Abstract
Progressive-ratio schedules are useful for studying reinforcing effects of drugs. Previous human laboratory studies showed that d-amphetamine significantly increased break points relative to placebo levels. However, the magnitude of the increase was modest, which may be attributable to rather high levels of placebo responding. We utilized novel response requirements under the modified progressive-ratio schedule and hypothesized that the altered range of response requirements would decrease responding for placebo and increase responding for d-amphetamine. Eight participants completed the study. The participants first sampled oral doses of d-amphetamine (0, 8, 16 and 24 mg). In subsequent sessions, participants were offered the opportunity to work for the sampled dose on a modified progressive-ratio procedure with response requirements ranging from 400 to 1800 mouse clicks. A battery of participant-rated drug-effect questionnaires, a performance measure and cardiovascular measures were included in orderto characterize more fully the effects of d-amphetamine. Placebo maintained low levels of responding. The intermediate dose of d-amphetamine increased responding significantly above placebo levels. d-Amphetamine produced prototypical subject-rated effects that were an orderly function of dose. The present data suggest that the modified response requirements resulted in lower levels of placebo taking and a larger separation between number of placebo and d-amphetamine capsules earned.
Keywords: Drug Reinforcement, Drug Self-Administration, Progressive Ratio, Subjective Effects, Human, Amphetamine
Introduction
The reinforcing effects of drugs are central to their abuse (Foltin and Fischman, 1991). Progressive-ratio schedules have been useful in assessing the reinforcing effects of abused drugs (Richardson and Roberts, 1996; Stoops, 2008). Under a progressive-ratio schedule, the response requirement (i.e., ratio) for obtaining each subsequent reinforcer is progressively increased, until the participant ceases to respond. The final ratio completed to obtain a reinforcer is termed as the break point (often the dependent measure), which presumably reflects the maximum effort that a participant will expend to receive the reinforcer (Richardson and Roberts, 1996). Commonly abused stimulants like cocaine, d-amphetamine, methamphetamine and methylphenidate dose-dependently increased breakpoints on progressive-ratio schedules in animals (Griffiths et al., 1975; 1979; Poncelet et al., 1983; Ranaldi and Wise 2000; Woolverton and Wang, 2004; Brebner et al., 2005). Progressive-ratio schedules are widely used in preclinical studies because they are thought to measure the reward strength or reinforcing efficacy of a drug dose (for reviews see Richardson and Roberts, 1996; Stafford et al., 1998).
Progressive-ratio schedules have also been adapted for use in humans. Laboratory studies conducted in humans have shown that progressive-ratio schedules are sensitive to the reinforcing effects of abused stimulants. For example, methylphenidate maintained responding under a progressive-ratio schedule and increased break points (i.e., the last ratio completed) significantly above placebo levels in humans (Stoops et al., 2004). Several previous studies have assessed reinforcing effects of oral d-amphetamine using a progressive-ratio schedule modified for use with humans (Rush et al., 2001; Stoops et al., 2004, 2007a,b). In the first two of our studies, the response requirement under the progressive-ratio schedule ranged from 50 to 6400 clicks (i.e., 50, 100, 200, 400, 800, 1600, 3200 and 6400 clicks) on a computer mouse (Rush et al., 2001; Stoops et al., 2004), whereas for the other studies the response ratios ranged from 25 to 3200 clicks (i.e., 25, 50, 100, 200, 400, 800, 1600 and 3200 clicks) (Stoops et al., 2007a,b). In each of these studies at least one dose of d-amphetamine increased break points significantly above placebo levels. However, the magnitude of the increase was modest. The small magnitude of effects of d-amphetamine observed in our previous studies may make it difficult to manipulate the reinforcing effects of d-amphetamine pharmacologically.
Also worth noting is that responding for placebo was relatively high in our previous studies that used a progressive-ratio procedure (Rush et al., 2001; Stoops et al., 2004, 2007a,b). On average, participants completed 3.3 of the 8 response ratios to receive placebo. Animal studies have shown that the ability to resolve the behavioral effects of d-amphetamine and related stimulants is blunted under conditions with high levels of baseline operant responding (Smith, 1964; McMillan, 1969). Therefore, the relatively low, albeit statistically significant, self-administration of d-amphetamine observed in our previous studies could be due, in part, to high levels of responding for placebo.
The present experiment was designed to refine the progressive-ratio schedule for assessing the reinforcing effects of d-amphetamine. In this experiment, we utilized novel response requirements (400–1800 clicks) under the modified progressive-ratio schedule. We hypothesized that the altered range of response requirements (i.e., higher “minimal” response requirements and lower “maximal” response requirements) would decrease responding for placebo and increase responding for d-amphetamine, which could enhance our ability to measure reinforcing effects of d-amphetamine. Refining laboratory procedures, such as progressive-ratio schedules, is important if these procedures are to be used for assessing the initial efficacy of potential pharmacotherapies for amphetamine dependence (Haney and Spealman, 2008; Stoops, 2008).
Methods
Participants
Nine healthy adult humans were recruited from the local community, via newspaper advertisements, flyers and word of mouth, to participate in this experiment. One of these participants was discharged from the study because his blood pressure exceeded our predetermined safety criteria (i.e., systolic blood pressure greater than 165 mm Hg) during the sampling session for the high d-amphetamine dose. Data from this participant were not included in the analyses. Eight participants (2 males, 6 females; 7 white, 1 black) completed the experiment. Participants were paid $40 at the end of each session for their participation, and received a $400 completion bonus (i.e., $40 for each completed session) at the end of entire experiment. Thus, participants received a total of $800 for completing the experiment.
