SUMMARY
Impaired lung function caused by decreased airway diameter (bronchoconstriction) is frequently observed whether body weight is abnormally high or low. That these opposite conditions affect the airways similarly suggests that the regulation of airway diameter and body weight are intertwined. We show here that, independently of its regulation of appetite, melanocortin pathway, or sympathetic tone, leptin is necessary and sufficient to increase airway diameter by signaling through its cognate receptor in cholinergic neurons. The latter decreases parasympathetic signaling through the M3 muscarinic receptor in airway smooth muscle cells, thereby increasing airway diameter without affecting local inflammation. Accordingly, decreasing parasympathetic tone genetically or pharmacologically corrects bronchoconstriction and normalizes lung function in obese mice regardless of bronchial inflammation. This study reveals an adipocyte-dependent regulation of bronchial diameter whose disruption contributes to the impaired lung function caused by abnormal body weight. These findings may be of use in the management of obesity-associated asthma.
INTRODUCTION
In vertebrates, the diameter of the airways determines, in part, the ability to breathe normally (Lumb, 2010). As a result, narrowing of the airways, i.e., bronchoconstriction, decreases airflow to the alveoli and ultimately leads to respiratory failure. Given the vital importance of maintaining proper airflow, it is not surprising that this process is tightly regulated. To date, the best-characterized regulation of airway diameter is the one fulfilled by the autonomic nervous system (Canning, 2006). While the parasympathetic nervous system signals in airway smooth muscle (ASM) cells to cause bronchoconstriction, the sympathetic nervous system increases airway diameter (bronchodilation) by antagonizing the action of the parasympathetic nervous system. It is not known, however, whether there is an endocrine regulation of airway diameter and, provided such a regulation exists, if it would impinge on the neuronal one described above. Those are questions of increasing importance, given the growing incidence of diseases affecting airway diameter and lung function (Wenzel, 2012).
Obesity, a disease that has reached epidemic proportions, often leads to bronchoconstriction and impaired lung function for poorly understood reasons (Dixon et al., 2010, 2011; Stenius-Aarniala et al., 2000). Remarkably, when body weight is abnormally low, the same airflow limitations and decreased lung function develop that are seen in obesity (Celedón et al., 2001; King et al., 2005). A common feature between these two otherwise opposite clinical situations is that they are caused, in part, by deregulation of appetite resulting in an abnormal body weight. This observation suggests that the control of appetite and/or body weight, and that of airway diameter, may make use of the same regulatory molecules. A major regulator of appetite is the hormone leptin that is also known to promote the activity of the sympathetic nervous system (Myers et al., 2009; Yadav et al., 2009). Thus, a mere decrease in leptin signaling could explain the aforementioned lung manifestations by affecting the sympathetic tone. On the other hand, the decrease in airflow seen in the case of high and low body weight could reveal a novel function and mode of action for leptin. If it exists, such regulatory pathway may have important therapeutic implications.
Airway narrowing or bronchoconstriction is a cardinal manifestation of a common lung disease, asthma (Lumb, 2010). In the most common form of asthma there is also local airway inflammation, which is thought to be the major cause of bronchoconstriction (Wenzel, 2012). In some rare circumstances, however, asthma develops in the absence of detectable local inflammation (Farah et al., 2011; Wenzel, 2012), an observation suggesting that the regulation of airway diameter may be independent of the local inflammatory status.
In trying to understand why high and low body weight both decrease airway diameter (King et al., 2005) and compromise lung function, we made five observations. The first is that leptin is necessary and sufficient to increase airway diameter. Second, this function occurs independently of leptin regulation of appetite and energy metabolism. Third, the positive influence of leptin on airway diameter occurs through its signaling in the brain to inhibit the parasympathetic outflow, an arm of the autonomic nervous system not classically associated with leptin. Fourth, this function of leptin occurs without causing immunological dysfunction. Fifth, under our experimental conditions, decreasing parasympathetic tone suffices to normalize airway diameter and lung function in obese WT mice as well as in mice with allergen-induced asthma. These new findings may have important implications for normalizing airflow in humans suffering from various conditions.
RESULTS
Obesity Causes Bronchoconstriction
A large body of evidence established that a deleterious consequence of obesity is bronchoconstriction. Moreover, when it develops in asthmatic patients, obesity exacerbates the severity of asthma and hampers its treatment through poorly understood mechanisms (Kattan et al., 2010; Peters-Golden et al., 2006). In an effort to elucidate the genetic and molecular bases of this relationship between obesity, airway diameter, and lung function, we asked what would be the consequences on lung function of a high-fat diet in WT mice.
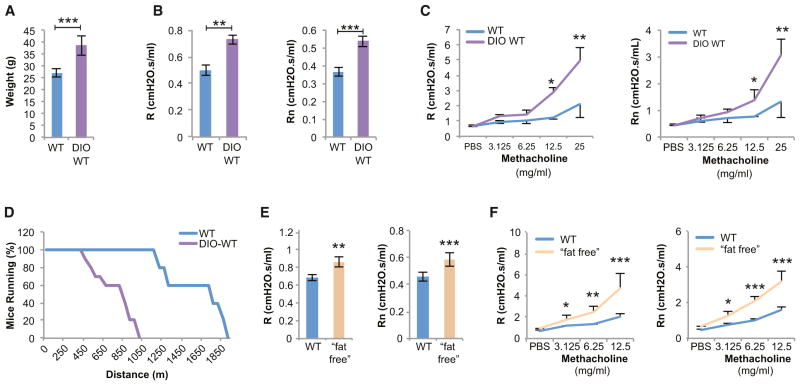
For that purpose, 3-week-old WT mice were fed a high-fat diet for 12 weeks (DIO-WT mice); this resulted in a 65% increase in body weight (Figure 1A). We then studied pulmonary mechanics by measuring total respiratory resistance (R), a parameter that takes into account the contribution of the lungs and chest wall, and Newtonian resistance (Rn), which represents the resistance to airflow in the airways (Vanoirbeek et al., 2010). DIO-WT mice displayed a significant increase in baseline R and Rn, i.e., a bronchoconstriction hampering their lung function (Figure 1B). DIO-WT mice also displayed innate airway hyperreactivity (AHR) following a challenge with a parasympathomimetic agent (methacholine), further indicating that lung function is compromised in these mice (Figure 1C). These findings are in agreement with a prior study, although in that case mice were fed a high-fat diet for more than 6 months (Johnston et al., 2008). DIO-WT mice showed considerably reduced endurance as compared to WT mice, as measured by their ability to run on a treadmill (Figure 1D). As shown below, this was due to their cardiopulmonary limitations, not to their weight (Figure 5G).
Figure 1. High and Low Fat Mass Results in Bronchoconstriction.
(A) Diet-induced obese (DIO) WT mice gain significant weight after 12 weeks on a high-fat diet.
(B) Total respiratory resistance (R) and airway resistance (Rn) are increased in DIO-WT mice.
(C) DIO-WT mice have innate airway hyperreactivity (AHR) to methacholine.
(D) Endurance is severely limited in DIO-WT mice when placed on a treadmill.
(E and F) Lypodystrophic, “fat-free,” mice have increased baseline R, Rn, and AHR.
For all panels, n = 10 per group. *p < 0.05, **p < 0.01, and ***p < 0.001. Error bars represent SEM. See also Figure S1.
