Abstract
NF-κB-inducing kinase (NIK, MAP3K14) is an essential kinase linking a subset of TNF receptor family members to the noncanonical NF-κB pathway. In order to assess the cell-intrinsic role of NIK in murine T cell function, we generated mixed bone marrow chimeras using bone marrow from NIK knockout (NIK KO) and wild type (WT) donor mice and infected the chimeras with lymphocytic choriomeningitis virus (LCMV). The chimeras possess an apparently normal immune system including a mixture of NIK KO and WT T cells, and the virus was cleared normally. Comparison of the NIK KO and WT CD4 and CD8 T cell responses at 8 days post-infection revealed modest but significant differences in the acute response. In both CD4 and CD8 compartments, there were relatively fewer activated (CD44hi) NIK KO T cells, but within the CD44hi population, a comparable percentage of the activated cells produced IFN-γ in response to ex vivo stimulation with antigenic LCMV peptides, although IL-7R expression was reduced in the NIK KO CD8 T cells. Assessment of the LCMV-specific memory at 65 days postinfection revealed many more LCMV-specific WT memory T cells than NIK KO memory T cells in both the CD4 and CD8 compartments, although the small number of surviving NIK KO memory T cells responded to secondary challenge with virus. These results demonstrate a cell-intrinsic requirement for NIK in the generation and/or maintenance of memory T cells in response to acute viral infection.
Introduction
Determining the signals and signaling pathways that shape effector T cell responses and generate long term T cell memory is essential for understanding the regulation of the adaptive immune response as well as for effective vaccine design. In addition to antigen recognition through the TCR and the initial costimulatory signal provided by CD28 ligands, the continued proliferation, survival, and differentiation to effector and memory T cells depends on the nature and availability of late costimulatory signals from receptors for soluble cytokines, such as IL-2, IL-21, IL-12, and IFN-α (1), and from costimulatory TNF receptor family members (TNFRs3) (2, 3), such as OX40 (CD134), 4-1BB (CD137), and CD27. These TNFRs engage multiple signaling pathways, including Akt/PI3K (4) and NF-κB (5, 6), but little is known about which pathways regulate differentiation and survival of memory and effector T cells.
The NF-κB family of transcription factors is essential for all arms of the immune system (7). The ancient canonical NF-κB pathway is required for antigen receptor, cytokine, and innate receptor signaling. In T cells deficient in essential components of this pathway, T cell development is curtailed and residual T cells are severely crippled. The canonical signal is transmitted within minutes and is rapidly inhibited by negative feedback mediated by the de novo expression of inhibitors of κB (IκBs), so this pathway is robust and rapid, but transient. In contrast, the noncanonical or alternative NF-κB pathway that operates downstream of a subset of TNFRs (8) is slower because it depends on new protein synthesis, and it lasts for hours or days because it is insensitive to rapid feedback inhibition by canonical IκBs. The noncanonical pathway is characterized by dependence on NF-κB-inducing kinase (NIK, MAP3K14) (9). When TNFRs are engaged, NIK accumulates and activates IKKα, which results in the processing of NF-κB2 from the inactive form (p100) to the transcriptionally active p52 subunit (10). Unprocessed NF-κB2 (p100) acts as an inhibitor of both the canonical and noncanonical pathways, so accumulation of NIK relieves inhibition by p100 in addition to generating the transcriptionally active p52:RelB heterodimers (11-14).
The noncanonical pathway has been shown to be activated by many costimulatory TNFRs overexpressed in cell lines (15), but only recently has the noncanonical NF-κB pathway been shown to play a T cell-intrinsic role in the T cell response to TNFR2 (16), 4-1BB (17), and OX40 ligation (6, 18). Based on our finding that NIK is necessary for the costimulatory activity of OX40 and for noncanonical but not canonical NF-κB activation by OX40, we proposed that activation of the noncanonical NF-κB pathway downstream of NIK is necessary in T cells to enable them to survive and acquire effector functions in response to late costimulatory signals delivered through OX40 and perhaps other TNFRs (6).
Mice with lesions in NIK or other components of the noncanonical NF-κB pathway have abnormal thymic structure and secondary lymphoid organs (owing to defective noncanonical NF-κB signaling downstream of the lymphotoxin-β receptor and other TNFRs (19, 20)), a severe deficit in mature B cells (owing to defective noncanonical signaling downstream of the B cell activating factor receptor (BAFFR) (21)), and abnormal dendritic cell functions (22-24), but T cell development and homeostasis is superficially normal, although NIK-deficient mice accumulate anergic, memory phenotype CD4 T cells that interfere with in vitro tests of T cell function (11, 25). T cell mediated autoimmunity and immunodeficiency in NIK-deficient mice has been attributed to defects in lymphoid organ structure (19, 26, 27) or other cell types (22, 23, 28, 29). However, T cell transfer studies have indicated an intrinsic requirement for NIK in T cells in mouse models of arthritis (30), experimental autoimmune encephalomyelitis (31), and graft versus host disease (6).
NIK-deficient T cells have not yet been examined in a system that allows assessment of cell-intrinsic defects in responses to infection and generation of memory. To do so, we generated mixed bone marrow chimeras using NIK-deficient (NIK KO) and wildtype (WT) bone marrow in WT hosts. In these animals, the T cells develop in a normal environment with normal lymphoid organs, B cells, and dendritic cells. The mixed chimeras are stable and possess mature naïve T cells from both NIK KO and WT donors. Following infection with LCMV Armstrong, infectious virus was undetectable in blood at day 8 post-infection, indicating that LCMV was controlled normally in the bone marrow chimeras. The NIK KO T cells made a robust primary LCMV-specific response, measured by MHC/peptide binding and cytokine production, although numbers of responding NIK KO T cells were lower than WT. However, at 65 days post-infection, we found dramatically fewer LCMV-specific NIK KO memory T cells than WT memory T cells in both the CD4 and CD8 compartments, although the small number of surviving NIK KO memory T cells responded to secondary challenge with virus. Thus we have demonstrated a cell-intrinsic requirement for NIK in the development and/or maintenance of T cell memory following an acute viral infection.
Materials and Methods
Mice
C57BL/6 mice and C57BL/6 congenic for CD45.1 or CD90.1 (Thy1.1) were purchased from The Jackson Laboratory (Bar Harbor, Maine) and bred in our facility. C57BL/6 NIK KO mice were a gift from Robert Schreiber (Washington University School of Medicine) (19) and CD45.1+ SMARTA mice (32) were a gift from Lindsay Whitton (The Scripps Research Institute). We crossed NIK KO mice with SMARTA transgenic mice. Briefly, NIK KO males were bred to CD45.1+ SMARTA+ females, and CD45.1+ SMARTA+ progeny were bred back to NIK KO males. CD4+ cells were isolated from the spleens of NIK KO, CD45.1+ SMARTA mice via negative selection, and 2×104 cells were transferred to Thy1.1+ mice with equal number of WT SMARTA T cells 24h before virus infection. Mice were used in accordance with National Institute of Health guidelines under an animal protocol approved by the Oregon Health & Science University Institutional Animal Care and Use Committee.
Generation of mixed bone marrow chimeras
Thy1.1+ or CD45.1+ mice were irradiated with 1,100 rads of gamma radiation (split dose) and reconstituted by transfer of 5×105 of a 1:1 mix of NIK KO and CD45.1+ or Thy1.1+ C57BL/6 bone marrow cells. To help protect against opportunistic infections the mice were treated with ciprofloxacin for 4 weeks following irradiation. The mice were then allowed to recover for an additional 4 weeks before use. Pre-infection chimerism was assessed by flow cytometry of blood mononuclear cells in all animals and in other organs by euthanizing a cohort of uninfected animals.
