Abstract
Background
Nuclear transfer (NT) technologies offer a means for producing the genetically modified pigs necessary to develop swine models for mechanistic studies of disease processes as well as to serve as organ donors for xenotransplantation. Most previous studies have used commercial pigs as surrogates.
Method and Results
In this study, we established a cloning technique for miniature pigs by somatic cell nuclear transfer (SCNT) using Nippon Institute for Biological Science (NIBS) miniature pigs as surrogates. Moreover, utilizing this technique, we have successfully produced an α-1, 3-galactosyltransferase knockout (GalT-KO) miniature swine. Fibroblasts procured from a NIBS miniature pig fetus were injected into 1312 enucleated oocytes. The cloned embryos were transferred to 11 surrogates of which five successfully delivered 13 cloned offspring; the production efficiency was 1.0% (13/1312). In a second experiment, lung fibroblasts obtained from neonatal GalT-KO MGH miniature swine were used as donor cells and 1953 cloned embryos were transferred to 12 surrogates. Six cloned offspring were born from five surrogates, a production efficiency of 0.3% (6/1953).
Conclusions
These results demonstrate successful establishment of a miniature pig cloning technique by SCNT using NIBS miniature pigs as surrogates. To our knowledge, this is the first demonstration of successful production of GalT-KO miniature swine using miniature swine surrogates. This technique could help to ensure a stable supply of the cloned pigs through the use of miniature pig surrogates and could expand production in countries with limited space or in facilities with special regulations such as specific pathogen-free or good laboratory practice.
Keywords: α-1,3-galactosyltransferase knockout; embryo transfer; GalT-KO MGH miniature; miniature pigs; Nippon Institute for Biological Science miniature pigs; somatic cell nuclear transfer
Introduction
Since the initial successes in commercial pig cloning by somatic cell nuclear transfer (SCNT) in 2000 [1,2], the nuclear transfer (NT) technique has produced transgenic pigs using genetically modified donor cells [3] and gene knockout pigs using gene-targeted donor cells [4–6]. Most of these studies have used commercial pigs as surrogates for NT, and the efficiency of SCNT was approximately 1 to 2% with this technique [7].
Biologically, pigs have favorable breeding characteristics, reproducing in 3 month gestation cycles, and are physiologically and anatomically similar to humans making them particularly well suited for biomedical research [8]. Partially inbred miniature swine are an especially attractive choice because of their size and subsequent ease of handling. The maximum adult weight of MGH miniature swine is approximately 120 kg (20 kg at 4 months of age), which is similar to that of humans, in contrast to domestic swine that can weigh in excess of 500 kg. Utilizing these advantages, several groups have succeeded in producing cloned miniature pigs using commercial pigs [5,6,9–20] or miniature pigs [13,14,21] as surrogates. We have developed a Nippon Institute for Biological Science (NIBS) miniature pig colony since 1993 for testing of medical devices and drug efficacy/toxicity tests as well as research in tissue engineering and xenotransplantation. The animals are routinely screened for several viruses (Japanese Encephalitis virus, Getah virus, Porcine Parvovirus, Transmissible Gastroenteritis virus, Porcine Epidemic diarrhea virus, Aujeszky’s disease virus, Swine fever virus, Porcine Reproductive and Respiratory virus syndrome virus); bacteria (Actinobacillus pleuropneumoniae (Serotypes: 1, 2, 5), Haemophilus parasuis (Serotypes: 2, 5), Bordetella bronchiseptica, Pasteurella multocida (toxin producing), Erysipelothrix rhusio-pathiae, Mycoplasma hyopneumoniae, Salmonella spp.), and ecto- and endoparasites (Toxoplasma gondii, Metastrongulus elomgatus, Ascaris suum, Balantidium coli) and are maintained free of these pathogens to minimize zoonosis after transplantation. In this study, we aimed to establish a cloning technique for miniature pigs by SCNT using NIBS miniature pigs as surrogates. To our knowledge, this is the first report demonstrating successful production of cloned α-1, 3-galactosyl-transferase knockout (GalT-KO) Massachusetts General Hospital (MGH) miniature swine using the same breed as surrogates.
