Abstract
Background
Chronic alcoholism is associated with increased incidence and severity of skin infection. Cutaneous dendritic cells (CDCs) play a pivotal role in skin immunity, and chronic ethanol (EtOH) feeding in mice has been shown to inhibit CDC migration to skin-draining lymph nodes (dLNs) following epicutaneous sensitization. Since CDC subsets differentially initiate T cell responses, it is important to determine how EtOH feeding affects migration of each subset and identify mechanisms responsible for observed defects.
Methods
Mice received EtOH in the drinking water for ≥16 weeks. Baseline numbers of CDC subsets and their migration to the dLNs following FITC sensitization were assessed by flow cytometry. Epidermal cell suspension and skin explant cultures were used to measure the impact of EtOH upon molecules that influence CDC migration. Cytokine arrays performed on explant culture supernatants assessed local production of inflammatory cytokines.
Results
Chronic EtOH feeding reduced migration of all CDC subsets to the dLNs following FITC sensitization. Reduced migration of dermal-resident CDCs did not correspond with reduced baseline numbers of these cells. For Langerhans cells (LCs), EtOH-induced migratory dysfunction corresponded with delayed down-regulation of E-cadherin, CCR1 and CCR6 and impaired upregulation of matrix metalloproteinases (MMPs) 2 and 9. In skin explant assays, EtOH blunted CDC mobilization following stimulation with CCL21/CPG 1826. No alteration in CD54 or CCR7 expression was observed, but production of skin-derived TNFα was reduced. Poor migratory responses in vitro could be improved by supplementing explant cultures from EtOH-fed mice with TNFα.
Conclusions
Chronic EtOH consumption does not alter baseline dermal-resident CDC numbers. However, like LCs, migratory responsiveness of dermal CDCs was decreased following FITC sensitization. Inefficient downregulation of both CCRs and adhesion molecules and the inability to upregulate MMPs indicates that EtOH impedes LC acquisition of a promigratory phenotype. These defects, combined with improvement of the migratory defect with in vitro TNFα replacement, demonstrate intrinsic as well as environmental contributions to defective CDC migration. These findings provide novel mechanisms to explain the observed increased incidence and severity of skin infections in chronic alcoholics.
Keywords: Langerhans cells, cutaneous dendritic cells, migration, TNFα, matrix metalloproteinases
INTRODUCTION
Chronic alcohol abuse exerts heavy stress upon immune competence in the skin. Alcoholism is associated with increased incidence of methicillin-resistant Staphylococcus aureus skin infection and is also a risk factor in septicemias due to Group A Streptococcus, Vibrio vulnificus, and Staphylococcus aureus, where skin is the primary site of infection (Kaech et al., 2006; Smith and Fenske, 2000). The precise immunologic lesions responsible for increased risk and severity of infection have not been defined; however it appears likely that ethanol-induced dendritic cell (DC) defects contribute to cutaneous immune deficiency. Supporting this view, DCs are functionally compromised by chronic ethanol (EtOH) consumption in both humans and mice (Fan et al., 2011; Laso et al., 2007; Lau et al., 2006; Mandrekar et al., 2004). Additionally, decreased numbers of cutaneous DCs (CDCs) and impaired migration following epicutaneous sensitization demonstrates their sensitivity to EtOH in vivo (Ness et al., 2008). Given the importance of CDC migration to the initiation and polarization of adaptive T cell responses in skin, an investigation into mechanisms by which EtOH impairs this process is warranted (Romani et al., 2012).
Skin immune responses to foreign antigen (Ag) are mediated in part by an epidermal and dermal CDC network (Romani et al., 2012). Immature CDCs capture and assess the pathogenicity of environmental Ags (Alvarez et al., 2008; Sparber et al., 2010). Activated CDCs then transport Ag from the skin to the draining lymph nodes (dLNs) where adaptive immunity is induced. In order to migrate, chemokine receptors (CCRs) and adhesion molecules (AMs) promoting CDC retention in skin are downregulated while those facilitating homing to dLNs are upregulated (Alvarez et al., 2008). Any diminution of these processes would be expected to increase susceptibility to skin infection.
Murine skin contains three major CDC populations. Langerhans cells (LCs), the only epidermal-resident CDC, can be distinguished from other CDCs by high expression of epithelial cell adhesion molecule (EpCAM). Dermal-resident DCs consist of Langerin− dermal dendritic cells (dDC) and Langerin+ dermal dendritic cells (LdDCs), which can be distinguished from LCs (also present in dermis) by low EpCAM expression (Nagao et al., 2009). Phenotypic heterogeneity among CDCs reflects functional specialization; thus a precise characterization of EtOH’s impact on CDC migration may ultimately prove to be therapeutically productive, particularly for vaccination strategies (Romani et al., 2010).
The objective of this study was to further clarify the impact of chronic EtOH feeding upon the migratory capacity of CDC subsets and to begin to characterize mechanisms responsible for observed changes. To permit long-term EtOH feeding without altering weight gain or invoking a confounding immunosuppressive stress hormone response, the Meadows-Cook EtOH-in-water model was utilized (Cook et al., 2007; Song et al., 2002). The results indicate that EtOH-induced reduction in total CDC migration from skin to dLNs reflects decreased migratory capacity of all CDC subsets. Unlike LCs, EtOH-induced migratory defects observed in dDCs and LdDCs do not correspond with an inability to maintain baseline populations in skin, demonstrating differential effects of EtOH upon epidermal vs. dermal DC. Additionally, the results provide evidence that defective CDC migration occurs as a result of both EtOH-induced intrinsic and environmental differences: EtOH impairs the ability of LCs to efficiently acquire the promigratory phenotype needed for migration out of skin, and EtOH reduces skin TNFα production--an environmental factor known to incite CDC migration (Wang et al., 1999a).
MATERIALS AND METHODS
Mice and Ethanol Administration
Six- to seven-week-old female C57BL/6 mice were purchased from National Cancer Institute (Fort Detrick, MD). Mice were maintained in specific pathogen free facilities with animal protocols approved by the Animal Care and Use Committee at University of Iowa. At approximately eight weeks of age, mice were separated into control and EtOH-consuming groups. Ethanol (AAPER Alcohol, Shelbyville, KY) was provided in the sole source of drinking water at 10% weight/volume for two days, 15% for five days and 20% on day seven and thereafter, for ≥16 weeks. Age-matched control mice were maintained on the same source of water as EtOH-consuming mice. In all cases mice were permitted ad libitum access to rodent chow and the appropriate fluid source. Mice treated using this Meadows-Cook model consume up to 40% of their calories from EtOH (Blank et al., 1991; Sipp et al., 1993). When C57Bl/6 mice are consuming 20% EtOH, they are visibly intoxicated in the early morning and have blood EtOH levels as high as 400 mg/dl at that time (Meadows et al., 1992; Song et al., 2002), with lower levels during the sleep period (41+/− 10 mg/dL for N=38 mice).