Participants ranged in age from 19 to 25 years (mean = 21), in education from 12 to 16 years (mean = 15) and in weight from 53 to 88 kg (mean = 66). All participants reported recreational stimulant use within the past year, but they did not meet DSM-IV criteria for stimulant or other drug use disorders. Five participants reported amphetamine use in the year prior to enrollment; four used Adderall® and one used ecstasy. One participant reported cocaine use in the year prior to enrollment. Two participants reported using amphetamine (Adderall®) and cocaine in the year prior to enrollment. All participants reported lifetime use of marijuana, reporting having used marijuana for 4.6 years on average prior to screening for the study. Five, three and two participants reported lifetime use of opioids, benzodiazepines and hallucinogens, respectively.
Prior to enrollment, all potential participants completed a standard comprehensive medical, physical and psychological screen. Routine clinical laboratory blood and urine chemistry tests. as well as an electrocardiogram. were conducted on all potential participants. Potential participants with histories of serious physical disease, current physical disease (e.g., impaired cardiovascular functioning, chronic obstructive pulmonary disease, etc.), seizure, head trauma or CNS tumors, or current or past histories of serious psychiatric disorder (i.e., Axis I, DSM-IV), including ADHD and substance abuse or dependence disorders (except nicotine), were excluded from participation. All participants were in good health with no contraindications to stimulant medications. Female participants had to report using an effective form of birth control in order to participate and must not have been pregnant. Female participants were also screened for pregnancy (urine HCG; Mainline Technology, Ann Arbor, MI) prior to each session to ensure that they did not continue in the study if pregnant. None of the female participants tested positive for pregnancy throughout the experimental protocol.
The Institutional Review Board of the University of Kentucky Chandler Medical Center approved this study and the informed consent document. Participants signed the informed consent after passing appropriate sobriety tests and prior to enrolling in the study.
General Procedures
Participants enrolled as outpatients at the Laboratory of Human Behavioral Pharmacology at the University of Kentucky Chandler Medical Center. They completed a total of 10 sessions (2 practice sessions and 8 experimental sessions).
Participants were informed that during their participation they would receive various drugs and that these could include placebo and d-amphetamine. Other than this general information, participants were blind to the type of drug administered. They were told that the purpose of the study was to see how different drugs affect mood and behavior and whether they would be willing to work to receive the drugs. Participants were given no instruction of what they were “supposed” to do or of what outcomes might be expected. They were asked to abstain from all drug (illicit and licit) use for the duration of the experiment, with the exception of non-steroidal anti-inflammatory analgesics and nicotine. In addition, participants were also asked not to ingest food or caffeine for 4 h prior to each experimental session, and alcohol for 12 h prior to and following each experimental session.
Experimental sessions were generally conducted daily, Monday through Friday. The time of day at which each session began was held constant for individual participants. On experimental session days, participants followed a daily routine. Each experimental session day participants were first provided a light breakfast. They then provided an expired air sample, which was assayed for the presence of alcohol using an Alco-Sensor breathalyzer (Intoximeters, St. Louis, MO), abd also underwent a field sobriety test. Participants had to provide a breath sample negative for alcohol and pass the field sobriety test to continue with the scheduled experimental session. At the beginning of each session, participants provided a urine sample that was screened for the presence of amphetamines, barbiturates, benzodiazepines, cocaine, opioids and THC. If a urine sample was positive for any drug, other than THC or compounds administered experimentally, the session was canceled until the participant provided a drug-free urine sample. No participants tested positive for the presence of drugs other than those administered experimentally or THC throughout the experimental protocol. Participants who reported the use of tobacco were permitted to smoke one tobacco cigarette midway through the experimental session. They were not able to smoke again until the experimental testing was completed.
Experimental design
Practice sessions
Before beginning the experiment proper, participants completed two ‘practice’ sessions. These practice sessions were used to familiarize participants with the modified progressive-ratio procedure, subject-rated drug-effect questionnaires, performance measure and daily laboratory routine, all of which are described below. No medications were administered on these days.
Experimental sessions
Participants completed the pre-session task between 08.30 h and 08.45 h, the modified progressive-ratio procedure (only on self-administration sessions) between 08.50 h and 10.00 h, ingested drug at approximately 10.00 h, and then completed the subject-rated drug-effect questionnaires and performance task at hourly intervals for 5 h. A minimum of 24 h generally separated all drug administrations. One to three participants were enrolled in this experiment at a time. Participants were instructed not to discuss their drug effects with each other during the sampling or self-administration sessions, or outside the laboratory.
Testing of each of the drug conditions described below consisted of two separate sessions: (i) a sampling session and (ii) a self-administration session. Sampling and self-administration sessions were conducted on separate days. Sampling sessions were immediately followed by self-administration sessions on the next experimental session day.
Sampling sessions
Sampling sessions were conducted to acquaint participants with the effects of each drug dose. After the pre-drug questionnaires were completed and cardiovascular measures recorded, participants were instructed to pay attention to and make notes about the effects of the drug because they would be offered the opportunity to work to receive that drug again in a future session. Participants then ingested eight identical capsules. Participants completed the subject-rated drug-effect questionnaires, cardiovascular measures and performance task at hourly intervals for 5 h. In addition to the standardized subject-rated drug-effect questionnaires, each participant was given a notebook in which they recorded individual comments concerning the effects of the drug dose.
