Abstract
Objective
Exercise has widely-documented cardioprotective effects but the mechanisms behind these effects are still poorly understood. Here, we test the hypothesis that aerobic training lowers cardiovascular sympathetic responses to and speeds recovery from challenge.
Methods
We conducted a randomized controlled trial contrasting aerobic versus strength training on indices of cardiac (pre-ejection period, PEP) and vascular (low-frequency blood pressure variability, LF-BPV) sympathetic responses to and recovery from psychological and orthostatic challenge in 149 young, healthy and sedentary adults.
Results
Aerobic and strength training did not alter PEP or LF-BPV reactivity to or recovery from challenge.
Conclusions
These findings, from a large randomized controlled trial using an intent-to-treat design, show that moderate aerobic exercise training has no effect on PEP and LF BPV reactivity to or recovery from psychological or orthostatic challenge. In healthy young adults, the cardioprotective effects of exercise training are unlikely to be mediated by changes in sympathetic activity.
It is well-established that aerobic exercise has significant cardioprotective effects for the general population. Recent meta-analyses have demonstrated that in 513,472 individuals, high and moderate levels of leisure time physical activity are associated with significant reduction in risk of heart disease (27% and 12% respectively) (Sofi, Capalbo, Cesari, Abbate, & Gensini, 2008) and that in 883,372 individuals followed for 20 years, physical activity was associated with a 35% reduction in the risk of cardiovascular mortality (Nocon et al., 2008). Consensus panels consistently recommend physical activity as an essential component of a healthy lifestyle (Haskell et al., 2007; Nelson et al., 2007; Pearson et al., 2003; Pearson et al., 2002).
Several biological mechanisms responsible for this cardioprotective effect are well understood, such as reduced metabolic demands on the myocardium and increased electrical stability (Scheuer & Tipton, 1977), but some remain more elusive. Modulation of responses to challenging events, both physical and psychological, is a pathophysiological mechanism that has received considerable attention. Although there is evidence to the contrary, a number of large cross-sectional and prospective studies have shown that greater reactivity to and prolonged recovery from psychological challenges predict future blood pressure status (Carroll, Phillips, Der, Hunt, & Benzeval, 2011; Steptoe & Marmot, 2005; Tuomisto, Majahalme, Kähönen, Fredrikson, & Turjanmaa, 2005), markers of atherosclerosis (Gianaros, Onyewuenyi, Sheu, Christie, & Critchley, 2011; Heponiemi et al., 2007; Jennings et al., 2004), and left ventricular mass (Kapuku et al., 1999). A recent meta-analysis confirms that greater responses to (relative risk (RR) = −.091, p <.001) and slower recovery (RR = −.096, p <.001) from laboratory challenges predict future cardiovascular disease (Chida & Steptoe, 2010). While these effects are small, they generally are consistent with the hypothesis.
Cardiovascular responses to and recovery from psychological challenge depend at least in part on the autonomic nervous system and in a recent report, we tested one element of this hypothesis. Healthy participants were randomized to a 12-week aerobic or strength training program. Indices of heart rate (HR) and cardiac parasympathetic modulation (HF-HRV) in response to and recovery from psychological and orthostatic challenge were collected before and after conditioning, as well as after 4 weeks of sedentary deconditioning. Contrary to expectation, aerobic conditioning produced no significant change in any of these indices. These findings suggest that aerobic training has its cardioprotective effects through some mechanism other than cardiac parasympathetic modulation of reactivity and recovery.
One candidate for this other mechanism is attenuated sympathetic nervous system (SNS) responses and recovery. The contribution of the SNS to cardiovascular disease and hypertension is unequivocal. Sympathetic nervous system dysfunction plays a significant role in the etiology of heart failure and hypertension (Parati & Esler, 2012) and in diabetes and metabolic syndrome (Straznicky et al., 2012). SNS activation is causally implicated in sudden cardiac death (Schwartz & Zipes, 2000). The increased incidence of myocardial infarction in the morning has been attributed to morning rise in SNS activity (Muller, 1999).
In addition, some evidence suggests that aerobic exercise training attenuates indices of SNS activity. For example, in post-myocardial infarction patients and untreated hypertensives, aerobic training significantly reduced muscle sympathetic nerve activity measured at rest (MSNA) (Laterza et al., 2007; Martinez et al., 2011). However, the effect of exercise training in normal participants may be different and indeed, some studies report that aerobic exercise training does not attenuate indices of SNS responses to psychological challenge (Cleroux, Peronnet, & de Champlain, 1985; Ray & Carter, 2010). However, these studies generally have had small and/or all male samples. In this paper, we examine the role of the SNS in reactivity to and recovery from psychological challenge in a large sample of sedentary, young and healthy men and women.
Impedance cardiography (ICG) is a non-invasive method of measuring cardiac hemodynamic indices and systolic time intervals. Among these, pre-ejection period (PEP) is often used to assess myocardial β-adrenergic activity (Kelsey, Alpert, Patterson, & Barnard, 2000; Richter, Baeriswyl, & Roets, 2012; Sherwood et al., 1990). PEP is defined as the time interval between ventricular depolarization and the opening of the aortic valve. Increases in sympathetically driven myocardial contractility result in decreases in PEP. In support of this understanding, Mezzacappa et al. demonstrated that administration of epinephrine in healthy subjects significantly decreased PEP compared to placebo (Mezzacappa, Kelsey, & Katkin, 1999). Blockade of β-adrenergic receptors leads to an increase of PEP (Schachinger, Weinbacher, Kiss, Ritz, & Langewitz, 2001), further validating it as a non-invasive index of cardiac sympathetic tone. Postural changes affect PEP as well, as myocardial contractility is dependent on preload. For example, head-up tilt has been shown to increase PEP duration (Stafford, Harris, & Weissler, 1970).
Some evidence suggests that it is also possible to non-invasively measure vascular sympathetic regulation. Like heart rate (HR), blood pressure exhibits periodic oscillations at high (0.15–0.50 Hz, HF) and low frequencies (0.04–0.15 Hz, LF), a result of the regulatory control systems having different response times to changes in blood pressure (Harald M Stauss, 2007). While HF blood pressure variability (HF-BPV) is a direct product of respiratory-induced mechanical fluctuations of cardiac output which change arterial pressure (deBoer, Karemaker, & Strackee, 1987), some studies suggest that LF-BPV reflects vascular sympathetic drive (Cevese, Grasso, Poltronieri, & Schena, 1995; Dimier-David, Billon, Costagliola, Jaillon, & Funck-Brentano, 1994; Ditor et al., 2005; Montano et al., 1992; Schächinger, Weinbacher, Kiss, Ritz, & Langewitz, 2001). For example, Schächinger et al. demonstrated that in healthy volunteers, systolic BPV in the low-frequency range increased during nitroprusside-induced hypotension and decreased during norepinephrine-induced hypertension, reflecting central SNS output (Schächinger et al., 2001). Also, significant increases in LF-BPV amplitude in response to orthostatic challenge have been observed (Cooke et al., 1999; Mukai & Hayano, 1995). These data are consistent with the view that LF-BPV reflects vascular sympathetic control. In this paper, we test the hypothesis that in contrast to strength training, aerobic exercise attenuates the sympathetic response to and shortens the recovery from psychological and orthostatic challenge.
Method
Study Design
The study was a randomized controlled trial of aerobic vs. strength training on cardiovascular autonomic regulation in response to and recovery from challenge. All subjects provided informed consent. The Institutional Review Boards of Columbia University Medical Center and St. John’s University approved this study.
Previously, we reported findings from this trial on the effects of aerobic training on cardiac parasympathetic reactivity to and recovery from psychological and orthostatic challenge (R. P. Sloan et al., 2011). Because that paper provided a comprehensive account of the research methods, below we present a less detailed description.
Study Participants
Study participants were healthy, sedentary young adults, 18–45 years of age. Participants were eligible if they did not exercise regularly or exceed American Heart Association standards for average fitness (VO2max ≤ 43 and 37 ml/kg/min for men and women respectively, established by cardiopulmonary exercise testing (CPET)). 149 participants met enrollment criteria and were randomized to either the aerobic (N=74) or strength training (N=75) group. Participants received a 6-month membership in a fitness facility and $300 for participation in the study.
Assessment of Aerobic Fitness
Maximum aerobic fitness (VO2max) was assessed by a 30 Watts (W) every 2 minute graded exercise test on an Ergoline 800S cycle ergometer (SensorMedics Corp., Anaheim, CA), until VO2max criteria (RQ ≥ 1.1, increases in ventilation without concomitant increases in VO2, achievement of maximum age-predicted heart rate, and/or volitional fatigue) were reached. The highest VO2 value attained was considered VO2max (Buchfuhrer et al., 1983). Minute ventilation was measured by a pneumotachometer connected to a FLO-1 volume transducer module (PHYSIO-DYNE Instrument Corp., Quogue, NY). Ventilatory gas analysis was assessed using paramagnetic O2 and infrared CO2 analyzers connected to a computerized system (MAX-1, PHYSIO-DYNE Instrument Corp.). All systems were calibrated against known medical grade gases.
