Abstract
Objective
Food reinforcement is cross-sectionally related to body mass index and energy intake in adults, and prospectively predicts weight gain in children, but there has not been any research studying food reinforcement as a predictor of adult weight gain.
Design and Methods
This study examined whether the relative reinforcing value of food versus sedentary activities, as measured on a progressive ratio schedule, predicts 12 month weight gain. Dietary disinhibition and dietary restraint were also examined as potential moderators of this relationship, in a sample of 115 non-obese (Body Mass Index< 30) adults.
Results
In a hierarchical regression controlling for baseline age and weight, dietary hunger, income, sex and minority status, food reinforcement significantly increased the variance from 6.3% to 11.7% (p = 0.01) and predicted weight gain (p = 0.01). Dietary disinhibition moderated this relationship (p = 0.02) and increased the variance an additional 4.7% (p = 0.02), such that individuals with high food reinforcement had greater weight gain if they were also high in disinhibition.
Conclusions
These results suggest that food reinforcement is a significant contributor to weight change over time, and food reinforcement may have the biggest effect on those who are most responsive to food cues.
Introduction
Obesity is a problem of energy imbalance with greater energy intake than expenditure. Excess energy slowly accumulates over meals to contribute to weight gain, as most adults show slow gains, with averages of 0.44 to 1.76 additional pounds each year (1–4). One determinant of energy intake may be the relative reinforcing value of food (5). Food reinforcement is associated energy intake (6) and energy intake mediates the relationship between food reinforcement and body weight (7). Food reinforcement is cross-sectionally related to body fat in children (8) and to body mass index in adults (9–11). Food reinforcement also prospectively predicts weight change in children (12), and sensitization of food reinforcement, which occurs after repeated and regular consumption of high energy dense foods (13, 14), predicts weight gain in adults (15).
Recent research has shown that various psychological factors may moderate the influence of food reinforcement on BMI. For example, we have shown that dietary disinhibition interacts with food reinforcement, such that those who are high in food reinforcement and dietary disinhibition have the highest BMI values (16). Dietary disinhibition also moderates the relationship between food reinforcement and energy intake, which is the primary mechanism for food reinforcement to increase body weight (16). Dietary disinhibition is thought to provide an index of responsivity to environmental cues, or the readiness to eat based on the environmental context, and high responsiveness to palatable food cues (17). In addition, dietary disinhibition is correlated with impulsivity (18), and high food reinforcement and impulsivity are cardinal characteristics of reinforcement pathology (19). Individuals with reinforcement pathology consume more food (20) and have more trouble losing weight than individuals with low food reinforcement or low impulsivity (21).
Dietary restraint also moderates the effect of food reinforcement on BMI (16), though the effect is not as strong as dietary disinhibition. Food is more reinforcing for restrained than non-restrained individuals (10), and BMI moderates the relationship between dietary restraint and the reinforcing value of food in women (22). Dietary disinhibition and restraint may not only interact with food reinforcement to cross-sectionally predict BMI, but may also interact with food reinforcement to prospectively predict weight change. This study was designed to examine the contribution of food reinforcement to weight change over one year in non-obese adults and assess the degree to which dietary disinhibition or restraint moderates this relationship.
Methods and Procedures
Participants
One hundred and fifteen non-obese adults (51 males, 64 females) were studied. The subjects were part of a larger study on food reinforcement and dopamine genetics, details of which are reported elsewhere (16). The focus of this study was weight gain in non-obese adults. We chose to study non-obese adults (BMI < 30) to provide information relevant to development of obesity, rather than study weight gain in those who are already obese. Participants were recruited from an existing family database, newspaper ads, flyers posted around the University at Buffalo campuses and in community settings, web based recruitment (e.g. ads on Craig’s list and on the department’s website) and direct mailings targeted to community residents between the ages of 18–50. Participants were excluded from the study if they were taking medications associated with loss of appetite, were smokers, had diabetes, had previously been diagnosed with an eating disorder or psychiatric disorder (e.g. anxiety, depression, attention deficit hyperactivity disorder), were allergic to the ingredients in the study foods, were currently dieting, and did not rate at least a moderate liking (≥4 on a 9 point Likert scale) for five out of the six study foods. Participants received a $50 gift certificate to local stores for completing the study. The study was approved by the University at Buffalo Health Sciences Institutional Review Board.
