Abstract
Objective
To develop a length of stay (LOS) model for extremely low birth weight (ELBW) infants.
Study Design
We included infants from the California Perinatal Quality Care Collaborative with birth weight 401–1,000 grams who were discharged to home. Exclusion criteria were congenital anomalies, surgery, and death. LOS was defined as days from admission to discharge. As patients who died or were transferred to lower level of care were excluded, we assessed correlation of hospital mortality rates and transfers torisk adjusted LOS.
Results
There were 2,012 infants with median LOS 79 days (range 23–219). Lower birth weight, lack of antenatal steroids, and lower Apgar score were associated with longer LOS. There was negligiblecorrelation between risk-adjusted LOS and hospital mortality rates (r = 0.0207) and transfer-out rates (r = 0.121).
Conclusion
Particularly because ELBW infants have extended hospital stays, identification of unbiased and informative risk-adjusted LOS for these infants is an important step in benchmarking best practice and improving efficiency in care.
Keywords: Length of stay, Extremely low birth weight
Introduction
Extremely low birth weight infants (ELBW) pose a heavy resource burden in perinatal healthcare.1, 2 While it is inevitable that ELBW infants would have relatively long neonatal intensive care unit (NICU) length of stay (LOS), there may be opportunities for improving and standardizing care, as unexplained variation in LOS has been described for premature infants.3, 4 Depending on the patient, hospital, and payer, there may be financial considerations that could influence practice in such a manner to discharge such infants sooner or later.5, 6 For these reasons, after baseline characteristics that contribute meaningful increments in explanatory power are accounted for, LOS may be considered a quality of care measurement. In order to use ELBW LOS as a quality measurement amongst hospitals, we need to reveal the impact of practice variation among providers via a model employing inclusion/exclusion criteria and baseline patient characteristics that are broadlyaccepted by relevant stakeholders.
Previous studies on modeling length of stay for premature infants have been limited due to being confined to one or a few centers or concerned only certain level NICUs.7, 8, 9, 10 A population-based study in Sweden evaluated moderately preterm infants 30–34 weeks.4 A study of 30 NICUs in the UK included a broad group of premature infants including those 23 to 32 weeks gestation and with birth weight up to 3000 grams.3 Our aim was to characterize LOS for the smallest, most vulnerable infants who draw especially heavily on NICU resources, across wide levels of hospital care.
The purpose of this study was to modelLOS for ELBW infants in order to examine provider variation and thus to inform quality assessmentwithin a population-based dataset. Because both widely heterogeneous patient care needs and patient care in more than one discrete system of care can obscure the true contribution of various independent risk factors to LOS, we studied ELBWs who survived and were cared for from birth or shortly after birth at a single institution until discharge to home. In particular, we considered: 1) type of risk adjustment model, 2) the potential for bias from excluding patients who died before discharge, and 3) the potential for bias from excluding patients who were transferred to a lower level hospital for convalescent care.
Methods
Data source, study population, and patient characteristics
We used data from the California Perinatal Quality Care Collaborative (CPQCC), a voluntary collective of 129 NICUs that report demographic and clinical data into a central repository. More than 90% of NICUs in California participated in this collaborative between January 1, 2008, and December 31, 2010. Member NICUs collect data on their patients in a prospective manner, and each record undergoes a variety of range, logic, and missing data checks.
The period of analysis included infants cared for in CPQCC NICUs for the three years, 2008 to 2010. Inclusion criteria were infants with birth weight 401–1,000 grams who were cared for at a CPQCC hospital from birth or within the first day after delivery, until discharge to home. Exclusion criteria were patients who died, those with congenital anomalies, and infants who had any surgery except circumcision.
The primary outcome ofinterest was length of stay (LOS), defined as the number of days from admission to discharge. For infants born at CPQCC hospitals, the day of birth counted as day one. For those infants not cared for at a CPQCC hospital on the first day, the first day of hospital stay at a CPQCC hospital counted as day one.
