Abstract
Recent clinical trials have raised concern that therapy with recombinant human erythropoietin (EPO) may increase cardiovascular disease risk, event rate, and mortality. Endothelial cell (EC) apoptosis has been implicated in both atherogenesis as well as in the destabilization and rupture of atheromatous plaques.
In the current study we observed that EPO and the EPO-mimetic peptide EMP-1 markedly suppressed lipopolysaccharide-induced apoptosis in EC monolayers. Therapeutic concentrations of EPO upregulated Bcl-2 expression while concurrently diminishing expression of Bax, resulting in a net decrease in the ratio of Bax to Bcl-2 protein concentrations. In vivo studies demonstrated that erythropoietin receptor (EPOR) is abundantly expressed in murine aorta and that EPO treatment for 10 weeks markedly decreased the ratio of Bax to Bcl-2 protein in the aortas of apolipoprotein E-deficient (APO E-KO) mice fed a high-fat diet. To our knowledge these data are the first to reveal a modulation of regulators of the apoptotic pathway in murine aorta by chronic EPO treatment. These observations imply that long-term administration of EPO may have the potential to affect plaque stability.
Keywords: Erythropoietin, Atherosclerosis, Apoptosis, Bcl-2, Bax, Apolipoprotein E-deficient mice
INTRODUCTION
The complications of accelerated atherosclerosis have become the leading cause of death among patients with chronic kidney disease (CKD). Cardiovascular mortality has been estimated to be approximately 30 times greater in these patients than in the general population.1 Recombinant human erythropoietin (EPO) has become the standard treatment of anemia that accompanies CKD. Despite its widespread use in a patient population prone to developing cardiovascular complications, the potentially varied effects of EPO on the vascular endothelium remain incompletely defined. Recent clinical studies suggest that EPO may have adverse effects on the cardiovascular system.2, 3 In a double-blinded randomized clinical trial involving more than 4,000 patients with diabetes, chronic kidney disease, and anemia, a significantly higher rate of stroke was observed in patients receiving an erythropoiesis stimulating agent compared to those treated with placebo.3 Reports from our group and others suggest that EPO may contribute to vascular dysfunction by upregulating expression of cytokine-induced monocyte chemoattractant protein-1 and by inhibiting the production of endothelial nitric oxide, effects fundamental to atherogenesis.4, 5
Endothelial cell apoptosis has been implicated in both atherogenesis as well as in the destabilization and subsequent rupture of atheromatous plaques. While the apoptotic pathways that are altered in atherosclerosis remain incompletely understood, EC apoptosis generally appears to play a detrimental role in plaque development and stability.6 Many atherogenic factors, including oxidized low density lipoproteins, angiotensin II, and oxidative stress can induce EC apoptosis which may result in increased permeability of the endothelial monolayer through loss of cells.7 This loss of integrity, in turn, may facilitate the migration of lipids, monocytes and vascular smooth muscle cells into the intima, further damaging the vasculature and propagating plaque development.8 Furthermore, EC apoptosis may critically contribute to plaque erosion which leads to thrombus formation and subsequent ischemia.9 In addition, apoptotic ECs have been shown to be procoagulant thereby promoting the accumulation of nonactivated platelets. 7, 10 The Bcl-2 family of proteins are potent regulators of apoptosis that control mitochondrial membrane permeability, thereby regulating release of apoptogenic factors from the intermembrane space into the cytoplasm. Anti-apoptotic Bcl-2 homologues such as Bcl-2, Bcl-xL, and A1 inhibit mitochondrial permeability transition pore opening, membrane depolarization, and cytochrome c release whereas members such as Bax, Balk, and Bok promote cell death.11 Bax opposes Bcl-2 bioactivity and triggers cytochrome release in vitro.12 Increased expression of Bax has been observed in endothelial cells in atherosclerotic lesions in allograft vasculopathy.13 Lipopolysaccharide (LPS) induces apoptosis in bovine and ovine endothelial cells in vitro and elicits human EC apoptosis in the absence of new gene expression.14 Induction of EC apoptosis by LPS in vitro results in decreased expression of Bcl-2 whereas overexpression of Bcl-2 in isolated mitochondria prevents apoptosis.15, 16 The LPS-induced decrease in Bcl-2 is accompanied by upregulation of Bax which has lead to the idea that such changes in the ratio of Bcl-2 and Bax trigger apoptosis.17, 18
Erythropoietin, the principal growth factor that promotes the survival, proliferation, and differentiation of erythroid progenitor cells, has been shown to repress apoptosis of murine erythroid progenitors by modulating the expression of Bcl-2 and Bcl-xL.19 Carlini et al. reported that EPO prevents LPS-induced apoptosis of endothelial cells but the mechanism by which EPO exerts this effect has not been investigated.20 Sekiguchi et al observed that EPO-treatment of ECs could reverse apoptosis induced by exposure to high concentrations of glucose. EPO-mediated inhibition of apoptosis was linked to phosphorylation of Akt which in turn resulted in inhibition of caspase 3.21, 22 However, these experiments employed EPO at a single concentration (100 U/ml) that is supraphysiologic by an order of magnitude. Chong et al. reported that EPO protects ECs from anoxia-induced apoptosis by activation of protein kinase B (Akt1) and inhibition of caspases 1, 3, and 9. 23
In the current study we explored the effect of physiologically relevant concentrations of EPO and the EPO-mimetic peptide EMP-1 on the apoptosis of EC monolayers. 4, 24, 25 This peptide bears no sequence homology with EPO but binds to EPOR and activates the same signaling pathways as EPO.25 To our knowledge, the present study is the first to demonstrate expression of EPOR in murine aorta and to assess the effect of chronic EPO treatment on the expression of members of the Bcl-2 family of proteins in an animal model of atherosclerosis. The dose of EPO used for our in vivo studies is equivalent to that used in humans and has been used in various animal models by numerous other laboratories underscoring the important implications that our in vivo findings may have for patients with cardiovascular morbidities.26–29
MATERIALS AND METHODS
Unless otherwise indicated, reagents were purchased from Sigma (St. Louis, MO). Recombinant human erythropoietin (Epogen) was obtained from Amgen (Thousand Oaks, CA). HUVECs and endothelial cell growth medium (EGM-2) were purchased from Clonetics (Walkersville, MD). EMP1 (GGTYSCHFGPLTWVCKPQGG-NH2) and its inactive analog iEMP1 (GGTYSSHFGPLTWVSKPQGG-NH2) were synthesized by the University of Michigan Protein Core Facility.25, 30, 31 LPS from Escherichia coli serotype 0111:B4 was used for all experiments.
