Abstract
CD40 stimulation has produced impressive results in early-stage clinical trials of patients with cancer. Further progress will be facilitated by a better understanding of how the CD40 receptor becomes activated and the subsequent functions of CD40-stimulated immune cells. This review focuses on two aspects of this subject. The first is the recent recognition that signaling by CD40 is initiated when the receptors are induced to cluster within the membrane of responding cells. This requirement for CD40 clustering explains the stimulatory effects of certain anti-CD40 antibodies and the activity of many-trimer, but not one-trimer, forms of CD40 ligand (CD40L, CD154). The second topic is the use of these CD40 activators to expand B cells (“CD40-B cells”). As antigen-presenting cells (APCs), CD40-B cells are as effective as dendritic cells, with the important difference that CD40-B cells can be induced to proliferate in vitro whereas DCs proliferate poorly if at all. As a result, the use of CD40-B cells as antigen-presenting cells (APCs) promises to streamline the generation of anti-tumor CD8+ T cells for the adoptive cell therapy (ACT) of cancer.
Keywords: CD40L, CD154, TNFSF5, CD40, B cell, T cell, dendritic cell, receptor, protein engineering, multimer, Acrp30, adiponectin, surfactant protein D, antibody therapy, cancer, FcgammaR
CD40 is a receptor in the TNF Receptor SuperFamily (TNFRSF) that is especially involved in activating immune cells. CD40 was initially discovered as a activating protein on the surface of B cells (1). Subsequent studies elucidated the presence of the ligand for CD40 (CD40L) on the surface of activated CD4+ helper T cells (2-4). Soon thereafter, an inherited lack CD40L was found to be responsible for the X-linked Hyper-IgM Syndrome (H-XIM), a severe form of both cellular and antibody immunodeficiency in humans (5, 6). The CD40L/CD40 system was subsequently shown to activate dendritic cells (DCs) and thereby “license” them to stimulate CD8+ T cell activation and proliferation (7-11). It was soon recognized that the CD40L/CD40 system is essential for prophylactic vaccines against cancer in mice (12). This and other studies (13-16) stimulated interest in testing CD40 agonists as an immunotherapy for cancer. Both recombinant soluble CD40L (17) and an agonistic anti-CD40 antibody (18, 19) were found to have significant anti-tumor effects in early stage clinical trials. Already by 2007, a National Cancer Institute Immunotherapy Agent Workshop gave the CD40L/CD40 system its highest priority as a co-stimulatory molecule and overall ranked it fourth (behind IL-15, anti-PD-1/anti-PD-L1, and IL-12) as a molecule with “high potential” for treating cancer (20).
CD40 receptor clustering is needed for cell signaling
Although the importance of CD40 was quickly recognized, it was less clear how it interacted with its ligand to convey a stimulus to responding cells. Crystallographic and molecular modeling studies produced a model in which the CD40L homotrimer fits into the three chains of the CD40 receptor (21, 22). However, the intracytoplasmic domain of CD40 does not interact with kinases or G proteins which raised questions about how ligand binding leads to downstream signaling. This problem was partially solved with the identification of adapter molecules called TNF Receptor Activation Factors or TRAFs (23-25). The cytoplasmic tails of CD40 can form a supramolecular signaling complex composed of many TRAFs that in turn leads to the activation of NF-κB and other transcription factors. The next question was how CD40L/CD40 engagement leads to these downstream events.
The current model of CD40 activation is based on the idea that clustering of the receptor is needed to assemble the supramolecular intracytoplasmic signaling complex. Hard evidence for this model comes from studies of Fas, a related TNFRSF receptor. Using cultured cells engineered to express a fusion protein between Fas and yellow fluorescent protein (YFP), Siegel et al. studied the effects of engaging Fas with Fas ligand (FasL, CD95L). Schneider et al had previously shown that the effects of membrane FasL could be replicated by FLAG-tagged trimers of soluble FasL, but only if the trimers were crosslinked by anti-FLAG antibody (26). Using Fas-YFP responder cells, Siegel et al showed that exposure to crosslinked FasL led to the rapid clustering of Fas-YFP into lipid rafts in the membrane. Under the fluorescent microscope, these receptor clusters were visualized as bright spots, reflecting the acronym given to them as Signaling Protein Oligomeric Transduction Structures or SPOTS (27).
While a similar experiment has not yet been conducted for CD40, compatible data has been provided by Spencer et al. These investigators engineered cells in which the full transmembrane CD40 molecule was replaced with an engineered protein lacking the entire CD40L-binding extracellular domain but instead expressing a membrane-anchoring motif, the intracellular domain of CD40 needed for binding TRAFs, and two FKBP-related motifs capable of binding an FK-506-like small molecule. A expression cassette for this construct was then transduced into cultured DCs using an adenoviral vector. Following this, the investigators added AP1903, a small dumbbell-shaped chemical containing two FK-506-like moieties. This chemical resulted in the inducible clustering of the engineered CD40 intracytoplasmic domains and led to full DC activation, obtained entirely without the CD40 extracellular domain or CD40L engagement (28). Combined with a tumor antigen, this system is now being tested in a clinical trial of prostate cancer immunotherapy (NCT00868595). In the context of this review, this work shows that clustering of the CD40 intracytoplasmic signaling domain is sufficient to activate DCs to initiates immune responses. Looking at the cell from the outside, clustering of CD40 receptor in the membrane by a ligand or antibody is the natural process whereby CD40 intracellular domains become clustered and capable of initiating downstream responses.
