Abstract
Renal cysts, pain and hematuria are common presentations of autosomal dominant polycystic kidney disease (ADPKD) in children. Renal function, however, is typically preserved in these patients despite increased renal volume. Since angiogenesis has been implicated in promotion of renal cyst growth in ADPKD we measured the serum level of various angiogenic factors and early renal structural changes and cardiovascular parameters in 71 patients with ADPKD with a mean age of 16 years. Renal structure and left ventricular mass index were measured by magnetic resonance imaging or by echocardiogram. Renal function was assessed by creatinine clearance, and urinary protein excretion. Serum growth factor levels were measured by enzyme-linked immunosorbent assay. Because of skewed distributions, the various parameters are reported as log10. Serum Log10 vascular endothelial growth factor was positively correlated with renal and cardiac structure, but negatively correlated with creatinine clearance. Serum angiopoietin 1 levels significantly correlated with structural change in both the kidney and the heart and with urinary protein. Thus, the correlation between angiogenic growth factors with both renal and cardiac disease severity is compatible with a possible role for angiogenesis in the early progression of disease in ADPKD.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) affects between 1 in 400 and 1 in 1000 individuals in the United States (1). Renal cysts are often evident on ultrasound imaging in children, who typically become symptomatic in young adulthood (2–4). However, we have previously shown that hypertensive (95th percentile for blood pressure) and borderline hypertensive (75th – 95th percentile for blood pressure) children have a higher left ventricular mass index (LVMI) than children with ADPKD and lower blood pressure (5). Moreover, hypertensive children with ADPKD have higher renal volumes than children with lower blood pressure (6). Thus careful clinical evaluation and monitoring of children with ADPKD is critical.
It is apparent that vascular changes including expansion and remodeling must occur in order to support the massive growth of cysts in ADPKD patients. Evidence for angiogenesis in the form of development of a well-defined vascular capsule around human renal cysts in ADPKD supports this tenet (7,8). Moreover, the identification of angiogenic growth factors including vascular endothelial growth factor (VEGF) in the fluid from both human renal and hepatic cysts (7–9) and upregulated expression of angiopoietin (Ang)-1, Ang-2 and their endothelial tyrosine kinase (Tie)-2 receptor in ADPKD cholangiocytes supports a role of angiogenic growth factors in mediating this process (10).
VEGF is a central mediator of angiogenesis inducing endothelial cell proliferation, sprouting and promoting vascular leakiness (11). The actions of the angiopoietins appear to be both cell type-specific and dependent upon the expression level of the respective growth factors. Ang–1 expressed by vascular smooth muscle cells and pericytes activates the Tie-2 receptor resulting in blood vessel stabilization (12). However, overexpression of Ang-1 has been associated with increased sprouting and formation of larger blood vessels in transgenic animal studies (13). In contrast Ang-2 causes destabilization of vessels, increased leakiness, and enables function of other cytokines including VEGF (12). In view of the potential opposing roles of Ang-1 and Ang-2, the Ang-1/Ang-2 ratio may also determine the biological activity (14–16). Several recent studies support an emerging role for an imbalance of angiogenic growth factor levels in disease processes including tumor growth, diabetes, chronic kidney disease and cardiovascular disease (12,17–20).
As angiogenesis may be an important process for renal cyst expansion, we hypothesized that the serum level of angiogenic growth factors may be linked to measures of disease progression in young patients with ADPKD. Children and young adults with ADPKD present an ideal study population as renal cysts are present and actively growing. But the disease is largely uncomplicated by features of advanced renal damage including fibrosis, glomerulosclerosis, and inflammation.
Results
Clinical and demographic characteristics of the study patients
A total of 71 young patients with age range 8 –26 years, who were enrolled in clinical studies at the University of Colorado at Denver, and who underwent clinical and radiological examination between 2000 and 2009 were included in the study. The patient characteristics are presented in Table 1. Serum VEGF levels were also assessed in 10 young patients (5 male and 5 female) with type 1 diabetes mellitus (serum hemoglobin A1c (%) 8.88 ± 1.63) matched for age (15.7 ± 2.3 years) and normal renal function (creatinine clearance 111 ±14 ml/min/1.73m2) to the young ADPKD patients. Mean serum VEGF level was significantly lower in the young diabetic patients compared to the ADPKD patients (617 ± 389 in diabetic patients compared with 2997 ± 5327 pg/ml in ADPKD patients, P = 0.0005).
Table 1.
Clinical and demographic characteristics of study population
| Parameter | N | Mean ± SD | Geometric Mean and 95% CI |
|---|---|---|---|
| Age at evaluation (years) | 71 | 16.3 ± 4.0 | |
| Male (%) | 29/71 (41%) | ||
| Height (cm) | 71 | 165 ± 14 | |
| Weight (Kg) | 71 | 66 ± 23 | |
| Body surface area (BSA; m2) | 71 | 1.71 ± 0.34 | |
| Hypertension (%) | 27/71 (38%) | ||
| Serum creatinine (mg/dl) | 70 | 0.74 ± 0.22 | 0.71 (0.66–0.76) |
| Ang-1 (ng/ml) | 71 | 35.52 ± 21.03 | 27.57 (22.56 – 33.69) |
| Ang-2 (pg/ml) | 71 | 2.35 ± 0.96 | 2.16 (1.96 – 2.39) |
| VEGF (pg/ml) | 70 | 2997 ± 5327 | 107.2 (42.78 – 268.6) |
| Urinary creatinine (mg/24h) | 68 | 1365.35 ± 609.50 | 1250.24 (1129.1 – 1384.4) |
| Urinary protein (g/24h) | 67 | 0.20 ± 0.28 | 0.14 (0.12 – 0.17) |
| Creatinine clearance (ml/min/1.73m2) | 70 | 130.24 ± 29.40 | 125.02 (118.85 - 139.73) |
| Total renal volume (mm3) | 61 | 653,795 ± 573,871 | 506,345 (423,634 – 604,464) |
| Total cyst volume (mm3) | 61 | 430,273 ± 534,914 | 254,174 (194,691 – 331,832) |
| Renal cyst count | 61 | 74.5 ± 42.6 | |
| LVMI (g/m2) | 66 | 61.81 ± 22.20 | 58.13 (53.29 – 63.41) |
Abbreviations: Ang, angiopoietin; BSA, body surface area; CI, confidence interval; LVMI, left ventricular mass index; VEGF, vascular endothelial cell growth factor.
