Abstract
Cranberry pomace is a byproduct of cranberry processing and is comprised of seeds, skins and stems of the cranberry fruit. While cranberry pomace contains beneficial polyphenols, including proanthocyanidins and anthocyanins, it is not a palatable source of these compounds and is typically discarded. In this study, we have developed and optimized a method to extract polyphenols from cranberry pomace using aqueous ethanol, a food grade solvent. Biochemical characterization of the pomace extract showed the presence of a broad range of polyphenols also present in cranberry juice concentrate. By co-drying cranberry pomace extract with a protein-rich food matrix, such as soy protein isolate (SPI), we have developed a method to produce a cranberry polyphenol-SPI complex (CBP-SPI) containing 10% cranberry polyphenols. Unlike dried cranberry pomace extract alone, proanthocyanidins, anthocyanins and total polyphenols were found to be highly stable at 37 °C in the CBP-SPI powder. The extraction and stabilization of cranberry pomace polyphenols using SPI provides an innovative approach for utilizing pomace in the development of novel food ingredients.
Keywords: cranberry pomace, polyphenols, proanthocyanidins, anthocyanins, soy protein isolate
1. Introduction
Cranberry fruits (Vaccinium macrocarpon Ait) are a rich source of type A proanthocyanidins, anthocyanins, flavonols, phenolic acids, benzoates, hydroxycinnamic acids, terpenes and organic acids (Pappas & Schaich, 2009). Research has associated several health benefits with the consumption of cranberry. Cranberries components are antimicrobial against Staphylococcus, Salmonella and Escherichia, which are responsible for food-borne illness and human disease (Puupponen-Pimia, Nohynek, Alakomi & Oksman-Caldentey, 2005). Cranberry polyphenols elicit antimicrobial activity via mechanisms independent of the acidic pH (Lacombe, Wu, Tyler & Edwards, 2010). Cranberry is used as a prophylactic for urinary tract infection (UTIs) (Barbosa-Cesnik, Brown, Buxton, Zhang, DeBusscher & Foxman, 2011; Epp et al., 2010; Jepson & Craig, 2008) and the presence of A-type proanthocyanidins has been associated with preventing the adhesion of uropathogenic E.coli to uroepithelial cells (Howell, Reed, Krueger, Winterbottom, Cunningham & Leahy, 2005; Sobota, 1984). In vitro studies have also shown that cranberry can prevent the adhesion of H. pylori to gastric mucosal cells (Burger, Ofek, Tabak, Weiss, Sharon & Neeman, 2000; Burger, Weiss, Sharon, Tabak, Neeman & Ofek, 2002; Shmuely et al., 2004) and in a clinical study the daily consumption of cranberry juice cocktail for 90 days was shown to decrease the H. pylori infection in adults compared to placebo (Zhang, Ma, Pan, Go, Chen & You, 2005).
Cranberries are commonly consumed as dried fruit or juice adulterated with large amounts of sugar. The sugar used to mask the tartness of cranberry adds excess calories and can counteract the beneficial effects of the fruit. Earlier, we developed a technology that uses protein-rich legume flours or powders, such as soy protein isolate (SPI), to sorb, concentrate and stabilize mid-polarity range cranberry phytochemicals from cranberry juice concentrate while separating them from excess water, sugar and lipid components (Roopchand et al., 2012). Cranberry pomace, a byproduct of the cranberry processing industry, is composed of dietary fiber from skins, seeds and stems (White, Howard & Prior, 2010) and is typically discarded. Due to its acidity and low protein content it has limited use as animal feed and disposal in landfills poses an environmental problem due to low pH. Cranberry pomace still contains an assortment of beneficial phytochemicals, such as polyphenols, which have a natural affinity for proteins. Soy protein isolate (SPI) made from soybeans is an inexpensive readily available protein that is frequently incorporated into foods and supplements. Compared to animal proteins such as casein, SPI was shown to be hypocholesterolemic (Nagata, Ishiwaki & Sugano, 1982), decrease the activity of lipogenic enzymes (Iritani, Nagashima, Fukuda, Katsurada & Tanaka, 1986), and reduce body fat in diet-induced obese rats and mice (Aoyama, Fukui, Takamatsu, Hashimoto & Yamamoto, 2000). Therefore we have developed a food-compatible method for the extraction of cranberry pomace and subsequent stabilization of cranberry phytochemicals via complexation to SPI to produce a novel phytochemical-enriched protein that can be used as a food ingredient.
2. Materials and methods
2.1. Optimization of method for cranberry pomace extraction
Frozen depectinized cranberry pomace (provided by BNK, Wisconsin Rapids, WI) was used in all analyses. Cranberry pomace (30 g wet wt.) was pureed in a Vitamix blender with 300 ml of water or 50% ethanol (10:1 extraction). Each puree was aliquoted into 40 ml volumes in 50 ml tubes and the pH was adjusted from 4 to 2, 3 or 5 with HCl or NaOH to investigate the effect of pH on extraction efficiency. The material was then extracted in loosely capped tubes in an 80 °C water bath for 2 h. The samples were centrifuged at 4,000 rpm for 10 min to separate solids from the liquid extract. The concentration of total polyphenols and proanthocyanidins in each extract was determined using the Folin-Ciocalteu (Singleton & Rossi, 1965) and 4-(dimethylamino)cinnamaldehyde (DMAC) methods (Prior et al., 2010), respectively.