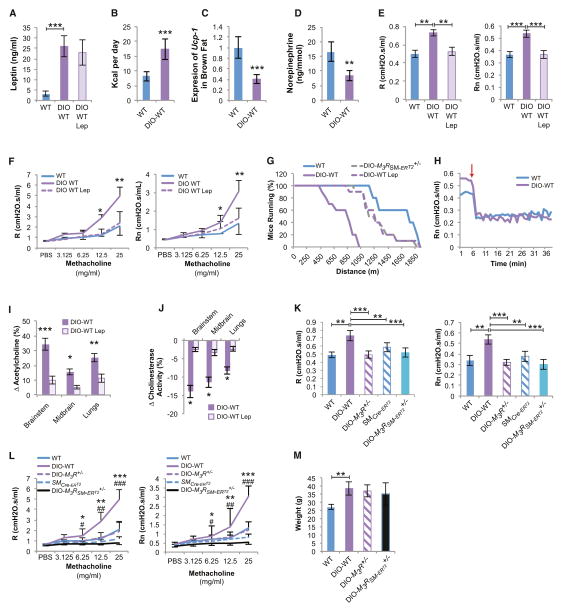
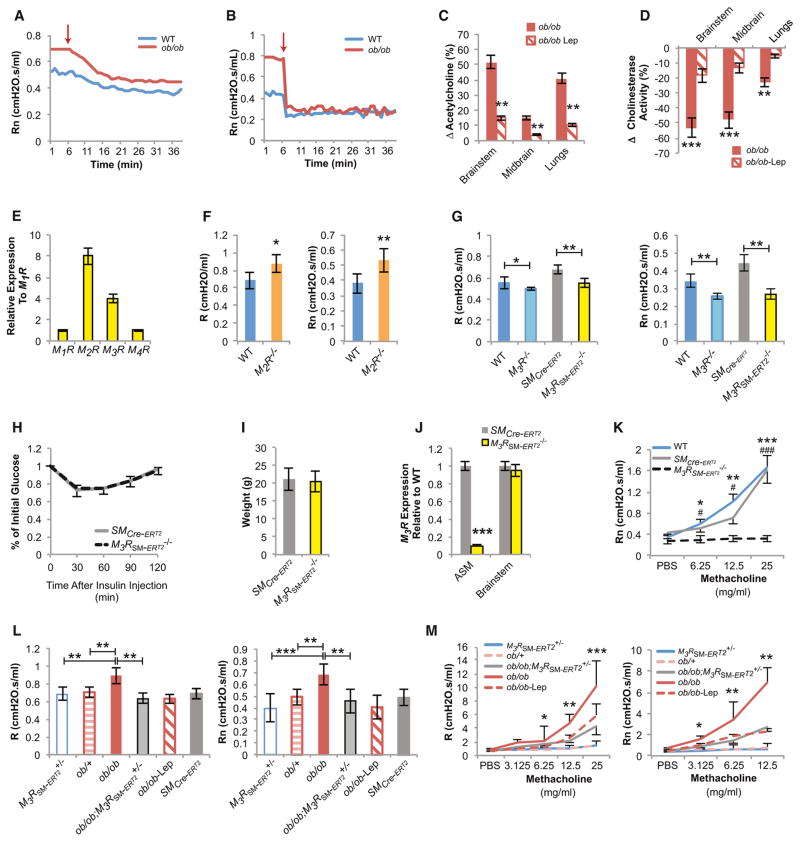
Figure 5. Central Leptin-Dependent Regulation of Bronchial Diameter in Diet-Induced Obese WT Mice.
(A–D) Diet-induced obese (DIO)-WT mice have increased levels of circulating leptin, food intake, and UCP-1 expression in brown fat and levels of urine norepinephrine, all suggestive of a state of leptin resistance (n = 8 per group).
(E and F) Increased baseline R, Rn, and AHR in DIO-WT mice reversed by leptin (Lep) ICV infusion (12 ng/hr for 5 days) (n = 8 per group, *DIO-WT versus DIO-WT Lep).
(G) Decreased endurance in DIO-WT mice reversed by leptin (Lep) ICV (12 ng/hr for 5 days) or inducible deletion of one allele of M3R in smooth muscle cells (M3RSM-ERT2+/−) (n = 8 per group).
(H) Bilateral cervical vagotomy causes a greater decrease in Rn in DIO-WT than in WT mice (n = 8 per group, arrow indicates time of vagotomy).
(I) High acetylcholine (Ach) levels in brain and lungs of DIO-WT mice corrected by leptin (Lep) ICV (12 ng/hr for 5 days) infusion (n = 6 per group).
(J) Decreased cholinesterase activity in DIO-WT improved by leptin ICV (n = 6 per group).
(K–M) Obese DIO-M3R+/− and DIO-M3RSM-ERT2+/− mice have baseline R, Rn, and AHR comparable to lean tamoxifen-treated SMCre-ERT2 and WT mice (n = 8 per group, *DIO-WT versus DIO-M3R+/−, #DIO-WT versus DIO-M3RSM-ERT2+/−).
For all panels, */#p < 0.05, **/##p < 0.01, and ***/###p < 0.001. Error bars represent the SEM. See also Figure S4.
Absence of Adipocytes Causes Bronchoconstriction
Obesity is a complex pathological state; therefore, the bronchoconstriction seen in DIO-WT mice could have multiple causes. Yet, many of the pathological manifestations triggered by obesity can be traced back to an increase in fat mass. This is important because patients with very low fat mass display airflow limitations similar to those of obese ones (Celedón et al., 2001; King et al., 2005). Thus, in the next set of experiments we asked whether decreasing adiposity would result in bronchoconstriction.
For that purpose, we studied a lipodystrophic mouse model, the “fat-free” mice that express in adipocyte progenitor cells a dominant-negative form of a leucine zipper protein termed A-ZIP/F that inhibits the DNA binding ability of both C/EBP and AP-1 proteins. As a result, “fat-free” mice have virtually no adipocytes and are lean (Moitra et al., 1998). Yet, these animals that had normal lung volumes showed the same significant increase in baseline R and Rn, and AHR as DIO-WT mice (Figures 1E and 1F, see Figure S1 online). Taken together, these two sets of experiments indicate that, in the mouse, abnormally low or high body weight and fat mass result in bronchoconstriction and decreased lung function.
Leptin Regulates Bronchial Diameter Independently of Its Effects on Appetite or Energy Metabolism
If adipocytes regulate bronchial diameter, such a regulation must occur through secreted molecules. The observation that both high and low fat masses cause bronchoconstriction led us to test whether leptin could be the adipocyte-derived signal regulating bronchial diameter. In support of this notion, obesity often leads to leptin resistance (Van Heek et al., 1997), whereas patients with low body mass and lipodystrophic mice both have very low leptin circulating levels (Mantzoros et al., 1997; Moitra et al., 1998).
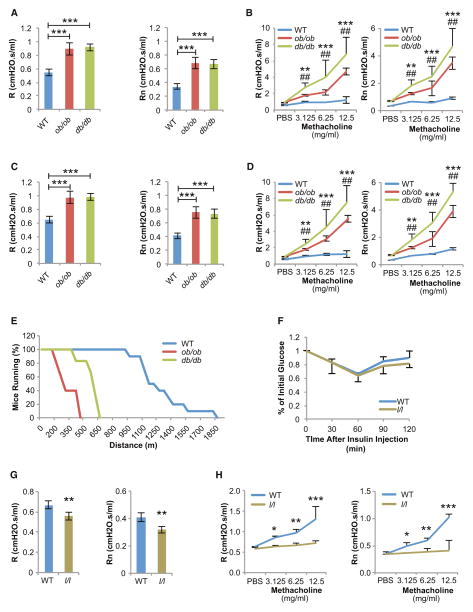
Consistent with this hypothesis, obese 8-week-old leptin-deficient (ob/ob) (body weight [BW] = 45 ± 3.6 g) and leptin receptor-deficient (db/db) (BW = 49 ± 4.5 g) mice demonstrated a severe increase in baseline R and Rn, and AHR compared to WT controls (BW = 25 ± 1.9 g) (Figures 2A and 2B). These findings are consistent with some but not all previous studies (Rivera-Sanchez et al., 2004; Shore et al., 2003). In fact, R, Rn, and AHR in ob/ob and db/db mice were more severely increased than in DIO-WT mice. We were concerned that these airway abnormalities might be a consequence of their severely increased body weight. To determine if this was the case or if instead leptin regulates airway diameter independently of body weight, we repeated this experiment using 5-week-old WT (BW = 15.5 ± 0.8 g), ob/ob (BW = 21.2 ± 1.7 g), and db/db (BW = 20.4 ± 2.3 g) mice that were only moderately overweight and obtained almost identical results (Figures 2C and 2D). Since the weight of these latter mice was not normal, we wanted to address the contention that leptin regulates bronchial diameter independently of its influence on body weight through another model. In that regard, the fact that “fat-free” mice, which have normal body weight but virtually no circulating leptin (Moitra et al., 1998), also display bronchoconstriction is important (Figures 1E and 1F). The functional consequences of the bronchoconstriction observed in 5-week-old ob/ob and db/db mice were analyzed using a treadmill apparatus. Just as it was the case for DIO-WT mice, endurance of 5-week-old, only moderately overweight, leptin signaling-deficient mice was severely hampered (Figure 2E).
