy (data not shown) although P. aeruginosa Gs isolated from mangrove sediments (Yin et al., 2005) and Pseudomonas sp. ST‐200 from soil (Doukyu and Aono, 1997) grow on indole.
Figure 3.
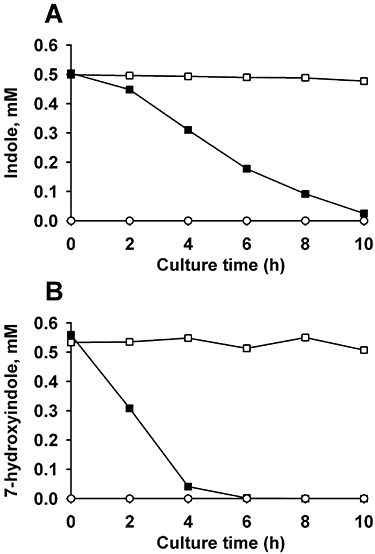
Degradation of indole and 7HI by P. aeruginosa. Pseudomonas aeruginosa degrades 0.5 mM indole (A) and 0.5 mM 7HI (B) in LB. The initial turbidity of cells was 1.0 at 600 nm. Closed square data (▪) are from live cells, open square data (□) are from autoclaved cells (dead cell control) and open circle data (○) are from live cells that lack added indole or 7HI. Each experiment was performed using two independent cultures, and one representative data set is shown.
In the whole‐transcriptome analysis, antABC was the most induced locus upon indole addition (Table 1); hence, we investigated if anthranilate dioxygenase is necessary for indole degradation. We found that the P. aeruginosa PAO1 antA mutant degraded indole to the same extent as the wild‐type strain; therefore, indole degradation is not related to anthranilate metabolism. Furthermore, as addition of the negative control IAA highly induced antA (Table 1), it appears both IAA and indole induce the ant locus due to some non‐specific interaction with a regulatory protein rather as substrates.
Indole and 7HI are less effective in the mexI and mvfR mutants
As the most highly repressed locus in the presence of indole and 7HI was mexGHI‐opmD (Table 1) and as mutation of mexI decreased the production of rhamnolipid and pyoverdine (Aendekerk et al., 2002), the effect of indole and 7HI was further investigated with P. aeruginosa PAO1 mexI. Consistent with a previous report (Aendekerk et al., 2002), the mexI mutation reduced rhamnolipid and pyoverdine by 10‐ and 5‐fold respectively (Fig. 1B). However, unlike with the wild‐type strain, indole did not decrease the production of PQS, rhamnolipid and pyoverdine in the mexI strain (Fig. 1B), and 7HI also did not decrease the production of rhamnolipid and pyoverdine in the mexI strain. These results suggest mexI is involved in a complex manner with the decrease of virulence factors with indole and 7HI. In contrast, indole and 7HI significantly decreased pyocyanin production in the mexI strain probably due to the presence of two different pathways for pyocyanin synthesis, the aro pathway and the phz pathway (Lau et al., 2004).
As the transcriptional regulator MvfR is required for full P. aeruginosa virulence and induces both mexGHI‐opmD and phenazine transcription (phz operon that is involved in pyocyanin biosynthesis) (Déziel et al., 2005), the effect of indole and 7HI was also investigated with the P. aeruginosa mvfR mutant. As expected, PQS and pyocyanin were decreased in the mvfR mutant compared with the wild‐type strain (Fig. 1C). However, unlike with the wild‐type strain, the addition of indole did not decrease the production of PQS, rhamnolipid and pyocyanin. These results suggest that indole requires MvfR to fully reduce virulence factors.
In addition, the P. aeruginosa PAO1 antA mutant was investigated as indole diminished PQS production (Fig. 1A) while inducing the antABC (Table 1); antABC encode proteins involved in the degradation of anthranilate, a precursor of PQS. As expected, the antA mutant produced about 1.8‐fold more PQS (Fig. S4) (as there is a larger pool of anthranilate for PQS). However, the response of the antA mutant upon addition of indole was similar to that of the wild‐type strain. Hence, antA is not directly involved in the indole mechanism to control virulence factors.
7HI reduces P. aeruginosa virulence in guinea pigs
As 7HI diminished virulence factors in P. aeruginosa more than indole (Table 1 and Fig. 1) and as 7HI abolished swarming motility (Fig. 2), we evaluated the ability of P. aeruginosa pre‐treated with 7HI or diluent dimethylformamide (DMF) as a control to colonize guinea pig lungs after infection. Pulmonary colonization was examined immediately after infection until 48 h post infection (Fig. 4A). Colonization of the lung by P. aeruginosa treated with 7HI was reduced by 25%, and 7HI‐treated bacteria are cleared more easily from the lungs for the first 4 h post infection, leading to a greater than 50% reduction in pulmonary bacteria by this time point. In the acute model, both the 7HI‐ and DMF‐treated P. aeruginosa continue to be expelled from the lungs, resulting in nearly complete clearance of the bacterial pneumonia by 48 h. As it was not possible to maintain 7HI levels with the bacteria in vivo, the differences between DMF‐ and 7HI‐treated bacteria decrease over the course of this experiment, as expected.
Figure 4.
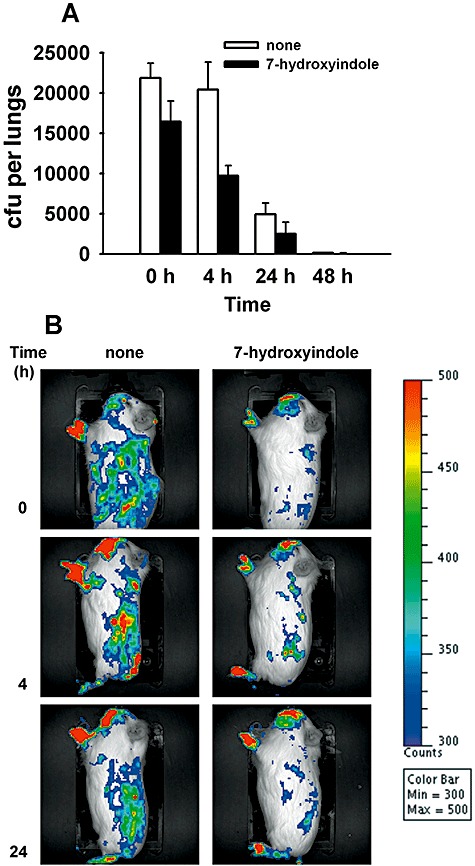
Reduction of virulence of P. aeruginosa in guinea pigs. A. Colonization and clearance of P. aeruginosa pre‐treated with 7HI or solvent (DMF) prior to infection of guinea pigs by aerosol with ∼2 × 105 cfu. Average of five replicates, and one standard deviation is shown. B. Real‐time analysis of P. aeruginosa pre‐treated with 7HI or solvent (DMF) in the acute guinea pig infection model (representative guinea pigs are shown for each group and are imaged laterally) using the Xenogen IVIS CCD camera. Colour bar represents the intensity of luminescent signal in photons s−1 cm−2 from low (blue) to high (red).
