Abstract
C-reactive protein (CRP) participates in the systemic response to inflammation. Previous studies report inconsistent findings regarding the relationship between plasma CRP and Alzheimer’s disease (AD). We measured plasma CRP in 203 subjects with AD, 58 subjects with mild cognitive impairment (MCI) and 117 normal aging subjects and administered annual mini-mental state examinations (MMSE) during a three year follow-up period to investigate CRP’s relationship with diagnosis and progression of cognitive decline. Adjusted for age, sex, and education, subjects with AD had significantly lower levels of plasma CRP than subjects with MCI and normal aging. However, there was no significant association between plasma CRP at baseline and subsequent cognitive decline as assessed by longitudinal changes in MMSE score. Our results support previous reports of reduced levels of plasma CRP in AD and indicate its potential utility as a biomarker for the diagnosis of AD.
Keywords: Alzheimer Disease, Mild Cognitive Impairment, C-Reactive Protein, Inflammation, Biological Markers
Introduction
Considerable evidence implicates inflammation in the pathophysiology of Alzheimer’s disease (AD). Studies of brain tissues from patients with AD consistently show evidence of inflammation, as indicated by the presence of activated microglia, activated complement factors, cytokines and other inflammatory proteins (1). Elevated levels of inflammatory proteins have also been found outside of the brain in patients with AD (2). C-reactive protein (CRP) is a member of the pentraxin family of proteins that participates in the systemic response to inflammation. It is transcriptionally regulated by IL-6 as well as by interleukin-1 beta (IL-1β) and measures of plasma CRP levels are used clinically as a biomarker of inflammation (3). Plasma CRP has been described to increase in diseases with chronic inflammation, including CNS diseases such as Creutzfeldt-Jakob disease (4). Both pro-inflammatory and anti-inflammatory effects of CRP have been described. Pro-inflammatory effects of CRP include agglutination, complement fixation, and scavenging material from damaged cells, whereas anti-inflammatory effects of CRP include inducing expression of interleukin-1 receptor antagonist (IL-1ra), an inhibitor of the pro-inflammatory cytokine interleukin-1 beta (IL-1β), and inducing expression of the anti-inflammatory cytokine interleukin-10 (IL-10) (5).
Previous studies reported inconsistent findings regarding the relationship between CRP and AD. Histopathological studies of AD brain tissue demonstrate widespread CRP immunoreactivity in areas with AD pathology (6). Mid-life elevations in plasma CRP have been reported as a risk factor for the development of AD in n the Honolulu-Asia Aging Study (1991–1996) (7). Paradoxically, however, recent cross-sectional studies by Nilsson et al. (8,9) and O’Bryant et al. (10) found that plasma CRP levels are reduced in patients with established AD. No clear explanation has been provided for why CRP might be low in established AD, despite the possible link between elevated mid-life CRP and the development of AD. Two other cross-sectional studies found no significant association between CRP and AD (2,11). In an unbiased multi-analyte discovery study measuring 189 proteins, peptides, and hormones using Luminex multi-analyte profiling, we recently identified low CRP as an AD biomarker candidate (12). The purpose of the present study was to validate this finding with a different assay platform and further investigate the association between plasma CRP and AD diagnosis and the prospective risk and rate of cognitive decline in a large community dwelling cohort.
Materials and Methods
University of Pennsylvania Alzheimer’s Disease Core Center (ADCC) research participants were recruited for plasma collection and longitudinal assessment of cognition. Subjects were eligible to participate if they were above the age of 50 and in good general health, without evidence of acute inflammatory conditions such as infections or malignancy. Written and verbal informed consent was obtained from all study participants at the time of enrollment and the study protocol was approved by the Institutional Review Board at the University of Pennsylvania. A subset of subjects included in our study cohort were previously studied in a multi-analyte biomarker discovery study conducted by our group (12). All subjects individually underwent cognitive and neurological examinations and were diagnosed at a consensus conference of neurologists, psychiatrists and geriatricians experienced in the evaluation of dementia. Only subjects belonging to one of the following three groups according to established criteria (13) were included in our analysis: normal aging, mild cognitive impairment (MCI), Alzheimer’s Disease (AD). We excluded subjects with other systemic or brain diseases that could account for a decline in cognition (e.g., medication induced, psychiatric, frontotemporal dementia, Lewy body dementia vascular, traumatic). All subjects were administered mini-mental state examinations (MMSE) at the beginning of the study and annually during a three year follow-up period. The MMSE is a 30-point questionnaire test providing a global assessment of cognitive function by evaluating orientation, registration, attention, recall, language, and constructional praxis.
