Abstract
Phytoecdysteroids, structurally similar to insect molting hormones, produce a range of effects in mammals, including increasing growth and physical performance. In skeletal muscle cells, phytoecdysteroids increase protein synthesis. In this study we show that in a mouse skeletal muscle cell line, C2C12, 20-hydroxyecdysone (20HE), a common phytoecdysteroid in both insects and plants, elicited a rapid elevation in intracellular calcium, followed by sustained Akt activation and increased protein synthesis. The effect was inhibited by a G-protein Coupled Receptor (GPCR) inhibitor, a phospholipase C (PLC) inhibitor, and a phosphoinositide kinase-3 (PI3K) inhibitor.
Keywords: ecdysteroid, skeletal muscle, Akt, GPCR, calcium, C2C12
Introduction
Ecdysteroids, polyhydroxylated ketosteroids with long carbon side chains, are produced primarily in insects and plants. Although the role of ecdysteroids as insect hormones and their involvement in development has been well studied, their role in mammals is less understood. Ecdysteroids have been reported to produce a wide range of effects in mammals [1]. One of the most interesting properties of ecdysteroids in mammals is their anabolic effect, behaving similar to anabolic steroids putatively without the androgenic effect. Recently, ecdysteroids were shown to increase muscle fibers in rat [2]. Ecdysteroids have been shown to increase growth and endurance in animals [1, 3] without negative side effects.
Using the murine skeletal muscle cell line C2C12, we have previously shown that treatment with ecdysteroids increases protein synthesis by up to 20% [4]. However, despite evidence showing both invivo and invitro anabolic effects of ecdysteroids, their cellular mode of action has not been elucidated. In mammals, there is no known nuclear receptor that is homologous to the ecdysone nuclear receptor (EcR) found in insects, ruling out the possibility that ecdysteroids function through similar pathways in both mammals and insects. Ecdysteroids do not appear to activate the nuclear androgen receptor (AR) since 20-hydroxyecdysone (20HE), the most common ecdysteroid, did not bind to the rat AR [4].
In addition to classical nuclear receptor responses, mammalian steroid hormones structurally related to ecdysteroids have been shown to elicit rapid non-genomic signaling events that mediate cell proliferation and survival. These responses may involve secondary signals such as altered Ca2+ or cAMP [5]. Mammalian steroid hormones increased Ca2+ influx in a variety of cell types including cardiac myocytes [6] and skeletal muscle [7]. Although some of these effects have been attributed to a new role for already identified nuclear receptors, such as the estrogen receptor (ER) and AR, there are data supporting the presence of distinct membrane-bound receptors that interact with these hormones. Estrogen and progesterone G-protein coupled receptors (GPCRs) have already been cloned and characterized and a putative membrane-bound AR has been described [8].
In addition to their classical nuclear receptor-mediated genomic response, ecdysteroids also produce many “non-genomic” effects in invertebrates [9, 10]. Although many of these effects may involve the classical nuclear EcR, a putative membrane-bound ecdysone receptor that has been described in silkworms may also be responsible [11]. A membrane-bound GPCR, DopEcR, has been identified in Drosophila which binds ecdysteroids in addition to dopamine [12]. A similar receptor may be responsible for the rapid effects of both androgens and ecdysteroids in mammals.
In this study we used the mouse skeletal muscle cell line, C2C12, to investigate the intracellular responses that may underlie the anabolic effects of ecdysteroids and compared them with the effects of Insulin-like Growth Factor 1 (IGF-1), a well characterized anabolic agent. Specifically, we investigated the ability of 20HE to affect intracellular calcium fluxes. We hypothesized that this might lead to Akt activation via a G-protein Coupled Receptor-Phospholipase C-Phosphoinositide-3-Kinase (GPCR-PLCPI3K)-mediated mechanism.
Experimental
Materials
1,2-bis-(o-aminophenoxy)-ethane-N,N,N’,N’-tetraacetic acid, tetraacetoxymethyl ester (BAPTA-AM), 1-[6-[((17β)-3-methoxyestra-1,3,5[10]-trien-17-yl)amino]hexyl]-1H-pyrrole-2,5-dione (U-73122), and 2-aminoethoxydiphenyl borate (2-APB) were purchased from Calbiochem (San Diego CA). Fluo-4 NW was purchased from Invitrogen (Carlsbad CA). Phospho-Akt and Akt antibodies were purchased from Cell Signaling (Danvers MA). 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY-294002), Bordetella pertussis toxin (PTX), ethylene glycol tetraacetic acid (EGTA), 20-hydroxyecdysone (20HE), and all other supplies were purchased from Sigma-Aldrich (St. Louis MO).
Cell Culture
A mouse skeletal muscle cell line, C2C12, (ATCC CRL-1772), was cultured as described previously [4]. Differentiated multinucleated myotubes were plated on 24 well plates for all studies with the exception of the Ca2+ flux assay where 96 well plates were used. All cells were serum-starved overnight prior to treatment. All treatments were delivered in ethanol at a final concentration of 0.1%
Determination of Intracellular Ca2+
Measurements of intracellular Ca2+ from C2C12 skeletal myotubes were determined using Fluo-4 NW based on Gee [13] according to manufacturer’s instructions (Invitrogen). C2C12 myotubes plated on 96 well plates were loaded with the Ca2+ fluophore, Fluo4-AM (Invitrogen) for 1 h prior to treatment. Cells were washed with either Ca2+ containing media (1 mM NaCl, 5 mM KCl, 2.6 mM CaCl2, 1 mM MgCl2, 10 mm HEPES-Na, 5.6 mM glucose) or Ca2+ free medium (1 mM NaCl, 5 mM KCl, 1.0 mM EGTA, 3.6 mM MgCl2, 10 mM HEPES-Na, 5.6 mM glucose). For inhibitor studies, cells were pretreated with inhibitors for 1 h before treatment. Baseline fluorescence was monitored prior to treatment. Cells were treated with 1 μl of 20HE dissolved in assay buffer to reach a final concentration of 1μM and fluorescence was measured using a Synergy™ HT Multi-Mode Microplate Reader (Biotek). Data were collected from three wells per treatment every 5 sec for 180 sec and analyzed using Gen5 Data Analysis software. The changes in fluorescent intensity over baseline (ΔF/F0) were averaged and plotted as a function of time. The study was performed in triplicate and the values averaged.