Procedures
Participants visited the laboratory for sessions that included baseline measurement of height and weight, assessment of food reinforcement, and collection of dietary disinhibition, dietary restraint and hunger using the Three-Factor Eating Questionnaire (TFEQ) (23), and a follow up session to collect height and weight approximately 1 year later. Baseline sessions were scheduled between the hours of 2PM and 5PM, during a normal period that individuals would consume additional calories outside of meal time for the food reinforcement task. Participants were asked to refrain from consuming food or drinking beverages, other than water, for at least 3 hours prior to the test session and to refrain from consuming the experimental foods in the 24 hours prior to the test session. Upon initial arrival to the laboratory, participants read and signed consent forms, completed a same day and 24 hour food recall and hunger questionnaires. Prior to the start of the food reinforcement session participants were provided a preload of a Luna Sunrise Blueberry Bliss, Strawberry Crumble or Vanilla Almond Breakfast bar (Clif Bar & Company; Berkeley, CA, 42g, 150kcal, 4g fat, 23g carbohydrates, 7g protein) to minimize the effects of hunger on energy intake and food reinforcement. The inclusion of a standard preload increases the ability to show individual differences in food reinforcement (24), focusing on hedonic, rather than homeostatic, hunger. The one year follow up session was scheduled at any time convenient for the participant, and no restrictions were placed on prior food consumption. Participants completed a questionnaire that assessed medication use, health history over the last year, intention to lose weight and self-reported change in weight (gained or lost at least 5 lbs). Participants then had their weight measured, were debriefed and completed payment forms.
Measurement
Demographics
The Hollingshead (25) demographics questionnaire was used to assess socioeconomic status on the basis of education level, income, occupation and race.
Height and weight
The participant’s weight and height were measured using a digital scale (TANITA Corporation of America Inc, Arlington Heights, IL) and a digital stadiometer (Measurement Concepts & Quick Medical, North Bend, WA). On the basis of height and weight data, body mass index (BMI) was calculated according to the following formula: BMI=kg/m2. Individuals were considered obese if their BMI was at least 30kg/m2 and non-obese if their BMI was less than 30kg/m2 (26). At one year, weight stability was defined as within <3% change in body weight for each individual (27). Weight loss was weight reduction more than 3% of baseline weight and weight gain was greater than 3% of baseline weight.
Food liking, hunger
Subjective ratings of hunger were collected pre and post intake of the pre-load and after both test sessions using a 10-point Likert-type scale. Food hedonics were also measured pre and post intake of the preload and after the session for the food reinforcement session. For hunger and fullness, 1 indicated not at all hungry or not at all full and 10 indicated extremely hungry or extremely full, while for hedonics 1 indicated not liking at all and 9 indicated liking very much.
TFEQ
Participants completed the Three Factor Eating Questionnaire (TFEQ) a validated instrument to detect dietary restraint (28) with three subscales that assess dietary restraint, hunger and disinhibition.
Food reinforcement task
The reinforcing value of food was measured by determining the number of responses participants made for food or food alternatives on progressive ratio schedules of reinforcement. The experimental environment included two computer stations that participants could go back and forth between. At one station, participants could earn points toward food and at the other station they could earn points for time to spend reading Time and Newsweek magazines. This alternative activity was provided to reduce the likelihood that participants would engage in responding out of boredom. Participants were instructed on how to use the computer task and given a practice session. Following the instructions for the task, the experimenter left the room. An intercom and closed circuit video system were present in the room so the experimenter could observe the participant and the participant could communicate with the experimenter.
The reinforcement task is similar to a slot machine with shapes that rotate on the screen and a point is earned each time the three shapes match in shape and color. For every five points earned, the subject was able to receive a 70–101 kcal (14 – 20 g) portion of his or her preferred snack food selected during the ad libitum eating session or 2 minutes of time to spend reading depending on which reward they were working for. The programmed reinforcement schedules for food and reading were progressive fixed ratio schedules with response requirements of 4, 8, 16, 32, 64, 128, 256, 512, 1,024, 2,048 and so forth for each point. Participants were instructed to perform one activity at a time (i.e. play the computer game, eat or read), and that the session would end when they no longer wished to earn points for access to food or time to spend reading. Water was provided ad libitum.
The food reinforcement task generates an overall response curve showing responding for food across the levels of reinforcement. The task also generates a total number of responses made for a reinforcer across all levels of reinforcement and was used to generate a breakpoint for responding, which was defined as the last reinforcement schedule completed for access to the food or non-food alternative. The reinforcing value of food and sedentary activity were calculated separately and the relative reinforcing value of food versus sedentary activities (RRVFOOD) was calculated by taking the breakpoint for food over the sum of the breakpoint for food and alternative reinforcers (BREAKPOINTFOOD/(BREAKPOINTFOOD+BREAKPOINTREADING).