Plasma was collected in 10 mL BD Vacutainer® K2EDTA tubes and centrifuged at 4°C into plasma and cellular components. Plasma was subsequently stored at −80°C in 1 mL polypropylene vial aliquots until the time of analysis. A commercial sandwich enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems Quantikine®) was used to determine CRP concentration following standard ELISA kit procedures. A 4-parameter logistic equation using the software program KC4® (Bio-Tek Instruments, Inc.) was used for the dose-response curve of this assay. The lower sensitivity limit of the assay was 0.01 ng/mL and our interassay coefficient of variation was 10.0%.
Statistics
CRP levels had a skewed distribution to the left and were therefore logarithmically transformed for all statistical analyses and are reported as log-transformed values. The associations of CRP with demographic and clinical confounder variables were examined using Pearson’s correlations for continuous variables and analysis of variance (ANOVA) for categorical variables. Potentially confounding variables were incorporated into subsequent regression analyses. Multinomial logistic regression analysis was used to determine the relationship between CRP levels and disease category membership, with adjustment for age, sex and education. Mixed-effects models (14) were used to test whether rate of decline on MMSE was predicted by baseline CRP level after controlling for age (centered at the mean), sex, and education. The mixed-effects model takes into account within-subject correlations from repeated measurements of MMSE scores in the same subjects and for missing data points. Our model specified the intercept and the regression coefficient for the follow-up time as random effects such that subjects have a unique intercept and slope characterizing their individual longitudinal trajectories. Population mean coefficient for the follow-up time was estimated by averaging across the subject-specific regression coefficients for the follow-up time. This population mean coefficient estimated the average annual change for the MMSE score over time. The interaction term “time x CRP baseline” represents the effect of the baseline CRP values on change in MMSE score over time. It can be interpreted as the annualized change in MMSE score for each one unit change in a given baseline CRP value. Chi-square, ANOVA, and logistic regression analyses were performed using JMP® 9.0 for Windows (SAS Institute Inc.). SAS software version 9.2 (SAS Institute Inc., Cary, North Carolina) was used for mixed-effects models. P-values <0.05 were considered significant and all statistical tests were two-sided.
Results
Of 450 ADCC participants who were initially enrolled in our study, 72 participants were excluded from analysis because of the presence of a systemic or brain disorder other than MCI or AD that could account for a decline in cognition. Of the remaining 378 participants, there were 203 subjects with AD, 58 with MCI, and 117 with normal cognitive aging. All subjects completed baseline cognitive testing, biofluid collection, and at least one year of annual follow-up clinical assessment. Baseline characteristics of the diagnostic groups are displayed in Table 1.
Table 1.
Baseline characteristics of participants
| Diagnostic Category | Normal | MCI | AD |
|---|---|---|---|
| Number | 117 | 58 | 203 |
| MMSE, baseline | 28.9 (1.5) | 25.7 (3.9) | 22.8 (4.5) |
| Male | 37 [32%] | 28 [48%] | 85 [42%] |
| Age | 69.9 (10.1) | 71.6 (8.4) | 74.5 (7.7) |
| Years of Education | 15.9 (3.5) | 12.7 (5.6) | 14 (4.0) |
| APOE ε4 | 30 (26%) | 25 (43%) | 125 (62%) |
Numbers in parentheses represent standard deviations.
Abbreviations: AD – Alzheimer’s Disease, MCI – Mild cognitive impairment
The average plasma CRP level for all patients in our study was 2.97 pg/ml (SD=0.43). To characterize the intra-individual stability across time and the reliability of plasma CRP as a biomarker, annual repeat plasma donations and CRP measurements were also obtained from willing participants. Two-hundred-twenty-two participants had at least one subsequent plasma donation and CRP measurement between baseline exam and the first year follow-up. The ratio of re-examination to baseline CRP measurement was 1.002 (Stdev 0.138), indicating that CRP tends to be stable within a given person over one year. There was no significant correlation between age and CRP (r=0.06, F[1,376]=1.25, p>0.05]. The average plasma CRP levels were higher for females, 3.02 (SD=0.41) compared to males, 2.90 (0.44) (t1,376=2.83, p<0.05). Additionally, years of education was modestly correlated with CRP levels (r=0.15, F[1, 375]=8.6, p<0.05).
