Abstract
Background
The effect of hyperoxygenation at reperfusion, particularly in the setting of cardiac arrest, remains unclear. This issue was studied in a prolonged cardiac arrest model consisting of 25 minutes cardiac arrest in a rat resuscitated with cardiopulmonary bypass (CPB). The objective of this study was to determine the effect of hyperoxygenation following prolonged cardiac arrest resuscitation on mitochondrial and cardiac function.
Methods
Male Sprague-Dawley rats (400–450 g) were anesthetized with ketamine and xylazine and instrumented for closed chest cardiopulmonary bypass (CPB). Following a 25-minute KCl-induced cardiac arrest, the animals were resuscitated by CPB with 100% oxygen. Three minutes after successful return of spontaneous circulation (ROSC), the animals received either normoxemic reperfusion (CPB with 40–50% oxygen) or hyperoxemic reperfusion (CPB with 100% oxygen) for one hour. Post-resuscitation hemodynamics, cardiac function, mitochondrial function and immunostaining of 3-nitrotyrosine were compared between the two different treatment groups.
Results
At one hour after ROSC, the hyperoxemic reperfusion group had a significant higher mean arterial pressure, less metabolic acidosis and better diastolic function than the normoxemic reperfusion group. Cardiac mitochondria from the hyperoxemic reperfusion group had a higher respiratory control ratio (RCR) and cardiac tissue showed less nitroxidative stress compared to the normoxemic reperfusion group.
Conclusions
One hour of hyperoxemic reperfusion after 25 minutes of cardiac arrest in an in vivo CPB model resulted in significant short-term improvement in myocardial and mitochondrial function compared with one hour of normoxemic reperfusion. This myocardial response may differ from previously reported post-arrest hyperoxia mediated effects following shorter arrest times.
Keywords: Hyperoxic reperfusion, Cardiopulmonary bypass, Cardiopulmonary resuscitation, Mitochondria, Nitroxidative stress, Oxygen
1. INTRODUCTION
Following an ischemic insult to the tissue, it is imperative to reintroduce blood flow and oxygen to the affected region for revitalization. However, reperfusion also provokes a cascade of pathological responses leading to reperfusion injury.1 Based upon this “oxygen paradox” theory, it is inferred that hyperoxygenation at reperfusion is harmful because of higher reactive oxygen species (ROS) production. The supportive evidence in the literature is controversial. For example, hyperoxemic reperfusion after cerebral global ischemia has demonstrated worse neurological outcome in pigs,2 dogs3,4 and rats.5 In a Langendorff buffer-perfused heart model, hearts with hyperoxic reperfusion (pO2 = 700 torr [93.3 kPa]) after 30-min ischemia had impaired recovery compared to hearts with lower oxygen tension at reperfusion (pO2 = 500 torr [66.7 kPa]).6 Other studies have reported similar adverse effects of hyperoxic reperfusion in kidneys7 and lungs.8 Recently in a clinical study, post cardiac arrest patients admitted to the intensive care units with initial hyperoxemia (PaO2 > 300 torr [40.0 kPa]) were noted to have a higher hospital mortality than patients with initial normoxia or hypoxia.9
Not all studies are in agreement with the deleterious effects of post ischemia hyperoxygenation. It has been shown that the infarct size of the heart does not increase with hyperoxic reperfusion in rabbits10 and rats11, and the incidence of lethal arrhythmias at reperfusion was less with hyperoxic reperfusion.11 In a canine coronary occlusion study, hyperoxic reperfusion demonstrated improved infarct size and left ventricular function.12 Recent clinical trials in acute myocardial infarction patients undergoing primary coronary intervention demonstrated no harmful and possibly beneficial effects with regional hyperoxemic reperfusion.13,14
In the clinical setting, patients who have been successfully resuscitated from cardiac arrest often remain on 100% oxygen immediately after resuscitation and while awaiting transfer to an intensive care unit. This may result in extended periods of hyperoxemia.15 In this study, a rat cardiac arrest model resuscitated with cardiopulmonary bypass (CPB) was used to examine the short-term cardiac functional recovery, cardiac mitochondrial respiration and swelling and the degree of nitro-oxidative stress in the heart after one-hour of hyperoxemic or normoxemic reperfusion. All but one of these measures were improved after 1 hour of hyperoxemic reperfusion compared with normoxemic reperfusion.