Analytic Plan
Participant characteristics were examined for differences between a tertiary split of food reinforcement and for differences between those who did and did not participate in the 1 year session, using analysis of variance for continuous variables and chi-square for categorical variables. Hierarchical regression analyses were done to examine the effect of food reinforcement on weight and BMI change over one year and dietary disinhibition and dietary restraint as potential moderators. In the first step control variables of income and baseline age, sex and weight (or BMI) were introduced. Incremental F-tests assessed if the steps significantly increased the variance accounted for (adjusted R2). The second step added food reinforcement as a predictor of weight and BMI change. The third step included adding dietary disinhibition or dietary restraint and the fourth step added the interaction of these variables with food reinforcement. These variables were chosen to be studied since in previous studies they moderated the effect of food reinforcement on body weight at baseline (19).
Results
One hundred and forty-eight participants were non-obese and eligible for follow up, and 115 completed the follow-up appointment (78%). There were no differences between those who participated or did not participate in the follow-up in terms of BMI, dietary disinhibition and restraint, food reinforcement, income or education; however, those who participated in follow-up were older (34.91 ± 1.01, F(1,146) = 11.52, p < 0.01) than those who did not participate in follow-up (27.65 ± 1.89) and those who participated in follow-up were more likely to be a non-minority (22% minority, χ2(1) = 7.31, p < 0.01) than those who did not participate (45% minority). Age and minority status were included as covariates in subsequent analyses to control for these variables.
Participant characteristics divided by those low (< 0.33, n = 48), average (≥ 0.33, <0.75, n = 42) and high (> 0.75, n = 25) in relative reinforcing value of food are shown in Table 1. An examination of a tertiary split by RRVFOOD revealed significant differences in dietary hunger (F(2,112) = 3.19, p = 0.04) and income (χ2(18) = 31.64, p = 0.02), with individuals with high food reinforcement having higher dietary hunger and lower proportions of high income individuals. As expected, weight change (F(2,112) = 3.86, p = 0.02) and food reinforcement (F(2,112) = 610.60, p < 0.01) also significantly different by group. Hypothesis testing on between group differences in in weight change revealed a significant difference between the combination of low and average groups versus the high group (F(1,112) = 5.67, p = 0.02), with no differences observed between low and average groups (p > 0.05). Similarly, a significant difference in percentage of participants who lost, were stable or gained weight was observed between the collapsed low and average versus high food reinforcement groups (χ2(2) = 8.24, p = 0.02), such that 44% of individuals with high food reinforcement gained weight versus 18% of individuals with low or average food reinforcement.
Table 1.
Participant Characteristics (± SEM)
| Food Reinforcementa | |||||
|---|---|---|---|---|---|
| Total | Low | Average | High | P Value | |
| N | 115 | 48 | 42 | 25 | |