With this in mind, it is now possible to understand how agonistic anti-CD40 antibodies act to stimulate immune cells. An early clue came from studies of anti-CD40 antibodies as a stimulus for B cell proliferation. Although these antibodies were known to produce partial stimulation of B cells (29), they did not lead to long-term B cell proliferation. Instead, Banchereau et al. found that it was necessary to add three components to the B cell cultures: anti-CD40 antibodies; a fibroblast line engineered to express the Fc receptor (FcR) for the antibody; and IL-4. Using this “B cell system,” cultured B cells could be massively expanded without the use of lectins or other artificial agents (30). Yet it remained unclear why FcR-bearing fibroblasts were needed in this culture system. Evidently, simple immobilization of anti-CD40 antibody on culture plates or beads is unable to convey the same type of stimulus as antibody mounted on the surface of the FcR-bearing cells.
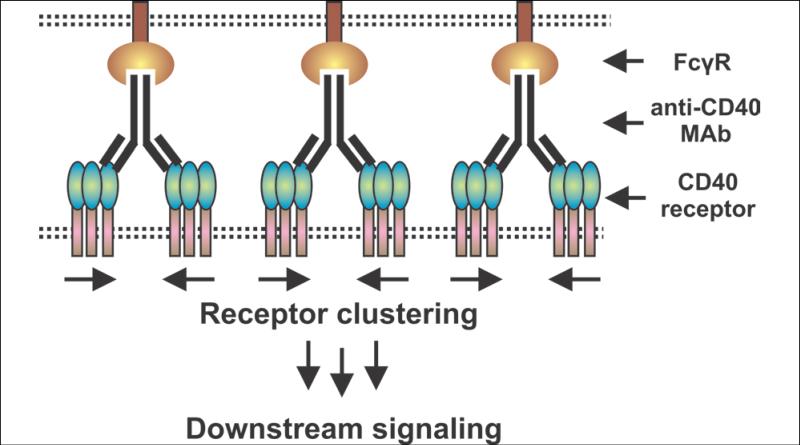
An important step in understanding agonistic anti-CD40 antibodies came in 2011 when the important role of FcRs was recognized. Similar to the Banchereau B cell system, four groups found that agonistic anti-CD40 antibodies only functioned in the presence of cells expressing IgG-binding FcγRs (31-33). Ironically, the best FcγR is FcγRIIB, which is usually thought of as an inhibitory FcγR. Anti-CD40 monoclonal antibodies (MAbs) that bind to FcγRIIB exhibit strong CD40-stimulating activity but only if a cell bearing FcγRIIB is adjacent to the CD40-bearing cell to be stimulated (Figure 1). This indicates an important spatial restriction on the effectiveness of anti-CD40 agonistic antibodies: a CD40-bearing cell that is not adjacent to an FcγR-bearing cell might not be effectively stimulated by these antibodies. Another scenario would be if the CD40-bearing cell itself also expressed FcγRIIB (a so-called cis effect) and it has been proposed that this would operate on B cells that are known to express both CD40 and FcγRIIB (34). However, the earlier results of Banchereau et al. in the B cell system (30) indicate that the FcR must be on an adjacent cell and not on the CD40-bearing B cell that is itself being stimulated by the agonistic anti-CD40 antibody.
Figure 1. Agonistic anti-CD40 MAbs require a nearby FcγR-bearing cell to cluster CD40 and induce a signal.
In the original Banchereau et al. B cell system, FcγR-expressing fibroblasts were needed in order for anti-CD40 antibody to stimulate B cell proliferation (30). Recent studies from three groups have shown that FcγRs, particularly FcγRIIB, are needed for anti-tumor immune effects in clinically relevant models of agonistic anti-CD40 antibodies (31-33) (drawing adapted from (32)). It has been proposed that APCs could express both CD40 and FcγRIIB so that a cis effect could occur (34) (not shown), but this was not reflected in the original Banchereau et al. B cell system in which B cells express both CD40 and FcγRIIB. Consequently, there may be spatial restrictions on where in the body agonistic anti-CD40 antibodies can exert their immune-stimulating effects. This spatial restriction would not be shared by soluble multimeric forms of CD40L which may be more effective for this reason.
A many-trimer, multimeric form of CD40L is needed to stimulate CD40
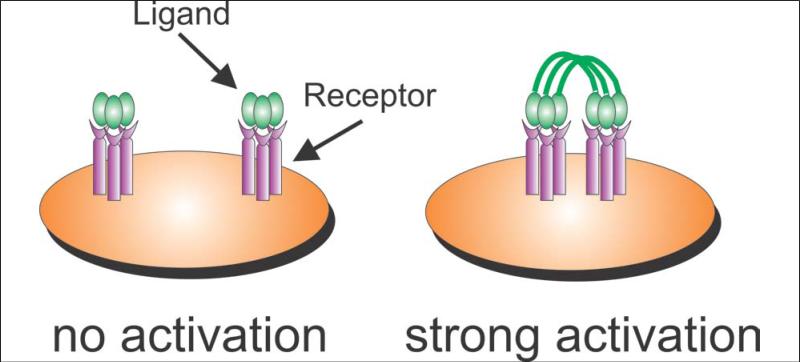
When CD40L is expressed as a membrane molecule on the surface of activated CD4+ T cells, it is effectively present as a many-trimer surface of ligand molecules. Consequently, direct contact of a CD40L-bearing cell (e.g., an activated CD4+ T cell) with a CD40-bearing APC allows CD40 clustering and immune activation. This model suggests that a single trimer of soluble CD40L (produced by proteolytic cleavage of CD40L from the cell surface or by genetic engineering) would be unable to provide full CD40 stimulation. This situation was shown for FasL where many-trimer membrane FasL rapidly induced apoptosis in Fas-bearing cells, yet 1-trimer soluble FasL was totally inactive (26). The formal proof of this effect for CD40L was provided by Haswell et al. These investigators prepared two forms of CD40L. One form was a single trimer composed of the CD40L extracellular domain (ECD). The other form was a 4-trimer protein prepared as a genetic fusion between the body of surfactant protein D (SP-D, a naturally self-assembling multimeric protein) and the CD40L ECD. The 1-trimer form of soluble CD40L was unable to stimulate B cell proliferation even at concentrations of 130 nM. In contrast, the 4-trimer protein was fully stimulatory for B cells at 30 nM (35). The conclusion is that a many-trimer multimeric form of CD40L is needed to provide full CD40 stimulation (see Figure 2).