Experimental Protocol
Both training programs were 12 weeks in length. Before training, all subjects met individually with a trainer to review their exercise regimens. After that, they exercised on their own, 3–4 times per week, in designated facilities. They were permitted to construct individualized exercise programs so long as they met the criteria below. Adherence to training programs was documented by weekly logs and computerized attendance records.
Subjects were tested on three occasions: before training, immediately after completion of training, and again after 4 weeks of sedentary deconditioning during which they were to abstain completely from any form of exercise. Data collection staff were blind to training group assignment.
Conditioning Programs
Aerobic conditioning
Subjects chose from a series of activities, e.g., cycling on a stationary ergometer, running on a treadmill, or climbing on a Stairmaster. Subjects were instructed to exercise at 70% of their maximum heart rate (220-age for men, 226-age for women). They were given an initial goal of at least 20 mins aerobic exercise per session and increased duration gradually over two to three weeks, up to 45–60 min.
Strength training
At the initial session, subjects established a level of effort that permitted them to complete three sets of 10 repetitions for each of the following exercises: bench presses, shoulder presses, quadriceps extensions, biceps curl, lateral pulls, triceps presses, and hamstring curls exercise. Subjects were instructed to increase the weight loads for these exercises by five pounds every two weeks.
Psychophysiology Testing Sessions
Testing sessions were scheduled for prior to randomization, after completion of training, and after completion of deconditioning. Participants were tested in the Behavioral Medicine Laboratory after eating a light breakfast and abstaining from caffeinated beverages. ECG electrodes were placed on the right shoulder, on the left anterior axillary line at the 10th intercostal space and in the right lower quadrant. Stretch bands were placed around the subject’s chest and abdomen for measurement of respiration. Band electrodes for impedance cardiography (ICG) were placed on the upper neck and the root of the neck and on the upper abdomen and at the level of the xiphoid process. A Finapres blood pressure cuff was placed on the middle finger of the non-dominant hand.
After instrumentation, the subject rested quietly in the seated position during a 10 min baseline period followed by a 5-min period for preparation of their public speaking task (see below), the 5-min public speaking period and a 5-min recovery period. Then the subject was placed in the supine position on a Midland electric tilt table, modified to suspend a computer monitor in the subject’s visual field for display of the psychological tasks. A numeric keypad, for responding to the arithmetic and Stroop tasks, was secured in a comfortable position relative to the dominant hand. The subject then rested quietly for 6 min of adaptation to position, followed by a two minute period for calibration of monitoring devices, and a second 10 min quiet, resting baseline. Subjects then, in fixed order, performed the 5-min mental arithmetic task, a 5-min recovery period, the 5-min Stroop color-word task, and another 5-min recovery period. Subjects were instructed to remain silent throughout the procedures. After the second recovery period, the tilt table was moved to the 70° head-up position and the monitoring devices were recalibrated. Physiological signals were collected for 10 min in the upright position.
Psychological Stressors
Public speaking task
Subjects selected one of five controversial topics, e.g., abortion, welfare. They were informed that their performance would be video-recorded for evaluation and a camera was placed prominently in their view. They then spent five min preparing for the speech followed by a 5-min period when they delivered it.
Mental arithmetic
The task required subjects to subtract serially by 7’s starting with a 4 digit number presented on the monitor. At one min intervals, subjects received verbal prompts from the laboratory technician, e.g., “please subtract faster.” Subjects were instructed to subtract as quickly and accurately as possible. If they made mistakes or lost their place, a new 4-digit starting number was provided.
Stroop color-word task
The computer presented color name words (blue, green, yellow, red) in a color which was either congruent or incongruent with the name. The task was to press a key on the keypad corresponding to the color of the letters, not the color name. The task was paced by the computer so that subjects achieved a 67% correct response rate. Thus, if they performed poorly, the presentation rate slowed and if they performed well, it increased.
Recovery periods
A five-minute recovery period followed each challenge.
Measurement of Physiological Signals
ECG and Impedance Cardiography Analog ECG signals were digitized at 500 Hz by a National Instruments 16 bit A/D conversion board and passed to a microcomputer. ICG data were collected using the Minnesota 304B system with no gain. The impedance signal (Z0) and the first derivative of pulsatile impedance acquisition (dZ/dt) were digitized at 250 Hz and 500 Hz respectively by the NI A/D board and collected by the microcomputer. Mindware software (MindWare Technologies LTD, Gahanna, Ohio) was used to analyze ECG and ICG signals in 60-second epochs. PEP was measured as the time interval between the Q wave of the ECG and the B point of the dZ/dt wave. Errors in marking of R waves in the ECG signal and B, Z and X points in the dZ/dt waveform were corrected by visual inspection. Epochs in which more than 20% of the ECG or ICG signals were unreadable due to artifact were not scored. Only baselines (10 min) with 6 60-second epochs of adequate data and other periods (5 min) with 3 60-second epochs of adequate data were submitted to analysis.
Low frequency blood pressure variability
The beat to beat BP waveform was captured using a Finapres noninvasive BP monitor. The analog BP waveform was captured by the NI A/D board and was sampled at 500 samples/sec. Systolic and diastolic values for each cardiac cycle were identified using custom-written software resulting in a BP-time series. Mean BP and spectral power in the low (0.04–0.15 Hz) frequency band of the blood pressure power spectrum for both SBP and DBP were computed from these time series. Because the servo adjustment of the Finapres monitor was enabled during the last minute of each 300 sec period, spectra were calculated on 240 second epochs using an interval method for computing Fourier transforms similar to that described by DeBoer, Karamaker, and Strackee (deBoer, Karemaker, & Strackee, 1984). Prior to computing Fourier transforms, the mean of the BP series was subtracted from each value in the series and the series then was filtered using a Hanning window (Harris, 1978) and the power, i.e., variance (in mmHg2), over the LF band was summed. Estimates of spectral power were adjusted to account for attenuation produced by this filter (Harris, 1978).
Computation of Reactivity and Recovery
For each variable, reactivity to each task was computed as the difference between the mean value during the task and the mean of the preceding baseline. Recovery was computed as the difference between challenge and recovery period. For each baseline, the two 300 sec epochs were averaged to yield a single value. To increase response stability, data from the arithmetic and Stroop tasks were averaged, as were the recovery periods that followed them (Kamarck et al., 1992). The speech task was treated separately because it was delivered in the seated, not the supine, position. To allow for complete equilibration to the upright position, data from the first 5 min epoch after tilt were excluded from analysis. No recovery data were collected after tilt.
Statistical Analysis
To examine relationships in the prediction of systolic LF BPV, diastolic LF BPV, and PEP, the data were analyzed by performing separate three-way analyses of variance. Each model examined the prediction by group (aerobic versus strength), session (baseline, post training, and after deconditioning), and period within session (baseline, public speaking, arithmetic, and Stroop tasks, tilt, and recovery), while controlling for gender and age. To model the correlation among repeated measures, an unstructured covariance matrix was used. This matrix was selected according to the Akaike criterion (Akaike, 1974). Comparisons regarding recovery from challenge were also treated separately for speech task and for combined math/Stroop tasks.
For all main effects, an alpha level of less than .05 was considered to be statistically significant. All analyses were conducted using mixed modeling software (SAS 9.2 Proc Mixed) to generate restricted maximum likelihood estimates, using all available data.
Results
Descriptive Data
A total of 149 healthy men (n = 58) and women (n = 91) were randomized and tested before training. Technically acceptable data for analysis of PEP and LF-BPV were available on 144 of these participants. The two groups were well balanced (see Table 1). A total of 101 participants completed training, yielding a dropout rate of 32%, and 87 participants completed deconditioning. The dropout rates were the same for both groups. Dropouts were significantly younger (28.42 years versus 32.17 years, p = .003) and there were significantly more women dropouts (42 versus 17, p = .037). However, they do not differ in other demographic and physical characteristics.