We used in vivo bioluminescence imaging to obtain a more comprehensive picture of the tissue colonization by DMF‐ and 7HI‐treated P. aeruginosa and to confirm differences in pulmonary colonization in guinea pigs (Fig. 4B). Guinea pigs infected by aerosol were colonized in the nasopharynx, lungs, liver and gastrointestinal area (Fig. 4B), similar to previous studies in mice (DiGiandomenico et al., 2007). As 7HI did not affect bioluminescence and as equal amounts of P. aeruginosa cells was used to infect the guinea pigs, the difference in colonization of P. aeruginosa was due to the treatment of 7HI. Similar to data obtained by counting colony‐forming units (cfu) in the guinea pig lungs, pulmonary clearance occurs rapidly for 7HI‐treated P. aeruginosa, with low bioluminescence signal observed in the pulmonary region by 4 h in this group (2.05 ± 0.04 × 105 photons s−1 cm−2) as compared with the DMF‐treated group (3.59 ± 0.47 × 105 photons s−1 cm−2). Lower colonization (P < 0.03) of all organs was observed for animals infected with 7HI‐treated bacteria (3.5 ± 0.1 × 106 photons s−1 cm−2) as compared with DMF‐treated bacteria (4.19 ± 0.08 × 106 photons s−1 cm−2). In addition, regional lymph nodes do not appear to be significantly involved, primarily with colonization of the gastrointestinal region. These observations could indicate that dissemination of P. aeruginosa to extrapulmonary and extra‐intestinal sites is also reduced, in addition to initial colonization. Further studies are needed to definitively identify the sites and tissues infected, despite these intriguing observations. Therefore, both cfu and bioluminescence imaging confirm that 7HI pre‐treatment of P. aeruginosa decreases colonization of guinea pig tissues.
Additionally, indole‐ and 7HI‐treated P. aeruginosa decreased cytotoxicity in human red blood cells by 40% and 57% respectively. These results imply that indole and 7HI made P. aeruginosa less virulent to red blood cells.
Discussion
Quorum sensing is related to virulence as has been shown by whole‐transcriptome studies of P. aeruginosa with 3O‐C12‐HSL and C4‐HSL (Schuster et al., 2003; Wagner et al., 2003) and PQS (Bredenbruch et al., 2006). Here, the addition of indole and 7HI resulted in an opposite pattern of gene expression of the mexGHI‐opmD multidrug efflux genes and many virulence genes compared with gene expression with the two exogenous AHLs. For example, the addition of the AHLs consistently induced the mexGHI‐opmD genes, genes involved in phenazine synthesis and PQS synthesis, and the flp–tad–rcp gene cluster (Schuster et al., 2003; Wagner et al., 2003), while indole and 7HI repressed these genes (Table 1). Similarly, the addition of PQS most significantly induced virulence genes involved in pyochelin synthesis (pch operon) (Bredenbruch et al., 2006) whereas indole repressed the pch operon. Genes involved in small molecule transport (PA2328, PA2329, PA2330 and PA2331) were also induced by AHLs (Schuster et al., 2003), while indole repressed them. Furthermore, the addition of pyocyanin, a physiological terminal signal molecule for the upregulation of QS‐controlled genes (Dietrich et al., 2006), induced mexGHI‐opmD and a possible regulator/putative monooxygenase for pyocyanin synthesis, PA2274 (Dietrich et al., 2006), while indole and 7HI repressed PA2274 (Table 1). In addition to the whole‐transcriptome data, our virulence factor assays (Fig. 1) clearly show that indole and 7HI inhibit QS‐controlled virulence factors. Taken together, indole and 7HI decrease the production of antimicrobial compounds and virulence factors in P. aeruginosa. A conceptual model of the interaction of indole and 7HI on cellular phenotypes of P. aeruginosa is shown in Fig. 5.
Figure 5.
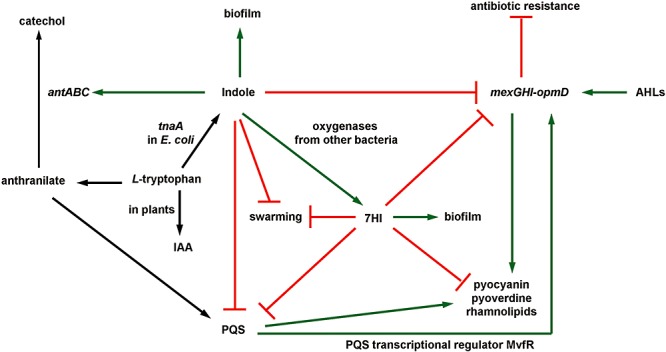
Summary of indole‐affected processes in P. aeruginosa→ indicates induction of gene expression or stimulation of a phenotype, ? indicates repression of gene expression or repression of a phenotype, and black arrows indicate reactions.
Genes encoding the MexGHI‐OpmD efflux pump were the most highly repressed genes in P. aeruginosa upon addition of indole and 7HI (Table 1), and antibiotic resistance was clearly enhanced in the presence of indole and 7HI (Fig. S3). With P. aeruginosa, inactivation of MexGHI‐OpmD reduces production of rhamnolipids, pyocyanin, pyoverdine, elastase and swarming motility (Aendekerk et al., 2002), impairs cell growth (Aendekerk et al., 2005), enhances antibiotic resistance (Aendekerk et al., 2005) and attenuates virulence via PQS (Aendekerk et al., 2005). As observed with mexI and opmD mutants (Aendekerk et al., 2005), indole and 7HI decreased the production of virulence factors without affecting the transcriptional levels of lasI and rhlI; hence, indole and 7HI probably inhibit virulence factors due to a similar mechanism, through PQS regulation at the post‐transcriptional level (Aendekerk et al., 2005). Therefore, the reduction of virulence factors, swarming motility and antibiotic sensitivity may be partially explained by the repression of this locus by indole and 7HI.
Also, the PQS transcriptional regulator MvfR induces the mexGHI‐opmD genes, pyocyanin synthesis (phz operon), PA0284, PA1914, PA2329 and PA2331 (Déziel et al., 2005), while both indole and 7HI repressed the mexGHI‐opmD genes and repressed PA2328, PA2329, PA2330 and PA2331, and indole repressed PA0284 (14‐fold) and 7HI repressed PA1914 (sixfold, Table 1). Interestingly, indole and 7HI were less effective for the production of virulence factors in the mvfR mutants (Fig. 1C), which suggested that MvfR was also required for the indole mechanism.
Swarming motility is related to QS (Köhler et al., 2000) and plays a role in the biofilm development of P. aeruginosa (Caiazza et al., 2007; Overhage et al., 2007). Overhage and colleagues (2007) showed a direct relationship between swarming motility and biofilm formation from the study of 5000 P. aeruginosa insertion mutants. In contrast, Caiazza and colleagues (2007) showed an inverse regulation of biofilm formation and swarming motility. In the present study, both indole and 7HI decrease slightly swimming motility, 7HI abolishes swarming motility (Fig. 2) and both lead to more biofilm formation (Fig. S1). Hence, we also observed an inverse relationship between biofilm formation and swarming motility with 7HI. In addition, the reduction of rhamnolipids by indole and 7HI (Fig. 1A) may be a partial reason for the reduced swarming motility (Fig. 2) as rhamnolipids modulate swarming motility patterns in P. aeruginosa (Caiazza et al., 2005).
