Abstract
Background
Anesthetics are widely used to induce unconsciousness, pain relief and immobility during surgery. It remains unclear whether the use of anesthetics has significant and long lasting effects on synapse development and plasticity in the brain. To address this question, we examined the formation and elimination of dendritic spines, postsynaptic sites of excitatory synapses, in the developing mouse cortex during and after anesthetics exposure.
Methods
Transgenic mice expressing yellow fluorescence protein in layer 5 pyramidal neurons were used in this study. Mice at 1 month of age underwent ketamine-xylazine and isoflurane anesthesia over a period of hours. The elimination and formation rates of dendritic spines and filopodia, the precursors of spines, were followed over hours to days in the primary somatosensory cortex using transcranial two-photon microscopy. 4–5 animals were examined under each experimental condition. Student's t-test and Mann-Whitney U-test were used to analyze the data.
Results
Administration of either ketamine-xylazine or isoflurane rapidly altered dendritic filopodial dynamics but had no significant effects on spine dynamics. Ketamine-xylazine increased filopodial formation while isoflurane decreased filopodial elimination during 4 hours of anesthesia. Both effects were transient and disappeared within a day after the animals woke up.
Conclusion
Our studies suggest that exposure to anesthetics transiently affects the dynamics of dendritic filopodia but has no significant effect on dendritic spine development and plasticity in the cortex of 1-month-old mice.
Introduction
General anesthetics are essential clinical tools to produce reversible loss of consciousness, block pain sensation, and prevent movement during surgery. While it is widely perceived that general anesthetics are safe, there is a growing concern about their long-lasting detrimental effects on brain structure and function, particularly in infant and juvenile populations.1-4 Studies in developing rodents and monkeys have found that exposure to anesthetics can result in widespread apoptotic neuronal degeneration and late cognitive impairment.5-10 While it is unclear whether the results of these animal studies can be extrapolated to humans, a recent population-based cohort study in children suggests that receiving multiple anesthetics may be a significant risk factor for later development of learning disabilities.11
General anesthetics have been shown to alter neuronal activity by affecting molecular targets including N-methyl-D-aspartate (NMDA) receptors, γ-aminobutyric acid type A receptors and K+ channels.12-14 Because neuronal activity plays a critical role in synaptogenesis,15-24 exposure to anesthetics may have a significant and long-lasting impact on neuronal connectivity in the developing brain and thereby contribute to learning and cognitive deficits later in life. Indeed, recent studies suggest that isoflurane exposure for two hours significantly reduces the synapse number in mouse hippocampus at postnatal day (PND) 5–7.25 In the mouse somatosensory cortex and hippocampus at PND 15 and 20, 5 hours of anesthesia with midazolam, propofol or ketamine causes a significant increase in the density of dendritic spines,26 which are the postsynaptic sites of the vast majority of excitatory axodendritic synapses in the brain.27,28 Furthermore, a substantial increase in dendritic spine density is observed in rat medial prefrontal cortex after exposure to isoflurane, sevoflurane or desflurane for 30–120 minutes at PND 16.29
Despite various effects of anesthetics on synaptogenesis in the rodent brain within the first 2–3 weeks after birth, little is known about the impact of anesthesia on synapse development in late postnatal life. In 1-month-old mice, dendritic spine remodeling in the cerebral cortex is substantially higher than that in adult mice (> 4 month old).30,31 A recent study on 1-month-old mice showed no change of dendritic spine density in different brain regions after 5-hour anesthesia with midazolam, propofol or ketamine.26 However, this study was performed with fixed brain preparations which only reveals the net change in spine number but does not provide information on the degree of spine formation and elimination. Thus, it remains unclear whether exposure to general anesthetics has any transient and/or long-lasting effects on dendritic spine development and plasticity in late postnatal life.
In this study, we used transcranial two-photon microscopy to examine individual dendritic spines of layer 5 pyramidal neurons in the somatosensory cortex of 1-month-old mice during and after exposure to two commonly used anesthetics: ketamine-xylazine (K-X) and isoflurane.31,32 We found that a 4-hour exposure to K-X or isoflurane altered the dynamics of dendritic filopodia, precursors of spines, but had no significant effects on dendritic spine dynamics. Furthermore, the effect of K-X or isoflurane on filopodia was transient and disappeared within a day after the animals woke up. These finding suggest that 4-hour exposure to K-X or isoflurane has no long-lasting effect on dendritic spine development in the mouse cortex during late postnatal life.
Materials and Methods
Experimental animals
Mice expressing yellow fluorescent protein in layer 5 pyramidal neurons (H-line)33 were purchased from the Jackson Laboratory (Bar Harbor, ME) and group-housed in the Skirball animal facilities. All experiments were done in accordance with institutional guidelines (NYU Medical Center Animal Care and Use Committee, New York, NY). 1-month-old animals were used for all the experiments.
Anesthesia Procedure
Animals were given an intraperitoneal injection (5.0 ml/kg body weight) of K-X mixture containing 17 mg/ml ketamine and 1.7 mg/ml xylazine in 0.9% sodium chloride solution. For continuous imaging with K-X, subcutaneous injections (2.5 ml/kg body weight) of this mixture were given to animals every 1.5 hours after the initial injection. For low dose K-X administration, the initial injection was given at the concentration of 2.5 ml/kg body weight. To determine the effect of the NMDA receptor antagonist MK801, MK801 (0.25 μg/g body weight) was injected into the peritoneum of awake mice right after the first imaging session. For isoflurane anesthesia, animals received 1.5% isoflurane through continuous oxygen flow for the induction of anesthesia and 1.0% isoflurane for the maintenance of anesthesia. During the experiment, a heating pad was used to maintain the animal's body temperature at ∼37°C. In a different group of animals, we measured arterial blood gases under spontaneous respiration after 3–4 h anesthesia with i-STAT system (Abbott Point of Care, Princeton, NJ). The analysis showed normal partial pressure of oxygen (K-X: 130 ± 4 mmHg; Isoflurane: 149 ± 2 mmHg), slight decreased pH (K-X: 7.27 ± 0.10; Isoflurane: 7.32 ± 0.07) and moderately elevated and normal partial pressure of carbon dioxide (K-X: 58.5 ± 23.7 mmHg; Isoflurane: 43.8 ± 8.5 mmHg) under both K-X and isoflurane anesthesia, respectively, which are consistent with previous studies.34,35
In vivo transcranial two-photon imaging
Surgical procedure for in vivo imaging
The surgical procedure for imaging anesthetized animals has been described previously32. During surgery mice were deeply anesthetized with K-X or isoflurane (see anesthesia procedure). The skull surface was exposed with a midline scalp incision and a small skull region (∼200 μm in diameter) was located over primary somatosensory cortex based on stereotaxic coordinates. A custom-made, stainless steel plate was glued (Ethyl cyanoacrylate) to the skull with a central opening over the cortical region of interest. In order to create a cranial window for imaging, a high-speed drill was used to carefully reduce the skull thickness by approximately 50% under a dissecting microscope. The skull was immersed in the artificial cerebrospinal fluid during drilling to avoid damage of the underlying cortex due to friction-induced heat. Skull thinning was completed by carefully scraping the cranial surface with a microsurgical blade to ∼20 μm in thickness. The entire surgical procedure usually took less than 30 minutes and the imaging took place immediately after the skull thinning. After imaging, the plate was gently detached from the skull, and the scalp was sutured with 6-0 silk.