Table 1.
| Group=Aerobic | Group=Strength | ||||||
|---|---|---|---|---|---|---|---|
| Variable | N | Mean | SE | N | Mean | SE | p-value |
| Age (years) | 71 | 30.28 | 0.83 | 73 | 31.29 | 0.92 | .42 |
| VO2max (mL/kg/min) | 71 | 34.07 | 0.73 | 73 | 33.10 | 0.65 | .32 |
| SBP (mmHg) | 71 | 109.82 | 1.26 | 72 | 110.25 | 1.40 | .82 |
| DBP (mmHg) | 71 | 71.11 | 0.93 | 72 | 71.22 | 1.01 | .94 |
| HR (bpm) | 71 | 70.97 | 1.07 | 73 | 71.84 | 1.07 | .57 |
| BMI (kg/m2) | 70 | 24.59 | 0.45 | 73 | 24.98 | 0.49 | .55 |
Effect of Aerobic Conditioning
Analysis revealed significant effects of group assignment (F1,503=3.83; P<.001), testing session (F2,503=68.95; P<.001), gender (F1,503=46.16; P<.001) and age (F1,503= 8.84; P<.001) on aerobic capacity. Women had lower VO2max than did men, which was consistent with the gender differences in the Heart Association fitness standards used as inclusion criteria. Body mass index and age were inversely related to VO2max. Most importantly, the group X session interaction was highly significant (F2,503=26.80; P<.001). Aerobic capacity increased after training and decreased after deconditioning only in the aerobic-conditioning group.
Impact of Training on PEP Reactivity to and Recovery from Challenge
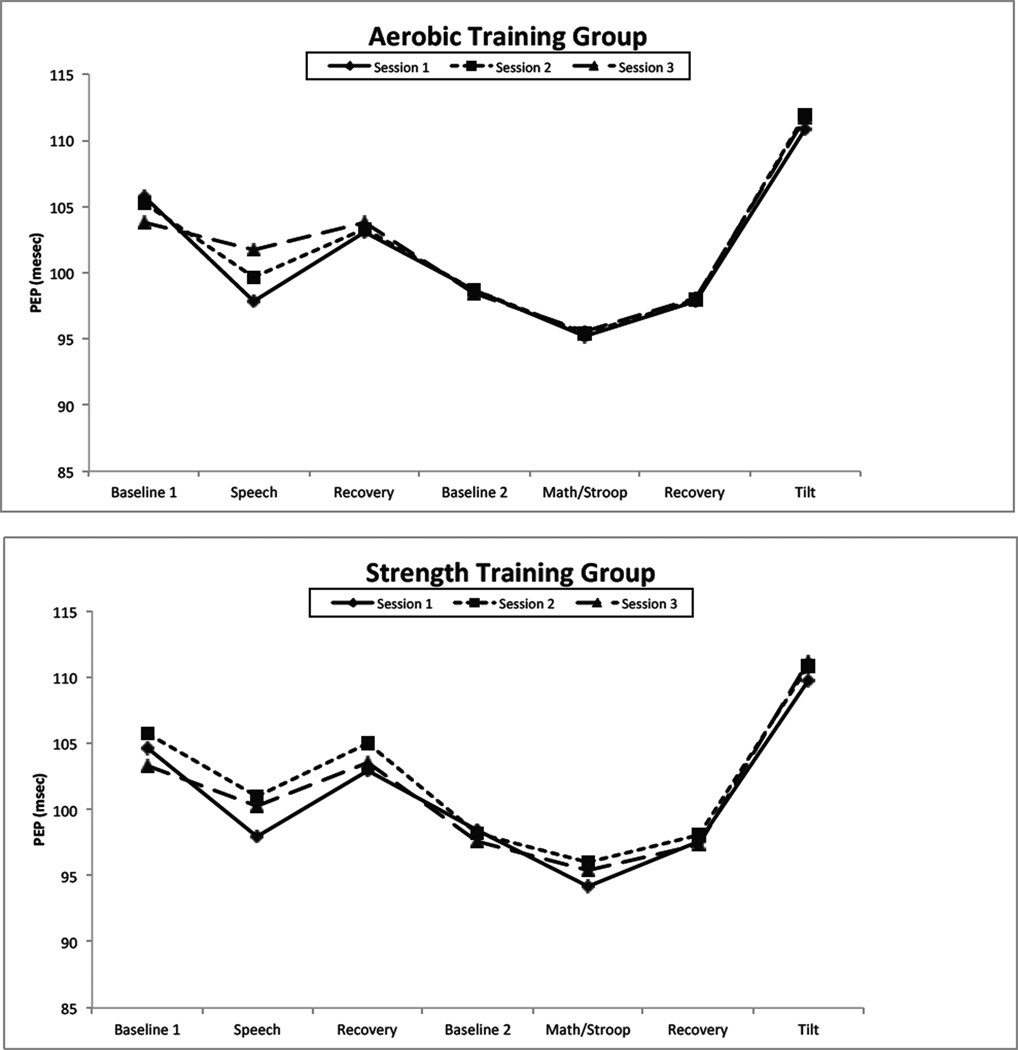
Figure 1 presents PEP values for the aerobic (top panel) and strength training (bottom panel) throughout the psychophysiology protocol. Table 2 presents the results of the statistical analysis. As the Table indicates and as Figure 1 makes clear, there was a significant effect of period on PEP, as expected. During the speech task, PEP shortened compared to the seated baseline and recovery period. Similarly, PEP shortened during the aggregated math/Stroop task relative to the supine baseline and recovery periods. Finally, Figure 1 illustrates the effect on PEP of moving from the supine to the 70° head up position. The absence of a significant group X session X period interaction indicates that aerobic training did not differ from strength training in the effect on PEP reactivity to or recovery from psychological or positional challenge.
Figure 1.
Table 2.
Type III F-Tests for Between-Time Models
| PEP | LF-SBPV | LF-DBPV | |
|---|---|---|---|
| Session | 1.21 | 0.42 | 3.47* |
| Group | .03 | 0.15 | 0.17 |
| Period | 126.98*** | 210.61*** | 180.77*** |
| Group*Session | .36 | 1.04 | 0.52 |
| Session*Period | 4.65*** | 1.30 | 1.59 |
| Group*Period | 0.66 | 2.80* | 2.68* |
| Group*Session*Period | 0.91 | 1.26 | 1.38 |
All models adjusted for age and gender.
p<.10
p<.05
p<.01
p<.0001
PEP = pre-ejection period; LF= low frequency; SBPV=Systolic Blood Pressure Variability; DBPV=Diastolic Blood Pressure Variability
Impact of Training on LF BPV Reactivity to and Recovery from Challenge
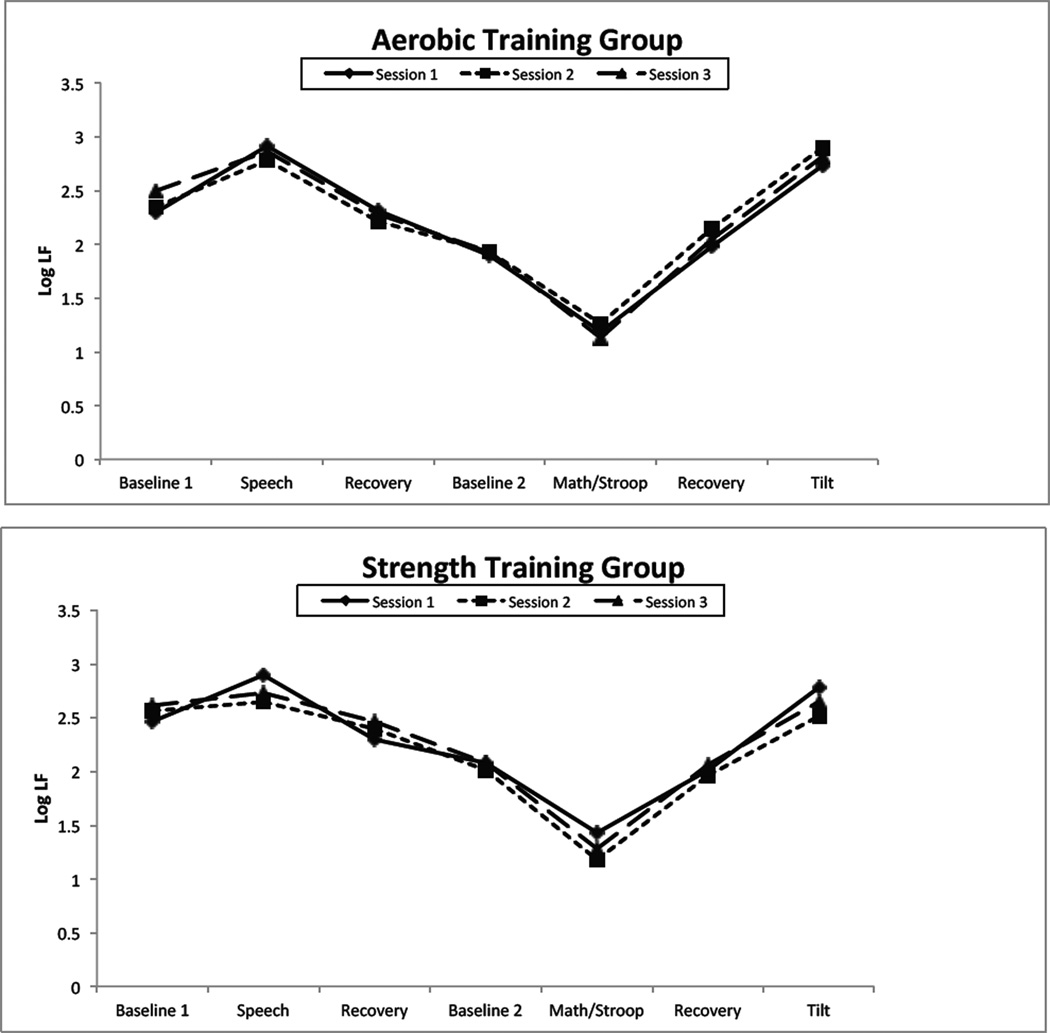
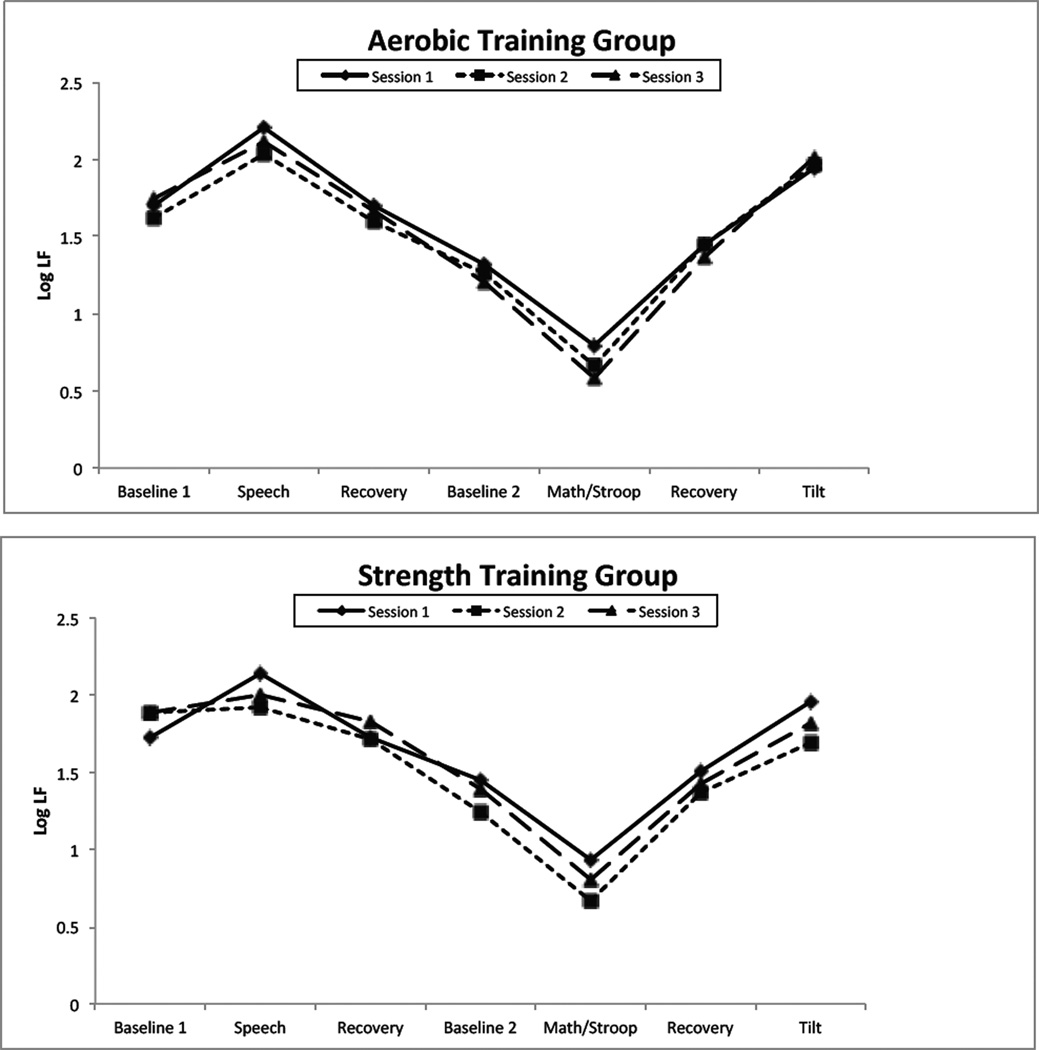
Figures 2 and 3 present low frequency systolic and diastolic pressure variability respectively. For both BPV indices, there was a highly significant effect of period, as there was for PEP. However, the group X session X period interaction failed to achieve significance, indicated that as for PEP, the two training conditions did not differ in their effect on BPV reactivity to and recovery from challenge.
Figure 2.
Figure 3.
Interestingly, however, as Figures 2 and 3 show, the effect of the speech task was different than that of the aggregated math and Stroop task. Post-hoc contrasts confirm that during the speech task, LF SBPV and DBPV increased compared to baseline (all ps < .01) except for non-significant increases in the strength-training group at times 2 and 3. In contrast, BPV fell in response to the aggregated math/Stroop task (all ps < .001) at in every session in each group.
To assess the relationship between PEP and LF-SBPV or LF-DBPV as putative SNS indices, we computed Pearson correlation coefficients for these variables for each period (14) and each session (3) throughout the study. Of the 84 such Pearsons, only 3 reached statistical significance.
Discussion