| Sex (n)(male/female) | 51/64 | 17/31 | 21/21 | 13/12 | 0.26 |
| Age | 34.91 ± 1.04 | 36.55 ± 1.54 | 33.78 ± 2.29 | 33.78 ± 2.29 | 0.42 |
| Restraint | 7.68 ± 0.48 | 8.04 ± 0.74 | 7.86 ± 0.81 | 6.68 ± 1.00 | 0.54 |
| Disinhibition | 5.25 ± 0.30 | 4.81 ± 0.46 | 5.71 ± 0.46 | 5.32 ± 0.58 | 0.40 |
| Hunger | 4.89 ± 0.31 | 4.19 ± 0.45 | 4.90 ± 0.50 | 6.20 ± 0.73 | 0.04 |
| Average Liking* | 7.08 ± 0.08 | 7.05 ± 0.12 | 7.22 ± 0.14 | 6.90 ± 0.16 | 0.31 |
| Liking of favorite food | 8.40 ± 0.06 | 8.42 ± 0.10 | 8.38 ± 0.10 | 8.40 ± 0.15 | 0.97 |
| RRVFOOD | 0.42 ± 0.03 | 0.13 ± 0.01 | 0.44 ± 0.02 | 0.93 ± 0.02 | <0.001 |
| BMI | 24.57 ± 0.28 | 24.29 ± 0.45 | 24.66 ± 0.46 | 24.96 ± 0.48 | 0.64 |
| BMI change | 0.25 ± 0.11 | 0.09 ± 0.16 | 0.11 ± 0.18 | 0.78 ± 0.23 | 0.03 |
| Baseline Height (in) | 66.96 ± 0.38 | 65.92 ± 0.58 | 67.54 ± 0.61 | 67.97 ± 0.80 | 0.06 |
| Baseline weight (lbs) | 157.68 ± 2.72 | 151.20 ± 4.30 | 160.70 ± 4.39 | 165.06 ± 5.77 | 0.11 |
| Weight change (lbs) | 1.71 ± 0.71 | 0.53 ± 0.99 | 0.88 ± 1.22 | 5.38 ± 1.58 | 0.02 |
| Weight changeb (%) | 0.07 | ||||
| Weight loss | 12% | 15% | 14% | 4% | |
| Weight stable | 64% | 70% | 64% | 52% | |
| Weight gain | 24% | 15% | 22% | 44% | |
| Education (%) | |||||
| Some college or more | 90% | 94% | 88% | 84% | 0.27 |
| Minority (%) | 22% | 19% | 26% | 20% | 0.68 |
| Income <$50,000 (%) | 57% | 48% | 62% | 64% | 0.02 |
Low food reinforcement <0.33, average food reinforcement ≥ 0.33, <0.75, high food reinforcement ≥ 0.75
Weight loss, baseline weight loss > 3% loss; Weight Stable, < 3% total change; Weight gain, > 3% gain
one unreported datapoint
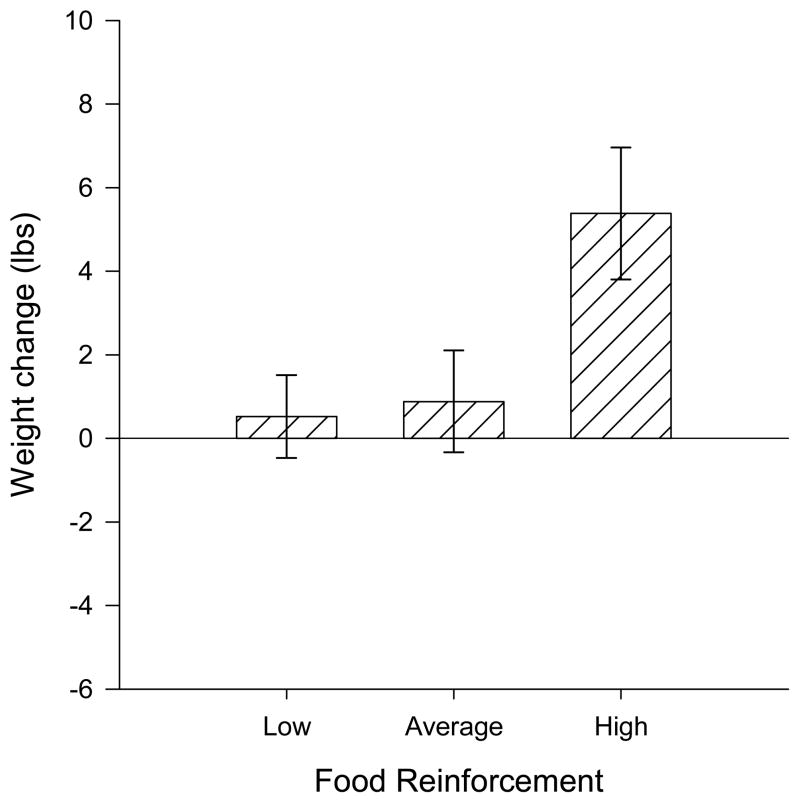
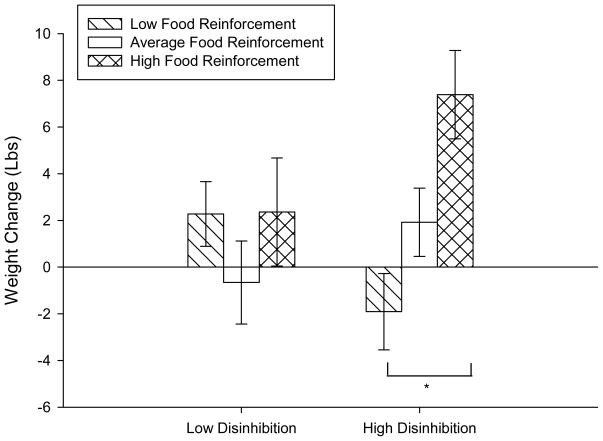
Both BMI and weight change showed similar results for the hierarchical regression analysis. Only weight change will be presented for simplicity. The first step in the model, included baseline age, weight, dietary hunger, sex, minority status and income and accounted for 6.33 percent of the variance in weight gain, with none of the variables being independent predictors of weight gain. Adding RRVFOOD was significantly associated with weight change (Beta = 5.79, p = 0.01) and increased the variance accounted for from 6.33 percent to 11.70 percent (FINC(1,108) = 6.59, p = 0.01) (see Table 2). As shown in Figure 1, a high RRVFOOD of greater than 0.75 was associated with a weight gain of 5.38 ± 1.50 lbs. (n = 25), while the average RRVFOOD group and low RRVFOOD group gain approximately 0.88 ± 1.15 lbs. (n = 42) and 0.53 ± 1.08 lbs (n = 48), respectively. Tests of the moderators of food reinforcement on weight gain showed that the addition of dietary disinhibition did not significantly increase the variance in the model (R2 = 11.99%, FINC(1,107) = 0.38, p = 0.54). The interaction between disinhibition and food reinforcement, however, significantly increased the variance to 16.69 percent (FINC(1,106) = 6.14, p = 0.01) and significantly predicted weight change (Beta = 1.78, p = 0.02). Significant interactions were examined using hypothesis testing examining a tertiary split of food reinforcement (<0.33, low; ≥0.33, <0.75, average; ≥0.75, high) and a median split of dietary disinhibition (<5, ≥5). Hypothesis testing examining each food reinforcement group showed that there were significant differences in