Renal structure
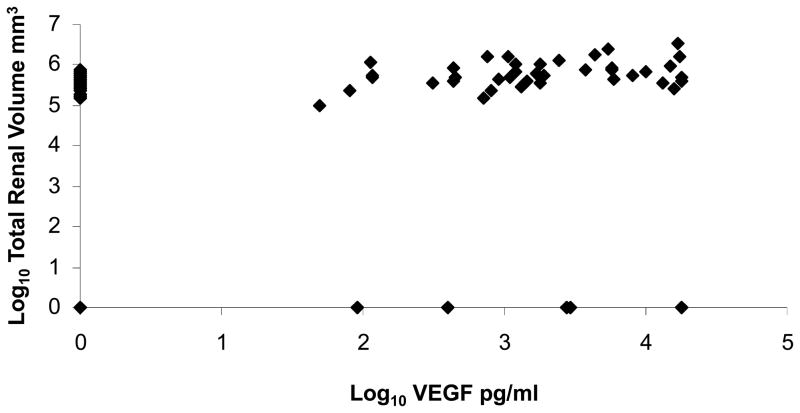
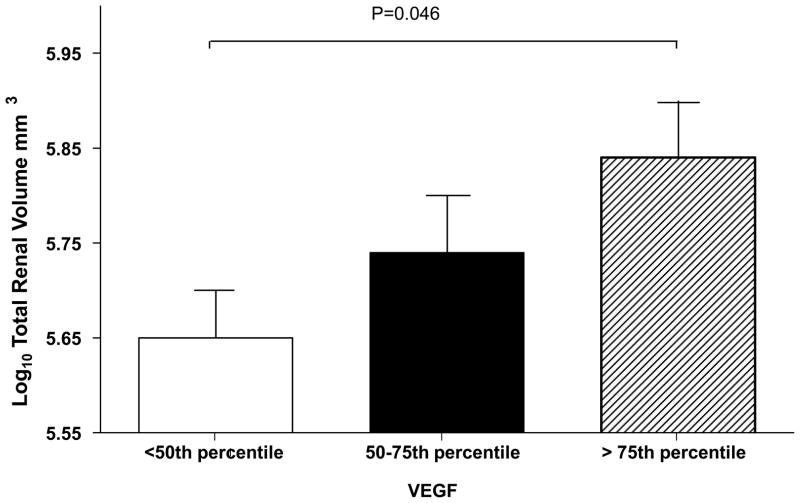
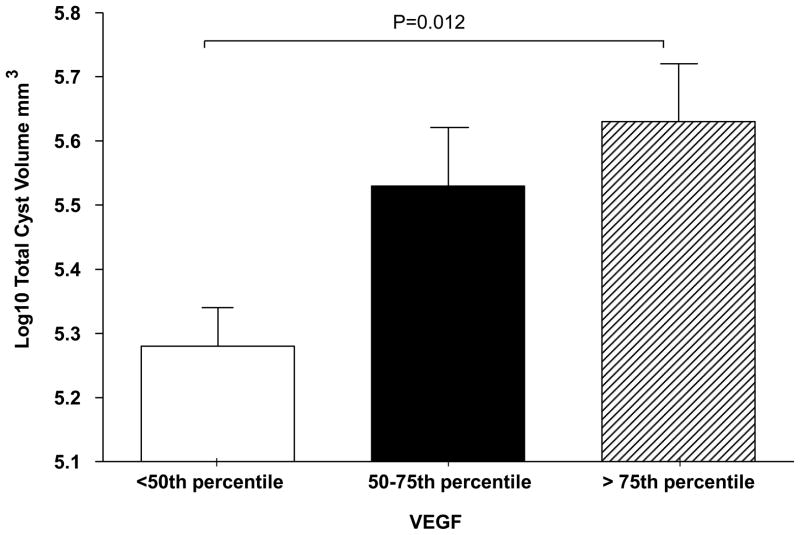
Serum VEGF was strongly positively correlated with log10 total renal and cyst volume (Table 2). The linear relationship between log10 VEGF and total renal volume is illustrated in Figure 1. Subjects with VEGF level > 75th percentile also had significantly th greater log 10 total renal and cyst volumes compared to subjects with VEGF level < 50 percentile after correction for age and sex based on a P-value adjusted by Tukey-Kramer correction for multiple comparisons (21) (Figures 2, 3) Serum Ang-1 was significantly positively correlated with log10 total renal volume (P = 0.04) after correction for age, sex and body surface area (Table 2). There were no significant relationships between renal structural parameters and Ang-2. However, the ratio of Ang-1/Ang-2 was significantly and positively correlated with log10 total renal volume, total cyst volume and cyst count.
Table 2.