Abundant evidence demonstrates that psychological stress increases vulnerability to cardiovascular events. Examples from the real world abound. For instance, on June 30, 1998, the day that England lost a World Cup football match to Argentina in a shoot-out, admission to English hospitals for acute myocardial infarction increased by 25%, compared to control days (Carroll, Ebrahim, Tilling, Macleod, & Smith, 2002). The risk of cardiac emergencies in Munich more than doubled on days when the German Football team was playing in the 2006 World Cup (Wilbert-Lampen et al., 2008). On the day of the 1994 Northridge earthquake in California, the number of sudden cardiovascular deaths increased 6-fold compared to a control period (Leor, Poole, & Kloner, 1996). In a patient undergoing 24-hour ambulatory blood pressure monitoring, systolic and diastolic pressure rose to 150 mm Hg and 122mm Hg respectively and heart rate rose to 150 bpm at the time of the strongest tremor from an earthquake that struck central Italy in March 1998. On the first day of the 1991 Iraqi missile attacks, total mortality in Israel increased by 58% largely due to cardiovascular events (Kark, Goldman, & Epstein, 1995).
Psychological stress in the laboratory, while less extreme, nonetheless produces physiological effects that may be in the causal pathway to CVD events. Laboratory challenges increase heart rate and blood pressure (Al'Absi et al., 1997) as well as sympathetic nervous system activity measured as norepinephrine measured in serum (Grillot et al., 1995; R.P. Sloan et al., 1996) or spillover rate, indicative of whole body sympathetic activity (Esler et al., 2004) and muscle sympathetic nerve activity (MSNA) (Anderson, Sinkey, & Mark, 1991; Hjemdahl et al., 1989). Mental challenges also lead to withdrawal of cardiac parasympathetic modulation (Gianaros, Van Der Veen, & Jennings, 2004; R. P. Sloan, Shapiro, & Gorman, 1990; R.P. Sloan, Korten, & Myers, 1991; Richard P. Sloan et al., 1997).
Because aerobic exercise is known to promote cardioprotection, we sought to examine whether it might do so by reducing sympathetically mediated responses to and recovery from laboratory challenge. In a randomized controlled trial of aerobic exercise vs. strength training in young, healthy, sedentary adults, we found no evidence that improvements in aerobic conditioning had an effect on myocardial sympathetic nervous system responses to or recovery from psychological or orthostatic challenge. Pre-ejection period, an index of sympathetically driven myocardial contractility, shortened as expected in response to the speech, math, and Stroop tasks, consistent with evidence that these tasks elicit sympathetic activation. However, there was no effect of treatment assignment, indicating that improvements in aerobic capacity did not attenuate the sympathetic response to and recovery from psychological challenge.
Seemingly paradoxically, PEP increased in response to tilt, suggesting a reduction in myocardial sympathetic activation. Positional change is widely recognized to elicit a powerful sympathetic response to compensate for the pooling of blood in the lower limbs (Cooke et al., 1999; Furlan et al., 2000). However, PEP is an unreliable index of myocardial sympathetic nervous system activity in response to postural shifts. Because moving from the supine to the upright position displaces blood to the lower limbs, it lowers cardiac preload. Through the Frank-Starling mechanism, myocardial contractile force is reduced, leading to a lengthening of PEP (Houtveen, Groot, & De Geus, 2005).
Low frequency blood pressure variability, a putative index of vascular sympathetic control, rose as expected in response to positional change. As indicated above, data suggest that laboratory-based psychological challenges also elicit sympathetic activation. While LF-BPV rose in response to and then recovered from speech, it unexpectedly fell in response to math and Stroop and then recovered. Improvements in aerobic capacity had no impact on this pattern of response and recovery.