Antibodies
Four and five color flow cytometric analyses were performed using the following antibodies: M5114.15.2 (rat anti-MHC Class II), G8.8 (rat anti-EpCAM; CD326) and YN-1 (rat anti-CD54). These antibodies were precipitated with (NH4)2SO4 and conjugated to fluorescein 5-isothiocyanate (FITC) (Molecular Probes, Carlsbad, CA) or cyanine 5.18 (Amersham, Piscataway, NJ). Commercial antibodies were obtained as follows: Cy5.5PE- or Cy7PE-anti-CD11c (N418), PE-anti-MHC Class II (M5114.15.2), PE-anti-Langerin (CD207; L31), Cy7PE-anti-CD205 (205yekta) and PE-anti-TNFα (TN3-19): eBioscience (San Diego, CA). FITC-anti-CCR6 (CD196; 140706) and biotin-anti-MMP 9 (116130): R&D Systems (Minneapolis, MN). FITC-anti-E-cadherin (CD324; 36/E-cadherin): BD Biosciences (San Jose, CA). Rabbit polyclonal anti-CCR1 (CD191): Imgenex (San Diego, CA). Rabbit polyclonal anti-MMP2: Sigma-Aldrich (St. Louis, MO). Biotin-anti-CCR7 (CD197; 4B12): Biolegend (San Diego, CA). Cy5-goat anti-rabbit IgG, Cy5–avidin (Jackson ImmunoResearch, West Grove, PA) or Cy7PE-avidin (eBioscience) detected biotinylated antibodies.
Cell Suspension Preparations
Epidermal cell suspensions (ECS)
Ear skin was split into ventral and dorsal surfaces and incubated in phosphate buffered saline (PBS) containing 6 mg/ml trypsin (AMRESCO, Solon, OH) (90 min, 37°C). Epidermal sheets were dissociated and passed through a 70-μm strainer.
Dermal cell suspensions
Dorsal ear surfaces were incubated in RPMI 1640 (Invitrogen, Carlsbad, CA) containing 2 mg/ml Dispase (Gibco, Grand Island, NY) (80 min, 37°C). Dermal sheets were incubated in RPMI containing 500 μg/ml collagenase type II (Gibco) (75 min, 37°C), dissociated and passed through a 70-μm strainer.
dLN cell suspensions
Minced LN were incubated in RPMI containing 25 μg/ml Liberase blenzyme III and 20 μg/ml DNase I (Roche, Indianapolis, IN) (20 min, 37°C).
FITC Sensitization
A 0.5% FITC solution was prepared in a 1:1 mixture of acetone:n-butyl phthalate. Twenty-five microliters of FITC solution was applied to the abdomen. Inguinal LN were harvested 24 and 72 hrs later.
Cell Culture
Epidermal and dermal cell suspension cultures
Epidermal cells were cultured in RPMI containing 10% fetal bovine serum, L-glutamine-penicillin-streptomycin and 2-mercaptoethanol (RPMIc) +/− 5 μg/ml CPG 1826 (Coley Pharmaceuticals, Wellesley, MA) for 24 or 48 hrs. For metalloproteinase analysis, GolgiPlug (BD Biosciences) was added for the final 4.5 hrs of culture. For intracellular cytokine staining experiments, ECS and dermal cell suspensions were cultured for 24-72 hrs + CCL21/CPG 1826. Brefeldin A (eBioscience) was added for the final 4 hrs.
Explant cultures
Ear skin was split into dorsal and ventral halves. Dorsal halves were pierced with an 18g needle and floated in RPMIc. Some explants were cultured with 50 ng/ml CCL21 (PeproTech Inc., Rocky Hill, NJ) and 5 μg/ml CPG 1826 +/− 50 U/ml recombinant TNFα (eBioscience). At 48 hrs, fresh media +/− stimuli was added; cultures continued an additional 24 hrs before emigrated CDC harvest.
Flow Cytometric Analyses
Following surface staining, cells were fixed in FACS lysing solution (BD Biosicences) and permeabilized using 0.5% saponin (Sigma-Aldrich). To block nonspecific binding to FcγR, cells were incubated with rat anti-mouse CD16/32 (FcγRIII/II; 2.4G2) and rat sera. For MMP2 staining both rat and goat sera were used (Pel-Freez Biologicals, Rogers, AR). For intracellular cytokine staining, cells were fixed and permeabilized prior to TNFα staining. Samples were collected on FACS Calibur or FACS Canto II (BD Biosciences) using CellQuestPro acquisition software and Flowjo data analysis software (TreeStar, Stanford, CA). Dead cells were excluded by low forward and side light scatter. Spectral overlaps were corrected by compensation on singly stained positive controls. In general 50,000 cells were collected per sample.
Cytokine Measurements From Culture Supernatants
Explant culture supernatants were incubated with multiplex cytokine plates (Millipore, Billerica, MA). Data were collected on a Luminex 100 (Luminex, Austin, TX) and analyzed with Bead View (Beadview, Toronto, ON) software.
Statistical Analysis
P values were calculated from two-tailed Student’s t-tests using InStat software (GraphPad Software, LaJolla, CA) to compare EtOH and control groups (cell number, MMP and cytokine comparisons). Two-tailed Wilcoxon matched pairs tests were performed on MFI values (CCR and AM comparisons).
RESULTS
Maintenance of dermal CDCs following chronic EtOH feeding
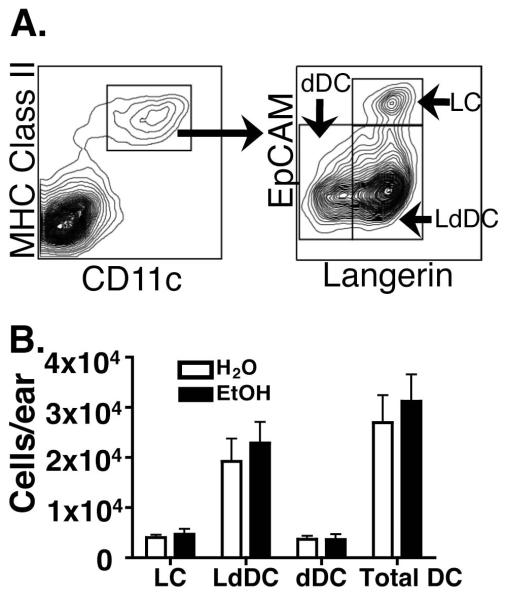
The goal of this study was to characterize mechanisms by which EtOH feeding decreases migratory capacity of the three CDC subsets: LCs, dDCs and LdDCs (Bursch et al., 2007; Ginhoux et al., 2007; Nagao et al., 2009; Poulin et al., 2007). We previously showed that EtOH feeding reduces baseline numbers of epidermal LCs and this lesion corresponded with decreased immigration to dLNs following FITC sensitization (Ness et al., 2008). With new information about dermal DC subsets, EtOH’s effect upon maintenance and migratory functions of these subsets could be investigated. To quantify baseline dermal DC numbers, CDC subsets were identified in dermal cell suspensions as shown in Figure 1A. After 16 weeks of EtOH feeding no evidence of an EtOH-induced impact upon dermal CDC numbers was observed (Figure 1B).
FIGURE 1.
Baseline number of dDCs in dermis does not differ between EtOH-fed and control mice. Fresh dermal cell suspensions from ear skin of mice chronically fed EtOH for 16 weeks or age matched water controls were counted and stained for flow cytometry. (A) CD11c+ MHC Class II+ CDC were further defined as (LC) CD207+EpCAM+, (LdDC) CD207+EpCAM− or (dDC) CD207−EpCAM−. (B) Number of CDCs per ear. Error bars represent standard error of the mean (SEM). N ≥4 mice/treatment group.