Virus
Naïve mice received 2×103 PFU of LCMV Armstrong intraperitoneally. Previously challenged mice received between 1×106 and 2×106 PFU of LCMV Armstrong intraperitoneally to assess recall responses.
Tetramer Staining
Tetramers were provided by the NIH Tetramer Core Facility (Emory Vaccine Center, Atlanta, GA). Staining with the gp66–77:I-Ab tetramer was performed at 37°C for 1 hr in RPMI 1640 containing 5% FCS, followed by washing and cell-surface staining for CD4, CD8, CD45.1, CD45.2, CD44 and Thy1.1. Tetramer fluorescence was normalized to samples stained with control tetramer, hCLIP:I-Ab. Staining with the NP396-404:Db tetramer was performed at 4°C for 1 hr in RPMI 1640 containing 10% FCS, followed by washing and cell-surface staining for CD4, CD8, CD45.1, CD45.2, CD44 and Thy1.1. Cells were kept on ice and stained with LIVE/DEAD fixable dead cell stain (Life Technologies, Grand Island, NY) and then fixed overnight at 4 degrees in 0.4% paraformaldehyde.
Ex vivo stimulation, flow cytometry and intracellular cytokine staining
For intracellular cytokine analysis, 106 splenocytes were cultured in the presence of brefeldin A and peptide at the indicated concentration for 5h at 37°C. Control cultures received media alone or were cultured with PMA (30nM) and 65ng/ml ionomycin. Cultures were then washed and stained for the surface antigens CD4 (clone RM4-5), CD8 (clone 53.6-7), CD45.1 (clone A20), CD45.2 (clone 104), CD44 (clone IM7) and Thy1.1 (clone HIS51) (eBioscience). The cells were stained for intracellular cytokines using the Cytofix/Cytoperm kit (BD/Pharmingen) according to the manufacturer's instructions. Antibodies used for intracellular cytokine detection were anti-IFN-γ (clone XMG1.2), and anti-TNF-α (clone MP6-XT22) (eBioscience). KLRG-1 was stained using a biotin labeled antibody (clone 2F1, BD Pharmigen) and streptavidin conjugated to APC (eBioscience). IL-7Rα was stained with anti-CD127 (clone A7R34) conjugated to PE (ebioscience). Foxp3 was stained with the eBioscience mouse regulatory T cell staining kit (eBioscience).
Results
Characterization of NIK KO:WT chimeras
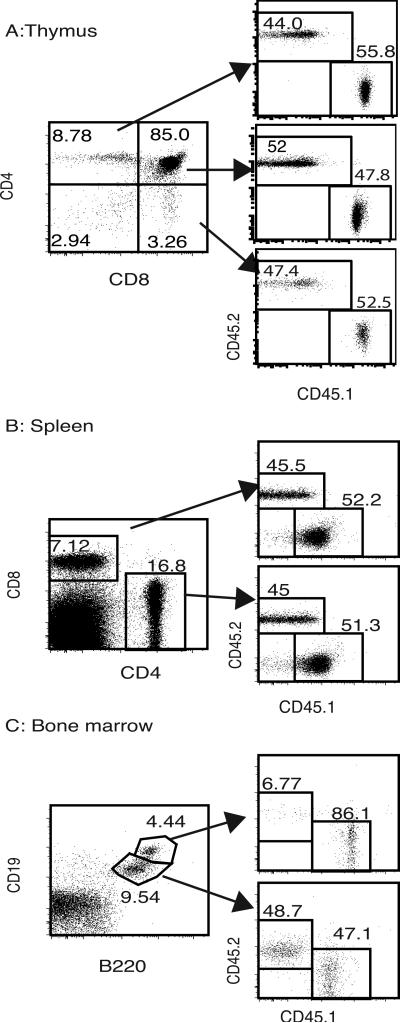
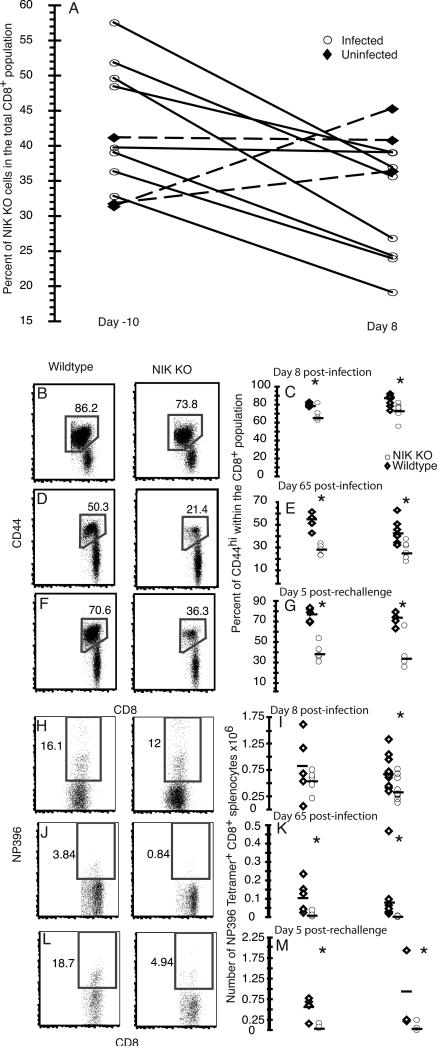
In order to test the T cell intrinsic role of NIK in response to a viral infection, we generated mixed bone marrow chimeras. Mice congenic for the Thy1.1 allele (or, in some experiments, mice heterozygous for CD45.1 and CD45.2) were lethally irradiated and rescued by transfer of an equal mixture of bone marrow cells from NIK KO (CD45.2) and WT B6 (congenic CD45.1) mice. The relative repopulation of the donor T cell compartment varied somewhat from experiment to experiment, as well as between mice within an experiment (Fig. 1 and Table I). Analysis of the mature B-cell (CD19hiB220hi) population in the bone marrow revealed a near absence of mature NIK KO B-cells (Fig. 1C and Table I), which we attributed to the requirement for NIK downstream of the BAFFR for survival of mature B-cells (21). The requirement for NIK and the non-canonical pathway for normal maturation and activation of professional antigen presenting cells, such as dendritic cells and macrophages, leads to dominant effects on T cell function. In our model the NIK KO cells made up less than 30% of the CD11c+/−CD11b+CD45+CD8− population in naïve chimeras (data not shown). We did note a larger contribution of surviving recipient cells to the CD11c+/−CD11b+CD45+ population than to the T and B cell populations in both infected and uninfected chimeras (data not shown).
FIGURE 1.
Characterization of chimeras. Thymus, spleen and bone marrow were harvested from mixed WT and NIK KO bone marrow chimeras 2 months following irradiation and bone marrow transplantation. Thymocytes (A) and splenocytes (B) were stained for CD8, CD4, Thy1.1 (recipient), CD45.1 (WT cells), and CD45.2 (NIK KO cells), and gated on Thy1.1negative donor cells. C) Bone marrow was stained for CD19, B220, CD45.1 and CD45.2. The percent NIK KO cells in the mature (CD19hiB220hi) and immature B-cells (CD19loB220lo) is shown.
TABLE I.
Contribution of NIK KO cells to reconstituted cell populations
| NIK KO * | ||||
|---|---|---|---|---|
| Thymus | AVERAGE | SEM | ||
| DP | 45.8 | 4.27 | ||
| DN | 50.2 | 6.38 | ||
| CD8SP | 46.7 | 3.43 | ||
| CD4SP | 43.4 | 3.63 | ||
|
Bone
marrow |
||||
| † | Mature B-cells | 8.3 | 3.34 | |
| Immature B-cells | 43.0 | 10.50 | ||
| Spleen | ||||
| CD4+ | 41.7 | 2.82 | ||
| CD8+ | 41.8 | 0.94 | ||
| † | B-cells | 7.3 | 1.21 | |
| CD4+CD44hi | 36.0 | 5.32 | ||
| § | CD8+CD44hi | 34.4 | 0.84 | |
Values are the percent NIK KO of the total donor cells in each subpopulation, derived from the average of five naïve mice assessed two months following reconstitution with same bone marrow cell mixture. The data are representative of four independent experiments.
