Introduction
With the introduction of highly active antiretroviral therapy (HAART), HIV is now considered a chronic and stable disease in effectively suppressed individuals on ART. Despite the fact that HAART can lead to undetectable levels of plasma HIV-RNA (<50 copies/ml), low-level viral replication persists in latently infected CD4+ T cells, which is believed to account for viral rebound on ART withdrawal [1, 2]. These viral reservoirs are a major challenge to HIV eradication. Research suggests that the CD4+ T cells harboring HIV are established early in the acute phase of infection and persist even with years of effective therapy [3, 4]. While the mechanisms responsible for HIV latency are poorly understood, the histone architecture within the latently infected T cell is believed to inhibit viral transcription, viral expression and the release of replication competent virus [5, 6]. In vitro, histone deacetylases (HDAC) inhibition has been shown to induce HIV-1 viral expression from resting CD4+ T cells [7]. HDAC inhibition has therefore been proposed as a novel strategy to purge the HIV reservoirs in ART-treated individuals, in whom treatment prevents integration of the released virus into naïve T cells [8]. Despite promising preliminary data using the HDAC inhibitor, valproic acid (VPA) [8], its role in the treatment of HIV-infected individuals remains unclear [9, 10], in part due to the small number of people studied and the varying treatments received. The CIHR Canadian HIV Trials Network (CTN) study 205 (CTN 205) was the first randomized controlled trial of VPA in HIV, undertaken to better characterize the effect of VPA on the HIV reservoir in ART-treated individuals. This multicentre study was initiated in 2006 and is now completed. The results of this randomized crossover study have recently been published [11]. Herein, the design features of CTN 205 are considered within established criteria for crossover designs [12], as a methodological contribution to the study of HIV therapeutics. In addition, we also consider the methodological challenges of the crossover design in the context of the extended study duration and the added logistical complexity.
Methods
The Crossover Design in Therapeutic HIV Trials
Given the long-term stability but heterogeneous nature of HIV disease among chronically infected people on ART, the crossover study may represent an efficient design in the early stage testing of adjuvant therapies. While used increasingly in medical intervention research [13, 14], the crossover design in HIV has historically been limited to pharmacokinetic studies of antiretroviral therapy [15, 16], although some HIV trials using a crossover study design have recently been reported [17–19]. Since the within-person variability of the HIV reservoir in ART-treated individuals has been characterized [20], the crossover design may be uniquely well-suited to evaluate the impact of HIV therapeutics, such as VPA, on the number of resting CD4+ T cells harboring HIV. As a therapeutic strategy that has the potential to reduce, but not clear the HIV reservoir [8], a crossover design in the study of VPA also affords an opportunity to better define treatment characteristics that enhance therapeutic efficacy, including optimal treatment duration and the durability of treatment effect following VPA withdrawal. The appropriateness of the crossover design in the study of VPA among people on ART is discussed below, using the following five important design features of the crossover study [12]: 1) minimal period and carryover effects; 2) randomized subject assignment; 3) time-dependent crossover rules and appropriate timing of measurement; 4) low dropout rates; 5) appropriate sample size estimates and analysis.
CTN 205 Design Features
In the first randomized trial of VPA in HIV, 56 effectively suppressed (<50 copies/ml) individuals on ART were randomly assigned (1:1) to one of two treatment sequences in this crossover study. Main eligibility criteria were HIV (+) men and women age ≥ 18 years, CD4+ ≥ 200 cells/μl on highly active antiretroviral therapy (HAART) for greater than 12 months. Patients were not eligible to participate if they had any bleeding disorders, liver disease, renal failure or were on daily anticoagulants. Further, study participants could not have received cytotoxic agents, systemic corticosteroids or immune modulators within four weeks of the baseline evaluation. If randomized to the first treatment arm (arm 1), study participants continued on ART and also received a therapeutic dose of VPA for 16 weeks, after which point they discontinued VPA and were followed for 32 weeks. The second treatment arm (arm 2) continued on ART alone during the first 16 weeks of the study and then had VPA added to their therapeutic regimen for a duration of 32 weeks. A modified crossover design was therefore employed to test two different durations of VPA treatment, in which the duration of the second treatment period was doubled (Figure 1). Study participants were monitored every four weeks during the study and underwent standard laboratory assessments and HIV-RNA and VPA serum concentration measurements (Figure 1). CD4+ T cell subsets were measured at baseline and then every 8 weeks, until week 48. Treatment effect, based on the frequency of resting CD4+ memory T cells carrying HIV-1 proviral DNA, was determined at the end of each treatment period at 16 and 48 weeks, respectively.