Figure 2. Leptin Regulates Bronchial Diameter Independently of Appetite and Body Weight.
(A and B) Severely obese 8-week-old ob/ob and db/db mice demonstrated increased R, Rn, and AHR (n = 10 per group, *db/db versus WT, #ob/ob versus WT).
(C and D) Moderately overweight 5-week-old ob/ob and db/db mice demonstrated increased R, Rn, and AHR (n = 12 per group, *db/db versus WT, #ob/ob versus WT).
(E) Endurance is decreased in 5-week-old ob/ob and db/db compared to WT mice (n = 8 per group).
(F) l/l mice have normal insulin sensitivity (n = 5 per group).
(G and H) Increased leptin signaling in l/l mice leads to a decrease in baseline R, Rn, and AHR compared to WT controls (n = 8 per group).
For all panels, */#p < 0.05, **/##p < 0.01, and ***/###p < 0.001. Error bars represent SEM. See also Figure S2.
The results presented above indicated that leptin signaling is necessary to maintain normal airway diameter. To determine if increasing leptin signaling is sufficient to increase airway diameter, we turned our attention to another mouse model, the l/l mice that display a partial gain-of-function mutation in the leptin receptor (Björnholm et al., 2007). This mutation does not affect appetite, body weight, circulating insulin levels, insulin sensitivity, or lung volumes in animals fed a normal diet (Figure 2F and Figures S2A–S2D). Nevertheless, and mirroring what we observed in leptin signaling-deficient mice, l/l mice demonstrated a decrease of baseline R and Rn and AHR compared to WT controls, indicating bronchodilation (Figures 2G and 2H). Taken together, these experiments indicate that a function of leptin is to increase airway diameter and that this regulation occurs independently of its regulation of appetite and body weight.
Leptin Acts Centrally to Increase Bronchial Diameter
In order to determine if the lack of leptin signaling is the cause of bronchoconstriction seen in DIO-WT mice, we first needed to identify mediators of leptin regulation of bronchial diameter.
In an organ bath assay, leptin did not cause constriction of WT tracheal rings as did acetylcholine (Ach), a positive control, nor did it affect WT tracheal ring constriction or relaxation by Ach or isoproterenol, respectively (Figures S3A–S3C). We also performed the same experiment using tracheal rings isolated from l/l mice that harbor a partial gain of function in the leptin receptor and still could not detect any direct effect of leptin on tracheal rings (Figures S3A–S3C). These results, consistent with those observed using bovine tracheal rings (Nair et al., 2008), indicate that leptin has no local effect on the regulation of bronchial diameter in several species.
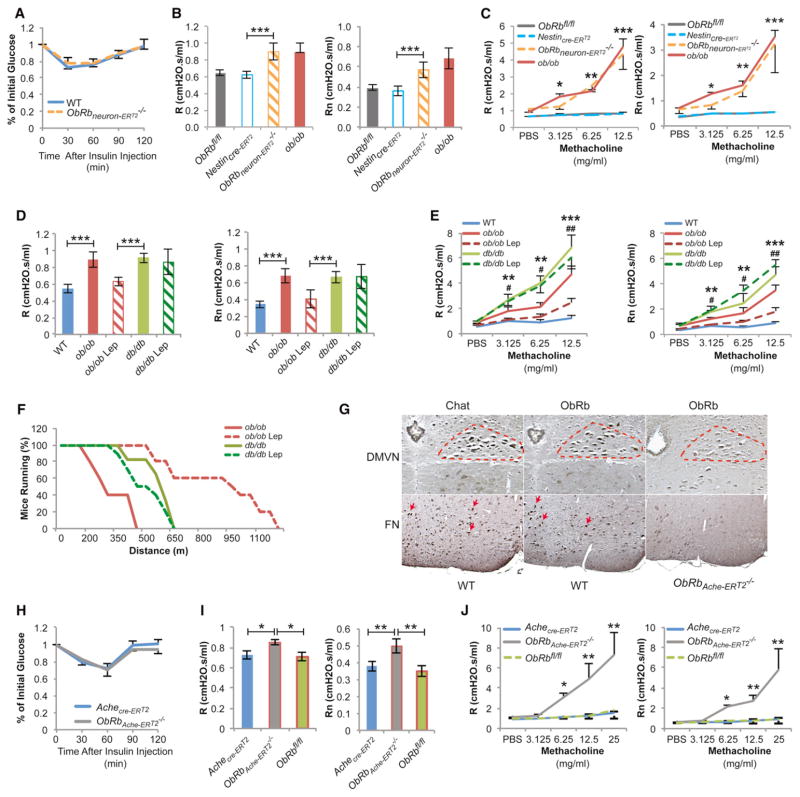
To demonstrate that leptin signals in the brain to affect airway diameter, we deleted ObRb, the signaling form of the leptin receptor, from all neurons (ObRbneurons-ERT2−/−) (Figure S3D). In order to avoid the confounding factors that obesity and insulin resistance represent, the ObRb deletion was generated 14 days prior to the analysis of lung function by using a tamoxifen-inducible Nestin-Cre driver. Five-week-old ObRbneurons-ERT2−/− mice, which were lean (BW = 15.8 ± 0.9 g) at the time of testing, and had normal circulating insulin levels, insulin sensitivity, and lung volumes (Figure 3A, and Figures S3E–S3G), showed the same increase in baseline R, Rn, and AHR that ob/ob, db/db, and “fat-free” mice did (Figures 3B and 3C). Moreover, leptin intracerebroventricular (ICV) infusion (4 ng/hr) for a short time (5 days) significantly decreased baseline R, Rn, and AHR in ob/ob, but not in db/db mice that lack the leptin receptor (Figures 3D and 3E). In addition, we tested whether leptin ICV infusions affected other physiologic functions under autonomic control. As one would expect, this short-term infusion increased heart rate and blood pressure (Figures S3H and S3I), two observations consistent with a decrease in parasympathetic tone. Leptin ICV infusion also rescued the ability of ob/ob mice to run in a treadmill test (Figure 3F). In each case we verified that leptin was not detectable in the general circulation following ICV infusion (Figure S3J). These experiments indicate that it is by signaling in the brain that leptin promotes bronchodilation and that this function is independent of leptin’s effect on insulin sensitivity.
Figure 3. Leptin Regulates Bronchial Diameter via a Central Pathway.
(A) Mice with deletion of ObRb in all neurons (ObRbneuron-ERT2−/−) have normal insulin sensitivity (n = 5 per group).
(B and C) ObRbneuron-ERT2−/− mice have decreased R, Rn, and AHR compared to tamoxifen-treated Nestincre-ERT2 and ObRbfl/fl controls (n = 8 per group, *ObRbneuron-ERT2−/− versus Nestincre-ERT2).
(D–F) Leptin (Lep) ICV infusion (4 ng/hr for 5 days) improves baseline R, Rn, and AHR, and endurance in ob/ob, not in db/db mice (n = 8 per group, *ob/ob versus WT, #ob/ob versus ob/ob-Lep).
(G) ObRb localizes in cholinergic-containing nuclei including the dorsal motor vagus (DMVN) (highlighted area) nucleus and facial motor nucleus (Mo7) (arrows) and is absent in mice with an inducible deletion of ObRb in Ach-producing neurons (ObRbAche-ERT2−/−).
(H–J) ObRbAche-ERT2−/− mice have normal insulin sensitivity and increased baseline R, Rn, and AHR compared to tamoxifen-treated Achecre-ERT2 and ObRbfl/fl controls (n = 10 per group). *ObRbAche-ERT2−/− versus Achecre-ERT2.