The following baseline characteristics were considered in risk adjustment: infant characteristics (sex, birth weight, gestational age, small for gestational age (SGA)), maternal and intrapartum variables (maternal age, race/ethnicity, prenatal care, location of birth (inborn/outborn), antenatal steroid use, multiple birth, mode of delivery, antenatal conditions), and delivery room variables (5 minute Apgar scores).
In considering SGA (defined as birth weight <10th percentile for gestational age), it was noted that SGA infants had shorter LOS, particularly those with gestational age >= 30 weeks. As birth weight < 1,000 grams was an inclusion criterion, we used an approach that accounted for the birth weight restriction of the eligible cohort. The SGA variable was categorized into 2 different groups: 1) SGA infants < 30 weeks of gestational age, and 2) SGA infants >= 30 weeks). We chose 30 weeks as the cut point because the 3rd percentile of 1,000 gram infants is approximately 30 weeks. The rationale was to distinguish small size by infants’ developmental stage in terms of gestational age. Previous research has shown a survival advantage for SGA neonates of < 31 weeks associated with cesarean delivery.11
Selection of explanatory variables
We examined collinearity among the model variables under consideration, as collinearity may influence estimation and inference for the primary parameters and lead to a loss of power, considering variance inflation factors and tolerance (see Appendix and Table e1 for details on this selection process). From this analysis, we included birth weight and SGA, but not gestational age as explanatory variables in models.
The empirical distribution of LOS showed poor approximation to standard theoretical normal distribution. Skewness in the empirical distribution with a long positive side tail suggested that the use of the non-parametric test (Kruskal-Wallis) would be preferable when comparing LOS between different levels in each risk factor.
Model selection
As the LOS of each infant cared for within a hospital may not be independentfrom that of another infant in the same hospital, even after adjusting for the fixed risk factors under study, we accounted for this hospital-level clustering by using generalized linear mixed models with hospital of care accounted for as a random effect.12
We considered a variety of models for LOS. We used a randomly selected 50% of the ELBW cohort (n=1,006) for model building, with the remaining 50% serving as a validation set. Further details of model selection are noted in the Appendix and online Table e2. In our review of the literature, we identified seven different approaches to modeling perinatal LOS. As described in the Appendix, these seven models were tested for goodness of fit and other characteristics. Model 6, a generalized linear model with negative binomial distribution and Model 2, a linear mixed model using the log scale were considered the two optimal LOS models. The results of primary analyses and tests for biases (described below) were similar for both models, and Model 2 formed the basis for the remainder of the study (see Appendix). In Model 2 we use a mixed model to account for clustering within NICUs and we use the log transformation of LOS to reconcile the non-normal distribution that results from the very long LOS of some ELBWs with modeling assumptions.
Final model construction
Having selected the linear mixed model using log transformation as the preferred solution to estimating risk adjustedLOS, the model was applied to the whole study cohort. We started from an unconditional means model, then added infant-level risk factors. Final risk factor selection for the model was determined by backward selection (alpha < 0.05 significance level). The model diagnostics and validation of model assumptions were checked again in the final model.
When residuals for the final model fitting were examined, there were two extreme outlier observations (with significantly higher DFFITS and Cook’s distance statistics).13 These two extreme data points were excluded from analysis in order to optimize model fit. As a linear mixed model does not generate an R2 statistic, we estimated R2 by using the same covariates in a model without random effects.
Based on infant-level estimates, hospital level results were then computed from our selected model for all hospitals. Risk adjusted LOS at each hospital was computed in the following fashion:14
(a) for each infant, estimate an expected LOS
(b) obtaina calibration factor and apply to infant expected LOS. The calibration factor was obtained by the overall ratio of (sum of actual LOS/sum of expected LOS) across the whole study cohort, as described in previous studies in order to center the estimate around 1.0.15
(c) sum infants’ actual LOS and their expected LOS obtained in the previous step by hospital
(d) divide hospitals’ actual LOS sum by expected LOS sum to obtain O/E ratio
(e) multiply hospitals’ O/E ratio by overall average LOS
In order to examine variation by hospital, we estimated risk-adjusted observed to expected LOS ratios for each hospital. In order to facilitate root cause analysis of a NICU’s infants with excessive LOS, outliers for LOS at the individual patient level were identified based on studentized residuals outside of the −2 to +2 range.16 These infants were considered individual outliers.