weight change for individuals with high disinhibition across low and high food reinforcement (F(1, 109) = 13.82, p < 0.01), but not for individuals with low disinhibition (F(1, 109) = 0.001, p = 0.97) (Figure 2). Within the high disinhibition group, individuals with low food reinforcement lost 1.91 ± 1.64 lbs, while those with high food reinforcement was associated with a gain of 7.39 ± 1.89 lbs. Low disinhibition individuals did not show a differential effect of food reinforcement, with both low and high food reinforcement gaining approximately 2.27 ± 1.38 lbs and 2.36 ± 2.32 lbs respectively. Dietary restraint did not moderate the effect of food reinforcement on body weight gain.
Table 2.
Hierarchical Regression Analysis
| Effect | R2 | Δ R2 | Beta | t | P value |
|---|---|---|---|---|---|
| Step 1 | 0.0633 | 0.30 | |||
| Baseline Age | 0.08 | 0.85 | 0.40 | ||
| Baseline Weight | 0.01 | 0.15 | 0.88 | ||
| Sex | −1.67 | −0.81 | 0.42 | ||
| Income | −0.95 | −1.98 | 0.05 | ||
| Minority status | −0.05 | −0.03 | 0.98 | ||
| Dietary hunger | −0.12 | −0.52 | 0.60 | ||
| Step 2 | 0.1170 | 0.0537 | |||
| Food reinforcement | 5.79 | 2.55 | 0.01 | ||
| Finc(1,108) = 6.59, p = 0.01 | |||||
| Step 3 | 0.1199 | 0.0029 | |||
| Dietary Disinhibition | −0.17 | −0.60 | 0.55 | ||
| FINC(1, 107) = 0.38, p = 0.54 | |||||
| Step 4 | 0.1669 | 0.0470 | |||
| Food reinforcement *Dietary Disinhibition | 1.78 | 2.43 | 0.02 | ||
| Finc(1, 106) = 6.14, p = 0.02 | |||||
Figure 1.
Weight change over 12 months (mean ± SEM) in relation to food reinforcement, divided into three groups, relative food reinforcement less than 0.33 (low), ≥0.33 and <0.75 (average), and greater than or equal to 0.75 (high). Food reinforcement significantly predicted weight change over one year (p = 0.01), with individuals having high food reinforcement gaining significantly more weight than those with low food reinforcement.
Figure 2.
Weight change over 12 months (mean ± SEM) in relation to food reinforcement and dietary disinhibition. Dietary disinhibition was divided into two groups based on a median split of less than 5 (n = 55) and greater than or equal to 5 (n = 60) and food reinforcement was divided into three groups, relative food reinforcement less than 0.33 (low; n = 48), ≥0.33 and <0.75 (average, n = 42), and greater than or equal to 0.75 (high, n = 25). There are significant differences in weight change across food reinforcement for individuals with high dietary disinhibition (*p < 0.01), but not low disinhibition (p = 0.97).
Discussion
Food reinforcement has been previously shown to be cross-sectionally related to BMI and obesity in adults (9–11). The results presented here extend the findings that food reinforcement predicts body fat gain in children (12) and sensitization of food reinforcement predicts weight gain in adults (15). Previous research has shown that food reinforcement is related to energy intake (6), energy intake mediates the relationship between food reinforcement and body weight (7), and food reinforcement predicts weight loss in children (21). Together with the data presented here, this suggests that food reinforcement may be an important determinant of weight gain and a target for prevention of obesity, which is strengthened by the parallel findings between weight and BMI change.