To image awake animals, the surgical procedure as described above was performed one day before the imaging session, except that the steel plate was mounted on top of the skull with both cyanoacrylate glue and dental acrylic cement to ensure the tight bond between the skull and the plate. The animals were then returned to their own cages to recover and to avoid lingering effects of anesthetics before the imaging session started. After the mice woke up from the surgery, animals were habituated to the imaging set up for a few times to minimize potential stress-related changes of spines and filopodia.
In vivo imaging of dendrites
To reduce respiration-induced cranial movements during imaging, the steel plate on the animal's head was screwed to two metal bars that were located on both sides of the animal's head and fixed to a solid metal base. The entire animal was placed under either a Bio-Rad multiphoton microscope (Bio-Rad Laboratories, Hercules, CA) or a custom-made two-photon microscope. The Ti-sapphire laser was tuned to the optimal excitation wavelength for yellow fluorescent protein (920 nm) while using low laser power (< 40 mW at the sample) to minimize phototoxicity. The images were acquired using a 60X water-immersion objective at zoom of 1.0–3.0. A stack of image planes within a depth of 100 μm from the pial surface was collected, yielding a full three-dimensional data set of dendrites in the area of interest. The step size was 2 μm for the initial low magnification image (no zoom) for relocation at later time points and 0.75 μm for all the other experiments (3.0X zoom).
Data quantification
Data analysis was performed as described previously30,31,36. Consistent with previous studies, we found that a sustained trend of dendritic plasticity could be acquired from 4–5 animals with 150–200 dendritic protrusions (spines and filopodia) quantified in each animal18,30,31,36. The formation and elimination rates of spines/filopodia were measured as the number of spines/filopodia formed or eliminated divided by the number of spines/filopodia existed in the initial image. To determine formation and elimination of dendritic protrusions over time, the same dendritic segments were identified from three-dimensional image stacks with high image quality (signal: background noise ratio > 4) taken from both time points. The number and location of dendritic protrusions (protrusion length > 1/3 dendritic shaft diameter) were identified in each view. Filopodia were identified as long, thin structures without enlarged heads (generally twice longer than the average spine length, head: neck diameter ratio < 1.2, length: neck diameter > 3). The rest of the protrusions were classified as spines. Spines or filopodia were considered the same (“stable”) between two views based on their spatial relationship to adjacent landmarks and their relative position to immediately adjacent spines. Spines or filopodia in the second view were considered different if they were more than 0.7 μm away from their expected positions based on the first view.
Statistics
All data were presented as mean ± S.D. SigmaPlot (Systat Software Inc, Chicago, IL) was used to conduct the statistical analysis. Tests for differences between populations were performed using two tailed student's t-tests with n being the number of animals. Significant levels were set at P ≤ 0.05. Use of Mann-Whitney U-test also confirmed all the conclusions.
Results
To study the effect of general anesthetics on synapse development, we measured the formation and elimination rates of dendritic spines in the primary somatosensory cortex of 1-month-old mice with or without K-X anesthesia (fig. 1A-D). In awake, control mice that had received K-X the day before imaging but none during the imaging session, we found that the rates of newly formed and eliminated dendritic spines were 1.0 ± 0.6% (mean ± S.D.) and 0.8 ± 0.7% over 1 h, respectively, and 1.5 ± 0.6% and 1.3 ± 1.1% over 4 h. K-X anesthesia for 1 h or 4 h had no significant effect on the formation and elimination of dendritic spines as compared with the controls (1 h K-X: 0.9 ± 0.3% formed, P > 0.6; 0.5 ± 0.9% eliminated, P > 0.6; 4 h K-X: 1.9 ± 0.7% formed, P > 0.4; 1.0 ± 1.1% eliminated, P > 0.7) (fig. 1A-D). In addition, the rate of spine formation was comparable to the rate of spine elimination over 1 or 4 h in mice with or without K-X anesthesia (P > 0.2). Together, these results suggest that exposure to K-X for 1–4 h has no significant effect on the dynamics or density of dendritic spines.
Fig. 1.