Materials and methods
Animals (surrogates)
A total of 23 NIBS miniature gilts and sows, aged 1 to 5 yr, were used as surrogates in this experiment. The NIBS miniature pig breed, which was originally derived from Pitman-Moore miniature pigs, Taiwanese small-ear pigs and Göttingen miniature pigs, was established in 1993 [22]. Prepubertal pigs begin to have an estrous cycle at approximately 4 months of age; they are placed in a breeding program at 8 to 12 months of age. Animals were individually housed in stainless steel cages (1600 × 1400 × 800 mm) or cement pens (70 × 1200 × 70 mm) and were given ad libitum access to fresh water in addition to 600 to 1200 g/ day of a commercial feed (Bremeal Maxim; Nippon Formula Feed Mfg Co., Ltd., Yokohama, Japan). Room temperature was maintained at 20 to 25 °C, and relative humidity was 40 to 70%. The animals were cared for and treated in accordance with the Regulation of Animal Experimentation of NIBS.
Donor cells (NT cells)
Fetal fibroblasts were harvested and isolated from a NIBS miniature pig on gestational day 30. The fetus was removed, and embryonic tissue was minced using fine scissors. The minced tissues were dissociated in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen Corp., Carlsbad, CA, USA) supplemented with 0.25% (w/v) trypsin and 1 mM EDTA for 1 h at 37 °C in a humidified atmosphere of 5% CO2. Trypsinized cells were washed once in Dulbecco’s phosphate buffered saline (DPBS; Invitrogen Corp.) by centrifugation at 300 g for 3 min and seeded onto a culture dish. Subsequently, cells were cultured for 8 to 10 days in DMEM supplemented with 10% (v/v) fetal bovine serum (FBS). Cultured cells at passages 2 to 8 were used for SCNT.
To produce cloned GalT-KO miniature swine, lung fibroblasts were obtained from a male neonatal GalT-KO MGH miniature swine (#17268) by the Organ Transplantation Tolerance and Xenotransplantation Laboratory, Transplantation Biology Research Center, MGH, USA. Isolated cells were frozen at −80 °C and then shipped to the NIBS, Japan. Thawed cultured cells at passages 3 to 10 were used for SCNT. A single-cell suspension of the fetal or lung fibroblasts in DPBS supplemented with 0.5% (v/v) FBS was prepared by trypsinization immediately prior to NT.
Oocytes for SCNT
All oocytes were prepared using the regimen described by Koo et al. [23]. Briefly, ovaries were procured from prepubertal gilts at two local slaughterhouses. Cumulus–oocyte complexes were cultured in Medium 199 Eagle’s (Medium 199; Invitrogen Corp.) supplemented with 10 ng/ml epidermal growth factor, 10 IU/ml equine chorionic gonadotropin (PMS; Nippon Zenyaku Kogyo Co., Ltd., Fukushima, Japan), 10 IU/ml human chorionic gonadotropin (Puberogen®; Novartis Animal Health K. K, Tokyo, Japan), and 10% (v/v) porcine follicular fluid at 39 °C in a humidified atmosphere of 5% CO2. After culturing for 22 h, cumulus–oocyte complexes were transferred to hormone-free medium and cultured for 15 to 22 h. Oocytes were freed from cumulus cells by repeated pipetting in 0.1% (w/v) hyaluronidase.
SCNT procedure
A micromanipulation system (NARISHIGE Group, Tokyo, Japan) attached to an inverted microscope (TE2000-S; Nikon Corporation, Tokyo, Japan) was used. A cumulus-free oocyte was held with a holding micropipette in HEPES-buffered Medium 199, and the first polar body and the metaphase-II chromosomes were removed with a 20-μm-inner-diameter injection micropipette. Single fetal or lung fibroblast cells with a smooth surface were collected using an injection micropipette and transferred into the perivitelline space of an enucleated oocyte. Enucleation was confirmed in medium supplemented with 5 mg/ml cytochalasin B and bisBenzimide (Hoechst 33342).
Cell–oocyte complexes were transferred to a pulsing medium containing 260 mM mannitol, 0.5 mM HEPES, 0.1 mM CaCl2, and 0.1 mM MgSO4 [23]. Two stainless steel wires (100-μm-diameter) were used as electrodes and attached to the micromanipulation system (as was carried out for SCNT). The single cell–oocyte-complex was sandwiched between the electrodes and oriented with the contact surface between the cytoplast and the donor cell perpendicular to the electrodes such that the two electrodes were 1 mm apart. The couplets were fused and simultaneously activated with a single 1.7 kV/cm DC pulse for 60 μs using an LF 101 Fusion Machine (Nepa Gene Co., Ltd., Chiba, Japan), and then cultured in porcine zygote medium (PZM-5; Research Institute for the Functional Peptides Co., Ltd., Yamagata, Japan) supplemented with 2 mM 6-dimethylaminopurine [24] for 1 h.