Figure 2. A many-trimer form of CD40L is needed to induce CD40 clustering and cell activation.
Haswell et al. showed that a single trimer of soluble CD40L is unable to stimulate B cells to proliferate (35). However, molecules engineered to express 2 (36) or 4 (35) CD40L trimers are highly stimulatory for B cells, reflecting their ability to cluster CD40 on responding cells. Note that unlike agonistic anti-CD40 antibody (Figure 1), many-trimer forms of soluble CD40L can stimulate cells without requiring an interaction with a second receptor on an adjacent cell type.
Given the need for a many-trimer form of CD40L to cluster CD40 and fully stimulate cells, it may seem odd that Immunex/Amgen produced a putative 1-trimer form of soluble CD40L (sCD40LT or Avrend®) that was highly effective for stimulating cells. Indeed, a Phase I clinical trial of sCD40LT in cancer patients had impressive effects. In one case, a man with stage IV metastatic laryngeal carcinoma previously treated with surgery, radiation, chemotherapy, and erbitux had a complete response to sCD40LT. Typical of many responses to immunotherapy agents, final resolution of the tumor was delayed by several months, but the patient remained free of disease for the subsequent four years of follow-up (17, 37).
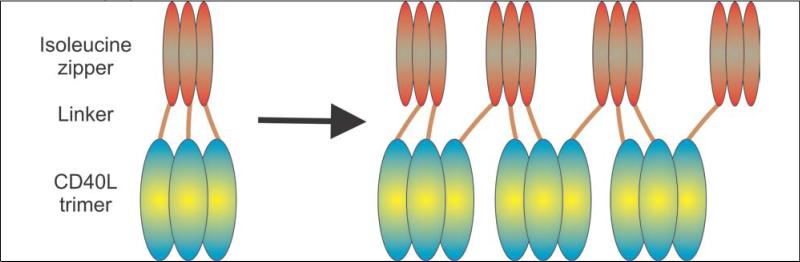
To explain these excellent results, it is necessary to probe a bit deeper into the structure of sCD40LT. This protein was engineered as a fusion between an isoleucine zipper domain that forms trimers and the CD40L ECD (38). This was done because it was assumed that the CD40L ECD does not spontaneously trimerize on its own. However, a subsequent study showed that the CD40L ECD is a naturally trimeric protein (39) and indeed many suppliers sell a 1-trimer CD40L ECD for laboratory use. The problem is that a protein with two trimerizing domains can form aggregates through a process called “domain swapping” (40) (see Figure 3). In this manner, a protein designed to be a 1-trimer form of CD40L can effectively form many-trimer multimers, albeit in an uncontrolled manner. Of note, while sCD40LT has been dropped from further development, aliquots are available from the Biological Resource Branch of the National Cancer Institute (Frederick, MD). The specification sheet for this frozen material produced in 1997 states that 10.4% of the protein is a “high molecular weight component.” This would be consistent with spontaneous aggregation of the protein into a many-trimer form that would be expected to have activity by clustering the CD40 receptor. Indeed, CD40 clustering has been observed with cells treated in vitro with sCD40LT (41).
Figure 3. Proposed domain-swapping mechanism for the formation of many-trimer multimers from sCD40LT.
sCD40LT (Avrend®) was engineered by genetically fusing an isoleucine zipper with the extracellular domain (ECD) of CD40L (with a linker to provide extra spacing in between). Because both ends in sCD40LT can spontaneously form trimers, there is a possibility for crossing of the molecules (“domain swapping”) to produce many-trimer multimers. This spontaneous multimerization would explain the clinical activity of sCD40LT given that 1-trimer forms of CD40L are poorly active in vitro.
Thus far, there have been no clinical studies that directly compare soluble CD40L with agonistic anti-CD40 antibodies in patients with cancer. However, both proteins have been tested in children with X-HIM, the CD40L deficiency disease. In the previous cancer study with sCD40LT, subjects were treated with 0.10 mg/kg/d given subcutaneously for 5 days and 28% developed elevated liver enzymes as a manifestation of liver toxicity. In contrast, a lower dose was used for these boys with X-HIM, 0.03 – 0.05 mg/kg subcutaneously every other day – and none developed signs of liver toxicity. However, there was a marked immune response in these X-HIM patients. The sCD40LT was given s.c. in the left forearm and there was partial restoration of lymph node architecture in the left axilla, but not in the contralateral right axilla which indicates a local and not systemic effect. In addition to the histological changes in lymph nodes, these boys developed the ability to respond to skin test antigens with a delayed-type hypersensitivity response and had restored function of their Th1 CD4+ T cells (42). This suggests that sCD40LT could be a useful and safe adjuvant for vaccines in humans. More recently, the same group of investigators tested an agonistic anti-CD40 antibody (CP-870,893, Pfizer) in X-HIM subjects. In this study, the immunological reconstitution produced by agonistic anti-CD40 antibody was less impressive than that produced by sCD40LT. In addition, the agonistic anti-CD40 antibody did not cause CD40 clustering but rather led to downregulation of CD40 from the surface of B cells in vitro (41). While there are many differences between these two X-HIM studies, the results imply that soluble forms of CD40L may be superior to agonistic anti-CD40 antibodies as a treatment for X-HIM and thus should continue to be developed as a cancer immunotherapy.