2. MATERIALS AND METHODS
2.1 The CPB resuscitation model
Male Sprague-Dawley rats (400–450 g) were cared for in accordance with the National Institutes of Health (NIH) guidelines and the Institutional Animal Care and Use Committee. Animals were anesthetized with an intraperitoneal injection of ketamine/xylazine (35/5 mg/kg). A tracheotomy was performed. Animals received mechanical ventilation with room air at a rate of 35 breaths/min, a tidal volume of 8 ml/kg, and a positive end expiratory pressure of 3 cm H2O (model 683, Harvard Apparatus, Holliston, MA). The left femoral artery and left femoral vein were cannulated with 22-gauge catheters for arterial pressure monitoring (HPA-410a, Digi-Med, Louisville, KY) and continuous anesthetic infusion during instrumentation (ketamine 18–36 mg/kg/hr + xylazine 0.4–0.8 mg/kg/hr, ketamine:xylazine ratio = 45:1). Volatile anesthetics were not chosen because of the concerns about mitochondrial depression16,17 and muscle relaxants were not used. The left jugular vein was cannulated by PE-60 tubing for central venous pressure monitoring (LPA, Digi-Med, Louisville, KY) and drug administration. The right carotid artery was cannulated with PE-25 (OD 0.91 mm, ID 0.46 mm) tubing and advanced into the left ventricle (LV) for hemodynamic monitoring (HPA-410a, Digi-Med, Louisville, KY). A 14-gauge catheter with 5 side holes was inserted into the right jugular vein and a 22-gauge catheter was inserted into the right femoral artery for the cardiopulmonary bypass circuit. The CPB circuit included a digital peristaltic pump (Masterflex Model 7550–90, Cole-Parmer, Vernon Hills, IL) and a membrane oxygenator (Martin Humbs, Ingenieurbuero für Feinwerktechnik, Germany). Core body temperature was maintained at 37 °C by a thermistor-controlled heat lamp. Peripheral oxygenation was measured with pulse oximetry (NPB-40, Nellcor, Boulder, CO). Cardiac rhythm was monitored using a three lead ECG with electrocardiograph leads inserted subcutaneously into the chest wall (Lifepak 9, Physio-Control, Redmond, WA). The total instrumentation time was about 1–1.5 hours.
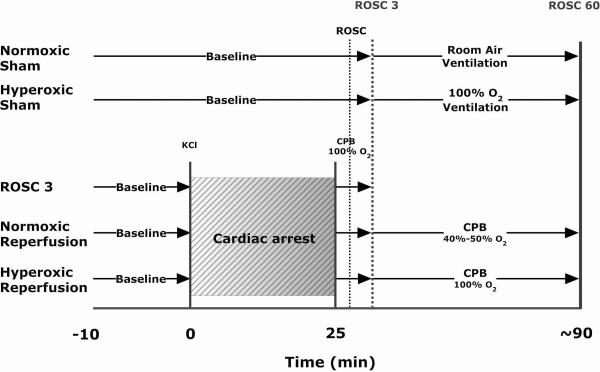
Following instrumentation, heparin (1000 U/kg) was administered 10 min before arrest and an arterial blood gas was measured. Animals were assigned to 5 groups: 1) sham arrest with 21% oxygen for the whole experiment (normoxemic sham, NS), 2) sham arrest with 100% oxygen for the last hour of the experiment (hyperoxemic sham, HS), 3) 25 min cardiac arrest, resuscitated by CPB with 100% oxygen until 3 minutes after return of spontaneous circulation (ROSC 3), 4) 25 min cardiac arrest, CPB with 100% oxygen until ROSC 3 and then switched to 40%–50% oxygen until 60 minutes after ROSC to maintain a normal PaO2 (normoxemic reperfusion, NR) and 5) 25 min cardiac arrest, CPB with 100 % oxygen until 60 minutes after ROSC (hyperoxemic reperfusion, HR) (Fig. 1). Cardiac arrest was induced with potassium chloride (KCl, 0.07mg/g) given via the jugular vein and ventilation was stopped. After 25 minutes of normothermic cardiac arrest, animals were resuscitated with CPB. The CPB circuit was primed with 20 ml of Plasma-lyte A, pH 7.4 (Baxter, Deerfield, IL) and oxygenated with 100% oxygen in the beginning. The flow rate for CPB was set at 120 ml/kg/min. ROSC was defined as the appearance of a regular, sustained pulsatile left ventricular wave form with a developed pressure > 40 mmHg as measured by the LV catheter. Once ROSC was obtained, ventilation was resumed at a reduced rate (25 breaths/min). Continuous intravenous phenylephrine infusion was titrated to maintain the mean arterial pressure at 60 mmHg. The maximal infusion dosage of phenylephrine was 1.5 μg/kg/min to minimize inotropic effects. Three minutes after ROSC, sodium bicarbonate (1 meq/kg) was given. Arterial blood gases were measured at 3, 15, 30 and 60 minutes after ROSC (GEM Premier 3000 Instrumentation Laboratory, IL). Intermittent boluses of Plasma-lyte A fluid were added to the reservoir to compensate for the vascular leakage into the interstitial tissue space and keep the bypass flow constant throughout the experiment. No additional anesthetic was needed after KCl-induced cardiac arrest in the three treatment groups, as all animals remained comatose. At the end of the protocol animals were euthanized with an additional bolus of anesthetic and surgical removal of the heart.