Relationship of VEGF, Ang-1, Ang-2, and Ratio of Ang-1/Ang-2 with renal structure
| Dependent Variable | |||
|---|---|---|---|
| Independent Variable | β | SE | P-value |
| Log10 Total Renal Volume | |||
| Log10VEGF | 0.0511 | 0.0183 | 0.0073 |
| Ang-1 | 0.0029 | 0.0014 | 0.0448 |
| Ang-2 | −0.00002 | 0.00003 | 0.5164 |
| Ang-1/Ang-2 | 0.1611 | 0.0763 | 0.0393 |
| Log10 Total Renal Cyst Volume | |||
| Log10VEGF | 0.0862 | 0.0273 | 0.0026 |
| Ang-1 | 0.004 | 0.0022 | 0.0680 |
| Ang-2 | −0.00001 | 0.00005 | 0.9039 |
| Ang-1/Ang-2 | 0.2616 | 0.1242 | 0.0397 |
| Log10 Total Cyst Number | |||
| Log10VEGF | −0.0199 | 0.0192 | 0.3027 |
| Ang-1 | 0.0025 | 0.0014 | 0.0789 |
| Ang-2 | −0.00003 | 0.00003 | 0.3547 |
| Ang-1/Ang-2 | 0.1923 | 0.0730 | 0.0109 |
Abbreviations: Ang, angiopoietin; VEGF, vascular endothelial cell growth factor.
Figure 1.
Scatter plot of serum log10 vascular endothelial growth factor (VEGF) and log10 total renal volume.
The plot depicts the uncorrected relationship between renal volume and serum VEGF. VEGF pg/ml and renal volume mm3.
Figure 2.
Total renal volume expressed by quartile of serum vascular endothelial growth factor (VEGF) level.
Renal volume was adjusted for age, sex, and body surface area. VEGF range < 50th percentile < 440 pg/ml; 50–75th percentile 224–2750 pg/ml; > 75th percentile 2950–18,000 pg/ml. All P-values were corrected for multiple comparisons by the Tukey-Kramer correction (21).
Figure 3.
Total cyst volume expressed by quartile of serum vascular endothelial growth factor (VEGF) level.
Cyst volume was adjusted for age and sex. VEGF range/quartile as presented in legend for Figure 2. All P-values were corrected for multiple comparisons by the Tukey-Kramer correction (21).
Renal function
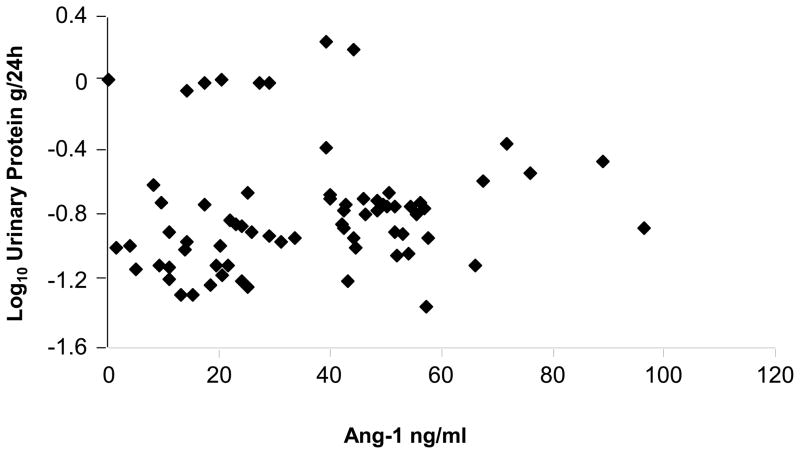
There were significant correlations between Ang-1 and log10 urinary protein excretion (P = 0.009) after correction for both age and sex (Table 3). The linear relationship is depicted in Figure 4. There were no significant relationships between serum VEGF or Ang-2 levels and urinary protein excretion. The ratio Ang-1/Ang2 was significantly correlated with log10 urinary protein excretion (Table 3).
Table 3.
Relationship of VEGF, Ang-1, Ang-2, and Ratio of Ang-1/Ang-2 with renal functional parameters including urinary protein and creatinine clearance
| Dependent Variable | |||
|---|---|---|---|
| Independent Variable | β | SE | p-value |
| Log10 Urinary Protein Excretion | |||
| Log10VEGF | 0.0401 | 0.0209 | 0.0592 |
| Ang-1 | 0.0042 | 0.0015 | 0.0093 |
| Ang-2 | 0.00002 | 0.00004 | 0.5658 |
| Ang-1/Ang-2 | 0.2050 | 0.0815 | 0.0145 |
| Log10 Creatinine Clearance | |||
| Log10VEGF | −0.0165 | 0.0070 | 0.0224 |
| Ang-1 | −0.0010 | 0.0005 | 0.3020 |
| Ang-2 | −0.00002 | 0.00001 | 0.1676 |
| Ang-1/Ang-2 | −0.0102 | 0.0291 | 0.7283 |
Abbreviations: Ang, angiopoietin; VEGF, vascular endothelial cell growth factor.
Figure 4.
Scatter plot of serum angiopoietin (Ang)-1 and log10 urinary protein excretion
The plot depicts the uncorrected relationship between 24h urinary protein excretion and serum Ang-1 level. Ang-1 ng/ml and urinary protein g/24h.
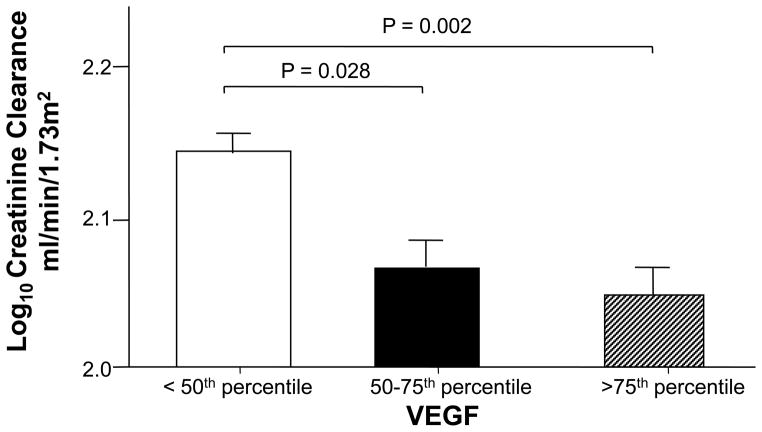
In this group of young patients with ADPKD and renal function largely preserved (creatinine clearance mean 130.24 ml/min/1.73 m2), there was a significant negative relationship between log10 VEGF and log10 creatinine clearance (P = 0.02; Table 3). Subjects with VEGF level > 75th percentile had significantly lower creatinine clearance compared to those with VEGF level < 50th percentile after correction for multiple comparisons (Figure 5). No significant relationships between Ang-1, Ang-2, Ang-1/Ang-2 and creatinine clearance were found.