Molecular designs for many-trimer forms of soluble CD40L
Three groups foresaw the need to construct many forms of CD40L. The first publication was by Haswell et al. and described a fusion between surfactant protein D (SP-D) and the extracellular domain (ECD) of CD40L to make SP-D-CD40L, a 4-trimer form of CD40L (35). A subsequent publication by Holler et al. described the fusion of Acrp30 (also called adiponectin) and the CD40L ECD to make Acrp30-CD40L, a 2-trimer form of CD40L (36). Both forms of CD40L were studied in vivo by Stone et al. (43). Of note, the earliest patent was filed by Richard Kornbluth and both proteins are now licensed exclusively to Multimeric Biotherapeutics, Inc. which is developing them further (see the Declaration of Interest Statement).
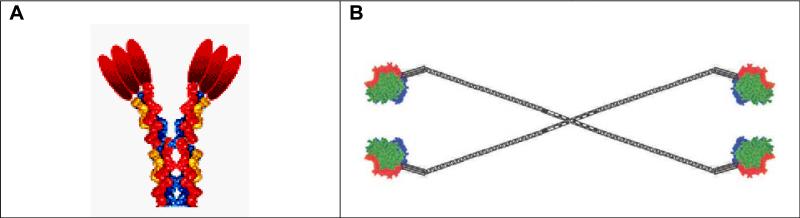
These two forms of multimeric soluble CD40L are shown in Figure 4. The 2-trimer Acrp30-CD40L has been tested extensively in academic labs and is described in 18 papers. For example, 2-trimer Acrp30-CD40L has been shown to activate human DCs (44) and stimulate B cell proliferation in vitro (36). Four-trimer SP-D-CD40L has also been shown to be a potent stimulus for B cell proliferation (35).
Figure 4. Structure of 2-trimer Acrp30-CD40L and 4-trimer SP-D-CD40L.
As described in the text, the extracellular domain (ECD) of CD40L was genetically fused to one of two protein scaffolds, Acrp30 (Panel A) or surfactant protein D (SP-D) (Panel B). Following expression of the protein in CHO cells, 2-trimer or 4-trimer proteins are produced. The 2-trimer Acrp30-CD40L protein is also called MegaCD40L™ or CD40L hexamer, whereas the 4-trimer SP-D-CD40L protein is also called UltraCD40L™.
Use of CD40L to grow B cells for cancer immunotherapy
Banchereau et al. were the first to show that human B cells could be grown using a system of anti-CD40 antibodies, fibroblasts engineered to express FcγR, and IL-4 (30). However, an important step forward came when Schultze et al. found that fibroblasts expressing CD40L could be used with IL-4 to generate large numbers of human B cells. They further showed that these “CD40-B cells” could be used as APCs to generate anti-tumor CD8+ T cells (45). As APCs, CD40-B cells are as strong as DCs for eliciting CD8+ T cell responses (46-49). Whereas DCs cannot be grown in vitro, CD40-B cells can be expanded to large numbers starting from less than 5 ml of blood, which makes them suitable for even pediatric immunotherapy studies (50). These CD40-B cells have been successfully used for generating anti-tumor CD8+ T cells (51-53).
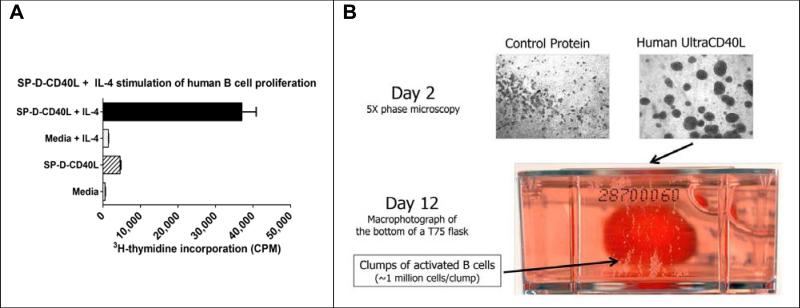
The standard system for generating CD40-B cells uses CD40L-expressing fibroblasts that have been irradiated to prevent them from overgrowing the cultures. This leads to apoptosis of the fibroblasts and a need to remove cell debris from the cultures. The use of multimeric soluble CD40L circumvents this problem by substituting a cell-free protein for CD40L-expressing fibroblasts (see Figure 5).
Figure 5. Proliferation of B cell APCs using multimeric soluble CD40L.
Panel A: B cells were purified from PBMCs using anti-CD19 immunomagnetic beads (Miltenyi). The cells were cultured in 96-well plates at 2 × 105 cells/ml in RPMI 1640, 10% FBS, and 10 ng/ml IL-4. Four days later, the cells were pulsed with 3H-Tdr and proliferation measured as thymidine incorporation. Acrp30-CD40L also induced B cell proliferation (not shown). Redrawn from Stone et al (Kornbluth group) (54).
Panel B: Visualization of growing CD40-B cells. By day 2 of culture in SP-D-CD40L-containing media, B cells expressed adhesion molecules and formed multicellular aggregates visible under 5X magnification. By day 12, these aggregates could be seen with the unaided eye. Here, a 75 cm2 flask was cultured vertically and the photograph was taken looking upward. (Photographs courtesy of Dr. John Mathison, The Scripps Research Institute, La Jolla, CA).