Decreasing rhamnolipid concentrations also promotes the clearance of P. aeruginosa biofilms in vivo (Jensen et al., 2007), which suggests a role for rhamnolipids in the reduced pulmonary colonization and increased clearance of P. aeruginosa by 7HI in the guinea pigs (Fig. 4). Colonization of multiple tissues by P. aeruginosa after aerosol infection is consistent with previous observations in mice demonstrating the greatest degree of infection as shown by in vivo imaging (DiGiandomenico et al., 2007). Both the number of bacteria present in the lungs and the bioluminescent signal produced after infecting guinea pigs with P. aeruginosa that have been pre‐treated with 7HI support this conclusion. Our in vivo bioluminescence imaging also suggests that dissemination of P. aeruginosa to extrapulmonary sites may be affected by 7HI pre‐treatment, but further studies are needed before this conclusion can be made. These observations suggest that hydroxylated indole represents a potential therapeutic approach or preventative measure for pulmonary infections caused by P. aeruginosa.
Previously, we reported that several hydroxyindoles affect biofilm formation of a pathogenic E. coli via different mechanisms; for example, doubly hydroxylated indole, isatin, enhanced E. coli O157:H7 biofilm by inducing flagellar genes and repressing genes encoding AI‐2 transporters lsrABCDFGKR (reminiscent of effects caused by AI‐2) and by repressing indole synthesis genes tnaABC, but indole, 5‐hydroxyindole and 7HI inhibited E. coli biofilm through cysteine (sulfur) metabolism (Lee et al., 2007b). Here, both indole and 7HI stimulate biofilm formation in P. aeruginosa, inhibit production of QS‐derived virulence factors, and enhance antibiotic resistance via similar mechanisms (Fig. 5) as both compounds differentially induced or repressed many common genes (Table 1). However, the impact of 7HI on the production of virulence factors was more significant than that with indole. Also, indole alone specifically induced several oxygenases, and 7HI alone repressed the type IVb pilus flp–tad–rcp gene cluster and abolished swarming motility of P. aeruginosa, suggesting a different physiological role of indole and hydroxylated indole. This result is additional support of the hypothesis that hydroxylation of abundant indole by non‐specific oxygenases (which are probably present in consortia) can lead to the formation of hydroxyindoles that alter cellular functions in P. aeruginosa as well as in E. coli in a manner different from indole (Lee et al., 2007b). The pattern of the modification of indole is important as the control compound IAA did not influence the expression of QS‐related genes (Table 1) or influence the production of virulence factors (Fig. 1).
In this study, we have demonstrated that the E. coli cell signal indole and hydroxylated indole (7HI) diminish several QS‐controlled virulence factors in P. aeruginosa, reduce its virulence in guinea pigs, enhance its biofilm formation, enhance its antibiotic resistance and are degraded readily (Fig. 5). Hence, we have shown that there is possibly interference of the E. coli signal indole by P. aeruginosa in that P. aeruginosa has a defence system to degrade indole. In a manner similar to the way P. aeruginosa may utilize AHLs as an interference strategy to preclude encroachment by competing bacteria (Kaufmann et al., 2005), E. coli may utilize indole to compete against P. aeruginosa (a pathogen that threatens its host) by decreasing the production of its siderophores (pyoverdine and pyochelin) and virulence factors. Although highly speculative, another possibility is that P. aeruginosa may use the indole to increase its biofilm formation in the intestinal tract to initially form a colony to avoid washout from the host; at this point the opportunistic pathogen downregulates virulence factors with the prevalent indole to avoid detection until a suitable cell density is reached (Williams, 2007). As AHLs have been shown to have other functions other than QS (Kaufmann et al., 2005), indole and 7HI may have functions other than inhibiting virulence factors in P. aeruginosa.
Given our results showing that indole diminishes virulence factors and virulence, that an indole derivative (CBR‐4830) has been shown to inhibit P. aeruginosa growth through a multidrug efflux pump, mexAB‐oprM (Robertson et al., 2007), and that some natural indole derivatives, such as indole‐3‐carbinol and 3,3′‐diindolylmethane derived from Cruciferous vegetables, show antimicrobial, antiviral and anticancer activity (Higdon et al., 2007), indole and indole derivatives appear to have pharmaceutical potential as treatments against pathogenic P. aeruginosa. Most importantly, indole and 7HI present an opportunity for antivirulence therapies (Lesic et al., 2007; Cegelski et al., 2008) which are also known as antipathogenic drugs (Rasmussen and Givskov, 2006). Antivirulence compounds are an important way to fight infectious diseases because unlike antimicrobials, antivirulence compounds like indole do not affect growth and so there is less chance of developing resistance (Hentzer et al., 2002; Lesic et al., 2007).
Experimental procedures
Bacterial strains, materials and growth rate measurements
All experiments were conducted at 37oC, and LB medium (Sambrook et al., 1989) was used except for the pyoverdine assay and swarming motility assay. Pseudomonas aeruginosa PAO1 used in this study was the sequenced Holloway strain (Stover et al., 2000), and P. aeruginosa PAO1 isogenic antA, mexI and mvfR mutants (Jacobs et al., 2003) were obtained from Professor Colin Manoil. Pseudomonas aeruginosa PA14 phzS, rhlR, pqsA and lasB mutants (Liberati et al., 2006) were used only as negative controls in virulence factor assays. The transposon mutations were confirmed (antA by us, mexI and mvfR by the source) by four PCR reactions using two primers in the ISphoA/hah or ISlacZ/hah transposon (5′‐CGGGTGCAGTAATATCGCCCT‐3′ and 5′‐GGG TAACGCCAGGGTTTTCC‐3′) and two primers in the target gene (5′‐GTGAGAACGCATGAACGCTA‐3′ and 5′‐CTGACG ATCTCGGTACGGTT‐3′ for antA, 5′‐ATCCGCCGCAACAAC TAC‐3′ and 5′‐GTAGACCTGGTCGAGCTTGC‐3′ for mexI, and 5′‐CTGCATGCTGGAATTGCTC‐3′ and 5′‐ACTGAA GATCTCCCGCTTCA‐3′ for mvfR respectively).
Indole, 7HI and IAA were used at 1.0, 0.5 and 1.0 mM, respectively, except for the biofilm dose–response and biodegradation experiments. Indole and 7HI were purchased from Fisher Scientific (Pittsburg, PA). IAA was purchased from USB Biochemicals (Cleveland, OH). The toxicity of indole, 7HI and IAA was evaluated using the specific growth rate with two independent cultures; these compounds were dissolved in DMF, and DMF was added as a control at 0.1 vol% for all experiments. To test for utilization of indole and 7HI as a carbon source by P. aeruginosa, cell numbers were measured for 4 days in M9 minimal medium (Rodriguez and Tait, 1983) with 1.0 and 2.0 mM indole and 0.5 and 2.0 mM 7HI.
Total RNA isolation and microarray analysis
For the microarray experiments, 10 g of glass wool (Corning Glass Works, Corning, NY) was used to form biofilms (Ren et al., 2004a) for 7 h in 250 ml in 1 l Erlenmeyer shake flasks which were inoculated with overnight cultures that were diluted 1:100. Indole (1 mM), 7HI (0.5 mM) or IAA (1.0 mM) in 250 µl of DMF, or 250 µl of DMF alone was added. Glass wool was used to increase the surface area so that RNA could be readily obtained for the microarrays. RNA was isolated from the biofilm cells as described previously (Ren et al., 2004a).