Embryo transfer and surrogate maintenance
Miniature gilts and sows received 10 mg/day altrenogest (Regumate® Porcine; Intervet International B.V., Boxmeer, the Netherlands) in the feed for 12 days, on estrous cycle days 10 to 21 [25]. One week after the final altrenogest treatment, the surrogates were anesthetized with an intramuscular injection of atropine sulfate (Atropine Sulfate Injection; Mitsubishi Tanabe Pharma Co., Osaka, Japan) and midazolam (Dormicum®; Astellas Pharma Inc., Tokyo, Japan), followed by inhaled isoflurane (Escain®; Mylan Seiyaku Ltd., Tokyo, Japan) and oxygen. The cloned embryos were directly transferred into the ampulla of the oviduct via exploratory laparotomy. Clinical status of the surrogates was monitored daily after embryo transfer (ET). If the sows had not experienced estrus at any time in the 42 days after SCNT, they were considered to be pregnant. The cloned piglets were delivered by cesarean section if they did not start to farrow on their own in several hours after drainage of fetal fluid and were then hand-raised.
Reagents
All reagents were obtained from Sigma-Aldrich Corp. (St. Louis, MO, USA) unless otherwise stated.
Microsatellite analysis
DNA analysis was performed on the cloned NIBS miniature pigs to confirm the successful NT technique. DNA was extracted from peripheral blood and donor cells. Twenty-three porcine strain-specific DNA microsatellite markers (SW286, SW840, SW957, SW133, SW274, SW373, SW491, SW741, SW839, SW742, SW1327, SW1311, SW122, SW435, SW540, SW942, SWR1021, SW1339, SW249, SWR426, SW524, SWR414, and SW717) were used [26].
PCR to determine GalT-KO genotype
Genomic DNA was isolated from either PBMC or cells from lungs of cloned GalT-KO animals using the DNeasy Kit (Qiagen, Tokyo, Japan) according to the manufacturer’s directions. PCR was performed using F527 (forward primer, GGTTGG CCACAAAGTCATC), RN1 (reverse primer, CCC TCAACCCAGAACAGATAAG) [6].
Immunohistochemistry to determine GalT-KO phenotype
To detect Gal expression, the immunoperoxidase technique with the biotin-labeled isolectin B4 (IB4; Vector Laboratories, Burlingame, CA, USA) was used as previously described with slight modifications [27]. Briefly, after treatment with 0.1% pepsin at 37 °C for 30 min, 4-μm sections of paraffin-embedded samples were incubated with biotin-labeled IB4 for 30 min at a predetermined optimal dilution. After washing with PBS, the sections were incubated with avidin/biotin peroxidase complex (Vectastain Elite ABC Kit; Vector) for 30 min, visualized using a 3,3′-diaminobenzidine substrate kit (Vector), and counterstained with hematoxylin.
Results
Successful production of cloned NIBS miniature pigs using NIBS miniature pig surrogates
In this study, treatment with the synthetic progestogen, altrenogest, led to successful estrus synchronization, and all surrogates had preovulatory follicles and/or ovulatory follicles at the time of ET. Based on synchronized estrus and normal ovulation, all surrogates underwent ET at the anticipated time of ovulation.
The production efficiency of cloned NIBS miniature pigs is shown in Table 1A. A total of 1312 embryos, constructed using NIBS fetal fibroblasts, were transferred to 11 synchronized NIBS surrogates. The average number of transferred embryos was 119. Five of the 11 surrogates successfully delivered a total of 13 cloned offspring, including five stillbirths. The success of the pregnancy was unrelated to parity. The production rate for cloned NIBS miniature pigs was 1.0% (cloned offspring/ embryo). All of the clones were female.
Table 1.
Production efficiency of cloned (A) Nippon Institute for Biological Science miniature pigs and (B) GalT-KO MGH miniature swine by somatic cell nuclear transfer
| No. of embryos | Average no. of embryos transferred | No. of surrogates | No. (%) of pregnancy state at day 42 | No. (%) of surrogates delivered | No. of offspring | No. (%) of offspring/embryos |
|---|---|---|---|---|---|---|
| (A) 1312 | 119.3 ± 17.1a | 11b | 5 (45.5) | 5 (45.5) | 13c | 13/1312 (1.0) |
| (B) 1953 | 162.8 ± 19.4a | 12b | 5 (41.7) | 5 (41.7) | 6d | 6/1953 (0.3) |
Value was shown as the mean ± standard deviation.
All surrogates had preovulatory follicles and/or ovulatory follicles in the ovary at embryo transfer.