Relatively few studies have examined the BPV response to psychological challenge and in those few, the findings are inconsistent. Mulder et al. reported that mental stress led to a reduction in BPV in the 0.01–0.06 Hz frequency band but did not alter power in the 0.07–0.14 Hz band (Mulder, Veldman, Ruddel, Robbe, & Muler, 1991). Tulen et al. (Tulen, 1999) also found that 0.07–0.14 Hz systolic pressure variability as well as total systolic pressure variability fell during a 10-min Stroop task administered in both the supine and seated positions, despite the fact that the task produced increases in both SBP (~11%) and DBP (~12%) (Tulen, 1999) but no change in plasma epinephrine and norepinephrine. Fauvel et al. (Fauvel et al., 2000) reported no change in the standard deviation of systolic pressure or in 0.07–0.14 Hz variability in response to a 5-min Stroop task administered to healthy men studied in the seated position and a systolic pressure increase of 22.4 mmHg (~14%). In contrast, Grillot et al. found that in subjects in supine position, a 10-min Stroop led to an increase in 0.07–0.14 Hz variability and in systolic pressure (31 mmHg, 25%) accompanied by significant increases in plasma epinephrine and norepinephrine. Moreover, the increases in blood pressure variability and epinephrine were significantly correlated (r = 0.69) (Grillot et al., 1995).
Competing views of the origins of LF-BPV exist. Some have suggested that it is the product of the feed-forward influence of HRV (Toska & Eriksen, 1993; Veerman, Imholz, Weiling, Karemaker, & van Montfrans, 1994). Others assert that it reflects a central sympathetic oscillator (Cevese et al., 1995; Montano et al., 1992). Still others argue that it merely is a resonance phenomenon, a product of the delay in the β-adrenergic vasoconstrictor response mediated by the baroreceptors (Bertram, Barres, Cuisinaud, & Julien, 1998; Cerutti, Barres, & Paultre, 1994; van de Borne et al., 2001) Hammer, 2005 #8134).
Our findings suggest that none of these accounts is complete and that LF BPV during psychological challenge reflects the interplay of two factors. Parasympathetically-mediated oscillations in HR and the associated changes in cardiac output, under certain conditions, can contribute to oscillations in BP (Toska & Eriksen, 1993; Veerman et al., 1994). Indeed, in the supine position, with relatively little SNS activity, most BPV is a product of the feed-forward influence of HRV (Saul et al., 1991).
During mental stress, HRV falls due to withdrawal of cardiac parasympathetic modulation. As HRV falls, so does the feed-forward contribution to oscillations in BP, thus reducing BPV. However, mental stress also causes LF-BPV-enhancing vascular sympathetic activation commensurate with the degree of stressfulness of the challenge. In our study, the math and Stroop tasks led to relatively small increases in SBP (10 mmHg, 8%), similar to the data of Tulen, who also reported a reduction in BPV. This modest SNS activation is insufficient to offset the feed-forward based reduction in BPV produced by the reduction in HRV.
In contrast, SBP increased substantially more (28 mmHg, 24%) in response to the speech task, suggesting that this task elicited considerably greater SNS activation and by this account, was sufficient to offset the loss of feed-forward influence of HRV on BPV. Thus, in response to this more evocative stress, LF-BPV rose. Grillot et al. similarly reported that LF-BPV rose during a Stroop task that was stressful enough to produce a 31 mmHg SBP increase (Grillot et al., 1995). This interpretation raises questions about the validity of LF-BPV as an unambiguous index of vascular sympathetic modulation, suggesting instead that it reflects both parasympathetic regulation of HR and sympathetic vascular regulation.
Previously, we reported that the PNS responses of subjects in this protocol (R. P. Sloan et al., 2011) and demonstrated that there were no training group differences in PNS reactivity to or recovery from psychological or orthostatic challenge. Here we report no training group effect on SNS activation during psychological challenge. Collectively, these findings support for the conclusion that the mechanism of exercise-induced cardioprotection is not attenuation of the autonomic response to or recovery from challenge, at least those that can be delivered in a laboratory. This conclusion is consistent with other evidence, too. Ray and Carter recently reported the results of a small study demonstrating that although aerobic training resulted in an 18% improvement in aerobic capacity, it had no effect on MSNA responses to mental arithmetic (Ray & Carter, 2010). In samples of similar aged and poorly conditioned participants, de Geus et al. found no effect of training on PEP (E. J. de Geus, van Doornen, & Orlebeke, 1993; E. J. C. De Geus, van Doornen, de Visser, & Orlebeke, 1990). In a small training study, the increased plasma epinephrine response to mental challenge was not altered by improvements in aerobic capacity. Plasma norepinephrine (NE) did not increase in response to mental challenge but as expected, moving to the upright position lead to an increase in NE but this effect was not changed by training (Cleroux et al., 1985). In a small sample of young Black men, aerobic training did not change BPV in the 0.04–0.15 Hz frequency band in response to the Stroop task (Bond et al., 2008).
Some studies have presented findings consistent with the hypothesis that improvements in aerobic fitness attenuate responses to psychological challenge. For example, Hamer and Steptoe reported that greater fitness was associated with smaller parasympathetically mediated reductions in HRV during mental stress. However, these data were based on cross-sectional analyses rather than from a randomized controlled trial (Hamer & Steptoe, 2007). In a six-week randomized trial of aerobic training vs. strength training or a no treatment control condition, the heart rate and rate pressure product response to and recovery from mental stress were reduced in the aerobic group but not in other two groups (Spalding, Lyon, Steel, & Hatfield, 2004). Other indices of autonomic responses to challenge were not collected in this study.
Limitations
Participants had limited oversight during the study. They received an initial training session with a research assistant but then exercised on their own. During training sessions, they measured their heart rate by palpation to determine if they were exercising at the recommended intensity. These limitations notwithstanding, the improvements in aerobic capacity in the aerobic but not the strength training group suggest that participants exercised as instructed.
It also is possible that PEP and LF-BPV are not valid indices of myocardial and vascular sympathetic drive, respectively. Supporting evidence differs for the two. In the case of PEP, pharmacological and clinical studies demonstrate that it provides an index of sympathetic modulation of myocardial contractility. Beta-adrenergic blockade lengthens PEP, as expected (Cacioppo et al., 1994; Winzer et al., 1999). In clinical conditions known to be associated with elevations in sympathetic activity such as diabetes (Berntson, Norman, Hawkley, & Cacioppo, 2008; Licht et al., 2010) and metabolic syndrome (Licht et al., 2010), PEP is reduced, as expected. Thus, it is unlikely that the failure to find an effect of aerobic training on myocardial sympathetic modulation is due to shortcomings of PEP as a valid index.
Evidence that LF-BPV is an index of vascular sympathetic activity is more limited. In rats, phentolamine decreased mid-frequency blood pressure variability, consistent with the hypothesis that it reflects β-adrenergic-mediated vasomotor function (Yoshimoto et al., 2011). Other blockade studies also support this view (Julien, Malpas, & Stauss, 2001; H. M. Stauss & Kregel, 1996; H. M. Stauss, Persson, Johnson, & Kregel, 1997; Zhang et al., 2002). Moreover, low frequency blood pressure oscillations are consistent with the time delays that characterize sympathetic modulation of vascular tone (Harald M Stauss, 2007). However, other studies suggest that LF-BPV is merely is a resonance phenomenon, a product of the delay in the β-adrenergic vasoconstrictor response mediated by the baroreceptors (Bertram et al., 1998; Cerutti et al., 1994; deBoer et al., 1987; Hammer & Saul, 2005; van de Borne et al., 2001). Evidence also suggests that under certain conditions, LF-BPV is the product of the feed-forward effects of RR interval variability. Thus, the status of LF-BPV as an index of cardiovascular sympathetic modulation is less well established than PEP.
Finally, because the psychological challenges were presented three times over the course of the study, it is possible that participants habituated to them, thus obscuring group differences in response to training. Examination of the figures reveals that the PEP response to the speech task was smaller during the second and third testing sessions. However, because the PEP responses to the math/Stroop task and the LF-BPV responses to all challenges were not appreciably different across the testing sessions, it is unlikely that habituation accounts for the failure to demonstrate training group differences in these indices.
To conclude, these findings, from a large randomized controlled trial using an intent-to-treat design, show that moderate aerobic exercise training has no effect on PEP and LF BPV reactivity to or recovery from psychological or orthostatic challenge. While this study raises questions about whether LF-BPV is an unambiguous index of vascular sympathetic activity, the PEP findings suggest that at least in healthy young adults, the cardioprotective effects of exercise training are unlikely to be mediated by attenuation of sympathetic nervous system responses to stress.
Acknowledgements
This study was supported in part by Independent Scientist Award K02 MH01491 from the National Institute of Mental Health, R01 HL61287 from the National Heart Lung and Blood Institute, M01-RR00645 from the General Clinical Research Centers Program of the National Institutes of Health, and the Nathaniel Wharton Fund.