Endothelial cell culture
Human umbilical vein endothelial cells (HUVECs) were grown at 37 °C in 5% CO2 on 150×25 mm plates as previously described.32 EGM-2 contains fetal bovine serum (FBS), hydrocortisone, recombinant human fibroblast growth factor (hFGF), vascular endothelial growth factor (VEGF), recombinant long release insulin-like growth factor (R3-IGF), ascorbic acid, recombinant human epidermal growth factor (hEGF), gentamycin sulfate and amphotericin B (GA-1000), and heparin. The cells were used between first and third passage.
Apolipoprotein E-deficient mice
Experiments were conducted in accordance with the NIH guidelines for the care and use of laboratory animals. Male apo E-deficient mice on a Balb/c background were generated as previously described.33 The animals used in the present study were F5 generation mice. The mice were housed at the University of Michigan unit for Laboratory Medicine (ULAM), subjected to fixed 12 hour light/dark cycles, and received a high fat diet (Adjusted Calories Diet TD88137, Harlan Teklad, Madison, WI) and water ad libitum. Starting at the age of 13 weeks, the mice were injected subcutaneously (s.c.) with Epogen (30 U) every other day for 10 weeks. Control mice were injected s.c. with an equivalent volume (200 µl) of saline. Following completion of the study, the mice were euthanized by an i.p. injection of pentobarbital. Harvested organs and plasma were snap-frozen in liquid nitrogen.
Treatment of HUVECs
The treatment schedule and sample abbreviations for all experiments are outlined in table 1. HUVECs were treated with EGM-2, EPO, or EPO and genistein, respectively for 24 hours. This was followed by a 4 hour serum starvation period. During this time the cells were incubated with EGM-2 in which FBS and VEGF had been omitted. Subsequently, cells were treated with either EGM-2 alone (without VEGF) or with addition of LPS (100 ng/ml) for 20 hours. For experiments with hydrogen peroxide, HUVECs were treated with medium or EPO respectively for 24 hours. The cells were then incubated in presence or absence of 250 µM of hydrogen peroxide for 30 minutes followed by another 24 period in presence of medium.
Table 1.
| Sample | M/M | M/LPS | EPO/M | EPO/LPS | EPO+ Genistein/ M |
EPO+ Genistein/ LPS |
H.I.EPO /LPS |
EMP1/ LPS |
iEMP1/LPS |
|---|---|---|---|---|---|---|---|---|---|
| 24 hours | EGM-2 | EGM-2 | EGM-2 + EPO (5 U/ml) |
EGM-2 + EPO (5 U/ml) |
EGM-2 + EPO (5 U/ml) + Genistein (10µM) |
EGM-2 + EPO (5 U/ml) + Genistein (10µM) |
EGM-2 + heat- inactivated EPO (5U/ml) |
EGM-2 + 0.1 µM EMP1 |
EGM-2 + 0.1 µM iEMP1 |
| 4 hours | EGM-2 without VEGF and FBS | ||||||||
| 20 hours | EGM-2 without VEGF |
EGM-2 without VEGF; LPS (100 ng/ml) |
EGM- without VEGF |
EGM-2 without VEGF; LPS (100 ng/ml) |
EGM-2 without VEGF |
EGM-2 without VEGF; LPS (100 ng/ml) |
EGM-2 without VEGF; LPS (100 ng/ml) |
EGM-2 without VEGF; LPS (100 ng/ml) |
EGM-2 without VEGF; LPS (100 ng/ml) |
Flow cytometric analysis of apoptotic cells
Apoptosis was determined by flow cytometry based on the cell surface expression of phosphatidylserine with Annexin V staining. Cell staining for flow-cytometric analysis was performed using an Annexin V-FITC Apoptosis Detection Kit I from BD Biosciences Pharmingen (San Diego, CA). Staining with propidium iodide was used to simultaneously monitor cell necrosis. Endothelial cells were treated as specified. The cells were trypsinized, centrifuged and washed twice with cold PBS. They were resuspended in 1×binding buffer at a concentration of 1×106 cells/ml. An aliquot of 100 µl of the solution (1×105 cells) was transferred to a 5 ml culture tube. After addition of Annexin V-FITC (5 µl) and PI (5µl), the cells were vortexed gently and incubated for 15 minutes at room temperature in the dark. Following addition of binding buffer (400 µl), the cells were analyzed with a Becton Dickinson FACS Scan (Becton Dickinson, Mountain View, CA) using a Macintosh G4 computer and CellQuest (Becton Dickinson) software for analysis.
Histone-associated-DNA-fragments
Histone-associated-DNA-fragments were measured using a Cell Death Detection ELISAPLUS (Roche Diagnostics GmbH, Penzberg, Germany). Briefly, endothelial cells were grown to confluence on a 96- well cell culture plate and treated as specified in table 1. The medium was removed and the cells were incubated in lysis buffer for 30 minutes at room temperature. The lysate was centrifuged at 200 g for 10 minutes and 20 µl of supernatant was transferred into the streptavidin coated microtiter plate provided in the kit. Following 2 hours of incubation, the microtiter plate was processed according to manufacturer’s directions. Absorbance at 405 nm with a reference wavelength of 490 nm (A405-A490) was measured using an automated microplate reader (ELx808, Bio-Tek Instruments, Winooski, VT).
Preparation of aortic lysates and HUVEC extracts
To prepare aortic lysates, previously snap-frozen tissue was immersed in ice-cold radioimmunoprecipitation (RIPA) buffer and homogenized at 4 °C. Protein determination was carried out by Micro BCA assay (Pierce, Rockford, IL). HUVEC extracts were prepared as previously described.17 Briefly, HUVECs were harvested by scraping and centrifuged in PBS for 5 minutes at 1200 rpm. The pelleted cells were incubated in lysis buffer (10 mM Tris-HCl, pH 8.0, 0.32 M sucrose. 5mM EDTA, 1% Triton X-100, 2 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride (PMSF) for 10 minutes on ice. Following centrifugation for 5 minutes, the supernatants were collected, subjected to a Micro BCA assay, and subsequently used for Western blots.