Cell Signaling Experiments
For the ecdysteroid dose response, C2C12 cells were washed with serum-free DMEM and treated for 2 h with increasing concentrations of 20HE or vehicle (0.1% ethanol), four wells per treatment. For the 20HE time course study, C2C12 cells were treated with 1 μM 20HE for 5 min to 24 h in serum-free media. For the inhibitor studies, C2C12 cells were pretreated with inhibitors or vehicle for 1 h prior to treatment with 1 μM 20HE or 100 ng/ml insulin-like growth factor 1 (IGF-1). For removal of free extra- or intracellular Ca2+, media was supplemented with either 3 mM EGTA or 25 μM BAPTA-AM for 30 min prior to treatment.
Western Blot Analysis
Western blot analysis was performed based on Ushio-Fukai [14]. After treatment, cells were lysed using 250 μl of cold lysis buffer per well. Lysis buffer consisted of 20 mM Tris, pH 7.4, 30 mM NaCl, 3.5 mM EDTA, 1 mM EGTA, 1.0% Triton X-100, 2.5 mM sodium pyrophosphate, 4 mM sodium fluoride, 1 mM sodium vanadate, 1mM phenylmethylsulfonyl fluoride and Protease Inhibitor Cocktail (Sigma) consisting of 2.0 μg/ml leupeptin and 2.0 μg/ml aprotinin. Two wells per treatment were pooled and whole cell lysates were sonicated for 15 seconds, boiled for 5 min, and centrifuged at 16,000 g for 5 min at 4 C. Protein content of the supernatant was quantified using a Nanodrop 1000 spectrophotometer according to manufacturer instructions (Thermo Scientific). 40 μg of protein were separated using 8% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Amersham). Membranes were blocked in Tris Buffered Saline (TBS) containing 5% nonfat dry milk and 0.01% Tween at room temperature for 1 h. Membranes were then incubated in 5% bovine serum albumin in TBS containing phospho-Akt (Ser473) antibody (1:1000) or total Akt antibody (1:1000) (Cell Signaling) overnight at 4 C. Membranes were rinsed and probed with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:2000) (Cell Signaling) in 5% nonfat dry milk in TBS. After incubation with secondary antibodies, phosphorylated or total Akt protein was detected by enhanced chemiluminescence (Amersham). Band intensity was quantified by densitometry of immunoblots using NIH ImageJ, version 1.38x, and phosphorylated Akt levels normalized to total Akt.
Protein Synthesis Assay
Protein synthesis was determined as described previously [4].
Statistical Analysis
Results are presented as the mean ± S.E. Data were analyzed using one-way ANOVA/Bonferroni method.
Results
Intracellular Ca2+
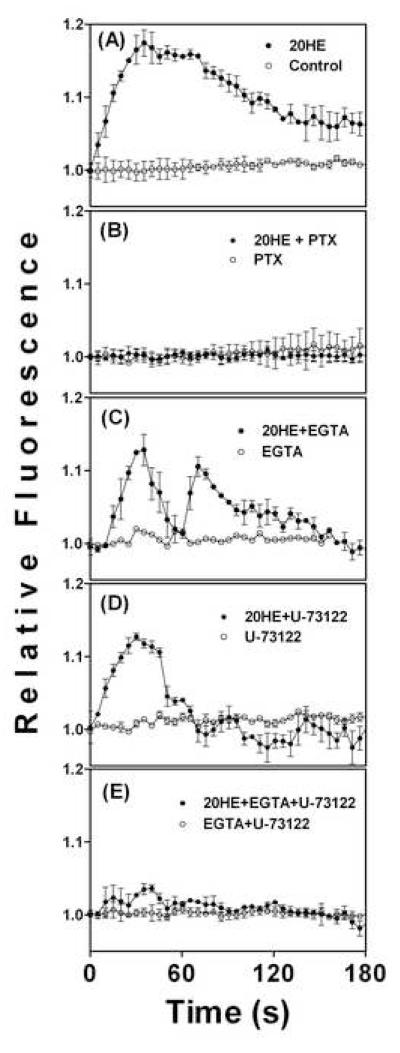
Treatment with 1 μM 20HE increased intracellular Ca2+ in C2C12 myotubes within 10 sec (Fig. 1A). Intracellular Ca2+ peaked 35 sec after treatment, and began to decline after 70 sec. The decrease was gradual with some elevation of intracellular Ca2+ still observed after 180 sec. This effect was completely abolished when cells were pretreated with 1 μg/ml PTX, 1 h prior to 20HE treatment (Fig. 1B). Ca2+ free media containing the extracellular Ca2+ chelator, 3 mM EGTA, slightly reduced and modified the 20HE response and decreased its duration (Fig. 1C).