Food reinforcement may differentially impact people based on their degree of dietary disinhibition, as individuals with a combination of high food reinforcement and high disinhibition had the most weight gain over one year. Disinhibition can be conceptualized as an increased responsivity to food cues, which is supported by laboratory results (29–31). Sensitivity to environmental cues for food may cause increased attention to food, which has been shown to be related to energy intake and weight change over time (32). Increased attention to food may provide additional opportunities to eat and individuals with low inhibitory control may be unable to prevent additional caloric intake (33). When combined with high food reinforcement, these individuals may have many more eating bouts or larger meals due to increased attention to food, resulting in weight gain over time. Dietary disinhibition has also been related to impulsivity (18), and previous studies have shown that a combination of high food reinforcement and impulsiveness predict greater energy intake (34) and higher BMI’s (20) for both obese and lean individuals. Theoretically this may be a combination of both being highly motivated to eat and unable to control one’s actions for future benefits, a concept we have termed reinforcement pathology (19, 35).
One implication of this data is that modifying the relative reinforcing value of food could be a target for prevention of obesity and obesity treatment programs. If individuals at risk for obesity are less motivated to eat, they will consume less food and gain less weight over time, preventing future obesity. Obese individuals may also have an easier time losing weight if they had lower food reinforcement and more enticing alternatives to food (21). One option to minimize the impact of high food reinforcement is to reduce access to highly reinforcing and energy-dense foods and work on methods for substituting healthier foods. It is well known in the behavioral economic literature that increasing price or limiting access leads to decreased purchasing, an idea can be translated in weight loss programs by modifying the food environment to limit access by stimulus or environmental control (36, 37). A complementary approach to increasing the cost of high energy dense foods is to focus on substitution of healthier, low energy dense or even non-food reinforcers in the place of high energy dense-foods (37). There may be individual differences in substitution that can be used to increase the propensity to substitute, which may ultimately decrease the reinforcing value of the originally preferred food. Food reinforcement for healthy foods may also be increased by consuming healthy foods following food deprivation, as food deprivation increases the reinforcing value of food (38).
In addition to interventions that target food reinforcement, it may be possible to target variables that moderate the effect of food reinforcement on body weight. Our data suggests that weight gain is the greatest for those who are high in both food reinforcement and dietary disinhibition, while those high in food reinforcement are not at great risk for weight gain if they are lower in dietary disinhibition. It may be possible to target reducing dietary disinhibition. As noted above, impulsivity is related to dietary disinhibition (29), and food reinforcement and impulsivity interact to predict energy intake (20). It is possible that techniques that reduce impulsivity, such as working memory training programs (39), may reduce food reinforcement. Dietary disinhibition is also related to responsivity to food cues, and studies have shown that attentional bias towards food words and images is predictive of weight gain over time (32). Individuals who attend to food stimuli may be more sensitive or prone to eating opportunities and may consume more calories. Modifying the relationship between food cues and eating by extinction methods to reduce cue control over eating, or modifying the allocation of attention toward food cues may be useful to reduce the influence of food reinforcement on body weight.
One limitation to this study includes the differences in age and minority status between individuals’ eligible for and completed follow up weight assessment. Individuals who completed follow up were older and more likely to be non-minorities, which may impact the generalizability of the study. However, previous studies have shown that food reinforcement predicts weight gain in children (12) suggesting that younger age groups also show this relationship. While it is possible that minority populations have additional factors that influence weight change, in addition to, or to the exclusion of food reinforcement, this has not been suggested by previous cross-sectional studies of food reinforcement (6).
There is a consistent body of research on cross-sectional and prospective relationships between food reinforcement and body weight. Additional research on how food reinforcement develops should be a priority, as research on food sensitization suggests that food reinforcement can change over time based on food consumption patterns (40). Developing a better understanding of how the motivation to eat develops and how to modify the motivation to eat provides a new pathway to alter body weight and obesity.
Acknowledgments
Appreciation is expressed to Lora G. Roba, Vida Rostami, Lauren Angelucci, Nicole Gens, Caitlin Hart and Kirstie Clune for data collection and data entry and assisting in the implementation of protocol. This research was funded in part by a grant from the National Institute of Drug Abuse, R01DA024883 awarded to Dr. Epstein. The funding agency was not involved in analysis or interpretation of the data.
Footnotes
Conflicts of Interest
None of the authors have conflicts of interest to report.