Tests for biases
As infants who died were excluded from the analysis, we wanted to examine the possibility that hospitals that may have shorter mean LOS could have higher rates of mortality, thereby having a less acutely ill surviving population of infants that would be biased toward shorter LOS. To examine this potential bias on average LOS, we examined the relationship of hospital mortality rate to average LOS. The mortality rate was computed from an established CPQCC algorithm, a modification of a Vermont Oxford Network model, using all ELBW infants excluding delivery room death in the CPQCC dataset between 2008–2010 (N=7,270).18 Observed to expected mortality ratios were computedand their relationship to hospital LOS was evaluated using weighted correlation. In this regression model, each hospital was assigned a weight of the number of eligible patients cared for at that hospital.
Another potential source of bias could be the exclusion of infants who are transferred out to lower levels of care. When ELBW infants cared for at Level III centers are transferred out for feeding and growing at lower levels of care, it is possible that the infants remaining in that NICU may present more complex cases who could be biased toward longer LOS. Similar to the analysis of mortality, we considered the relationship of transfer-out rates for feeding and growing with average LOS by hospital for NICUs with weighted correlation.
All statistical analyses were conducted using SAS version 9.3 (SAS, Cary, NC). The Stanford University Institutional Review Board approved this research.
Results
There were 2,012 ELBW infants in the eligible cohort cared for in 111 hospitals (Figure e1 – in online supplementary materials), with an average birth weight of 830 grams (SD 126) and gestational age 27.2 weeks (SD 2.0). The majority of patients were cared for in Community level NICUs (64%), with 32% of infants cared for in Regional NICUs, 1% in Intermediate NICUs, and 4% in non-classified NICUs. The median LOS was 79 days, with a range of 23 to 219 days (Table e3 – in online supplementary materials). Male infants and those with lower birth weight had longer mean LOS. Infants who had antenatal steroid exposure and were born by cesarean delivery had shorter mean LOS. Over the 3-year study period, LOS variation between years was not statistically significant (p=0.11).
The results of the risk adjusted LOS model are shown in Table 1, and the distribution of observed and predicted LOS is shown in Figure 1. Birth weight, gender, SGA, ethnicity, antenatal steroid use, fetal distress, maternal hypertension, and 5 minute Apgar score were significant risk factors for longer LOS. The random hospital intercept was strongly significant, indicating significant variation in LOS between hospitals. The model without hospital included as a random effect had R2 of 0.45.
Table 1.
Adjusted length of stay by subgroups.
| Subgroup | Category | Average adjusted length of stay | P |
|---|---|---|---|
| Birth weight (grams) | 499 and less | 117.58 | <.0001 |
| 500-599 | 96.70 | <.0001 | |
| 600-699 | 87.50 | <.0001 | |
| 700-799 | 78.59 | <.0001 | |
| 800-899 | 70.64 | <.0001 | |
| 900-999 | 63.37 | Reference | |
| Sex | Male | 86.12 | <.0001 |
| Female | 81.83 | Reference | |
| SGA status | SGA < 30 wks | 85.63 | <.0001 |
| SGA >= 30 wks | 70.16 | <.0001 | |
| Not SGA | 98.47 | Ref | |
| Ethnicity | Black | 80.48 | 0.0007 |
| Hispanic | 83.36 | 0.2139 | |
| White | 84.55 | Reference | |
| Asian/Pacific Islander | 85.29 | 0..6115 | |
| Native American | 85.15 | 0.9283 | |
| Others | 84.97 | 0.8775 | |
| Antenatal steroids | Yes | 82.55 | 0.0098 |
| No | 85.37 | Reference | |
| Fetal distress | Yes | 82.15 | <.0001 |
| No | 85.79 | Ref | |
| Maternal hypertension | Yes | 81.13 | <.0001 |
| No | 86.87 | Reference | |
| Apgar score at 5 minutes | 3 or less | 87.05 | 0.0003 |
| 4 to 7 | 84.12 | <.0001 | |
| 8 and higher | 80.79 | Reference |
SGA – small for gestational age
Figure 1.