The Genechip P. aeruginosa Genome Array (Affymetrix, P/N 900339) contains 5500 of the 5570 ORFs of P. aeruginosa (Whiteley et al., 2001). cDNA synthesis, fragmentation and hybridizations were as described previously (González Barrios et al., 2006). Hybridization was performed for 16 h at 50°C, and the total cell intensity was scaled to an average value of 500. The probe array images were inspected for any image artefact. Background values, noise values and scaling factors of both arrays were examined and were comparable. The intensities of polyadenosine RNA control were used to monitor the labelling process. For each binary microarray comparison of differential genes expression, if the gene with the larger transcription rate did not have a consistent transcription rate based on the 13 probe pairs (P‐value less than 0.05), these genes were discarded. A gene was considered differentially expressed when the P‐value for comparing two chips was lower than 0.05 (to assure that the change in gene expression was statistically significant and that false positives arise less than 5%) and when the expression ratio was higher (twofold) than the standard deviation for the whole microarrays (Ren et al., 2004b) (1.5‐fold for indole, 1.6‐fold for 7HI and 1.1‐fold for IAA). Gene functions were obtained from the Affymetrix‐NetAffx Analysis Center (https://www.affymetrix.com/analysis/netaffx/index.affx).
Reverse transcription polymerase chain reaction (RT‐PCR)
To corroborate the DNA microarray data, the transcription level of six prominent genes was quantified using RT‐PCR: mexG (forward primer 5′‐GGCGAAGCTGTTCGACTATC‐3′; reverse primer 5′‐AGAAGGTGTGGACGATGAGG‐3′), mexH (forward primer 5′‐GAAAAGCAATTTCTCCCTGGA C‐3′; reverse primer 5′‐GTTGATCTGTCCGGAAGTCACTA‐3′), mexI (forward primer 5′‐CTCTACCGGACCATGGAAGA‐3′; reverse primer 5′‐AGCGGTTGACGTAGTTCTCG‐3′), pvdS (forward primer 5′‐TAACCGTACGATCCTGGTGAAGA‐3′; reverse primer 5′‐ACGATCTGGAACAGGTAGCTGAG‐3′), ssuF (forward primer 5′‐CATCAACGTTCGTAACCAGTTCA‐3′; reverse primer 5′‐GATGGAGACCTCGGTGGACTT‐3′) and PA0284 (forward primer 5′‐ACCCTCAGAAGCCTGG ATG‐3′; reverse primer 5′‐GTTGCTGCAGACGGAATTTT‐3′). The expression level of the housekeeping gene proC (forward primer 5′‐CAGGCCGGGCAGTTGCTGTC‐3′; reverse primer 5′‐GGTCAGGCGCGAGGCTGTCT‐3′) (Savli et al., 2003) was used to normalize the expression data of interesting genes. An independent RNA sample using identical DNA microarray conditions were used for these studies (63 RT‐PCR reactions based on three RT‐PCR reactions for each of seven genes including the proC housekeeping gene, with DMF, indole and 7HI). RT‐PCR was performed in triplicate using a StepOne Real‐Time PCR system (Applied Biosystems, Foster City, CA). The relative fold changes for RT‐PCR were calculated from threshold cycle numbers measured using StepOne software v2.0 (Applied Biosystems). Using another housekeeping gene rpoN (forward primer 5′‐GAGCTCGACGAAGTGGAAGT‐3′; reverse primer 5′‐CG CATCAATTGGCTGTAGTC‐3′) and nine RT‐PCR reactions, similar results were obtained.
Crystal violet biofilm assay
This assay was adapted (Pratt and Kolter, 1998); overnight cultures diluted to an optical density at 600 nm of 0.05 and were incubated in polystyrene 96‐well plates for 24 h without shaking. The dye staining the biofilms (air–liquid interface biofilm as well as bottom liquid–solid biofilm) was dissolved in 95% ethanol, and an absorbance at 540 nm (OD540) was measured to quantify the total biofilm mass. Each data point was averaged from more than 12 replicate wells (six wells from two independent cultures).
Virulence factor assays
Except for the pyoverdine assay, overnight cultures were diluted 1:100 and contacted with indole (1 mM), 7HI (0.5 mM), IAA (1.0 mM) or diluent DMF (negative control). The pyocyanin assay was adapted (Essar et al., 1990); after growth for 7 h, supernatants were extracted with chloroform and 0.2 N HCl, and analysed spectrophotometrically. The phzS mutant was used as a negative control. The rhamnolipid assay was adapted (Wilhelm et al., 2007); after growth for 7 h, supernatants were assayed for rhamnolipids using the orcinol colorimetric assay (Wilhelm et al., 2007). The rhlR mutant was used as a negative control. The PQS assay was adapted (Attila et al., 2008a); after growth for 7 h, supernatants were extracted with acidified ethyl acetate and analysed by thin‐layer chromatography. As the standard, a synthetic PQS obtained from Professor Marvin Whiteley was used (Fig. S2), and the pqsA mutant was used as a negative control. The elastase assay was adapted (Ohman et al., 1980); after growth to a turbidity of 2 at 600 nm, supernatants were incubated with elastin‐Congo Red (MP Biomedicals, 101637), and the absorbance was measured at 495 nm to determine the elastase activity. The lasB mutant was used as a negative control. The pyoverdine assay was adapted (Ren et al., 2005); after growth in minimal succinate medium (Ren et al., 2005), cells were diluted to a turbidity of 0.05 at 600 nm in fresh minimal succinate medium and were grown for 8 h. The pyoverdine concentration was measured spectrophotometrically at 405 nm (Stintzi et al., 1998). Each experiment was performed using at least two independent cultures.
Antibiotic resistance assays
Overnight cultures were diluted 1:100 and grown to a turbidity of 1.5 at 600 nm with indole, 7HI or DMF. Antibiotics (0.06 mg ml−1 gentamicin, 10 mg ml−1 kanamycin, 0.4 mg ml−1 tetracycline and 5 mM VOSO4 · 2H2O) were mixed with cells and incubated for 60 min without shaking; cells were enumerated with LB agar plates. To test the antibiotic ampicillin, the MIC of ampicillin was determined using an LB agar dilution technique (Andrews, 2001). Cells were grown to mid‐log phase (turbidity of 1.5 at 600 nm) and 10 µl of diluted cells at 104 cells ml−1 were added to the surface of LB agar plates with indole or 7HI and 100–800 µg ml−1 ampicillin. All plates were incubated for 18 h. Two independent cultures were used for each strain.
Swimming, swarming and twitching motility
For swimming motility, 0.3% agar with 1% tryptone and 0.25% NaCl was used (Sperandio et al., 2002); for swarming motility, BM2 swarming medium (62 mM potassium phosphate buffer at pH 7, 2 mM MgSO4, 10 µM FeSO4, 0.4% glucose, 0.1% casamino acids and 0.5% agar) was used (Overhage et al., 2007); and for twitching motility, LB with 1.0% agar was used (Overhage et al., 2007). Briefly, strains were grown from diluted overnight cultures to a turbidity of 1.0 at 600 nm. Indole and 7HI dissolved in DMF were added to the motility agar. DMF (0.1%) was added as the negative control. The halo diameter at 24 h was measured for swimming motility. Each experiment was performed using two independent cultures.