The percent NIK KO is significantly lower than in the immature B-cell population in the bone marrow (p<0.01).
The percent NIK KO cells in this population is significantly lower than in the parent population (total CD8).
The NIK KO T cell population resembled the WT population in most respects before infection. The ratio of NIK KO to WT cells was the same in both the CD4+ and CD8+ populations (Table I), and the cells did not have aberrant expression of activation markers such KLRG-1 and CD11b, although there was a trend to lower IL-7R in the NIK KO CD8 T cells (discussed below). Notably, the NIK KO population had significantly fewer CD8+ CD44hi cells (Table I) and Foxp3+ CD4+ regulatory T cells than the WT controls (data not shown and S.E.M., manuscript submitted).
NIK KO CD4 T cells show a defective memory response upon acute viral infection
We challenged the chimeras with 2 ×105 pfu of LCMV Armstrong in order to induce a robust T cell response. Virus was undetectable in blood from chimeras at 8 and 65 days post-infection (data not shown), indicating that the presence of WT bone marrow-derived cells restored normal control of virus infection, which was shown to be defective in single NIK-deficient into WT bone marrow chimeras (29).
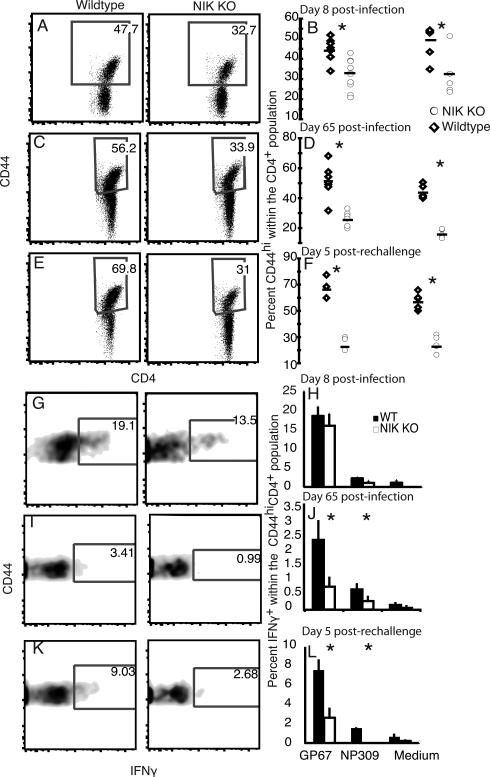
The relative contribution of NIK KO and WT donors to the total CD4 population was measured in the blood 10 days before infection and in the spleen at day 8 postinfection, and it did not change in a consistent or significant fashion following infection (data not shown). CD44 expression was used to distinguish naïve cells from effector/memory phenotype cells within the CD4 population. LCMV infection resulted in an increase in the percent CD44hi cells within the CD4+ T cell population compared to naïve controls. In four independent experiments, there was a significantly smaller percentage of CD44hi cells within the NIK KO CD4 population compared to WT cells on day 8 post-infection (Fig. 2A-B). When we examined the memory CD4+ T cells at day 65 post-infection, the percent CD44hi cells in the NIK KO population was further reduced compared to the WT population (Fig. 2C-D). The mice were rechallenged 60 days postinfection and the immune system was assessed at the peak of the recall response 5 days following rechallenge (33). The percent CD44hi cells was also significantly reduced 5 days following rechallenge (Fig. 2E & F). This indicates a significant decrease in the effector/memory phenotype population among NIK KO cell following an LCMV infection.
FIGURE 2.
The CD4 T-cell response to virus infection. Spleens were harvested from mixed bone marrow chimeras 8 or 65 days following infection with LCMV Armstrong. Rechallenged mice were injected with a ten fold greater dose of LCMV Armstrong at 60 days post-infection and their spleens were harvested 5 days later. A) Representative plots of donor CD4+Thy1.1negative splenocytes 8 days post infection. The splenocytes were stained directly ex vivo for CD45.1 (WT) and CD45.2 (NIK KO), and the percent CD44hi cells within each CD4 population was determined. B) Each symbol represents a single mouse 8 days post-infection, and the two cohorts represent two independent experiments. A significant difference (p<0.05) in the percent CD44hi within the CD4+ population between the WT and NIK KO populations is indicated by an asterisk. C) Representative splenocytes from a mouse at day 65 post-infection. D) The percent CD44hi cells within the CD4+ donor populations at day 65 post-infection. E) Representative splenocytes from a mouse 5 days following rechallenge on day 60. F) The percent CD44hi cells within the donor CD4+ populations 5 days following rechallenge on day 60. G) Splenocytes were cultured for 5 hours with GP67 in the presence of brefeldin A and stained for intracellular -γ. Dead cells were excluded by fixable vital dye and the cells were gated on the donor CD4+Thy1.1negativeCD44hi population. H) The percent IFN-γ+ cells within the CD44hi populations 8 days post infection following stimulation with LCMV peptides GP67, NP309 or medium alone. I) IFN-γ expression by ex vivo stimulated splenocytes 65 days post-infection. J) The percent IFN-γ+ cells within the CD44hi populations 65 days post infection. K) Percent IFN-γ+ cells within the CD4+CD44hi donor population 5 days following rechallenge on day 60. L) The percent IFN-γ+ cells within the CD44hi populations 5 days following rechallenge on day 60. Asterisks indicate significant differences (p<0.05) between NIK KO and WT T cells.
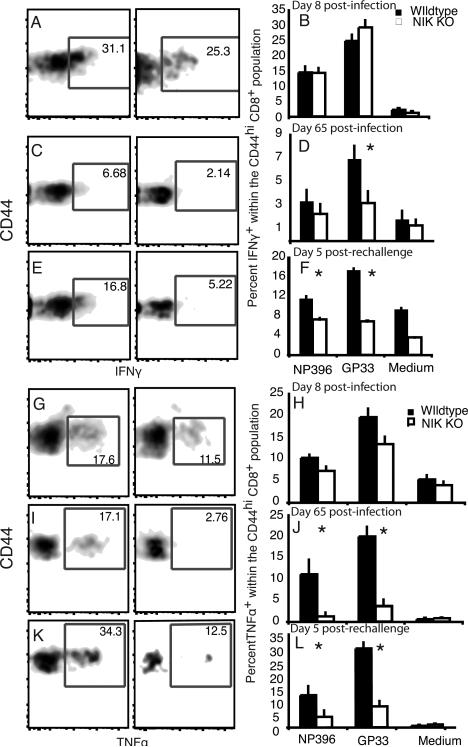
We next sought to determine whether this decrease was due to a lack of LCMV-specific effector/memory cells or to a NIK dependent modulation of CD44 expression on T cells. Ex vivo stimulation with LCMV-specific peptides as well as tetramer staining revealed that all the LCMV-specific cells were CD44hi (data not shown). Ex vivo stimulation with LCMV peptides allowed us to assess the function of LCMV-specific responders within the CD44hi population. Although there were fewer NIK KO CD44hi cells on day 8, there was not a significant difference in the frequency of NIK KO CD4+ CD44hi effector cells able to make IFN-γ in response to the dominant CD4 LCMV epitope, GP67, or a subdominant CD4 epitope, NP309 (34) (Fig. 2G-H). Stimulation of the polyclonal population with PMA and ionomycin also revealed no difference in the ability of effector/memory CD4 cells to make IFN-γ (Data not shown). However, despite the robust LCMV-specific response at 8 days post-infection there was a relative lack of CD4+ LCMV-specific responders within the CD44hi memory phenotype population at 65 days post-infection (Fig. 2I & J). Following rechallenge, the NIK KO memory phenotype population also had significantly fewer LCMV-specific responders as measured by IFN-γ production, although the frequencies of both wildtype and NIK KO GP67-specific T cells increased in response to rechallenge (Fig. 2K & L).