Figure 5.
Creatinine clearance expressed by quartile of serum vascular endothelial growth factor (VEGF) level.
Creatinine clearance was corrected for height and weight and VEGF range/quartile is presented in legend for Figure 2. All P-values were corrected for multiple comparisons by the Tukey-Kramer correction (21).
LVMI
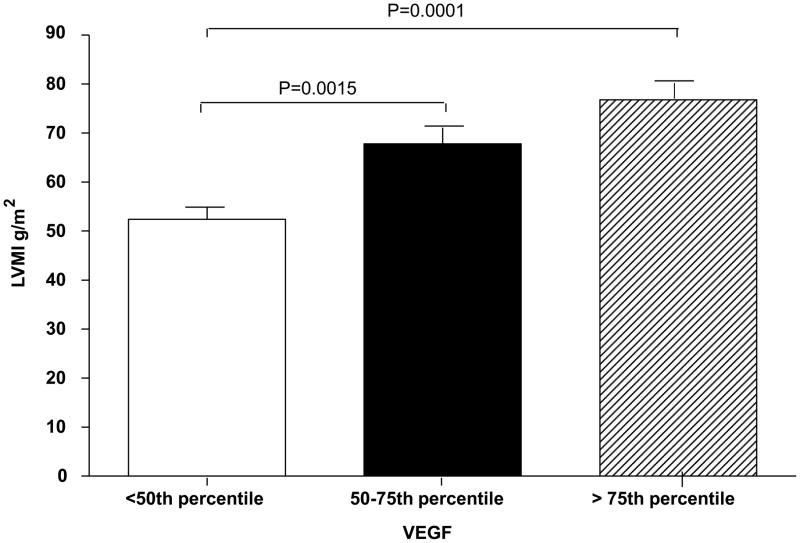
Serum VEGF and Ang-1 were positively correlated with log 10 LVMI after correction for age and sex (P ≤ 0.0001 and P =0.04; Table 4). There was a linear relationship between th log10 VEGF and log10 LVMI as shown in Figure 6. Subjects with VEGF level > 75 percentile had significantly higher LVMI compared with those with VEGF level between 50th and 75th percentile and those with VEGF < 50th percentile after correction for age, sex, and correction for multiple comparisons (Figure 7). Likewise, subjects with Ang-1 level > 75th percentile had significantly higher LVMI compared with those with Ang-1 level < 50th percentile (P = 0.013) after correction for age, sex, and correction for multiple comparisons (data not shown). There were no significant relationships between either Ang-2 level or the ratio Ang-1/Ang-2 and LVMI.
Table 4.
Relationship of VEGF, Ang-1, Ang-2, and Ratio of Ang-1/Ang-2 with LVMI
| Dependent Variable | |||
|---|---|---|---|
| Independent Variable | β | SE | p-value |
| Log10VEGF | 0.0409 | 0.0078 | < .0001 |
| Ang-1 | 0.0014 | 0.0007 | 0.0407 |
| Ang-2 | 0.00000 | 0.00002 | 0.8592 |
| Ang-1/Ang-2 | 0.0397 | 0.0344 | 0.2531 |
Abbreviations: Ang, angiopoietin; LVMI, left ventricular mass index; VEGF, vascular endothelial cell growth factor.
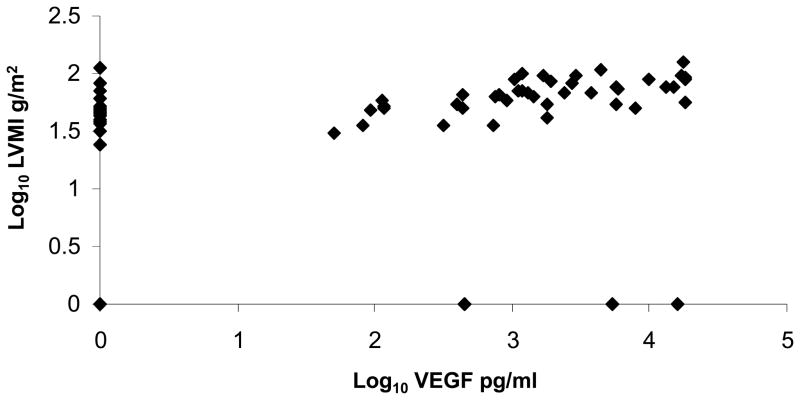
Figure 6.
Title: Scatter plot of serum log10 vascular endothelial growth factor (VEGF) and log10 left ventricular mass index (LVMI).
The plot depicts the relationship between serum VEGF and LVMI. VEGF pg/ml and LVMI g/m2.
Figure 7.
Left ventricular mass index (LVMI) expressed by quartile of serum vascular endothelial growth factor (VEGF) level.
LVMI is already adjusted for body surface area but age and sex were included as covariates. VEGF range/quartile as presented in legend for Figure 2. All P-values were corrected for multiple comparisons by the Tukey-Kramer correction (21).
Relationship between VEGF and Ang-1
Although the distribution of serum VEGF was highly skewed, log10 VEGF was significantly positively correlated with Ang-1 level (r = 0.37, P = 0.0019) but not with Ang-2 or with the ratio Ang-1/Ang-2. Forward selection was used in multiple linear regressions to determine independent effects of Ang-1, Ang-2, and VEGF on all dependent variables. Table 5 shows that VEGF was selected over Ang-1 in the regression of total renal volume, total cyst volume, LVMI and creatinine clearance. However, Ang-1 was selected over VEGF for urinary protein excretion. The correlation of VEGF to Ang-1 and their individual relationships to ADPKD phenotypes suggest a mediation effect of VEGF on Ang-1.