Degradation and detection of indole and 7HI
For the degradation of indole and 7HI, overnight cultures were diluted to a turbidity of 1.0 at 600 nm and were re‐grown with indole (0.5 mM) and 7HI (0.5 mM) at 250 r.p.m. As a negative control, autoclaved cells (turbidity of 1.0 at 600 nm) were contacted with indole or 7HI at the same conditions to confirm that there is no evaporation or adsorption of indole or 7HI. Extracellular concentrations of indole or 7HI were measured directly from filtered supernatants with reverse‐phase HPLC using a 100 × 4.6 mM Chromolith Performance RP‐18e column (Merck KGaA, Darmstadt, Germany) and gradient elution with H2O‐0.1% formic acid and acetonitrile as the mobile phases at a flow rate of 1 ml min−1 (65:35 for 0–5 min, 35:65 for 5–12 min and 65:35 at 12 min) (Lee et al., 2007b). Under these conditions, the retention times and the absorbance maxima were 3.6 min/264 nm for 7HI and 5.9 min/271 nm for indole. Each experiment was performed with two independent cultures. To determine the total protein, the Modified Lowry Protein Assay Kit from Pierce Biotechnology (Rockford, IL) was used. The protein content of P. aeruginosa was 0.255 mg protein (ml OD)−1.
Phenotype microarray
Phenotype PM1‐8 microarray plates (12111, 12112, 12121, 12131, 12141, 12181, 12182, 12183) (Biolog, Hayward, CA) were used to investigate 756 different phenotypes. Briefly, overnight cells were removed from BUG+B agar plates with a sterile swab and placed into IF‐O base buffer, and the cell turbidity at 600 nm was adjusted to 0.065. Bacterial inocula were prepared, and 100 µl of each cell suspension was inoculated into the plates.
Acute model of Pseudomonas infection in guinea pigs
Random bred Hartley strain guinea pigs weighing 200–300 g were obtained from Charles River Breeding Laboratories (Wilmington, MA). The animals were housed individually in polycarbonate cages in a temperature‐ and humidity‐controlled environment; ambient lighting was automatically controlled to provide 12 h light and 12 h dark cycles. Animals were given commercial chow and tap water ad libitum. All procedures were reviewed and approved by the Texas A&M University Laboratory Animal Care Committee. Pseudomonas aeruginosa was cultured with 7HI or DMF alone as a control in LB for 7 h, and guinea pigs were infected by aerosol with equal amounts of washed cells using a Madison chamber aerosol generation device calibrated to deliver between 104 and 105 cfu of P. aeruginosa into the lungs. At each time point (immediately after infection, 4, 24 and 48 h), five guinea pigs in each group were euthanized by overdose with sodium pentabarbitol and necropsy was performed to obtain total lung mass. Lungs were homogenized in saline and dilutions plated to determine the total cfu present at each time point.
In vivo infection imaging
In order to confirm differences observed in colonization of guinea pig tissues, four guinea pigs were infected in the same manner as for the acute model of infection and two animals from each group were examined by quantitative in vivo imaging. Dorsal and lateral images were acquired for each animal immediately after infection, at 4 h and at 24 h. Pseudomonas aeruginosa with the Photorhabdus luminescens luxCDABE operon stably inserted into the chromosome (single copy) was used (strain Xen41 from Xenogen, Alameda, CA); addition of 7HI did not change luminescence. Images were acquired using the luminescence settings on the Spectrum In Vivo Imaging System (IVIS) CCD camera and analysed with Living Image 3.0 software (Xenogen). Colonization of all organs was quantified by measurement of total photons from each animal and from the pulmonary region by measurement of total photons from the pulmonary region of each animal.
Cytotoxicity
Assays to determine cytotoxicity to red blood cells with indole and 7HI versus DMF alone were conducted as described previously (Attila et al., 2008b) with P. aeruginosa cultured for 7 h.
Microarray accession numbers
The expression data for biofilm samples with and without indole 7HI, and IAA are summarized in Table 1 and have been deposited in the NCBI Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) and are accessible through Accession No. GSE10065 (Edgar et al., 2002).
Acknowledgments
This research was supported by the NIH (5RO1EB003872‐05) and ARO (W911NF‐06‐1‐0408). We thank Professor Arul Jayaraman for insightful discussions, Professor Tim McDermott for providing P. aeruginosa PAO1, Professor Frederick M. Ausubel for providing P. aeruginosa PA14 transposon mutants, Professor Colin Manoil for providing the P. aeruginosa PAO1 antA, mexI and mvfR mutants Professor Marvin Whiteley for providing the PQS standard and for his assistance with the PQS assay and Dr Kevin Francis of Caliper Life Sciences for providing Xen41.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Indole influences P. aeruginosa metabolism. Metabolic phenotype based on Biolog arrays upon adding 1.0 mM indole versus no indole addition at 24 h. Optical density at 590 nm (OD590) indicates growth under these conditions.
Indole and 7HI increase P. aeruginosa biofilm formation. Biofilm formation in LB after 24 h in 96-well plates with indole (A), 7HI (B), and IAA (negative control) (C). Each experiment was performed two to four times with 6 wells each, and one standard deviation is shown. Structures of compounds are indicated as insets.
Indole and 7HI decrease PQS production. Culture components were purified and analyzed by thin layer chromatography. Included on the TLC plate are 500 ng of chemically synthesized PQS (arrow at lane 1), extract of P. aeruginosa wild-type culture (2), extract of P. aeruginosa wild-type culture with indole (1.0 mM) (3), extract of P. aeruginosa wild-type with 7HI (0.5 mM) (4), extract of P. aeruginosa mexI culture (5), and extract of P. aeruginosa PA14 pqsA as a negative control (6).
Indole and 7HI increase P. aeruginosa antibiotic resistance. Antibiotic resistance with 1.0 mM indole and 0.5 mM 7HI. Final concentrations were 0.4 mg/mL tetracycline, 0.06 mg/mL gentamicin, and 10 mg/mL kanamycin. Each experiment was performed with at least two independent cultures. Data show the average of the replicates, and one standard deviation is shown.