Self-administration sessions
Self-administration sessions were generally conducted 24 h following sampling sessions. Self-administration sessions differed from sampling sessions only in that participants had to earn capsules by responding on a modified progressive-ratio procedure. After the pre-drug questionnaires were completed and cardiovascular measures recorded, participants were given a sheet of paper that contained instructions regarding the self-administration session.
Modified progressive-ratio procedure
During each self-administration session, participants were given eight opportunities to respond on a standard Apple mouse (Apple, Cupertino, CA, USA) to earn all, or some, of the capsules that were administered during the preceding sampling session (i.e. the previous experimental session day). Before each of the eight opportunities to earn a capsule, participants were asked whether they wanted to work for one of yesterday’s capsules. If participants wanted to work, they responded by clicking on a button on the computer screen, labeled “Click here for 1 capsule”. Participants were required to click the computer mouse a predetermined number of times to earn a capsule. To earn the first capsule, participants had to click the mouse 400 times. The number of clicks required to earn each additional capsule was increased by 200 clicks (i.e., 600, 800, 1000, 1200, 1400, 1600 and 1800 clicks). To receive all eight capsules, subjects had to click the mouse a total of 8800 times. While completing each component of the progressive-ratio schedule, participants were also able to terminate the task by clicking on a button labeled stop. The dependent measure on this task was the number of capsules earned.
Participants ingested all of the capsules they earned immediately after completing the modified progressive-ratio schedule. Each capsule contained 12.5% of the total dose of the test drug administered during the preceding the sampling session. Thus, if a participant responded for all eight capsules during a self-administration session, he/she earned the total dose received during the preceding sampling session. After ingesting any capsules earned on the modified progressive-ratio procedure, participants completed the subject-rated drug-effect questionnaires and performance task at hourly intervals for 5 h. If a participant did not respond for any capsules, he/she still completed the subject-rated drug-effect questionnaires and performance task at hourly intervals for 5 h. Participants began completing the subject-rated drug-effects questionnaires and performance tasks at hourly intervals for 5 hours at a fixed time (i.e., approximately 11:00 am). This ensured that participants did not refuse to respond in an attempt to shorten the self-administration sessions.
Self-Report Questionnaires, Performance Task, Cardiovascular Measures
Three self-reported drug-effect questionnaires and a performance task were administered on an Apple Macintosh computer and were completed in a fixed order. These questionnaires were completed approximately 30 min before drug administration, and at hourly intervals for 5 h after drug administration.
Addiction Research Center Inventory (ARCI)
The short form of the ARCI consisted of 49 true/false questions and contained five major subscales: the morphine-benzedrine group (MBG; a measure of euphoria), the pentobarbital, chlorpromazine, alcohol group (PCAG; a measure of sedation), the lysergic acid diethylamide (LSD; a measure of dysphoria), and the benzedrine group and amphetamine scales (BG and A, respectively; Stimulant-Sensitive Scales) (Jasinski, 1977; Martin et al, 1971).
Adjective-Rating Scale
The Adjective-Rating Scale consisted of 32 items and contained two subscales: Sedative and Stimulant. These subscales are sensitive to the acute effects of orally administered sedative and stimulant drugs (Oliveto et al, 1992). Participants rated each item using the computer mouse to point to and select among one of five response options: Not at All, A Little Bit, Moderately, Quite a Bit, and Extremely (scored numerically from 0 to 4, respectively).
Drug-Effect Questionnaire
The questionnaire consisted of 20 items, which were presented on the computer screen one at a time (see Rush et al, 2003 for the items rated). Participants rated each item on a 0–100 scale anchored with “not at all” on the leftmost extreme and “extremely” on the rightmost extreme.
Digit-Symbol-Substitution Test (DSST)
A computerized version of the DSST, which has been described previously, was used in this experiment (McLeod et al, 1982). This measure is sensitive to the effects of orally administered sedative and stimulant drugs (Rush et al, 2003). Briefly, participants used a numeric keypad to enter a geometric pattern associated with one of nine digits displayed on a video screen. Participants had 90 s to enter as many geometric patterns as possible. The dependent measure was the number of patterns completed (i.e., trials completed) and number of patterns entered correctly (i.e., trials correct).
Heart rate and blood pressure
Heart rate and blood pressure were recorded, using an automated blood-pressure monitor (Spot Vital Signs LXi, Welch Allyn, Skaneateles Falls, NY), for approximately 30 min before drug administration and at hourly intervals for 5 h afterwards, and immediately before participants completed the self-reported drug-effect questionnaires and performance task.
Drug administration
All drug conditions were administered in a double-blind fashion. During each sampling session, participants orally ingested eight capsules with approximately 150 ml of water. During self-administration sessions, participants orally ingested the number of capsules earned during the modified progressive-ratio procedure with approximately 150 ml of water. Doses were prepared using commercially available d-amphetamine (Barr Labs, Pomona, NY, USA). Each d-amphetamine capsule contained 1 mg (8 mg dose), 2 mg (16 mg dose) or 3 mg (24 mg dose). Cornstarch was used to fill the remainder of all the capsules. Placebo capsules contained only cornstarch. The order of drug and dose administration was random, except that, for safety purposes, participants never received the highest dose (24 mg) in the first experimental session. Drug administration procedures were designed to ensure that participants swallowed the capsules and did not open them in their mouths and taste the contents (Abreu and Griffiths, 1996).