Fig 1. Intracellular calcium in C2C12 myotubes treated with 20-hydroxyecdysone (20HE) and various inhibitors.
Differentiated myotubes were pretreated with either (A) vehicle, (B) 1 μg/ml PTX, (C) 3 mM EGTA, (D) 10 μM U-73122, (E) 3 mM EGTA and 10 μM U-73122, for 1 h prior to treatment with 1 μM 20HE. Relative fluorescence was measured for 180 sec after treatment. Data represent the mean values ± S.E.M. of three experiments.
Pretreatment with the phospholipase C (PLC) inhibitor, 10 μM U-73122, for 1 h prior to 1 μM 20HE treatment also reduced the intensity and duration of the intracellular Ca2+ flux as compared to the 20HE treatment alone (Fig. 1D). The inositol 3-phosphate receptor (IP3R) inhibitor, 2-APB, produced similar results (data not shown). Both EGTA and U-73122 produced a more rapid, oscillating decline in Ca2+ that returned close to control levels in less than 120 s (Fig. 1C and D). When the PLC inhibitor, U-73122, was added to EGTA containing media, the 20HE-induced Ca2+ flux was almost completely abolished (Fig. 1E).
Akt Activation
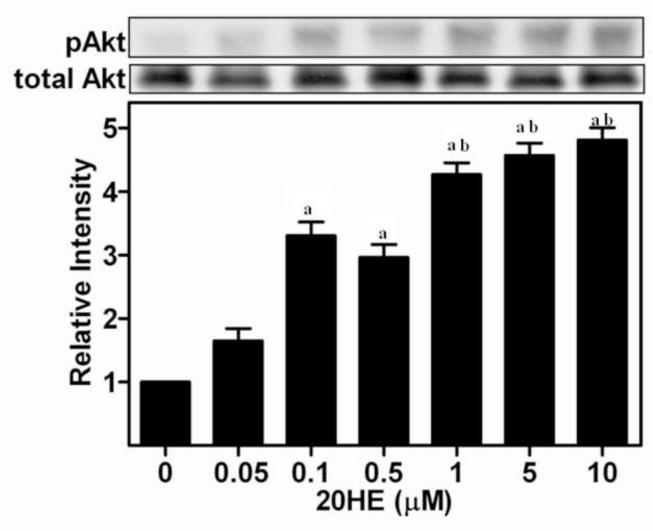
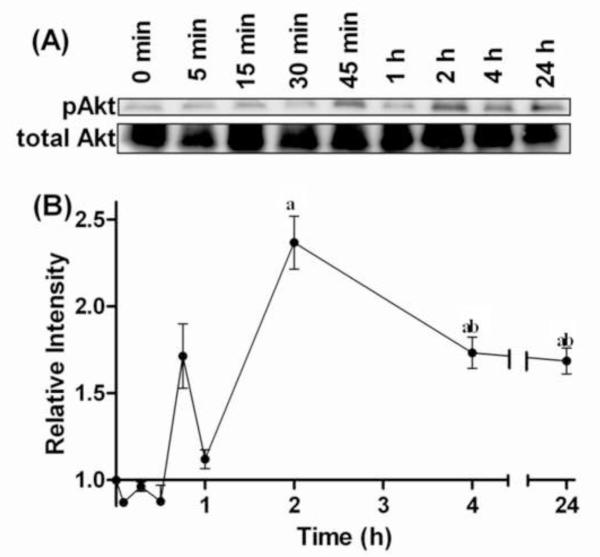
Exposure to 20HE activated Akt in C2C12 skeletal myotubes (Fig. 2). 0.1 μM 20HE significantly increased phosphorylation of Ser473 Akt by more than 3-fold. Activation of Akt continued to increase with greater concentrations of 20HE. Although maximal increases of approximately 5-fold were observed with the highest concentration of 20HE, and a clear dose response was observed at lower concentrations, with significant differences between 0.5 μM and 1 μM, there was no significant difference between 1 μM and higher concentrations. Treatment with 1 μM 20HE significantly increased Akt activation which peaked after 2 h, decreased somewhat after 4 h, but remained greater than initial levels even after 24 h with significant differences between 0 and 2 h, 2 and 4 h, and 0 and 24 h (Fig. 3A and 3B).
Fig. 2. Effect of increasing concentrations of 20-hydroxyecdysone (20HE) on phosphorylation of Akt in C2C12 myotubes.
Differentiated myotubes were treated for 2 h with increasing concentrations of 20HE or vehicle. Cell lysates were subjected to Western blotting and probed with either anti-pAkt or anti-Akt. Data represent the mean values of phosphorylated Akt normalized to total Akt ± S.E.M. of three experiments. a indicates p < 0.05 compared with control and b indicates p < 0.05 compared with 0.5 μM 20HE treatment (One way ANOVA/Bonferroni).
Fig. 3. Time course of Akt phosphorylation in C2C12 myotubes treated with 1 μM 20-hydroxyecdysone (20HE).
Differentiated myotubes were treated with 1 μM 20HE for 5 min to 24 h. (A) Cell lysates were subjected to Western blotting and probed with either anti-pAkt or anti-Akt. (B) Data represent the mean values of phosphorylated Akt normalized to total Akt ± S.E.M. of three experiments. a indicates p < 0.05 compared with control and b indicates p < 0.05 compared with 30 min treatment (One way ANOVA/Bonferroni).