FITC+ CDC migration into dLN is decreased following chronic EtOH feeding
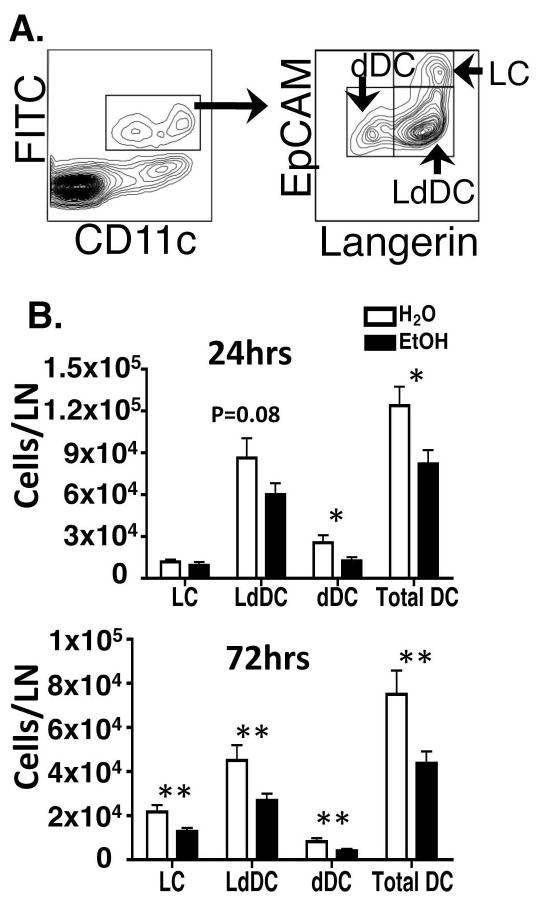
We previously showed that chronic EtOH feeding reduced Langerin+ and Langerin− DCs migration from skin to dLNs following FITC sensitization (Ness et al., 2008). In light of recent evidence that LC and LdDC are functionally distinct and developmentally unrelated (Bursch et al., 2007; Ginhoux et al., 2007; Nagao et al., 2009; Poulin et al., 2007) it was of interest to more precisely determine their migratory capacity in vivo following EtOH feeding. Inguinal LNs of EtOH-fed mice or controls were harvested after FITC sensitization, and FITC-bearing CDCs were identified (Figure 2A). Affirming previous work (Ness et al., 2008), EtOH feeding reduced the accumulation of total skin-derived FITC-bearing DCs in the dLNs at 24 and 72 hours post sensitization. Furthermore, EtOH feeding induced a statistically significant reduction or similar trend in LdDC and dDC at both time points (Figure 2B). In addition, LC migration was reduced at 72 hours—when meaningful numbers of LC have normally migrated (Kissenpfennig et al., 2005). These findings extend our previous report by showing that the EtOH-induced reduction of Langerin+ CDC migration is accompanied by decreased accumulation of LCs and LdDCs in the dLNs.
FIGURE 2.
Chronic EtOH feeding reduces CDC migration from skin to dLN. FITC solution was applied to abdominal skin of mice fed EtOH for 16 weeks or controls, and inguinal LN were subsequently harvested. (A) Gating strategy for identifying FITC+ CDC in dLN. (B) Comparison of FITC+ CDC enumeration in the LN of EtOH-fed mice or controls at 24 or 72 hours post FITC sensitization. (*)=P<0.05, (**)=P<0.01 for water vs. EtOH within each CDC subset. Error Bars represent SEM. N ≥ 4 mice/treatment group.
Loss of epidermal retaining proteins is impaired following chronic EtOH feeding
Defective CDC migration could be due to EtOH-induced inefficiencies in emigration out of skin and/or immigration into dLNs. Efficient epidermal egress requires that LCs down-regulate the CCR/AM profile facilitating epidermal retention and upregulate enzymes required to traverse the basement membrane (Ratzinger et al., 2002; Villablanca et al., 2008).
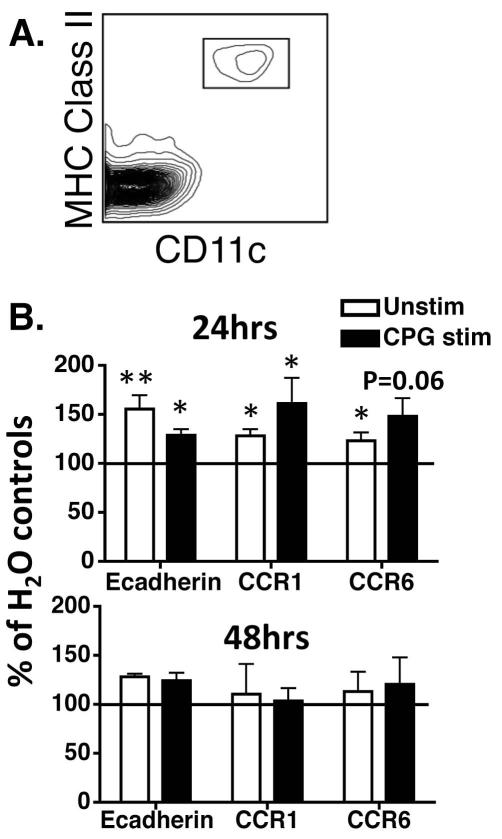
To determine if chronic EtOH consumption impedes efficient downregulation of surface proteins that sequester LCs in epidermis, ECS were prepared from EtOH-fed or control mice and cultured +/− TLR9 agonist CPG 1826 (Figure 3). CPG was chosen because it activates multiple DC populations including LCs (Ban et al., 2000). Also, chronic EtOH feeding renders splenic DCs hyporesponsive to CPG stimulation (Fan et al., 2011; Lau et al., 2006). In 24-hour cultures, LCs from EtOH-fed mice (identified as in Figure 3A) exhibited higher E-cadherin and CCR1 expression than water controls +/− CPG stimulation (Figure 3B, top panel). Also, EtOH feeding resulted in significantly higher CCR6 expression in unstimulated cultures relative to water controls, and a similar trend following CPG stimulation. By 48 hours, differences in CCR1, CCR6 and E-cadherin expression in EtOH-fed mice relative to controls were eliminated in stimulated and unstimulated cultures (Figure 3B, bottom panel). Thus EtOH feeding imposes a kinetic delay upon CCR/AM remodeling needed for epidermal egress.
FIGURE 3.
LCs from mice chronically fed EtOH exhibit increased expression of E-cadherin, CCR1 and CCR6 in both stimulated and unstimulated culture conditions. Mice fed EtOH for 16 weeks or controls were used to generate ECS cultures. Cells were cultured for 24 hours +/− CPG 1826 followed by flow cytometric analysis. (A) LC identification strategy. (B) Mean fluorescence intensity (MFI) values for CCR1, CCR6 and E-cadherin from EtOH-fed mice, normalized to those from controls (represented as 100%). Error bars represent SEM values calculated from normalized data. (*)=P≤0.05, (**)=P<0.01 for differences between EtOH and control mice within each group. N >5 mice/treatment group.