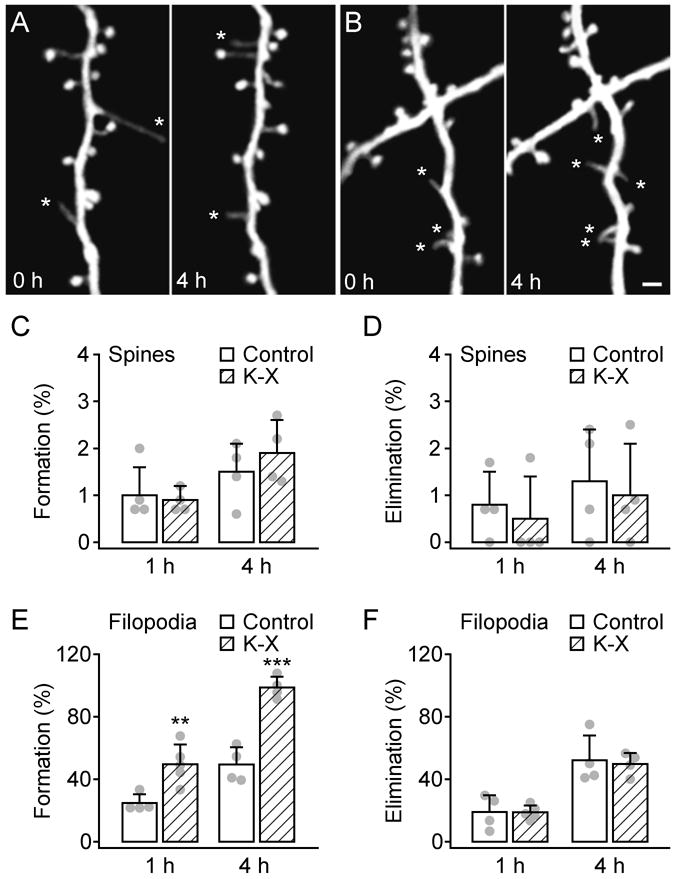
Administration of ketamine-xylazine rapidly increased the formation of dendritic filopodia but not spines over hours. A-B, In vivo time-lapse imaging of same dendritic segments over 4 h in the primary somatosensory cortex of 1-month-old animals that received no anesthesia (A) or ketamine-xylazine (K-X) anesthesia (B). Most dendritic spines on the same dendritic branches remained stable over 4 h whereas filopodia (asterisks) underwent rapid turnover. Scale bar, 2 μm. C-D, Percentage of newly formed (C) and eliminated (D) dendritic spines over 1 and 4 h. Administration of K-X did not alter spine dynamics. E-F, Percentage of newly formed (E) and eliminated (F) dendritic filopodia over 1 and 4 h. K-X anesthesia led to a rapid increase of filopodial formation but had no effect on filopodial elimination. Percentages were calculated as the number of spines/filopodia formed or eliminated divided by the number of pre-existing spines/filopodia. Each filled circle represents a single animal. Data are presented as mean ± S.D. ** P < 0.01; *** P < 0.001.
Dendrites in the developing cortex contain not only dendritic spines but also filopodia, which are long, thin protrusions lacking a bulbous head.37 Previous studies have shown that in 1-month-old mice, ∼15% of total dendritic protrusions are filopodia in the primary visual and somatosensory cortex.17,18,30,31 Furthermore, while spines persist over weeks to months, filopodia are highly dynamic and undergo rapid turnover within hours.30,38,39 In agreement with these studies, we found that in control mice that did not receive anesthesia, the formation and elimination rates of dendritic filopodia were high: 24.7 ± 5.7% and 19.0 ± 10.7% over 1 h, 49.3 ± 11.1% and 52.1 ± 15.8% over 4 h (fig. 1E-F). Notably, the rate of filopodial formation over 1 h was significantly higher in mice with K-X anesthesia (49.6 ± 12.6%) compared with mice without anesthesia (P < 0.01; fig. 1E). K-X anesthesia for 4 h further increased the formation of filopodia (98.6 ± 7.0%) as compared with the no-anesthesia control (P < 0.001; fig. 1E). On the other hand, there was no significant difference in the rate of filopodial elimination over 1 or 4 h between K-X anesthetized and non-anesthetized animals (P > 0.7; fig. 1F). We also found that the effect of K-X on filopodial formation was dose dependent: a lower dose (2.5 ml/kg initial injection) of K-X resulted in a lower formation rate of filopodia over 4 h (77.2 ± 18.7%, P < 0.05). Thus, while exposure to K-X has no significant effect on spine dynamics or density, it causes a significant increase in the formation rate of dendritic filopodia.
To determine whether K-X has long lasting effects on dendritic spines and filopodia, we imaged the same dendritic branches eight hours after the animals recovered from 4 h K-X anesthesia (fig. 2A). The animals appeared awake and reacted to visual and auditory stimuli between the 4 h and 12 h time points. We found no significant difference in spine formation (3.7 ± 1.3% versus 3.4 ± 0.5%, P > 0.5) and elimination (3.0 ± 0.8% versus 2.4 ± 0.5%, P > 0.2) over 12 h between animals with and without K-X anesthesia (fig. 2B-C). Moreover, the formation (77.2 ± 9.2% versus 71.3 ± 6.8%, P > 0.2) and elimination (78.5 ± 12.0% versus 73.8 ± 5.5%, P > 0.4) rates of filopodia over 12 h in anesthetized mice were comparable to those of non-anesthetized control (fig. 2D-E). These results suggest that 4 h K-X anesthesia has no significant long-lasting effect on spine and filopodial dynamics.
Fig. 2.
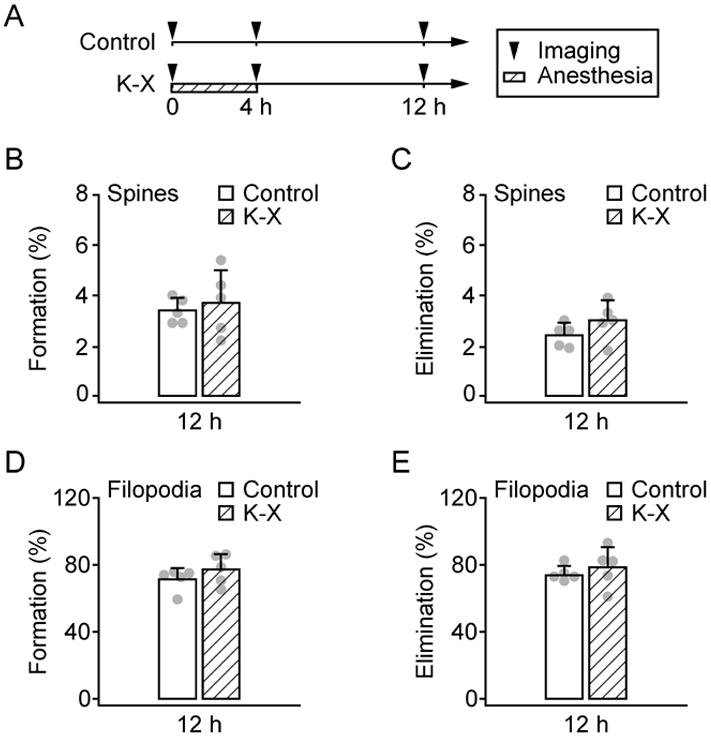
Ketamine-xylazine has no long lasting effects on the formation and elimination rates of dendritic spines and filopodia. A, Animals were under Ketamine-xylazine (K-X) anesthesia for the first 4 h and recovered for the next 8 h. B-C, Percentage of newly formed (B) and eliminated (C) dendritic spines over 12 h. D-E, Percentage of newly formed (D) and eliminated (E) dendritic filopodia over 12 h. There was no significant difference in spine or filopodial formation and elimination over 12 h between animals with and without K-X anesthesia. Percentages were calculated as the number of spines/filopodia formed or eliminated divided by the number of pre-existing spines/filopodia. Each filled circle represents a single animal. Data are presented as mean ± S.D.