Data analysis
Statistical analyses of group data were conducted to examine drug effects on the modified progressive-ratio procedure, subject-rated drug-effect questionnaires, performance task and cardiovascular measures. For all statistical analyses, effects were considered significant for p ≤ 0.05.
Number of capsules earned on the modified progressive-ratio procedure was analyzed using a repeated-measure ANOVA with Dose (0, 8, 16 and 24 mg) as the factor. If a significant effect of Dose was detected in the ANOVA, post hoc tests (Fisher’s Least Significant Difference) were conducted to compare each of the three doses of d-amphetamine with placebo.
Data from the sampling sessions were analyzed to assess the effects of d-amphetamine on the subject-rated drug-effect questionnaires, performance task and cardiovascular measures. Peak effect (i.e. the maximum value from 1 to 5 h following drug administration) was calculated for each participant. Area-under-the-time-action curve (AUC) was calculated for each participant using the trapezoidal method (Pollard, 1979). Peak effect and AUC data were analyzed in the same manner as data from the modified progressive-ratio procedure. The result of the analyses of peak effect and AUC data were similar; for the sake of brevity, only AUC data are presented. Because participants ingested varying amounts of drug during self-administration sessions, subject-rated drug-effect-questionnaire, performance and cardiovascular data from the self-administration sessions were not analyzed statistically.
Results
Modified Progressive-Ratio Performance
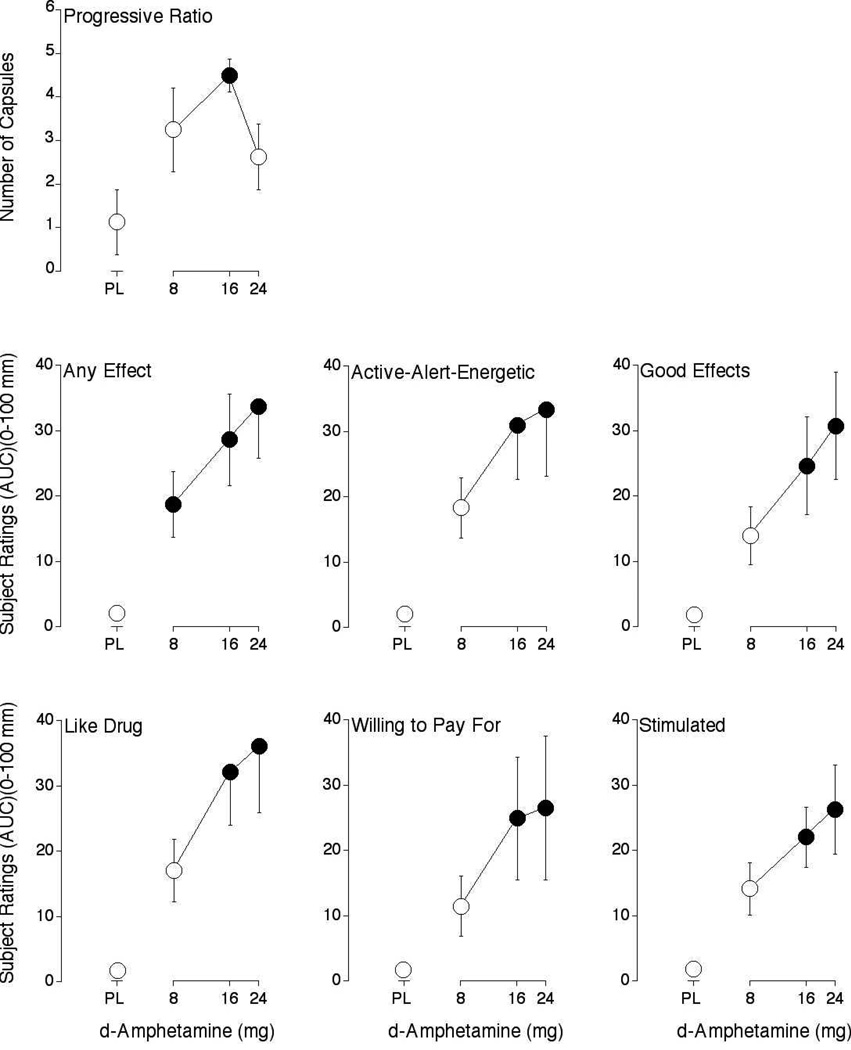
A main effect of Dose was found on number of capsules earned on the modified-progressive ratio procedure (F3,21 = 3.4 p < 0.05). Figure 1 shows that the intermediate dose of d-amphetamine, 16 mg, increased responding significantly above placebo levels.
Figure 1.
Dose-response functions for number of capsules earned on the progressive-ratio procedure, along with subjective ratings of Any Effect, Active-Alert-Energetic, Good Effects, Like Drug, Willing to Pay For and Stimulated from the Drug-Effect Questionnaire. Data are expresses as area-under-the-time-action curve (AUC). Horizontal axes: d-amphetamine dose in mg; data points above PL designate placebo values. Data points show means of 8 subjects; brackets show ± 1 S.E.M. Unidirectional brackets used in some instances for clarity. Filled symbols indicate those values that are significantly different from the placebo data point (p < 0.05, Fisher’s Protected Least Significant Difference post hoc test)
Subject-rated drug effects, performance and cardiovascular measures
ARCI
A significant effect of Dose was found on the A, BG and PCAG scale of the ARCI (F3,21 = 5.1, 3.7 and 5.2, respectively, p < 0.05). The high dose of d-amphetamine, 24 mg, increased responses on the A and BG scale significantly above placebo levels (Table 1). Each dose of d-amphetamine decreased responses on the PCAG scale significantly below levels observed with placebo (Table 1). d-Amphetamine did not affect responses on the MBG or LSD scale to a statistically significant degree (data not shown).