Expression of LC matrix metalloproteinases is decreased with chronic EtOH feeding
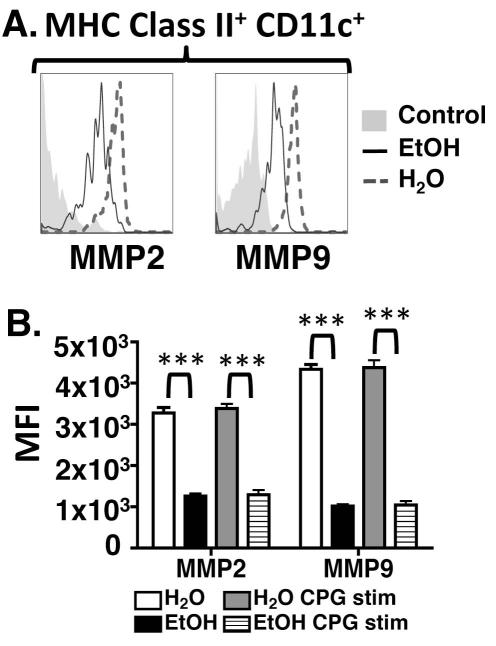
During LC mobilization, downregulation of CCRs/AMs is concomitant with upregulation of MMP2 and MMP9. Langerhans cells secrete these enzymes, that digest basement membrane collagen, to efficiently emigrate from epidermis (Alvarez et al., 2008). To determine if EtOH feeding impairs MMP upregulation, ECS cultures were established. Langerhans cells from EtOH-fed mice exhibited profoundly reduced expression of MMP2 and MMP9 in CPG-stimulated and unstimulated cultures (Figure 4). Thus defective LC migration in EtOH-fed mice is likely due in part to an inability to efficiently traverse the basement membrane and access dermal lymphatics.
FIGURE 4.
LCs from mice chronically fed EtOH exhibit reduced MMP2 and MMP9 production. ECS cultures were prepared from mice maintained on EtOH for 32 weeks or controls. Cells were cultured for 24 hours +/− CPG 1826 with addition of GolgiPlug at the end of the culture. (A) MMP2 and MMP9 expression were measured by flow cytometry on gated LC populations. Control for MMP2 (polyclonal unconjugated Ab) is unstained cells with appropriate secondary Ab, and control for MMP9 is isotype control. (B) MFIs from EtOH-fed and control groups for these enzymes. Error bars represent SEM. (***)=P<0.001 for differences between EtOH and control mice within each group. N = 4 mice/treatment group.
Cutaneous DC emigration is reduced by chronic EtOH feeding
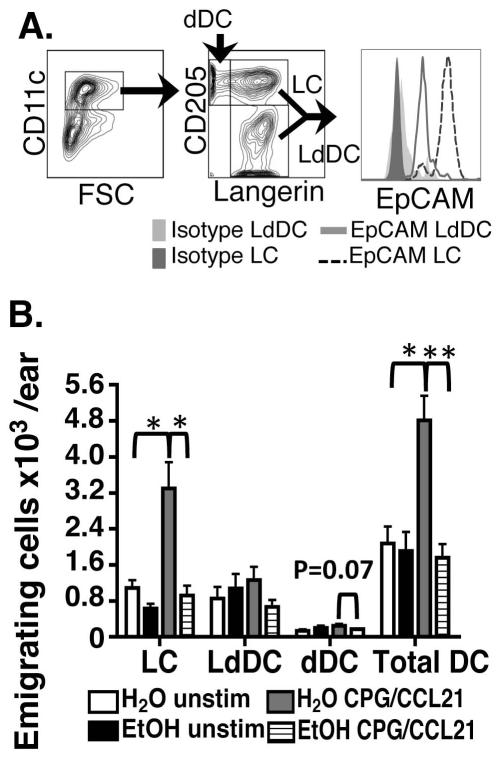
Figures 3 and 4 predict LC emigration from epidermis is delayed by chronic EtOH feeding, which contributes to the observed reduction in immigration into the dLNs (Figure 2). To test this hypothesis, and simultaneously study emigration rates of dermal DC out of skin, explant cultures were utilized. Dorsal ear halves were cultured +/− CPG and CCL21--exogenous and endogenous stimuli that promote CDC migration (Ban et al., 2000; Saeki et al., 1999). CDCs emigrating into culture media were defined by subsetting CD11c+ cells using Langerin and CD205 (Figure 5A). Consistent with Figures 1 and 2, CD205+Langerin+ LC had the highest EpCAM expression.
FIGURE 5.
Chronic EtOH feeding reduces CDC migration out of cultured ear explants following stimulation. CDCs emigrating from skin explant cultures after 72 hours +/− CCL21/CPG 1826 were stained for flow cytometry. (A) LC were defined as CD207+ CD205+, LdDC as CD207+ CD205+ and dDC as CD205+ CD207−. (B) Numbers of migrating CDCs/explant. Error bars represent SEM. (*)=P<0.05, (**)=P<0.01. N ≥3 mice/treatment group.
Paralleling steady state migration of LCs through dermis in vivo (Figure 1), unstimulated explant cultures yielded similar baseline CDC emigration for EtOH and control CDC (Figure 5). However, when explants were cultured with promigratory stimuli, increased CDC emigration was observed in control cultures but not in preparations from EtOH-fed mice. The negative impact of EtOH upon the migratory capacity of CDCs was most pronounced in LCs, which demonstrated a marked defect in their ability to emigrate out of skin explants treated for 72 hours with stimuli known to augment CDC migration i.e., CCL21/CPG (Ban et al., 2000; Saeki et al., 1999) (Figure 5b). This result was reminiscent of the EtOH-induced reduction in LC numbers immigrating into the dLNs 72 hours after FITC sensitization in vivo (Figure 2) and corresponds with the failure of EtOH exposed LCs to efficiently acquire a promigratory phenotype in vitro (Figures 3 and 4). Overall these results provide further evidence that EtOH feeding does not impair the steady-state migration process but impairs the migratory responsiveness of CDCs and LCs in particular to inflammatory stimuli.
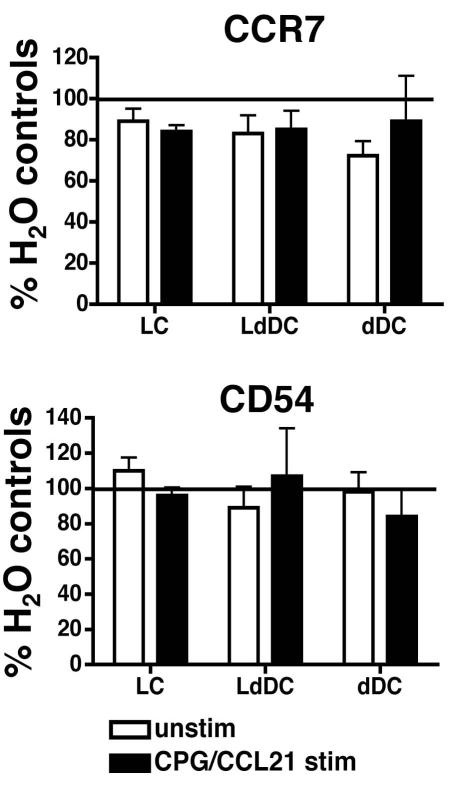
CCR7 and CD54 expression are not altered on CDC by chronic EtOH feeding
Upregulation of CCR7 is a hallmark of DC maturation. CCR7 guides DCs through CCL19 and CCL21 gradients, while CD54 mediates adhesive contacts needed for efficient lymphatic transendothelial migration (Alvarez et al., 2008). To determine whether EtOH-induced defects in CDC migration into dLNs are partially due to failure to acquire the phenotype associated with efficient intra-lymphatic migration, CCR7 and CD54 were measured on CDC recovered from explant cultures. In stimulated and unstimulated cultures, CDCs from EtOH-fed mice exhibited similar CCR7 and CD54 expression when compared with controls (Figure 6). Thus failure to achieve appropriate levels of CCR7 and CD54 does not explain the migratory defects induced by EtOH feeding.
FIGURE 6.