Previous studies have suggested that filopodia serve as the precursors of spines.22,30,40-42 To investigate the effect of K-X further, we identified new filopodia formed during the 4 h K-X anesthesia and examined the fate of these filopodia over next eight hours (fig. 3A). For filopodia formed within the 4 h K-X anesthesia, the majority of them (73.8 ± 4.8%) were eliminated over the next 8 h when the animals woke up. This elimination rate was comparable to that of filopodia formed without anesthesia (79.3 ± 5.7%). A small fraction of filopodia persisted over the next 8 h in mice with (16.7 ± 8.0%) or without (14.5 ± 7.1%) K-X anesthesia. Notably, 9.5 ± 3.7% of new filopodia formed during 4 h K-X anesthesia were transformed into spines 8 h later. This percentage of transformation from filopodia to spines was not significantly different from that of non-anesthetized animals (6.2 ± 4.1%, P > 0.2; fig. 3A). Because more filopodia were formed during the 4 h K-X anesthesia, there were ∼0.9% (fraction of total spines) more new spines transformed from filopodia in K-X anesthetized animals at the 12 h time point as compared with no K-X control mice. Thus, new filopodia formed during the 4 h K-X anesthesia are largely eliminated and result in only a slight increase (< 1%) in new spines 8 h later.
Fig. 3.
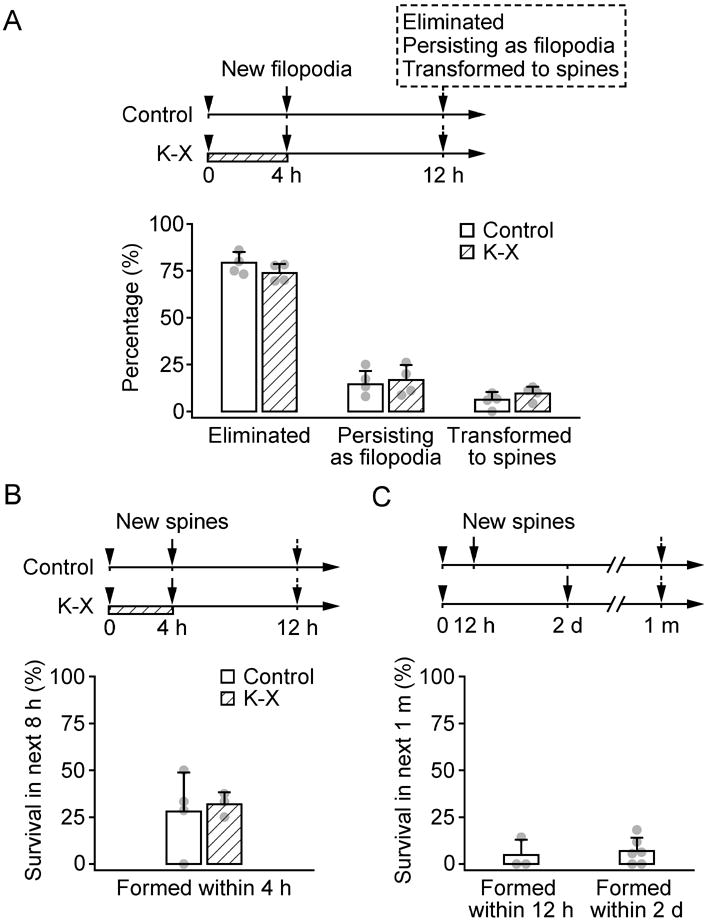
The majority of newly formed filopodia and spines do not persist. A, The percentage of new filopodia formed over the first 4 h that were eliminated, that persisted as filopodia, or that were transformed to spines over the next 8 h. The majority of filopodia were eliminated, a small percentage persisted, and less than 10% of filopodia were transformed to spines. There was no significant difference between filopodia formed with and without Ketamine-xylazine (K-X). B, Percentage of new spines persisting for 8 h. Less than half of new spines formed within the first 4 h persisted for the next 8 h. There was no significant difference between spines formed with and without K-X. C, Percentage of new spines persisting for 1 month. Less than 7% of new spines formed within 12 h or 2 days persisted over 1 month. Each filled circle represents a single animal. Data are presented as mean ± S.D.
It is important to note that new spines transformed from filopodia are largely unstable. In fact, more than half of new spines that were transformed from filopodia within 4 h were eliminated in the next 8 h, regardless of whether they were formed while awake or anesthetized (fig. 3B). Furthermore, when the fate of new spines formed over hours to days were followed over a period of one month, only 4.8 ± 8.2% of spines formed within 12 hours and 7.0 ± 7.0% of spines formed within 2 days persisted 1 month later (fig. 3C). These results are consistent with previous findings showing that the vast majority of new spines are eliminated over subsequent weeks to months18. Taken together, these findings suggest that 4 h K-X anesthesia has little or no effects on the formation of new dendritic spines over long periods of time.