Figure 1.
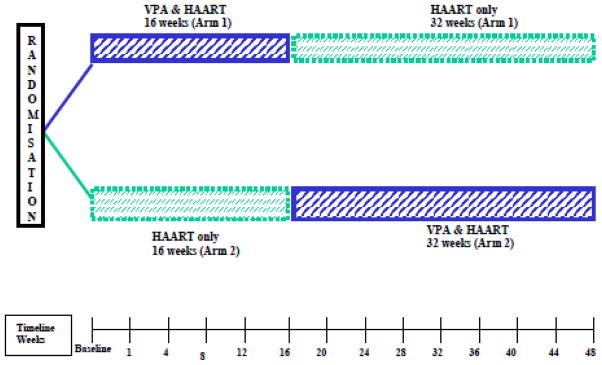
Study design
Period and Carryover Effects
Period effects (due to the passage of time) should be minimized in a crossover study in order to obtain an accurate estimate of the treatment effect. Stable diseases that are unlikely to progress spontaneously during the course of the study are therefore most appropriate for this within-subject design [12]. In the case of CTN 205, all study participants were required to have been effectively suppressed on ART (HIV-RNA< 50 copies/ml) for > 12 months as a measure of disease stability, thus making this ART-treated population well-suited for a crossover study.
Within the context of a crossover study, carryover effects from one period to the next can obscure treatment effects [12]. As the treatment characteristics of VPA were still under investigation at the time of study initiation, the second treatment period was lengthened in order to minimize the impact of potential carry over effects, as recommended by Jones and Kenward [21].
Subject Assignment
In study CTN 205 we compared the size of the viral reservoir in peripheral blood mononuclear cells (PBMC), based on the number of infectious units per log10 billion (IUPM) in ART-treated individuals with and without adjunctive VPA. Random assignment of study participants to the sequence of treatment (ART + VPA → ART alone OR ART alone→ ART + VPA) minimized the potential for selection and ascertainment bias. The random assignment of study participants to different treatment sequences likely also neutralized the impact of any natural history disease progression on the evaluation of treatment efficacy.
Crossover Rules and Timing of Measurements
The effectiveness of CTN 205’s study design was enhanced by specifying, a priori, a time-dependent crossover at 16 weeks. Sixteen weeks was chosen as the minimum duration of therapy necessary to achieve a decrease in the viral reservoir with VPA treatment based on existing data showing a treatment effect after 3–4 months of therapy [8]. Although not blinded, the objective nature of the primary outcome reduced the likelihood of ascertainment bias, as knowledge alone of the presence or absence of adjunctive therapy would be unlikely to influence measurements of the size of the viral reservoir.
In CTN 205, treatment effect was determined based on a comparison of the frequency of resting CD4+ memory T cells carrying HIV-1 proviral DNA in ART-treated individuals with and without VPA, measured at 16 and 48 weeks, respectively. The uneven duration of treatment periods in this study allowed for an assessment of the impact of extended VPA treatment (32 weeks) on the size of the viral reservoir in treatment arm 2, while frequent measurements of the serum VPA concentrations allowed for a better characterization of the effect of VPA treatment. The design also permitted an assessment of the durability of the effect on the reservoir following VPA withdrawal in treatment arm 1.