References
- 1.Rookus MA, Burema J, Van’t Hof MA, Deurenberg P, Van der Wiel-Wetzels WAM, Hautvast JGAJ. The development of the body mass index in young adults. I: Age-reference curves based on a four-year-mixed longitudinal study. Hum Biol. 1987;59:599–615. [PubMed] [Google Scholar]
- 2.Colditz GA, Willett W, Stampfer MJ, London SJ, Segal M, Speizer FE. Patterns of weight change and their relation to diet in a cohort of healthy women. Am J Clin Nutr. 1990;51:1100–1105. doi: 10.1093/ajcn/51.6.1100. [DOI] [PubMed] [Google Scholar]
- 3.Williamson DF. Descriptive epidemiology of body weight and weight change in US adults. Ann Intern Med. 1993;119:646–649. doi: 10.7326/0003-4819-119-7_part_2-199310011-00004. [DOI] [PubMed] [Google Scholar]
- 4.Yanovski JA, Yanovski SZ, Sovik KN, Nguyen TT, O’Neil PM, Sebring NG. A prospective study of holiday weight gain. N Engl J Med. 2000;342:861–867. doi: 10.1056/NEJM200003233421206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein LH, Leddy JJ, Temple JL, Faith MS. Food reinforcement and eating: A multilevel analysis. Psychol Bull. 2007;133:884–906. doi: 10.1037/0033-2909.133.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epstein LH, Carr KA, Lin H, Fletcher KD. Food reinforcement, energy intake, and macronutrient choice. Am J Clin Nutr. 2011;94:12–18. doi: 10.3945/ajcn.110.010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein LH, Carr KA, Lin H, Fletcher KD, Roemmich JN. Usual energy intake mediates the relationship between food reinforcement and BMI. Obesity (Silver Spring) 2012;20:1815–1819. doi: 10.1038/oby.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Temple JL, Legierski CM, Giacomelli AM, Salvy S-J, Epstein LH. Overweight children find food more reinforcing and consume more energy than do nonoverweight children. Am J Clin Nutr. 2008;87:1121–1127. doi: 10.1093/ajcn/87.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saelens BE, Epstein LH. Reinforcing value of food in obese and non-obese women. Appetite. 1996;27:41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- 10.Giesen JCAH, Havermans RC, Nederkoorn C, Strafaci S, Jansen A. Working harder to obtain more snack foods when wanting to eat less. Behav Res Ther. 2009;47:13–17. doi: 10.1016/j.brat.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Giesen JCAH, Havermans RC, Douven A, Tekelenburg M, Jansen A. Will work for snack food: the association of BMI and snack reinforcement. Obesity (Silver Spring) 2010;18:966–970. doi: 10.1038/oby.2010.20. [DOI] [PubMed] [Google Scholar]
- 12.Hill C, Saxton J, Webber L, Blundell J, Wardle J. The relative reinforcing value of food predicts weight gain in a longitudinal study of 7–10-y-old children. Am J Clin Nutr. 2009;90:276–281. doi: 10.3945/ajcn.2009.27479. [DOI] [PubMed] [Google Scholar]
- 13.Temple JL, Chappel A, Shalik J, Volcy S, Epstein LH. Daily consumption of individual snack foods decreases their reinforcing value. Eat Behav. 2008;9:267–276. doi: 10.1016/j.eatbeh.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Temple JL, Bulkley A, Badawy R, Krause N, McCann S, Epstein LH. Differential effects of daily snack food intake on food reinforcement in obese and non-obese women. Am J Clin Nutr. 2009;90:304–313. doi: 10.3945/ajcn.2008.27283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Temple J, Epstein L. Sensitization of food reinforcement is related to weight status and baseline food reinforcement. Int J Obes (Lond) 2011;36:1102–1107. doi: 10.1038/ijo.2011.210. [DOI] [PubMed] [Google Scholar]
- 16.Epstein LH, Lin H, Carr KA, Fletcher KD. Food reinforcement and obesity: Psychological Moderators. Appetite. 2012;58:157–162. doi: 10.1016/j.appet.2011.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryant EJ, King NA, Blundell JE. Disinhibition: its effects on appetite and weight regulation. Obes Rev. 2007;9:409–419. doi: 10.1111/j.1467-789X.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- 18.Yeomans MR, Leitch M, Mobini S. Impulsivity is associated with the disinhibition but not restraint factor from the Three Factor Eating Questionnaire. Appetite. 2008;50:469–476. doi: 10.1016/j.appet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Carr KA, Daniel TO, Lin H, Epstein LH. Reinforcement pathology and obesity. Curr Drug Abuse Rev. 2011;4:190–196. doi: 10.2174/1874473711104030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rollins BY, Dearing KK, Epstein LH. Delay discounting moderates the effect of food reinforcement on energy intake among non-obese women. Appetite. 2010;55:420–425. doi: 10.1016/j.appet.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Best JR, Theim KR, Gredysa DM, Stein RI, Welch RR, Saelens BE, et al. Behavioral economic predictors of overweight children’s weight loss. J Consult Clin Psychol. 2012;80:1086–1096. doi: 10.1037/a0029827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldfield GS, Lumb A. Effects of dietary restraint and body mass index on the relative reinforcing value of snack food. Eat Disord. 2009;17:46–62. doi: 10.1080/10640260802570106. [DOI] [PubMed] [Google Scholar]