CCR7 and CD54 expression are not altered on CDCs by chronic EtOH consumption. Explant cultures were prepared from ears of mice fed EtOH for 16 weeks and controls. Emigrating CDCs recovered after 72 hours of culture +/− CCL21/CPG 1826 were analyzed by flow cytometry (populations gated as in Figure 5A). MFIs from EtOH-fed mice were normalized to those from controls (represented as 100%). Error bars represent SEM calculated from normalized data. N ≥3 mice/treatment group.
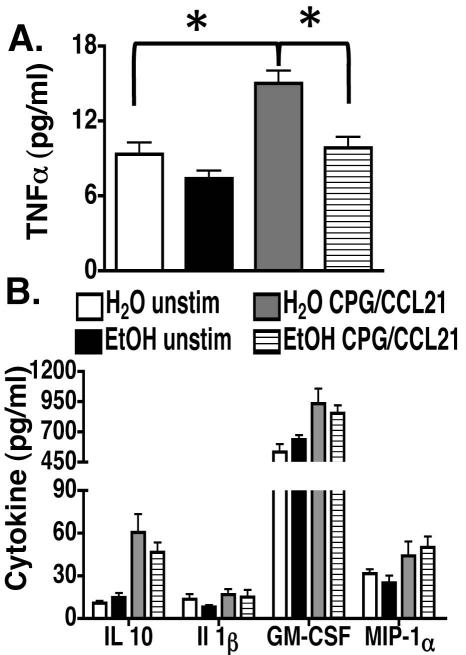
Effect of chronic EtOH feeding on promigratory cytokine signals
Environmental changes that initiate the CDC migratory program e.g., mechanical trauma, infection, etc. induce local production of TNFα and other proinflammatory molecules which in turn authorize CDC mobilization (Alvarez et al., 2008). To determine if chronic EtOH feeding alters production of skin-derived cytokines known to regulate CDC migration, levels of inflammatory cytokines in explant culture supernatants were measured by multiplex immunoassay. At baseline, equivalent levels of skin derived TNFα were detected in culture supernatants from EtOH-fed mice and controls (Figure 7A). In contrast, supernatants from CCL21/CPG-stimulated cultures, EtOH-fed mice exhibited significantly reduced TNFα concentrations. Unlike TNFα, measurements of multiple other inflammatory cytokines were not different between EtOH and control cultures (Figure 7B).
FIGURE 7.
Chronic EtOH feeding reduces TNFα levels in skin explant cultures stimulated with CCL21/CPG 1826. (A) TNFα and (B) other inflammatory cytokine production in 72 hour explant culture supernatants from ears of mice fed EtOH for 16 weeks and controls were measured with a customized multiplex immunoassay. Error bars represent SEM. (*)=P<0.05. N ≥3 mice/treatment group.
Multiple skin resident populations have the capacity to secrete TNFα (Wang et al., 1999a). Keratinocytes and dermal non-hematopoietic cells produce this cytokine and make up cellular majorities in the epidermis and dermis respectively (Bashir et al., 2009). Consistent with this, evaluation of TNFα production by intracellular cytokine staining of epidermal and dermal cells cultured with CCL21/CPG revealed the overwhelming majority of TNFα+ cells in epidermis and dermis were non-hematopoietic (Supplementary Figure S1). Thus it appears the EtOH-induced defect in TNFα production is extrinsic to CDCs.
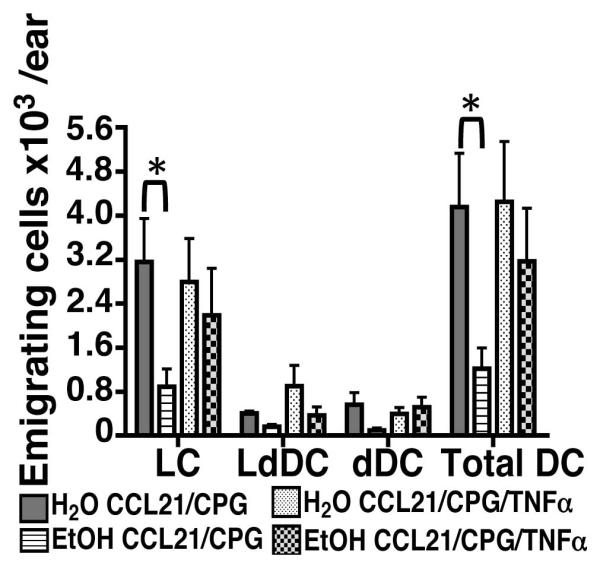
TNFα supplementation largely restores CDC migratory defects in vitro
Given the important role TNFα plays in the induction of CDC migration, the ability of exogenous recombinant TNFα to rescue the poor migratory responses of CDCs was evaluated in skin explant cultures prepared from EtOH-fed mice. As in Figure 5B, chronic EtOH feeding greatly impaired CDC mobilization out of explants in CCL21/CPG-stimulated cultures (Figure 8). In contrast, the number of emigrating CDCs, and LCs in particular, was similar between EtOH and control cultures supplemented with TNFα (Figure 8). The largely restored emigration of EtOH-exposed CDCs after TNFα supplementation provides further evidence that the limited availability of this key signal contributes to the poor CDC migratory responses observed in EtOH-fed mice.
FIGURE 8.
TNFα supplementation improves the migratory response of CDCs from EtOH-fed mice in skin explant cultures. As in Figure 5, explant cultures were prepared from ears of mice fed EtOH for 16 weeks and controls. CDCs emigrating out of ear skin stimulated with CCL21/CPG 1826 or CCL21/CPG 1826 + 50 U/ml recombinant TNFα were recovered after 72 hours and analyzed by flow cytometry (populations gated as in Figure 5A). Error bars represent SEM. (*)=P<0.05. N ≥5 mice/treatment group.
DISCUSSION
Chronic EtOH consumption results in immunosuppression, leading to increased infectious disease among alcoholics (Cook, 1998). The Meadows-Cook murine model of alcoholism has been productively employed to reproduce EtOH-induced immune deficiency and facilitate a better understanding of its cellular and molecular basis (Gurung et al., 2009; Meyerholz et al., 2008). Using this model system, EtOH-induced DC dysfunction has been observed in skin--a tissue that is commonly infected in human alcoholics (Smith and Fenske, 2000). The number of Langerin+ and Langerin− CDC able to migrate from skin to dLNs in response to inflammation is diminished in EtOH-fed mice (Ness et al., 2008). Given the propensity of alcoholics to suffer from skin infection and importance of CDC migration to cutaneous immunity (Alvarez et al., 2008) it is of interest to more precisely define how EtOH undermines this process.
To model immunologic effects of chronic alcoholism observed in humans, mice must be fed EtOH daily for at least several months (≥16 weeks in this study). Since mice cannot be maintained on the classic Lieber-DeCarli liquid diet for more than about four weeks without significant wasting, the Meadows-Cook model of EtOH administration was chosen for this study. Weight loss and elevated corticosterone levels seen in mice on the Lieber-DeCarli diet (Jerrells et al., 2007; Padgett et al., 2000) are not observed in the Meadows-Cook model (Cook et al., 2007). In addition, the latter model consistently reproduces multiple changes observed in the immune system of human alcoholic individuals. Thus it has become widely accepted among immunologists in the past decade as an appropriate model for studying the effects of chronic EtOH on murine immunity (D’Souza El-Guindy et al., 2010).