Three independent lots of frozen depectinized cranberry pomace were extracted to compare solvent to pomace ratio and percentage of ethanol on polyphenol extraction efficiency. A 10 g (wet wt.) sample was removed from each lot and the dry weight of each was determined by freeze drying. A 100 g (wet wt.) sample of each lot of cranberry pomace was pureed in a Vitamix blender with 1 l of 50% ethanol (10:1 extraction) or 500 ml of 50% ethanol (5:1 extraction). Each puree was adjusted to pH 2 with HCl and the material was then extracted in a rotary evaporation flask (without vacuum) in an 80 °C water bath for 2 h (Buchi, Switzerland). Any evaporated solvent collected in the catch flask was added back to the extraction flask. The samples were centrifuged at 4,000 rpm for 10 min to separate the solids from the extract, which was then filtered through miracloth (Calbiochem). Similarly, 100 g (wet wt.) of each lot of cranberry pomace was also extracted with 1 l of 75% ethanol (10:1 extraction) or 500 ml of 75% ethanol (5:1 extraction). The volumes of extract recovered were measured and the concentrations of total polyphenols and proanthocyanidins in each extract were quantified as described above. The dry weight of each extract was determined by vacuum drying 1 ml aliquots of liquid extract. These data were then used to calculate the percentage of total polyphenols and proanthocyanidins in the dried extract. Proanthocyanidins as a percentage of total polyphenols was also calculated.
2.2. Biochemical characterization of cranberry pomace extract
Cranberry pomace (50 g wet wt.) was blended with 250 ml of 50% ethanol, mixture was adjusted to pH 2 and then extracted in a flask at 80 °C for 2 h on a shaking water bath. The cranberry pomace extract was filtered (0.22 µm) prior to analysis. Samples were separated and analyzed with a Shimadzu LCMS-2010A high performance liquid chromatography/electrospray ionization/single quadrupole. The LCMS is equipped with two pumps (LC-10ADvp), controller (SCL-10Avp), autoinjector (SIL-10ADvp), column oven (CTO-10ACvp), photodiode array detector (SPD-M10Avp), and a single quadrupole analyzer. The HPLC column was a Widepore C5, 5 µm, 2.1×150 mm (Supelco, Bellefonte, PA). The mobile phase consisted of 0.1% formic acid in water (Solvent A) and 0.1% formic acid in acetonitrile (Solvent B) at a flow rate of 0.2 ml/min. The gradient used was: initially 90% A and 10% B using an isocratic flow for first 5 min; 85% A and 15% B over 15 min, followed by isocratic elution at 85% A and 15% B for 5 min; 100% A and 0% B over 2 min, followed by an isocratic elution at 100% A and 0% B for 5 min. There was a 13 min equilibration interval between injections.
For characterization of proanthocyanidins in the cranberry pomace extract, mass spectra were collected on a Bruker ULTRAFLEX®III MALDI TOF/TOF mass spectrometer (Billerica, MA, USA) equipped with delayed extraction and a SmartBeam® laser. All analyses were performed in positive linear mode. Spectra were the sum of 8–10 different locations in each well, accumulating a total of 400–500 shots to minimize intra-well variability and avoid heterogeneous co-crystallization spots. Threshold laser power was used to achieve optimal isotope patterns. The matrix was 2, 5-dihydroxybenzoic acid (DHB) at a concentration of 50 mg/ml in ethanol. FlexControl and FlexAnalysis (Bruker Daltonik GmbH, Bremen, Germany, version 3.0) were used for data acquisition and data processing, respectively. mMass (version 3.9.0) was used for spectra analysis. Based on previously described structures an equation was developed to predict the mass distribution of PAC in cranberry products (Reed, Krueger & Vestling, 2005). The equation is 290 + 288a − 2b + 23, where 290 represents the molecular weight of the terminal catechin/epicatechin unit, a is the degree of polymerization of catechin/epicatechin units, b is the number of A-type interflavan bonds, and 23 is the molecular weight of sodium.
2.3. Production of cranberry polyphenol-SPI complex
Cranberry pomace (300 g wet wt.) was extracted as described in Section 2.1 and 2.4 l of liquid extract was collected after separating the cranberry pomace solids; the concentrations of total polyphenols and proanthocyanidins were quantified in the extract. Three 1 ml aliquots of extract were dried in a speed vacuum and the average dry weight per ml was determined. A calculated amount of soy protein isolate (SPI; ADM, Decatur, IL) was added to the liquid cranberry pomace extract so that after solvent evaporation the result is a cranberry polyphenol-SPI complex (CBP-SPI) containing 10% total polyphenols. The dry weight of the 2.4 l of cranberry pomace extract was 19.1 g containing 3.04 g of total polyphenols. The final amount of CBP-SPI was therefore calculated to be 30.4 g to have the cranberry polyphenol content standardized to 10%. Therefore, the amount of SPI to be added to the cranberry extract was calculated as follows: 19.1 g + ×= 30.4 g; × = 11.3 g of SPI
After SPI was added to the cranberry pomace extract, ethanol was removed by rotary evaporation under vacuum (Büchi, Switzerland) with temperature set at 40 °C. The remaining liquid was removed by freeze-drying to yield CBP-SPI powder.