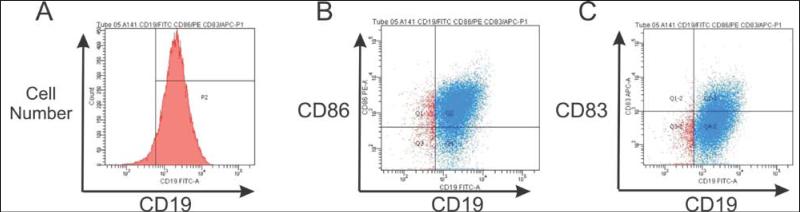
More recently, CD40-B cells grown with Acrp30-CD40L have been used to generate CD25+FoxP3+CD4+ regulatory T cells (55). Also, a recent report suggests that CD40-B cells can prime CD8+ T cells but do not generate memory CD8+ T cell responses (56). One factor that may be involved in these reports is the heterogeneity of the CD40-B cell population. For example, in CD40-B cells grown using SP-D-CD40L, only a minority of CD19+ CD40-B cells also express CD83, a maturation marker induced by CD40L on B cells (57) (see Figure 6). Indeed, recent studies have shown that some B cells can strongly inhibit the immune response (58-61). Under some circumstances, such regulatory B cells or “Bregs” (62) can suppress T cell responses to tumors in vivo (63-65). This suggests that further purification of CD40-B cells may be needed to select for those APCs most useful for generating CD8+ T cells for cancer immunotherapy.
Figure 6. Heterogeneity of CD40-B cells.
Panel A: Cultures like that shown in Figure 5 contain about 94-97% of cells that are positive for the CD19 B cell marker. Panel B: The majority of CD19+ cells are also CD86+ (75% in the example shown). Panel C: Some CD19+ B cells are also positive for the dendritic cell and longevity marker, CD83 (about 5.5% in the example shown).
Lastly, CD40-B cells express a pattern of adhesion molecules and chemokine receptors necessary for homing to secondary lymphoid organs (66, 67). This has led to the strategy of preparing CD40-B cells, electroporating them with tumor mRNAs to load them with antigens, and then injecting the antigen-charged APCs back into the host whereupon they migrate to lymph nodes and stimulate an anti-tumor immune response. Studies of this system in dogs with non-Hodgkin's lymphoma have shown promising results (68). This strategy resembles that of Sipuleucel-T (Provenge®, Dendeon) (69) and the DC vaccines currently being tested for cancer immunotherapy, except that only a small sample of venous blood is needed to prepare the CD40-B cell treatment vaccine.
Conclusions
CD40L and the CD40 receptor are exciting molecules that can be applied to induce powerful anti-tumor immune responses. A key to using CD40L protein for cancer immunotherapy is the recognition that a many-trimer soluble form is needed to fully stimulate CD40 on DCs and B cells. Likewise, agonistic anti-CD40 IgG antibodies only function if the antibodies are mounted on FcγR present on an adjacent cell, which allows movement of the IgG/FcγR complexes to cluster together in space and thereby cluster CD40 on the responding cell. A further use of CD40L is a method for growing B cells in vitro where the resulting CD40-B cells are excellent APCs for generating anti-tumor CD8+ T cells for adoptive cell therapy. Further studies of CD40-B cells are needed to define the best way to prepare them and a way to eliminate those cells capable of inhibiting an immune response. Taken together, knowledge of the CD40L/CD40 system and the initial clinical efficacy of soluble CD40L and agonistic anti-CD40 antibodies provide the basis for claiming that this molecular pathway will ultimately enable the development of an effective immunotherapy for cancer.
Acknowledgments
This work was supported by Public Health Service grants DA029435, AI073240, and AI63982 (for RSK), and AI063982 and the University of Miami Developmental Center for AIDS Research (AI073961) (for GWS). Assistance with flow cytometry and plasmid preparations was provided by the Flow Cytometry Core and Molecular Biology Core at the UC San Diego Center for AIDS Research (AI36214), the VA San Diego Health Care System, and the San Diego Veterans Medical Research Foundation.
Footnotes
Declaration of Interest Statement
Richard S. Kornbluth and Geoffrey W. Stone are listed as the inventors on patent applications related to the preparation and use of multimeric forms of soluble CD40L. Richard Kornbluth and Mariusz Stempniak are employees of Multimeric Biotherapeutics, Inc., La Jolla, CA, which is a company formed to develop these new forms of CD40L, including Acrp30-CD40L (MegaCD40L™) and SP-D-CD40L (UltraCD40L™).