References
- Akaike H. A new look at the statistical model identification. IEEE Trans Automatic Control, AC19. 1974:716–723. [Google Scholar]
- Al'Absi Mustafa, Bongard Stephan, Buchanan Tony, Pincomb Gwendolyn A, Licino Julio, Lovallo William R. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 1997;34:266–275. doi: 10.1111/j.1469-8986.1997.tb02397.x. [DOI] [PubMed] [Google Scholar]
- Anderson EA, Sinkey CA, Mark AL. Mental stress increases sympathetic nerve activity during sustained baroreceptor stimulation in humans. Hypertension. 1991;17(Suppl. II):III-43–III-49. doi: 10.1161/01.hyp.17.4_suppl.iii43. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Norman GJ, Hawkley LC, Cacioppo JT. Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology. 2008;45(4):643–652. doi: 10.1111/j.1469-8986.2008.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram Delphine, Barres Christian, Cuisinaud Guy, Julien Claude. The arterial baroreceptor reflex of the rat exhibits positive feedback properties at the frequency of Mayer waves. J Physiol (Lond) 1998;513(1):251–261. doi: 10.1111/j.1469-7793.1998.251by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond V, Bartels MN, Sloan RP, Millis RM, Zion AS, Andrews N, De Meersman RE. Exercise training favourably affects autonomic and blood pressure responses during mental and physical stressors in African-American men. J Hum Hypertens. 2008;23(4):267–273. doi: 10.1038/jhh.2008.125. 10.1038/jhh.2008.125. [DOI] [PubMed] [Google Scholar]
- Buchfuhrer MJ, Hansen JE, Robinson TE, Sue DY, Wasserman K, Whipp B. Optimizing the exercise protocol for cardiopulmonary assessment. Journal of Applied Physiology. 1983;55(5):1558–1564. doi: 10.1152/jappl.1983.55.5.1558. [DOI] [PubMed] [Google Scholar]
- Cacioppo John T, Berntson Gary G, Binkley Philip F, Quigley Karen S, Uchino Bert N, Fieldstone Annette. Autonomic cardiac control. II. Noninvasive indices and basal response as revealed by autonomic blockades. Psychophysiology. 1994;31(6):586–598. doi: 10.1111/j.1469-8986.1994.tb02351.x. [DOI] [PubMed] [Google Scholar]
- Carroll Douglas, Ebrahim Shah, Tilling Kate, Macleod John, Smith George Davey. Admissions for myocardial infarction and World Cup football: database survey. BMJ. 2002;325(7378):1439–1442. doi: 10.1136/bmj.325.7378.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll Douglas, Phillips Anna C, Der Geoff, Hunt Kate, Benzeval Michaela. Blood Pressure Reactions to Acute Mental Stress and Future Blood Pressure Status: Data From the 12-Year Follow-Up of the West of Scotland Study. Psychosomatic Medicine. 2011;73(9):737–742. doi: 10.1097/PSY.0b013e3182359808. [DOI] [PubMed] [Google Scholar]
- Cerutti C, Barres C, Paultre C. Baroreflex modulation of blood pressure and heart rate variabilities in rats: Assessment by spectral analysis. American Journal of Physiology. 1994;266:H1993–H2000. doi: 10.1152/ajpheart.1994.266.5.H1993. [DOI] [PubMed] [Google Scholar]
- Cevese A, Grasso R, Poltronieri R, Schena F. Vascular resistance and arterial pressure low-frequency oscillations in the anesthetized dog. American Journal of Physiology. 1995;37:H7–H16. doi: 10.1152/ajpheart.1995.268.1.H7. [DOI] [PubMed] [Google Scholar]
- Chida Yoichi, Steptoe Andrew. Greater Cardiovascular Responses to Laboratory Mental Stress Are Associated With Poor Subsequent Cardiovascular Risk Status: A Meta-Analysis of Prospective Evidence. Hypertension. 2010;55(4):1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Cleroux Jean, Peronnet Francois, de Champlain Jacques. Sympathetic indices during psychological and physical stimuli before and after training. Physiology & Behavior. 1985;35(2):271–275. doi: 10.1016/0031-9384(85)90349-x. [DOI] [PubMed] [Google Scholar]
- Cooke William H, Hoag Jeffrey B, Crossman Alexandria A, Kuusela Tom A, Tahvanainen Kari UO, Eckberg Dwain L. Human responses to upright tilt: A window on central autonomic integration. Journal of Physiology. 1999;517:617–628. doi: 10.1111/j.1469-7793.1999.0617t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Geus EJ, van Doornen LJ, Orlebeke JF. Regular exercise and aerobic fitness in relation to psychological make-up and physiological stress reactivity. Psychosom Med. 1993;55(4):347–363. doi: 10.1097/00006842-199307000-00003. [DOI] [PubMed] [Google Scholar]
- De Geus Eco JC, van Doornen Lorenz JP, de Visser Dianne C, Orlebeke Jacob F. Existing and Training Induced Differences in Aerobic Fitness: Their Relationship to Physiological Response Patterns During Different Types of Stress. Psychophysiology. 1990;27(4):457–477. doi: 10.1111/j.1469-8986.1990.tb02343.x. [DOI] [PubMed] [Google Scholar]
- deBoer RW, Karemaker JM, Strackee J. Comparing spectra of a series of point events, particularly for heart rate variability spectra. IEEE Transactions in Biomedical Engineering, BME-31. 1984:384–387. doi: 10.1109/TBME.1984.325351. [DOI] [PubMed] [Google Scholar]
- deBoer RW, Karemaker JM, Strackee J. Hemodynamic fluctuations and baroreflex sensitivity in humans: A beat-to-beat model. American Journal of Physiology. 1987;253:680–689. doi: 10.1152/ajpheart.1987.253.3.H680. [DOI] [PubMed] [Google Scholar]
- Dimier-David L, Billon N, Costagliola D, Jaillon P, Funck-Brentano C. Reproducibility of non-invasive measurement and of short-term variability of blood pressure and heart rate in healthy volunteers. Br J Clin Pharmacol. 1994;38(2):109–115. doi: 10.1111/j.1365-2125.1994.tb04333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditor DS, Kamath MV, Macdonald MJ, Bugaresti J, McCartney N, Hicks AL. Reproducibility of heart rate variability and blood pressure variability in individuals with spinal cord injury. Clin Auton Res. 2005;15(6):387–393. doi: 10.1007/s10286-005-0293-4. [DOI] [PubMed] [Google Scholar]
- Esler M, Lux A, Jennings G, Hastings J, Socratous F, Lambert G. Rilmenidine sympatholytic activity preserves mental stress, orthostatic sympathetic responses and adrenaline secretion. J Hypertens. 2004;22(8):1529–1534. doi: 10.1097/01.hjh.0000125453.28861.b8. [DOI] [PubMed] [Google Scholar]
- Fauvel Pierre Jean, Cerutti Catherine, Quelin Pierre, Laville Maurice, Gustin Paula Marie, Paultre Zacharie Christian, Ducher Michel. Mental stress-induced increase in blood pressure is not related to baroreflex sensitivity in middle-aged healthy men. Hypertension. 2000;35:887–891. doi: 10.1161/01.hyp.35.4.887. [DOI] [PubMed] [Google Scholar]
- Furlan Raffaello, Porta Alberto, Costa Fernando, Tank Jens, Baker Lemont, Schiavi Richard, Garcia-Mosqueda Rogelio. Oscillatory patterns in sympathetic neural discharge and cardiovascular variables during orthostatic stimulus. Circulation. 2000;101:886–892. doi: 10.1161/01.cir.101.8.886. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Onyewuenyi Ikechukwu C, Sheu Lei K, Christie Israel C, Critchley Hugo D. Brain systems for baroreflex suppression during stress in humans. Human Brain Mapping. 2011 doi: 10.1002/hbm.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Van Der Veen FM, Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology. 2004;41(4):521–530. doi: 10.1111/1469-8986.2004.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillot M, Fauvel JP, Cottet-Emard JM, Laville M, Peyrin L, Pozet N, Zech P. Spectral analysis of stress-induced change in blood pressure and heart rate in normotensive subjects. Journal of Cardiovascular Pharmacology. 1995;25:448–452. doi: 10.1097/00005344-199503000-00015. [DOI] [PubMed] [Google Scholar]
- Hamer Mark, Steptoe Andrew. Association Between Physical Fitness, Parasympathetic Control, and Proinflammatory Responses to Mental Stress. Psychosom Med. 2007;69(7):660–666. doi: 10.1097/PSY.0b013e318148c4c0. [DOI] [PubMed] [Google Scholar]