2.4. Temperature stability of polyphenols in CBP-SPI compared to cranberry pomace extract
Cranberry pomace extract was prepared as described in Section 2.1. A portion was used to make CBP-SPI containing 10% total polyphenols as described in Section 2.3 and a portion was dried to make cranberry pomace extract powder (CBP extract). The CBP-SPI and CBP extract were placed in a 37 °C incubator and aliquots of each sample were removed at indicated times. The dried cranberry pomace extract (10 mg) or CBP-SPI complex (100 mg) was eluted 3 times with 1 ml of 50% acetone. Proanthocyanidins were quantified using the DMAC method (Prior et al., 2010), anthocyanins were measured using the pH differential method (Lee, Durst & Wrolstad, 2005) and total polyphenols was measured by the Folin-Ciocalteu method (Singleton et al., 1965).
2.5. Statistics
Statistics were performed with STATISTICA v.10 (StatSoft). T-tests were performed between groups. One-way ANOVA was used to determine significance among three or more groups followed by the indicated post-hoc test.
3. Results
3.1. Optimization of cranberry pomace extraction using food-compatible solvents
The mean dry weight of three independent lots of frozen cranberry pomace was 26.7 ± 1.3 g per 100 g of wet weight. To determine the effect of pH on extraction efficiency a series of extractions were performed at 80 °C for 2 h in water or 50% ethanol using a 10:1 extraction ratio (solvent:wet pomace) at different pH values. For water extracts, pH 2 gave the highest concentration of total polyphenols (266 mg/l) and proanthocyanidins (114 mg/l) while raising the pH to 5 resulted in the lowest concentrations (Table 1). Extraction with 50% ethanol was more efficient than water resulting in a 3.6 fold greater yield of total polyphenols (963 mg/l) and a 2.7 fold greater yield of proanthocyanidins (306 mg/l) at pH 2. Yield became progressively lower as the pH was increased to 5 (Table 1).
Table 1.
pH dependence of polyphenols extracted from cranberry pomace in water or aq. ethanol (10:1, solvent:pomace)
| Solvent | pH | Total Polyphenols (mg/l) |
Proanthocyanidins (mg/l) |
|---|---|---|---|
| H20 | 2 | 266 | 114 |
| 3 | 104 | 47 | |
| 4 | 124 | 49 | |
| 5 | 78 | 25 | |
| 50% Ethanol | 2 | 964 | 306 |
| 3 | 591 | 181 | |
| 4 | 469 | 154 | |
| 5 | 408 | 123 | |
Total polyphenols were calculated as gallic acid and proanthocyanidins were calculated as proanthocyanidin A2 equivalents
The extraction of polyphenols and proanthocyanidins was also compared using the three lots of cranberry pomace, each extracted with 50% or 75% aqueous ethanol using either a 10:1 or 5:1 (solvent:wet pomace) extraction ratio. Each mixture was adjusted to pH 2 with HCl and extractions were performed at 80 °C with continuous mixing for 2 h. In the case of 50% ethanol a 10:1 solvent to solids ratio extracted a higher amount of total polyphenols (750 ± 19 mg) compared to the 5:1 ratio (507 ± 58 mg); however, there was no significant difference in the amount of proanthocyanidins extracted (Table 2). The 5:1 extractions performed with 50% ethanol resulted in the highest percentage of proanthocyanidins relative to total polyphenols (Table 2). The extractions performed with 75% ethanol resulted in higher amounts of proanthocyanidins when a 10:1 solvent ratio was used, but the amount of total polyphenols and the amount of proanthocyanidins as a percentage of total polyphenols was not significantly different between the 10:1 and 5:1 extractions (Table 2). The data indicated that optimal extraction conditions were a 5:1 (solvent:wet pomace) ratio of cranberry pomace in 50% ethanol adjusted to pH 2 incubated at 80 °C for 2 h with agitation. These conditions were best for obtaining an extract enriched in proanthocyanidins compared to total polyphenols while minimizing the volume of solvent therefore they were used for all subsequent extractions.
Table 2.
Comparison of 10:1 and 5:1 extraction of cranberry pomace with 50% and 75% aqueous ethanol
| Solvent | Volume (ml) |
TP (mg/l) | TP (mg) | % TP in dry extract |
PACs (mg/l) | PACs (mg) | % PACs in dry extract | PACs as % of TP |
|---|---|---|---|---|---|---|---|---|
| 50% EtOH (10:1) | 793 ± 40 | 947 ± 73.1 | 750 ± 19 ** | 15.1 ± 1.6 | 495 ± 73 | 391 ± 39 | 7.9 ± 1.3 | 52 ± 4.1 |
| 50% EtOH (5:1) | 328 ± 3 | 1545 ± 193 | 507 ± 58 | 12.6 ± 1.2 | 1170 ± 152 | 384 ± 47 | 9.6 ± 1.0 | 76 ± 7.0 ** |
| 75% EtOH (10:1) | 878 ± 45 | 863 ± 114 | 761 ± 139 | 11.8 ± 1.3 | 531 ± 31 | 466 ± 4.2 ** | 7.3 ± 1.5 | 63 ± 11 |
| 75% EtOH (5:1) | 418 ± 33 | 1610 ± 157 | 678 ± 118 | 15.7 ± 1.8 | 841 ± 11.5 | 352 ± 23 | 8.3 ± 1.5 | 53 ± 5.8 |
n=3 independent sample lots for each solvent condition.