Western Blot Analysis
HUVEC extracts (corresponding to 5 µg total protein) or aortic lysates (corresponding to 30 µg of total protein) were denatured in Tris-glycine sample buffer (Novex, San Diego, California) and electrophoresed on 4% to 20% Tris-glycine gels using Tris/Glycine/SDS running buffer (Bio-Rad, Minneapolis, Minnesota). Rainbow markers (Amersham) were used as molecular weight standards. The proteins were transferred to a nitrocellulose membrane using a mini-Trans blot for 6 hours at room temperature with 250 mA of current. Blots were blocked overnight with a solution of 1 % BSA in Tris-buffered saline (TBS) containing 0.1% Tween-20 (TBS-T). After 3 washes with TBS-T, the Bcl-2, Bax (Santa Cruz Biotechnology) or EPOR antibody (AF 1390, R&D Systems) was added (1:2500 dilution for Bax and Bcl-2 antibodies; 1:500 for EPOR antibody) and the blots were incubated overnight at 4 C°. Antibodies against Jak2, Stat 5 and their phosphorylated forms (catalog numbers 3230S, 3776S, 9351 and 9363) were purchased from Cell Signaling Technology (Danvers, MA) and were used according to directions from the manufacturer. Blots were washed rapidly 3 times in TBS-T. The blots were incubated for 20 minutes in TBS-T containing the detection antibody. Immunoreactive bands were visualized using an enhanced chemiluminescence light detecting kit (Pierce). Blots were stripped by incubating in stripping buffer (Pierce, Rockford, IL) and were reprobed with an antibody to β-actin (Sigma, St. Louis, MS).
Ribonuclease Protection Assay (RPA)
Total RNA was extracted from endothelial cells using Tri Reagent according to manufacturer’s instructions. The RPA was performed as previously described.34
Real-time quantitative polymerase chain reaction (qRT-PCR)
Isolation of total RNA from murine aorta was performed using the RNeasy kit from Qiagen (Hilden, Germany). Subsequently RT PCR reactions were performed with the QuantiTect SYBR Green RT-PCR Kit from Qiagen (catalog number 204243) with 50 ng of RNA and 0.5 μM of each primer per reaction. Sequences of the primers used were as follows:
Murine EPOR forward primer: 5’ AAG TCA GAC GGG CAG GAA GAT 3’
Murine EPOR reverse primer: 5’AGT GAG CAT GCC CAG GAC A 3’
Murine β-actin forward primer: 5’ GCT ACA GCT TCA CCA CCA CA 3’
Murine β-actin reverse primer: 5’ TCT CCA GGG AGG AAG AGG AT 3’
Densitometric Scanning
Autoradiographs from ribonuclease protection assays and Western blots were scanned using Polaroid PhotoMAX Pro and integrated densities were determined using Scion Image for windows (Scion Corporation, Frederick, MD). To adjust for differences in sample loading between wells in ribonuclease protection assays, Bcl-2 mRNA levels were expressed as ratios of integrated densities for Bcl-2 to GAPDH products.
Hematocrit Measurements
Blood from the retro-orbital plexus was collected into microcapillary tubes and hematocrits were determined using a CritSpin microhematocrit centrifuge with digital reader (Iris Intl., Westwood, MA).
Statistical Analysis
Statistical analysis was performed using Statistics for Windows, version 2.0. All values are expressed as the means ± standard error. Data were analyzed using Friedman two way analysis of variance (ANOVA) with Bonferroni comparison of means. Probability (p) values of < 0.05 were considered significant.
RESULTS
EPO suppresses LPS-induced apoptosis of HUVECs
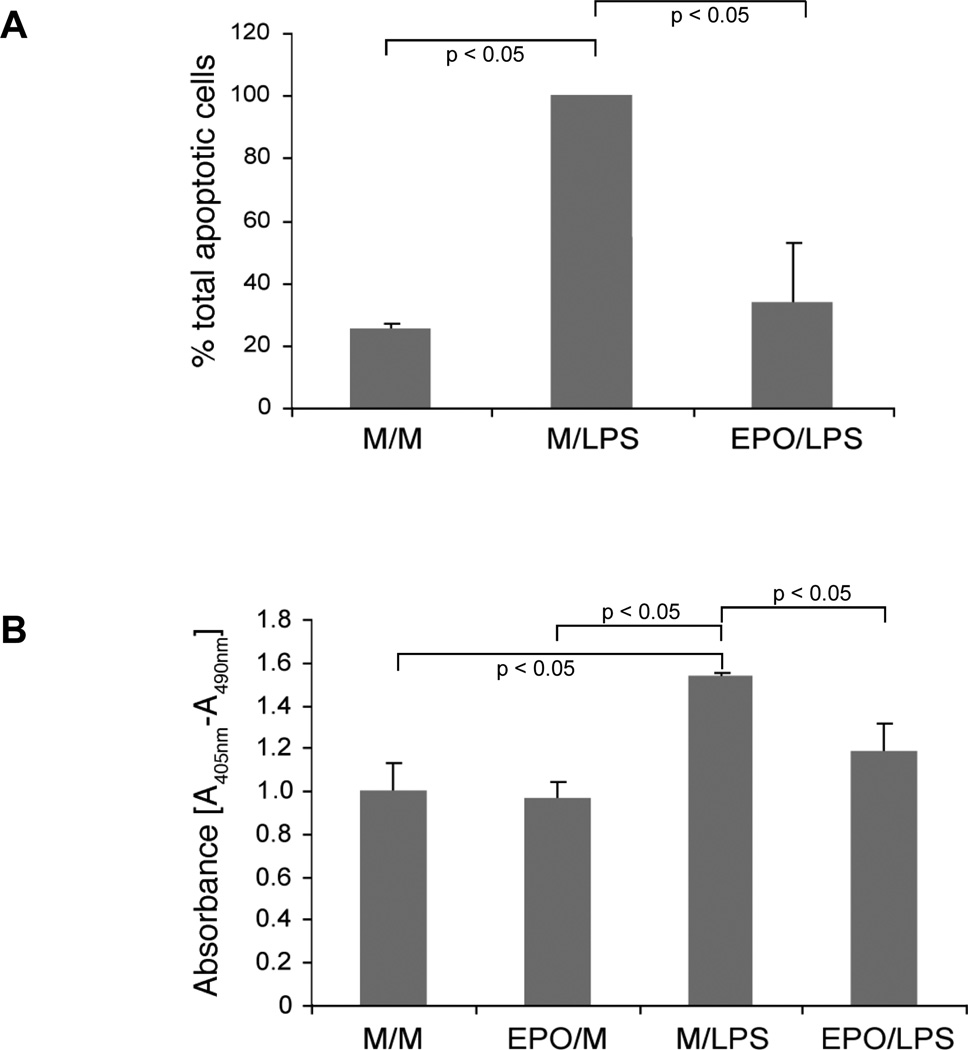
LPS-induced apoptosis was assessed by two independent methods. Using a flow cytometry based assay that measures the cell surface expression of phosphatidylserine with Annexin V staining we observed that LPS increased the fraction of cells undergoing apoptosis by 75 % when compared to media-treated HUVECs (figure 1A). Pre-incubation with EPO for 24 hours reduced LPS-induced apoptosis by 66 % compared to HUVECs pretreated with medium alone. Heat-inactivated EPO had no effect on LPS-induced endothelial cell apoptosis (data not shown). Our second approach to quantify EC apoptosis utilized an enzyme-linked immunoassay (ELISA) that measures the accumulation of histone-associated DNA fragments. LPS exposure of HUVECs resulted in a 54 % increase in absorbance readings when compared to media-treated endothelial cells. EPO pretreatment of LPS-stimulated ECs reduced the absorbance at 405 nm by 23 % when compared to cells pretreated with medium (figure 1B).