Average birth weight of offspring containing five stillbirths was 351 ± 85.17 g, and they were female.
Average birth weight of offspring containing four stillbirths was 330 ± 108.6 g, and they were male.
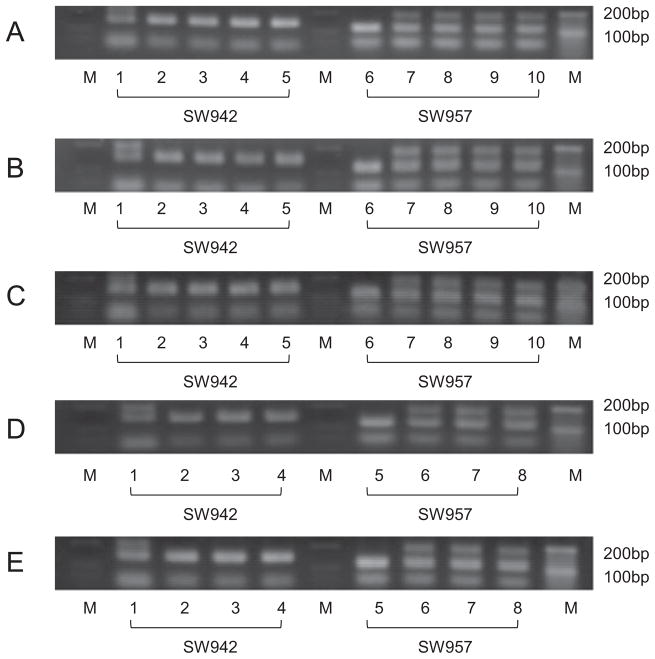
The results of representative PCR analysis of two microsatellite makers verified that the donor cells were the source of the genetic material used to produce the cloned NIBS miniature pigs. The genomic patterns of 3 (Fig. 1A), 3 (Fig. 1B), 3 (Fig. 1C), 2 (Fig. 1D), and 2 (Fig. 1E) littermate clones showed perfect identity with the donor cells and differed from the surrogate. Figure 2 shows three littermate clones shortly after birth. The average birth weight of cloned NIBS offspring was 351 ± 85.17 g.
Fig. 1.
(A–C) Representative polymerase chain reaction analysis of microsatellite markers (SW942: 1 to 5 and SW957: 6 to 10) in genomic DNA from Nippon Institute for Biological Science (NIBS) surrogate (1 and 6), clones (2 to 4 and 7 to 9) and donor cells used for nuclear transfer (NT; 5 and 10) in NIBS miniature pigs. (D, E) Representative polymerase chain reaction analysis of microsatellite markers (SW942: 1 to 4 and SW957: 5 to 8) in genomic DNA from NIBS surrogate (1 and 5), clones (2 to 3 and 6 to 7) and donor cells used for NT (4 and 8) in NIBS miniature pigs.
Fig. 2.

Picture of three cloned Nippon Institute for Biological Science (NIBS) miniature pigs shortly after birth. They were littermates and were identified individually with a black marker. The body weights were 380 g (head), 380 g (abdomen), and 420 g (no mark).
Successful production of cloned GalT-KO MGH miniature swine using as NIBS miniature pig surrogates
We next attempted to produce genetically manipulated cloned pigs using the methods described above. Because (i) swine are thought to be potential donors for clinical xenotransplantation and (ii) a major breakthrough in xenotransplantation was the production of GalT-KO swine, we chose to produce GalT-KO swine using our technique with NIBS surrogates. Lung fibroblasts obtained from a male neonatal GalT-KO MGH miniature swine isolated by the Organ Transplantation Tolerance and Xenotransplantation Laboratory, Transplantation Biology Research Center, MGH, USA, were used for SCNT.
Six cloned GalT-KO miniature swine, including four stillbirths, were produced (Table 1B). An average of 163 cloned embryos was transferred to each of 12 synchronized surrogates. Five of the 12 surrogates delivered six male piglets, and the production efficiency was 0.3%. The average birth weight of cloned GalT-KO piglets was 330 ± 108.6 g. Two of six GalT-KO swine grew well without adverse events for more than 4 months. One (GalT-KO #3) will be maintained as a future sire and is currently 11 months old (Fig. 3). The other (GalT-KO #4) provided xenogeneic kidneys for transplantation into two cynomolgous monkeys at 4 and 6 months of age. Both recipients of these GalT-KO kidneys maintained normal creatinine levels for the first 14 days post-transplant without signs of rejection. Details of these xenogeneic kidney transplants will be reported separately (K. Yamada, unpublished data). The remaining four swine died early; two died before delivery (GalT-KO #2, 6), one died at delivery (GalT-KO #1), and one died at day 1 (GalT-KO #5). The numbering of the GalT-KO pigs is based upon the date of birth.