Following the recent discovery of LdDCs (Bursch et al., 2007; Ginhoux et al., 2007; Nagao et al., 2009; Poulin et al., 2007), it was important to delineate the impact of EtOH upon CDC subset maintenance in dermis. We previously showed that loss of epidermal LCs occurs after 4 weeks of EtOH feeding and becomes increasingly severe by 16 weeks (Ness et al., 2008). In contrast, baseline numbers of all dermal CDCs remain intact at 16 weeks of EtOH feeding (Figure 1), which corroborates our previous finding that the number of CD11c+ cells in the dermis of skin tissue sections was unaltered by EtOH (Ness et al., 2008). These data are not surprising given the distinct pathways that maintain epidermal and dermal DCs. LdDCs and dDCs are replaced throughout life by bone marrow precursors whereas local progenitors renew LCs (Helft et al., 2010). Additionally, unaltered numbers of LCs in the dermis of EtOH-fed mice indicate the magnitude of steady-state LC migration remains similar despite their reduced frequency in the epidermis (Ness et al., 2008).
Having established baseline numbers of CDCs in EtOH-fed and control mice, we evaluated EtOH’s impact upon stimulated migratory capacity of these subsets in vivo. Analysis of dLNs at time points corresponding with peaks of LdDC and LC immigration after FITC sensitization (24 and 72 hours respectively) revealed a reduction in total CDC immigrants and a significant reduction or similar trend in the number of immigrants from each subset (Figure 2). These results confirm our previous report that EtOH feeding reduces the accumulation of Langerin+ CDCs in the dLNs following FITC sensitization and clarifies the relative impact of EtOH upon LC vs. LdDC migration (Ness et al., 2008). The modest effect of EtOH upon LC immigration after 24 hours likely reflects the fact that most LC immigration following sensitization occurs between 72-96 hours (Kissenpfennig et al., 2005). Thus, it is likely that the majority of LCs reaching the dLNs after 24 hours acquired FITC during steady-state migration, which is not substantially impacted by EtOH. In contrast to LCs, the decrease in LdDC immigrants does not correspond with a reduction in their baseline numbers in skin and therefore unambiguously identifies an EtOH-induced defect in the migratory responsiveness of these cells. Either a kinetic or total decrease in CDC migration would limit timely transport of Ag into the dLNs and predictably impair cutaneous immunity. Considering that EtOH hampers the ability of splenic DCs to prime naïve T cells (Fan et al., 2011), robust activation of adaptive immunity during skin infection may be challenged by a quantitative deficit in CDC mobilization and a qualitative defect in the ability of EtOH-exposed CDCs to interact appropriately with T cells.
A growing body of evidence suggests CDC subsets have specialized roles as initiators of T cell immunity in vivo. For example, LCs are prodigious inducers of Ag-specific Th17 responses following bacterial and fungal infection (Haley et al., 2012; Igyártó et al., 2011), whereas Ag cross-presentation and Th1 induction are functions of LdDCs (Bedoui et al., 2009; Igyártó et al., 2011; Stoitzner et al., 2006). The precise role of dDCs in the initiation and polarization of effector T cell responses remains unclear, however their ability to activate T cells is suggested by normal hypersensitivity responses in mice constitutively deficient in LCs or LdDCs (Kissenpfennig et al., 2005). Considering the critical contributions of Th17 cells and IL-17 to anti-S. aureus immunity, EtOH-induced LC migratory dysfunction may contribute to the susceptibility of alcoholics to this pathogen (Cho et al., 2010; Kaech et al., 2006; Lin et al., 2009; Smith and Fenske, 2000).
The EtOH-induced lesions underlying defective CDC migration remain unknown. Langerhans cells from EtOH-fed mice exhibited transiently higher expression of CCR1, CCR6, and E-cadherin relative to controls in CPG-stimulated cultures (Figure 3). This EtOH-induced kinetic alteration in CCR/AM remodeling occurs alongside a diminished capacity of LCs from EtOH-fed mice to produce MMP2 and MMP9 (Figure 4). Taken together, these results show that chronic EtOH feeding negatively impacts the acquisition of phenotypic and functional attributes associated with LC epidermal egress, and provide mechansims to explain the migratory dysfunction observed in vivo.
The modest level of steady-state CDC migration is dramatically increased in response to inflammatory stimuli (Tripp et al., 2008). Skin explant cultures are well suited for study of CDC migration because they recapitulate this dynamic (Ban et al., 2000; Nagao et al., 2009), and permit evaluation of CDC emigration from skin independent of immigration into the dLNs. In explant cultures from control mice, total CDC emigration was sharply increased by CCL21/CPG stimulation. In contrast, these stimuli did not increase CDC emigration in cultures from EtOH-fed mice (Figure 5). The negative impact of EtOH in stimulated cultures was most impressively evident in LCs. Thus, the EtOH-induced defects in LC migration in vivo are reproduced in vitro and correspond with multiple defects that would inhibit their migratory responsiveness. When considered with Figure 2, clearly all CDC subsets show impaired migration as a result of EtOH feeding.
While the early maturation events and corresponding phenotypic modifications supporting dermal DC migration are less well characterized than for LCs, both populations depend upon CCR7 and its ligands for efficient transit to the dLNs (Gunn et al., 1999; Ohl et al., 2004). Consistent with our previous observation that after entering the dLNs, CCR7 expression was similar on FITC-bearing DCs from EtOH-fed and control animals (Ness et al., 2008), EtOH did not alter CCR7 expression on CDCs migrating out of skin explants (Figure 6). Thus, EtOH-induced migratory defects do not occur through a CDC-intrinsic failure to upregulate CCR7. Considering that deficiency of either CCR7 or its ligands blunts CDC migration, it remains possible that EtOH disrupts this process by virtue of an environmental alteration such as suppressed CCL19/CCL21 production within lymphatic vessels (Gunn et al., 1999; Ohl et al., 2004).
The current CDC migration paradigm indicates that in addition to chemokine-directed chemotaxis, efficient transendothelial trafficking involves adhesive interactions between CDCs and lymphatic endothelial cells (Alvarez et al., 2008). CD54 is upregulated on migrating CDCs and its ability to facilitate transendothelial migration is suggested by the reduced accumulation of CDCs in the dLNs of FITC-sensitized mice deficient in CD54 or treated with neutralizing antibodies (Ban et al., 2000; Ma et al., 1994; Xu et al., 2001). The similar expression of CD54 on CDCs emigrants from EtOH-fed and control mice suggests EtOH-induced migratory dysfunction in CDCs is not due to their inability to execute CD54-mediated adhesions (Figure 6). Overall, the data suggest EtOH selectively interferes with the capacity of CDCs to mobilize out of skin rather than traffic through afferent lymphatics. A cell autonomous mechanism has not yet been identified to explain why LdDC and dDC from EtOH-fed mice fail to migrate appropriately to dLN.
Skin-derived cytokines are critical initiators of CDC migration (Wang et al., 1999a). Of special interest is TNFα, which is critically involved in CDC maturation and migration following epicutaneous sensitization (Cumberbatch and Kimber, 1995; Nishibu et al., 2007; Wang et al., 1997). When TNFα activity is eliminated in explant culture by neutralizing antibodies, CDC migration is dramatically reduced (Stoitzner et al., 1999). Additionally, CDCs fail to accumulate in dLNs following FITC sensitization in TNF receptor 2 deficient mice (Wang et al., 1997). Our finding that TNFα production is reduced in stimulated skin explant cultures from EtOH-fed mice provides evidence that the EtOH-induced defects in CDC migration may be due in part to an altered skin cytokine milieu (Figure 7). The significance of this alteration was demonstrated by the ability of TNFα supplementation to rescue migratory responses of EtOH-exposed CDCs, and LCs in particular, to CCL21/CPG stimulation (Figure 8). Given that CCR/AM remodeling and MMP upregulation in LCs occurs downstream of TNFα stimulation, the efficacy of TNFα supplementation upon LC migration in vitro may be a consequence of the ability of TNFα to induce these processes (Alvarez et al., 2008).