Figure 1.
(A) EPO suppresses LPS-induced apoptosis as assessed by EC surface expression of phosphatidyl serine. HUVECs were treated as outlined in table 1 and subjected to flow cytometric analysis of cell surface phosphatidyl serine. These data represent mean ± s.e. of three independent experiments. (B) Effect of EPO on LPS-induced formation of histone-associated DNA fragments as assessed by enzyme immunoassay. HUVECs were treated as described in “METHODS” and as specified in table 1. Cell lysates were prepared and analyzed with a cell death detection ELISA kit. These data represent mean ± s.e. of three separate experiments.
EPO upregulates the expression of Bcl-2 at the protein but not the mRNA level
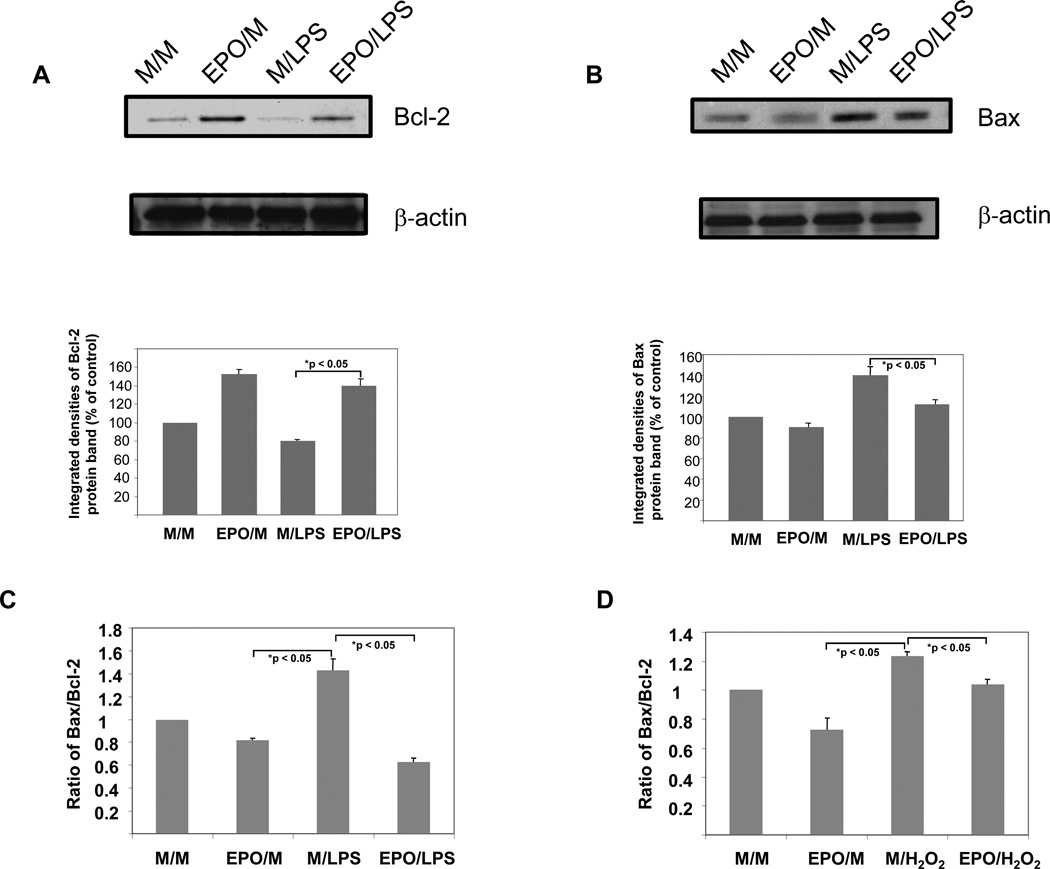
LPS reduced the protein expression of bcl-2 by 20 % when compared to media-treated control cells (figure 2A). Conversely, a 24 hour incubation in presence of EPO (5 U/ml) increased Bcl-2 expression by 53 % over media-treated controls. When cells were treated with EPO for 24 hours followed by 20 hours incubation in presence of LPS, Bcl-2 expression was increased by 75 % when compared to HUVECs exposed to medium for 24 hours prior to LPS stimulation. As previously reported by Haendeler et al.,17 no effect of LPS was seen on Bcl-2 mRNA concentrations in our experiments (table 2). Furthermore, preincubation with EPO did not alter Bcl-2 mRNA concentrations (table 2). To investigate whether the EPO-induced upregulation of Bcl-2 was accompanied by changes in Bax concentrations we performed a second set of Western blot analyses using the same samples. In ECs incubated in presence of LPS a 40 % increase in Bax concentrations was measured compared to cells treated with medium alone (figure 2B). When cells were treated with EPO for 24 hours followed by 20 hours incubation in presence of LPS, Bax expression was decreased by 20 % when compared to cells exposed to medium for 24 hours prior to LPS stimulation. Taken collectively, the data presented in figures 2A and 2B suggest that EPO upregulates Bcl-2 while downregulating Bax expression. In HUVECs treated with EPO prior to LPS stimulation, the net Bax/Bcl-2 ratio was decreased by 54% when compared to cells treated with medium prior to LPS challenge (from 1.75 to 0.8, respectively, figure 2C). Similarly, when hydrogen peroxide was used as an apoptotic stimulus, the Bax/Bcl-2 ratio decreased by 16 % in cells pretreated with EPO (figure 2D). These data indicate that in HUVECs EPO is able to lower the Bax/Bcl-2 ratio with two different apoptotic stimuli suggesting that Bax and Bcl-2 are common targets for the antiapoptotic activity of EPO.