Table 5.
Stepwise Linear Regression Models using Forward Selection to determine independent effects of Ang-1, Ang-2 and VEGF
| Dependent Variable | |||
|---|---|---|---|
| Independent Variable | Partial R2 | Model R2 | p-value |
| Log10 Total Renal Volume | |||
| Age | 0.2863 | 0.2863 | <.0001 |
| Sex | 0.1379 | 0.4242 | 0.0006 |
| Log10VEGF | 0.0723 | 0.4965 | 0.0068 |
| Ang-1 | 0.0163 | 0.5128 | 0.1846 |
| Ang-2 | 0.0065 | 0.5193 | 0.4013 |
| Log10 Total Renal Cyst Volume | |||
| Age | 0.2722 | 0.2722 | <.0001 |
| Sex (Male) | 0.1314 | 0.4036 | 0.0010 |
| Log10VEGF | 0.0929 | 0.4966 | 0.0026 |
| Ang-1 | 0.0070 | 0.5036 | 0.3908 |
| Log10 Total Cyst Number | |||
| Sex | 0.0941 | 0.0941 | 0.0181 |
| Age | 0.0698 | 0.1638 | 0.0349 |
| Ang-1 | 0.0490 | 0.2128 | 0.0697 |
| Log10VEGF | 0.0539 | 0.2668 | 0.0513 |
| Ang-2 | 0.0150 | 0.2818 | 0.2973 |
| Log10 LVMI | |||
| Log10VEGF | 0.3965 | 0.3965 | <.0001 |
| Age | 0.1446 | 0.5412 | <.0001 |
| Sex | 0.0764 | 0.6175 | 0.0009 |
| Log10 Urinary Protein Excretion | |||
| Sex | 0.1943 | 0.1943 | 0.0002 |
| Ang-1 | 0.0989 | 0.2932 | 0.0042 |
| Log10VEGF | 0.0143 | 0.3075 | 0.2616 |
| ANG2 | 0.0062 | 0.3137 | 0.4607 |
| Log10 Creatinine Clearance | |||
| Log10VEGF | 0.0801 | 0.0801 | 0.0224 |
| Ang-2 | 0.0308 | 0.1108 | 0.1480 |
Abbreviations: Ang, angiopoietin; VEGF, vascular endothelial cell growth factor.
Note that all models included Log10VEGF, Ang-1, Ang-2 and appropriate covariates. Ratio of Ang-1/Ang-2 was not included owing to significant colinearity with Ang-1 and Ang-2. Entries represent a summary of forward selection with variables that remained in the model and the order of selection. P < 0.15 was required to remain in the model.
Discussion
In this study we examined the relationship between the angiogenic growth factors VEGF, Ang-1, and Ang-2 and renal and cardiovascular disease progression in young patients with ADPKD. Significant relationships were observed between levels of VEGF, Ang-1, and Ang-1/Ang-2 and renal structural disease, including total renal volume, total cyst volume, and total cyst number (Table 2). These results are indicative of a potential role for upregulated angiogenesis in early renal cyst progression. Indeed, evidence of angiogenesis has previously been demonstrated on the surface of renal cysts from patients with ADPKD (7,8).
Both human liver and renal ADPKD cyst fluids contain VEGF, and a role for angiogenic growth factors in the growth of liver cysts in ADPKD has previously been demonstrated (6,8). Expression of VEGF, VEGF receptor 1, Ang-1, and Tie2 are strongly upregulated in liver cholangiocytes from ADPKD patients (10), and inhibition of the VEGF receptor is known to inhibit liver cyst growth in the pkd2(WS25/−) mouse model of ADPKD2, supporting a role for angiogenesis in liver cyst growth (22). However, the current study to the best of our knowledge, is the first to support a relationship between increased VEGF and angiopoietin with structural renal disease progression in human ADPKD. In view of the potential antagonistic effect of Ang-2 on Ang-1 activity (14–16), the Ang-1/Ang-2 ratio may impact biological activity. We therefore also assessed the affect of the ratio on renal and cardiac disease severity. Ang-1/Ang-2 was significantly related to total cyst volume and notably the only measure significantly, albeit weakly, related to total cyst number (P = 0.01).
The serum level of VEGF and Ang-2 also appears to be higher in young ADPKD patients compared to previously reported values for healthy young subjects or those with pathological conditions associated with elevated angiogenic growth factors. In the current study the mean serum VEGF level was 2997 pg/ml compared to a literature reported mean of 306 pg/ml in healthy children (23). In order to assess whether the elevation in serum VEGF reflected impaired renal clearance due to mild renal functional impairment or loss of renal reserve, we compared serum VEGF levels in young diabetic patients who were matched by age, sex, and renal function with the ADPKD patients. Mean serum VEGF level was significantly lower in the diabetic patients compared with the level in the ADPKD patients. Similarly, Ang-2 level in ADPKD was 2350 pg/ml compared to a literature reported control level of 68 (68–1330) pg/ml (14). Mysliwiec et al (24) reported a serum VEGF level of 312 (0–803) pg/ml in young patients with diabetic retinopathy and a healthy control level of 94 (13–328) pg/ml. This is also lower than the levels observed in ADPKD patients in the current study. It should also be noted that renal function was still within the normal range in the young ADPKD subjects in the current study. Serum angiogenic growth factor levels were also significantly related to renal function. Ang-1 levels were significantly correlated with urinary protein excretion (P = 0.009; Table 3). Previous animal studies have demonstrated strong time-dependent expression of Ang-1 in glomeruli of a diabetic rat model, and correlation with glomerular volume, glomerular area, and urinary protein (25). Serum VEGF level was correlated with creatinine clearance (P = 0.022; Table 3). This result indicates that serum VEGF may indicate very early renal changes as kidney function is essentially preserved in young patients with ADPKD. The mean creatinine clearance in the current study was 130.24 ± 29.4 ml/min/1.73 m2 (Table 1).