Figure 1.
Experimental protocol. ROSC = return of spontaneous circulation; CPB = cardiopulmonary bypass.
2.2 Isolation of heart mitochondria
Mitochondria were isolated from the excised whole heart as previously described18 except that type XXIV protease was replaced by 250 U (1mg) collagenase19 (type II, Invitrogen, Carlsbad, CA) in 1ml buffer without EGTA (sucrose 250 mM, HEPES 10 mM, pH 7.2). The protein concentration of isolated mitochondria was determined by an UV absorption method on a UV-VIS spectrophotometer (HP 8452a, Agilent Technologies, Santa Clara CA) after mitochondrial protein samples were dissolved in 0.02% n-dodecyl β-D-maltoside phosphate buffered saline (PBS) buffer.20
2.3 Mitochondrial respiration and swelling assay
Mitochondrial respiration was measured using well defined methods.18 Mitochondrial swelling was measured by incubating 0.25 mg of mitochondrial protein in 1 ml swelling assay buffer (250 mM sucrose, 10 mM HEPES, 6 mM succinate, 1.2 μM rotenone, and 250 μM CaCl2, pH 7.4 at room temperature). The change of light absorbance at 520 nm, indicating calcium-induced swelling in energized mitochondria, was measured with a UV-VIS spectrophotometer at room temperature (HP 8452a, Agilent Technologies, Santa Clara CA) every 10 min over a one-hour period.
2.4 Immunohistochemical staining in the heart
Immunohistochemical staining for 3-nitrotyrosine was performed for identification of nitroxidative stress in the myocardium. At the end of the protocol, two hearts from each group was fixed in 10% buffered formalin for 24 hours, embedded in paraffin, and then sectioned with a Leica RM2125 RT microtome. Multiple 4 μm-thick sections from each heart were deparaffinized, rehydrated and blocked with 10% normal goat serum (Vector Laboratories, Burlingame, CA) for one hour. Sections were then incubated in primary rabbit anti-nitrotyrosine antibody (1.33 μg/ml in serum, Millipore, Billerica, MA) or rabbit IgG (Vector Laboratories) at the same concentration for negative control, overnight at 4° C in a humid chamber. After PBS washes, all sections were incubated in secondary biotinylated goat anti-rabbit IgG (7.5 μg/ml in serum, Vector Laboratories) for 30 minutes at room temperature. After PBS washes, all sections were incubated with ABC-HRP solution (Vector Laboratories) for 30 minutes, washed and then incubated with the HRP substrate 3,3'-diaminobenzidine (Kirkegaard and Perry Laboratories, Gaithersburg, MD) in the dark for 8 minutes. All sections were then rinsed in distilled water and counterstained with hematoxylin-2 (Richard-Allan Scientific, Kalamazoo, MI). Sections were dehydrated in a graded series of ethanol washes, cleared in Clear-Rite 3 (Richard-Allan Scientific) and cover slipped with Permount (Thermo Fisher Scientific, Fair Lawn, NJ). Slides were photographed with a Zeiss Axioskop 40 inverted microscope coupled to an AxioCam MRc5 camera, using AxioVision LE software.
2.5 Statistical Methods
All data were expressed as mean ± SEM. Statistical analyses were carried out with one-way ANOVA and followed by Tukey's post hoc analysis. Significance was assigned at P < 0.05.
3. RESULTS
3.1 Resuscitation and arterial blood gases
The CPB resuscitation time, defined as the start of CPB circuit to ROSC, was comparable in both treatment groups (NR group: 138 ± 32 sec, HR group: 132 ± 32 sec, n = 8/group). The total volume of Plasma-lyte A added during the post ROSC period was similar between groups (NR group: 122 ± 17 ml, HR group: 119 ± 10 ml, n = 8/group). Based on arterial blood gases, both groups showed the same change in blood pH and PaCO2. The HR group maintained an arterial PaO2 above 400 torr [53.3 kPa] while the NR group had an arterial PaO2 of approximately 100 torr [13.3 kPa] at 15, 30 and 60 minutes after ROSC (Table 1). During the one-hour reperfusion, the HR group had a significantly higher base excess level than the NR group at 30 and 60 minutes after ROSC, suggesting a decreased level of metabolic acidosis in the HR group (Table 1). Both groups maintained a hemoglobin level above 6 g/dL throughout the experiment (NR group: 6.4 ± 0.2 g/dL, HR group: 6.5 ± 0.4 g/dL at ROSC 60 minutes).
Table 1.