Figure 2.
(A): Upregulation of Bcl-2 protein expression by EPO as assessed by Western blotting. HUVECs were treated as described in “METHODS” and as specified in table 1. HUVEC extracts were subjected to Western blotting using a mouse monoclonal antibody to quantitate Bcl-2 protein expression. All lanes in the gel were loaded with equal amounts of total protein and integrated densities (mean ± s.e.) of the Bcl-2 protein bands obtained from scannings of autoradiographs from two independent experiments are displayed in the histogram. The blot was stripped and reprobed with an antibody directed against ß-actin (B) Downregulation of Bax protein expression by EPO as assessed by Western blotting. HUVEC extracts used for experiments in figure 2A were subjected to Western blotting using a mouse monoclonal antibody to quantitate Bax protein expression. All lanes in the gel were loaded with equal amounts of total protein and integrated densities (mean ± s.e.) of the Bax protein bands obtained from scannings of autoradiographs from two independent experiments are displayed in the table. The blot was stripped and reprobed with an antibody directed against ß-actin (C) Effect of EPO treatment on Bax/Bcl-2 ratio in LPS-stimulated HUVECs. Integrated densities of Bax protein bands were divided by corresponding densities for Bcl-2 protein bands. Data shown is representative of three independent experiments. (D) Effect of EPO treatment on Bax/Bcl-2 ratio in hydrogen peroxide-stimulated HUVECs. Integrated densities of Bax protein bands were divided by corresponding densities for Bcl-2 protein bands. Data shown is representative of three independent experiments.
Table 2.
Effect of EPO on Bcl-2 mRNA expression as assessed by ribonuclease protection assay. HUVECs were treated as outlined in table 1. Total RNA was extracted and utilized in a ribonuclease protection assay as described in “METHODS”. Integrated density ratios of the Bcl-2 mRNA bands to GAPDH (housekeeping) mRNA products (mean ± s.e.) are displayed. These data are representative of 2 independent experiments.
| Treatment | Ratio of integrated densities (Bcl-2/L32) |
|---|---|
| EPO/M | 98 ± 15 |
| EPO/LPS | 97 ± 8 |
| M/M | 100 ± 12 |
| M/LPS | 98 ± 9 |
| EPO+Genistein/M | 97 ± 7 |
| EPO+Genistein/LPS | 98 ± 10 |
The tyrosine kinase inhibitor genistein reverses the effect of EPO on EC apoptosis and on the Bax/Bcl-2 ratio
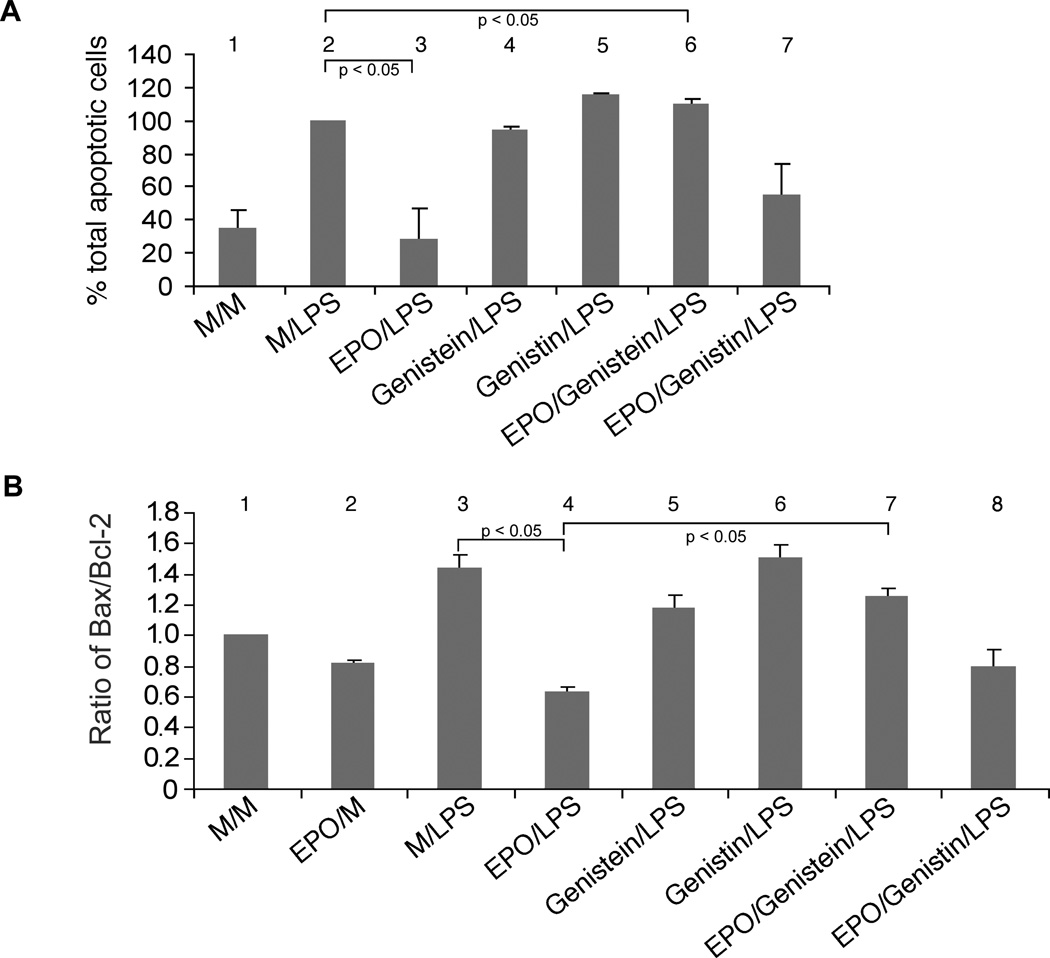
The soy isoflavone genistein is a tyrosine kinase inhibitor that has been shown to reverse the effects of EPO on endothelial cells and neurons.31, 35 Using the flow cytometry based cell surface phosphatidyl serine-annexin V binding assay, we also observed that pretreatment with genistein but not its inactive analog genistin, abrogated the antiapoptotic effect of EPO (figure 3A). Whereas EPO treatment prior to LPS exposure reduced the fraction of apoptotic cells by 72% when compared to media-preteated cells, addition of genistein returned the fraction of apoptotic cells to approximately that of controls (HUVECs treated with medium followed by LPS).
