Abstract
Enteric oxalate secretion that correlated with reductions in urinary oxalate excretion was previously reported in a mouse model of Primary Hyperoxaluria, and in wild type (WT) mice colonized with a wild rat strain (OXWR) of Oxalobacter (Am J Physiol 300: G461-G469, 2011). Since a human strain of the bacterium is more likely to be clinically used as a probiotic therapeutic, we tested the effects of HC-1 in WT. Following artificial colonization of WT mice with HC-1, the bacteria were confirmed to be present in the large intestine and, unexpectedly, detected in the small intestine for varying periods of time. The main objective of the present study was to determine whether the presence of HC-1 promoted intestinal secretion in the more proximal segments of the gastrointestinal tract. In addition, we determined whether HC-1 colonization led to reductions in urinary oxalate excretion in these mice. The results show that the human Oxalobacter strain promotes a robust net secretion of oxalate in the distal ileum as well as in the caecum and distal colon and these changes in transport correlate with the beneficial effect of reducing renal excretion of oxalate. We conclude that OXWR effects on intestinal oxalate transport and oxalate homeostasis are not unique to the wild rat strain and that, mechanistically, HC-1 has significant potential for use as a probiotic treatment for hyperoxaluria especially if it is also targeted to the upper and lower gastrointestinal tract.
Keywords: Jejunum, ileum, caecum, distal colon, urinary oxalate
INTRODUCTION
Previously we reported on the beneficial effects of a wild rat strain of Oxalobacter (OXWR) on oxalate homeostasis and oxalate transport across the rat and mouse large intestine [1, 2]. Oxalobacter colonization of the mouse caecum and colon was correlated with the promotion of intestinal oxalate secretion leading to a significant reduction in urinary oxalate excretion in healthy wild type mice. Furthermore, high levels of oxalate in both plasma and urine were normalized in a mouse model of Primary Hyperoxaluria, type 1 that was colonized with OXWR indicating that Oxalobacter can derive oxalate from endogenous sources by promoting an enteric shunt [2]. Those results supported our initial hypothesis that OXWR has a dual action in that it can derive oxalate from systemic sources in addition to degrading dietary sources of oxalate. A relevant question we addressed here was whether the human Oxalobacter strain (HC-1) [3] can similarly induce enteric oxalate secretion leading to lower urinary oxalate excretion since administration of a human strain as a probiotic is more likely to be developed for therapeutic use in patients with oxalate-associated diseases.
Especially important, and the focus of the present study, was the serendipitous detection of Oxalobacter in the distal ileum of mice colonized with HC-1 which prompted an examination of the oxalate transport characteristics of the small intestine in the presence of the bacteria. The results of this study show that Oxalobacter survives in the upper part of the small intestine in the short-term and the presence of HC-1 is correlated with changes in both the small and large intestinal oxalate transport. The presence and effect of Oxalobacter on oxalate transport in the small intestine is a novel finding that could have significant implications for clinical use of a probiotic therapy using Oxalobacter to reduce urinary oxalate excretion in a variety of hyperoxaluric conditions. For example, therapeutic preparations should now be targeted for release into both the small and large intestine in light of these new findings and, since the bacteria do not appear to be retained in the small intestinal segments for as long as they are retained in the large intestine, these studies are important in terms of addressing the frequency of probiotic delivery.
MATERIALS AND METHODS
Animals
The animal experiments were conducted in accordance with the University of Florida and the NIH Guide for the Care and Use of Laboratory Animals. Both male and female C57BL/6 (WT) mice (25–30 g) used in these studies were purchased from Charles River Laboratories and were given free access to a standard mouse chow (Harlan Teklad #2018S) and drinking water before initiating the colonization procedure. The mice were euthanized by 100% CO2 inhalation at the time of the flux studies and the entire intestinal tract which was removed was divided into different segments. Prior to rinsing each segment with chilled isotonic saline, the intestinal contents were sampled as mentioned in the Colonization Studies section below. Segments of jejunum (a 3-cm portion 4 cm distal to the pyloric sphincter), mid-ileum (a 3-cm portion ~ 20 cm distal to the pyloric sphincter and ~ mid-way along the small intestine), distal ileum (a 3-cm portion proximal to the ileo-caecal valve), the entire caecum, and a 3 cm section of the most distal colon were removed for the flux studies.