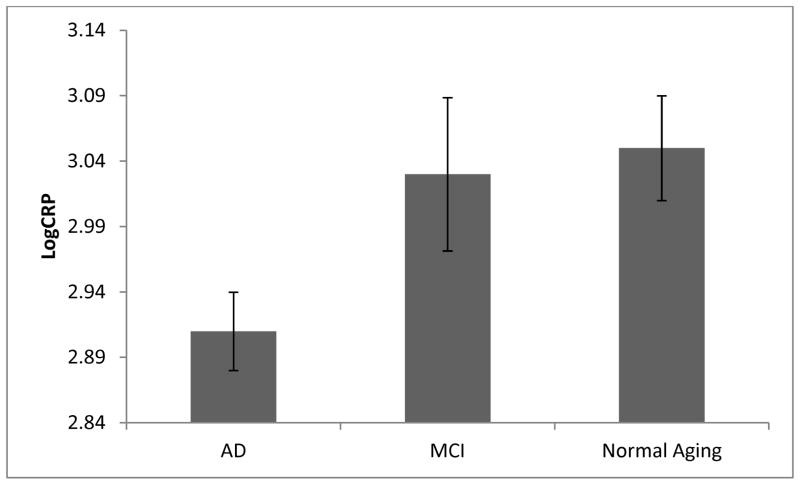
Baseline CRP levels differed significantly among the three diagnostic groups (F[2,375]=4.5, p<0.05). The AD group had the lowest average plasma CRP levels, whereas similar plasma CRP levels were observed in the MCI and normal aging groups. The mean CRP levels for participants with established AD was 2.91 pg/ml (SD=0.43), as compared to 3.03 pg/ml (0.45) and 3.05 pg/ml (0.40), for the MCI and normal aging groups, respectively (see figure 1). The differences in CRP levels between the diagnostic groups remained significant after adjusting these CRP values for age, sex, and education in linear regression analysis (F[5,371]=6.49, p<0.05). The differences in plasma CRP levels were further examined with individual group comparisons. Plasma CRP was significantly lower in the AD group compared to the MCI group (F[4,257]=2.2, p<0.05) and normal aging group (F[4,316]=3.6, p<0.05). Normal and MCI groups did not differ (F[4,171]=0.97, p>0.05).
Figure 1.
Cross sectional analysis demonstrating significantly lower plasma CRP levels in established AD as compared to normal or MCI
Error bars represent standard error of the mean. Abbreviations: AD – Alzheimer’s Disease, MCI – Mild cognitive impairment
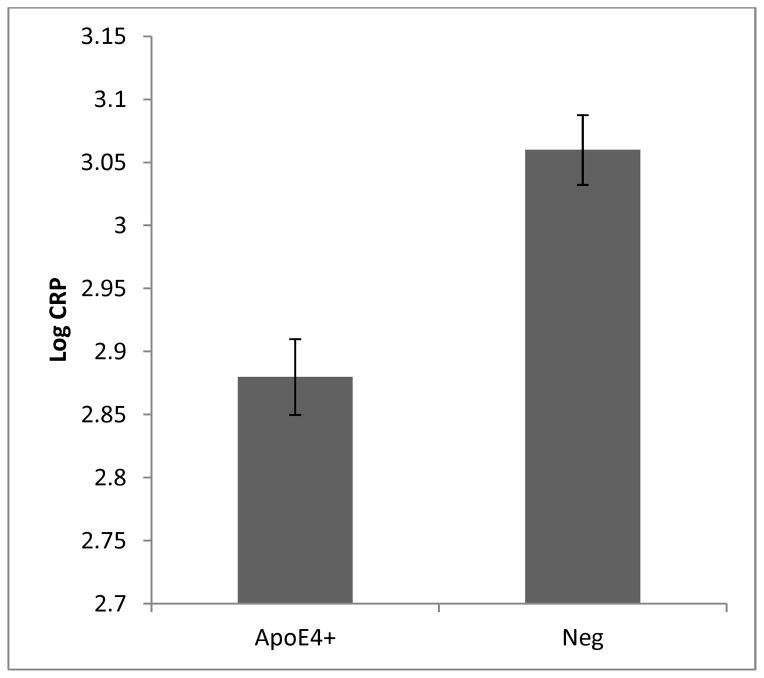
Apolipoprotein E4 (APOE ε4) has been linked previously to lower plasma CRP levels (15), and this genotype is also highly associated with AD (16). To determine if CRP levels are associated with AD independently of APOE ε4, the relationship between CRP and AD was evaluated separately in carriers and non-carriers of the APOE ε4 alleles. AD was more prevalent among carriers of the APOE ε4 genotype as expected (X2[2]=39.0, p<0.05) and CRP levels were significantly reduced among carriers of the APOE ε4 genotype (F[1,376]=14.2, p<0.05). Among APOE ε4 carriers (defined as one or more copies of the APOE ε4 allele, n=180), the mean plasma CRP levels were 2.89 (SD=0.46) compared to 3.05 (0.37) for non-carriers (n=198) (see figure 2). In the subset of APOE ε4 carriers, there were no significant differences in plasma CRP levels (adjusted for age, sex, and education) between the diagnostic groups (F[4,175] = 1.40, p>0.05). However, in the subset of APOE ε4 non-carriers, there were significant differences in adjusted plasma CRP levels between the three diagnostic groups (F[4,193]=3.1, p<0.05).