Significant positive correlations between LVMI and Ang-1 and VEGF were also demonstrated in the current study. In particular, log10 transformed VEGF level was strongly related to LVMI after correction for age and sex (P < 0.0001) (Table 4, Figure 7). This is of particular relevance as patients with ADPKD are at an increased risk for left ventricular hypertrophy (LVH) (26). Moreover, higher serum VEGF level in young ADPKD patients in the current study was associated with elevated LVMI even in the absence of overt hypertension. We have previously shown increased LVMI in children with ADPKD who are hypertensive or who have borderline hypertension (blood pressure between 75th and 95th percentile for age, height, and sex) (5). Identification of children at risk for cardiovascular complications is important because cardiac magnetic resonance imaging and/or echocardiography are not routinely performed on young patients with ADPKD. However, future studies to investigate the role of angiogenic growth factors in the etiology of cardiac damage in ADPKD will be necessary
Previous studies have demonstrated increased plasma and platelet levels of Ang-1 and VEGF in hypertension that decreased with treatment (19). In the current study 26 subjects were hypertensive (blood pressure ≥ 95th percentile for age, height, and sex) and 45 were normotensive. However, there was no effect of Ang-1 (odds ratio = 0.993 (0.969–1.018), P = 0.59) and VEGF (odds ratio= 0.765 (0.548–1.068), P = 0.1159) on the presence of hypertension. Moreover, it has previously been shown that hypertensive patients with target organ damage (stroke, myocardial infarction, angina, left ventricular hypertrophy, and mild renal failure) had higher levels of VEGF and Ang-1 (20). This report agrees with the findings of our current study and indicates that both Ang-1 and VEGF may have potential roles in the early stages of renal and cardiac damage in ADPKD.
In the current study, a relationship between VEGF level and Ang-1 was demonstrated. Furthermore, forward selection in multiple linear regression used to assess independent effects of Ang-1 and VEGF on all dependent variables suggest a mediation effect of VEGF on Ang-1 (Table 5). Stimulation of Ang-1 by VEGF has previously been demonstrated in bovine retinal pericytes (27). In addition a recent study demonstrated that VEGF activates Tie2 via proteolytic cleavage of the Tie1 tyrosine kinase resulting in transphosphorylation and activation of Tie2 (28). This suggests a synergistic action of the angiopoietin and VEGF growth factors. However, while VEGF appeared to have a stronger relationship to renal structural parameters and LVMI, notably each growth factor had a unique relationship between specific renal functional parameters as evidenced by the relationship between VEGF and creatinine clearance and Ang-1 with urinary protein excretion.
Activation of the renin-angiotensin system occurs early in ADPKD (29–32) and may in part explain the upregulated expression of both VEGF and angiopoietins. In rats increased renal expression of VEGF, Ang-1, and Ang-2 following infusion of angiotensin has previously been demonstrated (33,34). Furthermore angiotensin II has been shown to stimulate division of human endothelial cells in culture and to upregulate expression of VEGF (35). These effects were blocked by candesartan implicating involvement of the angiotensin I receptor.
In conclusion VEGF level increases with expanding kidney volume and rise in LVMI, possibly reflecting a role of this growth factor in early renal and cardiac disease progression in ADPKD. Future animal and cell studies that focus on angiogenesis in ADPKD models will be required to unravel the involved molecular mechanisms, but may provide potential new therapeutic targets for ADPKD.
Methods
Patient description and clinical evaluation
All patients gave informed consent to a protocol that was approved by the Colorado Multiple Institute Review Board. Patients were recruited from those participating in ongoing clinical studies of ADPKD at the University of Colorado Denver between 1990–2009. ADPKD diagnosis was based on radiographic imaging and Ravine’s cirteria (36); all patients included in this study had a positive family history of ADPKD. All patients were studied during a 2-day in-patient visit at the Pediatric Clinical Translational Research Center at the Children’s Hospital in Denver Colorado as described previously (5). Serum and urine creatinine, height, and weight were used to calculate creatinine clearance for all subjects. Blood was drawn for serum chemistries and growth factor assay. Two 24h urines were collected for urinary protein excretion and the value reported is the mean for both 24h urines. Urinary protein was determined in the hospital core laboratory by the pyrogallol red method. Left ventricular mass was determined by standard two-dimensional and Doppler echocardiogram as previously described (5) or by cardiac magnetic resonance imaging on a 1.5 T Siemens system (Siemens, Malvern, PA, USA). Cardiac-gated breath-hold 2 dimensional true-Fast Imaging with Steady State Precession (Fast Imaging Employing Steady-state Aquisition) short-axis cine images were obtained to cover the left ventricle atrioventricular ring to apex (10 mm slice thickness, no gap, field of view 250–320 mm; 10–15 breath holds to cover the whole ventricle). The imaging protocol consisted of localizers in three orthogonal planes (axial, coronal and sagital). After the data were acquired a Leonardo (Siemens Medical System) workstation was used to manually contour endocardial and epicardial contours of the left ventricle in end-diastole and end-systole and left ventricular mass was calculated. Left ventricular mass was corrected by body surface area and reported as LVMI in g/m2.
Angiopoietin and VEGF assay
Patient sera were stored at –800C before assay and all were samples assayed in duplicate. Ang-1 and VEGF were assayed by enzyme-linked immunosorbent assay using kits from Raybiotech (Norcross, GA, USA). The intra- and inter-assay coefficient of variability of the Ang-1 assay was < 10% and < 12%, respectively, and lower limit of detection 30pg/ml. The VEGF intra- and inter-assay coefficient of varaibility was < 10 and <12%, respectively, with lower limit of detection 20 pg/ml. Serum Ang-2 was assayed by Quantikine enzyme-linked immunosorbent assay from R & D Systems (Minneapolis, MN, USA). The intra- and inter-assay reproducibility was 7 and 10.4%, respectively, with lower limit of detection, 21.3 pg/ml.