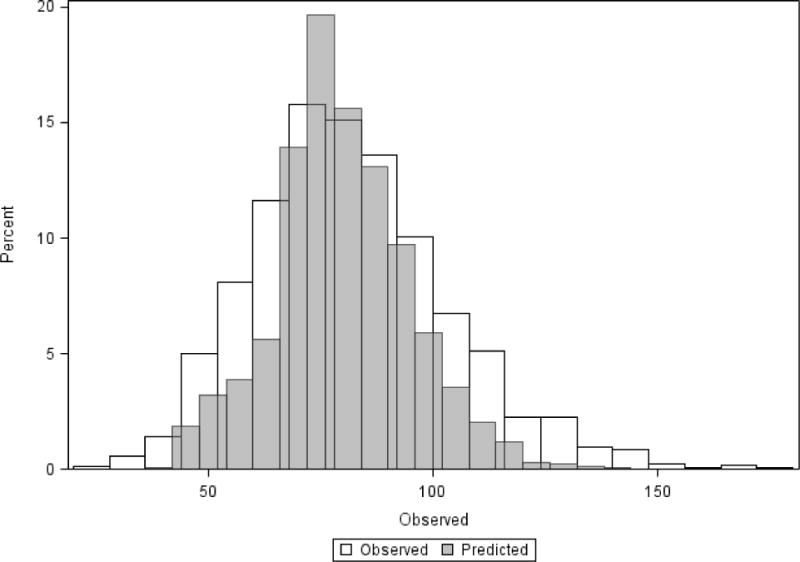
Distribution of crude and predicted length of stay for extremely low birth weight infants.
In order to assess the possibility of bias for NICUs that had higher mortality rates leading to shorter LOS, we investigated the relationship between risk-adjusted mortality to the mean risk-adjusted LOS in hospitals (Figure 2). The mean mortality rate at the NICU level was 15.3% with interquartile range: 8.7% to 22.4% (risk adjusted mortality rate mean 16.0%, interquartile range 9.9% to 23.5%). The regression fit between LOS and risk adjusted mortality rate was poor with a slope of 0.00351and R = 0.0207, indicating no relationship between mortality rate and length of stay by NICU.
Figure 2.
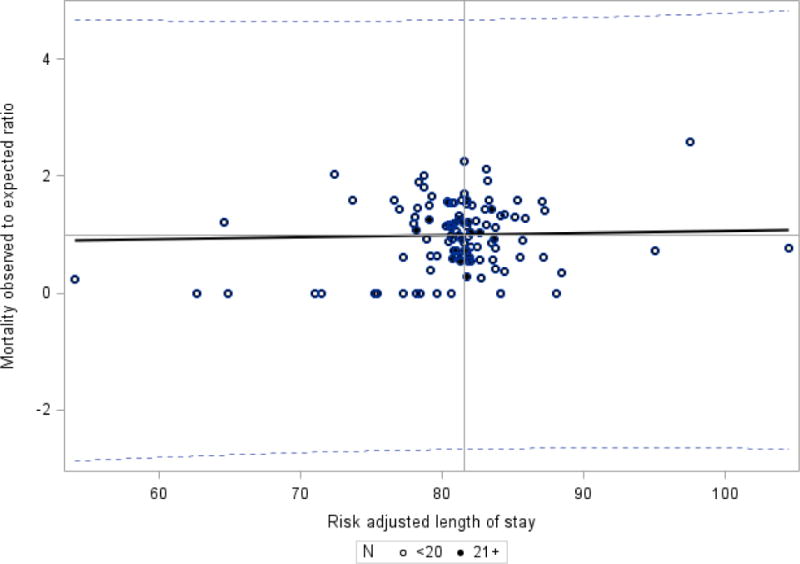
Mortality observed to expected ratio and risk adjusted length of stay – CPQCC Member Hospitals.