Urine collection and analytical methods
Mice were housed in metabolic cages for the 24-h collections (2 mice/urine pool) which were made under mineral oil into vessels containing 10 μl of 2% sodium azide as a preservative. Urinary oxalate was determined in acidified (HCl) samples collected from all of the mice over a 24-h period on several occasions using a kit assay (Trinity Biotech #591, St. Louis, MO). Urinary creatinine was determined using a modification of the Jaffé reaction as previously described [4].
Colonization Studies
The protocol for Oxalobacter colonization was outlined previously [#1]. The human strain of Oxalobacter, HC-1, was generously provided by Dr. Milt Allison at Iowa State University, Ames, Iowa. Mice were primed by administering a 1.5% oxalate-supplemented diet (0.5% calcium, Harlan Teklad 89222) for approximately 5 days prior to an esophageal gavage of a 0.5 ml inoculum containing an average of 35 mg wet weight of bacteria from a 24-h culture of HC-1. Two days later the mice were similarly inoculated a second time. Fresh fecal specimens were collected approximately 7 days after the second gavage (G+7) for the detection of Oxalobacter which was routinely determined by an anaerobic culture method [1]. At G+12 days, the oxalate-supplemented diet was replaced with the standard chow. Mice were defined as colonized if Oxalobacter was detected in a fresh fecal specimen collected directly from the animal. Prior to starting the study, all animals were confirmed non-colonized. Colonization status was also confirmed at the time the flux studies were conducted by sampling the intestinal contents in each of the segments described above at the time of dissection. Care was taken during the sampling process to avoid cross-contamination of the contents from one segment to another.
Flux Studies
The magnitude and direction of the net flux ( ) was determined by calculating the difference between two unidirectional fluxes (mucosal to serosal, and serosal to mucosal, ) of oxalate measured using 14C-oxalate (Amersham, Piscataway, NJ, USA) as previously described [5] and are presented as picomoles per square centimeter per hour. The radiochemical purity of 14C-oxalate was confirmed by comparing 14C activity in samples before and after hydrolysis with the substrate specific enzyme oxalate decarboxylase following volatilization of 14CO2. The transport studies were conducted between 7 and 11 days following the gavage procedure. The specific activity of the radioisotope, which remained constant throughout the flux period, was determined at the beginning and end of the experiment by sampling (50 μl) from the labeled compartment. Samples were dissolved in 5 ml of Ecoscint A (National Diagnostics, Atlanta, GA) and isotopic activity was measured by liquid scintillation spectrometry with quench correction using a Beckman LS 6500 (Beckman Coulter, Fullerton, CA). The electrical parameters of the tissue were also recorded at 15 min intervals throughout the entire experiment. Tissue conductance (GT, mS·cm−2) was calculated as the ratio of the open-circuit potential (mV) to the short-circuit current (Isc, μA ·cm−2) and net fluxes were determined on conductance matched tissues.
Statistical analyses
Statistical analysis of the data was performed using a paired or unpaired t-test for the comparison of two means. Multiple means were compared by a one-way ANOVA followed by Bonferroni’s t-test. For all analyses, differences were considered significant if P ≤ 0.05.