For all panels, */#p < 0.05, **/##p < 0.01, and ***/###p < 0.001. Error bars represent SEM. See also Figure S3.
Leptin Uses Autonomic Signaling to Favor Bronchodilation
To identify the pathway used by leptin signaling in the brain to favor bronchodilation, we made use of the knowledge available regarding the mediation of leptin’s other functions.
The melanocortin pathway is a prominent peripheral mediator of leptin regulation of appetite, and expression of the melanocortin 4 receptor (MC4R) is regulated by leptin (Myers et al., 2009). Yet, mice lacking MC3R (MC3R−/−) or MC4R (MC4R−/−) had normal baseline R, Rn, and AHR (Figures S3K and S3L), thus ruling out the melanocortin pathway as a mediator of leptin inhibition of bronchoconstriction. Since MC4R−/− mice are obese, hyperinsulinemic, and insulin resistant (Huszar et al., 1997), this experiment indicates that obesity, or insulin resistance, per se cannot induce bronchoconstriction. This observation is consistent with the fact that the leptin regulation of bronchial diameter occurs independently of its influence on appetite and body weight.
Another peripheral mediator of leptin signaling is the autonomic nervous system, which represents a key regulator of airway diameter. To determine if any arm of the autonomic nervous system was mediating leptin regulation of bronchial diameter, we generated, also in an inducible manner, mutant mice lacking ObRb in autonomic neurons (ObRbAch-ERT2) by using the Chat-Cre mice as the Cre driver (Madisen et al., 2010). We first verified that we had achieved efficient deletion of ObRb selectively in cholinergic neurons. Immunohistochemical analyses revealed that Chat and ObRb localized to the para-brachial nuclei, motor nuclei of the trigeminal system (Mo5), facial motor nuclei (Mo7), and more importantly the dorsal motor nuclei of the vagus nerve, where preganglionic parasympathetic fibers originate (Figure 3G and data not shown) (Loewy and Spyer, 1990). We failed to detect ObRb in ObRbAch-ERT2−/− mice in the Chat containing nuclei (Figure 3G). We also verified that ObRbAch-ERT2−/− mice had a normal body weight, normal circulating insulin levels, and insulin sensitivity (Figure 3H and Figures S3M and S3N). ObRbAch-ERT2−/− mice that had normal lung volumes demonstrated the same significant increase in baseline R and Rn, and AHR than leptin signaling-deficient mice do (Figures 3I and 3J, and Figure S3O), indicating that the autonomic nervous system mediates leptin regulation of bronchial diameter and that this regulation occurs independently of metabolic perturbations.
Since Chat-Cre deletes gene in preganglionic sympathetic and all parasympathetic neurons, this experiment could not determine which arm of the autonomic nervous system is used by leptin in this context. In the absence of specific Cre-drivers designed to address this question properly, we relied on a variety of other tests. Hexamethonium blocks nicotinic acetylcholine receptors in autonomic ganglia and thereby hinders all autonomic influence on airway tone. Thus, if bronchial diameter is equally controlled by the sympathetic and parasympathetic nervous system, hexamethonium treatment should have no effect. However, if bronchial diameter was primarily controlled by the sympathetic or parasympathetic nervous system, hexamethonium treatment in ob/ob mice should cause bronchoconstriction or bronchodilation, respectively. We found that hexamethonium within minutes in both WT and ob/ob mice decreased Rn, i.e., caused bronchodilation, indicating that the parasympathetic, but not the sympathetic nervous system, mediates leptin regulation of bronchial diameter (Figure 4A).
Figure 4. Leptin Regulates Bronchial Diameter by Inhibiting Parasympathetic Nervous System Signaling in ASM Cells through the M3R.
(A) Autonomic nervous system blockade by hexamethonium causes a greater decrease in Rn in ob/ob than in WT mice (n = 6 per group, arrow indicates time of hexamethonium administration).
(B) Bilateral cervical vagotomy causes a greater decrease in Rn in ob/ob than in WT mice (n = 6 per group, arrow indicates time of vagotomy).
(C) Elevated acetylcholine (Ach) levels in ob/ob brain and lungs are decreased by leptin (Lep) ICV infusion (4 ng/hr for 5 days) (n = 5 per group).
(D) Decreased cholinesterase activity in ob/ob brain and lungs is improved with leptin (Lep) ICV (n = 5 per group).
(E) M2R and M3R muscarinic receptors are the highest expressed cholinergic receptors in the mouse lung.
(F) M2R−/− mice have increased R and Rn compared to WT controls (n = 6 per group).
(G) Global M3R−/− and smooth muscle-specific inducible deletion of M3R (M3RSM-ERT2−/−) decrease baseline R and Rn in mice compared to WT and tamoxifen-treated SMMHC-CreERT2 control mice, respectively (n = 8 per group).
(H and I) M3RSM-ERT2−/− mice have normal insulin sensitivity and weight compared to tamoxifen-treated SMMHC-CreERT2 control mice (n = 5 per group).
(J) Primary M3RSM-ERT2−/− ASM cells have a marked reduction in M3R expression levels compared to control ASM cells, but not in the brainstem (n = 6 per group).
(K) M3RSM-ERT2−/− mice have no response to methacholine compared to tamoxifen-treated SMMHC-CreERT2 and WT littermate controls, confirming M3R deletion in ASM cells (n = 8 per group, *WT versus M3RSM-ERT2−/−, #SMMHC-CreERT2 versus M3RSM-ERT2−/−).
(L and M) Removing one allele of M3R in smooth muscle of ob/ob mice normalizes their baseline R, Rn, and AHR, as leptin ICV does (n = 8 per group, *ob/ob versusob/ob; M3RSM-ERT2−/−).
For all panels, */#p < 0.05, **/##p < 0.01, and ***/###p < 0.001. Error bars represent SEM. See also Figure S4.
To rule out conclusively any involvement of the sympathetic nervous system, we studied adrenergic receptor expression in ASM cells and observed that β2 adrenergic receptor (β2R) was by far the most highly expressed adrenergic receptor subtype in ASM cells (Figures S4A and S4B). If leptin causes bronchodilation by increasing the sympathetic tone, β2R−/− mice should have the same lung phenotype as ob/ob mice. Instead, R and Rn at baseline were decreased in β2R−/− compared to WT mice (Figure S4C). Nadolol, a β2-antagonist, did not prevent the beneficial effect of leptin ICV infusion in ob/ob and increased leptin signaling in l/l mice, further dissociating leptin regulation of bronchodilation and sympathetic tone (Figures S4D–S4G). These observations, consistent with the recently described role ascribed to β2Rs in airway epithelium (Nguyen et al., 2009), ruled out the possibility that sympathetic signaling through β2Rs in ASM cells mediates the leptin regulation of bronchial diameter.
Next, the involvement of the parasympathetic tone in mediating leptin regulation was confirmed by several in vivo assays. First, bilateral vagotomies, which abolish parasympathetic signaling as determined by an increase in heart rate and blood pressure (Figures S4H and S4I), immediately normalized Rn in ob/ob mice (Figure 4B). Second, when measured in the brainstem, midbrain, and lungs, Ach content was significantly higher in ob/ob than in WT mice (Figure 4C). Third, cholinesterase activity was lower in brainstem, midbrain, and lungs of ob/ob compared to WT mice (Figure 4D). Fourth and more directly, leptin ICV infusion in ob/ob mice corrected all the abnormalities presented above (Figures 4C and 4D). Taken together, these experiments point toward a pathway in which leptin increases bronchial diameter by inhibiting parasympathetic signaling.