Arterial blood gases of the normoxic reperfusion (NR) and hyperoxic reperfusion (HR) groups at different time points.
| Group | pH | pO2 (torr) | Base Excess (mEq/L) | Hemoglobin (mg/dL) | |
|---|---|---|---|---|---|
| Baseline | NR | 7.46 ± 0.01 | 99 ± 5 | 0.2 ± 0.6 | 13.0 ± 0.2 |
| HR | 7.47 ± 0.01 | 95 ± 4 | −0.4 ± 0.6 | 12.9 ± 0.4 | |
|
| |||||
| ROSC3 | NR | 7.16 ± 0.02 | 409 ± 17 | −20.4 ± 0.6 | 6.9 ± 0.5 |
| HR | 7.15 ± 0.03 | 442 ± 12 | −19.6 ± 0.4 | 6.6 ± 0.4 | |
|
| |||||
| ROSC 15 | NR | 7.31 ± 0.02 | 94 ± 10 | −16.5 ± 0.7 | 7.2 ± 0.3 |
| HR | 7.35 ± 0.04 | 395 ± 16† | −14.6 ± 0.9 | 7.0 ± 0.2 | |
|
| |||||
| ROSC 30 | NR | 7.40 ± 0.02 | 106 ± 8 | −15.4 ± 1.0 | 7.1 ± 0.3 |
| HR | 7.41 ± 0.03 | 437 ± 20† | −13.0 ± 0.5* | 6.4 ± 0.4 | |
|
| |||||
| ROSC 60 | NR | 7.48 ± 0.03 | 112 ± 12 | −13.1 ± 1.1 | 6.4 ± 0.2 |
| HR | 7.52 ± 0.02 | 441± 25† | −10.4 ± 0.4* | 6.5 ± 0.4 | |
Data expressed as mean ± SEM, n=8/group,
p < 0.05,
p < 0.001
3.2 Hemodynamics
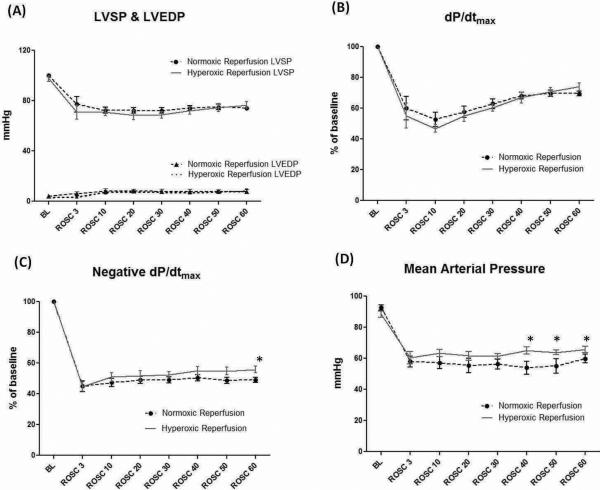
Following the onset of ROSC, a pulsatile arterial pressure was seen in all hearts during CPB. Both normoxemic and hyperoxemic reperfusion groups showed similar functional recovery of left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP), and dP/dtmax (Fig. 2A & Fig. 2B). However, the HR group had a significantly higher negative dP/dtmax compared to the NR group at ROSC 60 minutes (55.9 ± 2.1% vs. 49.1 ± 1.7%, n = 8/group, p < 0.05), indicating better diastolic function (relaxation) (Fig. 2C). The HR group had a significantly higher mean arterial pressure than the NR group despite the same degree of vasopressor support (Fig. 2D). In both groups, six out of eight animals received the maximal infusion dose of phenylephrine during the reperfusion period.
Figure 2.
A) There were no differences between groups in left ventricular systolic pressures (LVSP), end-diastolic pressures (LVEDP) and B) dP/dtmax•. C) Negative dP/dtmax was significantly higher at ROSC 60 min in the hyperoxemic reperfusion group compared to the normoxemic reperfusion group. D) The hyperoxemic reperfusion group had a significantly higher MAP than the normoxemic reperfusion group at ROSC 40, 50 and 60 min.
BL = baseline; ROSC = return of spontaneous circulation. (Data expressed as mean ± SEM, n = 8/group, *p < 0.05)
3.3 Mitochondrial swelling assay
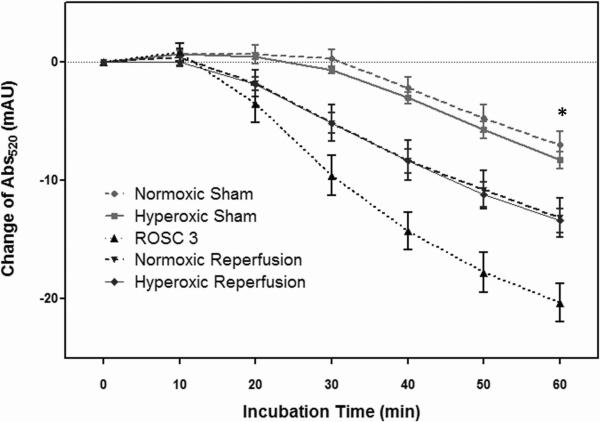
Among all groups, mitochondria from the ROSC 3 group showed the least resistance to calcium-induced swelling over the 60 min incubation period, implying that the ROSC 3 group had the most damaged mitochondria (Fig. 3, n=6/group). After one hour of normoxemic or hyperoxemic reperfusion, the severity of calcium-induced swelling was significantly improved as compared with the ROSC 3 group (p < 0.01). There was no statistical difference in mitochondrial swelling between the HR and NR groups, or between the sham groups.