RESULTS
HC-1 Colonization of WT (C57BL/6) Mice
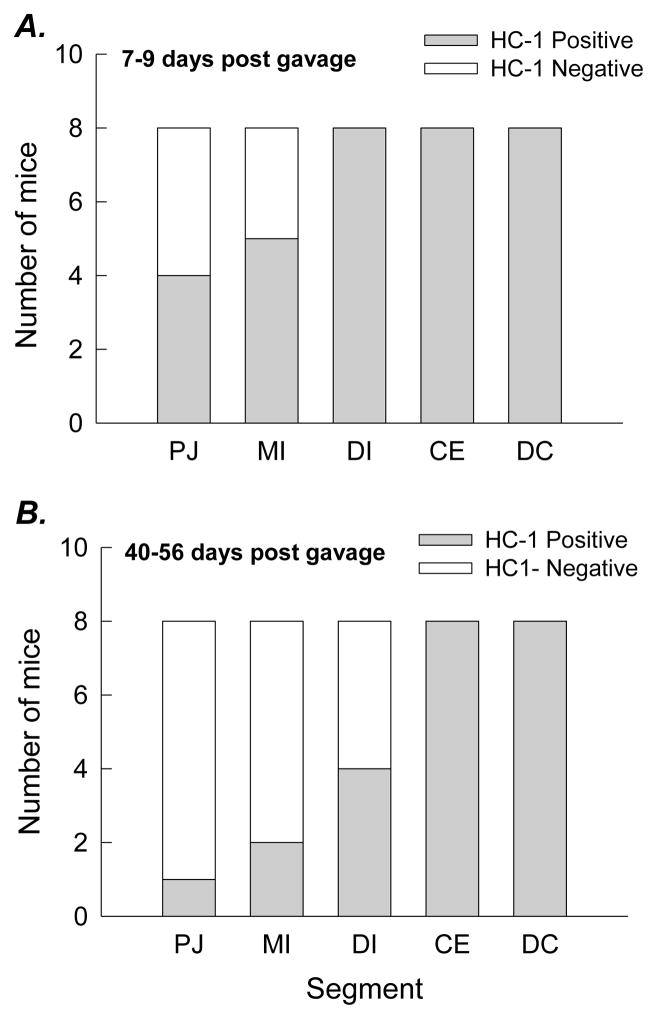
WT mice were found to be readily (100%) colonized by HC-1 and, in a sub-set of animals (n=8), we determined that colonization of the entire large intestine (caecum, proximal, and distal colon) was sustained for at least 70 days following gavage in the absence of dietary oxalate supplementation. HC-1 was detected in the distal ileum and, upon further examination; the bacteria were also detected in the proximal jejunum and mid-small intestinal segment, but not in all mice (Figure 1A). Between 7 and 9 days after gavage (G+7 to G+9), HC-1 was detected in the proximal jejunum and mid intestine in ~50% of the mice examined while the bacteria were detected in the distal ileum, caecum, and distal colon in all mice in this group (n=8). When another group of mice (n=8) were similarly examined between G+40 and G+56 days (Figure 1B), HC-1 was detected in the small intestinal segments in fewer mice but remained present in the large intestine of all mice in this group.
Figure 1.
Distribution of HC-1 along the length of the WT mouse intestine between 7 and 9 days after gavage (G+7 to G+9, n=8, in Figure 1A) and between G+40 to G+56, n=8, in Figure 1B). PJ = Proximal jejunum; MI = Mid-ileum; DI = Distal ileum; CE = Caecum; DC = Distal colon.
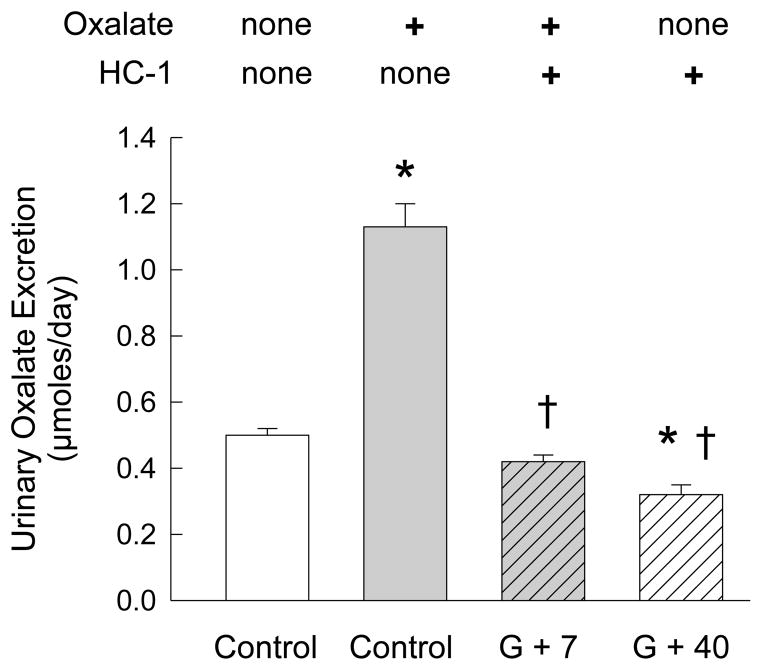
The effect of HC-1 colonization on urinary oxalate excretion in mice primed with an oxalate-supplemented (1.5% oxalate) diet was examined and the results confirmed that urinary oxalate excretion is significantly increased over 2-fold with dietary oxalate supplementation compared to the standard chow (Figure 2). At G+7, urinary oxalate excretion in a sub-group of mice (n=11) fed the oxalate diet and colonized with HC-1, was > 60% lower than the non-colonized group (n=18) on the same oxalate-supplemented diet. And, at G+40, urinary oxalate excretion in the mice (n=18) was ~ 40% lower than the baseline value when compared to oxalate excretion in mice on the standard chow without oxalate. These significant reductions in renal excretion of oxalate clearly correlate with the presence of intestinal HC-1. There were no changes in urinary creatinine excretion which was determined at the same time (3.22 ± 0.17, n=18; 3.10 ± 0.21, n=18; 3.20 ± 0.10, n=11; 3.62 ± 0.22, n=18, in each group, respectively, left to right in Figure 2).