Dropout Rate and Study Duration
The design efficiency of the within-subject design can be offset by high dropout rates in crossover studies, where study participants receive sequential treatments. As an adjuvant trial of an oral therapy in ART-treated individuals, the longer study duration (48 weeks) was not expected to result in high dropout rates, given that all study participants would have been on long-term ART regardless of study participation. In CTN 205, the number of study withdrawals was only slightly higher in the second treatment period (5 vs 7), despite its longer duration [11]. To evaluate the impact of longer durations of VPA treatment on participant apathy, the serial measurements of VPA serum concentrations were used as a measure of treatment adherence. In the group receiving vaproic acid for the first 16 weeks, there was a trend for the VPA serum concentrations to go down over time (slope = −2.97/week, p=0.06), although the trend reversed for the group receiving 32 weeks of VPA in the second treatment period (slope=1.54/week, p=0.03). As such, there was no consistent evidence to suggest that compliance to VPA was decreasing over time based on serial serum VPA measurements.
While treatment compliance was acceptable for study participants completing the study, adverse events leading to study withdrawal were common during VPA treatment (n=8) and increased with treatment duration (16 weeks VPA=3, 32 weeks VPA=5) [11]. This finding may have implications for the tolerability of extended VPA treatment and, more broadly, on the feasibility of crossover studies evaluating the impact of treatment regimens of different duration in adjunctive therapies with time-dependent toxicity profiles.
Statistical Considerations
By eliminating the impact of between subject variability on the assessment of treatment efficacy, the crossover study represents an efficient study design often requiring fewer study participants than parallel group designs [22]. Based on 20 evaluable participants per treatment arm and an intra-patient variability in the viral reservoir of the IUPM of 0.7log [20], CTN 205 was designed to have 75% power to detect a 0.3 log difference in the IUPM in the resting CD4+ memory cells, under the assumption of a 0.5 correlation between measures within the same person using a paired t-test and a 2-sided test of statistical significance (p=0.05). A minimum difference of 0.3 log in the size of the viral reservoir was chosen based on the inter-assay variance of the assay used to quantitate proviral DNA [9]. Importantly, as a parallel-group design, over 3 times as many evaluable study participants per treatment arm (74 vs 20) would have been required to achieve the same power, given the statistical criteria described above. Under the assumption of a 20% drop-out rate, CTN 205 set out to enroll 50 study participants, in order the achieve the necessary number of evaluable participants per treatment arm. As recently reported [11], a total of 56 participants were enrolled to the trial, with 22 and 20 study participants completing the follow-up period in treatment arms 1 and 2, respectively.
The modified crossover design of CTN 205 allowed for the evaluation of two different durations of treatment, was permissive to unknown carryover effects, and enabled an evaluation of the sustained effect of VPA on the reservoir following VPA withdrawal in treatment arm 1. As recently reported [11], however, the lack of effect of VPA treatment on the size of the viral reservoir following either 16 or 32 weeks of treatment precluded an assessment of these treatment characteristics.
Methodologic Challenges
In the study of adjuvant therapies among ART-treated individuals, the crossover design represents an efficient and practical approach to early stage evaluations of clinical efficacy. In CTN 205 we applied a modified form of the standard crossover design to carry out a within-subject comparison of the HIV viral reservoir in ART-treated individuals with and without VPA therapy. Application of this unique study design posed administrative and logistical challenges which deserve mention and may have implications for the use of this design in future HIV trials. Although logistically more complex than a parallel-group study design, the unequal treatment periods of this modified crossover study added additional complexity in study management and the scheduling of clinical assessments, thereby increasing the likelihood of human error. In addition, the uneven duration of treatment also created additional administrative burdens and cost in the execution of the trial, such as the development of treatment arm specific case report forms (CRF). Finally, the long duration of the crossover study, which evaluated study participants both on and off VPA, increased the study burden to personnel and study participants, given the frequency of evaluations. Despite the extended duration of the study (48 weeks), however, there were only three participant withdrawals not precipitated by an adverse event among the 56 participants enrolled and no one was lost to follow up. Further, based on serial serum VPA measurements, the duration of treatment did not affect compliance (p=0.25), which was acceptable in both treatment arms (mean serum VPA = 362.3 mcg/ml in the 16 week group and 384.9 mcg/ml in the 32 week group).