- 23.Stunkard A, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 24.Reiss S, Havercamp S. The sensitivity theory of motivation: Implications for psychopathology. Behav Res Ther. 1996;34:621–632. doi: 10.1016/0005-7967(96)00041-1. [DOI] [PubMed] [Google Scholar]
- 25.Hollingshead A. Thesis. New Haven, CT: Yale University; 1975. Four factor index of social status. [Google Scholar]
- 26.NHLBI Obesity Education Initiative Expert Panel. Clinical Guidelines on the identification, evaluation, and treatment of overweight and obesity in adults - The evidence report. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 27.Stevens J, Truesdale K, McClain J, Cai J. The definition of weight maintenance. Int J Obs (Lond) 2005;30:391–399. doi: 10.1038/sj.ijo.0803175. [DOI] [PubMed] [Google Scholar]
- 28.Allison DB, Kalinsky L, Gorman B. A comparision of the psychometric properties of three measures of dietary restraint. Psychol Assess. 1992;4:391–398. [Google Scholar]
- 29.Yeomans MR, Tovey HM, Tinley EM, Haynes CJ. Effects of manipulated palatability on appetite depend on restraint and disinhibition scores from the Three-Factor Eating Questionnaire. Int J Obes Relat Metab Disord. 2004;28:144–151. doi: 10.1038/sj.ijo.0802483. [DOI] [PubMed] [Google Scholar]
- 30.Tapper K, Pothos EM, Fadardi JS, Ziori E. Restraint, disinhibition and food-related processing bias. Appetite. 2008;51:335–338. doi: 10.1016/j.appet.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Yeomans MR, Mobini S, Bertenshaw EJ, Gould NJ. Acquired liking for sweet-paired odours is related to the disinhibition but not restraint factor from the Three Factor Eating Questionnaire. Physiol Behav. 2009;96:244–252. doi: 10.1016/j.physbeh.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Yokum S, Ng J, Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity (Silver Spring) 2011;19:1775–1783. doi: 10.1038/oby.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loeber S, Grosshans M, Korucuoglu O, Vollmert C, Vollstädt-Klein S, Schneider S, et al. Impairment of inhibitory control in response to food-associated cues and attentional bias of obese participants and normal-weight controls. Int J Obes (Lond) 2011;36:1334–1339. doi: 10.1038/ijo.2011.184. [DOI] [PubMed] [Google Scholar]
- 34.Appelhans BM, Woolf K, Pagoto SL, Schneider KL, Whited MC, Liebman R. Inhibiting food reward: Delay discounting, food reward sensitivity, and palatable food intake in overweight and obese women. Obesity (Silver Spring) 2011;19:2175–2182. doi: 10.1038/oby.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Epstein LH, Salvy S-J, Carr KA, Dearing KK, Bickel WK. Food reinforcement, delay discounting and obesity. Physiol Behav. 2010;100:438–445. doi: 10.1016/j.physbeh.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Epstein LH, Paluch RA, Kilanowski CK, Raynor HA. The effect of reinforcement or stimulus control to reduce sedentary behavior in the treatment of pediatric obesity. Health Psychol. 2004;23:371–380. doi: 10.1037/0278-6133.23.4.371. [DOI] [PubMed] [Google Scholar]
- 37.Epstein LH, Jankowiak N, Nederkoorn C, Raynor HA, French SA, Finkelstein E. Experimental research on the relation between food price changes and food-purchasing patterns: a targeted review. Am J Clin Nutr. 2012;95:789–809. doi: 10.3945/ajcn.111.024380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raynor HA, Epstein LH. The relative-reinforcing value of food under differing levels of food deprivation and restriction. Appetite. 2003;40:15–24. doi: 10.1016/s0195-6663(02)00161-7. [DOI] [PubMed] [Google Scholar]
- 39.Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol Psychiatry. 2011;69:260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark EN, Dewey AM, Temple JL. Effects of daily snack food intake on food reinforcement depend on body mass index and energy density. Am J Clin Nutr. 2010;91:300–308. doi: 10.3945/ajcn.2009.28632. [DOI] [PubMed] [Google Scholar]