Weighted regression – each dot represents one hospital and is assigned a weight of number of eligible infants. Hospitals with greater than 20 eligible patients are noted with a dark circle; hospitals with 20 or less patients are noted with an open circle. Slope =0.00351, R = 0.0207, R2 =0.0004, p=0.829
As our study included only infants who were discharged home, another potential bias that we considered was that hospitals that transferred more infants to lower levels of care for feeding and growing may have higher LOS. The average transfer-out rate for growth and discharge planning was 7.5% overall for ELBW infants, with range between 0 to 31%. The mean adjusted LOS for NICUs with transfer out rate < 10% (n=96 NICUs) was 80.7 days (interquartile range 79.1 – 83.0) while the mean adjusted LOS for NICUs with transfer out rate greater than or equal to 10% (n=15 NICUs) was 82.5 days (interquartile range 80.4 – 83.5). Figure 3 displays the relationship between transfer-out rate and risk-adjusted LOS. The fitted regression line has a slope of 0.365 and R = 0.121. Overall, the impact of transfer-out rate on our risk-adjusted LOS appeared limited.
Figure 3.
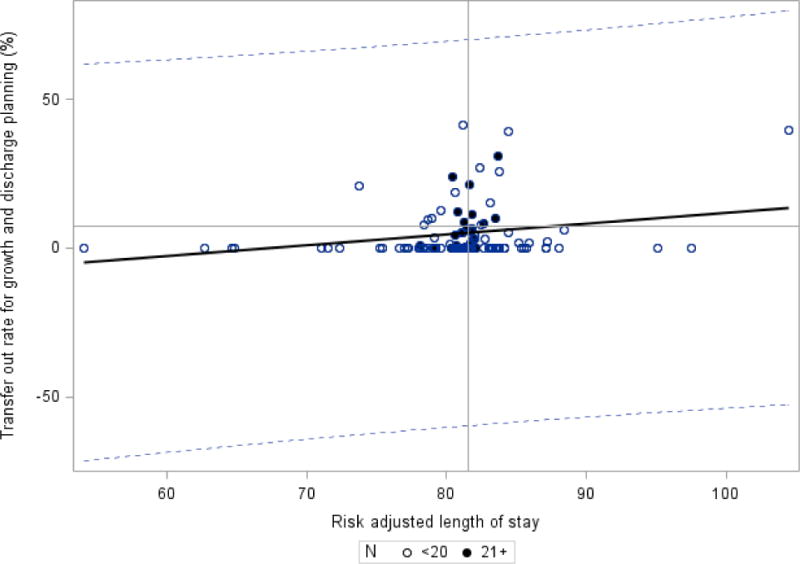
Rate of transfer-out and risk adjusted length of stay – CPQCC Member Hospitals.
Weighted regression – each dot represents one hospital and is assigned a weight of number of eligible infants. Hospitals with greater than 20 eligible patients are noted with a dark circle; hospitals with 20 or less patients are noted with an open circle. Slope =0.365, R = 0.121, R2 = 0.0146, p=0.207
Figure 4 shows the distribution of adjusted LOS by hospital. After risk adjustment, the mean NICU LOS among 27 NICUs with more than 20 eligible patientswas 81.3 days with an interquartile range of 80.7–81.8 days and a total range of 78.2–83.7 days. For the 84 centers with ≤ 20 eligible infants, the mean adjusted LOS was 81.8 days with a hospital interquartile range of 78.7–83.7 days and a total range of 54.0–104.5 days. If these smaller NICUs were considered a single unit, the observed to expected ratio would be 1.003. At the patient level, there were 91individual outliers identified of which 56 had observed LOS exceeding predicted.
Figure 4.