Statistical analysis
All variables were checked for the distributional assumption of normality. Owing to the highly skewed distributions of all outcome variables, log10 transformations were applied and the transformed variables were used in all analyses. Log10 transformations were also applied to VEGF and the ratio of Ang-1/Ang-2. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA). Descriptive statistics are presented as mean, s.d., and 95% confidence interval or geometric mean and 95% confidence interval. Univariate linear regression was used to test the relationship of VEGF, Ang-1, Ang-2, and the ratio of Ang-1/Ang-2 on ADPKD phenotypes including total renal volume, total cyst number, total cyst volume, creatinine clearance, LVMI, and urinary protein excretion. Stepwise linear regression was used with the forward selection method to determine independent effects of Ang-1, Ang-2, and VEGF on all dependent variables. No correction for multiple endpoints was applied; however, the primary hypothesis was that Ang-1 would be related to kidney volume measures. In linear regression, renal volume was adjusted for age, sex and body surface area, renal cyst volume was adjusted for age and sex. Creatinine clearance was corrected for body surface area, and proteinuria were corrected for age and sex. LVMI was already adjusted for body surface area, but age and sex were included as covariates. Logistic regression was used to test the relationship of VEGF, Ang-1, Ang-2, and ratio Ang-1/Ang-2 with hypertension. In analysis of total renal volume, total cyst volume, creatinine clearance, and LVMI by quartile of VEGF; all P-values were corrected for multiple comparisons by the Tukey-Kramer correction (21).
Acknowledgments
This research was supported by grant numbers M01RR00051, M01RR00069 General Research Centers Program, National Center for Research Resources (NCRR)/NIH; by NIH/NCRR Colorado CTSI grant number ULI RR025780 by grants DK34039 and DK090005 from NIH(NIDDK) and by the Zell Family Foundation. The content of this publication are the authors sole responsibility and do not necessarily represent the official NIH views,
Footnotes
Disclosure
All the authors declared no competing interests.
References
- 1.Ecder T, Fick-Brosnahan G, Schrier RW. Polycystic kidney disease. In: Schrier RW, editor. Diseases of the Kidney and Urinary Tract. Vol. 1. Lipincott, Williams and Wilkins; Philadelphia: 2007. pp. 502–539. [Google Scholar]
- 2.Chapman AB, Guay-Woodford LM, Grantham JJ, et al. Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int. 2003;64:1035–1045. doi: 10.1046/j.1523-1755.2003.00185.x. [DOI] [PubMed] [Google Scholar]
- 3.Seeman T, Dusek J, Vondrichova H, et al. Ambulatory blood pressure correlates with renal volume and number of renal cysts in children with autosomal dominant polycystic kidney disease. Blood Press Monit. 2003;8:107–110. doi: 10.1097/01.mbp.0000085762.28312.4a. [DOI] [PubMed] [Google Scholar]
- 4.Fick-Brosnahan GM, Tran ZV, Johnson AM, et al. Progression of autosomal dominant polycystic kidney disease in children. Kidney Int. 2001;5:1654–1662. doi: 10.1046/j.1523-1755.2001.0590051654.x. [DOI] [PubMed] [Google Scholar]
- 5.Cadnapaphornchai MA, McFann K, Strain JD, et al. Increased left ventricular mass in children with autosomal dominant polycystic kidney disease and borderline hypertension. Kidney Int. 2008;74:1192–1196. doi: 10.1038/ki.2008.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cadnapaphornchai M, McFann K, Strain JD, et al. Prospective changes in renal volume and function in children with ADPKD. Clin J Am Soc Nephrol. 2009;4:820–829. doi: 10.2215/CJN.02810608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bello-Reuss E, Holubec K, Rajaraman S, et al. Angiogenesis in autosomal dominant polycystic kidney disease. Kidney Int. 2001;60:37–45. doi: 10.1046/j.1523-1755.2001.00768.x. [DOI] [PubMed] [Google Scholar]
- 8.Wei W, Popov V, Walocha JA, et al. Evidence of angiogenesis and microvascular regression in autosomal-dominant polycystic kidney disease kidneys: A corrosion cast study. Kidney Int. 2006;70:1261–1268. doi: 10.1038/sj.ki.5001725. [DOI] [PubMed] [Google Scholar]
- 9.Nichols MT, Gidney E, Matzakos T, et al. Secretion of cytokines and growth factors into ADPKD liver cyst fluid. Hepatology. 2004;40:836–846. doi: 10.1002/hep.20401. [DOI] [PubMed] [Google Scholar]
- 10.Fabris L, Cadamuro M, Fiorotto R, et al. Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver disease. Hepatology. 2004;43:1001–1012. doi: 10.1002/hep.21143. [DOI] [PubMed] [Google Scholar]
- 11.Otrock ZK, Mahfouz RAR, Makarem JA, et al. Understanding the biology of angiogenesis: Review of the most important molecular mechanisms. Blood Cells Mol Dis. 2007;39:211–220. doi: 10.1016/j.bcmd.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Augustin HG, Koh GY, Thurston G, et al. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nature Reviews Mol Cell Biol. 2009:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 13.Suri C, McClain J, Thurston G, et al. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282:468–471. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- 14.Lovegrove FE, Tangpukdee N, Opoka RO, et al. Serum Angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PlosOne. 2009;4:e4912, 2009. doi: 10.1371/journal.pone.0004912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fielder U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27:552–558. doi: 10.1016/j.it.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Bolin M, Wiberg-Itzel E, Wilkstrom AK, et al. Angiopoietin-1/angiopoietin-2 ratio for prediction of preeclampsia. Am J Hypertens. 2009;22:891–895. doi: 10.1038/ajh.2009.97. [DOI] [PubMed] [Google Scholar]