Figure 3.
Mitochondrial swelling assay. After 60 min of incubation, the ROSC 3 group had the greatest decline of Abs520 compared to other groups (*p < 0.01). Both HR and NR groups had significantly worse mitochondrial swelling than the sham groups (*p < 0.01). However, there was no statistical difference between the HR and NR groups, nor between the sham groups. (Data expressed as mean ± SEM, n = 6/group)
3.4 Mitochondrial respiration
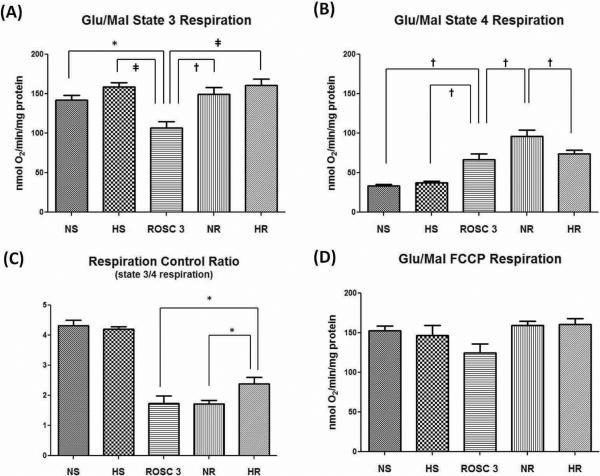
The NAD-linked respiration, using glutamate/malate as substrates in isolated mitochondria demonstrated that both NR and HR groups had comparable state 3 respiration (Fig. 4A) but the NR group had a significantly higher state 4 respiration (Fig. 4B), which resulted in a lower respiration control ratio (RCR) (Fig. 4C, n=6/group). These findings suggest that the mitochondria in the NR group were less coupled to phosphorylation than those in the HR group. The ROSC 3 group had the lowest state 3 respiration (Fig. 4A) suggesting that complex I was reversibly compromised (low RCP) 3 min after ROSC (ROSC 3 group) and improved during the 60 min reperfusion period in the HR group but not the NR group (Fig 4C). There were no significant group differences with the uncoupler carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP)-stimulated respiration (Fig. 4D).
Figure 4.
Mitochondrial respiration using glutamate/malate (10mM/ 5mM) as substrates. (A) State 3 respiration. The ROSC 3 group had the lowest state 3 respiration and was statistically significant from any other group (*p < 0.05, †p < 0.01, ‡p < 0.001); (B) State 4 respiration. The NR group had a higher state 4 respiration than the HR group (†p < 0.01); (C) Respiration control ratio (RCR). The HR group had a significantly higher RCR than the NR group. (*p < 0.05); (D) FCCP-stimulated respiration. There was no statistical difference among the groups. (Data expressed as mean ± SEM, n=6/group)
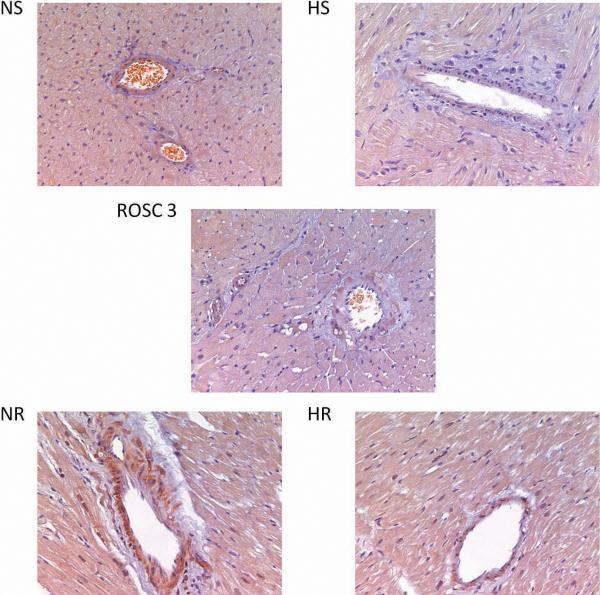
3.5 Immunohistochemical staining of 3-nitrotyrosine
Staining of 3-nitrotyrosine residues was seen in all groups. However, the NR group had the most prominent staining especially in the perivascular regions (Fig. 5). These findings suggest that the NR group experienced more nitro-oxidative stress than the HR group. The perivascular smooth muscle cells seem to be the major site of nitroxidative stress as the staining of 3-nitrotyrosine is always darker in the perivascular smooth muscle layer.