Leptin Favors Bronchodilation by Inhibiting Parasympathetic Signaling through the M3 Muscarinic Receptor Expressed in Airway Smooth Muscle Cells
Ach mediates its peripheral parasympathomimetic actions via stimulation of different muscarinic receptor subtypes. To identify the muscarinic receptor mediating the leptin-dependent parasympathetic regulation of bronchial diameter, we analyzed the pattern of expression of all muscarinic receptors in the lung. We observed that M2 and M3 muscarinic receptors (M2R and M3R) are the most highly expressed (Figure 4E) and that they both localize to ASM cells and the airway epithelium (Figure S4J) (Coulson and Fryer, 2003; Fisher et al., 2004). Signaling through M2R exerts an inhibitory role in prejunctional parasympathetic nerve terminals by preventing Ach release (Canning and Spina, 2009; Coulson and Fryer, 2003). Consistent with this notion, M2R−/− mice showed an increase in R and Rn at baseline (Figure 4F), thus distinguishing signaling through this receptor from leptin regulation of bronchodilation.
In contrast, mice lacking M3R throughout the body (M3R−/−mice) demonstrated a significant decrease in baseline R and Rn when compared to WT littermates (Figure 4G). To ascertain that this was due to the expression of M3Rs in ASM cells, we generated mice lacking M3Rs only in smooth muscle cells, again in an inducible manner (M3RSM-ERT2−/− mice). These mutant mice demonstrated the same decrease in baseline R and Rn as did M3R−/− mice, when compared to the appropriate controls (Figure 4G), indicating that parasympathetic signaling through M3Rs expressed in ASM cells decreases bronchial diameter. Yet, as observed with other inducible cell-specific gene deletion models used in this study, M3RSM-ERT2−/− mice had normal appetite and body weight, as well as normal circulating insulin levels and insulin sensitivity (Figures 4H and 4I, and Figures S4K and S4L). Cell-specific deletion of the M3R was confirmed by the near-complete absence of M3R expression in primary ASM cell cultures and by the absence of a response to methacholine, a parasympathomimetic agent (Figures 4J and 4K). Since M3RSM-ERT2+/− mice have normal lung function (Figures 4L and 4M), we tested the hypothesis that leptin increases bronchial diameter via parasympathetic signaling in ASM cells by removing one allele of M3R in ob/ob mice (ob/ob;M3RSM-ERT2+/−). This manipulation normalized baseline R, Rn, and AHR in ob/ob;M3RSM-ERT2+/− mice without affecting lung volume (Figures 4L and 4M, and Figure S4M). The ob/ob;M3RSM-ERT2+/− mice were overweight, hyperinsulinemic, and insulin resistant but had normal lung function, further distinguishing leptin regulation of bronchial diameter from its effects on energy metabolism (Figures S4N–S4P).
Leptin Resistance Is a Cause of Bronchoconstriction in DIO-WT Mice
Having identified a pathway through which leptin regulates bronchial diameter allowed us to test whether the bronchoconstriction induced by a high-fat diet in WT mice is caused, at least partially, by a state of leptin resistance leading to an increase in parasympathetic signaling through the M3R in ASM cells.
Despite their high circulating leptin levels, DIO-WT mice were hyperphagic and had decreased sympathetic tone, as indicated by decreased Ucp1 expression in brown fat and reduced urine catecholamine elimination (Figures 5A–5D). These manifestations were suggestive of a state of leptin resistance that would cause bronchoconstriction. Consistent with this idea, leptin ICV infusion for only 5 days normalized R, Rn, and AHR in DIO-WT mice as well as their ability to run on a treadmill (Figures 5E–5G). Since these beneficial effects occurred without overt modification of body weight, the reduced endurance of DIO-WT mice is most likely a direct consequence of the cardio-pulmonary deficiency caused by leptin resistance and not due to changes in body weight (Figure 5G).
Five pieces of experimental evidence support the notion that the leptin resistance observed in DIO-WT mice led to a bronchoconstriction phenotype because of an increase in parasympathetic signaling through M3R in ASM cells. First, hexamethonium treatment and bilateral vagotomy normalized Rn in ob/ob mice (Figure 5H and Figure S5). Second, Ach content was increased in brainstem, midbrain, and lungs of DlO-WT mice (Figure 5I). Third, cholinesterase activity was decreased in brainstem, midbrain, and lungs of DlO-WT compared to lean WT mice (Figure 5J). These abnormalities of parasympathetic tone were less severe in DlO-WT than in leptin signaling-deficient mice, which explains why lung function was also less severely affected in the former model. Fourth, leptin ICV infusions normalized all features of increased parasympathetic activity in DlO-WT mice (Figures 5E–5G, 5I, and 5J). Fifth, and even more directly, DIO-WT mice lacking one allele of M3R (DIO-M3R+/−) in all cells or only in smooth muscle cells (DIO-M3RSM-ERT2+/−) showed no evidence of bronchoconstriction and behaved normally in an endurance test despite being obese (Figures 5G and 5K–5M).
Leptin Regulates Bronchial Diameter Regardless of the Inflammatory Status
Bronchoconstriction is a hallmark of asthma. In the vast majority of asthma cases, bronchoconstriction is thought to result from airway inflammation. Thus, we were interested to test whether the effect of leptin on airway diameter was affected by local airway inflammation.
Studies with DIO-WT, ob/ob, “fat-free,” or db/db mice failed to detect any manifestations of airway inflammation such as those detected in ovalbumin-sensitized and challenged WT (WTova) mice. Histological and bronchoalveolar lavage (BAL) analyses did not reveal goblet cell hyperplasia or eosinophilia or changes in the BAL content of inflammatory cytokines including IL-5 and IL-13 in DIO-WT, ob/ob, “fat-free,” or db/db mice compared to WT mice, while they were readily detectable in WTova mice (Figures 6A and 6B, and Figure S6A). These results, which confirm and extend the BAL analysis in ob/ob, db/db, and DIO-WT mice (Lu et al., 2006; Rivera-Sanchez et al., 2004; Shore et al., 2003), indicated that, in an environmentally controlled mouse facility, the lack of leptin signaling leads to bronchoconstriction either before or without triggering bronchial inflammation.
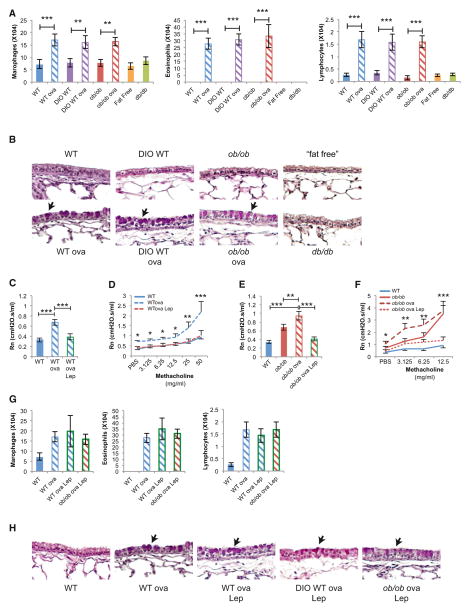
Figure 6. Leptin Regulation of Bronchial Diameter Occurs Independently of Local Inflammation.
(A) Bronchoalveolar lavage analysis demonstrates no difference in inflammatory cells in DIO-WT, ob/ob, “fat-free,” or db/db compared to the ovalbumin (ova)-sensitized and challenged mice (n = 8 per group).
(B) Periodic acid-Shiff staining fails to detect goblet cell (arrows) hyperplasia in DIO-WT, ob/ob, ‘fat-free,” or db/db mice compared to ova mice.
(C–F) Leptin (Lep) ICV infusion in WTova and ob/obova mice decreased Rn and AHR compared to control (n = 12 per group, D-*WTova versus WTova Lep, F-*ob/obova versus ob/obova Lep).
(G) Leptin ICV infusion failed to change the levels of inflammatory cells in WTova, DIO-WTova, ob/obova mice (n = 8 per group).
(H) Leptin ICV infusion failed to change the goblet cell (arrows) hyperplasia in WTova, DIO-WTova, ob/obova mice.
For all panels, *p < 0.05, **p < 0.01, and ***p < 0.001. Error bars represent the SEM. See also Figure S5.