Beyond its direct impact on DCs, TNFα may also facilitate migratory responses through additional environmental alterations. Given that TNFα increases CCL21 expression by lymphatic endothelial cells, suboptimal TNFα induction would likely render the afferent lymphatics inadequately primed for CDC migration (Martin-Fontecha et al., 2003). GM-CSF and IL1-β have also been shown to promote CDC maturation and migration (Alvarez et al., 2008; Wang et al., 1999a). Equivalent production of these cytokines indicates EtOH feeding does not cause a global defect in the elaboration of promigratory cytokines while similar IL-10 induction suggests EtOH does not oppose CDC migration by elevating a cytokine signal that promotes CDC retention within skin (Wang et al., 1999b). Taken together, these findings indicate the selective inhibition of TNFα response in skin is an important mechanism by which EtOH impairs migratory responses of CDCs (Figures 7 & 8).
In summary, this study shows chronic EtOH feeding compromises the normal migratory response of all CDC subsets to proinflammatory stimuli in vivo and/or in vitro. Newly identified mechanisms to account for this dysfunction in LCs are 1) inefficient downregulation of CCR1, CCR6 and E-cadherin and 2) diminished production of MMP2 and MMP9. EtOH feeding was not found to affect the capacity for lymphatic transit via upregulation of CCR7 and CD54 on migrating CDCs. Finally, EtOH feeding resulted in diminished production of the potent promigratory cytokine TNFα from skin-resident cells, and restoration of this cytokine improved CDC migration in vitro. These findings provide new mechanisms by which dysfunctional LCs could contribute to the observed increase in skin infections in alcoholics. Additional studies are underway to determine mechanisms underlying dysfunctional dermal DC migration, and to further understand environmental changes as a result of EtOH feeding that may impact CDC migration.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Ruth Coleman for expert management of EtOH administration to mice, and Dr. Thomas Waldschmidt for critical review of the manuscript.
This work was supported by NIH R01 AA014405 and AA014406, and the Carver College of Medicine Department of Pathology.
REFERENCES
- Alvarez D, Vollman EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29:325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban E, Dupré L, Hermann E, Rohn W, Vendeville C, Quatannens B, Ricciardi-Castagnoli P, Capron A, Riveau G. CpG motifs induce Langerhans cell migration in vivo. Int Immunol. 2000;12:737–745. doi: 10.1093/intimm/12.6.737. [DOI] [PubMed] [Google Scholar]
- Bashir MM, Sharma MR, Werth VP. TNF-alpha production in the skin. Arch Dermatol Res. 2009;301:87–91. doi: 10.1007/s00403-008-0893-7. [DOI] [PubMed] [Google Scholar]
- Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, Brooks AG, Heath WR. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- Blank SE, Duncan DD, Meadows GG. Suppression of natural killer cell activity by ethanol consumption and food restriction. Alcohol Clin Exp Res. 1991;15:16–22. doi: 10.1111/j.1530-0277.1991.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, Hogquist KA. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RT. Alcohol abuse, alcoholism, and damage to the immune system--a review. Alcohol Clin Exp Res. 1998;22:1927–42. [PubMed] [Google Scholar]
- Cook RT, Schlueter AJ, Coleman RA, Tygrett L, Ballas ZK, Jerrells TR, Nashelsky MB, Ray NB, Haugen TH, Waldschmidt TJ. Thymocytes, pre-B cells, and organ changes in a mouse model of chronic ethanol ingestion--absence of subset-specific glucocorticoid-induced immune cell loss. Alcohol Clin Exp Res. 2007;31:1746–1758. doi: 10.1111/j.1530-0277.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberbatch M, Kimber I. Tumour necrosis factor-alpha is required for accumulation of dendritic cells in draining lymph nodes and for optimal contact sensitization. Immunology. 1995;84:31–35. [PMC free article] [PubMed] [Google Scholar]
- D’Souza El-Guindy NB, Kovacs EJ, De Witte P, Spies C, Littleton JM, de Villiers WJS, Lott AJ, Plackett TP, Lanzke N, Meadows GG. Laboratory models available to study alcohol-induced organ damage and immune variations: choosing the appropriate model. Alcohol Clin Exp Res. 2010;34:1489–1511. doi: 10.1111/j.1530-0277.2010.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Moore MR, Turner LE, Cook RT, Legge KL, Waldschmidt TJ, Schlueter AJ. Mechanisms by which chronic ethanol feeding limits the ability of dendritic cells to stimulate T-cell proliferation. Alcohol Clin Exp Res. 2011;35:47–59. doi: 10.1111/j.1530-0277.2010.01321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, Helft J, Ochando J, Kissenpfennig A, Malissen B, Grisotto M, Snoeck H, Randolph G, Merad M. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT, Nakano H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med. 1999;189:451–460. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P, Young BM, Coleman RA, Wiechert S, Turner LE, Ray NB, Waldschmidt TJ, Legge KL, Cook RT. Chronic ethanol induces inhibition of antigen-specific CD8+ but not CD4+ immunodominant T cell responses following Listeria monocytogenes inoculation. J Leukoc Biol. 2009;85:34–43. doi: 10.1189/jlb.0208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley K, Igyártó BZ, Ortner D, Bobr A, Kashem S, Schenten D, Kaplan DH. Langerhans cells require MyD88-dependent signals for Candida albicans response but not for contact hypersensitivity or migration. J Immunol. 2012;188:4334–4339. doi: 10.4049/jimmunol.1102759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helft J, Ginhoux F, Bogunovic M, Merad M. Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol Rev. 2010;234:55–75. doi: 10.1111/j.0105-2896.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- Igyártó BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, Zurawski SM, Malissen B, Zurawski G, Berman J, DH K. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35:260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells TR, Pavlik JA, DeVasure J, Vidlak D, Costello A, Strachota JM, Wyatt TA. Association of chronic alcohol consumption and increased susceptibility to and pathogenic effects of pulmonary infection with respiratory syncytial virus in mice. Alcohol. 2007;41:357–369. doi: 10.1016/j.alcohol.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech C, Elzi L, Sendi P, Frei R, Laifer G, Bassetti S, Fluckiger U. Course and outcome of Staphylococcus aureus bacteraemia: a retrospective analysis of 308 episodes in a Swiss tertiary-care centre. Clin Microbiol Infect. 2006;12:345–352. doi: 10.1111/j.1469-0691.2005.01359.x. [DOI] [PubMed] [Google Scholar]