References
- 1.Clark EA, Ledbetter JA. Activation of human B cells mediated through two distinct cell surface differentiation antigens, Bp35 and Bp50. Proc Natl Acad Sci U S A. 1986;83:4494–8. doi: 10.1073/pnas.83.12.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lederman S, Yellin MJ, Inghirami G, Lee JJ, Knowles DM, Chess L. Molecular interactions mediating T-B lymphocyte collaboration in human lymphoid follicles. Roles of T cell-B-cell-activating molecule (5c8 antigen) and CD40 in contact-dependent help. J Immunol. 1992;149:3817–26. [PubMed] [Google Scholar]
- 3.Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci U S A. 1992;89:6550–4. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lane P, Traunecker A, Hubele S, Inui S, Lanzavecchia A, Gray D. Activated human T cells express a ligand for the human B cell-associated antigen CD40 which participates in T cell-dependent activation of B lymphocytes. Eur J Immunol. 1992;22:2573–8. doi: 10.1002/eji.1830221016. [DOI] [PubMed] [Google Scholar]
- 5.Allen RC, Armitage RJ, Conley ME, Rosenblatt H, Jenkins NA, Copeland NG, Bedell MA, Edelhoff S, Disteche CM, Simoneaux DK, et al. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science. 1993;259:990–3. doi: 10.1126/science.7679801. [see comments] [DOI] [PubMed] [Google Scholar]
- 6.Aruffo A, Farrington M, Hollenbaugh D, Li X, Milatovich A, Nonoyama S, Bajorath J, Grosmaire LS, Stenkamp R, Neubauer M, et al. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993;72:291–300. doi: 10.1016/0092-8674(93)90668-g. [DOI] [PubMed] [Google Scholar]
- 7.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–8. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 8.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–80. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 9.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–3. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 10.Lanzavecchia A. Immunology. Licence to kill. Nature. 1998;393:413–4. doi: 10.1038/30845. [DOI] [PubMed] [Google Scholar]
- 11.Mackey MF, Barth RJ, Jr., Noelle RJ. The role of CD40/CD154 interactions in the priming, differentiation, and effector function of helper and cytotoxic T cells. J Leukoc Biol. 1998;63:418–28. doi: 10.1002/jlb.63.4.418. [DOI] [PubMed] [Google Scholar]
- 12.Mackey MF, Gunn JR, Ting PP, Kikutani H, Dranoff G, Noelle RJ, Barth RJ., Jr. Protective immunity induced by tumor vaccines requires interaction between CD40 and its ligand, CD154. Cancer Res. 1997;57:2569–74. [PubMed] [Google Scholar]
- 13.Grossmann ME, Brown MP, Brenner MK. Antitumor responses induced by transgenic expression of CD40 ligand. Hum Gene Ther. 1997;8:1935–43. doi: 10.1089/hum.1997.8.16-1935. [DOI] [PubMed] [Google Scholar]
- 14.Nakajima A, Kodama T, Morimoto S, Azuma M, Takeda K, Oshima H, Yoshino S, Yagita H, Okumura K. Antitumor effect of CD40 ligand: elicitation of local and systemic antitumor responses by IL-12 and B7. J Immunol. 1998;161:1901–7. [PubMed] [Google Scholar]
- 15.van Mierlo GJ, den Boer AT, Medema JP, van der Voort EI, Fransen MF, Offringa R, Melief CJ, Toes RE. CD40 stimulation leads to effective therapy of CD40(−) tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc Natl Acad Sci U S A. 2002;99:5561–6. doi: 10.1073/pnas.082107699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stumbles PA, Himbeck R, Frelinger JA, Collins EJ, Lake RA, Robinson BW. Cutting edge: tumor-specific CTL are constitutively cross-armed in draining lymph nodes and transiently disseminate to mediate tumor regression following systemic CD40 activation. J Immunol. 2004;173:5923–8. doi: 10.4049/jimmunol.173.10.5923. [DOI] [PubMed] [Google Scholar]
- 17.Vonderheide RH, Dutcher JP, Anderson JE, Eckhardt SG, Stephans KF, Razvillas B, Garl S, Butine MD, Perry VP, Armitage RJ, Ghalie R, Caron DA, Gribben JG. Phase I study of recombinant human CD40 ligand in cancer patients. J Clin Oncol. 2001;19:3280–7. doi: 10.1200/JCO.2001.19.13.3280. [DOI] [PubMed] [Google Scholar]
- 18.Khalil M, Vonderheide RH. Anti-CD40 agonist antibodies: preclinical and clinical experience. Update Cancer Ther. 2007;2:61–5. doi: 10.1016/j.uct.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, Sullivan P, Mahany JJ, Gallagher M, Kramer A, Green SJ, O'Dwyer PJ, Running KL, Huhn RD, Antonia SJ. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25:876–83. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 20.NCI National Cancer Institute Immunotherapy Agent Workshop. 2007 2007 Jul 12; [Google Scholar]
- 21.Bajorath J, Marken JS, Chalupny NJ, Spoon TL, Siadak AW, Gordon M, Noelle RJ, Hollenbaugh D, Aruffo A. Analysis of gp39/CD40 interactions using molecular models and site-directed mutagenesis. Biochemistry. 1995;34:9884–92. doi: 10.1021/bi00031a009. [DOI] [PubMed] [Google Scholar]
- 22.Bajorath J. Detailed comparison of two molecular models of the human CD40 ligand with an x-ray structure and critical assessment of model-based mutagenesis and residue mapping studies. J Biol Chem. 1998;273:24603–9. doi: 10.1074/jbc.273.38.24603. [DOI] [PubMed] [Google Scholar]
- 23.Cheng G, Cleary AM, Ye ZS, Hong DI, Lederman S, Baltimore D. Involvement of CRAF1, a relative of TRAF, in CD40 signaling. Science. 1995;267:1494–8. doi: 10.1126/science.7533327. [DOI] [PubMed] [Google Scholar]
- 24.Kehry MR. CD40-mediated signaling in B cells. Balancing cell survival, growth, and death. J Immunol. 1996;156:2345–8. [PubMed] [Google Scholar]
- 25.Bishop GA, Moore CR, Xie P, Stunz LL, Kraus ZJ. TRAF proteins in CD40 signaling. Adv Exp Med Biol. 2007;597:131–51. doi: 10.1007/978-0-387-70630-6_11. [DOI] [PubMed] [Google Scholar]