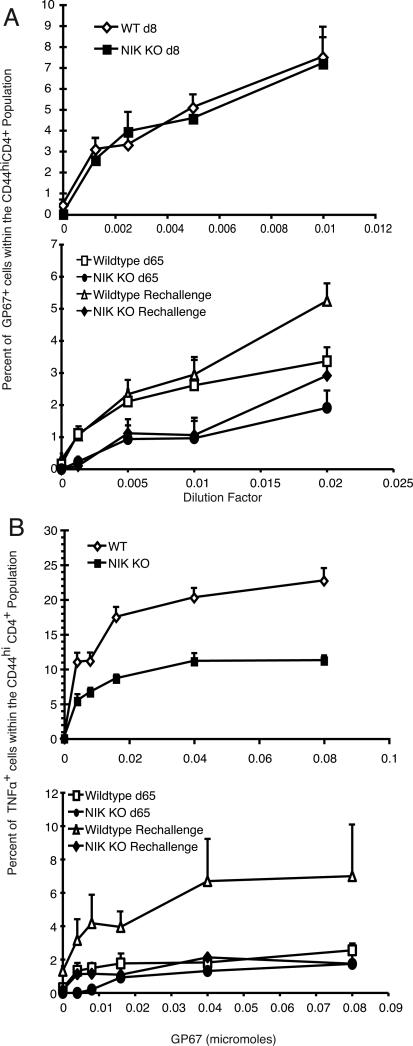
The ability of the CD4 responder population to survive as memory cells has been previously linked to the relative avidity of the cells for peptide/MHC complexes (35). The dominant CD4 epitope during an LCMV response in B6 mice is GP67 (32, 34). Using GP67-MHC tetramers, we measured the relative avidity of the polyclonal population for the GP67-MHC peptide complex (Fig. 3A, upper panel). At day 8 postinfection, the NIK KO and WT CD4+CD44hi population had nearly identical avidity for the peptide/MHC complex, and the proportion of NIK KO GP67-specific cells was comparable to WT, although the efficiency of detection with tetramers was substantially lower than by intracellular IFN-γ. It was also important to determine if the lack of NIK KO cytokine producers at 65 days post-infection (Fig. 2) was due to a lack of LCMV-specific cells or an inability of the cells to make IFN-γ. Perhaps NIK KO LCMV specific cells differentiated into non-IFN-γ producing cells. Using GP67 tetramers in titration we also noted that the relative numbers of GP67-specific CD4 cells in the CD44hi population was significantly reduced in the NIK KO population compared to the WT memory population (Fig. 3A, lower panel). Therefore, the NIK KO LCMV-specific clones did generate effectors but did not persist as memory cells.
FIGURE 3.
Tetramer binding and TNF-α production by CD4 T cells responding to virus infection. A) GP67 tetramer binding by splenocytes was measured at the tetramer dilutions indicated on the x axis, and the values were adjusted for nonspecific tetramer binding. The percent tetramer+ cells within the CD44hiCD4+ donor cells at each tetramer concentration was determined at 8 days post-infection (upper panel) and at 65 days postinfection and 5 days following rechallenge (lower panel). B) Splenocytes were cultured for 5h with brefeldin A in the presence of varying concentrations of GP67, and TNF-α production was determined by intracellular staining. Cells were gated on live CD4+CD44hi donor cells. The percent TNF-α+ cells within CD4+CD44hi populations was determined at 8 days and 65 days following infection and at 5 days following rechallenge.
We next compared the quality and sensitivity of the NIK KO cells to the WT cells by comparing the cytokine response to different levels of peptides. We measured the ability of CD4 cells to make TNF-α 8 and 65 days following infection. Titration of the GP67 peptide demonstrated that we had reached a saturating concentration. While there was no difference within the CD44hi population in IFN-γ production (Fig. 2) or tetramer binding (Fig. 3A) at day 8, there were fewer TNF-α-producing cells among the NIK KO T cells (Fig. 3B). TNF-α production at day 65 post-infection and day 5 following rechallenge was also significantly lower in NIK KO T cells in all experiments. We also observed that the NIK KO memory cells at day 65 post-infection were unresponsive to the lower concentrations of peptide (Fig. 3B), suggesting a selective loss or anergy in high affinity T cells.
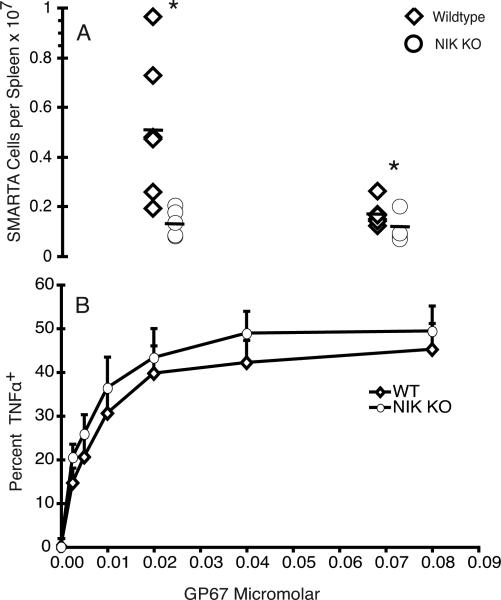
Could the differences we observed up until this point be due to a lower precursor frequency of LCMV reactive clones within the naïve NIK KO T cell compartment owing to a shift in the T cell repertoire of the NIK KO T cells? To answer this question we crossed NIK KO mice with SMARTA transgenic mice in which all the CD4+ T cells express a TCR specific for the LCMV peptide GP67. We then adoptively transferred purified SMARTA cells of both genotypes together into naïve mice and challenged the mice. After injection of equal numbers of NIK KO and naïve SMARTA cells, the NIK KO SMARTA population was significantly smaller than the WT SMARTA population on day 8 following infection (Fig. 4A). However, we found that the NIK KO cells all upregulated CD44, bound tetramer with the same efficiency as WT, and produced IFN-γ and TNF-α as efficiently as WT cells (Fig. 4B and data not shown). Thus the NIK KO SMARTA CD4 cells do not proliferate as well or do not survive as well as WT upon challenge, and the differences we observed in the polyclonal responses on day 8 were not likely to be owing to differences in TCR repertoire. Despite repeated attempts, we were unable to recover either wildtype or NIK KO SMARTA T cells on day 30 following infection, so we could not assess memory cell development.
FIGURE 4.
The deficit in the NIK KO CD4 response is not due to changes in the clonal repertoire. A) Naïve NIK KO and WT SMARTA cells were transferred to congenically marked naïve mice in a 1:1 mixture. At 24h post-transfer the mice were challenged with LCMV Armstrong, and expansion of the SMARTA cells was assessed 8 days postinfection. Each symbol represents a single mouse, and the two cohorts represent two independent experiments. There were significantly fewer NIK KO SMARTA cells than WT SMARTA cells. B) Splenocytes were cultured for 5h with brefeldin A and GP67 peptide, and stained for intracellular cytokines. There was not a significant decrease in cytokine production in NIK KO SMARTA cells compared to WT cells.