Figure 2.
Comparison of 24-h urinary oxalate excretion in WT mice (n=18 pools) before and after gavage (G) with HC-1. HC-1, is a human strain of Oxalobacter. Urinary oxalate excretion is significantly increased from basal levels (Control-no Oxalate) before the mice are fed a diet supplemented with 1.5% oxalate (Control+Oxalate, *p < 0.05, n=18). Following colonization, at G+7 days, there is a significant reduction in oxalate excretion, in a sub-group of n=11 mice, compared to the Control+Oxalate group (†, p ≤ 0.05). At G+12 days after gavage, the standard chow replaced the oxalate-supplemented diet and at G+40 urinary oxalate excretion (n=18) is significantly lower compared to both the Control-no Oxalate group (*, p ≤ 0.05) and the Control+Oxalate group values (†, p ≤ 0.05).
Intestinal Oxalate Transport
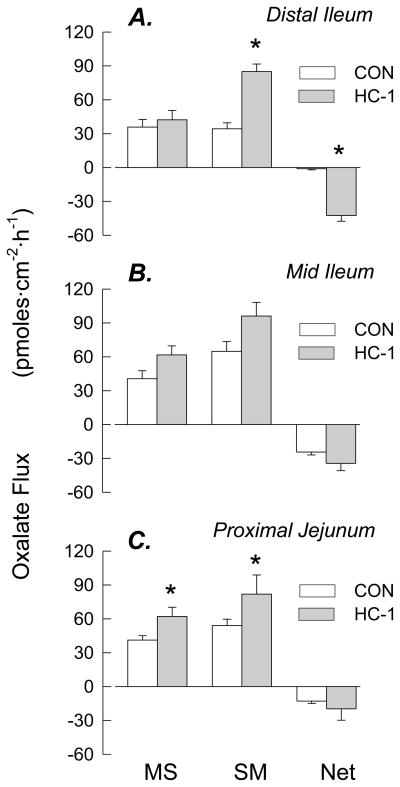
Following the serendipitous detection of Oxalobacter in the distal segment of the small intestine colonized with HC-1, a series of flux experiments was conducted to examine the effects of Oxalobacter on oxalate transport in this segment and the results are presented in Figure 3. These results show that HC-1 promotes a substantial net secretory flux across the distal ileum of WT mice, Figure 3A, as a consequence of a 2.5-fold increase in . There was also a significant increase in Isc across this tissue suggesting a change in the movement of another ion/charged solute (whose identity is not yet determined), but there was no effect on tissue conductance (GT). Oxalate fluxes were also examined across colonized tissues removed from the two other more proximal segments of the small intestine (see Figures 3B and 3C). While both of these segments exhibit a basal net secretion of oxalate in the absence of HC-1 and this net secretion is enhanced in the presence of the bacteria but the increases do not reach a level of statistical significance. It is notable that in both of the more proximal segments, there are also increases in the magnitude of the unidirectional fluxes associated with increases in GT (indicative of changes in the passive permeability of these segments).
Figure 3.