- 26.Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A, Tschopp J. Conversion of membrane-bound Fas (CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med. 1998;187:1205–13. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegel RM, Muppidi JR, Sarker M, Lobito A, Jen M, Martin D, Straus SE, Lenardo MJ. SPOTS: signaling protein oligomeric transduction structures are early mediators of death receptor-induced apoptosis at the plasma membrane. J Cell Biol. 2004;167:735–44. doi: 10.1083/jcb.200406101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanks BA, Jiang J, Singh RA, Song W, Barry M, Huls MH, Slawin KM, Spencer DM. Re-engineered CD40 receptor enables potent pharmacological activation of dendritic-cell cancer vaccines in vivo. Nat Med. 2005;11:130–7. doi: 10.1038/nm1183. [DOI] [PubMed] [Google Scholar]
- 29.Heath AW, Chang R, Harada N, Santos-Argumedo L, Gordon J, Hannum C, Campbell D, Shanafelt AB, Clark EA, Torres R, et al. Antibodies to murine CD40 stimulate normal B lymphocytes but inhibit proliferation of B lymphoma cells. Cell Immunol. 1993;152:468–80. doi: 10.1006/cimm.1993.1305. [DOI] [PubMed] [Google Scholar]
- 30.Banchereau J, de Paoli P, Valle A, Garcia E, Rousset F. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science. 1991;251:70–2. doi: 10.1126/science.1702555. [DOI] [PubMed] [Google Scholar]
- 31.White AL, Chan HT, Roghanian A, French RR, Mockridge CI, Tutt AL, Dixon SV, Ajona D, Verbeek JS, Al-Shamkhani A, Cragg MS, Beers SA, Glennie MJ. Interaction with FcgammaRIIB is critical for the agonistic activity of anti-CD40 monoclonal antibody. Journal of immunology. 2011;187:1754–63. doi: 10.4049/jimmunol.1101135. [DOI] [PubMed] [Google Scholar]
- 32.Wilson NS, Yang B, Yang A, Loeser S, Marsters S, Lawrence D, Li Y, Pitti R, Totpal K, Yee S, Ross S, Vernes JM, Lu Y, Adams C, Offringa R, Kelley B, Hymowitz S, Daniel D, Meng G, Ashkenazi A. An Fcgamma receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer Cell. 2011;19:101–13. doi: 10.1016/j.ccr.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Li F, Ravetch JV. Inhibitory Fcgamma receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 2011;333:1030–4. doi: 10.1126/science.1206954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smyth MJ, Kershaw MH. Immunology. The adjuvant effects of antibodies. Science. 2011;333:944–5. doi: 10.1126/science.1210801. [DOI] [PubMed] [Google Scholar]
- 35.Haswell LE, Glennie MJ, Al-Shamkhani A. Analysis of the oligomeric requirement for signaling by CD40 using soluble multimeric forms of its ligand, CD154. Eur J Immunol. 2001;31:3094–100. doi: 10.1002/1521-4141(2001010)31:10<3094::aid-immu3094>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 36.Holler N, Tardivel A, Kovacsovics-Bankowski M, Hertig S, Gaide O, Martinon F, Tinel A, Deperthes D, Calderara S, Schulthess T, Engel J, Schneider P, Tschopp J. Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex. Mol Cell Biol. 2003;23:1428–40. doi: 10.1128/MCB.23.4.1428-1440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vonderheide RH. Prospect of targeting the CD40 pathway for cancer therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:1083–8. doi: 10.1158/1078-0432.CCR-06-1893. [DOI] [PubMed] [Google Scholar]
- 38.Morris AE, Remmele RL, Jr., Klinke R, Macduff BM, Fanslow WC, Armitage RJ. Incorporation of an isoleucine zipper motif enhances the biological activity of soluble CD40L (CD154). J Biol Chem. 1999;274:418–23. doi: 10.1074/jbc.274.1.418. [DOI] [PubMed] [Google Scholar]
- 39.Matsuura JE, Morris AE, Ketchem RR, Braswell EH, Klinke R, Gombotz WR, Remmele RL., Jr. Biophysical characterization of a soluble CD40 ligand (CD154) coiled-coil trimer: evidence of a reversible acid-denatured molten globule. Arch Biochem Biophys. 2001;392:208–18. doi: 10.1006/abbi.2001.2454. [DOI] [PubMed] [Google Scholar]
- 40.Guo Z, Eisenberg D. Runaway domain swapping in amyloid-like fibrils of T7 endonuclease I. Proc Natl Acad Sci U S A. 2006;103:8042–7. doi: 10.1073/pnas.0602607103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan X, Upadhyaya B, Wu L, Koh C, Santin-Duran M, Pittaluga S, Uzel G, Kleiner D, Williams E, Ma CA, Bodansky A, Oliveira JB, Edmonds P, Hornung R, Wong DW, Fayer R, Fleisher T, Heller T, Prussin C, Jain A. CD40 agonist antibody mediated improvement of chronic Cryptosporidium infection in patients with X-linked hyper IgM syndrome. Clin Immunol. 2012;143:152–61. doi: 10.1016/j.clim.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jain A, Kovacs JA, Nelson DL, Migueles SA, Pittaluga S, Fanslow W, Fan X, Wong DW, Massey J, Hornung R, Brown MR, Spinner JJ, Liu S, Davey V, Hill HA, Ochs H, Fleisher TA. Partial immune reconstitution of X-linked hyper IgM syndrome with recombinant CD40 ligand. Blood. 2011;118:3811–7. doi: 10.1182/blood-2011-04-351254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stone GW, Barzee S, Snarsky V, Kee K, Spina CA, Yu XF, Kornbluth RS. Multimeric soluble CD40 ligand and GITR ligand as adjuvants for human immunodeficiency virus DNA vaccines. J Virol. 2006;80:1762–72. doi: 10.1128/JVI.80.4.1762-1772.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miconnet I, Pantaleo G. A soluble hexameric form of CD40 ligand activates human dendritic cells and augments memory T cell response. Vaccine. 2008;26:4006–14. doi: 10.1016/j.vaccine.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 45.Schultze JL, Michalak S, Seamon MJ, Dranoff G, Jung K, Daley J, Delgado JC, Gribben JG, Nadler LM. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. 1997;100:2757–65. doi: 10.1172/JCI119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janeway CA, Jr., Ron J, Katz ME. The B cell is the initiating antigen-presenting cell in peripheral lymph nodes. J Immunol. 1987;138:1051–5. [PubMed] [Google Scholar]