Previous studies have shown that ketamine is an antagonist of NMDA receptors and produces unconsciousness with analgesia.43 Xylazine is an agonist of α2-adrenergic receptors and serves as an adjunct to ketamine anesthesia with sedative and muscle relaxant activities.44 To test whether the increased rate of filopodial formation during K-X anesthesia could be due to NMDA receptor blockade, we administered an intraperitoneal injection of MK801 (0.25 μg/g), another NMDA receptor antagonist, in awake animals. We observed that MK801 injection caused a high rate of filopodial formation (115.5 ± 7.5% versus 49.3 ± 11.1%; fig 4A) comparable to that in animals anesthetized with K-X for 4 h (fig. 1E). Over 12 h, the formation rate of filopodia was comparable between MK801 and saline injected control animals (76.4 ± 9.5% versus 71.3 ± 6.8%, P > 0.4; fig. 4A), suggesting the effect of MK801 on filopodial formation is transient. The elimination rates of filopodia over 4 h (47.1 ± 12.9% versus 52.1 ± 15.8%, P > 0.6) and 12 h (68.6 ± 8.5% versus 73.8 ± 5.5%, P > 0.3) were unaffected by MK801 administration (fig. 4B). Together, these results suggest that the transient effect of 4 h K-X anesthesia on filopodial dynamics is likely mediated by NMDA receptor blockade.
Fig. 4.
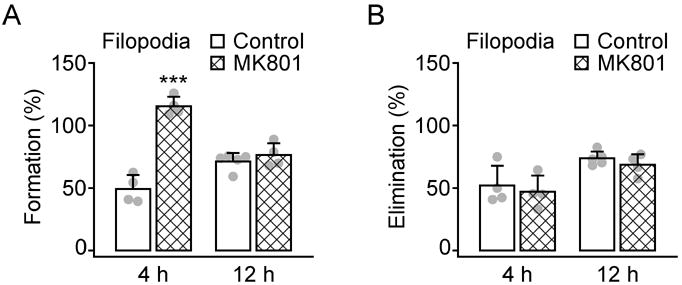
Systemic administration of MK801 mimics Ketamine-xylazine induced filopodial formation. A, Percentage of newly formed dendritic filopodia over 4 and 12 h. Animals were injected with MK801 after the first imaging session and re-imaged 4 and 12 h later. MK801 injection caused a rapid increase of filopodial formation over 4 but not 12 h. B, Percentage of eliminated dendritic filopodia over 4 and 12 h. MK801 had no significant effects on filopodial elimination. Percentages were calculated as the number of filopodia formed or eliminated divided by the number of pre-existing filopodia. Each filled circle represents a single animal. Data are presented as mean ± S.D. ***P < 0.001.
To further investigate the effect of anesthetics on synapse development, we examined the dynamics of dendritic spines and filopodia after the animals were exposed to isoflurane anesthesia (fig. 5). Similar to K-X, we found that isoflurane had no significant effect on the formation (0.9 ± 0.4% versus 1.5 ± 0.6%, P > 0.2) and elimination (0.8 ± 0.8% versus 1.3 ± 1.1%, P > 0.5) of dendritic spines over 4 h (fig. 5A-C). Interestingly, the rate of filopodia elimination was significantly lower in isoflurane anesthetized mice than in non-anesthetized control mice (18.7 ± 3.4% versus 52.1 ± 15.8%, P < 0.05; fig. 5E). The rate of filopodial formation over 4 h in isoflurane anesthetized mice was also lower than that in control mice, although not statistically significant (32.2 ± 10.1% versus 49.3 ± 11.1%, P = 0.1; fig. 5D). After the animals woke up for 8 h, there was no difference in filopodial elimination between animals with and without isoflurane (70.8 ± 4.5% versus 73.8 ± 5.5%, P > 0.4), suggesting that similar to K-X, isoflurane also has a transient effect on filopodial dynamics.
Fig. 5.
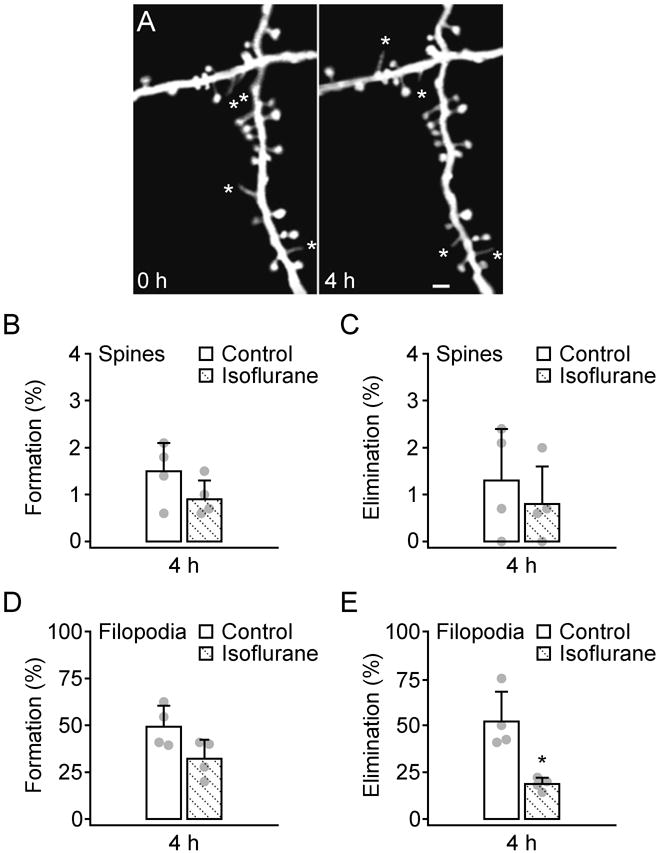
Administration of isoflurane affects the dynamics of dendritic filopodia but not spines. A, In vivo time-lapse imaging of the same dendritic segments over 4 h in 1-month-old, isoflurane anesthetized animals. Most dendritic spines remained stable over 4 h whereas filopodia (asterisks) underwent rapid turnover. Scale bar, 2 μm. B-C, Percentage of newly formed (B) and eliminated (C) dendritic spines over 4 h. Administration of isoflurane did not alter spine formation and elimination during this time period. D-E, Percentage of newly formed (D) and eliminated (E) dendritic filopodia over 4 h. Isoflurane anesthesia decreased the elimination of filopodia but had no significant effect on the formation of filopodia over 4 h. Percentages were calculated as the number of spines/filopodia formed or eliminated divided by the number of pre-existing spines/filopodia. Each filled circle represents a single animal. Data are presented as mean ± S.D. * P < 0.05.