Fig. 3.

Picture of cloned GalT-KO MGH miniature swine (#3) weighing 25 kg at 10 months of age.
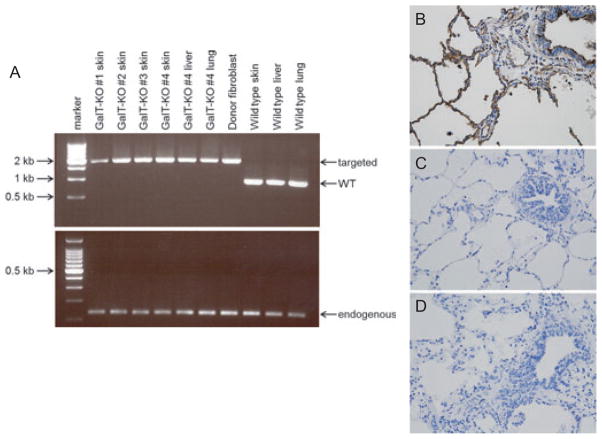
To confirm complete elimination of Gal expression, frozen samples of skin, lung, or liver were tested by PCR (GalT-KO #1-4). In the remaining two swine (GalT-KO #5 and #6), from which frozen samples were not available, isolectin B4 (IB4) staining was performed using paraffin-embedded lung sections. Both PCR (Fig. 4A) and immunohistochemistry (Fig. 4B–D) confirmed the absence of Gal epitopes in the cloned GalT-KO MGH miniature swine.
Fig. 4.
(A) PCR analysis of genomic DNA isolated from GalT-KO swine, donor cells, and wild-type swine. A PCR product of approximately 2 kb was amplified from the targeted GalT gene, while a 0.867 kb product was amplified from wild-type GalT gene. CytB was included as a control. (B–D) Isolectin B4 (IB4) staining of paraffin-embedded lungs. The same magnification was used for all pictures (×400). Positive IB4 staining (brown) in alveolar and vascular endothelium and bronchial ciliated cells in wild-type swine (B). Negative IB4 staining in the lung from GalT-KO animal #5 (C) and animal #6 (D).
Discussion
The results in this study indicate that miniature pigs can serve as surrogates and are ideal for situations where there is limited space available, such as closed specific pathogen-free (SPF) or good laboratory practice (GLP) facilities, which is the major advantage over commercial recipients. For all SCNT produced swine, the possibility of introducing undesired biological agents from the oocyte source is unavoidable in any practical scenario, although this can be reduced using washed oocytes. In addition, commercial recipients potentially introduce other agents because these animals are generally not derived from SPF herds. One disadvantage of miniature pigs is the relatively small litter size, which is of particular concern due to the large numbers of embryos that are sometimes necessary for the production of genetically cloned pigs The results of the present study, however, demonstrated that with our cloning technique, NIBS miniature pigs can be successfully used as surrogates in SCNT with an efficiency similar to that of commercial pig surrogates.
It is generally accepted that commercial pigs are preferable as surrogates for production of cloned commercial or miniature pigs due to their ability to accommodate more fetuses [14]. The average litter size of the NIBS breed is 4.2 [22] and comparatively smaller than the other miniature pig breeds (5 to 7) [25,28–30]. Despite of this smaller litter size, we demonstrated that our final production efficiency of 1% was comparable with that of commercial surrogates (1 to 2%) [7] and higher than previously reported with other miniature pigs (0.2 to 0.9%) [14,21]. In addition, production of GalT-KO MGH miniature swine using NIBS surrogates in this study (0.3%) was at least comparable with, if not slightly higher than, results reported using commercial pigs as surrogates (0.2%) [6]. Because we anticipated that highly accurate synchronization between the cloned embryos and ovarian cycles of the surrogates could increase clone production, we used altrenogest to inhibit the release of gonadotrophin- releasing hormone for estrus synchronization in this study. All surrogates had preovulatory follicles and/or ovulatory follicles at the time of ET, and cloned embryos were transferred at the anticipated time of ovulation. As a result, more than the 40% of the surrogates successfully delivered cloned NIBS miniature pigs or GalT-KO miniature swine which likely contributed to the high rate of clone production.