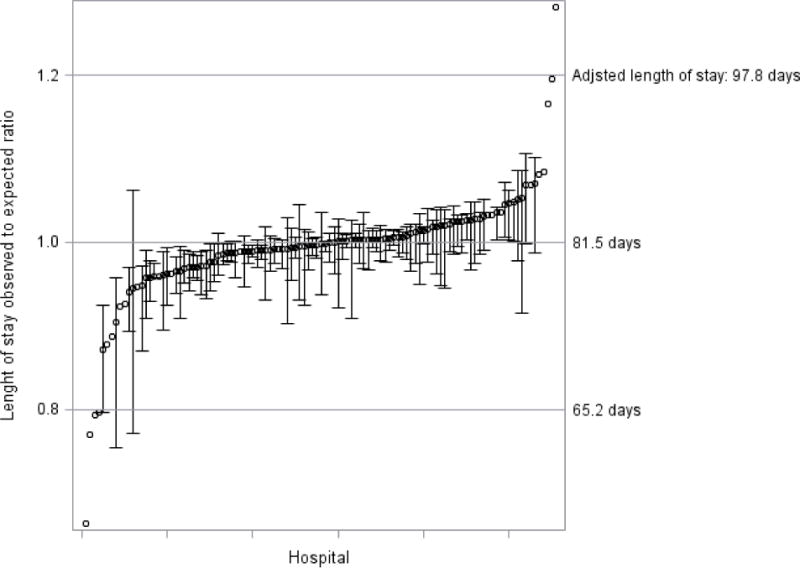
Hospital length of stay with 95% confidence intervals using linear mixed model with log scale.
Observed to expected ratio for all 111 centers are shown; no confidence interval is shown if the hospital cared for less than 5 eligible infants.
Discussion
Using a large population dataset, we developed a risk adjustment model for NICU LOS for ELBW infants, for estimating individual patient LOS, and for comparing NICU variation. The methodology employed in this study also forms a basis for further development of LOS models for other NICU sub-populations.
There was wide variance in LOS by birth weight, by gestational age, and other factors (Table e3). These differences should be noted as various stakeholders may consider LOS to be a benchmark of quality of care, highlighting the need for the development of optimal risk adjustment models.
Because of the non-normality of LOS, we tried several forms of transformation as well as different conditional distribution models to fit the data. Most models performed well, and had similar cross-validation results. The log-scale model was chosen as the primary model due to favorable model characteristics. The negative binomial model had similar characteristics and could be an alternative model choice.
We made certain exclusions in order to get a group of ELBW infants that would best represent the typical LOS of a patient in this NICU subgroup. First, we excluded deaths. As deaths can occur soon after birth and all the way up to several weeks or months of age, the “noise” in this group of patients would make assessment of NICU LOS difficult. However, by excluding deaths, there was the possibility that NICUs that perform poorly in survival could be perceived as having lower LOS, as their sickest patients would not be counted in the analysis. In a study by Cotten et al. examining infants born at or before 28 weeks gestational age, there was noted to be an inverse relationship of LOS and mortality, with centers having less infants with prolonged LOS more likely to have higher mortality.19 In contrast, across 111 NICUs that included regional, community, and intermediate NICUs, and caring for a few to many ELBW patients, we found less concern for this potential bias, as there was no correlation between LOS and risk-adjusted mortality. In the study by Cotten et al., the cohort included births during 1998 to 2001, and mortality rate was higher overall at 28.5% vs. 15.3% in our cohort. Although we have addressed one aspect of the question of how deaths are incorporated into analyses on LOS, this is a complex issue that will require ongoingstudy to develop optimal strategies for assessment, as well as study in other settings where the death to LOS relationship may differ.
On the other hand, NICUs that transfer many of their less acute patients to NICUs with lower level of care could falsely have an increased LOS, as their sickest patients would be over-represented in the analysis. Some centers may have a networkof several lower level NICUs at outlying hospitals for transferring convalescing infants, whereas other NICUs may not have that capacity. Within our heterogeneous group of NICUs, we did not find a significant relationship between risk-adjusted LOS and transfer-out rates for feeding and growing, alleviating this concern. However, as our analysis only concerned ELBW infants, these potential biases would need to be further tested for other NICU populations. Further work in evaluating models that include transferred patients, both transferred in to a higher level of care, as well as those transferred out to lower level of care may increase the potential to assess larger systems of care.