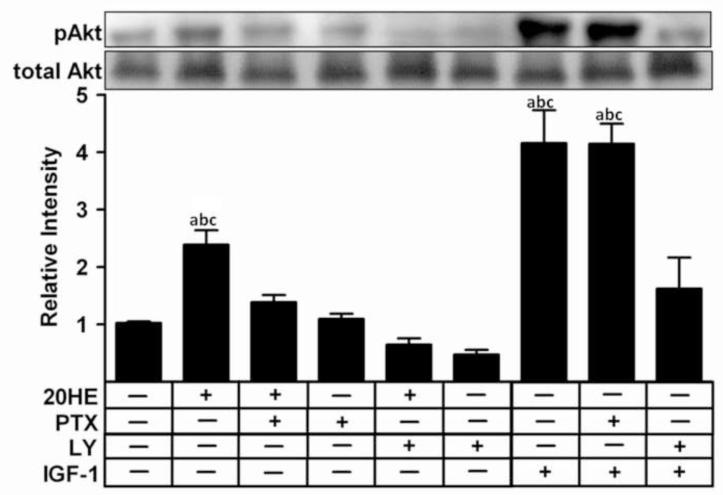
Pretreatment with either 10 μM LY, a PI3K inhibitor, or 1 μg/ml PTX, a G-protein inhibitor, for 1 h inhibited the 20HE-induced Akt activation in C2C12 myotubes (Fig. 4). In contrast, activation of Akt by 100 ng/ml IGF-1 was inhibited by LY but not PTX.
Fig. 4. Effect of G-protein and PI3K inhibitors on Akt phosphorylation in C2C12 myotubes treated with 20-hydroxyecdysone (20HE).
Differentiated myotubes were pretreated for 1 h with either 1 μg/ml PTX, 10 μM LY, or vehicle prior to treatment with either 1 μM 20HE or 100 ng/ml IGF-1 for 2 h. Cell lysates were subjected to Western blotting and probed with either anti-pAkt or anti-Akt. Data shown represent the mean values of phosphorylated Akt normalized to total Akt ± S.E.M. of three experiments. a indicates p < 0.05 compared with control, b indicates p < 0.05 compared with PTX treatment alone and c indicates p < 0.05 compared with LY treatment alone (One way ANOVA/Bonferroni).
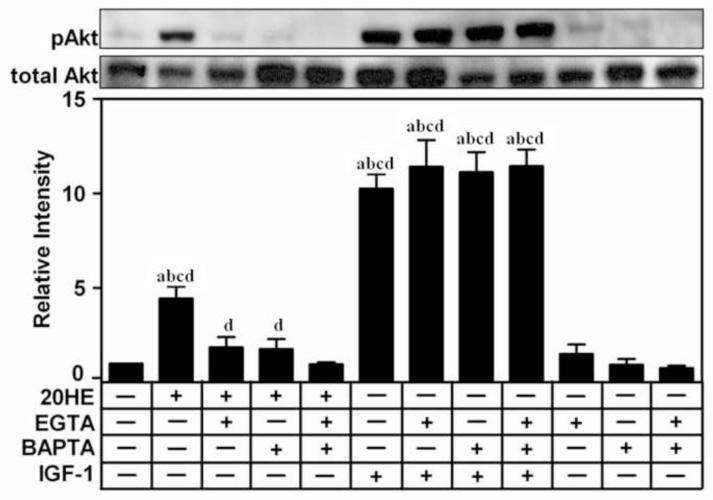
Removal of available Ca2+ also inhibited the 20HE-induced activation of Akt. Treatment with either the extracellular chelator, 3mM EGTA, or the intracellular chelator, 25μM BAPTA-AM, 1 h prior to treatment reduced 20HE-induced Akt phosphorylation (Fig. 5). Pretreatment with both EGTA and BAPTA-AM together completely abolished the 20HE-induced phosphorylation of Akt, although the response was not significantly different than either EGTA or BAPTA-AM alone. Although 20HE treatment was significantly greater than the EGTA, BAPTA-AM, or control groups, there was no significant difference between either EGTA or BAPTA-AM with the control. IGF-1 induced activation of Akt was not affected by pretreatment with EGTA, BAPTA-AM, or both.
Fig. 5. Effect of extra- and intracellular calcium chelators on 20-hydroxyedysone (20HE) induced Akt phosphorylation in C2C12 myotubes.
Differentiated myotubes were pretreated with either 3 mM EGTA, 25 μM BAPTA-AM, alone or in combination, or vehicle, for 1 h prior to treatment with 1 μM 20HE or 100 ng/ml IGF-1 for 2 h. Cell lysates were subjected to Western blotting and probed with either anti-pAkt or anti-Akt. Data shown represent the mean values of phosphorylated Akt normalized to total Akt ± S.E.M. of three experiments. a indicates p < 0.05 compared with control, b indicates p < 0.05 compared with EGTA treatment alone, c indicates p < 0.05 compared with BAPTA-AM treatment alone, and d indicates p < 0.05 compared with EGTA + BAPTA-AM treatment (One way ANOVA/Bonferroni).