Figure 5.
Representative slides of 3-nitrotyrosine immunohistochemical staining which show the most prominent staining in the NR group, particularly in the perivascular regions.
4. DISCUSSION
In the present study, a rat CPB resuscitation model was used to evaluate the effects of hyperoxygenation in the post cardiac-arrest period. After a prolonged normothermic cardiac arrest (25 min), hyperoxemic reperfusion for one hour with CPB showed significant improvement of systemic hemodynamics, less metabolic acidosis, better mitochondrial respiratory function and less nitroxidative stress when compared with normoxemic reperfusion. This is in contrast to previous studies done by Fiskum et al which demonstrated the deleterious effects of hyperoxemic reperfusion on brain neurons after a 10-min cardiac arrest and resuscitation.4,5 These studies focused on CNS injury, utilized a much shorter cardiac arrest time and did not examine the post-resuscitation cardiac function. The results of the current study also differ from our previous work in which we utilized a shorter cardiac arrest time (6.5 min in the rat) resuscitated with CPR and then ventilated with one hour of hyperoxia or normoxia ventilation after ROSC. In this earlier study, cardiac mitochondrial function was depressed following hyperoxia when compared with one hour of normoxia.21
For most in vivo cardiac arrest animal studies, the cardiac arrest time is quite limited because of the difficulties of obtaining ROSC by conventional cardiopulmonary resuscitation. The use of CPB in our cardiac arrest model enabled us to extend the arrest time to 25 minutes and still achieve ROSC. Although, incorporation of chest compressions preceding CPB more closely approximates the clinical scenario of using CPB in refractory cardiac arrest patients,22 no CPR was done prior to starting CPB in order to minimize the variability introduced during reperfusion by chest compression generated blood flow. To our knowledge, this is the first invivo cardiac arrest study to report the change of cardiac mitochondrial function after a comparable length of ischemia as applied in Langendorff models. Mitochondrial dysfunction, a distinctive feature in cardiac ischemia/reperfusion injury,23 has been demonstrated in Langendorff isolated heart models after ischemia times of 25–30 minutes with further deterioration following reperfusion.24–26 In this study, our data suggests improved mitochondrial function with one hour of reperfusion. To our knowledge, this is the first in vivo cardiac arrest study to report the change of cardiac mitochondrial function after a comparable length of ischemia as applied in Langendorff models. Moreover, hyperoxemic reperfusion seems to improve the mitochondrial function when compared with normoxemic reperfusion despite the absence of mitochondrial swelling. Based on these findings, it can be inferred that 1) hyperoxemic reperfusion in this model induces less mitochondrial uncoupling and 2) the mitochondrial permeability transition pore is not differentially affected by the oxygen level at reperfusion.
Nitration of protein tyrosine residues is a marker of nitroxidative stress, which is derived from the imbalance of oxidant-antioxidant homeostasis.27 Although 3-nitrotyrosine is produced by peroxynitrite and heme peroxidase-dependent nitration, both pathways are regulated by the production rate of superoxide and nitric oxide.27 Our results showed that hyperoxemic reperfusion produced less nitroxidative stress than normoxemic reperfusion. In regard to the complexity of interplay between superoxide and nitric oxide in reperfusion,28 more studies are warranted to elucidate the exact molecular mechanism behind our observation.
We surmise that the conflicting cardiac mitochondrial results in our model with hyperoxemic reperfusion following short21 and long cardiac arrest times (current study) exemplifies the heterogeneous nature of post-ischemia reperfusion conditions. During the ischemia/reperfusion process, tissue re-oxygenation not only depends on the arterial oxygen tension but also on the degree of vascular injury. After long periods of ischemia, the “no reflow phenomenon”, elicited by endothelial dysfunction, microvascular thrombosis and inflammation29 can prevent the tissue from adequate oxygenation despite normal oxygen content in the blood. In such an environment, a higher oxygen gradient may allow for greater oxygen diffusion to the ischemic tissue. However, when the endothelial function is preserved and the microcirculation is intact, i.e. shorter ischemia times, hyperoxemic reperfusion may actually increase redox mediated reperfusion injury. Further studies are required to examine this hypothesis.