Figure 2.
Plasma CRP levels among carriers and non-carriers of the apolipoprotein E4 genotype
Error bars represent standard error of the mean.
All 378 participants underwent repeated cognitive and neurological examinations one year following baseline examination, and 227 (60%), and 133 (35%) participants also completed follow-up assessments at two and three years after baseline examination, respectively. The average duration of follow up for subjects in our sample was 2.0 years. For all subjects, the average annualized cognitive decline as assessed by the MMSE was a change in MMSE score of −1.5 (SD=2.28), which was largely due to changes in the MMSE scores in the AD subjects. Diagnosis of AD at study baseline (adjusted for age, sex, and education) was highly associated with subsequent cognitive decline (F[4,374]=51.4, p<0.05). The mean annualized MMSE change for subjects with a diagnosis of AD at study baseline was −2.43 (SD=2.68), as compared to −0.85 (1.23) and −0.18 (0.37) for subjects with a diagnosis of MCI and normal aging, respectively.
The relationship between plasma CRP and subsequent cognitive decline as assessed by longitudinal change in the MMSE was determined among all subjects using mixed-effects models. Table 2 displays the results of mixed-effects analyses. We found no significant association between reduced plasma CRP at baseline and prevalence and severity of subsequent cognitive decline among subjects with after adjusting for age, sex, and education (F[1,374]= 3.27, p>0.05). Similar results were obtained for subjects with normal aging, MCI, and AD, as well as APOE ε4 positive and negative subjects (data not shown). We further investigated whether low plasma CRP was a risk factor for “conversion” to AD among subjects with MCI at study baseline. In the MCI group (n=58), there were 13 subjects who converted to AD and 45 subjects who did not convert. There was no association between baseline plasma CRP and conversion from MCI to AD within this group (F[4, 53]=0.49, p>0.05).
Table 2.