Table 1.
Summary of Mean Values
| Measure | Placebo | d-Amphetamine (8 mg) |
d-Amphetamine (16 mg) |
d-Amphetamine (24 mg) |
|---|---|---|---|---|
| Progressive-ratio | ||||
| Number of capsules earned | 1.1 | 3.3 | 4.5 | 2.6 |
| Addiction Research Center Inventory | ||||
| A | 1.9 | 2.5 | 2.9 | 3.7 |
| BG | 5.4 | 6.1 | 6.3 | 7.0 |
| PCAG | 3.5 | 2.8 | 2.5 | 2.5 |
| Adjective-Rating Scale | ||||
| Stimulant Scale | 4.8 | 7.5 | 9.9 | 10.9 |
| Drug-Effect Questionnaire | ||||
| Any Effect | 2.0 | 18.7 | 28.6 | 33.7 |
| Active-Alert-Energetic | 2.0 | 18.3 | 30.9 | 33.3 |
| Good Effects | 1.8 | 13.9 | 24.6 | 30.7 |
| Like Drug | 1.7 | 17.0 | 32.1 | 36.1 |
| Willing to Pay For | 1.7 | 11.4 | 24.9 | 26.5 |
| Stimulated | 1.8 | 14.1 | 22.0 | 26.2 |
| Talkative-Friendly | 1.9 | 7.2 | 26.9 | 25.6 |
| Rush | 1.7 | 7.2 | 9.5 | 17.5 |
| Bad Effects | 1.7 | 7.3 | 5.5 | 6.9 |
| Irregular-Racing Heartbeat | 1.7 | 3.6 | 8.0 | 8.8 |
| Restless | 1.7 | 10.0 | 12.2 | 13.1 |
| Shaky-Jittery | 1.5 | 4.3 | 7.8 | 11.4 |
| Digit-Symbol-Substitution-Task | ||||
| Number of Trials Correct | 64.4 | 65.2 | 68.0 | 67.9 |
| Cardiovascular Measures | ||||
| Systolic Pressure | 115.7 | 122.8 | 128.6 | 131.5 |
| Diastolic Pressure | 73.4 | 78.7 | 80.7 | 83.2 |
Mean values are not included for a particular measure if the repeated-measure ANOVA failed to reveal a significant effect of Dose. Bold and underlined values indicate significant difference (p ≤ .05) from placebo according to post-hoc tests. A = Amphetamine; BG = Benzedrine Group; PCAG = Pentobarbital, Chlorpromazine, Alcohol Group.
Adjective-Rating Scale
A significant effect of Dose was found on the Stimulant Scale of the Adjective-Rating Scale (F3,21 = 9.4 p < 0.005). Each dose of d-amphetamine increased responses on this scale significantly above levels observed with placebo (Table 1). d-Amphetamine did not affect responses on the Sedative Scale of the Adjective-Rating Scale to a statistically significant degree (data not shown).
Drug-Effect Questionnaire
A significant effect of Dose was found in 12 items from the Drug-Effect Questionnaire: Any Effect (F3,21 = 7.5, p < 0.002), Active-Alert-Energetic (F3,21 = 5.2, p < 0.01), Good Effects (F3,21 = 5.2, p < 0.01), Like Drug (F3,21 = 5.7, p < 0.01), Willing to Pay For (F3,21 = 3.1 p < 0.05), Stimulated (F3,21 = 5.3, p < 0.01), Talkative-Friendly (F3,21 = 4.1, p < 0.02), Rush (F3,21 = 3.1, p < 0.05), Bad Effects (F3,21 = 3.0, p ≤ 0.05), Irregular-Racing Heartbeat (F3,21 = 3.8, p < 0.05), Restless (F3,21 = 4.3, p < 0.02), Shaky-Jittery (F3,21 = 5.4, p < 0.01). Figure 1 shows the effects of d-amphetamine on six representative items: Any Effect, Active-Alert-Energetic, Good Effects, Like Drug, Willing to Pay For and Stimulated. Each dose of d-amphetamine increased subject ratings of Any Effect significantly above levels observed with placebo, while the intermediate and high dose of increased subject ratings of Active-Alert-Energetic, Good Effects, Like Drug, Willing to Pay For and Stimulated.
DSST
A significant effect of Dose was found on number of trials correct on the DSST (F3,21 = 3.5 p < 0.05). The intermediate and high dose of d-amphetamine increased the number of trials completed correctly significantly above placebo levels (Table 1). d-Amphetamine did not affect the total number of trials completed to a statistically significant degree (data not shown).
Heart Rate and Blood Pressure
d-Amphetamine increased systolic and diastolic blood pressure as an orderly function of dose (F3,21 = 14.7 and 16.4, respectively, p < 0.001). Each dose of d-amphetamine increased systolic and diastolic blood pressure significantly above levels observed with placebo (Table 1). d-Amphetamine did not increase heart rate to a statistically significant degree (data not shown).