Figure 3.
(A) Effects of genistein and genistin on EC apoptosis as assessed by surface expression of phosphatidyl serine. HUVECs were treated as outlined in table 1 and subjected to flow cytometric analysis of cell surface phosphatidyl serine. These data represent mean ± s.e. of three independent experiments. Asterisk* denotes a statistically significant difference (p<0.05) from HUVECs treated with EPO followed by LPS (EPO/LPS). (B) Effects of genistein and genistin on Bax/Bcl-2 ratio as assessed by Western blot. HUVECs were treated as described in “METHODS” and as specified in table 1. Cytoplasmic extracts were subjected to Western blotting using a mouse monoclonal antibody to quantitate Bax. The same blot was stripped and reprobed using an antibody to quantitate Bcl-2 protein expression. All lanes in the gel were loaded with equal amounts of total protein. Ratios of integrated densities (mean ± s.e.) of the Bcl-2 to Bax protein bands obtained from scannings of autoradiographs from 2 independent experiments are displayed in the table. Asterisk* denotes a statistically significant difference (p<0.05) from HUVECs treated with EPO followed by LPS (EPO/LPS).
In HUVECs treated with EPO prior to LPS stimulation, the net Bax/Bcl-2 ratio was decreased by 56 % when compared to cells treated with medium prior to LPS challenge (figure 3B). Pretreatment with neither genistein nor genistin had any effect on LPS-induced changes in the Bax/Bcl-2 ratio.31 When HUVECs were incubated simultaneously in presence of EPO and genistein for 24 hours, the effect of EPO on the Bax/Bcl-2 ratio was reversed (figure 3). Treatment of HUVECs with genistin and EPO prior to LPS exposure did not alter the Bax/Bcl-2 ratio when compared to cells treated with EPO and LPS (figure 3B).
EPO-mimetic peptide (EMP1) reduces LPS-induced apoptosis of HUVECs measured by a flow-cytometric assay
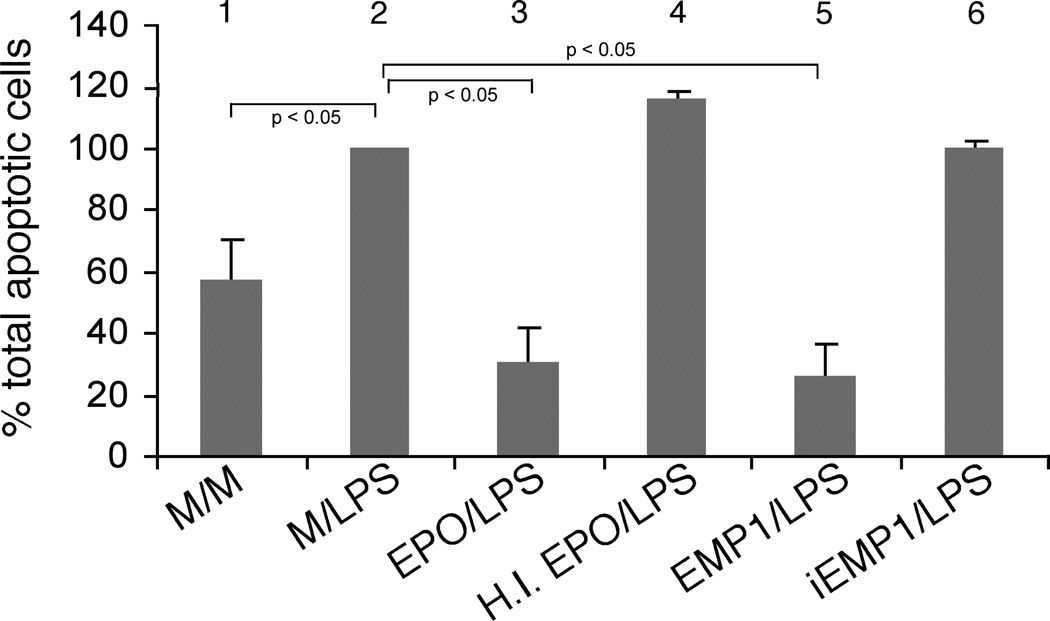
We sought to determine whether the antiapoptotic effect of EPO could be mimicked by a peptide that bears no sequence homology with EPO but binds to the EPO receptor and activates the same signaling pathways as EPO. As shown in figure 4, EMP1 was as effective as EPO in suppressing LPS-induced apoptosis of endothelial cells. A 24 hour pretreatment with 0.1 µM of EMP1 resulted in 74 % reduction in the number of apoptotic cells while EPO suppressed apoptosis by 70 %. Inactive peptide (iEMP1) in which both of the cysteine residues of EMP1 have been substituted by serine residues,31 did not suppress LPS-induced apopoptosis. As expected, heat-inactivated EPO was ineffective in suppressing LPS-induced endothelial cell apoptosis (figure 4).
Figure 4.
Effect of EMP1 on apoptosis assessed by surface expression of phosphatidyl serine. HUVECs were subjected to a 24 hour pretreatment with either medium (samples 1 and 2), EPO (5 U/ml) (sample 3), heat-inactivated EPO (sample 4), EMP1 (sample 5) or iEMP1 (sample 6), respectively. Following 4 hours of serum starvation the cells were incubated for 20 hours with medium alone (sample 1) or in presence of LPS (100 ng/ml; samples 2-6) and subjected to flow cytometric analysis of cell surface phosphatidyl serine as described in “METHODS”. These data represent mean ± s.e. of three independent experiments. Asterisk* denotes a statistically significant difference (p<0.05) from positive control (M/LPS).