Protein Synthesis
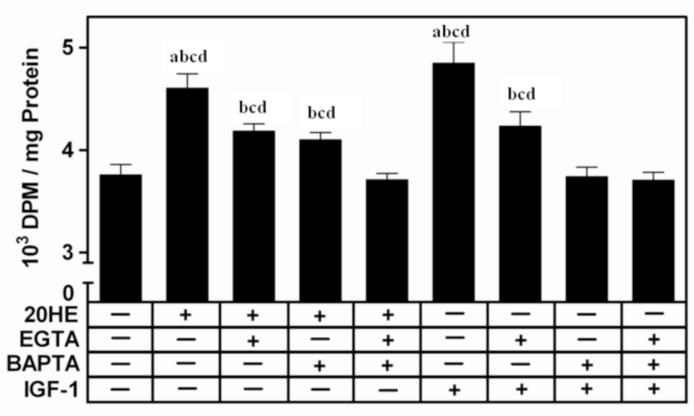
Removal of extracellular Ca2+ partially abrogated the 20HE-induced increase in protein synthesis in C2C12 myotubes (Fig 6). When cells were incubated in media containing 3 mM EGTA to chelate free Ca2+, the increase in protein synthesis was only 8%, significantly lower than the 16% when cells were exposed to 20HE in Ca2+ containing media. The response to 20HE was similarly attenuated by pretreatment with 25μM BAPTA-AM for 1 h. When cells were pretreated for 1 h with both EGTA and BAPTAAM together, the 20HE-induced increase was completely abolished producing a response not significantly different from either EGTA and/or BAPTA-AM pretreatments or control while significantly lower then 20HE pretreated with either EGTA or BAPTA-AM. The IGF-1 induced increase in protein synthesis was also inhibited by both EGTA and BAPTA-AM. While IGF-l still increased protein synthesis in cells pretreated with EGTA, IGF-1 produced no increase in cells pretreated with BAPTA-AM. Treatment with only EGTA and/or BAPTA-AM did not alter protein synthesis (data not shown).
Fig. 6. [3H] Leucine incorporation in C2C12 myotubes pretreated with the calcium chelators, EGTA and/or BAPTA-AM.
Differentiated myotubes were pretreated with either 3 mM EGTA or 25 μM BAPTAAM, alone or in combination or vehicle for 1 h before treatment with 1 μM 20HE, 100 ng/ml IGF-1, or vehicle. DPM were normalized by total protein. Data represent the mean values ± S.E.M. of three experiments. a indicates p < 0.05 compared with control, b indicates p < 0.05 compared with EGTA treatment alone, c indicates p < 0.05 compared with BAPTA-AM treatment alone, and d indicates p < 0.05 compared with EGTA + BAPTA-AM treatment (One way ANOVA/Bonferroni).
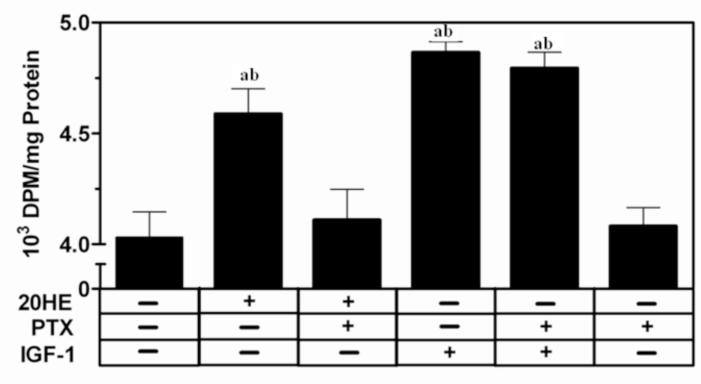
Pretreatment with 1 μg/ml PTX, a G protein inhibitor, for 1 h completely abolished the 20HE-induced increase in protein synthesis in C2C12 myotubes (Fig. 7). In contrast, the IGF-1 induced increase in protein synthesis was unchanged after PTX pretreatment.
Fig. 7. [3H] leucine incorporation in C2C12 myotubes pretreated with G-protein inhibitor, PTX.
Differentiated myotubes were pretreated with the G protein inhibitor, 1 μg/ml PTX, or vehicle for 1 h before treatment with 1 μM 20HE, 100 ng/ml IGF-1, or vehicle. DPM were normalized by total protein. Data represent the mean values ± S.E.M. of three experiments. a indicates p < 0.05 compared with control and b indicates p < 0.05 compared with PTX treatment alone (One way ANOVA/Bonferroni).
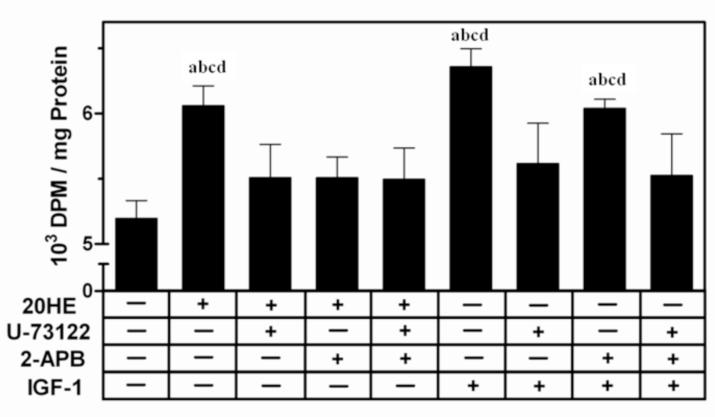
Pretreatment with either 10 μMU-73122, a PLC inhibitor, or 10 μM2-APB, an IP3R inhibitor, for 1 h reduced the protein synthesis response to 20HE, but the effect was not additive when both inhibitors were combined (Fig. 8). Pretreatment with U-73122 significantly reduced the IGF-1 induced increase in protein synthesis while 2-APB caused only a slight decrease. Treatment with only U-73122 and/or 2-APB did not alter protein synthesis (data not shown).