Based on the results presented herein, the evaluation of modest adjunctive therapies within a trial of extended duration and multiple evaluations does not appear to result in participant fatigue in an ART-treated population. In such trials, the biggest threat to the validity of the study results are withdrawals due to treatment-related adverse events, which in the case of CTN 205 resulted in 8 withdrawals in participants on VPA therapy [11]. Given that withdrawals and losses to follow-up reduce the design efficiency of the crossover trial, application of this design in the testing of HIV therapeutics would best be undertaken in adjunctive therapies with good toxicity profiles. Although within the projected drop-out rate, the large number of withdrawals due to treatment-related adverse events was somewhat unexpected given the limited in vivo data available at the time of study initiation [8].
Conclusion
Attempts to purge the HIV reservoirs have, to date, proven unsuccessful [23, 24]. VPA has recently been advanced as a novel strategy to purge the HIV reservoir in ART-treated individuals, by increasing viral gene expression and allowing the killing of HIV infected cells [8]. Since the initial case series, conflicting results have been reported on a small number of subjects under varying conditions of treatment duration [9, 10]. CTN 205 was the first study to evaluate VPA in people with HIV using a randomized design. In the early stage testing of novel HIV therapeutics, efficient clinical trials with reduced sample size requirements can streamline the testing of adjunctive therapies. Given the stable but heterogeneous nature of the disease among ART-treated individuals, the crossover design is an appealing alternative to the parallel-group design in the study of HIV therapeutics.
The strength of the crossover design in the study of HIV therapeutics lies in the reduced sample size of this approach, which in the case of CTN 205 decreased the required number of study participants by two thirds. As the smaller sample size requirements of the crossover design is achieved by eliminating the impact of inter-subject variability (by evaluating study participants both on and off therapy) a detailed consideration of therapeutic factors that may threaten the ability of study participants to complete the trial is required, as this will offset the statistical benefits this study design. Most important among these therapeutic factors in the study of novel HIV therapeutics are adverse events leading to participant withdrawal and unpleasant treatment regimens leading to participant apathy over time. In addition, other investigator determined crossover design characteristics, such as the total duration of the study and the frequency of study visits, can also affect participant study completion rates and should be considered for their potential impact on participant withdrawal. Given these criteria, in the study of HIV therapeutics, we feel that unpleasant treatment regimens of short duration (i.e. 2–3 weekly injections) or longer less involved regimens (i.e. daily pills) with a reasonable follow-up period and limited assessments are most appropriate for the crossover design.
In CTN 205, doubling of the second period not only permitted an assessment of the impact of an extended duration of VPA treatment on the size of the HIV reservoir in the second treatment arm, but also allowed for an evaluation of the stability of treatment effects on VPA withdrawal in the first treatment arm. While the absence of a treatment effect in CTN 205 [11] precluded this evaluation, this methodology could be employed in the early testing of other novel therapeutics in HIV, with poorly-defined treatment characteristics. Of note, there are important considerations specific to the analysis of data from crossover studies pertaining to the order of the treatments received. Indeed, a review article by Hill [25] provides a detailed account of the methods for testing for period effects in a two-period crossover trial and the statistical manipulations required to obtain an unbiased estimate of the treatment effect, in the presence of a period by treatment interaction.