HUVECs and aortas of apo E-KO mice express EPOR protein
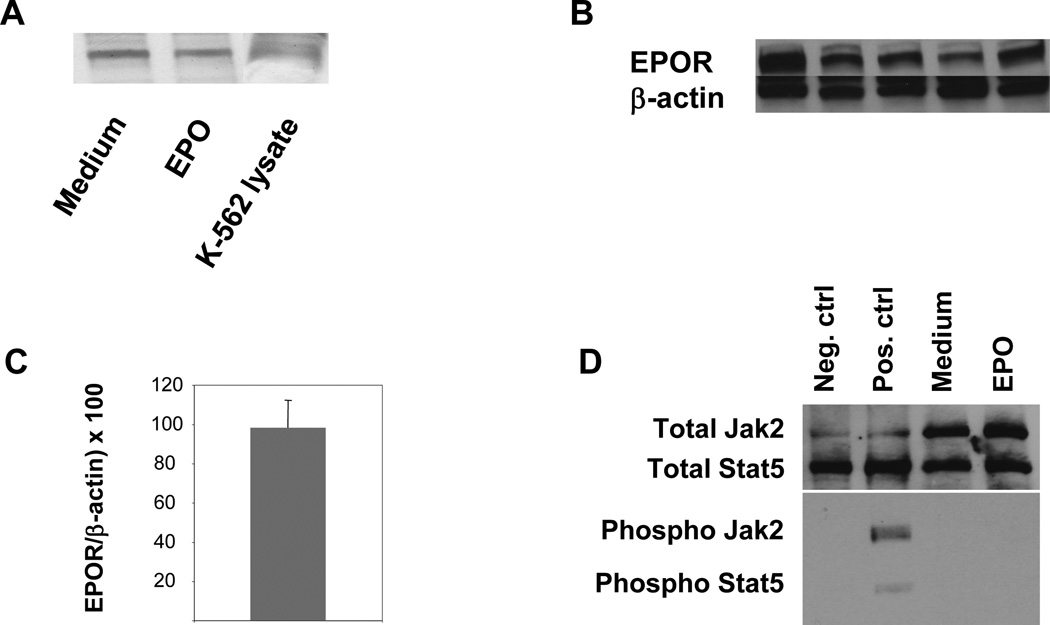
Anagnostou et al. were the first to report expression of EPOR in endothelial cells by PCR and immunohistochemical techniques.36 Subsequently, expression of EPOR has been demonstrated in a number of cells types and organs.37–39 Many but not all EPO-mediated effects in these various cell types have been associated with phosphorylation of an EPOR-associated Janus kinase 2 (JAK 2) with resultant downstream activation of the transcription factor Stat 5.40 As depicted in figure 5A, EPOR was expressed in the human erythromyeloblastoid leukemia cell line K-562 and in HUVECs. In HUVECs its expression was not significantly altered following 24 hours of EPO-treatment. Importantly, we detected abundant expression of EPOR in aortas of apo E-KO mice both at the protein (figure 5B) and the RNA level (figure 5C). Although JAK2 and Stat5 proteins were abundantly expressed in HUVECs, we did not detect their phosphorylation following treatment with EPO (figure 5D).
Figure 5.
(A) Expression of EPOR in HUVECs treated with medium alone or EPO (5U/ml) for 24 hours as determined by Western blotting. K-562 lysate was used as a positive control. Data shown is representative of three independent experiments. (B) Expression of EPOR protein in murine aorta. Aortas were harvested from 10 individual mice and whole aortic lysate from each animal was subjected to Western blotting. These data depict 5 of the total of 10 aortic lysates analyzed in this experiment. (C) Expression of EPOR in murine aorta measured by qRT-PCR. These data represent mean ± s.e. of a total of 10 mice. (D) Expression of total Jak2, Stat5 and their phosphorylated forms, respectively, in HUVECs treated with either Medium or EPO for 24 hours as determined by Western blotting. Total HeLa cell extracts (prepared with our without interferon-α treatment and purchased from the manufacturer of the Jak 2 and Stat5 antibodies) were used as positive or negative controls.
EPO treatment of apo E-knockout mice fed a high fat diet results in downregulation of Bax/Bcl-2 ratio in the aorta
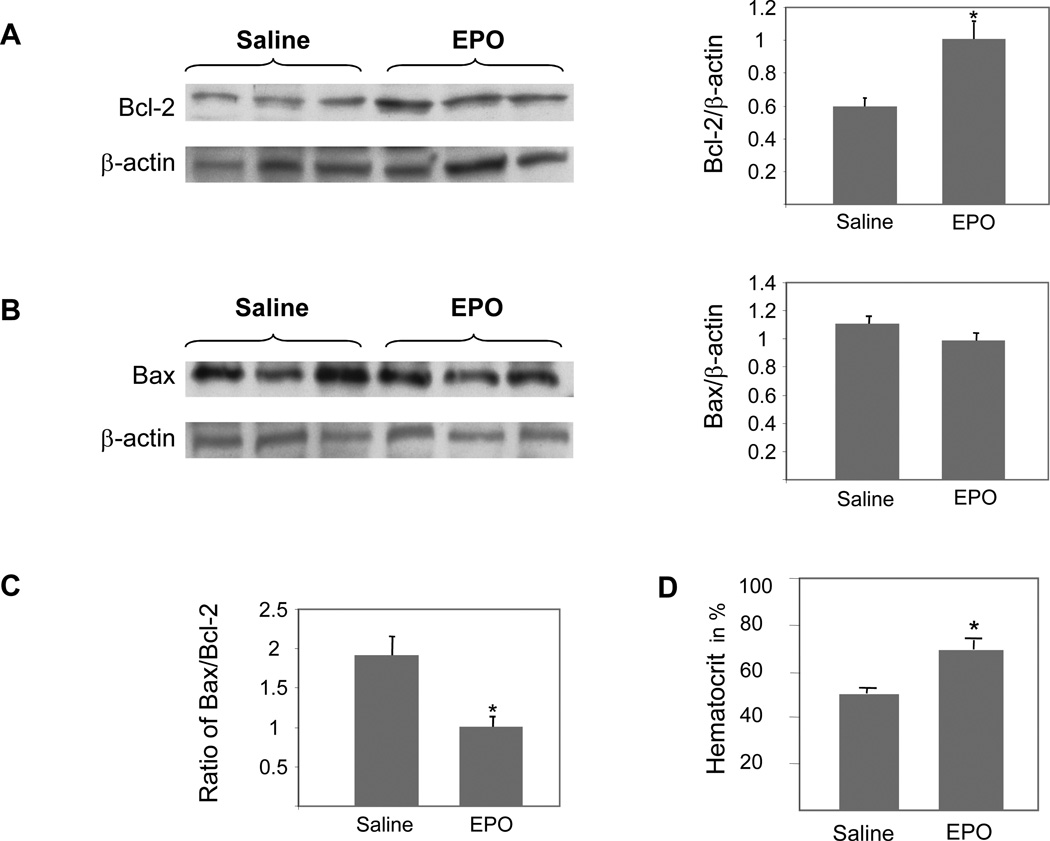
Apolipoprotein-E-deficient (apo E-KO) mice are a well-characterized model of atherosclerosis. Following 10 weeks of EPO administration (30 U injected subcutaneously every other day), we harvested aortas to characterize expression of Bcl-2 and Bax. While no change in Bax expression was detected (figure 6A), there was a 69% increase in the expression of Bcl-2 protein in the aorta of EPO-treated apo E-KO mice (figure 6B) resulting in a significant decrease in the Bax/Bcl-2 ratio (figure 6C). Hematocrit measurements confirmed the erythropoiesis-stimulating activity of EPO at the chosen dose (figure 6D).