NIK KO CD8 T cells show a defective memory response upon acute viral infection
The percent NIK KO cells in the total donor CD8 population was measured before infection in the blood and at the peak of the LCMV CD8 response in the spleen. Following infection there was a significant decrease in the percent NIK KO cells in the total CD8 population (Fig. 5A). The ratio of NIK KO to WT cells in uninfected mice was not significantly altered over the period (Fig. 5A). The LCMV response was reduced in the NIK KO population compared to the WT population at 8 days post-infection as measured by the percent CD44hi cells following infection (Fig. 5B & C). At 65 days following infection the percent CD44hi cells within the total NIK KO CD8 population had fallen back to levels close to or below that found in naïve animals (Fig. 5D & E). However, within the CD44hi population at 8 days post-infection, the percentage of LCMV-specific responders as measured by NP396 tetramer staining was comparable between genotypes (Fig. 5H). The absolute number of NP396 CD8 NIK KO responders was less than WT at day 8 (Fig. 5I), but this difference reached statistical significance only in a single experiment, owing to variation in the degree of relative chimerism from animal to animal. When we examined the memory cells, we found a dramatic drop in the relative number of the NIK KO NP396+ cells at day 65 post-infection. The number of NP396 specific responders in the WT population was 7 and 7.5 times lower on day 65 than on day 8, whereas the average number of NIK KO NP396 responders on day 65 was 34 and 83 times lower than on day 8 as determined by tetramer binding in the two experiments presented (Fig. 5H–K). The magnitude of this difference varied from experiment, but in all cases the decrease in the NIK KO cells was between 5 and 10 times greater than the decrease in the WT cells between day 8 and day 65 post-infection. Following rechallenge there was an increase in both WT and NIK KO NP396+ responders in the spleen, and the fold change was comparable between the two populations (Fig. 5J–M).
FIGURE 5.
The CD8 response to virus infection. A) The percent NIK KO (CD45.2+) cells within the CD8+ donor population was determined in blood 10 days prior to infection and in the spleen 8 days post-infection. B) Splenocytes were stained directly ex vivo and gated on CD8+Thy1.1negative donor cells. WT and NIK KO populations were determined based on the expression of CD45.1 and CD45.2 respectively. C) The percent CD44hi CD8+ was determined at day 8 post-infection. Each symbol represents a single mouse, and the two cohorts represent separate experiments. Significance (p<0.05) was determined by comparing the percent CD44hi within the CD8+ population between the wildtype and NIK KO populations. D) Representative splenocytes at day 65 postinfection. E) Percent CD44hi cells within the CD8+ population at 65 days post-infection. F) Representative splenocytes 5 days following rechallenge. G) Percent CD44hi cells within the CD8+ population 5 days following rechallenge. H) NP396 tetramer was used to identify LCMV specific responders. Cells were gated on live CD8+Thy1.1negativeCD44hi donor cells and the percent NP396+ cells at 8 days following infection was determined. I) A comparison of the absolute number of NP396+ CD8+ cells from each genotype in two experiments 8 days following infection. J) The percentage of NP396+ cells in the NIK KO and wildtype populations 65 days post infection. K) The absolute number of NP396+ CD8 cells of each genotype 65 days following infection. L) The percentage of NP396 specific cells in the activated CD8+ population 5 days following rechallenge. M) The absolute number of NP396+ CD8 cells from each genotype 5 days following rechallenge.
We next sought to examine the ability of CD8 cells to produce cytokines in response to LCMV-specific peptides. We found that although the contribution of NIK KO cells to the total CD44hi CD8+ population was reduced at day 8 post-infection compared to the WT population (Fig. 5), within CD44hi cells, the NIK KO CD8 response to LCMV was nevertheless robust and appeared to reflect the same pattern of clonal dominance as the WT population (Fig. 6A & B). However, within the memory phenotype population at 65 days post-infection there were far fewer LCMV-specific responders within the NIK KO population compared to the WT population as measured by the number IFN-γ producing cells 65 days post-infection and 5 days following rechallenge (Fig. 6C–F), taking into account the lower proportion of NIK KO CD44hi cells (Fig. 5D–G). As with the CD4 population, we found that the CD8+ NIK KO T cells appear to be more deficient in their ability to differentiate into TNF-α producing cells than IFN-γ following ex vivo stimulation with LCMV-specific peptide (compare Fig. 6B, D, & F with Fig. 6H, J, & L). These data demonstrate that NIK is required for the generation and/or maintenance of T cell memory during an LCMV infection.
FIGURE 6.
Cytokine production in the CD8 T cell response to virus infection. A) IFN-γ production by CD8 cells was assessed by intracellular staining following a 5hr incubation with GP33 and brefeldin A. Samples were gated on live CD8+CD44hi donor cells and the percent IFN-γ producing cells in the activated WT and NIK KO CD8 populations was determined. B) The percent IFN-γ producing cells in the activated CD8 population in response to ex vivo culture with the dominant CD8 peptides 8 days following infection. Data are representative of 4 independent experiments (n=8). C, D) The percent IFN-γ producing cells in the activated CD8 population 65 days following infection. E, F) The percent IFN-γ producing cells in the activated CD8 population 5 days following rechallenge. G—L) The percent TNF-α producing cells was determined by culturing splenocytes from the same mice assayed in A—F for 5h with LCMV peptides. The analysis was conducted on events within the live CD8+CD44hi donor population. Data are representative of 2 experiments.
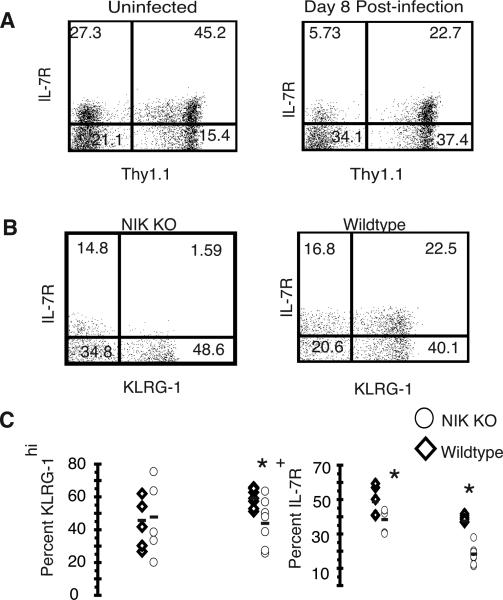
Decreased IL-7R expression among activated NIK KO CD8 cells
At day 8 post LCMV infection, IL-7R is a marker of CD8 memory cell precursors, while KLRG-1 marks terminally differentiated effector cells (36). To determine whether the selective loss of NIK KO CD8+ memory cells we saw at day 65 might be reflected in a loss of IL-7Rhi, KLRG-1lo memory cell precursors at day 8, we assessed IL-7R and KLRG-1 expression among CD8+ cells in naïve mice and 8 days post-infection (Fig. 7A). We observed lower expression of IL-7R in NIK KO (Thy1.1neg) CD8 T cells compared to the WT population in naïve mice and at day 8 post-infection, however, the difference was not significant in naïve mice (Fig. 7A). The lower IL-7R expression in NIK KO CD8 T cells at day 8 post-infection occurred without a corresponding increase in KLRG-1hi short-lived effector cells. Instead, in three of four experiments (two experiments are not shown), there were also significantly fewer KLRG-1hi cells in the NIK KO population. Low IL-7R expression may account at least in part for the disappearance of most of the NIK KO memory CD8 cells by day 65.
FIGURE 7.
Decreased IL-7R expression among activated NIK KO cells. Splenocytes were stained for CD8, CD45.1, Thy1.1, IL-7R, CD44 and KLRG-1. A) Two representative plots illustrate the difference in IL-7R expression on CD8+ cells between NIK KO (Thy1.1−) and wildtype (Thy1.1+) cells. B) NIK KO (left panel) and wildtype (right panel) CD44hiCD8+ cells were analyzed for the presence of IL7R and KLRG-1 8 days post-infection. C) There was a significant (p<0.05) decrease in the percent IL-7R expressing cells within the CD44hiCD8+ cells 8 days post-infection, and a significant decrease in the percent KLRG-1hi within the CD44hiCD8+ 8 days post infection in one of two experiments shown, and in three of four total experiments.