Mixed-effects Model of longitudinal change in MMSE
| Fixed Effect | Estimate | Std Err | F Value | P Value |
|---|---|---|---|---|
| Intercept | 20.0464 | 1.7244 | ||
| CRP Baseline | 0.5854 | 0.3542 | 2.73 [1,374] | 0.0992 |
| Time | −3.0987 | 1.0404 | 8.87 [1,375] | 0.0031 |
| CRP baseline * Time | 0.6248 | 0.3457 | 3.27 [1,374] | 0.0715 |
| Age (years) | −0.07196 | 0.01658 | 18.85 [1,374] | <.0001 |
| Education (years) | 0.5698 | 0.03526 | 261.11 [1,374] | <.0001 |
| Sex (F) | 0.8481 | 0.3060 | 7.68 [1,374] | 0.0059 |
Discussion
In this cross-sectional and longitudinal evaluation of plasma CRP as a potential biomarker for AD, we observed a significant association between low plasma CRP values and the diagnosis of AD, even after adjusting for several potential confounds described above. These results are consistent with the previous studies by Nilsson et al. (8,9) and O’Bryant et al. (10). The association between low CRP and AD is not fully explained by the APOE ε4 genotype because we observe significant differences in plasma CRP by diagnosis among non-carriers of the APOE ε4 genotype. Since prior cross-sectional studies have consistently identified elevations in other peripheral inflammatory signals in established AD, including CRP’s transcriptional promoter, IL-6 (2,11), there may be a specific reduction in CRP in AD as opposed to an overall reduction in peripheral inflammatory signaling. Reduced CRP levels may represent a trait biomarker of the risk or presence of AD or possibly the state of activity of AD in the brain. The underlying cause of decreased plasma CRP in AD warrants further investigation.
Using mixed effects models’ testing, we found no significant association between baseline CRP and subsequent cognitive decline as assessed by longitudinal change in the MMSE. Since a diagnosis of AD at study baseline was highly associated with MMSE decline, and low CRP was highly associated with an AD diagnosis, it is surprising that there was no significant association between CRP and progression of cognitive decline. One explanation of this finding is that an association between low plasma CRP and cognitive decline was confounded by subjects with an inflammatory condition contributing independently to higher CRP and cognitive decline. Although subjects with inflammatory conditions or cognitive impairment other than AD or amnestic MCI were excluded from our analysis, cognitive decline was most likely multifactorial in many subjects. Future studies including more subjects with longer follow-up times and more sensitive measures of cognitive decline are warranted to further evaluate CRP as a biomarker for cognitive decline.
Strengths of this study include a robust and well-characterized clinical setting sample and prospective data on well documented clinical end points for a subset of our study sample. Although we controlled for multiple possible covariates, there are other potential confounders of the observed associations that were not controlled in our study. Another limitation of our study is that longitudinal plasma CRP levels were measured in only a subset of participants. However, we confirmed the intra-subject stability of spot CRP plasma levels as a biomarker by repeat CRP measurement in many subjects, and we found a strong correlation between baseline and subsequent CRP measures suggesting that spot CRP is a reliable marker. In conclusion, we observe that patients with established AD have significantly lower levels of plasma CRP than those with normal cognition or MCI, and however we find no significant association between plasma CRP levels and subsequent cognitive decline. If the described association between low plasma CRP and AD is validated in other studies of similar cohorts, we speculate that low CRP levels could become readily accessible and inexpensive peripheral biomarkers that could aid in AD diagnosis and may stimulate additional investigation of the role of CRP and inflammation in the pathophysiology of AD.
Acknowledgments
This study has been supported by NIH grant AG10124, the Penn-Pfizer Research Alliance at the University of Pennsylvania, the Marian S. Ware Alzheimer’s Program, and the Allen H. and Selma W. Berkman Charitable Trust. We thank Xiaoyan Han, M.S., for her help with the statistical programming of the mixed-effects model analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mark Yarchoan, Department of Internal Medicine, University of Pennsylvania, Philadelphia, PA.
Natalia Louneva, Department of Psychiatry, University of Pennsylvania, Philadelphia, PA.
Sharon X. Xie, Department of Biostatistics & Epidemiology, University of Pennsylvania, hiladelphia, PA
Frank J Swenson, Pfizer Diagnostics, PharmaTX Precision Medicine, Groton, CT.
William Hu, Emory Alzheimer’s Disease Research Center, Emory University, Atlanta, GA.
Holly Soares, Pfizer Global Research and Development, Groton, CT.
John Q. Trojanowski, Center for Neurodegenerative Disease Research, University of Pennsylvania, Philadelphia, PA
Virginia M.-Y. Lee, Center for Neurodegenerative Disease Research, University of Pennsylvania, Philadelphia, PA
Mitchel A. Kling, Behavioral Health Service, Philadelphia VA Medical Center, Philadelphia, PA
Leslie M. Shaw, Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA
Alice Chen-Plotkin, Department of Neurology, University of Pennsylvania, Philadelphia, PA.