A number of important mechanistic questions remain which warrant further work. First, although we carefully controlled arterial oxygen partial pressure during the reperfusion period, we do not know the actual oxygen tension exposure at the tissue or mitochondrial level. As postulated, conditions of ischemia (duration of arrest) and reperfusion (microcirculatory changes) may allow for cellular hypoxia despite arterial normoxemia. Due to the prolonged duration of cardiac arrest, it was not possible to wean animals from bypass and maintain uniform reperfusion conditions during the 60 minute period. To control reperfusion between groups, a significant amount of crystalloid fluid was administered to keep bypass flow constant and phenylephrine was used to standardize arterial pressure. It is difficult to extrapolate the short-term benefits seen into long-term outcome because of the non-survival nature of the experimental design. The anemia which resulted from hemodilution was similar between groups. It has been shown that, in Sprague-Dawley rats, the oxygen delivery is relatively constant when the hemoglobin level is greater than 6 g/dL. The critical hemoglobin level is estimated to be 2.4 g/dL.30 In our model, the hemoglobin levels of both groups at ROSC 60 remained above 6 g/dL, presumably enough for adequate oxygen delivery. Whether the benefits of hyperoxygenation will remain when the anemia is corrected is unclear. Finally, the duration of hyperoxemia, likely an important determinant of outcome, was not addressed in this study.
5. CONCLUSION
In conclusion, in this in vivo prolonged cardiac arrest model, one hour of hyperoxemic reperfusion after a prolonged cardiac arrest of 25 minutes with controlled reperfusion using CPB resulted in a significant short-term improvement in myocardial and mitochondrial function compared with one hour of normoxemic reperfusion. This myocardial response to post-arrest hyperoxia may differ from previously reported post-arrest hyperoxia mediated neurologic and cardiac effects in models of shorter cardiac arrest duration.
ACKNOWLEDGMENTS
We greatly thank Dr. Tomas Drabek and Jason Stezoski from the Safar Center for Resuscitation Research of the University of Pittsburgh for the technical instruction of the instrumentation for the CPB model.
FUNDING The study was performed at the Ohio State University and supported by American Heart Association (Yeh, 09PRE2170046; Angelos, 60015791) and National Institutes of Health (Wilgus, CA127109).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST There are no commercial relations involving any of the authors that might pose a conflict of interest in connection with this manuscript.
REFERENCES
- 1.Hoffman JW, Jr., Gilbert TB, Poston RS, et al. Myocardial reperfusion injury: etiology, mechanisms, and therapies. J Extra Corpor Technol. 2004;36:391–411. [PubMed] [Google Scholar]
- 2.Douzinas EE, Andrianakis I, Pitaridis MT, et al. The effect of hypoxemic reperfusion on cerebral protection after a severe global ischemic brain insult. Intensive Care Med. 2001;27:269–75. doi: 10.1007/s001340000796. [DOI] [PubMed] [Google Scholar]
- 3.Zwemer CF, Whitesall SE, D'Alecy LG. Cardiopulmonary-cerebral resuscitation with 100% oxygen exacerbates neurological dysfunction following nine minutes of normothermic cardiac arrest in dogs. Resuscitation. 1994;27:159–70. doi: 10.1016/0300-9572(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 4.Balan IS, Fiskum G, Hazelton J, et al. Oximetry-guided reoxygenation improves neurological outcome after experimental cardiac arrest. Stroke. 2006;37:3008–13. doi: 10.1161/01.STR.0000248455.73785.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hazelton JL, Balan I, Elmer GI, et al. Hyperoxic reperfusion after global cerebral ischemia promotes inflammation and long-term hippocampal neuronal death. J Neurotrauma. 2010;27:753–62. doi: 10.1089/neu.2009.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaneda T, Ku K, Inoue T, et al. Postischemic reperfusion injury can be attenuated by oxygen tension control. Jpn Circ J. 2001;65:213–18. doi: 10.1253/jcj.65.213. [DOI] [PubMed] [Google Scholar]
- 7.Zwemer CF, Shoemaker JL, Jr., Hazard SW, III, et al. Hyperoxic reperfusion exacerbates postischemic renal dysfunction. Surgery. 2000;128:815–21. doi: 10.1067/msy.2000.109117. [DOI] [PubMed] [Google Scholar]
- 8.Ellman PI, Alvis JS, Tache-Leon C, et al. Hyperoxic ventilation exacerbates lung reperfusion injury. J Thorac Cardiovasc Surg. 2005;130:1440, e1–8. doi: 10.1016/j.jtcvs.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 9.Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303:2165–71. doi: 10.1001/jama.2010.707. [DOI] [PubMed] [Google Scholar]