Previous reports in cloned calves indicated that large birth weight and placental abnormality are common [31–33]; cloned mouse placentas were also larger than normal [34]. In our study, no morphological abnormalities were observed in any of the cloned miniature pigs, including the stillborn animals. Moreover, the birth weights of cloned NIBS in this study were comparable with those of normal sex-matched NIBS pigs. The average birth weight of cloned NIBS offspring (351 ± 85.17 g) was comparable with that of female NIBS pigs (403 ± 75.3 g, unpublished data).
One approach to overcoming the limited supply of transplantable human organs is xenotransplantation [35,36]. Pigs have many advantages as donors. Firstly, the supply of pigs is essentially inexhaustible. Biologically, pigs have favorable breeding characteristics, relatively short gestation time (3 to 4 months), and are physiologically and anatomically similar to humans. Although the pig currently is viewed as the most likely source of organs for future xenotransplantation, organs from commercial pigs are incompatibly sized for humans. Partially inbred MGH miniature swine, which have been developed by David H. Sachs and pedigree-bred for the past 35 yr, are a particularly attractive choice [8,36]. Their maximum adult weights are approximately 120 kg, which is similar to that of humans, in contrast to commercial pigs which can weigh in excess of 500 kg. Despite these advantages, hyperacute rejection (HAR) caused by natural preformed xenoreactive antibodies directed against the sugar α1,3 Gal on porcine endotherial cells [37,38] was a major obstacle to xenotransplantation. To avoid this problem, two groups, including our colleagues, produced knockout pigs (GalT-KO) which do not express the Gal epitope in 2002 [4–6]. The use of GalT-KO MGH swine as kidneys donors in baboons has avoided HAR [39]. Thus far, all research centers that have successfully produced GalT-KO swine have used commercial pigs as SCNT surrogates [4–6,20,40]. To our knowledge, our study is the first demonstration of successful production of GalT-KO miniature swine by SCNT using the same breed surrogates. Use of miniature pigs as SCNT surrogates will facilitate production through SCNT in facilities with limited spaces In addition, PCR as well as pathological examination have confirmed the elimination of Gal antigens thus further demonstrating the successful application of this method using miniature pig surrogates. Finally, we have also confirmed that kidneys from our cloned GalT-KO swine maintained stable renal function in vivo for more than 3 weeks without hyperacute or accelerated humoral/vascular xenograft rejection (K. Yamada et al., unpublished data).
In conclusion, we have successfully established a miniature pig cloning technique by SCNT using the NIBS miniature pigs as surrogates. This is the first report demonstrating successful production of cloned GalT-KO MGH miniature swine using the same breed as surrogates. This technique could help to ensure a stable supply of the cloned pigs through the use of miniature pig surrogates and could expand production in countries with limited space or in facilities with special regulations such as SPF or GLP facilities.
Acknowledgments
We thank Dr. Byeong-Chun Lee (College of Veterinary Medicine, Seoul National University) for technical advice and Mr. Scott Arn for breeding and maintenance of the MGH GalT-KO miniature swine heard. We also thank Dr. Robert Hawley, Dr. Isabel Hanekamp and Dr. Masayuki Tasaki for their critical review of this manuscript. This work was supported by Grant-in-Aid for Scientific Research (A) from the Ministry of Education, Science, Sports, Culture and Technology of Japan (KY) and 2P01AI45897-11A1 from NIH/NIAID (DHS and KY).