Unidirectional and net transepithelial fluxes of oxalate across isolated, short-circuited segments of distal ileum (n=13 vs 7), mid-ileum (n=6 vs 7), and proximal jejunum (n=15 vs 5) of WT mice not colonized vs mice artificially colonized with HC-1. An asterisk indicates a significant difference between the two groups, p ≤ 0.05. Transepithelial conductance (GT) was not significantly affected by colonization in any segment (GT = 34.2 ± 3.4 vs 36.9 ± 1.5 mS/cm2, across distal ileum, GT = 35.3 ± 1.9 vs 40.3 ± 4.9 mS/cm2 mid-ileum, and GT = 27.9 ± 1.7 vs 39.0 ± 7.7 mS/cm2 in proximal jejunum of non-colonized vs colonized mice, respectively). With the exception of the distal ileum, Isc was not affected by colonization (Isc = 2.9 ± 0.5 vs 6.9 ± 0.8* μEq/cm2 in distal ileum, Isc = 5.6 ±0.5 vs 4.8 ±0.7 μEq/cm2 in mid-ileum, and Isc = 2.5 ± 0.3 vs 2.9 ± 0.7 μEq/cm2 in the proximal jejunum of non-colonized mice vs colonized mice, respectively).
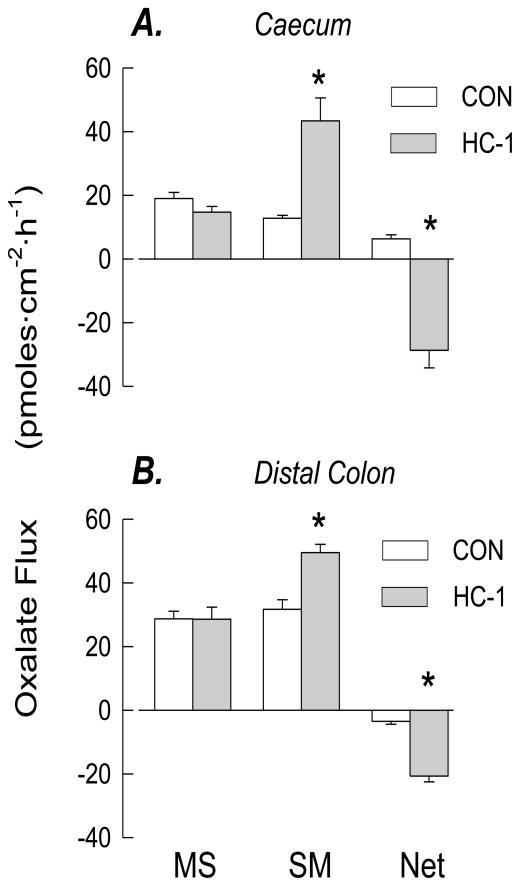
Oxalate fluxes across the caecum and distal colon of HC-1 colonized mice compared to their non-colonized counterparts are presented in Figure 4. Colonization of both segments of the large intestine was correlated with a robust net secretory flux of oxalate. In the caecum, there was a reversal in the direction of the net flux from oxalate absorption to a large net secretion in colonized tissues. In the distal colon, basal net secretion was significantly enhanced compared to non-colonized tissues. There were no changes in the electrical parameters of either segment.
Figure 4.
Unidirectional and net transepithelial fluxes of oxalate across isolated, short-circuited segments of caecum (n=11 vs 7) and distal colon (n=8 vs 10) of WT mice (not colonized vs mice artificially colonized with HC-1. An asterisk indicates a significant difference between the two groups p ≤ 0.05. Transepithelial conductance (GT) was not significantly affected by colonization in any segment (GT = 19.5 ± 1.7 vs 20.8 ± 1.2 mS/cm2, across caecum, GT = 15.5 ± 2.0 vs 16.5 ± 1.3 mS/cm2 in distal colon of non-colonized mice vs colonized mice, respectively). Isc was not affected by colonization (Isc = 0.5 ± 0.1 vs 0.5 ± 0.1 μEq/cm2 in caecum and Isc = 0.7 ± 0.1 vs 1.3 ±0.1 μEq/cm2 in distal colon of non-colonized mice vs colonized mice, respectively).