Discussion
The present study examined the reinforcing effects of d-amphetamine using novel response ratios in a modified progressive-ratio procedure. The results indicate that placebo maintained low levels of responding and the intermediate dose of d-amphetamine increased responding significantly above placebo levels. Moreover, d-amphetamine produced prototypical subject-rated drug effects that were orderly functions of dose. The present data suggest that the modified response requirements resulted in lower levels of placebo taking and a larger separation between the number of placebo and d-amphetamine capsules earned.
Human laboratory studies have shown that progressive-ratio schedules are sensitive to the reinforcing effects of a number of drugs, including heroin, marijuana, pentobarbital, caffeine and cocaine (McLeod and Griffiths, 1983; Griffith et al., 1989; Comer et al., 1997; Haney et al., 1997; 1998). The present findings are concordant with the previous findings from our laboratory showing that d-amphetamine functions as a reinforcer under a progressive-ratio schedule (Rush et al., 2001; Stoops et al., 2004, 2007a,b). These earlier studies used response requirements ranging from either 50 to 6400 or 25 to 3200 mouse clicks under the progressive-ratio procedure and at least one dose of d-amphetamine increased break points significantly above placebo levels. However, the responding for placebo was relatively high in these previous studies, that is, on average participants completed 3.3 of the 8 response ratios to earn placebo capsules. Participants completed on average 4.5, 5.2 and 3.1 of the 8 response ratios to earn small (8 or 10 mg), medium (16 or 20 mg) and large (24 mg) doses of d-amphetamine, respectively (Rush et al., 2001; Stoops et al., 2004, 2007a,b). Thus, the high responding for placebo resulted in modest separations between the number of placebo and d-amphetamine capsules earned. In the present experiment, we utilized novel response requirements (400–1800 clicks) under the modified progressive-ratio schedule. We observed decreased responding for placebo relative to our previous studies, likely due to the higher initial response requirement. The decreased placebo responding resulted in a larger separation between the number of placebo and d-amphetamine capsules earned, which may enhance our ability to detect a shift in the d-amphetamine dose-response curve. Thus, the present procedure could be useful for assessing pharmacological modification of the reinforcing effects of amphetamines.
The results of this experiment are also concordant with the findings from animal studies that demonstrated that d-amphetamine and related stimulants function as reinforcers under progressive-ratio schedules (Griffiths et al., 1975; 1979; Poncelet et al., 1983; Ranaldi and Wise 2000; Woolverton and Wang, 2004; Brebner et al., 2005). Several studies conducted with animals have shown that stimulants produce inverted-U shaped dose-response curves under progressive-ratio schedules (e.g., Griffiths et al., 1979; Martelle et al., 2008). However, in humans ethical and safety concerns limit the feasibility of studying large doses of abused drugs, and therefore reduce the possibility of obtaining inverted-U shaped dose-response curves (see Fischman and Johanson, 1998). Worth noting is that an inverted-U shaped dose-response curve of d-amphetamine was obtained using the progressive-ratio schedule in the present and a previous human laboratory study (Stoops et al., 2004). Some theorists have suggested that ascending and descending limbs of the inverted-U shaped curves involve distinct receptor mechanisms (Kenakin, 1987; Rowlett, 2000; Collins et al., 2005). Therefore, the current procedure could be a useful non-invasive tool for studying mechanisms of effects of potential pharmacotherapies that shift the ascending and/or descending limbs of the d-amphetamine dose-response curve.
Subject-rated effects are the most widely used behavioral measures in human laboratory experiments for assessing the abuse-related effects of stimulants. In the present study, d-amphetamine produced an array of positive subject-rated effects across the range of doses tested (e.g., increased ratings of Good Effects, Like Drug). The dose-dependent enhancement observed in the subject-rated effects of d-amphetamine is consistent with previous reports (Martin et al., 1971; Rush et al., 1998; Sevak et al., 2009). The potency of d-amphetamine for enhancing the positive subject-rated effects was also similar to that reported in earlier studies (Stoops et al., 2004, 2007a). Moreover, concordant with the findings from previous studies, d-amphetamine enhanced the number of trials completed correctly on the DSST, indicating improvements in motor performance (Rush et al., 2003; Stoops et al., 2004). Thus, in agreement with the published findings, the results of the current study indicate that d-amphetamine increases positive subject-rated effects as an orderly function of dose.
The present results indicate that the reinforcing and subject-rated effects of drugs are not isomorphic. The intermediate dose of d-amphetamine increased the number of capsules earned above placebo levels, whereas in general, the two higher doses of d-amphetamine produced prototypical stimulant-like subject-rated effects. These findings are consistent with previous research showing that positive subject-rated effects and reinforcing effects are dissociable (Johanson and Uhlenhuth, 1980; 1981; Lamb et al., 1991; Haney et al., 1999). Thus, the present data suggest that positive subject-rated effects of d-amphetamine do not entirely account for the drug-taking behavior in the laboratory and that these two behavioral measures do not assess the same mechanism that is related to the abuse of d-amphetamine.