The ant locus is not responsible for indole signaling. Production of virulence factors with 1.0 mM indole with P. aeruginosa PAO1 antA. For clarity, values of the wild-type strain are not shown so bars indicate the relative amount of each compound made compared to no indole addition to the wild-type strain. Each experiment was performed with at least two independent cultures. Data show the average of the replicates and one standard deviation is shown.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Aendekerk S., Ghysels B., Cornelis P., Baysse C. Characterization of a new efflux pump, MexGHI‐OpmD, from Pseudomonas aeruginosa that confers resistance to vanadium. Microbiology. 2002;148:2371–2381. doi: 10.1099/00221287-148-8-2371. [DOI] [PubMed] [Google Scholar]
- Aendekerk S., Diggle S.P., Song Z., Høiby N., Cornelis P., Williams P. The MexGHI‐OpmD multidrug efflux pump controls growth, antibiotic susceptibility and virulence in Pseudomonas aeruginosa via 4‐quinolone‐dependent cell‐to‐cell communication. Microbiology. 2005;151:1113–1125. doi: 10.1099/mic.0.27631-0. et al. [DOI] [PubMed] [Google Scholar]
- Andrews J.M. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48(1):5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- Attila C., Ueda A., Wood T.K. PA2663 (PpyR) increases biofilm formation in Pseudomonas aeruginosa PAO1 through the psl operon and stimulates virulence and quorum‐sensing phenotypes. Appl Microbiol Biotechnol. 2008a;78:293–307. doi: 10.1007/s00253-007-1308-y. [DOI] [PubMed] [Google Scholar]
- Attila C., Ueda A., Cirillo S.L.G., Cirillo J.D., Chen W., Wood T.K. Pseudomonas aeruginosa PAO1 virulence factors and poplar tree response in the rhizosphere. Microbial Biotech. 2008b;1:17–29. doi: 10.1111/j.1751-7915.2007.00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal T., Englert D., Lee J., Hegde M., Wood T.K., Jayaraman A. Differential effects of epinephrine, norepinephrine, and indole on Escherichia coli O157:H7 chemotaxis, colonization, and gene expression. Infect Immun. 2007;75:4597–4607. doi: 10.1128/IAI.00630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredenbruch F., Geffers R., Nimtz M., Buer J., Häussler S. The Pseudomonas aeruginosa quinolone signal (PQS) has an iron‐chelating activity. Environ Microbiol. 2006;8:1318–1329. doi: 10.1111/j.1462-2920.2006.01025.x. [DOI] [PubMed] [Google Scholar]
- Caiazza N.C., Shanks R.M., O'Toole G.A. Rhamnolipids modulate swarming motility patterns of Pseudomonas aeruginosa. J Bacteriol. 2005;187:7351–7361. doi: 10.1128/JB.187.21.7351-7361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazza N.C., Merritt J.H., Brothers K.M., O'Toole G.A. Inverse regulation of biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J Bacteriol. 2007;189:3603–3612. doi: 10.1128/JB.01685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cegelski L., Marshall G.R., Eldridge G.R., Hultgren S.J. The biology and future prospects of antivirulence therapies. Nat Rev Microbiol. 2008;6:17–27. doi: 10.1038/nrmicro1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chant E.L., Summers D.K. Indole signalling contributes to the stable maintenance of Escherichia coli multicopy plasmids. Mol Microbiol. 2007;63:35–43. doi: 10.1111/j.1365-2958.2006.05481.x. [DOI] [PubMed] [Google Scholar]
- Clemons K.V., Gadberry J.L. Increased indole detection for Pasteurella multocida. J Clin Microbiol. 1982;15:731–732. doi: 10.1128/jcm.15.4.731-732.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C.D. Effect of pyochelin on the virulence of Pseudomonas aeruginosa. Infect Immun. 1982;36:17–23. doi: 10.1128/iai.36.1.17-23.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I.P., Yanofsky C. On the separation of the tryptophan synthetase of Escherichia coli into two protein components. Proc Natl Acad Sci USA. 1958;44:1161–1170. doi: 10.1073/pnas.44.12.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard I., Høi L., Siebeling R.J., Dalsgaard A. Indole‐positive Vibrio vulnificus isolated from disease outbreaks on a Danish eel farm. Dis Aquat Organ. 1999;35:187–194. doi: 10.3354/dao035187. [DOI] [PubMed] [Google Scholar]
- DeMoss R.D., Moser K. Tryptophanase in diverse bacterial species. J Bacteriol. 1969;98:167–171. doi: 10.1128/jb.98.1.167-171.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Déziel E., Lépine F., Milot S., Villemur R. rhlA is required for the production of a novel biosurfactant promoting swarming motility in Pseudomonas aeruginosa: 3‐(3‐hydroxyalkanoyloxy)alkanoic acids (HAAs), the precursors of rhamnolipids. Microbiology. 2003;149:2005–2013. doi: 10.1099/mic.0.26154-0. [DOI] [PubMed] [Google Scholar]
- Déziel E., Gopalan S., Tampakaki A.P., Lépine F., Padfield K.E., Saucier M. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: multiple quorum sensing‐regulated genes are modulated without affecting lasRIrhlRI or the production of N‐acyl‐l‐homoserine lactones. Mol Microbiol. 2005;55:998–1014. doi: 10.1111/j.1365-2958.2004.04448.x. et al. [DOI] [PubMed] [Google Scholar]
- Dietrich L.E., Price‐Whelan A., Petersen A., Whiteley M., Newman D.K. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol Microbiol. 2006;61:1308–1321. doi: 10.1111/j.1365-2958.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- DiGiandomenico A., Rao J., Harcher K., Zaidi T.S., Gardner J., Neely A.N. Intranasal immunization with heterologously expressed polysaccharide protects against multiple Pseudomonas aeruginosa infections. Proc Natl Acad Sci USA. 2007;104:4624–4629. doi: 10.1073/pnas.0608657104. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle S.P., Cornelis P., Williams P., Cámara M. 4‐Quinolone signalling in Pseudomonas aeruginosa: old molecules, new perspectives. Int J Med Microbiol. 2006;296:83–91. doi: 10.1016/j.ijmm.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Domka J., Lee J., Wood T.K. YliH (BssR) and YceP (BssS) regulate Escherichia coli K‐12 biofilm formation by influencing cell signaling. Appl Environ Microbiol. 2006;72:2449–2459. doi: 10.1128/AEM.72.4.2449-2459.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doukyu N., Aono R. Biodegradation of indole at high concentration by persolvent fermentation with Pseudomonas sp. ST‐200. Extremophiles. 1997;1:100–105. doi: 10.1007/s007920050021. [DOI] [PubMed] [Google Scholar]
- Duan K., Dammel C., Stein J., Rabin H., Surette M.G. Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol Microbiol. 2003;50:1477–1491. doi: 10.1046/j.1365-2958.2003.03803.x. [DOI] [PubMed] [Google Scholar]