T-test, ** p< 0.01
TP = total polyphenols calculated as gallic acid equivalents. PACs = proanthocyanidins calculated as proanthocyanidin A2 equivalents
3.2. Biochemical characterization of cranberry pomace extract
Cranberries contain three types of flavonoids: flavonols, anthocyanins and proanthocyanidins (PAC) (Puski & Francis, 1967; Hong & Wrolstad, 1990; Wang, Du & Francis, 1978). The flavonols in cranberries are glycosides of quercetin, myricetin and kaempferol (Hong & Wrolstad, 1990; Bilyk & Sapers, 1986). Anthocyanins present are glycosides of cyanidin and peonidin (Hong & Wrolstad, 1990). Proanthocyanidins are polymers of flavan-3-ols and flavans linked through A-type and B-type interflavan carbon bonds (Howell, Reed, Krueger, Winterbottom, Cunningham & Leahy, 2005; Porter, Krueger, Wiebe, Cunningham & Reed, 2001; Neto et al., 2006; Reed, Krueger, Vestling, 2005; Foo, Lu, Howell, Vorsa, 2000). Cranberry pomace was extracted using the optimal method, described in Section 3.1, for compound characterization by LC-MS and MALDI-TOF MS. Table 3 summarizes putatively identified compounds from cranberry pomace extract, which included anthocyanins, quercetin and quercetin glycosides. According to relative peak mass area, peonidin glycosides were the most abundant compounds detected, followed by cyanidin glycosides and quercetin. Quercetin was 10–17 times more abundant than quercetin glycosides. These findings are in agreement with previously reported data for cranberry pomace (White et al., 2010).
Table 3.
Compounds extracted from cranberry pomac
| Compounds | Peak mass area (× 106) |
MS (m/z) ESI |
Rt (min) | |
|---|---|---|---|---|
| [M]+ | [M-H]+ | |||
| Cyanidin-3-O-galactoside | 16.5 | 449 | 4.48 | |
| Cyanidin-3-O-arabinoside | 19.7 | 419 | 4.91 | |
| Peonidin -3-O-galactoside | 37.6 | 463 | 5.81 | |
| Peonidin-3-O-arabinoside | 35.0 | 433 | 7.33 | |
| Quercetin | 19.5 | 301 | 23.03 | |
| Quercetin-3-O-galactoside | 1.46 | 463 | 12.3 | |
| Quercetin-3-O-arabinoside | 1.98 | 433 | 15.03; 15.57 | |
| Quercetin-3-O-rhamnoside | 1.13 | 447 | 17.63 | |
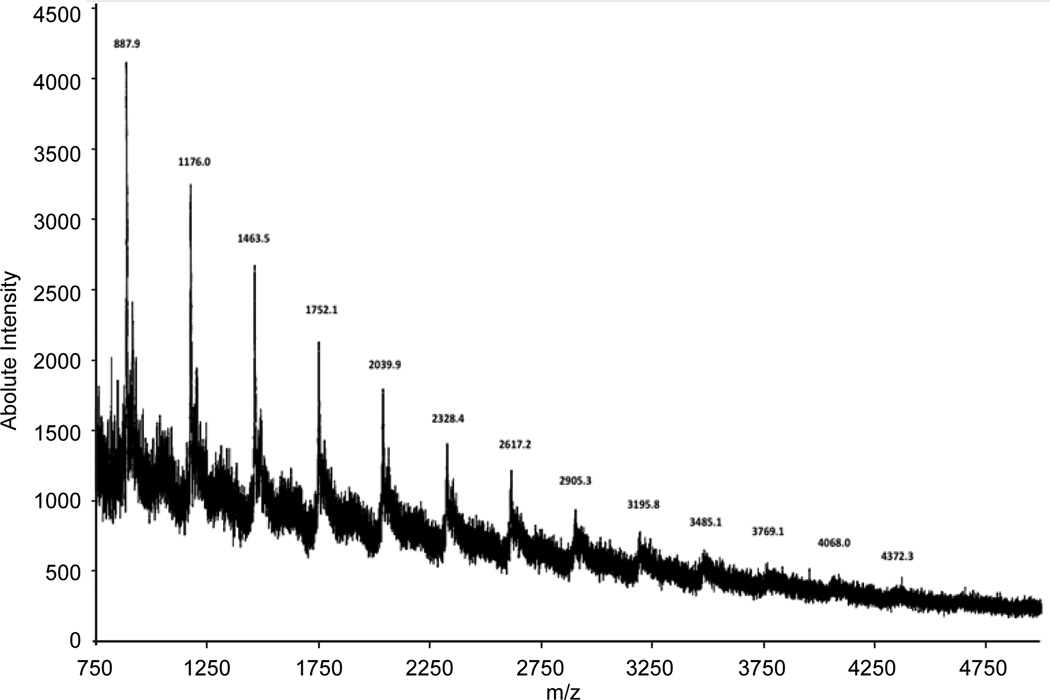
MALDI-TOF MS methods have been developed and applied to characterize structural features of proanthocyanidins and other tannins in fruits (Feliciano, Krueger, Shanmuganayagam, Vestling & Reed, 2012; Howell, Reed, Krueger, Winterbottom, Cunningham & Lehy, 2005; Reed, Krueger & Vestling 2005; Krueger, Dopke, Treichel, Folts & Reed, 2000; Krueger, Vestling & Reed, 2003; Krueger, Vestling & Reed, 2004, Afaq, Saleem, Krueger, Reed & Mukhtar, 2005; Neto et al 2006; Li et al 2010). MALDI-TOF MS is ideally suited for characterizing polydispersed oligomers and is considered the mass spectral method of choice for analysis of proanthocyanidins, which exhibit large structural heterogeneity (Reed, Krueger & Vestling, 2005; Hanton; 2001). MALDI-TOF MS produces a singly charged molecular ion for each parent molecule allowing precise detection of high mass (Montaudo, 2002) and baseline detection of a broad range of cranberry proanthocyanidin oligomers over the degree of polymerization (DP) range of 2 to 23 (Reed, Krueger, & Vestling, 2000). MALDI-TOF MS analysis of the cranberry pomace extract confirmed a series of masses corresponding to a proanthocyanidin series [M + Na]+ from three to fifteen degrees of polymerization (m/z 887.9 to m/z 4372.3), which have previously been described (Reed et al., 2005) in cranberry products (Figure 1). Therefore extraction of cranberry pomace using our described method efficiently captures a broad range of compounds representative of cranberry fruit.