Although the present study made an advance in attempts to separate the placebo and stimulant drug self-administration in the progressive-ratio paradigm in humans, only one dose of d-amphetamine increased the number of capsules earned significantly above placebo levels. Notably, this result indicated an improvement from the earlier findings (in our previous studies the average separation between the placebo and d-amphetamine [16 mg] self-administration was 1.8 response ratios, whereas in the current study that was 3.4 responses ratios). However, future modifications and refinements (e.g., changes in response requirements, dose selections) are needed to further enhance the sensitivity of the procedure. Worth noting is the inherent difficulty in separating placebo and stimulant drug self-administration under progressive-ratio procedures, or other models of drug-taking in humans, without the extensive training that can be imposed in nonhuman animals. Notably, human laboratory studies involving extensive training before test sessions (e.g., drug-discrimination) demonstrate a large separation between placebo and stimulant drug effects (Jones et al., 2001), and also improve outcomes of subject-rated behavioral measures (Rush et al., 1998; 2003; Sevak et al., 2009). Future progressive-ratio studies incorporating this methodological improvement (i.e., extensive training before test sessions) may enhance the sensitivity of the procedure to better separate the placebo and stimulant drug effects. Nonetheless, progressive-ratio procedures can still be useful for assessing pharmacological modifications of behavioral effects of stimulants with improved methodology and enhanced sensitivity that can detect a large magnitude of reinforcing effects (Stoops, 2008). To that end, we believe the present study provides an important contribution to a laboratory model of drug taking, which should be further refined to obtain more sensitive human laboratory procedures for evaluating potential medications for the management of stimulant dependence.
In summary, the present data suggest that the modified response requirements under the progressive-ratio procedure resulted in lower levels of placebo taking and a larger separation between the number of placebo and d-amphetamine capsules earned, which may enhance our ability to detect a shift in the d-amphetamine dose-response curve. Thus, the present procedure could be well suited for assessing pharmacological modification of the reinforcing effects of amphetamines. Reinforcing effects are central to the abuse of amphetamines and related stimulants; hence, a drug that modifies the reinforcing effects of amphetamines can be expected to be effective for the management of amphetamine dependence (Foltin and Fischman, 1991). The findings from the present study may therefore facilitate the human laboratory evaluations of potential medications for managing methamphetamine dependence.
Acknowledgements
We thank Frances Wagner, RN, for her expert managerial and nursing support. We thank Bryan Hall and Jennifer Schmedes, Claire Kunkle and Sarah Wells for their skilled technical assistance.
Source of Support: Supported by grants from the National Institute on Drug Abuse (DA017711, DA025591 and DA025032) awarded to Craig R. Rush.
References
- Abreu ME, Griffiths RR. Drug tasting may confound human drug discrimination studies. Psychopharmacology (Berl) 1996;125:255–257. doi: 10.1007/BF02247336. [DOI] [PubMed] [Google Scholar]
- Brebner K, Ahn S, Phillips AG. Attenuation of d-amphetamine self-administration by baclofen in the rat: behavioral and neurochemical correlates. Psychopharmacology (Berl) 2005;177:409–417. doi: 10.1007/s00213-004-1968-6. [DOI] [PubMed] [Google Scholar]
- Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J, Woods JH. Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. J Pharmacol Exp Ther. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Fischman MW. Choice between money and intranasal heroin in morphine-maintained humans. Behav Pharmacol. 1997;8:677–690. doi: 10.1097/00008877-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Johanson CE. Ethical and practical issues involved in behavioral pharmacology research that administers drugs of abuse to human volunteers. Behav Pharmacol. 1998;9:479–498. doi: 10.1097/00008877-199811000-00002. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Assessment of abuse liability of stimulant drugs in humans: a methodological survey. Drug Alcohol Depend. 1991;28:3–48. doi: 10.1016/0376-8716(91)90052-z. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bradford LD, Brady JV. Progressive ratio and fixed ratio schedules of cocaine-maintained responding in baboons. Psychopharmacology (Berl) 1979;65:125–136. doi: 10.1007/BF00433038. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Findley JD, Brady JV, Dolan-Gutcher K, Robinson WW. Comparison of progressive-ratio performance maintained by cocaine, methylphenidate and secobarbital. Psychopharmacologia. 1975;43:81–83. doi: 10.1007/BF00437619. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Liebson IA. Reinforcing effects of caffeine in coffee and capsules. J Exp Anal Behav. 1989;52:127–140. doi: 10.1901/jeab.1989.52-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. Psychopharmacology (Berl) 143: 102-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Comer SD, Ward AS, Foltin RW, Fischman MW. Factors influencing marijuana self-administration by humans. Behav Pharmacol. 1997;8:101–112. [PubMed] [Google Scholar]
- Haney M, Foltin RW, Fischman MW. Effects of pergolide on intravenous cocaine self-administration in men and women. Psychopharmacology (Berl) 1998;137:15–24. doi: 10.1007/s002130050588. [DOI] [PubMed] [Google Scholar]