In view of these observations, we then asked whether leptin ICV infusion could correct allergen-induced asthma in lean or obese mice. To that end, WT and ob/ob mice were sensitized and challenged with ovalbumin (WTova, ob/obova) to induce an allergy model of asthma associated with bronchial inflammation, bronchoconstriction, and AHR. Both types of mice were then infused ICV with leptin for 5 days. This manipulation decreased baseline R, Rn, and AHR but did not correct any of the allergic manifestations induced by ovalbumin (Figures 6C–6H, and Figures S6B–S6F). Taken together, these results indicate that leptin regulation of airway diameter occurs independently of the local inflammatory status. We took advantage of this feature to test the therapeutic implications of our findings.
Anticholinergic Agents Correct Bronchoconstriction in Obese Mice
An implication of the results presented above is that it should be possible to improve bronchoconstriction in leptin signaling-deficient mice or in obese WT mice by decreasing the activity of the parasympathetic nervous system.
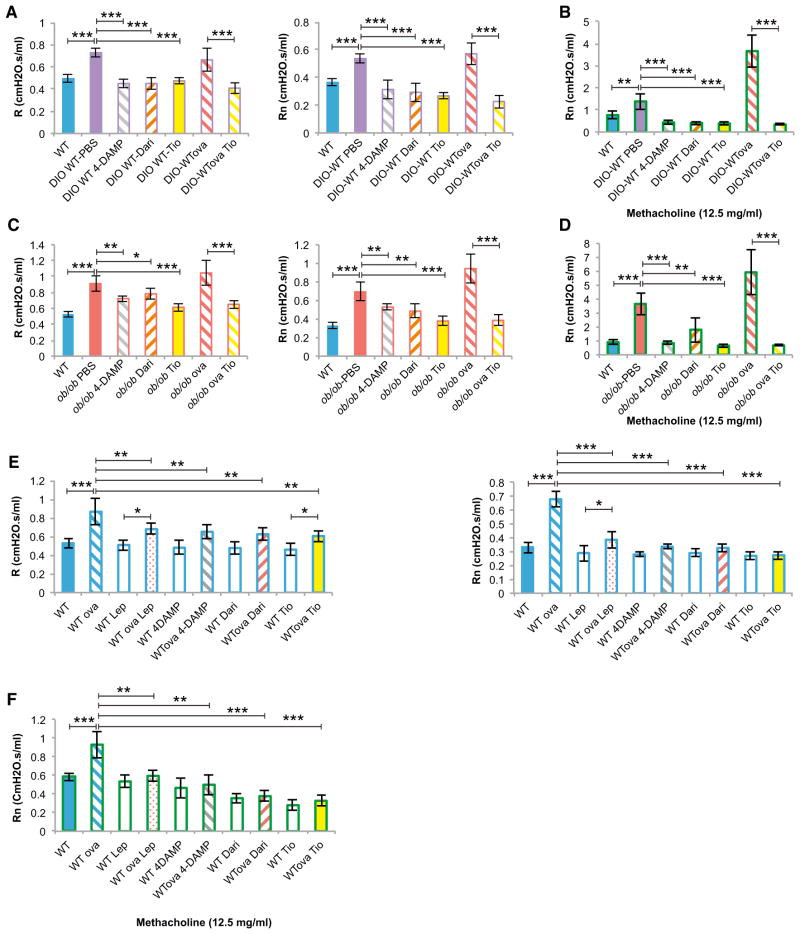
To test this contention, we treated ob/ob and DlO-WT mice with nebulized tiotropium bromide (200 μl of 50 μg/ml solution once a day for 2 days), a long-acting anticholinergic agent that is functionally selective for the M3R (Barnes, 2000), or with one dose of nebulized 4-DAMP (10 μl of 100 μg/ml solution) or darifenacin (200 μl of 100 μg/ml solution), two M3R-preferring antagonists (Yamanishi et al., 2000). 4-DAMP-, darifenacin-, or tiotropium-treated DlO-WT and ob/ob mice had baseline R and Rn values comparable to WT mice fed a normal diet and as expected showed no response to methacholine, indicating adequate drug administration (Figures 7A–7D). The finding that the same doses of anticholinergic agents caused a stronger decrease in R and Rn in DlO-WT than in ob/ob mice (Figures 7A and 7C) further supports the concept that parasympathetic tone is increased in ob/ob mice. In addition, tiotropium normalized baseline R and Rn in ovalbumin-sensitized and challenged DIO-WT and ob/ob mice (Figures 7A and 7C). These results suggest that, in the mouse, anticholinergic agents can treat the bronchoconstriction caused by obesity even when lung disease is allergen induced. In view of the ability of leptin to affect airway diameter regardless of the degree of inflammation, we also asked whether anticholinergic agents were effective in treating the allergen-induced bronchoconstriction in WTova mice. Indeed, tiotropium-, 4-DAMP-, or darifenacin-treated WTova mice had normal baseline R and Rn and showed no response to methacholine (Figures 7G–7J).
Figure 7. Single Anticholinergic Agent Improves Airway Resistance in Obese Mice.
(A, C, and E) Nebulized tiotropium (Tio) (200 μl of 50 μg/ml solution once a day for 2 days), 4-diphenylacetoxy-N-methylpiperidine methiodide (4-DAMP) (200 μl of 100 μg/ml solution 30 min prior to airway measurements), and darifenacin (Dari) (200 μl of 100 μg/ml solution 30 min prior to airway measurements) therapies normalize R and Rn in DIO-WT, DIO-WTova, ob/ob, ob/obova, and WTova male mice (n = 6 per group).
(B, D, and F) Anticholinergic therapy blocked the response to nebulized methacholine (20 μl of 12.5 mg/ml solution) in DIO-WT, DIO-WTova, ob/ob, ob/obova, and WTova-treated compared to control mice, indicating proper therapeutic drug delivery (n = 6 per group).
For all panels, *p < 0.05, **p < 0.01, and ***p < 0.001. Error bars represent the SEM.
DISCUSSION
By addressing the intriguing finding that obesity and anorexia affect lung function in a similar fashion, we identified leptin as a regulator of bronchial diameter. Surprisingly, this function of leptin is independent of its regulation of appetite and energy metabolism, and requires leptin signaling in cholinergic neurons to inhibit parasympathetic signaling in airway smooth cells. The absence of leptin signaling explains why WT mice rendered obese develop bronchoconstriction that could be rescued by anticholinergic agents.
Influence of Body Weight on Lung Function
Obesity and anorexia are complex pathological conditions that affect a multitude of functions including the ability to breathe, which is often reduced in both diseases (Celedón et al., 2001; King et al., 2005). To understand how these two diseases that affect body weight in an opposite manner can have the same deleterious consequence on lung functions, we used the mouse as model organism to test the hypothesis that this was due to the absence of a circulating molecule that favors bronchodilation and that this molecule was leptin. The results presented here indicate that, in the mouse, leptin does favor bronchodilation independently of its regulation of appetite or energy metabolism. Although further work is needed to extend these results beyond the mouse, our work points toward a molecular basis for the fact that either very low or very high fat mass hampers lung function (data summarized in Table S1).
Leptin Uses Different Pathways to Regulate Appetite and Bronchial Diameter
As it does for most of its functions, leptin signals in the brain to affect lung function. Unexpectedly, given that the influence of body weight revealed this function, leptin regulates bronchial diameter independently of appetite and through a different pathway. Indeed, while genetic experiments ruled out any overt involvement of the melanocortin-signaling pathway, they revealed that leptin signaling involving cholinergic neurons eventually causes bronchodilation. A combination of experimental strategies including hexamethonium tests, bilateral vagotomies experiments, and studies with muscarinic and adrenergic receptor mutant mice clearly demonstrated that leptin favors bronchodilation by signaling outside the hypothalamus in cholinergic neurons to eventually inhibit parasympathetic signaling through the M3R in ASM cells. Consistent with this hypothesis, removing one allele of M3R from smooth muscle cells in ob/ob mice normalized their lung function. This manipulation had the same effect in DIO-WT mice, further supporting the notion that the leptin resistance noticed in these latter mice is a significant cause of their bronchoconstriction. Remarkably, the effect of leptin on airway diameter is quite fast, since a 5-day-long leptin infusion sufficed to normalize this parameter in ob/ob mice, while such treatment had only a marginal effect on body weight.