There may be several limitations to our analysis in regard to the data available for modeling. There may be some socio-demographic factors that were not accounted for in the dataset, such as family income. Although we accounted in part for severity of illness by including variables such as birth weight and Apgar score in the model, other measures obtained at time points such as admission to the NICU or in the early hospital course could potentially strengthen the model fit.9, 19 On the other hand, as antenatal and delivery room management could impact both severity of illness and length of stay, the ultimate purpose of the LOS assessment may guide inclusion of such variables in modeling.
In our random effects model, the hospital intercept was highly significant, indicating that there are opportunities for reducing the variation of ELBW LOS across NICUs. When the hospital effect was removed from the model, the explanatory power of the model (R2 = 0.45) was comparable to that reported in a previous study of NICU All Patient Diagnosis-Related Groups, a classification system for inpatient hospital care.20 As our model incorporated both clinically and statistically significant maternal and neonatal factors for risk adjustment, it may be useful as a basis for assessing and comparing NICU LOS for an individual center across time, as well as between centers.
Conclusion
LOS is an important issue for quality and cost of healthcare. We have developed a patient-level risk-adjusted model for assessing LOS for ELBW infants, which additionally accounts for clustering of outcomes by NICU (hospital). Our study also allows hospitals to assess their risk adjusted LOS and will give the ability for each NICU to identify infants whose LOS are extreme and could serve as a starting point for root cause analysis of excessive LOS. This work forms the basis for developing models for LOS for other NICU sub-populations. Further research will focus on optimal methods to compare hospitals in LOS for the purpose of quality improvement, public reporting, and optimizing payment structures. This work also forms the foundation for studying the relationship of LOS to short- and long-term clinical outcomes, and future research on how family resources and competency, NICU processes, and hospital factors such as level of care may influence risk-adjusted LOS.
Supplementary Material
* Discharged home with eligibility criteria (inborn or outborn admitted within 24 hours from birth)
** Transfer or discharge home cases where eligibility criteria were not met
Acknowledgments
The project described was supported by Grant Number K23HD068400 from the Eunice Kennedy Shriver National Institute of Child Health & Human Developmentand the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR000004. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
Abbreviations
- AIC
Aikake Information Criterion
- BIC
Bayesian Information Criterion
- CPQCC
California Perinatal Quality Care Collaborative
- LOS
length of stay
- NICU
neonatal intensive care unit
- RSME
root mean squared prediction error
- SGA
small for gestational age
- VIF
variance inflation factor
Footnotes
Financial disclosure: No financial relationships relevant to this study.
Conflicts of interest: None
Contributor’s statement
All above-listed authors meet the following criteria:
1) Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data.
2) Drafting the article or revising it critically for intellectual content; and
3) Final approval of the version to be published.
References
- 1.Tyson JE, Younes N, Verter J, Wright LL. Viability, morbidity, and resource use among newborns of 501- to 800-g birth weight. National Institute of Child Health and Human Development Neonatal Research Network. JAMA : the journal of the American Medical Association. 1996;276(20):1645–1651. [PubMed] [Google Scholar]
- 2.Economic outcome for intensive care of infants of birthweight 500–999 g born in Victoria in the post surfactant era. The Victorian Infant Collaborative Study Group. Journal of paediatrics and child health. 1997;33(3):202–208. [PubMed] [Google Scholar]
- 3.Manktelow B, Draper ES, Field C, Field D. Estimates of length of neonatal stay for very premature babies in the UK. Archives of disease in childhood Fetal and neonatal edition. 2010;95(4):F288–292. doi: 10.1136/adc.2009.168633. [DOI] [PubMed] [Google Scholar]