Abbreviations
- ET
embryo transfer
- GLP
good laboratory practice
- IB4
isolectin B4
- MGH
Massachusetts General Hospital
- SCNT
somatic cell nuclear transfer
- SPF
specific pathogen-free
- PBMC
peripheral blood mononuclear cells
- NIBS
Nippon Institute for Biological Science
References
- 1.Onishi A, Iwamoto M, Akita T, et al. Pig cloning by microinjection of fetal fibroblast nuclei. Science. 2000;289:1188–1190. doi: 10.1126/science.289.5482.1188. [DOI] [PubMed] [Google Scholar]
- 2.Polejaeva IA, Chen SH, Vaught TD, et al. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature. 2000;407:86–90. doi: 10.1038/35024082. [DOI] [PubMed] [Google Scholar]
- 3.Zaidi A, Schmoeckel M, Bhatti F, et al. Life-supporting pig-to-primate renal xenotransplantation using genetically modified donors. Transplantation. 1998;65:1584–1590. doi: 10.1097/00007890-199806270-00008. [DOI] [PubMed] [Google Scholar]
- 4.Dai Y, Vaught TD, Boone J, et al. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol. 2002;20:251–255. doi: 10.1038/nbt0302-251. [DOI] [PubMed] [Google Scholar]
- 5.Kolber-Simonds D, Lai L, Watt SR, et al. Production of α-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA. 2004;101:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai L, Kolber-Simonds D, Park K, et al. Production of α-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 7.Lai L, Prather RS. Creating genetically modified pigs by using nuclear transfer. Reprod Biol Endocrinol. 2003;1:82. doi: 10.1186/1477-7827-1-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sachs DH. MHC Homozygous miniature swine. In: Swindle MM, Moody DC, Phillips LD, editors. Swine as Models in Biomedical Research. Ames, IA: Iowa State University Press; 1992. pp. 3–15. [Google Scholar]
- 9.Ahn KS, Kim YJ, Kim M, et al. Resurrection of an alpha-1,3-galactosyltransferase gene-targeted miniature pig by recloning using postmortem ear skin fibroblasts. Theriogenology. 2011;75:933–939. doi: 10.1016/j.theriogenology.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Estrada JL, Collins B, York A, et al. Successful cloning of the Yucatan minipig using commercial/occidental breeds as oocyte donors and embryo recipients. Cloning Stem Cells. 2008;10:287–296. doi: 10.1089/clo.2008.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoshino Y, Uchida M, Shimatsu Y, et al. Developmental competence of somatic cell nuclear transfer embryos reconstructed from oocytes matured in vitro with follicle shells in miniature pig. Cloning Stem Cells. 2005;7:17–26. doi: 10.1089/clo.2005.7.17. [DOI] [PubMed] [Google Scholar]
- 12.Kawarasaki T, Uchiyama K, Hirao A, et al. Profile of new green fluorescent protein transgenic Jinhua pigs as an imaging source. J Biomed Opt. 2009;14:054017. doi: 10.1117/1.3241985. [DOI] [PubMed] [Google Scholar]
- 13.Koo OJ, Park HJ, Kwon DK, et al. Effect of recipient breed on delivery rate of cloned miniature pig. Zygote. 2009;17:203–207. doi: 10.1017/S0967199409005267. [DOI] [PubMed] [Google Scholar]
- 14.Kurome M, Ishikawa T, Tomii R, et al. Production of transgenic and non-transgenic clones in miniature pigs by somatic cell nuclear transfer. J Reprod Dev. 2008;54:156–163. doi: 10.1262/jrd.19165. [DOI] [PubMed] [Google Scholar]
- 15.Lee SL, Kang EJ, Maeng GH, et al. Developmental ability of miniature pig embryos cloned with mesenchymal stem cells. J Reprod Dev. 2010;56:256–262. doi: 10.1262/jrd.09-196a. [DOI] [PubMed] [Google Scholar]
- 16.Liu HB, Lv PR, He RG, et al. Cloned Guangxi Bama minipig (Sus scrofa) and its offspring have normal reproductive performance. Cell Reprogram. 2010;12:543–550. doi: 10.1089/cell.2009.0094. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Liu J, Dai J, et al. Production of cloned miniature pigs by enucleation using the spindle view system. Reprod Domest Anim. 2010;45:608–613. doi: 10.1111/j.1439-0531.2008.01311.x. [DOI] [PubMed] [Google Scholar]
- 18.Miyoshi K, Inoue S, Himaki T, Mikawa S, Yoshida M. Birth of cloned miniature pigs derived from somatic cell nuclear transferred embryos activated by ultrasound treatment. Mol Reprod Dev. 2007;74:1568–1574. doi: 10.1002/mrd.20730. [DOI] [PubMed] [Google Scholar]
- 19.Hao YH, Yong HY, Murphy CN, et al. Production of endothelial nitric oxide synthase (eNOS) over-expressing piglets. Transgenic Res. 2006;15:739–750. doi: 10.1007/s11248-006-9020-8. [DOI] [PubMed] [Google Scholar]