Figure 1. MALDI-TOF mass spectrum of cranberry pomace extract.
Data was generated in positive linear mode and shows a proanthocyanidin series [M + Na]+ from 3 degrees of polymerization (DP; m/z 887.9) to DP 15 (m/z 4372.3).
3.3. Temperature stability of proanthocyanidins and anthocyanins in CBP-SPI
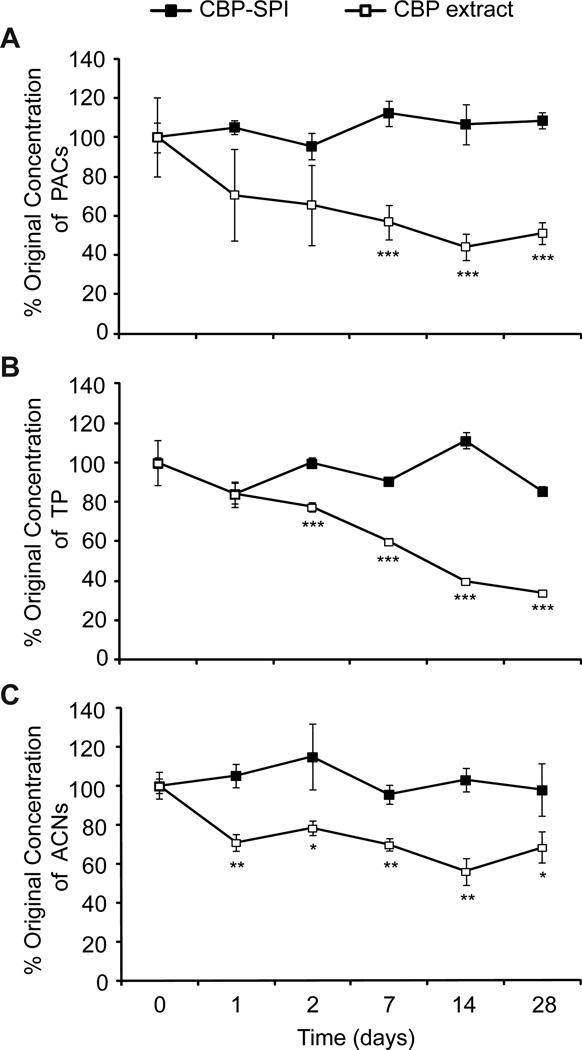
Polyphenol-protein complexes are known to form through hydrophobic and hydrogen bond interactions that depend on the structure of the protein and polyphenol molecules (Bandyopadhyay, Ghosh & Ghosh, 2012). We explored the utility of complexing cranberry polyphenols to SPI to create a novel food ingredient. Cranberry pomace was extracted as described in Section 3.1 and polyphenols in the cranberry pomace extract were complexed with SPI as described in Section 2.3 to produce cranberry polyphenol-SPI complex (CBP-SPI) containing 10% total polyphenols (Figure 2). Lyophilized cranberry pomace extract was also prepared, as described in Section 2.4. CBP-SPI and dried cranberry pomace extract were incubated at 37 °C and the stability of proanthocyanidins, anthocyanins and total polyphenols was evaluated over the course of 28 days. Prior to incubation at 37 °C (Day 0), the polyphenol concentrations were quantified in the dried pomace extract or CBP-SPI after eluting the powders three times with 50% acetone. Aliquots of dried pomace extract or CBP-SPI were removed after incubation at 37 °C on the indicated days and subjected to three rounds of elution with 50% acetone. Proanthocyanidins, anthocyanins and total polyphenols were measured as described in methods and expressed as a percentage of the original amounts that were eluted on day 0. The levels of proanthocyanidins, anthocyanins and total polyphenols in the CBP-SPI powder remained remarkably stable after the 28-day incubation period at 37 °C (Fig. 3). In contrast, on day 28 the levels of proanthocyanidins, anthocyanins and total polyphenols in the dried cranberry pomace extract declined significantly by 49%, 32% and 66%, respectively (Fig. 3). Anthocyanins are known to be highly unstable at elevated temperature (Buckow, Kastell, Terefe & Versteeg, 2010; Kechinski, Guimaraes, Norena, Tessaro & Marczak, 2010), therefore the data suggest that complexation with SPI protects them from degradation at 37 °C. Proanthocyanidins on the other hand are known to be stable (Grace, Massey, Mbeunkui, Yousef & Lila, 2012) therefore the decline in acetone-extractable proanthocyanidins was rather unexpected. One possible explanation is that at elevated temperature proanthocyanidins in the CBP extract rearrange to form insoluble complexes that cannot be measured by the colorimetric DMAC assay. In contrast proanthocyanidins in complex with SPI appear to be protected from such rearrangements at 37 °C and are thus more likely to retain their biological effects. Compared to CBP-SPI, the levels of all polyphenols decreased in the dried cranberry pomace extract at the elevated temperature (Fig. 3C), suggesting that complexation with SPI has a stabilizing effect on all polyphenols.