Discussion
Based on our finding that NIK is necessary in CD4 T cells for both noncanonical NF-κB activation and for costimulation of survival and effector cytokine production downstream of OX40 in CD4 T cells (6), we proposed that NIK may be more generally necessary in costimulatory signaling pathways in T cells leading to optimal immune responses to infection. A previous attempt to characterize the immune response to LCMV in bone marrow chimeras made with NIK-deficient bone marrow identified defects in the antibody response attributed to B cell deficiency (29), but failed to identify a cell intrinsic role for NIK in T cells. Using mixed bone marrow chimeras, which possess WT APCs and B cells, a normal thymus, and normal secondary lymphoid organs, we have identified a cell-intrinsic role for NIK in T cells in response to LCMV infection. Although NIK KO T cells participated in the acute response to virus infection, numbers of responding NIK KO T cells were somewhat reduced compared to WT T cells in the same animals (Fig. 2 and Figs. 4–6). As expected for a lesion in late costimulatory pathways, the most dramatic effects of NIK deficiency in T cells occurred during the memory phase of the immune response. The magnitude of the decrease in the number of LCMV specific CD8 WT between 8 and 65 days post-infection varied from experiment to experiment and varied slightly depending on the epitope and means of analysis. For example in the data presented in Fig. 5, the number of NP396 tetramer positive cells decreased by a factor 7 and 7.5 respectively in the WT cells between 8 and 65 days post infection. In contrast, the reduction in the average number of LCMV-specific NIK KO T cells was much greater, decreasing by a factor 34 and 83 respectively.
Our finding that NIK is necessary in T cells for the generation of a robust memory response raises the question of the identity of the upstream and downstream components of the NIK-dependent signal. Beginning upstream, costimulatory members of the TNFR family are major players in enabling T cell memory responses. Members of this family expressed on T cells include 4-1BB, OX40, GITR, CD27, DR3, HVEM, TNFR2, TL1A, and CD30 (2, 3). Most of them have been shown to activate the NIK-dependent noncanonical NF-κB pathway when overexpressed in a cell line (15), and TNFR2, 4-1BB, and OX40 have been shown to do so in primary T cells (6, 16–18). Ligation of TNFRs, specifically CD27, 4-1BB, and OX40, on activated CD8 T cells promotes long-term CD8-mediated immunity (37-43). CD8 T cells lacking 4-1BB or CD27 differentiate into effectors and appear to carry out effector functions, but just as we found with NIK KO cells, their expansion was several fold less than that of WT cells (39, 44). CD4 and CD8 T cells in CD27 KO mice accumulate poorly compared to WT controls following infection with influenza, but the surviving cells make cytokines and effectors molecules just as efficiently as their CD27-sufficient counterparts (39). CD27 KO CD8 T cells also failed to persist as memory cells following an influenza infection, and CD8 memory cells transferred into 4-1BBL KO host also failed to persist (45, 46). The role of TNFRs is not limited to CD8s, as 4-1BB and OX40 have been found to play a role in the generation or maintenance of CD4+ memory T cells (47-50). Thus, the phenotype of NIK KO T cells during and following acute viral infection in our mixed chimeras resembles the phenotypes of cells lacking a TNFR family member.
However, while the various TNFR KOs appear to have similar phenotypes, they are not redundant (47). The various TNFRs and their ligands are expressed by and/or available to T-cells at different times during the immune response, allowing T cells to continue to monitor and respond to other cells in the environment (2). For example CD27 is expressed on naïve T cells and down regulated on active CTLs, but 4-1BB, GITR and OX40 expression is induced by TCR ligation or IL-15 (46, 51, 52). Knocking out both OX40L and 4-1BBL (47) or both CD27 and OX40L or 4-1BBL (53) has synergistic effect on the T cell response. Like our NIK KO T cells, the double KO mice differ mildly but significantly (in some but not all organs) from the WT during the peak of a viral infection, but the magnitude of the difference between KOs and WT mice is much greater during the memory phase of the T cell response (53). Additionally many of the aforementioned KO studies showed changes in only a single population, CD4 or CD8, indicating a different requirement for TNFR ligands between the two populations. Our study showed dramatic, similar differences in both CD4 and CD8 T cells. Thus, we believe NIK may serve as a nexus for all the complementary costimulatory TNFR signals.
Looking downstream of NIK, the best characterized target is p100, the product of the NF-κB2 gene. The cleavage of p100 by NIK-dependent IKKα activity has multiple effects on NF-κB signaling. First, full length p100 is an inhibitor of both canonical and noncanonical NF-κB signaling (11-14, 54). Second, cleavage of p100 produces the active p52 subunit, which pairs preferentially with RelB to produce active, nuclear p52:RelB heterodimers, and potentially other active heterodimers (55). Downstream of NF-κB is a host of cytokines, chemokines, additional effector molecules, cytokine receptors, survival factors, transcription factors, and chromatin remodeling machinery that may account for many of the costimulatory effects of the TNFR family members (8). Our own recent study found that overexpression of NIK in T cells alone was sufficient to cause a potent and lethal inflammatory disease, owing to hyperactive effector T cells and poorly suppressive Foxp3+ regulatory T cells (6). A recent paper examining a lesion in the pathway that degrades NIK found that the accumulation of NIK in T cells leads to CD28-independent activation through the TCR and lethal inflammation in the face of Toxoplasma gondii challenge owing to overexpression of inflammatory cytokines (14).
Although we believe for the reasons stated above that the dominant effect of NIK KO and NIK overexpression in T cells is owing to modulation of NF-κB downstream of costimulatory TNFRs, other possible upstream regulators and downstream targets of NIK should be noted (56). NIK has been implicated in the costimulatory activity of CD28 leading to IL-2 transcription (57, 58), and NIK has been shown recently to play a role in the activation of the STAT3 pathway in T cells downstream of TCR and IL-6R leading to Th17 differentiation (31) and in epithelial cells downstream of HVEM (59). NIK activity has been shown to be necessary for proper T cell homing in response to chemokines (60). Akt/PKB and other signaling pathways have been identified and are likely to be necessary downstream of costimulatory TNFRs (3). Further work will be required to link the physiological effects of lack of NIK activity in T cells to decreased signaling from costimulatory TNFRs to NF-κB.
NIK and noncanonical NF-κB are known to be essential for naive B cell homeostasis and survival of plasmablasts downstream of the BAFF receptors (21, 61), but their role in T cells was not appreciated. Here we show that NIK is a necessary part of the cell-intrinsic molecular program for generation of both CD4 and CD8 T cell memory in response to acute viral infection. We suggest that NIK may be a common, necessary component of late costimulatory signals through TNFRs and perhaps other cytokines (62) that shape T cell responses and enable the responding T cells to survive as memory cells.
Acknowledgements
We thank Fanny Polesso and Katelyn Gardner Toren for excellent technical assistance, and Robert Schreiber and Lindsay Whitton for generously providing the knockout and transgenic mice.
Disclosures
The authors have no financial conflicts of interest.
Footnotes
Footnotes
This work was supported by the National Institute for Allergy and Infectious Diseases (R21-AI077032 and R01-AI092080 to D.C.P. and R01-AI098723 to M.K.S.). A.R. was supported in part by the Molecular Hematology training grant (T32-HL07781).
Abbreviations used in this article: LCMV (lymphocytic choriomeningitis virus); NIK (NF-κB inducing kinase); KO (knockout); TNFRs (TNF receptor family members); WT (wild type, mice or cells that possess an unaltered C57Bl/6 genome excepting the congenic markers CD90.1 (Thy1.1) or CD45.1); IκBs (inhibitors of κB).