- 17.Lim HS, Lip GYH, Blann AD. Angiopoietin-1 and angiopoietin-2 in diabetes mellitus: relationship to VEGF, glycaemic control, endothelial damage/dysfunction and atherosclerosis. Atherosclerosis. 2005;180:113–118. doi: 10.1016/j.atherosclerosis.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Sascha D, Kumpers P, Hellpap J, et al. Angiopoietin 2 and cardiovascular disease in dialysis and kidney transplantation. Am J Kid Dis. 2009;53:770–778. doi: 10.1053/j.ajkd.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 19.Nadar SK, Blann AD, Lip GYH. Plasma and platelet-derived vascular endothelial growth factor and angiopoietin-1 in hypertension: effects of antihypertensive therapy. J Int Med. 2004;256:331–337. doi: 10.1111/j.1365-2796.2004.01367.x. [DOI] [PubMed] [Google Scholar]
- 20.Nadar SK, Blann A, Beevers DG, et al. Abnormal angiopoietins 1 & 2, angiopoietin receptor Tie-2 and vascular endothelial growth factor levels in hypertension: relationship to target organ damage [a sub-study of the Anglo-Scandanavian Cardiac Outcomes Trial (ASCOT)] J Int Med. 2004;258:336–343. doi: 10.1111/j.1365-2796.2005.01550.x. [DOI] [PubMed] [Google Scholar]
- 21.Kramer CY. Extension of multiple range tests to group means with unequal numbers of replications. Biometrics. 1966;12:307–310. [Google Scholar]
- 22.Amura CA, Brodsky KS, Groff R, et al. VEGF receptor inhibition blocks liver cyst growth in pkd(WS25/−)mice. Am J Cell Physiol. 2007;293:C419–C428. doi: 10.1152/ajpcell.00038.2007. [DOI] [PubMed] [Google Scholar]
- 23.Heshmat NM, El-Kerdany TH. Serum levels of vascular endothelial growth factor in children and adolescents with sytemic lupus erythematosus. Pediatr Allergy Immunol. 2007;18:346–353. doi: 10.1111/j.1399-3038.2006.00510.x. [DOI] [PubMed] [Google Scholar]
- 24.Mysliwiec M, Balcerska A, Zorena K, Mysliwiec J, Lipowski P, Raczynska K. The role of vascular endothelial growth factor, tumor necrosis factor alpha and interleukin-6 in pathogenesis of diabetic retinopathy. Diabetes Care Clin Practice. 2008;79:141–146. doi: 10.1016/j.diabres.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Yang YB, Liu F, Huang SM. The expression and significance of the angiopoietin-1 in the kidney of diabetic rats. Sichuan Da Xue Xue Bao Yi Xue Ban. 2007;38:93–96. [PubMed] [Google Scholar]
- 26.Chapman AB, Johnson AM, Rainguet S, et al. Left ventricular hypertrophy in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1997;8:1292–1297. doi: 10.1681/ASN.V881292. [DOI] [PubMed] [Google Scholar]
- 27.Park YS, Kim NH, Jo I. Hypoxia and vascular endothelial growth factor acutely up-regulate angiopoietin-1 and Tie2 mRNA in bovine retinal pericytes. Microvasc Res. 2003;65:125–131. doi: 10.1016/s0026-2862(02)00035-3. [DOI] [PubMed] [Google Scholar]
- 28.Singh H, Milner CS, Aguilar Hernandez MM, et al. Vascular endothelial growth factor activates the tie family of receptor tyrosine kinases. Cell Signal. 2009;21:1346–1350. doi: 10.1016/j.cellsig.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Chapman AB, Johnson AM, Gabow P, et al. The renin-angiotensin-aldosterone system and autosomal polycystic kidney disease. N Eng J Med. 1990;323:1091–1096. doi: 10.1056/NEJM199010183231602. [DOI] [PubMed] [Google Scholar]
- 30.Schrier RW. Renal volume, renin-angiotensin-aldosterone system, hypertension, and left ventricular hypertrophy in patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2009;20:1888–1893. doi: 10.1681/ASN.2008080882. [DOI] [PubMed] [Google Scholar]
- 31.Barrett BJ, Foley R, Morgan J, et al. Differences in hormonal and renal vascular responses between normotensive patients with autosomal dominant polycystic kidney disease and unaffected family members. Kidney Int. 1994;46:1118–1123. doi: 10.1038/ki.1994.374. [DOI] [PubMed] [Google Scholar]
- 32.Harrap SB, Davies DL, Macnicol AM, et al. Renal, cardiovascular and hormonal characteristics of young adults with autosomal dominant polycystic kidney disease. Kidney Int. 1991;40:501–508. doi: 10.1038/ki.1991.238. [DOI] [PubMed] [Google Scholar]
- 33.Rizkalla B, Forbes JM, Cooper ME, et al. Increased renal vascular endothelial growth factor and angiopoietins by angiotensin II infusion is mediated by both AT1 and AT2 receptors. J Am Soc Nephrol. 2003;14:3061–3071. doi: 10.1097/01.asn.0000099374.58607.c9. [DOI] [PubMed] [Google Scholar]
- 34.Kitayama H, Maeshima Y, Takazawa Y, et al. Regulation of angiogenic factors in angiotensin II infusion model in association with tubulointerstitial injuries. Am J Hypertens. 2006;19:718–727. doi: 10.1016/j.amjhyper.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 35.Herr D, Rodewald M, Fraser HM, et al. Regulation of endothelial proliferation by the renin-angiotensin system in human umbilical vein endothelial cells. Reproduction. 2008;136:125–130. doi: 10.1530/REP-07-0374. [DOI] [PubMed] [Google Scholar]
- 36.Ravine D, Gibson RN, Walker RG, et al. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney 1. Lancet. 1994;343:824–827. doi: 10.1016/s0140-6736(94)92026-5. [DOI] [PubMed] [Google Scholar]