- Hammer PE, Saul JP. Resonance in a mathematical model of baroreflex control: arterial blood pressure waves accompanying postural stress. Am J Physiol Regul Integr Comp Physiol. 2005;288(6):R1637–R1648. doi: 10.1152/ajpregu.00050.2004. [DOI] [PubMed] [Google Scholar]
- Harris FJ. On the use of windows for harmonic analysis with the discrete Fourier transform. Proceedings of the IEEE. 1978;66(1):51–83. [Google Scholar]
- Haskell William L, Lee I-Min, Pate Russell R, Powell Kenneth E, Blair Steven N, Franklin Barry A, Bauman Adrian. Physical Activity and Public Health: Updated Recommendation for Adults From the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- Heponiemi T, Elovainio M, Pulkki L, Puttonen S, Raitakari O, Keltikangas-Jarvinen L. Cardiac autonomic reactivity and recovery in predicting carotid atherosclerosis: the cardiovascular risk in young Finns study. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2007;26(1):13–21. doi: 10.1037/0278-6133.26.1.13. [DOI] [PubMed] [Google Scholar]
- Hjemdahl P, Fagius J, Freyschuss U, Wallin BG, Daleskog M, Bohlin G, Perski A. Muscle sympathetic activity and norepinephrine release during mental challenge in humans. Am J Physiol. 1989;257(5 Pt 1):E654–E664. doi: 10.1152/ajpendo.1989.257.5.E654. [DOI] [PubMed] [Google Scholar]
- Houtveen Jan H, Groot Paul FC, De Geus Eco JC. Effects of variation in posture and respiration on RSA and pre-ejection period. Psychophysiology. 2005;42(6):713–719. doi: 10.1111/j.1469-8986.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- Jennings JRichard, Kamarck Thomas W, Everson-Rose Susan A, Kaplan George A, Manuck Stephen B, Salonen JT. Exaggerated Blood Pressure Responses During Mental Stress Are Prospectively Related to Enhanced Carotid Atherosclerosis in Middle-Aged Finnish Men. Circulation. 2004;110(15):2198–2203. doi: 10.1161/01.CIR.0000143840.77061.E9. [DOI] [PubMed] [Google Scholar]
- Julien C, Malpas SC, Stauss HM. Sympathetic modulation of blood pressure variability. J Hypertens. 2001;19(10):1707–1712. doi: 10.1097/00004872-200110000-00002. [DOI] [PubMed] [Google Scholar]
- Kamarck TW, Jennings JR, Debski TT, Glickman-Weiss E, Johnson PS, Eddy JJ, Manuck SB. Reliable measures of behaviorally-evoked cardiovascular reactivity from a PC-based test battery: Results from student and community samples. Psychophysiology. 1992;29:17–28. doi: 10.1111/j.1469-8986.1992.tb02006.x. [DOI] [PubMed] [Google Scholar]
- Kapuku GK, Treiber FA, Davis HC, Harshfield GA, Cook BB, Mensah GA. Hemodynamic function at rest, during acute stress, and in the field: predictors of cardiac structure and function 2 years later in youth. Hypertension. 1999;34:1026–1031. doi: 10.1161/01.hyp.34.5.1026. [DOI] [PubMed] [Google Scholar]
- Kark JD, Goldman S, Epstein L. Iraqi missile attacks on Israel: The association of mortality with a life-threatening stressor. JAMA. 1995;273:1208–1210. doi: 10.1001/jama.273.15.1208. [DOI] [PubMed] [Google Scholar]
- Kelsey RM, Alpert BS, Patterson SM, Barnard M. Racial differences in hemodynamic responses to environmental thermal stress among adolescents. Circulation. 2000;101(19):2284–2289. doi: 10.1161/01.cir.101.19.2284. [DOI] [PubMed] [Google Scholar]
- Laterza Mateus C, de Matos Luciana DNJ, Trombetta Ivani C, Braga Ana MW, Roveda Fabiana, Alves Maria JNN, Rondon Maria UPB. Exercise Training Restores Baroreflex Sensitivity in Never-Treated Hypertensive Patients. Hypertension. 2007;49(6):1298–1306. doi: 10.1161/HYPERTENSIONAHA.106.085548. [DOI] [PubMed] [Google Scholar]
- Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. New England Journal of Medicine. 1996;334:413–419. doi: 10.1056/NEJM199602153340701. [DOI] [PubMed] [Google Scholar]
- Licht Carmilla MM, Vreeburg Sophie A, van Reedt Dortland Arianne KB, Giltay Erik J, Hoogendijk Witte JG, DeRijk Roel H, Penninx Brenda WJH. Increased Sympathetic and Decreased Parasympathetic Activity Rather Than Changes in Hypothalamic-Pituitary-Adrenal Axis Activity Is Associated with Metabolic Abnormalities. Journal of Clinical Endocrinology & Metabolism. 2010;95(5):2458–2466. doi: 10.1210/jc.2009-2801. [DOI] [PubMed] [Google Scholar]
- Martinez Daniel G, Nicolau Jos√© C, Lage Rony L, Toschi-Dias Edgar, de Matos Luciana DNJ, Alves Maria Janieire NN, Rondon Maria UPB. Effects of Long-Term Exercise Training on Autonomic Control in Myocardial Infarction Patients. Hypertension. 2011;58(6):1049–1056. doi: 10.1161/HYPERTENSIONAHA.111.176644. [DOI] [PubMed] [Google Scholar]
- Mezzacappa ES, Kelsey RM, Katkin ES. The effects of epinephrine administration on impedance cardiographic measures of cardiovascular function. Int J Psychophysiol. 1999;31(3):189–196. doi: 10.1016/s0167-8760(98)00058-0. [DOI] [PubMed] [Google Scholar]
- Montano N, Lombardi F, Ruscone TG, Contini M, Finocchiaro ML, Baselli G, Malliani A. Spectral analysis of sympathetic discharge, R-R interval and systolic arterial pressure in decerebrate cats. Journal of the Autonomic Nervous System. 1992;40:21–32. doi: 10.1016/0165-1838(92)90222-3. [DOI] [PubMed] [Google Scholar]
- Mukai S, Hayano J. Heart rate and blood pressure variabilities during graded head-up tilt. Journal of Applied Physiology. 1995;78:212–216. doi: 10.1152/jappl.1995.78.1.212. [DOI] [PubMed] [Google Scholar]
- Mulder LJM, Veldman JbP, Ruddel H, Robbe HWJ, Muler G. On the usefulness of finger blood-pressure measurements for studies on mental workload. Homeostasis. 1991;33:47–60. [PubMed] [Google Scholar]
- Muller James E. Circadian variation and triggering of acute coronary events. American Heart Journal. 1999;137(4) Supplement 1:S1–S8. doi: 10.1016/s0002-8703(99)70390-x. [DOI] [PubMed] [Google Scholar]
- Nelson Miriam E, Rejeski WJack, Blair Steven N, Duncan Pamela W, Judge James O, King Abby C, Castaneda-Sceppa Carmen. Physical Activity and Public Health in Older Adults: Recommendation From the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1094–1105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- Nocon Marc, Hiemann Theresa, Müller-Riemenschneider Falk, Thalau Frank, Roll Stephanie, Willich Stefan N. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. European Journal of Cardiovascular Prevention & Rehabilitation. 2008;15(3):239–246. doi: 10.1097/HJR.0b013e3282f55e09. [DOI] [PubMed] [Google Scholar]
- Parati Gianfranco, Esler Murray. The human sympathetic nervous system: its relevance in hypertension and heart failure. European Heart Journal. 2012;33(9):1058–1066. doi: 10.1093/eurheartj/ehs041. [DOI] [PubMed] [Google Scholar]
- Pearson Thomas A, Bazzarre Terry L, Daniels Stephen R, Fair Joan M, Fortmann Stephen P, Franklin Barry A, Taubert Kathryn A. American Heart Association Guide for Improving Cardiovascular Health at the Community Level: A Statement for Public Health Practitioners, Healthcare Providers, and Health Policy Makers From the American Heart Association Expert Panel on Population and Prevention Science. Circulation. 2003;107(4):645–651. doi: 10.1161/01.cir.0000054482.38437.13. [DOI] [PubMed] [Google Scholar]
- Pearson Thomas A, Blair Steven N, Daniels Stephen R, Eckel Robert H, Fair Joan M, Fortmann Stephen P, Taubert Kathryn A. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. Circulation. 2002;106(3):388–391. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- Ray Chester A, Carter Jason R. Effects of aerobic exercise training on sympathetic and renal responses to mental stress in humans. Am J Physiol Heart Circ Physiol. 2010;298(1):H229–H234. doi: 10.1152/ajpheart.00880.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M, Baeriswyl E, Roets A. Personality effects on cardiovascular reactivity: need for closure moderates the impact of task difficulty on engagement-related myocardial beta-adrenergic activity. Psychophysiology. 2012;49(5):704–707. doi: 10.1111/j.1469-8986.2011.01350.x. [DOI] [PubMed] [Google Scholar]