- Haney M, Collins ED, Ward AS, Foltin RW, Fischman MW. Effect of a selective dopamine D1 agonist (ABT-431) on smoked cocaine self-administration in humans. 1999 doi: 10.1007/s002130050925. [DOI] [PubMed] [Google Scholar]
- Jasinski D. Assessment of the abuse potentiality of morphine-like drugs (methods used in man) In: Martin WR, editor. Drug Addiction I. New York: Springer-Verlag New York Inc.; 1977. pp. 197–258. [Google Scholar]
- Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: d-amphetamine. Psychopharmacology (Berl) 1980;71:275–279. doi: 10.1007/BF00433062. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: repeated assessment of d-amphetamine. Pharmacol Biochem Behav. 1981;14:159–163. doi: 10.1016/0091-3057(81)90237-9. [DOI] [PubMed] [Google Scholar]
- Jones HE, Garrett BE, Griffiths RR. Reinforcing effects of oral cocaine: contextual determinants. Psychopharmacology (Berl) 2001;154:143–152. doi: 10.1007/s002130000626. [DOI] [PubMed] [Google Scholar]
- Kenakin TP. Pharmacologic analysis of drug-receptor interaction. New York: Raven Press; 1987. [Google Scholar]
- Lamb RJ, Preston KL, Schindler CW, Meisch RA, Davis F, Katz JL, Henningfield JE, Goldberg SR. The reinforcing and subjective effects of morphine in post-addicts: a dose-response study. J Pharmacol Exp Ther. 1991;259:1165–1173. [PubMed] [Google Scholar]
- Martelle JL, Czoty PW, Nader MA. Effect of time-out duration on the reinforcing strength of cocaine assessed under a progressive-ratio schedule in rhesus monkeys. Behav Pharmacol. 2008;19:743–746. doi: 10.1097/FBP.0b013e3283123c6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR. Human progressive-ratio performance: maintenance by pentobarbital. Psychopharmacology (Berl) 1983;79:4–9. doi: 10.1007/BF00433007. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling JE. An automated version of the digit symbol substitution test (DSST) Behav Res Methods Instr. 1982;14:463–466. [Google Scholar]
- McMillan DE. Effects of d-amphetamine on performance under several parameters of multiple fixed-ratio, fixed-interval schedules. J Pharmacol Exp Ther. 1969;167:26–33. [PubMed] [Google Scholar]
- Oliveto AH, Bickel WK, Hughes JR, Shea PJ, Higgins ST, Fenwick JW. Caffeine drug discrimination in humans: acquisition, specificity and correlation with self-reports. J Pharmacol Exp Ther. 1992;261:885–894. [PubMed] [Google Scholar]
- Pollard JH. A Handbook of Numerical and Statistical Techniques: With Examples Mainly from the Life Sciences. Cambridge: University Press; 1979. [Google Scholar]
- Poncelet M, Chermat R, Soubrie P, Simon P. The progressive ratio schedule as a model for studying the psychomotor stimulant activity of drugs in the rat. Psychopharmacology (Berl) 1983;80:184–189. doi: 10.1007/BF00427967. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Wise RA. Intravenous self-administration of methamphetamine-heroin (speedball) combinations under a progressive-ratio schedule of reinforcement in rats. Neuroreport. 2000;11:2621–2623. doi: 10.1097/00001756-200008210-00003. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rowlett JK. A labor-supply analysis of cocaine self-administration under progressive-ratio schedules: antecedents, methodologies, and perspectives. Psychopharmacology (Berl) 2000;153:1–16. doi: 10.1007/s002130000610. [DOI] [PubMed] [Google Scholar]
- Rush CR, Essman WD, Simpson CA, Baker RW. Reinforcing and subject-rated effects of methylphenidate and d-amphetamine in non-drug-abusing humans. J Clin Psychopharmacol. 2001;21:273–286. doi: 10.1097/00004714-200106000-00005. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kollins SH, Pazzaglia PJ. Discriminative-stimulus and participant-rated effects of methylphenidate, bupropion, and triazolam in d-amphetamine-trained humans. Exp Clin Psychopharmacol. 1998;6:32–44. doi: 10.1037//1064-1297.6.1.32. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR, Glaser PE, Hays LS. Risperidone attenuates the discriminative-stimulus effects of d-amphetamine in humans. J Pharmacol Exp Ther. 2003;306:195–204. doi: 10.1124/jpet.102.048439. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Stoops WW, Hays LR, Rush CR. Discriminative stimulus and subject-rated effects of methamphetamine, d-amphetamine, methylphenidate, and triazolam in methamphetamine-trained humans. J Pharmacol Exp Ther. 2009;328:1007–1018. doi: 10.1124/jpet.108.147124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CB. Effects of d-amphetamine upon operant behavior of pigeons: Enhancement by reserpine. J Pharmacol Exp Ther. 1964;146:167–174. [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology (Berl) 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Stoops WW. Reinforcing effects of stimulants in humans: sensitivity of progressive-ratio schedules. Exp Clin Psychopharmacol. 2008;16:503–512. doi: 10.1037/a0013657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Glaser PE, Fillmore MT, Rush CR. Reinforcing, subject-rated, performance and physiological effects of methylphenidate and d-amphetamine in stimulant abusing humans. J Psychopharmacol. 2004;18:534–543. doi: 10.1177/0269881104047281. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Robbins CG, Martin CA, Rush CR, Kelly TH. The reinforcing, subject-rated, performance, and cardiovascular effects of d-amphetamine: influence of sensation-seeking status. Addict Behav. 2007a;32:1177–1188. doi: 10.1016/j.addbeh.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Vansickel AR, Lile JA, Rush CR. Acute d-amphetamine pretreatment does not alter stimulant self-administration in humans. Pharmacol Biochem Behav. 2007b;87:20–29. doi: 10.1016/j.pbb.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z. Relationship between injection duration, transporter occupancy and reinforcing strength of cocaine. Eur J Pharmacol. 2004;486:251–257. doi: 10.1016/j.ejphar.2004.01.003. [DOI] [PubMed] [Google Scholar]