Discussion
There is increasing evidence that anesthetics induce changes in the developing brain3,4. However, the extent of such changes either as a function of the anesthetic, the developmental age of an animal, or the length of the exposure has not been well established. Delineation of these relationships is essential to the development of clinically relevant strategies which would minimize the effect of anesthetic exposure in neonates and during early childhood.
In the present study, we used in vivo two-photon microscopy to examine whether exposure to general anesthetics has long lasting effects on the development and plasticity of dendritic spines in the primary somatosensory cortex of mice at one month of age. This intravital imaging approach allows monitoring of the same dendritic spines over extended periods of time in the living mouse cortex, and therefore provides a powerful tool to determine the extent of anesthetic-induced structural changes in neural circuits. By comparing dendritic spine plasticity in mice with or without anesthesia, we have found that exposure to K-X and isoflurane for 4 h has no significant effect on dendritic spine formation and elimination but transiently alters the dynamics of dendritic filopodia, the precursors of dendritic spines. The effect of both anesthetics on filopodial dynamics is transient such that the formation and elimination rates of filopodia return to the control level 8 h after animals recover from anesthesia. Furthermore, there is no significant difference in spine formation and elimination over 12 h between mice with and without 4 h anesthesia. Together, our results suggest that exposure to general anesthetics for 4 h has no significant long-lasting effect on synaptic connectivity in the mouse cortex during late postnatal development.
Our studies show that, during late postnatal development (1 month of age), K-X anesthesia leads to transient increase of dendritic filopodia over 4 h and a slight increase (< 1%) of new spines over the next 8 h. It might be argued that this small increase in new spines could have important functional consequences on the development of neural circuits. However, recent in vivo two photon imaging studies have shown that in 1-month-old mice, ∼6–7% of spines were eliminated and formed over a two-day interval in barrel and primary motor cortices under normal conditions.18 Sensory enrichment and motor skill learning led to a further 5–7% increase in new spine formation over 1–2 days in barrel and motor cortex respectively.18 Thus, the population of new spines associated with K-X exposure is much smaller than the population of new spines formed over 1–2 days under normal and enriched environments, suggesting that 4 h exposure to K-X has less impact on the development of neuronal connections than daily sensory or motor experience. Furthermore, consistent with previous studies,18 the vast majority of new spines formed over days were eliminated over subsequent weeks and months (fig. 3). Taken together, our results suggest that long-lasting effects of anesthesia on synaptic connections are negligible in 1-month-old mice.
Recent studies from fixed brain preparation have shown that exposure to ketamine45 and isoflurane25 decreases synapse or spine density in hippocampus of neonatal rodents at PND 5–13. 5 h exposure to ketamine26 caused a significant increase in dendritic spine density in the somatosensory cortex of PND 15 and 20, but not PND 30 mice and 2 h exposure to isoflurane29 increased the spine density in the prefrontal cortex of PND 16 rats. These findings suggest that general anesthesia has a significant effect on the number of dendritic spines during early, but not late postnatal development. However, because these studies were based on single-time-point observations, it is not known whether K-X or isoflurane may have significant impact on the rate of dendritic spine formation and elimination without affecting the net number of spines in late postnatal life. Using transcranial two-photon imaging to follow spine dynamics in the living mouse cortex, we found that K-X and isoflurane have only transient effects on dendritic filopodial dynamics without significant long-lasting effect on the dynamics and density of spines in the primary somatosensory cortex of 1-month-old mice. It is important to note that previous studies did not follow the fate of dendritic spines and filopodia associated with anesthesia in the 2–3 week old animals.26,29 Therefore, it remains to be determined whether exposure to anesthetics in early postnatal development also has a transient, but not long-lasting, effect on the plasticity of dendritic filopodia and spines.
The mechanisms underlying K-X and isoflurane effects on dendritic filopodia remain unclear. Anesthetics alter neural activity and metabolism,46 and have variable effects on blood pressure and cardiac output.34,35,47-49 It has been shown in both rats47 and mice35 that K-X decreases arterial pH, increases partial arterial pressure of carbon dioxide and decreases partial arterial pressure of oxygen. It is possible that the changes of neuronal activity and various physiological parameters all contribute to the alteration of dendritic filopodial dynamics in the brain. A wide variety of experimental evidence has shown that neuronal activity/experience plays a vital role in regulating synaptogenesis in developing neural circuits.16,17,20,21,23 It has been shown that ketamine inhibits glutamatergic signaling via blockade of NMDA receptors. Isoflurane also affects glutamatergic transmission by blocking release of glutamate50,51, in addition to enhancing γ-aminobutyric acid and glycine receptor transimission.13,14 Thus, K-X and isoflurane likely modulate neuronal activity through different mechanisms and this differential modulation of neuronal activity may affect dendritic filopodial dynamics differently. Consistent with this notion, our studies showed that over a 4 h anesthesia interval, K-X preferentially increased the rate of filopodial formation whereas isoflurane mainly decreased the rate of filopodial elimination. MK801, an antagonist of NMDA receptors, produced a similar effect on filopodial dynamics as K-X, suggesting that the effect of K-X is due, at least in part, to blockade of NMDA receptor activity.
To our knowledge, our studies are the first to evaluate the effect of general anesthetics on the dynamics of synaptic structures in the living animals using transcranial two-photon microscopy. Despite the advantage of being able to repetitively image the neuronal structures in the intact cortex of live animals, it is important to mention some potential limitations of using in vivo two photon microscopy to study the effect of anesthetics on synapse development and plasticity and the strategies to overcome such limitations. First, prior surgery and related anesthesia is unavoidable during the animal preparation, and the awake, head restraining situation may induce extra stress and cause changes in spine dynamics. It is important to minimize the potential effect of prior anesthesia by performing awake animal imaging at least one day after anesthesia exposure and surgical preparation. It is also desirable to habituate animals to the imaging setup a few times on the day before imaging to minimize stress. Second, surgery may induce an inflammatory response and contribute to the alteration of spine and filopodial dynamics. While skull thinning is a minor and noninvasive surgery, extra care must be taken such that the skull is not over thinned for imaging. Our previous study has demonstrated that carefully performed skull thinning does not induce the activation of microglia, the innate immune cells in the brain.36 Finally, it is important to point out that our study focused on the formation and elimination of dendritic spines of layer 5 pyramidal cells in the superficial layer of mouse somatosensory cortex. Future studies of the effect of general anesthetics on other cell types and brain regions will be needed in order to obtain a more comprehensive understanding of the effects of anesthetics on brain structure and function.