- 10.Shnier CB, Cason BA, Horton AF, et al. Hyperoxemic reperfusion does not increase myocardial infarct size. Am J Physiol. 1991;260:H1307–12. doi: 10.1152/ajpheart.1991.260.4.H1307. [DOI] [PubMed] [Google Scholar]
- 11.Mariero LH, Rutkovskiy A, Stenslokken KO, et al. Hyperoxia during early reperfusion does not increase ischemia/reperfusion injury. Eur J Cardiothorac Surg. 2012;41:149–153. doi: 10.1016/j.ejcts.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly RF, Hursey TL, Parrillo JE, et al. Effect of 100% oxygen administration on infarct size and left ventricular function in a canine model of myocardial infarction and reperfusion. Am Heart J. 1995;130:957–65. doi: 10.1016/0002-8703(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 13.Bartorelli AL. Hyperoxemic perfusion for treatment of reperfusion microvascular ischemia in patients with myocardial infarction. Am J Cardiovasc Drugs. 2003;3:253–63. doi: 10.2165/00129784-200303040-00004. [DOI] [PubMed] [Google Scholar]
- 14.O'Neill WW, Martin JL, Dixon SR, et al. Acute Myocardial Infarction with Hyperoxemic Therapy (AMIHOT): a prospective, randomized trial of intracoronary hyperoxemic reperfusion after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50:397–405. doi: 10.1016/j.jacc.2007.01.099. [DOI] [PubMed] [Google Scholar]
- 15.Kuisma M, Boyd J, Voipio V, et al. Comparison of 30 and the 100% inspired oxygen concentrations during early post-resuscitation period: a randomised controlled pilot study. Resuscitation. 2006;69:199–206. doi: 10.1016/j.resuscitation.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Hall GM, Kirtland SJ, Baum H. The inhibition of mitochondrial respiration by inhalational anaesthetic agents. Br J Anaesth. 1973;45:1005–09. doi: 10.1093/bja/45.10.1005. [DOI] [PubMed] [Google Scholar]
- 17.Hanley PJ, Ray J, Brandt U, et al. Halothane, isoflurane and sevoflurane inhibit NADH:ubiquinone oxidoreductase (complex I) of cardiac mitochondria. J Physiol. 2002;544:687–93. doi: 10.1113/jphysiol.2002.025015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeh ST, Lee HL, Aune SE, Chen CL, Chen YR, Angelos MG. Preservation of mitochondrial function with cardiopulmonary resuscitation in prolonged cardiac arrest in rats. J Mol Cell Cardiol. 2009;47:789–97. doi: 10.1016/j.yjmcc.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toth PP, Ferguson-Miller SM, Suelter CH. Isolation of highly coupled heart mitochondria in high yield using a bacterial collagenase. Methods Enzymol. 1986;125:16–27. doi: 10.1016/s0076-6879(86)25004-1. [DOI] [PubMed] [Google Scholar]
- 20.Whitaker JR, Granum PE. An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal Biochem. 1980;109:156–59. doi: 10.1016/0003-2697(80)90024-x. [DOI] [PubMed] [Google Scholar]
- 21.Angelos MG, Yeh ST, Aune SE. Post cardiac-arrest hyperoxia and mitochondrial function. Resuscitation. 2011;82(Suppl 2):S48–S51. doi: 10.1016/S0300-9572(11)70151-4. [DOI] [PubMed] [Google Scholar]
- 22.Chen YS, Yu HY, Huang SC, et al. Extracorporeal membrane oxygenation support can extend the duration of cardiopulmonary resuscitation. Crit Care Med. 2008;36:2529–35. doi: 10.1097/CCM.0b013e318183f491. [DOI] [PubMed] [Google Scholar]
- 23.Lesnefsky EJ, Moghaddas S, Tandler B, et al. Mitochondrial dysfunction in cardiac disease: ischemia--reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001;33:1065–89. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 24.Lucas DT, Szweda LI. Declines in mitochondrial respiration during cardiac reperfusion: age-dependent inactivation of alpha-ketoglutarate dehydrogenase. Proc Natl Acad Sci U S A. 1999;96:6689–93. doi: 10.1073/pnas.96.12.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paradies G, Petrosillo G, Pistolese M, et al. Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circ Res. 2004;94:53–59. doi: 10.1161/01.RES.0000109416.56608.64. [DOI] [PubMed] [Google Scholar]
- 26.Bosetti F, Baracca A, Lenaz G, et al. Increased state 4 mitochondrial respiration and swelling in early post-ischemic reperfusion of rat heart. FEBS Lett. 2004;563:161–64. doi: 10.1016/S0014-5793(04)00294-7. [DOI] [PubMed] [Google Scholar]
- 27.Peluffo G, Radi R. Biochemistry of protein tyrosine nitration in cardiovascular pathology. Cardiovasc Res. 2007;75:291–302. doi: 10.1016/j.cardiores.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 28.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 29.Adams JA. Endothelium and cardiopulmonary resuscitation. Crit Care Med. 2006;34:S458–S465. doi: 10.1097/01.CCM.0000246012.68479.49. [DOI] [PubMed] [Google Scholar]
- 30.Torres Filho IP, Spiess BD, Pittman RN, et al. Experimental analysis of critical oxygen delivery. Am J Physiol Heart Circ Physiol. 2005;288:H1071–H1079. doi: 10.1152/ajpheart.00884.2004. [DOI] [PubMed] [Google Scholar]