- Edgar R., Domrachev M., Lash A.E. Gene expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essar D.W., Eberly L., Hadero A., Crawford I.P. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol. 1990;172:884–900. doi: 10.1128/jb.172.2.884-900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher L.A., McKnight S.L., Kuznetsova M.S., Pesci E.C., Manoil C. Functions required for extracellular quinolone signaling by Pseudomonas aeruginosa. J Bacteriol. 2002;184:6472–6480. doi: 10.1128/JB.184.23.6472-6480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambello M.J., Iglewski B.H. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol. 1991;173:3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González Barrios A.F., Zuo R., Hashimoto Y., Yang L., Bentley W.E., Wood T.K. Autoinducer 2 controls biofilm formation in Escherichia coli through a novel motility quorum‐sensing regulator (MqsR, B3022) J Bacteriol. 2006;188:305–316. doi: 10.1128/JB.188.1.305-316.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett D.J., Charniga L., Bean K., Ohman D.E., Cohen M.S. Response of Pseudomonas aeruginosa to pyocyanin: mechanisms of resistance, antioxidant defenses, and demonstration of a manganese‐cofactored superoxide dismutase. Infect Immun. 1992;60:328–336. doi: 10.1128/iai.60.2.328-336.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentzer M., Riedel K., Rasmussen T.B., Heydorn A., Andersen J.B., Parsek M.R. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology. 2002;148:87–102. doi: 10.1099/00221287-148-1-87. et al. [DOI] [PubMed] [Google Scholar]
- Higdon J.V., Delage B., Williams D.E., Dashwood R.H. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55:224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa H., Inazumi Y., Masaki T., Hirata T., Yamaguchi A. Indole induces the expression of multidrug exporter genes in Escherichia coli. Mol Microbiol. 2005;55:1113–1126. doi: 10.1111/j.1365-2958.2004.04449.x. [DOI] [PubMed] [Google Scholar]
- Jacobs M.A., Alwood A., Thaipisuttikul I., Spencer D., Haugen E., Ernst S. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2003;100:14339–14344. doi: 10.1073/pnas.2036282100. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P.Ø., Bjarnsholt T., Phipps R., Rasmussen T.B., Calum H., Christoffersen L. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum‐sensing‐controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology. 2007;153:1329–1338. doi: 10.1099/mic.0.2006/003863-0. et al. [DOI] [PubMed] [Google Scholar]
- Kahnert A., Vermeij P., Wietek C., James P., Leisinger T., Kertesz M.A. The ssu locus plays a key role in organosulfur metabolism in Pseudomonas putida S‐313. J Bacteriol. 2000;182:2869–2878. doi: 10.1128/jb.182.10.2869-2878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann G.F., Sartorio R., Lee S.H., Rogers C.J., Meijler M.M., Moss J.A. Revisiting quorum sensing: discovery of additional chemical and biological functions for 3‐oxo‐N‐acylhomoserine lactones. Proc Natl Acad Sci USA. 2005;102:309–314. doi: 10.1073/pnas.0408639102. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler T., Curty L.K., Barja F., Van Delden C., Pechère J.C. Swarming of Pseudomonas aeruginosa is dependent on cell‐to‐cell signaling and requires flagella and pili. J Bacteriol. 2000;182:5990–5996. doi: 10.1128/jb.182.21.5990-5996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont I.L., Martin L.W. Identification and characterization of novel pyoverdine synthesis genes in Pseudomonas aeruginosa. Microbiology. 2003;149:833–842. doi: 10.1099/mic.0.26085-0. [DOI] [PubMed] [Google Scholar]
- Lau G.W., Hassett D.J., Ran H., Kong F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol Med. 2004;10:599–606. doi: 10.1016/j.molmed.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Lee J., Jayaraman A., Wood T.K. Indole is an inter‐species biofilm signal mediated by SdiA. BMC Microbiol. 2007a;7:42. doi: 10.1186/1471-2180-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Bansal T., Jayaraman A., Bentley W.E., Wood T.K. Enterohemorrhagic Escherichia coli biofilms are inhibited by 7‐hydroxyindole and stimulated by isatin. Appl Environ Microbiol. 2007b;73:4100–4109. doi: 10.1128/AEM.00360-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Zhang X.S., Hegde M., Bentley W.E., Jayaraman A., Wood T.K. Indole cell signaling occurs primarily at low temperatures in Escherichia coli. ISME J. 2008;2:1007–1023. doi: 10.1038/ismej.2008.54. [DOI] [PubMed] [Google Scholar]
- Lesic B., Lépine F., Déziel E., Zhang J., Zhang Q., Padfield K. Inhibitors of pathogen intercellular signals as selective anti‐infective compounds. PLoS Pathog. 2007;3:1229–1239. doi: 10.1371/journal.ppat.0030126. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewenza S., Falsafi R.K., Winsor G., Gooderham W.J., McPhee J.B., Brinkman F.S. Construction of a mini‐Tn5‐luxCDABE mutant library in Pseudomonas aeruginosa PAO1: a tool for identifying differentially regulated genes. Genome Res. 2005;15:583–589. doi: 10.1101/gr.3513905. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati N.T., Urbach J.M., Miyata S., Lee D.G., Drenkard E., Wu G. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc Natl Acad Sci USA. 2006;103:2833–2838. doi: 10.1073/pnas.0511100103. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Mee B.J., Mulgrave L. Identification of clinical isolates of indole‐positive Klebsiella spp., including Klebsiella planticola, and a genetic and molecular analysis of their β‐lactamases. J Clin Microbiol. 1997;35:2365–2369. doi: 10.1128/jcm.35.9.2365-2369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashburn L.M., Whiteley M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature. 2005;437:422–425. doi: 10.1038/nature03925. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Tateda K., Miyazaki S., Furuya N., Ohno A., Ishii Y. Effect of antiflagellar human monoclonal antibody on gut‐derived Pseudomonas aeruginosa sepsis in mice. Clin Diagn Lab Immunol. 1999;6:537–541. doi: 10.1128/cdli.6.4.537-541.1999. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J.M., Neely A., Stintzi A., Georges C., Holder I.A. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect Immun. 1996;64:518–523. doi: 10.1128/iai.64.2.518-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael B., Smith J.N., Swift S., Heffron F., Ahmer B.M. SdiA of Salmonella enterica is a LuxR homolog that detects mixed microbial communities. J Bacteriol. 2001;183:5733–5742. doi: 10.1128/JB.183.19.5733-5742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel L., González N., Jagdeep S., Nguyen‐Ngoc T., Reimmann C. PchR‐box recognition by the AraC‐type regulator PchR of Pseudomonas aeruginosa requires the siderophore pyochelin as an effector. Mol Microbiol. 2005;58:495–509. doi: 10.1111/j.1365-2958.2005.04837.x. [DOI] [PubMed] [Google Scholar]
- Nikaido E., Yamaguchi A., Nishino K. AcrAB multidrug efflux pump regulation in Salmonella enterica serovar Typhimurium by RamA in response to environmental signals. J Biol Chem. 2008 doi: 10.1074/jbc.M804544200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner U.A., Reiser J. Autoinducer‐mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:6424–6428. doi: 10.1073/pnas.92.14.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman D.E., Cryz S.J., Iglewski B.H. Isolation and characterization of Pseudomonas aeruginosa PAO mutant that produces altered elastase. J Bacteriol. 1980;142:836–842. doi: 10.1128/jb.142.3.836-842.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overhage J., Lewenza S., Marr A.K., Hancock R.E. Identification of genes involved in swarming motility using a Pseudomonas aeruginosa PAO1 mini‐Tn5‐lux mutant library. J Bacteriol. 2007;189:2164–2169. doi: 10.1128/JB.01623-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L.A., Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen T.B., Givskov M. Quorum‐sensing inhibitors as anti‐pathogenic drugs. Int J Med Microbiol. 2006;296:149–161. doi: 10.1016/j.ijmm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Ren D., Bedzyk L.A., Thomas S.M., Ye R.W., Wood T.K. Gene expression in Escherichia coli biofilms. Appl Microbiol Biotechnol. 2004a;64:515–524. doi: 10.1007/s00253-003-1517-y. [DOI] [PubMed] [Google Scholar]