References
- 1.Curtsinger J, Mescher M. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watts T. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 3.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.So T, Choi H, Croft M. OX40 complexes with phosphoinositide 3-kinase and protein kinase B (PKB) to augment TCR-dependent PKB signaling. J Immunol. 2011;186:3547–3555. doi: 10.4049/jimmunol.1003156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song J, So T, Croft M. Activation of NF-kappaB1 by OX40 contributes to antigen-driven T cell expansion and survival. J Immunol. 2008;180:7240–7248. doi: 10.4049/jimmunol.180.11.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray S, Polesso F, Rowe A, Basak S, Koguchi Y, Toren K, Hoffmann A, Parker D. NF-kappaB-inducing kinase plays an essential T cell-intrinsic role in graft-versus-host disease and lethal autoimmunity in mice. J Clin Invest. 2011;121:4775–4786. doi: 10.1172/JCI44943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beinke S, Ley S. Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem J. 2004;382:393–409. doi: 10.1042/BJ20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun S. The noncanonical NF-kappaB pathway. Immunol Rev. 2012;246:125–140. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao G, Harhaj E, Sun S. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell. 2001;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 10.Senftleben U, Cao Y, Xiao G, Greten F, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun S, Karin M. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 11.Ishimaru N, Kishimoto H, Hayashi Y, Sprent J. Regulation of naive T cell function by the NF-kappaB2 pathway. Nat Immunol. 2006;7:763–772. doi: 10.1038/ni1351. [DOI] [PubMed] [Google Scholar]
- 12.Basak S, Kim H, Kearns J, Tergaonkar V, O'Dea E, Werner S, Benedict C, Ware C, Ghosh G, Verma I, Hoffmann A. A fourth IkappaB protein within the NF-kappaB signaling module. Cell. 2007;128:369–381. doi: 10.1016/j.cell.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Legarda-Addison D, Ting A. Negative regulation of TCR signaling by NF-kappaB2/p100. J Immunol. 2007;178:7767–7778. doi: 10.4049/jimmunol.178.12.7767. [DOI] [PubMed] [Google Scholar]
- 14.Giardino Torchia M, Conze D, Jankovic D, Ashwell J. Balance between NF-kappaB p100 and p52 regulates T cell costimulation dependence. J Immunol. 2013;190:549–555. doi: 10.4049/jimmunol.1201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauer J, Puschner S, Ramakrishnan P, Simon U, Bongers M, Federle C, Engelmann H. TNF receptor (TNFR)-associated factor (TRAF) 3 serves as an inhibitor of TRAF2/5-mediated activation of the noncanonical NF-kappaB pathway by TRAF-binding TNFRs. Proc Natl Acad Sci U S A. 2005;102:2874–2879. doi: 10.1073/pnas.0500187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauert H, Wicovsky A, Muller N, Siegmund D, Spindler V, Waschke J, Kneitz C, Wajant H. Membrane tumor necrosis factor (TNF) induces p100 processing via TNF receptor-2 (TNFR2) J Biol Chem. 2010;285:7394–7404. doi: 10.1074/jbc.M109.037341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McPherson AJ, Snell LM, Mak TW, Watts TH. Opposing roles for TRAF1 in the alternative versus classical NF-kappaB pathway in T cells. J Biol Chem. 2012;287:23010–23019. doi: 10.1074/jbc.M112.350538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao X, Balasubramanian S, Liu W, Chu X, Wang H, Taparowsky E, Fu Y, Choi Y, Walsh M, Li X. OX40 signaling favors the induction of T(H)9 cells and airway inflammation. Nat Immunol. 2012;13:981–990. doi: 10.1038/ni.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin L, Wu L, Wesche H, Arthur C, White J, Goeddel D, Schreiber R. Defective lymphotoxin-beta receptor-induced NF-kappaB transcriptional activity in NIK-deficient mice. Science. 2001;291:2162–2165. doi: 10.1126/science.1058453. [DOI] [PubMed] [Google Scholar]
- 20.Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger J, Matsumoto M, Nitta T, Takahama Y, Inoue J. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29:423–437. doi: 10.1016/j.immuni.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Claudio E, Brown K, Park S, Wang H, Siebenlist U. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nat Immunol. 2002;3:958–965. doi: 10.1038/ni842. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann J, Mair F, Greter M, Schmidt-Supprian M, Becher B. NIK signaling in dendritic cells but not in T cells is required for the development of effector T cells and cell-mediated immune responses. J Exp Med. 2011;208:1917–1929. doi: 10.1084/jem.20110128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lind E, Ahonen C, Wasiuk A, Kosaka Y, Becher B, Bennett K, Noelle R. Dendritic cells require the NF-kappaB2 pathway for cross-presentation of soluble antigens. J Immunol. 2008;181:354–363. doi: 10.4049/jimmunol.181.1.354. [DOI] [PubMed] [Google Scholar]
- 24.Tamura C, Nakazawa M, Kasahara M, Hotta C, Yoshinari M, Sato F, Minami M. Impaired function of dendritic cells in alymphoplasia (aly/aly) mice for expansion of CD25+CD4+ regulatory T cells. Autoimmunity. 2006;39:445–453. doi: 10.1080/08916930600833390. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto M, Yamada T, Yoshinaga S, Boone T, Horan T, Fujita S, Li Y, Mitani T. Essential role of NF-kappa B-inducing kinase in T cell activation through the TCR/CD3 pathway. J Immunol. 2002;169:1151–1158. doi: 10.4049/jimmunol.169.3.1151. [DOI] [PubMed] [Google Scholar]
- 26.Kajiura F, Sun S, Nomura T, Izumi K, Ueno T, Bando Y, Kuroda N, Han H, Li Y, Matsushima A, Takahama Y, Sakaguchi S, Mitani T, Matsumoto M. NF-kappa B-inducing kinase establishes self-tolerance in a thymic stroma-dependent manner. J Immunol. 2004;172:2067–2075. doi: 10.4049/jimmunol.172.4.2067. [DOI] [PubMed] [Google Scholar]
- 27.Lakkis F, Arakelov A, Konieczny B, Inoue Y. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. 2000;6:686–688. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- 28.Yamada T, Mitani T, Yorita K, Uchida D, Matsushima A, Iwamasa K, Fujita S, Matsumoto M. Abnormal immune function of hemopoietic cells from alymphoplasia (aly) mice, a natural strain with mutant NF-kappa B-inducing kinase. J Immunol. 2000;165:804–812. doi: 10.4049/jimmunol.165.2.804. [DOI] [PubMed] [Google Scholar]