- 4.Altman M, Vanpee M, Cnattingius S, Norman M. Moderately preterm infants and determinants of length of hospital stay. Archives of disease in childhood Fetal and neonatal edition. 2009;94(6):F414–418. doi: 10.1136/adc.2008.153668. [DOI] [PubMed] [Google Scholar]
- 5.Miller RH, Luft HS. Managed care plan performance since 1980. A literature analysis. JAMA : the journal of the American Medical Association. 1994;271(19):1512–1519. [PubMed] [Google Scholar]
- 6.Chaix-Couturier C, Durand-Zaleski I, Jolly D, Durieux P. Effects of financial incentives on medical practice: results from a systematic review of the literature and methodological issues. International journal for quality in health care : journal of the International Society for Quality in Health Care/ISQua. 2000;12(2):133–142. doi: 10.1093/intqhc/12.2.133. [DOI] [PubMed] [Google Scholar]
- 7.Bannwart Dde C, Rebello CM, Sadeck LS, Pontes MD, Ramos JL, Leone CR. Prediction of length of hospital stay in neonatal units for very low birth weight infants. J Perinatol. 1999;19(2):92–96. doi: 10.1038/sj.jp.7200134. [DOI] [PubMed] [Google Scholar]
- 8.Berry MA, Shah PS, Brouillette RT, Hellmann J. Predictors of mortality and length of stay for neonates admitted to children’s hospital neonatal intensive care units. J Perinatol. 2008;28(4):297–302. doi: 10.1038/sj.jp.7211904. [DOI] [PubMed] [Google Scholar]
- 9.Hintz SR, Bann CM, Ambalavanan N, Cotten CM, Das A, Higgins RD. Predicting time to hospital discharge for extremely preterm infants. Pediatrics. 2010;125(1):e146–154. doi: 10.1542/peds.2009-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bender GJ, Koestler D, Ombao H, McCourt M, Alskinis B, Rubin LP, et al. Neonatal intensive care unit: predictive models for length of stay. Journal of perinatology : official journal of the California Perinatal Association. 2013;33(2):147–153. doi: 10.1038/jp.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HC, Gould JB. Survival rates and mode of delivery for vertex preterm neonates according to small- or appropriate-for-gestational-age status. Pediatrics. 2006;118(6):e1836–1844. doi: 10.1542/peds.2006-1327. [DOI] [PubMed] [Google Scholar]
- 12.Schulman J. Studying determinants of length of hospital stay. J Perinatol. 2006;26(4):243–245. doi: 10.1038/sj.jp.7211478. [DOI] [PubMed] [Google Scholar]
- 13.Rahman SMAK, Sathik MM, Senthamarai KK. Multiple linear regression models in outlier detection. International Journal of Research in Computer Science. 2012;2(2):23–28. [Google Scholar]
- 14.Thomas JW. Hospital cost efficiency measurement: Methodological approaches: Pacific Business Group on Health. 2006. [Google Scholar]
- 15.Jin R, Furnary AP, Fine SC, Blackstone EH, Grunkemeier GL. Using Society of Thoracic Surgeons risk models for risk-adjusting cardiac surgery results. The Annals of thoracic surgery. 2010;89(3):677–682. doi: 10.1016/j.athoracsur.2009.10.078. [DOI] [PubMed] [Google Scholar]
- 16.Rawlings JO, Pantula SG, Dickey DA. Applied Regression Analysis: A Research Tool. 2. Springer; New York: p. 2001. [Google Scholar]
- 17.Parry GJ, Gould CR, McCabe CJ, Tarnow-Mordi WO. Annual league tables of mortality in neonatal intensive care units: longitudinal study. International Neonatal Network and the Scottish Neonatal Consultants and Nurses Collaborative Study Group. BMJ. 1998;316(7149):1931–1935. doi: 10.1136/bmj.316.7149.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horbar JD, Soll RF, Edwards WH. The Vermont Oxford Network: a community of practice. Clinics in perinatology. 2010;37(1):29–47. doi: 10.1016/j.clp.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Cotten CM, Oh W, McDonald S, Carlo W, Fanaroff AA, Duara S, et al. Prolonged hospital stay for extremely premature infants: risk factors, center differences, and the impact of mortality on selecting a best-performing center. J Perinatol. 2005;25(10):650–655. doi: 10.1038/sj.jp.7211369. [DOI] [PubMed] [Google Scholar]
- 20.Muldoon JH. Structure and Performance of Different DRG Classification Systems for Neonatal Medicine. Pediatrics. 1999;103(Supplement E1):302–318. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
* Discharged home with eligibility criteria (inborn or outborn admitted within 24 hours from birth)
** Transfer or discharge home cases where eligibility criteria were not met