- Kissenpfennig A, Henri S, Dubois B, Laplace-Builhé C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, Saeland S, Davoust J, Malissen B. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;225:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Laso FJ, Vaquero JM, Almeida J, Marcos M, Orfao A. Chronic alcohol consumption is associated with changes in the distribution, immunophenotype, and the inflammatory cytokine secretion profile of circulating dendritic cells. Alcohol Clin Exp Res. 2007;31:846–854. doi: 10.1111/j.1530-0277.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- Lau AH, Abe M, Thomson AW. Ethanol affects the generation, cosignaling molecule expression, and function of plasmacytoid and myeloid dendritic cell subsets in vitro and in vivo. J Leukoc Biol. 2006;79:941–953. doi: 10.1189/jlb.0905517. [DOI] [PubMed] [Google Scholar]
- Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, Fu Y, French SW, Edwards JEJ, Spellberg B. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009;5:e1000703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Wang JH, Guo YJ, Sy MS, Bigby M. In vivo treatment with anti-ICAM-1 and anti-LFA-1 antibodies inhibits contact sensitization-induced migration of epidermal Langerhans cells to regional lymph nodes. Cell Immunol. 1994;158:389–399. doi: 10.1006/cimm.1994.1285. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Dolganiuc A, Kodys K, Szabo G. Inhibition of myeloid dendritic cell accessory cell function and induction of T cell anergy by alcohol correlates with decreased IL-12 production. J Immunol. 2004;173:3398–3407. doi: 10.4049/jimmunol.173.5.3398. [DOI] [PubMed] [Google Scholar]
- Martin-Fontecha A, Sebastiani S, Höpken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows GG, Wallendal M, Kosugi A, Wunderlich J, Singer DS. Ethanol induces marked changes in lymphocyte populations and natural killer cell activity in mice. Alcoholism: Clinical & Experimental Research. 1992;16:474–479. doi: 10.1111/j.1530-0277.1992.tb01403.x. [DOI] [PubMed] [Google Scholar]
- Meyerholz DK, Edsen-Moore M, McGill J, Coleman RA, Cook RT, Legge KL. Chronic alcohol consumption increases the severity of murine influenza virus infections. J Immunol. 2008;181:641–648. doi: 10.4049/jimmunol.181.1.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao K, Ginhoux F, Leitner WW, Motegi S, Bennett CL, Clausen BE, Merad M, Udey MC. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc Natl Acad Sci U S A. 2009;106:3312–3317. doi: 10.1073/pnas.0807126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness KJ, Fan J, Wilke WW, Coleman RA, Cook RT, Schlueter AJ. Chronic ethanol consumption decreases murine Langerhans cell numbers and delays migration of Langerhans cells as well as dermal dendritic cells. Alcohol Clin Exp Res. 2008;32:657–668. doi: 10.1111/j.1530-0277.2007.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibu A, Ward BR, Boes M, Takashima A. Roles for IL-1 and TNFalpha in dynamic behavioral responses of Langerhans cells to topical hapten application. J Dermatol Sci. 2007;45:23–30. doi: 10.1016/j.jdermsci.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, Forster R. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Padgett EL, Sibley DA, Jerrells TR. Effect of adrenalectomy on ethanol-associated changes in lymphocyte cell numbers and subpopulations in thymus, spleen, and gut-associated lymphoid tissues. Int J Immunopharmacol. 2000;22:285–298. doi: 10.1016/s0192-0561(99)00083-1. [DOI] [PubMed] [Google Scholar]
- Poulin LF, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204:3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzinger G, Stoitzner P, Ebner S, Lutz MB, Layton GT, Rainer C, Senior RM, Shipley JM, Fritsch P, Schuler G, Romani N. Matrix metalloproteinases 9 and 2 are necessary for the migration of Langerhans cells and dermal dendritic cells from human and murine skin. J Immunol. 2002;168:4361–4367. doi: 10.4049/jimmunol.168.9.4361. [DOI] [PubMed] [Google Scholar]
- Romani N, Brunner PM, Stingl G. Changing views of the role of Langerhans cells. J Invest Dermatol. 2012;132:872–881. doi: 10.1038/jid.2011.437. [DOI] [PubMed] [Google Scholar]
- Romani N, Thurnher M, Idoyaga J, Steinman RM, Flacher V. Targeting of antigens to skin dendritic cells: possibilities to enhance vaccine efficacy. Immunol Cell Biol. 2010;88:424–430. doi: 10.1038/icb.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki H, Moore AM, Brown MJ, Hwang ST. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol. 1999;162:2472–2475. [PubMed] [Google Scholar]
- Sipp TL, Blank SE, Lee EG, Meadows GG. Plasma corticosterone response to chronic ethanol consumption and exercise stress. Proc Soc Exp Biol Med. 1993;204:184–190. doi: 10.3181/00379727-204-43650. [DOI] [PubMed] [Google Scholar]
- Smith KE, Fenske NA. Cutaneous manifestations of alcohol abuse. J Am Acad Dermatol. 2000;43:1–16. doi: 10.1067/mjd.2000.104512. [DOI] [PubMed] [Google Scholar]
- Song K, Coleman RA, Zhu X, Alber C, Ballas ZK, Waldschmidt TJ, Cook RT. Chronic ethanol consumption by mice results in activated splenic T cells. J Leukoc Biol. 2002;79:941–953. [PubMed] [Google Scholar]
- Sparber F, Tripp CH, Hermann M, Romani N, Stoitzner P. Langerhans cells and dermal dendritic cells capture protein antigens in the skin: possible targets for vaccination through the skin. Immunobiol. 2010;215:770–779. doi: 10.1016/j.imbio.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoitzner P, Tripp CH, Eberhart A, Price KM, Jung JY, Bursch L, Ronchese F, Romani N. Langerhans cells cross-present antigen derived from skin. Proc Natl Acad Sci U S A. 2006;103:7783–7788. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoitzner P, Zanella M, Ortner U, Lukas M, Tagwerker A, Janke K, Lutz MB, Schuler G, Echtenacher B, Ryffel B, Koch F, Romani N. Migration of langerhans cells and dermal dendritic cells in skin organ cultures: augmentation by TNF-alpha and IL-1beta. J Leukoc Biol. 1999;66:462–470. [PubMed] [Google Scholar]
- Tripp CH, Haid B, Flacher V, Sixt M, Peter H, Farkas J, Gschwentner R, Sorokin L, Romani N, Stoitzner P. The lymph vessel network in mouse skin visualised with antibodies against the hyaluronan receptor LYVE-1. Immunobiol. 2008;213:715–728. doi: 10.1016/j.imbio.2008.07.025. [DOI] [PubMed] [Google Scholar]
- Villablanca EJ, Russo V, Mora J. Dendritic cell migration and lymphocyte homing imprinting. Histol Histopathol. 2008;23:897–910. doi: 10.14670/HH-23.897. [DOI] [PubMed] [Google Scholar]
- Wang B, Amerio P, Sauder DN. Role of cytokines in epidermal Langerhans cell migration. J Leukoc Biol. 1999a;66:33–39. doi: 10.1002/jlb.66.1.33. [DOI] [PubMed] [Google Scholar]
- Wang B, Fujisawa H, Zhuang L, Kondo S, Shivji GM, Kim CS, Mak TW, Sauder DN. Depressed Langerhans cell migration and reduced contact hypersensitivity response in mice lacking TNF receptor p75. J Immunol. 1997;159:6148–6155. [PubMed] [Google Scholar]
- Wang B, Zhuang L, Fujisawa H, Shinder GA, Feliciani C, Shivji GM, Suzuki H, Amerio P, Toto P, Sauder DN. Enhanced epidermal Langerhans cell migration in IL-10 knockout mice. J Immunol. 1999b;162:277–283. [PubMed] [Google Scholar]
- Xu H, Guan H, Zu G, Bullard D, Hanson J, Slater M, Elmets CA. The role of ICAM-1 molecule in the migration of Langerhans cells in the skin and regional lymph node. Eur J Immunol. 2001;10:3085–3093. doi: 10.1002/1521-4141(2001010)31:10<3085::AID-IMMU3085>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.