Regulation of Bronchial Diameter and Local Inflammation
A second unexpected result of this study is that disruption of lep-tin signaling affects bronchial diameter without having any overt influence on local bronchial inflammation. These results support that, in the housing conditions of a confined mouse facility, bronchoconstriction can develop in the absence of local bronchial inflammation or before it is detectable (Farah et al., 2011; Wenzel, 2012). Therefore, our findings support the existence of means to regulate airway diameter independently of airway inflammation. Accordingly, leptin infusion could correct bronchoconstriction and improve lung function in obese mice, but also in WT mice with allergic asthma. Our results do not deny the importance of local inflammation in the development of most forms of asthma, they simply point toward a regulation of airway diameter independent of the inflammatory status of the bronchi. Consistent with our findings is the clinical observation that anti-inflammatory agents are not effective in the treatment of obesity-associated airflow limitation or asthma (Peters-Golden et al., 2006). As such, they imply that differences may exist between the pathogenesis of allergen-induced and appetite disorder-induced asthma. It is conceivable, for instance, that in the case of obesity and outside the artificial environment that a clean mouse facility represents, two mechanisms concur to the development of an asthma-like phenotype. One may be the decrease in leptin signaling leading to bronchoconstriction, and the other one could be the well-known bronchial inflammation that itself results in airflow limitations. Yet, as illustrated in this study, improving one of these two causes of lung dysfunction may have significant beneficial effects.
We are well aware of differences in autonomic nervous system innervations and anatomy between mice and humans (Canning, 2006); however, it is now important to determine if the findings presented here can be extended to humans. Indeed, if it were the case, it could lead to a simpler and more adapted therapy for the bronchoconstriction seen in obese individuals. In broader terms, the present study provides an example of how important novel biological information can be gathered by studying unexplained pathophysiological conditions, when aided by the use of model organisms that allow systematic genetic and molecular analyses of complex physiological processes. Insights obtained from such studies are likely to lead to improved therapies for various important human diseases.
EXPERIMENTAL PROCEDURES
Animals
All animal procedures were approved by the Columbia University Institutional Animal Care and Use Committee. Nestin-CreERT2 (B6129SF1/J), Chat-CreERT2 (B6129SF1/J), ob/ob (C57BL/6J), MC4R−/− (C57BL/6J), and DIO-WT (C57BL/6J) mice were purchased from Jackson Laboratories. The β2R−/− (C57BL/6J), l/l (C57BL/6J), lipodystrophy “fat-free” (FVB/NJ), MC3R−/− (C57BL/6J), M2R−/− (C57BL/6J), SMMHC-CreERT2 (C57BL/6J), M3R−/− (C57BL/6J), M3Rfl/fl (C57BL/6J), and ObRbfl/fl (B6129SF1/J) mice were previously described (Björnholm et al., 2007; Butler et al., 2000; Chruscinski et al., 1999; Gautam et al., 2006; Gomeza et al., 1999; McMinn et al., 2004; Moitra et al., 1998; Wirth et al., 2008; Yamada et al., 2001). ObRbfl/fl mice were crossed with Nestin-CreERT2 and Chat-CreERT2 to generate ObRbneuron-ERT2−/− and ObRbAch-ERT2−/− mice. M3Rfl/fl mice were crossed with SMMHC-CreERT2 to generate M3RSM-ERT2−/− mice. Inducible gene deletion was performed by daily tamoxifen (1 mg) i.p. injections for 5 days with analysis performed 15 days postinduction at 5 weeks of age.
Leptin ICV
Mice were ICV infused using osmotic pumps (Alzet, CA) connected to a 28-gauge cannula (Brain Infusion Kit II, Alzet) implanted into the third ventricle according to the following coordinates: midline, −0.3 AP, 3 mm ventral (0 point bregma). The pumps delivered either phosphate-buffered saline (PBS) at 0.25 μl/hr or leptin (4 ng/hr in WT, ob/ob, and db/db mice, and 12 ng/hr in DIO-WT mice) (Sigma, MO) in PBS at 0.25 μl/hr for 5 days.
Pulmonary Mechanics, Heart Rate, and Blood Pressure Measurements
Measurements of pulmonary mechanics were done using the forced oscillation technique with a Flexivent (Scireq, Ca), as previously described (Huang et al., 2008; Johnston et al., 2008). The mice were sedated with an i.p. injection of pentobarbital (50 mg/kg). A tracheostomy was performed with an 18 G cannula, and the mouse was connected to the Flexivent. EKG leads were placed on the extremities, and the right carotid artery was cannulated and connected to the blood pressure transducer connected to the Flexivent. The mice were ventilated at 150 Hz, tidal volume (VT) 10 ml/kg, and positive end-expiratory pressure (PEEP) level of 3 cmH2O. A higher ventilator frequency of 180 Hz was used in ob/ob and db/db mice because of their increased respiratory rate. In obese mice over 30 g, a VT of 0.3 ml was used to avoid hyperinflation of the lung due to VT calculated from their exaggerated body weights. To ensure identical volume history profiles, the lungs were first inflated to three times the VT and ventilated for 10 min. Mice were then paralyzed with an i.p. injection of 0.05 ml of 9 mg/ml succinylcholine. All experiments were performed on closed-chest male mice, as no significant difference in pulmonary mechanics compared to open-chest ob/ob mice has been observed (Huang et al., 2008).
Drug Treatments
Mice were placed in a 2 l chamber with a constant fresh airflow of 2 l/min. Tiotropium (Boehringer, CT), 200 μl of 50 μg/ml solution, was nebulized with an ultrasonic nebulizer (Aeroneb, Aerogen, Ireland) over 20 min once a day for 2 days and pulmonary mechanics performed at least 24 hr later. Darifenacin (BioTang, MA), 200 μl of 100 μg/ml solution, was nebulized 30 min prior to airway measurements as described above. 4-diphenylacetoxy-N-methylpi-peridine methiodide (4-DAMP) (Sigma, MO), 10 μl of 100 μg/ml solution, was nebulized intratracheally 15 min prior to airway measurements. Nadolol (72 mg/kg) was given as a single i.p. injection 15 min prior to airway measurements (Callaerts-Vegh et al., 2004). Control mice were treated equally with the same volume of vehicle.
Statistical Analyses
Comparisons of baseline R and Rn, weights, and BAL parameters were assessed using factorial ANOVA, using genotype, age, and treatment as the main effects. To determine the significance of differences between individual groups, the Fisher’s least significant difference test was used as a follow-up. Repeated-measures ANOVA was used to assess the changes in R and Rn during methacholine challenge within the same age groups. Comparisons of serum markers, urine cathecolamines, biochemical assays, and lung volumes were made by unpaired Student’s two-tailed t test. The results are expressed as means ± SEM, except where noted. Values were considered statistically significant at p < 0.05.
Additional experimental procedures are included in the Supplemental Information.
Supplementary Material
Acknowledgments
We thank Drs. S. Offermanns and R. Cone for providing SMMHC-creERT2 and MC3R−/− mice, respectively; Dr. J. D’Armiento for experimental support; and Dr. P. Ducy for critical review of the manuscript. This work was supported by the John M. Driscoll, Jr., M.D. Children’s Fund Scholars and the Irving Institute/Clinical Trials Office Pilot Award (to E.A.-S.), and by the NIH (to G.K.). This study is dedicated to Antoine Karsenty. E.A.-S. and G.K. planned and designed the study; C.W.E. provided reagents and expertise; J.W. and C.V. provided reagents. E.A.-S. performed all the experiments except for insulin levels and tolerance tests performed by T.Z. The manuscript was written by E.A.-S. and G.K.
Footnotes
Supplemental Information includes six figures, one table, Supplemental Experimental Procedures, and Supplemental References and can be found with this article at http://dx.doi.org/10.1016/j.cmet.2012.12.004.