In an era where accrual to clinical trials is difficult, the crossover design may be a useful alternative in the early stage testing of novel HIV therapeutics with good toxicity profiles and acceptable treatment regimens, given its reduced sample size requirements. The participant burden and logistical complexity of this approach combined with the longer duration of these studies are, however, important factors which must be also be considered when choosing the study design. Given the chronic nature of HIV treatment, the extended duration of the crossover design may not negatively impact dropout rates among ART-treated individuals who would otherwise still be on treatment and should, therefore, be considered in the early stage testing of novel HIV therapeutics, including the newer HDAC inhibitors [26].
Acknowledgments
We are thankful to M. D. deB. Edwardes for advice in the study design, and nurses and coordinators for their invaluable assistance in patient recruitment at all study sites and also Jacquie Sas and Jim Pankovich from CTN staff. We are also grateful to the laboratory staff for technical assistance and reservoir assessments and to Dr. Mohamed-Rachid Boulassel for his assistance in the design and execution of the study. Routy J-P is a clinician-scientist supported by Fonds de la recherche en santé du Québec (FRSQ). Angel, JB is an Ontario HIV Treatment Network Career Scientist.
Grant Support: This project was funded in part by The American Foundation for AIDS Research (amfAR#106722-40RGRL); and the Canadian Foundation for AIDS Research (CANFAR #017-718); and the CIHR Canadian HIV Trials Network (CTN); and Abbott Canada
Funding
This project was funded in part by The American Foundation for AIDS Research (amfAR#106722-40RGRL); and the Canadian Foundation for AIDS Research (CANFAR #017-718); and the CIHR Canadian HIV Trials Network (CTN); and Abbott Canada.
Footnotes
Clinical trials.gov identifier: NCT00289952.
Declaration of Conflicting Interests
The authors declare that there is no conflict of interest.
References
- 1.Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 3.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection. Proc Natl Acad Sci USA. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1 even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 5.Sheridan PL, Mayall TP, Verdin E, Jones KA. Histone acetyltransferases regulate HIV-1 enhancer activity in vitro. Genes Dev. 1997;11:3327–3340. doi: 10.1101/gad.11.24.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benkirane M, Chun RF, Xiao H, Ogryzko VV, Howard BH, Nakatani Y, et al. Activation of integrated provirus requires histone acetyltransferase. p300 and P/CAF are coactivators for HIV-1 Tat. J Biol Chem. 1998;273:24898–24905. doi: 10.1074/jbc.273.38.24898. [DOI] [PubMed] [Google Scholar]
- 7.Ylisastigui L, Coull JJ, Rucker VC, et al. Polyamides reveal a role for repression in latency within resting T cells of HIV-infected donors. J Infect Dis. 2004;190:1429–1437. doi: 10.1086/423822. [DOI] [PubMed] [Google Scholar]
- 8.Lehrman G, Hogue IB, Palmer S, et al. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005;366:549–555. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Archin NA, Eron JJ, Palmer S, et al. Standard ART and valproic acid have limited impact on the persistence of HIV infection in resting CD4+ T cells. AIDS. 2008;22:1131–1135. doi: 10.1097/QAD.0b013e3282fd6df4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sagot-Lerolle N, Lamine A, Chaix ML, et al. Prolonged valproic acid treatment does not reduce the size of the latent HIV reservoir. AIDS. 2008;22:1125–1129. doi: 10.1097/QAD.0b013e3282fd6ddc. [DOI] [PubMed] [Google Scholar]