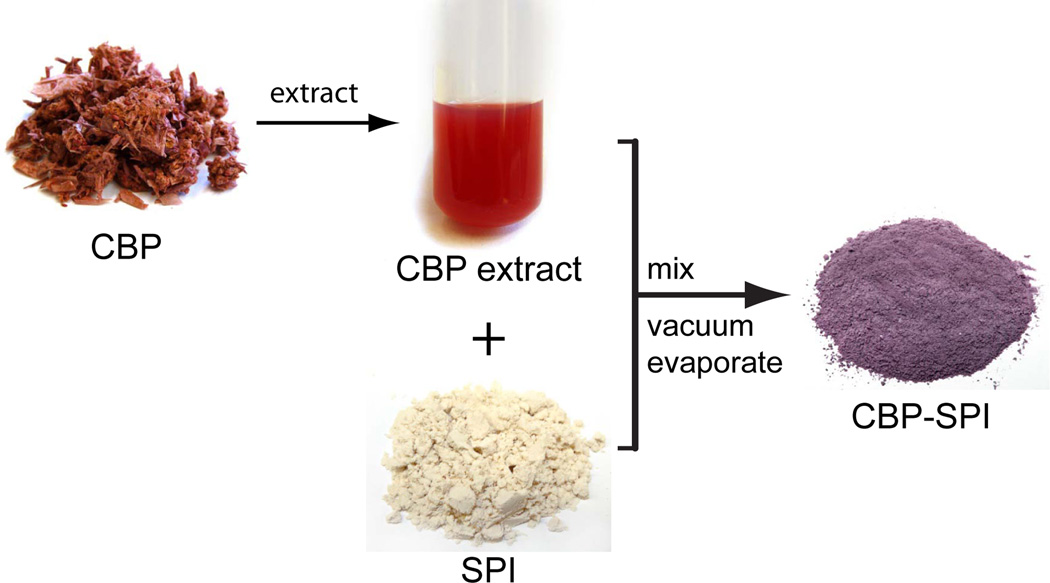
Figure 2. Schematic for production of CBP-SPI complex.
Cranberry pomace was extracted in 50% ethanol at 80 °C at pH 2 for 2 h. After the total polyphenols in the cranberry pomace extract (CBP extract) was quantified, a calculated amount of soy protein isolate (SPI) is mixed into the extract to yield a final product containing up to 10% total polyphenols. Vacuum evaporation of 50% ethanol followed by freeze drying yielded the cranberry polyphenol-SPI complex (CBP-SPI).
Figure 3. Stability of cranberry polyphenols bound and unbound to SPI.
Cranberry (A) proanthocyanidins, (B) total polyphenols and (C) anthocyanins expressed as a percentage of the original concentration measured on day 0 (before incubation at 37 °C) and after 1, 2, 7, 14 and 28 days at 37 °C.
4. Discussion
Our method of extracting phytochemicals from cranberry pomace and stabilizing extracted compounds via complexation with SPI offers a novel strategy to capture, stabilize and deliver valuable phytochemicals that are usually wasted in the cranberry juicing process. Extraction of cranberry pomace with 50% ethanol likely releases more high molecular weight oligomeric tannins than would be expected in water-extracted or juiced cranberries. The resulting CBP-SPI matrix is a phytochemically-enriched protein ingredient that can be incorporated into foods and dietary supplements.
Work on polyphenol-protein interactions have been recently reviewed (Bandyopadhyay et al., 2012; Santos-Buelga & Scalbert, 2000). In addition to solution parameters such as pH and temperature, polyphenol-protein complexation depends on the three dimensional distributions of different amino acid residues on protein surfaces or cavities and the size of the cavity to accommodate large or small polyphenol molecules. In general interactions are mediated by flexible proline-rich proteins that possess an open conformation allowing hydrophobic interactions with polyphenols, which can be reinforced by hydrogen bonds between phenolic hydroxyl groups (proton donors) and carbonyl groups of peptide bonds (proton acceptor) (Hagerman & Butler, 1980; Hagerman & Butler, 1981). Polyphenols-protein interactions can cause either unfolding of a protein chain or induce proteins to go from an unfolded to folded state (Bandyopadhyay et al., 2012). Non-polar tannins (eg. pentagalloylglucose; PGG) were found to precipitate bovine serum albumin (BSA) by forming a hydrophobic coat around the protein while polar tannins (eg. epicatechin16(4–8) catechin; EC16-C) formed hydrogen bonds between protein molecules (Hagerman, Rice & Ritchard, 1998). Ethanol suppressed the interaction between BSA and non-polar PGG, but not the interaction with more polar EC16-C (Hagerman et al., 1998). In our process, the 50% ethanol used for extraction is evaporated after addition of SPI therefore polyphenol-protein complexes should form without disruption.
The stabilizing effect of SPI on cranberry pomace polyphenols at 37 °C is an advantage that will also have to be further evaluated at higher temperatures and in finished food products that incorporate the CBP-SPI ingredient. Based on our preliminary taste assessments we observed that complexation of CBP polyphenols with SPI masks the astringent taste associated with tannins as they are not available to interact with proteins in saliva; however, this remains to be formally evaluated in a sensory panel.