Our findings show that exposure to general anesthetics such as K-X and isoflurane has transient effects on filopodial (spine precursors) dynamics but does not affect dendritic spine plasticity in 1-month-old mice. Because these effects rapidly disappeared upon recovery, 4 h exposure to K-X or isoflurane does not appear to have a long-lasting detrimental impact on synaptic connections in adolescent rodents. The relevance of these findings to clinical anesthesia in infant and juvenile patients remains unclear, largely due to the substantial differences in neurodevelopmental time courses between rodents and humans. Although it is difficult to extrapolate the developmental stage of mouse brains to that of human brains, the time line of synaptogenesis may offer a hint. In the cerebral cortex of mammals, including that of rodents and humans, rapid synaptogenesis during early postnatal life is followed by an up-to-50% loss of synapses that extends through late postnatal development.52-56 In mouse somatosensory cortex, for example, the synapse density peaks around 2 weeks of age and decreases to adult level at ∼2 months of age18,30. In human visual cortex, the rapid synapse production ends at about postnatal age 8 months and the subsequent synapse elimination extends at ∼3 years of age.56 Assuming K-X and isoflurane have similar effects on synapse plasticity during the period of synapse pruning in both rodents and humans, our results would suggest that exposure to anesthetics for hours is likely safe for pediatric patients after toddler stage.
MS #201012148 – Final Boxed Summary Statement.
What we already know about this topic
Animal studies suggest that exposure of the developing brain to general anesthetics can have persistent effects on neurological function, but whether anesthetics produce long-lasting effects on synapse structure is unclear
What this article tells us that is new
Exposure of late postnatal mice to ketamine/xylazine or isoflurane for 4 h altered dynamics of dendritic filopodia, precursors of spines, but not of dendritic spines
Permanent changes in spine structure were not observed following anesthesia in juvenile mice
Acknowledgments
Supported by National Institutes of Health grant, Bethesda, MD (R01 NS047325; to Dr. Gan); Alzheimer's Association, Chicago, IL (The Investigator-Initiated Research Grant; to Dr. Gan); and Department of Anesthesiology, New York University Medical Center, New York, NY.
The authors thank Drs. Yong-sheng Li (M.D., Assistant Professor, Department of Neurology, New York University Medical Center, New York, NY) and Michael Haile (M.D. Assistant Professor, Department of Anesthesiology, New York University Medical Center, New York, NY) for their help on the blood gas measurement and Dr. Conor Liston (M.D., Ph.D., DeWitt Wallace Research Fellow, Department of Psychiatry, Weill Cornell Medical College, New York, NY) for his critical comments on the manuscript.
Footnotes
The work is attributed to Department of Anesthesiology and Skirball Institute, New York University Medical Center, New York, NY.
Summary Statement: Exposure to general anesthetics has no significant effect on dendritic spine dynamics in the living mouse cortex but transiently alters the dynamics of dendritic filopodia, spine precursors.
References
- 1.Kalkman CJ, Peelen L, Moons KG, Veenhuizen M, Bruens M, Sinnema G, de Jong TP. Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology. 2009;110:805–12. doi: 10.1097/ALN.0b013e31819c7124. [DOI] [PubMed] [Google Scholar]
- 2.Anand KJ, Soriano SG. Anesthetic agents and the immature brain: Are these toxic or therapeutic? Anesthesiology. 2004;101:527–30. doi: 10.1097/00000542-200408000-00033. [DOI] [PubMed] [Google Scholar]
- 3.Perouansky M, Hemmings HC., Jr Neurotoxicity of general anesthetics: Cause for concern? Anesthesiology. 2009;111:1365–71. doi: 10.1097/ALN.0b013e3181bf1d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creeley CE, Olney JW. The young: Neuroapoptosis induced by anesthetics and what to do about it. Anesth Analg. 2010;110:442–8. doi: 10.1213/ANE.0b013e3181c6b9ca. [DOI] [PubMed] [Google Scholar]
- 5.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–4. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 6.Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–37. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 7.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815–27. doi: 10.1016/j.neuroscience.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 9.Yoshizawa K, Oishi Y, Matsumoto M, Nyska A. Ischemic brain damage after ketamine and xylazine treatment in a young laboratory monkey (Macaca fascicularis) Contemp Top Lab Anim Sci. 2005;44:19–24. [PubMed] [Google Scholar]
- 10.Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT, Dai R. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–48. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 11.Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–14. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- 13.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–20. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 14.Yamakura T, Bertaccini E, Trudell JR, Harris RA. Anesthetics and ion channels: Molecular models and sites of action. Annu Rev Pharmacol Toxicol. 2001;41:23–51. doi: 10.1146/annurev.pharmtox.41.1.23. [DOI] [PubMed] [Google Scholar]
- 15.Gan WB, Lichtman JW. Synaptic segregation at the developing neuromuscular junction. Science. 1998;282:1508–11. doi: 10.1126/science.282.5393.1508. [DOI] [PubMed] [Google Scholar]
- 16.Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci. 1996;16:3274–86. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuo Y, Yang G, Kwon E, Gan WB. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005;436:261–5. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]
- 18.Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–4. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kakizawa S, Yamasaki M, Watanabe M, Kano M. Critical period for activity-dependent synapse elimination in developing cerebellum. J Neurosci. 2000;20:4954–61. doi: 10.1523/JNEUROSCI.20-13-04954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 21.Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–89. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- 22.Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Annu Rev Physiol. 2009;71:261–82. doi: 10.1146/annurev.physiol.010908.163140. [DOI] [PubMed] [Google Scholar]