- Saul JP, Berger RD, Albrecht P, Stein SP, Chen MH, Cohen RJ. Transfer function analysis of the circulation: Unique insights into cardiovascular regulation. American Journal of Physiology. 1991;30:H1231–H1245. doi: 10.1152/ajpheart.1991.261.4.H1231. [DOI] [PubMed] [Google Scholar]
- Schachinger H, Weinbacher M, Kiss A, Ritz R, Langewitz W. Cardiovascular indices of peripheral and central sympathetic activation. Psychosom Med. 2001;63(5):788–796. doi: 10.1097/00006842-200109000-00012. [DOI] [PubMed] [Google Scholar]
- Schächinger Hartmut, Weinbacher Markus, Kiss Alexander, Ritz Rudolf, Langewitz Wolf. Cardiovascular Indices of Peripheral and Central Sympathetic Activation. Psychosomatic Medicine. 2001;63(5):788–796. doi: 10.1097/00006842-200109000-00012. [DOI] [PubMed] [Google Scholar]
- Scheuer J, Tipton CM. Cardiovascular adaptations to physical training. Annu Rev Physiol. 1977;39:221–251. doi: 10.1146/annurev.ph.39.030177.001253. [DOI] [PubMed] [Google Scholar]
- Schwartz Peter J, Zipes Douglas P. Autonomic modulation of cardiac arrhythmias. In: Zipes DP, Jaffe JH, editors. Cardiac Electrophysiology: From Cell to Bedside. Philadelphia: W.B. Saunders; 2000. pp. 300–314. [Google Scholar]
- Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJ. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27(1):1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Sloan RP, Shapiro PA, DeMeersman RE, Bagiella E, Brondolo EN, McKinley PS, Myers MM. Impact of aerobic training on cardiovascular reactivity to and recovery from challenge. Psychosomatic Medicine. 2011;73(2):134–141. doi: 10.1097/PSY.0b013e31820a1174. 10.1097/PSY.0b013e31820a1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan RP, Shapiro PA, Gorman JM. Psychophysiological reactivity in cardiac transplant recipients. Psychophysiology. 1990;27:187–194. doi: 10.1111/j.1469-8986.1990.tb00369.x. [DOI] [PubMed] [Google Scholar]
- Sloan RP, Korten JB, Myers MM. Components of heart rate reactivity during mental arithmetic with and without speaking. Physiology and Behavior. 1991;50:1039–1045. doi: 10.1016/0031-9384(91)90434-p. [DOI] [PubMed] [Google Scholar]
- Sloan RP, Shapiro PA, Bagiella E, Bigger JT, Lo ES, Gorman JM. Relationship between circulating catecholamines and low frequency heart period variability as indices of cardiac sympathetic activity during mental stress. Psychosomatic Medicine. 1996;58:25–31. doi: 10.1097/00006842-199601000-00005. [DOI] [PubMed] [Google Scholar]
- Sloan Richard P, DeMeersman Ronald E, Shapiro Peter A, Bagiella Emilia, Kuhl John P, Paik Myunghee, Myers Michael M. Cardiac autonomic control is inversely related to blood pressure variability responses to psychological challenge. American Journal of Physiology. 1997;272:H2227–H2232. doi: 10.1152/ajpheart.1997.272.5.H2227. [DOI] [PubMed] [Google Scholar]
- Sofi Francesco, Capalbo Andrea, Cesari Francesca, Abbate Rosanna, Gensini Gian Franco. Physical activity during leisure time and primary prevention of coronary heart disease: an updated meta-analysis of cohort studies. European Journal of Cardiovascular Prevention & Rehabilitation. 2008;15(3):247–257. doi: 10.1097/HJR.0b013e3282f232ac. [DOI] [PubMed] [Google Scholar]
- Spalding TW, Lyon LA, Steel DH, Hatfield BD. Aerobic exercise training and cardiovascular reactivity to psychological stress in sedentary young normotensive men and women. Psychophysiology. 2004;41(4):552–562. doi: 10.1111/j.1469-8986.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- Stafford RW, Harris WS, Weissler AM. Left ventricular systolic time intervals as indices of postural circulatory stress in man. Circulation. 1970;41(3):485–492. doi: 10.1161/01.cir.41.3.485. [DOI] [PubMed] [Google Scholar]
- Stauss HM, Kregel KC. Frequency response characteristic of sympathetic-mediated vasomotor waves in conscious rats. Am J Physiol. 1996;271(4 Pt 2):H1416–H1422. doi: 10.1152/ajpheart.1996.271.4.H1416. [DOI] [PubMed] [Google Scholar]
- Stauss HM, Persson PB, Johnson AK, Kregel KC. Frequency-response characteristics of autonomic nervous system function in conscious rats. Am J Physiol. 1997;273(2 Pt 2):H786–H795. doi: 10.1152/ajpheart.1997.273.2.H786. [DOI] [PubMed] [Google Scholar]
- Stauss Harald M. Identification Of Blood Pressure Control Mechanisms By Power Spectral Analysis. Clinical and Experimental Pharmacology and Physiology. 2007;34(4):362–368. doi: 10.1111/j.1440-1681.2007.04588.x. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Marmot M. Impaired cardiovascular recovery following stress predicts 3-year increases in blood pressure. J Hypertens. 2005;23(3):529–536. doi: 10.1097/01.hjh.0000160208.66405.a8. [DOI] [PubMed] [Google Scholar]
- Straznicky Nora E, Grima Mariee T, Sari Carolina I, Eikelis Nina, Lambert Elisabeth A, Nestel Paul J, Lambert Gavin W. Neuroadrenergic Dysfunction Along the Diabetes Continuum: A Comparative Study in Obese Metabolic Syndrome Subjects. Diabetes. 2012;61(10):2506–2516. doi: 10.2337/db12-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toska K, Eriksen M. Respiration-synchronous fluctuations in stroke volume, heart rate and arterial pressure in humans. Journal of Physiology. 1993;472:501–512. doi: 10.1113/jphysiol.1993.sp019958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulen JHM, Boomsma F, Man AJ. Cardiovascular control and plasma catecholamines during rest and mental stress: effects of posture. Clinical Science. 1999;96:567–576. [PubMed] [Google Scholar]
- Tuomisto Martti T, Majahalme Silja, Kähönen Mika, Fredrikson Mats, Turjanmaa Väinö. Psychological Stress Tasks in the Prediction of Blood Pressure Level and Need for Antihypertensive Medication: 9–12 Years of Follow-Up. Health Psychology. 2005;24(1):77–87. doi: 10.1037/0278-6133.24.1.77. [DOI] [PubMed] [Google Scholar]
- van de Borne Philippe, Rahnama Mohsen, Mezzetti Silvia, Montano Nicola, Porta Alberto, Degaute Jean Paul, Somers Virend K. Contrasting effects of phentolamine and nitroprusside on neural and cardiovascular variability. Am. J. Physiol. 2001;281(2):H559–H565. doi: 10.1152/ajpheart.2001.281.2.H559. [DOI] [PubMed] [Google Scholar]
- Veerman DP, Imholz BPM, Weiling W, Karemaker JM, van Montfrans GA. Effects of aging on blood pressure variability in resting conditions. Hypertension. 1994;24:120–130. doi: 10.1161/01.hyp.24.1.120. [DOI] [PubMed] [Google Scholar]
- Wilbert-Lampen U, Leistner D, Greven S, Pohl T, Sper S, Volker C, Steinbeck G. Cardiovascular events during World Cup soccer. N Engl J Med. 2008;358(5):475–483. doi: 10.1056/NEJMoa0707427. 10.1056/NEJMoa0707427. [DOI] [PubMed] [Google Scholar]
- Winzer Alexandra, Ring Christopher, Carroll Douglas, Willemsen Gonneke, Drayson Mark, Kendall Martin. Secretory immunoglobulin A and cardiovascular reactions to mental arithmetic, cold pressor, and exercise: Effects of beta-adrenergic blockade. Psychophysiology. 1999;36(5):591–601. [PubMed] [Google Scholar]
- Yoshimoto Takahiko, Eguchi Kunihiro, Sakurai Hiroki, Ohmichi Yusuke, Hashimoto Tatsuyuki, Ohmichi Mika, Kumazawa Takao. Frequency components of systolic blood pressure variability reflect vasomotor and cardiac sympathetic functions in conscious rats. The Journal of Physiological Sciences. 2011:1–11. doi: 10.1007/s12576-011-0158-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Rong, Iwasaki Kenichi, Zuckerman Julie H, Behbehani Khosrow, Crandall Craig G, Levine Benjamin D. Mechanism of blood pressure and R-R variability: insights from ganglion blockade in humans. J Physiol (Lond) 2002;543(1):337–348. doi: 10.1113/jphysiol.2001.013398. [DOI] [PMC free article] [PubMed] [Google Scholar]