- 47.Ahmadi T, Flies A, Efebera Y, Sherr DH. CD40 Ligand-activated, antigen-specific B cells are comparable to mature dendritic cells in presenting protein antigens and major histocompatibility complex class I- and class II-binding peptides. Immunology. 2008;124:129–40. doi: 10.1111/j.1365-2567.2007.02749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee J, Dollins CM, Boczkowski D, Sullenger BA, Nair S. Activated B cells modified by electroporation of multiple mRNAs encoding immune stimulatory molecules are comparable to mature dendritic cells in inducing in vitro antigen-specific T-cell responses. Immunology. 2008 doi: 10.1111/j.1365-2567.2008.02833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu C, Liu Y, Zhao Q, Chen G, Chen J, Yan X, Zhou YH, Huang Z. Soluble CD40 ligand-activated human peripheral B cells as surrogated antigen presenting cells: A preliminary approach for anti-HBV immunotherapy. Virol J. 2010;7:370. doi: 10.1186/1743-422X-7-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coughlin CM, Vance BA, Grupp SA, Vonderheide RH. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood. 2004;103:2046–54. doi: 10.1182/blood-2003-07-2379. [DOI] [PubMed] [Google Scholar]
- 51.von Bergwelt-Baildon MS, Vonderheide RH, Maecker B, Hirano N, Anderson KS, Butler MO, Xia Z, Zeng WY, Wucherpfennig KW, Nadler LM, Schultze JL. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood. 2002;99:3319–25. doi: 10.1182/blood.v99.9.3319. [DOI] [PubMed] [Google Scholar]
- 52.Kondo E, Gryschok L, Schultze JL, von Bergwelt-Baildon MS. Using CD40-activated B cells to efficiently identify epitopes of tumor antigens. J Immunother. 2009;32:157–60. doi: 10.1097/CJI.0b013e31819031a2. [DOI] [PubMed] [Google Scholar]
- 53.Lapointe R, Bellemare-Pelletier A, Housseau F, Thibodeau J, Hwu P. CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 2003;63:2836–43. [PubMed] [Google Scholar]
- 54.Stone GW, Barzee S, Snarsky V, Spina CA, Lifson JD, Pillai VK, Amara RR, Villinger F, Kornbluth RS. Macaque multimeric soluble CD40 ligand and GITR ligand constructs are immunostimulatory molecules in vitro. Clin Vaccine Immunol. 2006;13:1223–30. doi: 10.1128/CVI.00198-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tu W, Lau YL, Zheng J, Liu Y, Chan PL, Mao H, Dionis K, Schneider P, Lewis DB. Efficient generation of human alloantigen-specific CD4+ regulatory T cells from naive precursors by CD40-activated B cells. Blood. 2008;112:2554–62. doi: 10.1182/blood-2008-04-152041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mathieu M, Cotta-Grand N, Daudelin JF, Boulet S, Lapointe R, Labrecque N. CD40-activated B cells can efficiently prime antigen-specific naive CD8+ T cells to generate effector but not memory T cells. PloS one. 2012;7:e30139. doi: 10.1371/journal.pone.0030139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kretschmer B, Kuhl S, Fleischer B, Breloer M. Activated T cells induce rapid CD83 expression on B cells by engagement of CD40. Immunol Lett. 2011;136:221–7. doi: 10.1016/j.imlet.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 58.Blair PA, Chavez-Rueda KA, Evans JG, Shlomchik MJ, Eddaoudi A, Isenberg DA, Ehrenstein MR, Mauri C. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol. 2009;182:3492–502. doi: 10.4049/jimmunol.0803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morva A, Lemoine S, Achour A, Pers JO, Youinou P, Jamin C. Maturation and function of human dendritic cells are regulated by B lymphocytes. Blood. 2012;119:106–14. doi: 10.1182/blood-2011-06-360768. [DOI] [PubMed] [Google Scholar]
- 60.Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, Mauri C, Coussens LM, Balkwill FR. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proc Natl Acad Sci U S A. 2011;108:10662–7. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, Hall RP, St Clair EW, Tedder TF. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–41. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mauri C, Ehrenstein MR. The ‘short’ history of regulatory B cells. Trends Immunol. 2008;29:34–40. doi: 10.1016/j.it.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 63.Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4:627–30. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 64.Wijesuriya R, Maruo S, Zou JP, Ogawa M, Umehara K, Yamashita M, Ono S, Fujiwara H, Hamaoka T. B cell-mediated down-regulation of IFN-gamma and IL-12 production induced during anti-tumor immune responses in the tumor-bearing state. Int Immunol. 1998;10:1057–65. doi: 10.1093/intimm/10.8.1057. [DOI] [PubMed] [Google Scholar]
- 65.Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4 T cells to T-regulatory cells. Cancer Res. 2011;71:3505–15. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.von Bergwelt-Baildon M, Shimabukuro-Vornhagen A, Popov A, Klein-Gonzalez N, Fiore F, Debey S, Draube A, Maecker B, Menezes I, Nadler LM, Schultze JL. CD40-activated B cells express full lymph node homing triad and induce T-cell chemotaxis: potential as cellular adjuvants. Blood. 2006;107:2786–9. doi: 10.1182/blood-2004-01-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kondo E, Gryschok L, Klein-Gonzalez N, Rademacher S, Weihrauch MR, Liebig T, Shimabukuro-Vornhagen A, Kochanek M, Draube A, von Bergwelt-Baildon MS. CD40-activated B cells can be generated in high number and purity in cancer patients: analysis of immunogenicity and homing potential. Clin Exp Immunol. 2009;155:249–56. doi: 10.1111/j.1365-2249.2008.03820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sorenmo KU, Krick E, Coughlin CM, Overley B, Gregor TP, Vonderheide RH, Mason NJ. CD40-activated B cell cancer vaccine improves second clinical remission and survival in privately owned dogs with non-Hodgkin's lymphoma. PloS one. 2011;6:e24167. doi: 10.1371/journal.pone.0024167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]