- 23.Buonomano DV, Merzenich MM. Cortical plasticity: From synapses to maps. Annu Rev Neurosci. 1998;21:149–86. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 24.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–8. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 25.Head BP, Patel HH, Niesman IR, Drummond JC, Roth DM, Patel PM. Inhibition of p75 neurotrophin receptor attenuates isoflurane-mediated neuronal apoptosis in the neonatal central nervous system. Anesthesiology. 2009;110:813–25. doi: 10.1097/ALN.0b013e31819b602b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Roo M, Klauser P, Briner A, Nikonenko I, Mendez P, Dayer A, Kiss JZ, Muller D, Vutskits L. Anesthetics rapidly promote synaptogenesis during a critical period of brain development. PLoS One. 2009;4:e7043. doi: 10.1371/journal.pone.0007043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shepherd GM. The dendritic spine: A multifunctional integrative unit. J Neurophysiol. 1996;75:2197–210. doi: 10.1152/jn.1996.75.6.2197. [DOI] [PubMed] [Google Scholar]
- 28.Yuste R, Bonhoeffer T. Genesis of dendritic spines: Insights from ultrastructural and imaging studies. Nat Rev Neurosci. 2004;5:24–34. doi: 10.1038/nrn1300. [DOI] [PubMed] [Google Scholar]
- 29.Briner A, De Roo M, Dayer A, Muller D, Habre W, Vutskits L. Volatile anesthetics rapidly increase dendritic spine density in the rat medial prefrontal cortex during synaptogenesis. Anesthesiology. 2010;112:546–56. doi: 10.1097/ALN.0b013e3181cd7942. [DOI] [PubMed] [Google Scholar]
- 30.Zuo Y, Lin A, Chang P, Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46:181–9. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–6. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- 32.Yang G, Pan F, Parkhurst CN, Grutzendler J, Gan WB. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat Protoc. 2010;5:201–8. doi: 10.1038/nprot.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 34.Janssen BJ, De Celle T, Debets JJ, Brouns AE, Callahan MF, Smith TL. Effects of anesthetics on systemic hemodynamics in mice. Am J Physiol Heart Circ Physiol. 2004;287:H1618–24. doi: 10.1152/ajpheart.01192.2003. [DOI] [PubMed] [Google Scholar]
- 35.Erhardt W, Hebestedt A, Aschenbrenner G, Pichotka B, Blumel G. A comparative study with various anesthetics in mice (pentobarbitone, ketamine-xylazine, carfentanyletomidate) Res Exp Med (Berl) 1984;184:159–69. doi: 10.1007/BF01852390. [DOI] [PubMed] [Google Scholar]
- 36.Xu HT, Pan F, Yang G, Gan WB. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat Neurosci. 2007;10:549–51. doi: 10.1038/nn1883. [DOI] [PubMed] [Google Scholar]
- 37.Harris KM, Kater SB. Dendritic spines: Cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–71. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- 38.Portera-Cailliau C, Pan DT, Yuste R. Activity-regulated dynamic behavior of early dendritic protrusions: Evidence for different types of dendritic filopodia. J Neurosci. 2003;23:7129–42. doi: 10.1523/JNEUROSCI.23-18-07129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jontes JD, Smith SJ. Filopodia, spines, and the generation of synaptic diversity. Neuron. 2000;27:11–4. doi: 10.1016/s0896-6273(00)00003-9. [DOI] [PubMed] [Google Scholar]
- 40.Dailey ME, Smith SJ. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci. 1996;16:2983–94. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 42.Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci. 1998;18:8900–11. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomson AM, West DC, Lodge D. An N-methylaspartate receptor-mediated synapse in rat cerebral cortex: A site of action of ketamine? Nature. 1985;313:479–81. doi: 10.1038/313479a0. [DOI] [PubMed] [Google Scholar]
- 44.Wright M. Pharmacologic effects of ketamine and its use in veterinary medicine. J Am Vet Med Assoc. 1982;180:1462–71. [PubMed] [Google Scholar]
- 45.Tan H, Ren RR, Xiong ZQ, Wang YW. Effects of ketamine and midazolam on morphology of dendritic spines in hippocampal CA1 region of neonatal mice. Chin Med J (Engl) 2009;122:455–9. [PubMed] [Google Scholar]
- 46.Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–86. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 47.Wixson SK, White WJ, Hughes HC, Jr, Lang CM, Marshall WK. The effects of pentobarbital, fentanyl-droperidol, ketamine-xylazine and ketamine-diazepam on arterial blood pH, blood gases, mean arterial blood pressure and heart rate in adult male rats. Lab Anim Sci. 1987;37:736–42. [PubMed] [Google Scholar]
- 48.Yang XP, Liu YH, Rhaleb NE, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol. 1999;277:H1967–74. doi: 10.1152/ajpheart.1999.277.5.H1967. [DOI] [PubMed] [Google Scholar]
- 49.Menke H, Vaupel P. Effect of injectable or inhalational anesthetics and of neuroleptic, neuroleptanalgesic, and sedative agents on tumor blood flow. Radiat Res. 1988;114:64–76. [PubMed] [Google Scholar]
- 50.Maclver MB, Mikulec AA, Amagasu SM, Monroe FA. Volatile anesthetics depress glutamate transmission via presynaptic actions. Anesthesiology. 1996;85:823–34. doi: 10.1097/00000542-199610000-00018. [DOI] [PubMed] [Google Scholar]
- 51.Schlame M, Hemmings HC., Jr Inhibition by volatile anesthetics of endogenous glutamate release from synaptosomes by a presynaptic mechanism. Anesthesiology. 1995;82:1406–16. doi: 10.1097/00000542-199506000-00012. [DOI] [PubMed] [Google Scholar]
- 52.Lubke J, Albus K. The postnatal development of layer VI pyramidal neurons in the cat's striate cortex, as visualized by intracellular Lucifer yellow injections in aldehyde-fixed tissue. Brain Res Dev Brain Res. 1989;45:29–38. doi: 10.1016/0165-3806(89)90004-7. [DOI] [PubMed] [Google Scholar]
- 53.Markus EJ, Petit TL. Neocortical synaptogenesis, aging, and behavior: Lifespan development in the motor-sensory system of the rat. Exp Neurol. 1987;96:262–78. doi: 10.1016/0014-4886(87)90045-8. [DOI] [PubMed] [Google Scholar]
- 54.Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–5. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- 55.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 56.Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H. Synaptogenesis in human visual cortex--evidence for synapse elimination during normal development. Neurosci Lett. 1982;33:247–52. doi: 10.1016/0304-3940(82)90379-2. [DOI] [PubMed] [Google Scholar]