David A. Wolk, Department of Neurology, University of Pennsylvania, Philadelphia, PA
Steven E. Arnold, Departments of Psychiatry and Neurology, University of Pennsylvania, Philadelphia, PA
References
- 1.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and Alzheimer’s disease. Neurobiology of Aging Elsevier. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, Van Swieten JC, et al. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Archives of neurology. 2004 May;61(5):668–72. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- 3.Black S, Kushner I, Samols D. C-reactive Protein. The Journal of biological chemistry. 2004 Nov 19;279(47):48487–90. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 4.Völkel D, Zimmermann K, Zerr I, Lindner T, Bodemer M, Poser S, et al. C-reactive protein and IL-6: new marker proteins for the diagnosis of CJD in plasma? Transfusion. 2001 Dec;41(12):1509–14. doi: 10.1046/j.1537-2995.2001.41121509.x. [DOI] [PubMed] [Google Scholar]
- 5.Kravitz MS, Pitashny M, Shoenfeld Y. Protective molecules--C-reactive protein (CRP), serum amyloid P (SAP), pentraxin3 (PTX3), mannose-binding lectin (MBL), and apolipoprotein A1 (Apo A1), and their autoantibodies: prevalence and clinical significance in autoimmunity. Journal of clinical immunology. 2005 Nov;25(6):582–91. doi: 10.1007/s10875-005-7828-2. [DOI] [PubMed] [Google Scholar]
- 6.Iwamoto N, Nishiyama E, Ohwada J, Arai H. Demonstration of CRP immunoreactivity in brains of Alzheimer’s disease: immunohistochemical study using formic acid pretreatment of tissue sections. Neuroscience letters. 1994 Aug 15;177(1–2):23–6. doi: 10.1016/0304-3940(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Annals of neurology. 2002 Aug;52(2):168–74. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson K, Gustafson L, Hultberg B. C-reactive protein: vascular risk marker in elderly patients with mental illness. Dementia and geriatric cognitive disorders. 2008 Jan;26(3):251–6. doi: 10.1159/000160957. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson K, Gustafson L, Hultberg B. C-reactive protein level is decreased in patients with Alzheimer’s disease and related to cognitive function and survival time. Clinical biochemistry. 2011 Oct;44(14–15):1205–8. doi: 10.1016/j.clinbiochem.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 10.O’Bryant SE, Waring SC, Hobson V, Hall JR, Moore CB, Bottiglieri T, et al. Decreased C-reactive protein levels in Alzheimer disease. Journal of geriatric psychiatry and neurology. 2010 Mar;23(1):49–53. doi: 10.1177/0891988709351832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Licastro F, Pedrini S, Caputo L, Annoni G, Davis LJ, Ferri C, et al. Increased plasma levels of interleukin-1, interleukin-6 and α-1-antichymotrypsin in patients with Alzheimer’s disease: peripheral inflammation or signals from the brain? Journal of Neuroimmunology. 2000 Feb;103(1):97–102. doi: 10.1016/s0165-5728(99)00226-x. [DOI] [PubMed] [Google Scholar]
- 12.Hu WT, Holtzman DM, Fagan AM, Shaw LM, Perrin R, Arnold SE, et al. Plasma multianalyte profiling in mild cognitive impairment and Alzheimer disease. Neurology. 2012 Aug 28;79(9):897–905. doi: 10.1212/WNL.0b013e318266fa70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work group under the auspices of department of health and human services task force on Alzheimer’s diesease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 14.Laird NM, Ware JH. Random-effects Models for Longitudinal Data. Biometrics. 1982;38(4):963–74. [PubMed] [Google Scholar]
- 15.März W, Scharnagl H, Hoffmann MM, Boehm BO, Winkelmann BR. The apolipoprotein E polymorphism is associated with circulating C-reactive protein (the Ludwigshafen risk and cardiovascular health study) European heart journal. 2004 Dec;25(23):2109–19. doi: 10.1016/j.ehj.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Berry-Kravis E, Arnold SE. Amyloid mediates the association of apolipoprotein E e4 allele to cognitive function in older people. Journal of neurology, neurosurgery, and psychiatry. 2005 Sep;76(9):1194–9. doi: 10.1136/jnnp.2004.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]