References
- Barnes PJ. The pharmacological properties of tiotropium. Chest. 2000;117(2, Suppl):63S–66S. doi: 10.1378/chest.117.2_suppl.63s. [DOI] [PubMed] [Google Scholar]
- Björnholm M, Münzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, Jones JC, Ishida-Takahashi R, Bjørbaek C, Myers MG., Jr Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest. 2007;117:1354–1360. doi: 10.1172/JCI30688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- Callaerts-Vegh Z, Evans KL, Dudekula N, Cuba D, Knoll BJ, Callaerts PF, Giles H, Shardonofsky FR, Bond RA. Effects of acute and chronic administration of beta-adrenoceptor ligands on airway function in a murine model of asthma. Proc Natl Acad Sci USA. 2004;101:4948–4953. doi: 10.1073/pnas.0400452101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ. Reflex regulation of airway smooth muscle tone. J Appl Physiol. 2006;101:971–985. doi: 10.1152/japplphysiol.00313.2006. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Spina D. Sensory nerves and airway irritability. Handb Exp Pharmacol. 2009;194:139–183. doi: 10.1007/978-3-540-79090-7_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celedón JC, Palmer LJ, Litonjua AA, Weiss ST, Wang B, Fang Z, Xu X. Body mass index and asthma in adults in families of subjects with asthma in Anqing, China. Am J Respir Crit Care Med. 2001;164:1835–1840. doi: 10.1164/ajrccm.164.10.2105033. [DOI] [PubMed] [Google Scholar]
- Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, Kobilka BK. Targeted disruption of the beta2 adrenergic receptor gene. J Biol Chem. 1999;274:16694–16700. doi: 10.1074/jbc.274.24.16694. [DOI] [PubMed] [Google Scholar]
- Coulson FR, Fryer AD. Muscarinic acetylcholine receptors and airway diseases. Pharmacol Ther. 2003;98:59–69. doi: 10.1016/s0163-7258(03)00004-4. [DOI] [PubMed] [Google Scholar]
- Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, Celedón JC, Shore SA American Thoracic Society Ad Hoc Subcommittee on Obesity Lung Disease. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7:325–335. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, Garudathri J, Raymond D, Poynter ME, Bunn JY, Irvin CG. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128:508–515. e1–e2. doi: 10.1016/j.jaci.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah CS, Kermode JA, Downie SR, Brown NJ, Hardaker KM, Berend N, King GG, Salome CM. Obesity is a determinant of asthma control independent of inflammation and lung mechanics. Chest. 2011;140:659–666. doi: 10.1378/chest.11-0027. [DOI] [PubMed] [Google Scholar]
- Fisher JT, Vincent SG, Gomeza J, Yamada M, Wess J. Loss of vagally mediated bradycardia and bronchoconstriction in mice lacking M2 or M3 muscarinic acetylcholine receptors. FASEB J. 2004;18:711–713. doi: 10.1096/fj.03-0648fje. [DOI] [PubMed] [Google Scholar]
- Gautam D, Han SJ, Hamdan FF, Jeon J, Li B, Li JH, Cui Y, Mears D, Lu H, Deng C, et al. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2006;3:449–461. doi: 10.1016/j.cmet.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, Wess J. Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:1692–1697. doi: 10.1073/pnas.96.4.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Rabold R, Abston E, Schofield B, Misra V, Galdzicka E, Lee H, Biswal S, Mitzner W, Tankersley CG. Effects of leptin deficiency on postnatal lung development in mice. J Appl Physiol. 2008;105:249–259. doi: 10.1152/japplphysiol.00052.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Johnston RA, Theman TA, Lu FL, Terry RD, Williams ES, Shore SA. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J Appl Physiol. 2008;104:1727–1735. doi: 10.1152/japplphysiol.00075.2008. [DOI] [PubMed] [Google Scholar]
- Kattan M, Kumar R, Bloomberg GR, Mitchell HE, Calatroni A, Gergen PJ, Kercsmar CM, Visness CM, Matsui EC, Steinbach SF, et al. Asthma control, adiposity, and adipokines among inner-city adolescents. J Allergy Clin Immunol. 2010;125:584–592. doi: 10.1016/j.jaci.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GG, Brown NJ, Diba C, Thorpe CW, Muñoz P, Marks GB, Toelle B, Ng K, Berend N, Salome CM. The effects of body weight on airway calibre. Eur Respir J. 2005;25:896–901. doi: 10.1183/09031936.05.00104504. [DOI] [PubMed] [Google Scholar]
- Loewy AD, Spyer KM. Central Regulation of Autonomic Functions. New York: Oxford University Press; 1990. [Google Scholar]
- Lu FL, Johnston RA, Flynt L, Theman TA, Terry RD, Schwartzman IN, Lee A, Shore SA. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am J Physiol Lung Cell Mol Physiol. 2006;290:L856–L865. doi: 10.1152/ajplung.00386.2005. [DOI] [PubMed] [Google Scholar]
- Lumb AB. Nunn’s Applied Respiratory Physiology. 7. Edinburgh: Churchill Livingstone/Elsevier; 2010. [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzoros C, Flier JS, Lesem MD, Brewerton TD, Jimerson DC. Cerebrospinal fluid leptin in anorexia nervosa: correlation with nutritional status and potential role in resistance to weight gain. J Clin Endocrinol Metab. 1997;82:1845–1851. doi: 10.1210/jcem.82.6.4006. [DOI] [PubMed] [Google Scholar]
- McMinn JE, Liu SM, Dragatsis I, Dietrich P, Ludwig T, Eiden S, Chua SC., Jr An allelic series for the leptin receptor gene generated by CRE and FLP recombinase. Mamm Genome. 2004;15:677–685. doi: 10.1007/s00335-004-2340-1. [DOI] [PubMed] [Google Scholar]
- Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, et al. Life without white fat: a transgenic mouse. Genes Dev. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Jr, Münzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 2009;9:117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair P, Radford K, Fanat A, Janssen LJ, Peters-Golden M, Cox PG. The effects of leptin on airway smooth muscle responses. Am J Respir Cell Mol Biol. 2008;39:475–481. doi: 10.1165/rcmb.2007-0091OC. [DOI] [PubMed] [Google Scholar]
- Nguyen LP, Lin R, Parra S, Omoluabi O, Hanania NA, Tuvim MJ, Knoll BJ, Dickey BF, Bond RA. Beta2-adrenoceptor signaling is required for the development of an asthma phenotype in a murine model. Proc Natl Acad Sci USA. 2009;106:2435–2440. doi: 10.1073/pnas.0810902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006;27:495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- Rivera-Sanchez YM, Johnston RA, Schwartzman IN, Valone J, Silverman ES, Fredberg JJ, Shore SA. Differential effects of ozone on airway and tissue mechanics in obese mice. J Appl Physiol. 2004;96:2200–2206. doi: 10.1152/japplphysiol.00960.2003. [DOI] [PubMed] [Google Scholar]
- Shore SA, Rivera-Sanchez YM, Schwartzman IN, Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol. 2003;95:938–945. doi: 10.1152/japplphysiol.00336.2003. [DOI] [PubMed] [Google Scholar]
- Stenius-Aarniala B, Poussa T, Kvarnström J, Grönlund EL, Ylikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ. 2000;320:827–832. doi: 10.1136/bmj.320.7238.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD, Davis HR., Jr Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest. 1997;99:385–390. doi: 10.1172/JCI119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoirbeek JA, Rinaldi M, De Vooght V, Haenen S, Bobic S, Gayan-Ramirez G, Hoet PH, Verbeken E, Decramer M, Nemery B, Janssens W. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am J Respir Cell Mol Biol. 2010;42:96–104. doi: 10.1165/rcmb.2008-0487OC. [DOI] [PubMed] [Google Scholar]
- Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- Wirth A, Benyó Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horváth B, Maser-Gluth C, Greiner E, et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Miyakawa T, Duttaroy A, Yamanaka A, Moriguchi T, Makita R, Ogawa M, Chou CJ, Xia B, Crawley JN, et al. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001;410:207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
- Yamanishi T, Chapple CR, Yasuda K, Chess-Williams R. The role of M(2)-muscarinic receptors in mediating contraction of the pig urinary bladder in vitro. Br J Pharmacol. 2000;131:1482–1488. doi: 10.1038/sj.bjp.0703719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.