- 29.Karrer U, Althage A, Odermatt B, Hengartner H, Zinkernagel R. Immunodeficiency of alymphoplasia mice (aly/aly) in vivo: structural defect of secondary lymphoid organs and functional B cell defect. Eur J Immunol. 2000;30:2799–2807. doi: 10.1002/1521-4141(200010)30:10<2799::AID-IMMU2799>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 30.Aya K, Alhawagri M, Hagen-Stapleton A, Kitaura H, Kanagawa O, Novack D. NF-(kappa)B-inducing kinase controls lymphocyte and osteoclast activities in inflammatory arthritis. J Clin Invest. 2005;115:1848–1854. doi: 10.1172/JCI23763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin W, Zhou X, Yu J, Cheng X, Sun S. Regulation of Th17 cell differentiation and EAE induction by MAP3K NIK. Blood. 2009;113:6603–6610. doi: 10.1182/blood-2008-12-192914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oxenius A, Bachmann M, Zinkernagel R, Hengartner H. Virusspecific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur J Immunol. 1998;28:390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 33.Murali-Krishna K, Altman J, Suresh M, Sourdive D, Zajac A, Miller J, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 34.Homann D, Lewicki H, Brooks D, Eberlein J, Mallet-Designe V, Teyton L, Oldstone M. Mapping and restriction of a dominant viral CD4+ T cell core epitope by both MHC class I and MHC class II. Virology. 2007;363:113–123. doi: 10.1016/j.virol.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams M, Ravkov E, Bevan M. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–545. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180:1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 37.Dong H, Franklin N, Roberts D, Yagita H, Glennie M, Bullock T. CD27 stimulation promotes the frequency of IL-7 receptor-expressing memory precursors and prevents IL-12-mediated loss of CD8(+) T cell memory in the absence of CD4(+) T cell help. J Immunol. 2012;188:3829–3838. doi: 10.4049/jimmunol.1103329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bullock T, Yagita H. Induction of CD70 on dendritic cells through CD40 or TLR stimulation contributes to the development of CD8+ T cell responses in the absence of CD4+ T cells. J Immunol. 2005;174:710–717. doi: 10.4049/jimmunol.174.2.710. [DOI] [PubMed] [Google Scholar]
- 39.Hendriks J, Gravestein L, Tesselaar K, van Lier R, Schumacher T, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 40.Matter M, Claus C, Ochsenbein A. CD4+ T cell help improves CD8+ T cell memory by retained CD27 expression. Eur J Immunol. 2008;38:1847–1856. doi: 10.1002/eji.200737824. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi C, Mittler RS, Vella AT. Cutting edge: 4-1BB is a bona fide CD8 T cell survival signal. J Immunol. 1999;162:5037–5040. [PubMed] [Google Scholar]
- 42.Mousavi S, Soroosh P, Takahashi T, Yoshikai Y, Shen H, Lefrancois L, Borst J, Sugamura K, Ishii N. OX40 costimulatory signals potentiate the memory commitment of effector CD8+ T cells. J Immunol. 2008;181:5990–6001. doi: 10.4049/jimmunol.181.9.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowley T, Al-Shamkhani A. Stimulation by soluble CD70 promotes strong primary and secondary CD8+ cytotoxic T cell responses in vivo. J Immunol. 2004;172:6039–6046. doi: 10.4049/jimmunol.172.10.6039. [DOI] [PubMed] [Google Scholar]
- 44.Tan J, Whitmire J, Ahmed R, Pearson T, Larsen C. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J Immunol. 1999;163:4859–4868. [PubMed] [Google Scholar]
- 45.Peperzak V, Xiao Y, Veraar E, Borst J. CD27 sustains survival of CTLs in virus-infected nonlymphoid tissue in mice by inducing autocrine IL-2 production. J Clin Invest. 2010;120:168–178. doi: 10.1172/JCI40178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pulle G, Vidric M, Watts T. IL-15-dependent induction of 4-1BB promotes antigen-independent CD8 memory T cell survival. J Immunol. 2006;176:2739–2748. doi: 10.4049/jimmunol.176.5.2739. [DOI] [PubMed] [Google Scholar]
- 47.Dawicki W, Bertram E, Sharpe A, Watts T. 4-1BB and OX40 act independently to facilitate robust CD8 and CD4 recall responses. J Immunol. 2004;173:5944–5951. doi: 10.4049/jimmunol.173.10.5944. [DOI] [PubMed] [Google Scholar]
- 48.Maxwell JR, Weinberg A, Prell RA, Vella AT. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J Immunol. 2000;164:107–112. doi: 10.4049/jimmunol.164.1.107. [DOI] [PubMed] [Google Scholar]
- 49.Kopf M, Ruedl C, Schmitz N, Gallimore A, Lefrang K, Ecabert B, Odermatt B, Bachmann M. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL responses after virus infection. Immunity. 1999;11:699–708. doi: 10.1016/s1074-7613(00)80144-2. [DOI] [PubMed] [Google Scholar]
- 50.Evans DE, Prell RA, Thalhofer CJ, Hurwitz AA, Weinberg AD. Engagement of OX40 enhances antigen-specific CD4− T cell mobilization/memory development and humoral immunity: comparison of anti-OX-40 with anti-CTLA-4. Journal of Immunology. 2001;167:6804–6811. doi: 10.4049/jimmunol.167.12.6804. [DOI] [PubMed] [Google Scholar]
- 51.Mendel I, Shevach E. Activated T cells express the OX40 ligand: requirements for induction and costimulatory function. Immunology. 2006;117:196–204. doi: 10.1111/j.1365-2567.2005.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snell L, Lin G, Watts T. IL-15-dependent upregulation of GITR on CD8 memory phenotype T cells in the bone marrow relative to spleen and lymph node suggests the bone marrow as a site of superior bioavailability of IL-15. J Immunol. 2012;188:5915–5923. doi: 10.4049/jimmunol.1103270. [DOI] [PubMed] [Google Scholar]
- 53.Hendriks J, Xiao Y, Rossen J, van der Sluijs K, Sugamura K, Ishii N, Borst J. During viral infection of the respiratory tract, CD27, 4-1BB, and OX40 collectively determine formation of CD8+ memory T cells and their capacity for secondary expansion. J Immunol. 2005;175:1665–1676. doi: 10.4049/jimmunol.175.3.1665. [DOI] [PubMed] [Google Scholar]
- 54.Tucker E, O'Donnell K, Fuchsberger M, Hilton A, Metcalf D, Greig K, Sims N, Quinn J, Alexander W, Hilton D, Kile B, Tarlinton D, Starr R. A novel mutation in the Nfkb2 gene generates an NF-kappa B2 “super repressor”. J Immunol. 2007;179:7514–7522. doi: 10.4049/jimmunol.179.11.7514. [DOI] [PubMed] [Google Scholar]
- 55.Shih V, Tsui R, Caldwell A, Hoffmann A. A single NFkappaB system for both canonical and non-canonical signaling. Cell Res. 2011;21:86–102. doi: 10.1038/cr.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thu Y, Richmond A. NF-kappaB inducing kinase: a key regulator in the immune system and in cancer. Cytokine Growth Factor Rev. 2010;21:213–226. doi: 10.1016/j.cytogfr.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, Sedwick C, Hu J, Altman A. Role for protein kinase Ctheta (PKCtheta) in TCR/CD28-mediated signaling through the canonical but not the non-canonical pathway for NF-kappaB activation. J Biol Chem. 2005;280:1217–1223. doi: 10.1074/jbc.M409492200. [DOI] [PubMed] [Google Scholar]
- 58.Sanchez-Valdepenas C, Martin A, Ramakrishnan P, Wallach D, Fresno M. NF-kappaB-inducing kinase is involved in the activation of the CD28 responsive element through phosphorylation of c-Rel and regulation of its transactivating activity. J Immunol. 2006;176:4666–4674. doi: 10.4049/jimmunol.176.8.4666. [DOI] [PubMed] [Google Scholar]
- 59.Shui J, Larange A, Kim G, Vela J, Zahner S, Cheroutre H, Kronenberg M. HVEM signalling at mucosal barriers provides host defense against pathogenic bacteria. Nature. 2012;488:222–225. doi: 10.1038/nature11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fagarasan S, Shinkura R, Kamata T, Nogaki F, Ikuta K, Tashiro K, Honjo T. Alymphoplasia (aly)-type nuclear factor kappaB-inducing kinase (NIK) causes defects in secondary lymphoid tissue chemokine receptor signaling and homing of peritoneal cells to the gut-associated lymphatic tissue system. J Exp Med. 2000;191:1477–1486. doi: 10.1084/jem.191.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karnell J, Ettinger R. The interplay of IL-21 and BAFF in the formation and maintenance of human B cell memory. Front Immunol. 2012;3:2. doi: 10.3389/fimmu.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang C, Murti A, Pfeffer S, Fan M, Du Z, Pfeffer L. The role of TRAF2 binding to the type I interferon receptor in alternative NF kappaB activation and antiviral response. J Biol Chem. 2008;283:14309–14316. doi: 10.1074/jbc.M708895200. [DOI] [PMC free article] [PubMed] [Google Scholar]