DISCUSSION
The results of the present study examining the human strain of Oxalobacter, HC-1, demonstrate that WT mice are readily colonized by an esophageal gavage of these live bacteria and that the physiological interactions and effects of this bacterial species on mouse intestinal transport of oxalate shown previously [1, 2] are not unique to the wild rat strain, OXWR. A major finding of the present study was the detection and physiological effects of HC-1 in the small intestine. Another unexpected finding was the detection of the bacteria in the more proximal segments of the small intestine, albeit for limited periods of time and not in all mice. Although the factors involved in both the colonization and/or the elimination of Oxalobacter from any segment of the intestine are presently poorly characterized, other than the effects of certain antibiotics [6–8] and intraluminal calcium concentrations [1], the results indicate Oxalobacter can reside in the upper gastrointestinal tract, at least temporarily. The results obtained from this study are novel and demonstrate the significant potential of HC-1 for use as a probiotic treatment for hyperoxaluria especially if the therapeutic is frequently targeted to both the upper and lower gastrointestinal tract. These studies also underscore the need for more information about the factors affecting Oxalobacter colonization.
HC-1 and intestinal oxalate transport
As shown here, the presence of HC-1 in segments of both the small and large intestine of WT mice is consistently associated with the promotion or enhancement of enteric oxalate secretion in a segment-specific manner. In the small intestine, the most significant effect of HC-1 was observed in the distal ileum which exhibited a marked increase in the serosal to mucosal flux of oxalate leading to a very robust net secretion across the tissue. While we observed increases of ~ 40% and ~ 50% in net oxalate secretion in the mid-ileum and proximal jejunum, respectively, these changes did not reach a level of statistical significance. It should be noted here that the while we can detect the presence of Oxalobacter in luminal contents and in fecal material, we do not have any information regarding the relative abundance of the bacteria, or numbers of bacteria present in any of the segments examined. It is possible, perhaps, that while the bacteria are detected in the upper small intestine, their abundance may not have been sufficient to impact in a statistically significant manner. Currently, we are in the process of developing methods to quantify numbers of bacteria within each intestinal segment in order to be able to interpret these kinds of results in a more meaningful way. Nonetheless, it can be concluded from the results that HC-1 promotes an overall enteric elimination of oxalate and the contribution of the small intestine in excreting oxalate in the presence of HC-1 is likely to be very substantial in vivo.
The segmental pattern of oxalate transport in the caecum and distal colon colonized with HC-1 was similar to that observed in OXWR colonized mice [2] with both of these tissue segments supporting a substantial net secretory flux of oxalate when Oxalobacter of either strain is present. In the caecum, a basal net absorptive flux is reversed to a substantial net secretory flux in the presence of Oxalobacter and basal net secretion of oxalate across the distal colon is markedly enhanced by the bacteria. In both segments, the changes in net flux occurred via a significant increase in the serosal to mucosal unidirectional flux. The proximal colon was not examined in the present study since we have not previously observed Oxalobacter effects on oxalate transport of this segment in either the mouse [1] or rat [2] species.
Although a similar inoculum of HC-1 and OXWR was delivered to each mouse in terms of wet weight of bacterial mass, we have no information regarding the numbers of viable bacteria present/active along the length of the alimentary tract. As a consequence, a direct comparison of the magnitude of the responses elicited by the two different Oxalobacter strains is not a valid approach. It is notable, however, that the magnitude of the net secretory fluxes induced by HC-1 (present study) and OXWR [2] in the two studies involving the large intestine appears to be quite comparable.
HC-1 and urinary oxalate excretion
The results of the present study also confirmed that mice colonized with HC-1 excreted significantly lower amounts (~ 40% and 70% reduction at G+ 40, respectively) of urinary oxalate compared to basal levels in the same mice fed either the standard chow or the oxalate-supplemented diet. Previously [2], we also reported progressive reductions in urinary oxalate with OXWR colonization when colonized mice were assessed over a longer time period (~ 11 weeks) but we did not examine this specific aspect in the present study. In addition, we cannot directly compare absolute values for urinary oxalate excretion between the effects of HC-1 and OXWR in mice in the two different studies for the same reasons as stated above.