We have previously reported that defatted soybean flour (DSF) can be used to sorb polyphenols from cranberry juice concentrate while leaving behind excess sugars/lipids in the supernatant (Roopchand et al., 2012). However, the sorption of polyphenols from cranberry concentrate is limited by the sorption capacity of the DSF or other protein-rich matrix and is inversely related to the amount of DSF added to a given dilution of cranberry concentrate. For example, mixing 50 g/L protein-rich DSF matrix with cranberry concentrate (50° Brix) diluted 2 times with water will sorb 49% of the proanthocyanidins from the juice to produce a cranberry polyphenol-enriched DSF (CB-DSF) matrix containing 3% proanthocyanidins (29 mg/g) while adding 5 g/L of DSF to similarly diluted cranberry concentrate will sorb 17% of proanthocyanidins from the juice, but produce a CB-DSF matrix containing 10% proanthocyanidins (100 mg/g) (Roopchand et al., 2012). While the lower ratio of DSF to juice produces a more concentrated CB-DSF product, the scale is not favorable for manufacturing large quantities of product. In the process described in this study, the co-drying of cranberry pomace extract with the protein matrix allows production of a CBP-SPI product containing up to 10% total polyphenols by simply adjusting the amount of protein matrix added to the liquid extract prior to co-drying. Our food-compatible method of extracting polyphenols from fruit and vegetable byproducts and creating polyphenol-enriched protein or flour ingredients may be a practical way of utilizing agricultural byproducts and increasing the level of beneficial polyphenols in the diet.
Highlights.
Cranberry pomace polyphenols are most efficiently extracted at pH 2.
Cranberry pomace extract contains compounds present in cranberry juice.
Cranberry polyphenols are stabilized when complexed to soy protein isolate.
Acknowledgements
We thank Brandon Mirda for technical assistance. DER designed experiments, performed statistical analysis and wrote the manuscript. CGK did MALDI-TOF and LC-MS analysis. KM performed stability study. MAL and BF were consultants to the work. IR is the principal investigator of the laboratory where the research was performed. All authors read and approved the final manuscript.
Funding This work was supported in part by SBIR grant 1 R43DK092104-01A1 from NIAID, NIH training grant T32 AT004094 (supporting DER) and P50AT002776-01 from the National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements. MALDI-TOF MS work was performed on the Bruker Ultraflex III MALDI TOF/TOF MS which was partially funded by NIH NCRR 1S10RR024601-01.
ABBREVIATIONS
- SPI
soy protein isolate
- CBP-SPI
cranberry polyphenol-SPI complex
- ACN
anthocyanin
- PAC
proanthocyanidin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement DER, BF, MAL and IR have equity in Nutrasorb LLC, which has interest in developing polyphenol sorption technology.
References
- Aoyama T, Fukui K, Takamatsu K, Hashimoto Y, Yamamoto T. Soy protein isolate and its hydrolysate reduce body fat of dietary obese rats and genetically obese mice (yellow KK) Nutrition. 2000;16(5):349–354. doi: 10.1016/s0899-9007(00)00230-6. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay P, Ghosh AK, Ghosh C. Recent developments on polyphenol-protein interactions: effects on tea and coffee taste, antioxidant properties and the digestive system. Food Funct. 2012;3(6):592–605. doi: 10.1039/c2fo00006g. [DOI] [PubMed] [Google Scholar]
- Barbosa-Cesnik C, Brown MB, Buxton M, Zhang L, DeBusscher J, Foxman B. Cranberry juice fails to prevent recurrent urinary tract infection: results from a randomized placebo-controlled trial. Clin Infect Dis. 2011;52(1):23–30. doi: 10.1093/cid/ciq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckow R, Kastell A, Terefe NS, Versteeg C. Pressure and temperature effects on degradation kinetics and storage stability of total anthocyanins in blueberry juice. J Agric Food Chem. 2010;58(18):10076–10084. doi: 10.1021/jf1015347. [DOI] [PubMed] [Google Scholar]
- Burger O, Ofek I, Tabak M, Weiss EI, Sharon N, Neeman I. A high molecular mass constituent of cranberry juice inhibits helicobacter pylori adhesion to human gastric mucus. FEMS Immunol Med Microbiol. 2000;29(4):295–301. doi: 10.1111/j.1574-695X.2000.tb01537.x. [DOI] [PubMed] [Google Scholar]
- Burger O, Weiss E, Sharon N, Tabak M, Neeman I, Ofek I. Inhibition of Helicobacter pylori adhesion to human gastric mucus by a high-molecular-weight constituent of cranberry juice. Crit Rev Food Sci Nutr. 2002;42(3 Suppl):279–284. doi: 10.1080/10408390209351916. [DOI] [PubMed] [Google Scholar]
- Epp A, Larochelle A, Lovatsis D, Walter JE, Easton W, Farrell SA, Girouard L, Gupta C, Harvey MA, Robert M, Ross S, Schachter J, Schulz JA, Wilkie D, Ehman W, Domb S, Gagnon A, Hughes O, Konkin J, Lynch J, Marshall C. Recurrent urinary tract infection. J Obstet Gynaecol Can. 2010;32(11):1082–1090. doi: 10.1016/S1701-2163(16)34717-X. [DOI] [PubMed] [Google Scholar]