- 20.Zhao J, Ross JW, Hao Y, et al. Significant improvement in cloning efficiency of an inbred miniature pig by histone deacetylase inhibitor treatment after somatic cell nuclear transfer. Biol Reprod. 2009;81:525–530. doi: 10.1095/biolreprod.109.077016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakai T, Sugimura S, Yamanaka K, et al. Production of viable cloned miniature pig embryos using oocytes derived from commercial pig ovaries. Cloning Stem Cells. 2008;10:249–262. doi: 10.1089/clo.2007.0045. [DOI] [PubMed] [Google Scholar]
- 22.Nunoya T, Shibuya K, Saitoh T, et al. Use of miniature pig for biomedical research, with reference to toxicologic studies. J Toxicol Pathol. 2007;20:125–132. [Google Scholar]
- 23.Koo OJ, Jang G, Kwon DK, et al. Electrical activation induces reactive oxygen species in porcine embryos. Theriogenology. 2008;70:1111–1118. doi: 10.1016/j.theriogenology.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 24.Im GS, Samuel M, Lai L, Hao Y, Prather RS. Development and calcium level changes in pre-implantation porcine nuclear transfer embryos activated with 6-DMAP after fusion. Mol Reprod Dev. 2007;74:1158–1164. doi: 10.1002/mrd.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimatsu Y, UCHIDA M, Niki R, Imai H. Effects of a synthetic progestogen, altrenogest, on oestrus synchronisation and fertility in miniature pigs. Vet Rec. 2004;155:633–635. doi: 10.1136/vr.155.20.633. [DOI] [PubMed] [Google Scholar]
- 26.Rohrer GA, Alexander LJ, Hu Z, Smith TP, Keele JW, Beattie CW. A comprehensive map of the porcine genome. Genome Res. 1996;6:371–391. doi: 10.1101/gr.6.5.371. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu A, Yamada K, Sachs DH, Colvin RB. Mechanisms of chronic renal allograft rejection. II. Progressive allograft glomerulopathy in miniature swine. Lab Invest. 2002;82:673–686. doi: 10.1097/01.lab.0000017370.74529.89. [DOI] [PubMed] [Google Scholar]
- 28.Bouchard G, McLaughlin RM, Ellersieck MR, Krause GF, Franklin C, Reddy CS. Retrospective evaluation of production characteristics in Sinclair miniature swine–44 years later. Lab Anim Sci. 1995;45:408–414. [PubMed] [Google Scholar]
- 29.Conley AJ, Jung YC, Schwartz NK, et al. Influence of SLA haplotype on ovulation rate and litter size in miniature pigs. J Reprod Fertil. 1988;82:595–601. doi: 10.1530/jrf.0.0820595. [DOI] [PubMed] [Google Scholar]
- 30.Panepinto LM, Phillips RW, Wheeler LR, Will DH. The Yucatan miniature pig as a laboratory animal. Lab Anim Sci. 1978;28:308–313. [PubMed] [Google Scholar]
- 31.Hill JR, Roussel AJ, Cibelli JB, et al. Clinical and pathologic features of cloned transgenic calves and fetuses (13 case studies) Theriogenology. 1999;51:1451–1465. doi: 10.1016/s0093-691x(99)00089-8. [DOI] [PubMed] [Google Scholar]
- 32.Kato Y, Tani T, Tsunoda Y. Cloning of calves from various somatic cell types of male and female adult, newborn and fetal cows. J Reprod Fertil. 2000;120:231–237. [PubMed] [Google Scholar]
- 33.Wells DN, Misica PM, Tervit HR. Production of cloned calves following nuclear transfer with cultured adult mural granulosa cells. Biol Reprod. 1999;60:996–1005. doi: 10.1095/biolreprod60.4.996. [DOI] [PubMed] [Google Scholar]
- 34.Wakayama T, Yanagimachi R. Cloning of male mice from adult tail-tip cells. Nat Genet. 1999;22:127–128. doi: 10.1038/9632. [DOI] [PubMed] [Google Scholar]
- 35.Sachs DH, Sykes M, Robson SC, Cooper DK. Xenotransplantation. Adv Immunol. 2001;79:129–223. doi: 10.1016/s0065-2776(01)79004-9. [DOI] [PubMed] [Google Scholar]
- 36.Sachs DH. The pig as a potential xenograft donor. Vet Immunol Immunopathol. 1994;43:185–191. doi: 10.1016/0165-2427(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 37.Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique natural human IgG antibody with anti-alphagalactosyl specificity. J Exp Med. 1984;160:1519–1531. doi: 10.1084/jem.160.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galili U. Anti-alpha galactosyl (anti-Gal) antibody damage beyond hyperacute rejection. In: Cooper DKC, Kemp E, Platt JL, White DJG, editors. Xenotransplantation. Heidelberg: Springer; 1997. pp. 95–103. [Google Scholar]
- 39.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of α-1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 40.Cowan PJ, Chen CG, Shinkel TA, et al. Knock out of α1,3-galactosyltransferase or expression of α1,2-fucosyl-transferase further protects CD55- and CD59-expressing mouse hearts in an ex vivo model of xenograft rejection. Transplantation. 1998;65:1599–1604. doi: 10.1097/00007890-199806270-00010. [DOI] [PubMed] [Google Scholar]