- 11.Routy JP, Tremblay CL, Angel JB, et al. Valproic Acid in association with highly active antiretroviral therapy for reducing systemic HIV-1 reservoirs: results from a multicentre randomized clinical study. HIV Medicine. 2012;13 (5):291–296. doi: 10.1111/j.1468-1293.2011.00975.x. [DOI] [PubMed] [Google Scholar]
- 12.Louis TA, Lavori PW, Bailar JC, Polansky M. Crossover and Self-Controlled Designs in Clinical Research. NEJM. 1984;310(1):24–31. doi: 10.1056/NEJM198401053100106. [DOI] [PubMed] [Google Scholar]
- 13.Young JM, Florkowski CM, Molyneux SL, et al. A randomized, double-blind, placebo-controlled crossover study of coenzyme Q(10) therapy in hypertensive patients with the metabolic syndrome. Am J Hypertension. 2011;25(2):261–70. doi: 10.1038/ajh.2011.209. [DOI] [PubMed] [Google Scholar]
- 14.Dashti-Khavidaki S, Chamani N, Khalili H, et al. Comparing effects of clonazopam and zolpidem on sleep quality of patients on maintenance hemodialysis. Iran J Kidney Dis. 2011;5(6):404–409. [PubMed] [Google Scholar]
- 15.Samineni D, Desai PB, Sallans L, Fichtenbaum CJ. Steady-state pharmacokinetic interactions of Darunavir/Ritonavir with Lipid-Lowering agent rosuvastatin. Clin Pharmacol. 2012;52(6):922–31. doi: 10.1177/0091270011407494. [DOI] [PubMed] [Google Scholar]
- 16.Innes S, Norman J, Smith P, Smuts M, Capparelli E, Rosenkranz B, Cotton Bioequivalence of dispersed stavudine: opened versus closed capsule dosing. Antivir Ther. 2011;16(7):1131–1134. doi: 10.3851/IMP1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston C, Saracino M, Kuntz S, et al. Standard-dose and high-dose daily antiviral therapy for short episodes of genital HSV-2 reactivation: three randomized, open-label, cross-over trials. Lancet. 2012;379 (9816):641–7. doi: 10.1016/S0140-6736(11)61750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mugwanya K, Baeten JM, Mugo NR. High-dose valacyclovir HSV-2 suppression results in greater reduction in plasma HIV-1 levels compared with standard dose acyclovir among HIV-1/HSV-2 coinfected persons: a randomized, crossover trial. J Infect Dis. 2011;204(12):1912–7. doi: 10.1093/infdis/jir649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen A, Calmy A, Delhumeau C, et al. A randomized cross-over study to compare raltegravir and efavirenz (SWITCH-ER study) AIDS. 2011;25(12):1481–7. doi: 10.1097/QAD.0b013e328348dab0. [DOI] [PubMed] [Google Scholar]
- 20.Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 21.Jones B, Kenward MG. Design and Analysis of Cross-Over Trials: Chapter 2. Chapman and Hall; 1989. [Google Scholar]
- 22.Woods JR, Williams JG, Tavel M. The Two-Period Crossove Design in Medical Research. Annals of Internal Medicine. 1989;110(7):560–566. doi: 10.7326/0003-4819-110-7-560. [DOI] [PubMed] [Google Scholar]
- 23.Emery S, Capra WB, Cooper DA, Mitsuyasu RT, Kovacs JA, Vig P, et al. Pooled analysis of 3 randomized, controlled trials of interleukin-2 therapy in adult human immunodeficiency virus type 1 disease. J Infect Dis. 2000;182:428–34. doi: 10.1086/315736. [DOI] [PubMed] [Google Scholar]
- 24.Dybul M, Hidalgo B, Chun TW, Belson M, Migueles SA, Justement JS, et al. Pilot study of the effects of intermittent interleukin-2 on human immunodeficiency virus (HIV)-specific immune responses in patients treated during recently acquired HIV infection. J Infect Dis. 2002;185:61–68. doi: 10.1086/338123. [DOI] [PubMed] [Google Scholar]
- 25.Hills M, Armitage P. The two-period cross-over clinical trial. Br J Clin Pharmac. 1979;8:7–20. doi: 10.1111/j.1365-2125.1979.tb05903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasmussen T, Søgaard O, Melchjorsen J, et al. The Histone Deacetylase Inhibitor (HDACi) panobinostat (LBH589) Stimulates HIV-1 Expression More Potently than Other HDACi in Clinical Use and Disrupts HIV Latency at Clinically Achievable Concentrations (Paper #370). CROI; Seattle, Washington. March 5–8, 2012. [Google Scholar]