Fig. 8. [3H] leucine incorporation in C2C12 myotubes pretreated with phospholipase C (PLC) or inositol-3-phosphate receptor (IP3R) inhibitors.
Differentiated myotubes were pretreated with either the PLC inhibitor, 10 μM U-73122, and/or the IP3R inhibitor, 10 μM 2-APB, or vehicle for 30 min before treatment with either 1 μM 20HE, 100 ng/ml IGF-1, or vehicle. DPM were normalized by total protein. Data represent the mean values ± S.E.M. of three experiments. a indicates p < 0.05 compared with control, b indicates p < 0.05 compared with U-73122 treatment alone, c indicates p < 0.05 compared with 2-APB treatment alone, and d indicates p < 0.05 compared with U-73122 + 2-APB treatment (One way ANOVA/Bonferroni).
Discussion
20HE produced rapid responses in C2C12 myotubes, including increasing Ca2+ flux in seconds (Fig. 1A) and phosphorylating Akt within 2 h (Fig. 3 A and B). The G-protein inhibitor, PTX completely abolished the 20HE-induced increase in both Ca2+ (Fig. 1A) and protein synthesis (Fig. 8), and significantly reduced Akt activation (Fig. 5), suggesting a G-protein-dependant pathway is involved in both the rapid and long term response. Increased intracellular Ca2+, which is involved in GPCR signaling may come from either extracellular Ca2+ or internal stores [15]. The extracellular Ca2+ chelator EGTA only partially inhibited the 20HE effect (Fig. 6), suggesting that both extracellular and internal stores of Ca2+ may be involved.
Steroids can rapidly alter Ca2+ levels by affecting both influx and intracellular stores. This effect is steroid- and cell-type specific. In Serotoli cells, testosterone induced Ca2+ intake was due to PLC-mediated closure of K+−ATP channels [16]. In T cells, while estradiol produced rapid Ca2+ flux from both extracellular and intracellular pools [17], testosterone induced only extracellular Ca2+ flux [18]. Testosterone enhanced Ca2+ transport by opening T-type Ca2+ channels in rabbit kidney [19]. Chronic testosterone treatment increased the current size and single channel activity of L-type Ca2+ channels in rat cardiomyocytes [20]. However, acute testosterone decreased activity of T-type Ca2+ channels [21]. [22] described androgen-induced Ca2+ oscillations in neurite cells which were blocked by the G protein inhibitor, PTX. This oscillation also required both intra- and extracellular Ca2+. In human granulosa luteinizing cells, androstenedione increased Ca2+ through voltage-dependent Ca2+ channels in the plasma membrane and PLC activation via a PTX-sensitive G protein [23].
An important pathway for release of internal Ca2+ in skeletal muscle is the PLC-IP3R pathway [24]. The membrane-bound enzyme, PLC, breaks down phosphoinisotol 3 phosphate (PIP3), producing inositol 3-phosphate (IP3) which travels to the sarcoplasmic reticulim (SR) and binds to the IP3 receptor (IP3R). When activated by IP3, the IP3R releases Ca2+ from the SR into the cytoplasm. Our data show that the inhibition of PLC with U-73122 or the IP3R using 2-APB shortened the duration of the Ca2+ increase following 20HE treatment but did not completely abolish it (Fig. 1 and data not shown). This suggests that, although the PLC pathway may be involved, it is not the only source of intracellular Ca2+. GPCR activated Ca2+ channels, which have been described in LNCaP prostate cancer cells [25] may also be activated by 20HE, allowing extracellular Ca2+ to flow into the cell.
20HE activated Akt in C2C12 skeletal myotubes in a dose- and time-dependant manner. Although ecdysteroid-induced Akt phosphorylation has been described previously in pro-B lymphocyte cells [26] and RKO cells [27], this is the first example in skeletal muscle. Previous studies involved long treatment periods of 48 h, suggesting a non-specific response. Our study shows a more rapid response, with significant activation after 2 h. However, this is still a much slower response then that produced by other activators like IGF-1, which phosphorylate Akt within 10 min.
Ca2+ has been shown to be an important regulator of Akt activation [28]. Inhibiting PLC or chelating intracellular Ca2+ abolished the EGF-induced activation of Akt in mammary epithelial cells. The present study provides further support for a role of Ca2+ in Akt activation by demonstrating that removal of free Ca2+ reduces 20HE-induced Akt phosphorylation (Fig. 5). Removal of either extracellular Ca2+ using EGTA or intracellular Ca2+ with BAPTA-AM also reduced the 20HE effect on protein synthesis (Fig. 6). This suggests Ca2+ flux is a necessary signal in the 20HE-induced response leading to increased protein synthesis. However, although the induced Ca2+ flux represents a rapid response, the increase in protein synthesis is not observed until after 2 h [4]. While many Akt activators such as IGF-1 phosphorylate Akt within minutes, 20HE-induced activation of Akt was a much slower response with a minor statistically insignificant increase after 45 min and a significant increase after 2 h. Therefore, the delayed Akt response may explain the delay in protein synthesis. We previously showed that treatment with the PI3K inhibitor, LY, completely abolished the 20HE-induced increase in protein synthesis [4]. In this study, we have shown 20HE treatment activates Akt and that blocking Akt activation also blocks increased protein synthesis. These data further support the key role of Akt in the 20HE-induced increase in protein synthesis in skeletal muscle.