The effect of HC-1 in reducing urinary oxalate excretion in mice in the present study is consistent with observations reported by Hoppe et al. [9] following the administration of a paste preparations of IxOC-2, or a capsule, IxOC-3, which presumably contain a human strain of Oxalobacter, to a small number of patients with Primary Hyperoxaluria type 1 (PH1). Whether IxOC-2 and IxOC-3 are formulations of HC-1, or another human strain, is not clear and colonization was transient despite daily dosing for a 4-week period. In fact, in all (n=16) but one patient in Hoppe’s study [9], colonization was lost within 2 weeks of stopping the treatment which is in contrast to our experience with WT mice that sustain colonization for at least 10–11 weeks (there has been no assessment beyond this time period) in the absence of repeated treatments(present study and [2]). Of note here also, is that we have observed differences between how long WT mice and a mouse model of PH1 (Agxt) sustain OXWR colonization which was quickly lost in the Agxt mice at ~G+22 days [2]. Taken together, the results from the patient studies by Hoppe et al. and from the mouse studies in our laboratory would suggest the possibility that the intraluminal environment in PH1 is not conducive for sustaining Oxalobacter colonization. Further studies are warranted to investigate the factors that impact intestinal Oxalobacter activity and colonization in health and in disease states.
In conclusion, this study is the first to show that a human strain of Oxalobacter can be detected in the small intestinal environments where it also alters oxalate handling and can significantly contribute to overall enteric oxalate excretion leading to beneficial reductions in urinary oxalate excretion. These physiologically beneficial effects of Oxalobacter as a probiotic therapy need to be confirmed in various hyperoxaluric patient populations.
Acknowledgments
Sources of Support: DKO81624, DK088892 and the Oxalosis & Hyperoxaluria Foundation.
The technical assistance of Shreya Mishra, Kristina Fernandez, and Tara Braun is greatly appreciated.
Footnotes
DISCLOSURE
The authors declared no competing interest.
References
- 1.Hatch M, Cornelius J, Allison M, Sidhu H, Peck A, Freel RW. Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int. 2006;69:691–8. doi: 10.1038/sj.ki.5000162. [DOI] [PubMed] [Google Scholar]
- 2.Hatch M, Gjymishka A, Salido EC, Allison MJ, Freel RW. Enteric oxalate elimination is induced and oxalate is normalized in a mouse model of primary hyperoxaluria following intestinal colonization with Oxalobacter. Am J Physiol Gastrointest Liver Physiol. 2010;300:G461–9. doi: 10.1152/ajpgi.00434.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allison MJ, Dawson KA, Mayberry WR, Foss JG. Oxalobacter formigenes gen. nov., sp. nov.: oxalate-degrading anaerobes that inhabit the gastrointestinal tract. Arch Microbiol. 1985;141:1–7. doi: 10.1007/BF00446731. [DOI] [PubMed] [Google Scholar]
- 4.Green ML, Hatch M, Freel RW. Ethylene glycol induces hyperoxaluria without metabolic acidosis in rats. Am J Physiol Renal Physiol. 2005;289:F536–43. doi: 10.1152/ajprenal.00025.2005. [DOI] [PubMed] [Google Scholar]
- 5.Freel RW, Hatch M, Green M, Soleimani M. Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol Gastrointest Liver Physiol. 2006;290:G719–28. doi: 10.1152/ajpgi.00481.2005. [DOI] [PubMed] [Google Scholar]
- 6.Kharlamb V, Schelker J, Francois F, Jiang J, Holmes RP, Goldfarb DS. Oral antibiotic treatment of Helicobacter pylori leads to persistently reduced intestinal colonization rates with Oxalobacter formigenes. J Endourol. 2011;25:1781–5. doi: 10.1089/end.2011.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mittal RD, Kumar R, Bid HK, Mittal B. Effect of antibiotics on Oxalobacter formigenes colonization of human gastrointestinal tract. J Endourol. 2005;19:102–6. doi: 10.1089/end.2005.19.102. [DOI] [PubMed] [Google Scholar]
- 8.Lange JN, Wood KD, Wong H, Otto R, Mufarrij PW, Knight J, Akpinar H, Holmes RP, Assimos DG. Sensitivity of human strains of Oxalobacter formigenes to commonly prescribed antibiotics. Urology. 2012;79:1286–9. doi: 10.1016/j.urology.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoppe B, Beck B, Gatter N, von Unruh G, Tischer A, Hesse A, Laube N, Kaul P, Sidhu H. Oxalobacter formigenes: a potential tool for the treatment of primary hyperoxaluria type 1. Kidney Int. 2006;70:1305–11. doi: 10.1038/sj.ki.5001707. [DOI] [PubMed] [Google Scholar]