- Grace MH, Massey AR, Mbeunkui F, Yousef GG, Lila MA. Comparison of health-relevant flavonoids in commonly consumed cranberry products. J Food Sci. 2012;77(8):H176–H183. doi: 10.1111/j.1750-3841.2012.02788.x. [DOI] [PubMed] [Google Scholar]
- Hagerman AE, Butler LG. Determination of protein in tannin-protein precipitates. Journal of Agricultural and Food Chemistry. 1980;28:944–947. doi: 10.1021/jf60231a010. [DOI] [PubMed] [Google Scholar]
- Hagerman AE, Butler LG. The specificity of proanthocyanidin-protein interactions. J Biol Chem. 1981;256(9):4494–4497. [PubMed] [Google Scholar]
- Hagerman AE, Rice ME, Ritchard NT. Mechanisms of protein precipitation for two tannins, pentagalloyl glucose and epicatechin(16) (4 -> 8) catechin (procyanidin) Journal of Agricultural and Food Chemistry. 1998;46(7):2590–2595. [Google Scholar]
- Howell AB, Reed JD, Krueger CG, Winterbottom R, Cunningham DG, Leahy M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry. 2005;66(18):2281–2291. doi: 10.1016/j.phytochem.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Iritani N, Nagashima K, Fukuda H, Katsurada A, Tanaka T. Effects of Dietary Proteins on Lipogenic Enzymes in Rat-Liver. Journal of Nutrition. 1986;116(2):190–197. doi: 10.1093/jn/116.2.190. [DOI] [PubMed] [Google Scholar]
- Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;(1):CD001321. doi: 10.1002/14651858.CD001321.pub4. [DOI] [PubMed] [Google Scholar]
- Kechinski CP, Guimaraes PV, Norena CP, Tessaro IC, Marczak LD. Degradation kinetics of anthocyanin in blueberry juice during thermal treatment. J Food Sci. 2010;75(2):C173–C176. doi: 10.1111/j.1750-3841.2009.01479.x. [DOI] [PubMed] [Google Scholar]
- Lacombe A, Wu VCH, Tyler S, Edwards K. Antimicrobial action of the American cranberry constituents; phenolics, anthocyanins, and organic acids, against Escherichia coli O157:H7. International Journal of Food Microbiology. 2010;139(1–2):102–107. doi: 10.1016/j.ijfoodmicro.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Lee J, Durst RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int. 2005;88(5):1269–1278. [PubMed] [Google Scholar]
- Nagata Y, Ishiwaki N, Sugano M. Studies on the mechanism of antihypercholesterolemic action of soy protein and soy protein-type amino acid mixtures in relation to the casein counterparts in rats. J Nutr. 1982;112(8):1614–1625. doi: 10.1093/jn/112.8.1614. [DOI] [PubMed] [Google Scholar]
- Pappas E, Schaich KM. Phytochemicals of Cranberries and Cranberry Products: Characterization, Potential Health Effects, and Processing Stability. Critical Reviews in Food Science and Nutrition. 2009;49(9):741–781. doi: 10.1080/10408390802145377. [DOI] [PubMed] [Google Scholar]
- Prior RL, Fan E, Ji H, Howell A, Nio C, Payne MJ, Reed J. Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. J Sci Food Agric. 2010;90(9):1473–1478. doi: 10.1002/jsfa.3966. [DOI] [PubMed] [Google Scholar]
- Puupponen-Pimia R, Nohynek L, Alakomi HL, Oksman-Caldentey KM. Bioactive berry compounds - novel tools against human pathogens. Applied Microbiology and Biotechnology. 2005;67(1):8–18. doi: 10.1007/s00253-004-1817-x. [DOI] [PubMed] [Google Scholar]
- Reed JD, Krueger CG, Vestling MM. MALDI-TOF mass spectrometry of oligomeric food polyphenols. Phytochemistry. 2005;66(18):2248–2263. doi: 10.1016/j.phytochem.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Roopchand DE, Grace MH, Kuhn P, Cheng DM, Plundrich N, Pouleva A, Howell A, Fridlender B, Lila MA, Raskin I. Efficient sorption of polyphenols to soybean flour enables natural fortification of foods. Food Chemistry. 2012;131:1193–1200. doi: 10.1016/j.foodchem.2011.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Buelga C, Scalbert A. Proanthocyanidins and tannin-like compounds - nature, occurrence, dietary intake and effects on nutrition and health. Journal of the Science of Food and Agriculture. 2000;80(7):1094–1117. [Google Scholar]
- Shmuely H, Burger O, Neeman I, Yahav J, Samra Z, Niv Y, Sharon N, Weiss E, Athamna A, Tabak M, Ofek I. Susceptibility of Helicobacter pylori isolates to the antiadhesion activity of a high-molecular-weight constituent of cranberry. Diagn Microbiol Infect Dis. 2004;50(4):231–235. doi: 10.1016/j.diagmicrobio.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- Sobota AE. Inhibition of Bacterial Adherence by Cranberry Juice - Potential Use for the Treatment of Urinary-Tract Infections. Journal of Urology. 1984;131(5):1013–1016. doi: 10.1016/s0022-5347(17)50751-x. [DOI] [PubMed] [Google Scholar]
- White BL, Howard LR, Prior RL. Proximate and polyphenolic characterization of cranberry pomace. J Agric Food Chem. 2010;58(7):4030–4036. doi: 10.1021/jf902829g. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ma J, Pan K, Go VL, Chen J, You WC. Efficacy of cranberry juice on Helicobacter pylori infection: a double-blind, randomized placebo-controlled trial. Helicobacter. 2005;10(2):139–145. doi: 10.1111/j.1523-5378.2005.00301.x. [DOI] [PubMed] [Google Scholar]