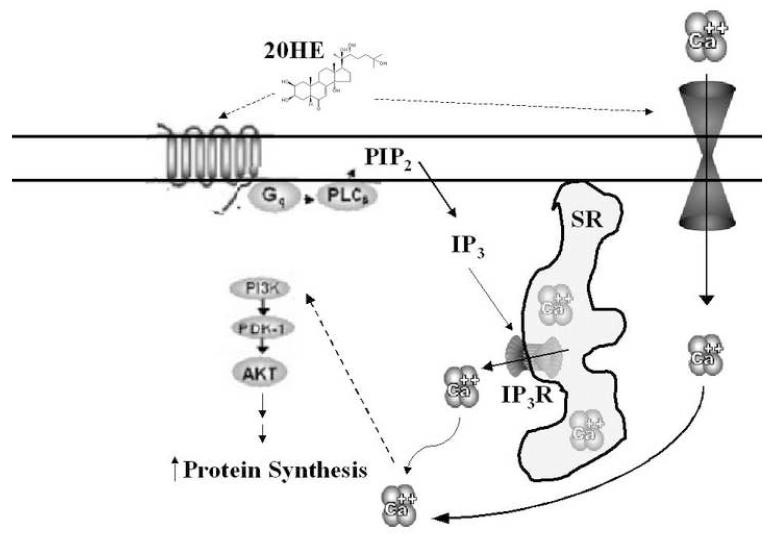
Although the mode of action of 20HE in skeletal muscle is still largely a mystery, it is tempting to hypothesize a 20HE activated membrane bound GPCR, similar to the one found in insects. High affinity membrane binding sites have already been described in rat erythrocytes using 20HE bound to magnetic nanoparticles [29]. Although there is as of yet no direct evidence of its existence, a putative 20HE GPCR may activate the PLC-IP3 pathway as well as open Ca2+ channels, leading to G-αi protein-dependant activation of PI3K/Akt and increased protein synthesis (Fig. 9).
Fig. 9. Schematic representation of the proposed mode of action of 20HE in skeletal muscle.
The proposed pathway of 20HE includes activation of a putative membrane bound GPCR. This leads to PLC activation producing IP3. IP3 activates IP3R in the SR releasing intracellular stores of Ca2+ into the cytoplasm. 20HE may also elicit the opening of extracellular Ca2+ channels. The total Ca2+ flux leads to phosphorylation of Akt which leads to increase protein synthesis. Hypothetical signaling is described with dashed arrows.
Acknowledgments
Research supported by Fogarty International Center of the NIH under U01 TW006674 for the International Cooperative Biodiversity Groups; NIH Center for Dietary Supplements Research on Botanicals and Metabolic Syndrome, grant # 1-P50 AT002776-01; Rutgers University, and Phytomedics Inc. (Jamesburg NJ, USA);
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lafont R, Dinan L. Effects and applications of arthropod steroid hormones (ecdysteroids) in mammals. J Endocrinol. 2006;191:1–8. doi: 10.1677/joe.1.06900. [DOI] [PubMed] [Google Scholar]
- [2].Toth N, Szabo A, Kacsala P, Heger J, Zador E. 20-Hydroxyecdysone increases fiber size in a muscle specific fashion in rat. Phytomedicine. 2008;15:691–698. doi: 10.1016/j.phymed.2008.04.015. [DOI] [PubMed] [Google Scholar]
- [3].Syrov VN, Kurmukov AG. Anabolic activity of phytoecdysone-ecdysterone isolated from Rhaponticum carthamoides (Willd.) Iljin. Farmakol Toksikol. 1976;39:690–693. [PubMed] [Google Scholar]
- [4].Gorelick-Feldman J, Maclean D, Ilic N, Poulev A, Lila MA, Cheng D, et al. Phytoecdysteroids increase protein synthesis in skeletal muscle cells. J Agric Food Chem. 2008;56:3532–3537. doi: 10.1021/jf073059z. [DOI] [PubMed] [Google Scholar]
- [5].Losel RM, Falkenstein E, Feuring M, Schultz A, Tillmann HC, Rossol-Haseroth K, et al. Nongenomic steroid action: controversies, questions, and answers. Physiol Rev. 2003;83:965–1016. doi: 10.1152/physrev.00003.2003. [DOI] [PubMed] [Google Scholar]
- [6].Vicencio JM, Ibarra C, Estrada M, Chiong M, Soto D, Parra V, et al. Testosterone induces an intracellular calcium increase by a nongenomic mechanism in cultured rat cardiac myocytes. Endocrinology. 2006;147:1386–1395. doi: 10.1210/en.2005-1139. [DOI] [PubMed] [Google Scholar]
- [7].Estrada M, Espinosa A, Muller M, Jaimovich E. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology. 2003;144:3586–3597. doi: 10.1210/en.2002-0164. [DOI] [PubMed] [Google Scholar]
- [8].Rahman F, Christian HC. Non-classical actions of testosterone: an update. Trends Endocrinol Metab. 2007;18:371–378. doi: 10.1016/j.tem.2007.09.004. [DOI] [PubMed] [Google Scholar]
- [9].Schlattner U, Vafopoulou X, Steel CG, Hormann RE, Lezzi M. Non-genomic ecdysone effects and the invertebrate nuclear steroid hormone receptor EcR--new role for an “old” receptor? Mol Cell Endocrinol. 2006;247:64–72. doi: 10.1016/j.mce.2005.12.051. [DOI] [PubMed] [Google Scholar]
- [10].Iga M, Iwami M, Sakurai S. Nongenomic action of an insect steroid hormone in steroid-induced programmed cell death. Mol Cell Endocrinol. 2007;263:18–28. doi: 10.1016/j.mce.2006.08.005. [DOI] [PubMed] [Google Scholar]
- [11].Elmogy M, Iwami M, Sakurai S. Presence of membrane ecdysone receptor in the anterior silk gland of the silkworm Bombyx mori. Eur J Biochem. 2004;271:3171–3179. doi: 10.1111/j.1432-1033.2004.04249.x. [DOI] [PubMed] [Google Scholar]