- Ren D., Bedzyk L.A., Ye R.W., Thomas S.M., Wood T.K. Differential gene expression shows natural brominated furanones interfere with the autoinducer‐2 bacterial signaling system of Escherichia coli. Biotechnol Bioeng. 2004b;88:630–642. doi: 10.1002/bit.20259. [DOI] [PubMed] [Google Scholar]
- Ren D., Zuo R., Wood T.K. Quorum‐sensing antagonist (5Z)‐4‐bromo‐5‐(bromomethylene)‐3‐butyl‐2(5H)‐furanone influences siderophore biosynthesis in Pseudomonas putida and Pseudomonas aeruginosa. Appl Microbiol Biotechnol. 2005;66:689–695. doi: 10.1007/s00253-004-1691-6. [DOI] [PubMed] [Google Scholar]
- Robertson G.T., Doyle T.B., Du Q., Duncan L., Mdluli K.E., Lynch A.S. A novel indole compound that inhibits Pseudomonas aeruginosa growth by targeting MreB is a substrate for MexAB‐OprM. J Bacteriol. 2007;189:6870–6881. doi: 10.1128/JB.00805-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R.L., Tait R.C. Benjamin/Cummings Publishing; 1983. [Google Scholar]
- Rui L., Reardon K.F., Wood T.K. Protein engineering of toluene ortho‐monooxygenase of Burkholderia cepacia G4 for regiospecific hydroxylation of indole to form various indigoid compounds. Appl Microbiol Biotechnol. 2005;66:422–429. doi: 10.1007/s00253-004-1698-z. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Savli H., Karadenizli A., Kolayli F., Gundes S., Ozbek U., Vahaboglu H. Expression stability of six housekeeping genes: a proposal for resistance gene quantification studies of Pseudomonas aeruginosa by real‐time quantitative RT‐PCR. J Med Microbiol. 2003;52:403–408. doi: 10.1099/jmm.0.05132-0. [DOI] [PubMed] [Google Scholar]
- Schuster M., Lostroh C.P., Ogi T., Greenberg E.P. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum‐controlled genes: a transcriptome analysis. J Bacteriol. 2003;185:2066–2079. doi: 10.1128/JB.185.7.2066-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio V., Torres A.G., Kaper J.B. Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two‐component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol Microbiol. 2002;43:809–821. doi: 10.1046/j.1365-2958.2002.02803.x. [DOI] [PubMed] [Google Scholar]
- Stewart V., Yanofsky C. Evidence for transcription antitermination control of tryptophanase operon expression in Escherichia coli K‐12. J Bacteriol. 1985;164:731–740. doi: 10.1128/jb.164.2.731-740.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzi A., Evans K., Meyer J.M., Poole K. Quorum‐sensing and siderophore biosynthesis in Pseudomonas aeruginosalasR/lasI mutants exhibit reduced pyoverdine biosynthesis. FEMS Microbiol Lett. 1998;166:341–345. doi: 10.1111/j.1574-6968.1998.tb13910.x. [DOI] [PubMed] [Google Scholar]
- Stover C.K., Pham X.Q., Erwin A.L., Mizoguchi S.D., Warrener P., Hickey M.J. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. et al. [DOI] [PubMed] [Google Scholar]
- Stull T.L., Hyun L., Sharetzsky C., Wooten J., McCauley J.P., Jr, Smith A.B., 3rd Production and oxidation of indole by Haemophilus influenzae. J Biol Chem. 1995;270:5–8. doi: 10.1074/jbc.270.1.5. [DOI] [PubMed] [Google Scholar]
- Wagner V.E., Bushnell D., Passador L., Brooks A.I., Iglewski B.H. Microarray analysis of Pseudomonas aeruginosa quorum‐sensing regulons: effects of growth phase and environment. J Bacteriol. 2003;185:2080–2095. doi: 10.1128/JB.185.7.2080-2095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Ding X., Rather P.N. Indole can act as an extracellular signal in Escherichia coli. J Bacteriol. 2001;183:4210–4216. doi: 10.1128/JB.183.14.4210-4216.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Rochon M., Lamprokostopoulou A., Lunsdorf H., Nimtz M., Römling U. Impact of biofilm matrix components on interaction of commensal Escherichia coli with the gastrointestinal cell line HT‐29. Cell Mol Life Sci. 2006;63:2352–2363. doi: 10.1007/s00018-006-6222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley M., Bangera M.G., Bumgarner R.E., Parsek M.R., Teitzel G.M., Lory S. Gene expression in Pseudomonas aeruginosa biofilms. Nature. 2001;413:860–864. doi: 10.1038/35101627. et al. [DOI] [PubMed] [Google Scholar]
- Wilhelm S., Gdynia A., Tielen P., Rosenau F., Jaeger K.E. The autotransporter esterase EstA of Pseudomonas aeruginosa is required for rhamnolipid production, cell motility, and biofilm formation. J Bacteriol. 2007;189:6695–6703. doi: 10.1128/JB.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. Quorum sensing, communication and cross‐kingdom signalling in the bacterial world. Microbiology. 2007;153:3923–3938. doi: 10.1099/mic.0.2007/012856-0. [DOI] [PubMed] [Google Scholar]
- Xavier K.B., Bassler B.L. Interference with AI‐2‐mediated bacterial cell–cell communication. Nature. 2005;437:750–753. doi: 10.1038/nature03960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin B., Gu J.‐D., Wan N. Degradation of indole by enrichment culture and Pseudomonas aeruginosa Gs isolated from mangrove sediment. Int Biodeterior BioDegradation. 2005;56:243–248. [Google Scholar]
- Zulianello L., Canard C., Kohler T., Caille D., Lacroix J.S., Meda P. Rhamnolipids are virulence factors that promote early infiltration of primary human airway epithelia by Pseudomonas aeruginosa. Infect Immun. 2006;74:3134–3147. doi: 10.1128/IAI.01772-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Indole influences P. aeruginosa metabolism. Metabolic phenotype based on Biolog arrays upon adding 1.0 mM indole versus no indole addition at 24 h. Optical density at 590 nm (OD590) indicates growth under these conditions.
Indole and 7HI increase P. aeruginosa biofilm formation. Biofilm formation in LB after 24 h in 96-well plates with indole (A), 7HI (B), and IAA (negative control) (C). Each experiment was performed two to four times with 6 wells each, and one standard deviation is shown. Structures of compounds are indicated as insets.
Indole and 7HI decrease PQS production. Culture components were purified and analyzed by thin layer chromatography. Included on the TLC plate are 500 ng of chemically synthesized PQS (arrow at lane 1), extract of P. aeruginosa wild-type culture (2), extract of P. aeruginosa wild-type culture with indole (1.0 mM) (3), extract of P. aeruginosa wild-type with 7HI (0.5 mM) (4), extract of P. aeruginosa mexI culture (5), and extract of P. aeruginosa PA14 pqsA as a negative control (6).
Indole and 7HI increase P. aeruginosa antibiotic resistance. Antibiotic resistance with 1.0 mM indole and 0.5 mM 7HI. Final concentrations were 0.4 mg/mL tetracycline, 0.06 mg/mL gentamicin, and 10 mg/mL kanamycin. Each experiment was performed with at least two independent cultures. Data show the average of the replicates, and one standard deviation is shown.
The ant locus is not responsible for indole signaling. Production of virulence factors with 1.0 mM indole with P. aeruginosa PAO1 antA. For clarity, values of the wild-type strain are not shown so bars indicate the relative amount of each compound made compared to no indole addition to the wild-type strain. Each experiment was performed with at least two independent cultures. Data show the average of the replicates and one standard deviation is shown.