- [12].Srivastava DP, Yu EJ, Kennedy K, Chatwin H, Reale V, Hamon M, et al. Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. J Neurosci. 2005;25:6145–6155. doi: 10.1523/JNEUROSCI.1005-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gee KR, Brown KA, Chen WN, Bishop-Stewart J, Gray D, Johnson I. Chemical and physiological characterization of fluo-4 Ca(2+)-indicator dyes. Cell Calcium. 2000;27:97–106. doi: 10.1054/ceca.1999.0095. [DOI] [PubMed] [Google Scholar]
- [14].Ushio-Fukai M, Alexander RW, Akers M, Yin Q, Fujio Y, Walsh K, et al. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J Biol Chem. 1999;274:22699–22704. doi: 10.1074/jbc.274.32.22699. [DOI] [PubMed] [Google Scholar]
- [15].Berridge MJ, Bootman MD, Lipp P. Calcium--a life and death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- [16].Loss ES, Jacobsen M, Costa ZS, Jacobus AP, Borelli F, Wassermann GF. Testosterone modulates K(+)ATP channels in Sertoli cell membrane via the PLC-PIP2 pathway. Horm Metab Res. 2004;36:519–525. doi: 10.1055/s-2004-825753. [DOI] [PubMed] [Google Scholar]
- [17].Benten WP, Lieberherr M, Giese G, Wunderlich F. Estradiol binding to cell surface raises cytosolic free calcium in T cells. FEBS Lett. 1998;422:349–353. doi: 10.1016/s0014-5793(98)00039-8. [DOI] [PubMed] [Google Scholar]
- [18].Benten WP, Lieberherr M, Sekeris CE, Wunderlich F. Testosterone induces Ca2+ influx via non-genomic surface receptors in activated T cells. FEBS Lett. 1997;407:211–214. doi: 10.1016/s0014-5793(97)00346-3. [DOI] [PubMed] [Google Scholar]
- [19].Couchourel D, Leclerc M, Filep J, Brunette MG. Testosterone enhances calcium reabsorption by the kidney. Mol Cell Endocrinol. 2004;222:71–81. doi: 10.1016/j.mce.2004.05.001. [DOI] [PubMed] [Google Scholar]
- [20].Er F, Michels G, Brandt MC, Khan I, Haase H, Eicks M, et al. Impact of testosterone on cardiac L-type calcium channels and Ca2+ sparks: acute actions antagonize chronic effects. Cell Calcium. 2007;41:467–477. doi: 10.1016/j.ceca.2006.09.003. [DOI] [PubMed] [Google Scholar]
- [21].Michels G, Er F, Eicks M, Herzig S, Hoppe UC. Long-term and immediate effect of testosterone on single T-type calcium channel in neonatal rat cardiomyocytes. Endocrinology. 2006;147:5160–5169. doi: 10.1210/en.2006-0186. [DOI] [PubMed] [Google Scholar]
- [22].Estrada M, Uhlen P, Ehrlich BE. Ca2+ oscillations induced by testosterone enhance neurite outgrowth. J Cell Sci. 2006;119:733–743. doi: 10.1242/jcs.02775. [DOI] [PubMed] [Google Scholar]
- [23].Machelon V, Nome F, Tesarik J. Nongenomic effects of androstenedione on human granulosa luteinizing cells. J Clin Endocrinol Metab. 1998;83:263–269. doi: 10.1210/jcem.83.1.4523. [DOI] [PubMed] [Google Scholar]
- [24].Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol. 2007;213:589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- [25].Sun YH, Gao X, Tang YJ, Xu CL, Wang LH. Androgens induce increases in intracellular calcium via a G protein-coupled receptor in LNCaP prostate cancer cells. J Androl. 2006;27:671–678. doi: 10.2164/jandrol.106.000554. [DOI] [PubMed] [Google Scholar]
- [26].Constantino S, Santos R, Gisselbrecht S, Gouilleux F. The ecdysone inducible gene expression system: unexpected effects of muristerone A and ponasterone A on cytokine signaling in mammalian cells. Eur Cytokine Netw. 2001;12:365–367. [PubMed] [Google Scholar]
- [27].Oehme I, Bosser S, Zornig M. Agonists of an ecdysone-inducible mammalian expression system inhibit Fas Ligand- and TRAIL-induced apoptosis in the human colon carcinoma cell line RKO. Cell Death Differ. 2006;13:189–201. doi: 10.1038/sj.cdd.4401730. [DOI] [PubMed] [Google Scholar]
- [28].Deb TB, Coticchia CM, Dickson RB. Calmodulin-mediated activation of Akt regulates survival of c-Myc-overexpressing mouse mammary carcinoma cells. J Biol Chem. 2004;279:38903–38911. doi: 10.1074/jbc.M405314200. [DOI] [PubMed] [Google Scholar]
- [29].Mykhaylyk OM, Kotsuruba AV, Buchanevich OM, Korduban AM, Mengel EF, Gulaya NM. Signal transduction of erythrocytes after specific binging of ecdysterone and cholesterol immobilized on nanodispersed magnetite. J Magn Magn Mater. 2001;225:226–234. [Google Scholar]