Figure 6.
Expression of Bcl-2 (A) and Bax (B) in aortas of apo E-KO mice injected subcutaneously with EPO (30 U every other day) or an equivalent volume of vehicle (saline) for 10 weeks. (C) Ratio of Bax/Bcl-2 proteins in aortas of apo E-KO mice.(D) Hematocrits of apo E-KO mice following 10 weeks of treatment with saline or EPO. The data displayed represent mean ± s.e. of a total of 10 mice run in triplicate. Asterisk* represents a statistically significant difference (p < 0.05) between the two groups.
DISCUSSION
Despite its widespread use in this population which is at a staggeringly high risk of developing cardiovascular complications, the effects of EPO on the vascular endothelium are largely unknown. Both vascular endothelial and smooth muscle cells have been shown to secrete EPO and to express its receptor (EPOR).39 The current study is the first to demonstrate that EPOR is expressed in the aorta of apo E-KO mice. Recent data from our laboratory suggest that EPO may contribute to vascular dysfunction by upregulating the expression of monocyte chemoattractant protein-1, a chemokine pivotal to the recruitment, adhesion, and transendothelial migration of monocytes during atherogenesis.4, 41, 42 EPO also downregulates basal and acetycholine-induced NO production by HUVECs.5 Administration of EPO to mice results in elevated concentrations of asymmetric dimethylarginine (ADMA), an endogenous inhibitor of NOS. 29 Studies of EPO-induced hypertension in rats with chronic renal failure suggest that EPO may induce resistance to the vasodilatory response of arteries to endogenous NO.43
While these data suggest a potentially detrimental effect of EPO on the vasculature, numerous recent reports have revealed that EPO may exert neuroprotective activity.44–47 Several studies have implicated an antiapoptotic mechanism as being primarily responsible for the neuroprotective activity of EPO.44 Importantly, the antiapoptotic effect of EPO has been demonstrated in a variety of cell types.48, 49
Cumulatively, these data illustrate the potentially varied effects that EPO may have on different organ systems, cell types, and in different pathological states. It should, however, be emphasized that in most studies that have attributed neuroprotective activity to EPO, the drug was only given acutely, in some studies only once prior to induction of ischemia. There is dearth of studies that explore the effects of chronic EPO administration.
The ratio of Bax to Bcl-2 is thought to be a critical determinant of survival or death following an apoptotic stimulus.50 Oxidized LDL has been shown to modulate Bcl-2 expression in human coronary endothelial cells.51, 52 Mildly oxidized LDL generated by incubation with oxygen-radical producing xanthine/xanthine oxidase (X/XO) induces apoptosis in primary cultures of human coronary endothelial and smooth muscle cells.53 In endothelial but not smooth muscle cells, activation of apoptotic Fas and TNF receptors is accompanied by an increase in proapoptotic and a decrease in antiapoptotic proteins of the Bcl-2 family, suggesting that in endothelial cells mildly oxidized LDL may contribute to apoptosis by shifting the equilibrium between pro-and antiapoptotic members of the Bcl-2 family.53 Saxena et al. recently studied human endarterectomy and atherectomy specimens and observed increased apoptosis in the smooth muscle cells present in atheromatous plaques.54 In apoptotic cells, there was increased expression of proapoptotic Bax and Bak coupled with paucity of Bcl-2 and lack of Bcl-xL proteins.54
In the current study we observed that at therapeutically relevant concentrations, EPO suppresses LPS-induced apoptosis of HUVECs, upregulates the protein expression of Bcl-2, and downregulates Bax expression, resulting in a decreased Bax/Bcl-2 ratio. Molostov et al. observed that it is this ratio of proapoptotic Bax to antiapoptotic Bcl-2 that controls cell death in primary ECs.18 An intriguing finding of the present study is that suppression of LPS-induced EC apoptosis is also observed with EMP1, a peptide mimetic recently developed by Wrighton et al.25 Although EMP1 has no sequence homology with EPO it possesses EPO mimetic action both in vitro and in vivo.25 Several lines of evidence suggest that the biological activity of EMP1 is mediated through interaction with EPO receptor (EPOR). EMP1 has been shown to compete with EPO in receptor binding assays and induces cellular proliferation of cell lines engineered to respond to EPO. Both EPO and EPM1 induce a similar cascade of phosphorylation events and cell cycle progression in EPO responsive cells. Furthermore, EMP1 has significant erythropoietic effects in mice indicated by in vivo assays of nascent red blood cell proliferation.25 Livnah et al. have described the crystal structure of EMP1 with the extracellular domain of EPO receptor at 2.8 Å resolution revealing that a peptide dimer induces dimerization of the receptor.30 These observations imply that the suppression of EC apoptosis by EPO as well as by EMP1 observed in the current study is a direct result of dimerization of the EPO receptor and subsequent activation of receptor-coupled signal transduction pathways.
Our experiments with the tyrosine kinase inhibitor genistein were conceived on the basis of reports by several investigators indicating that treatment of endothelial and smooth muscle cells with EPO results in an augmentation of intracellular calcium concentrations and in the activation of tyrosine kinase-mediated signaling pathways.35, 55 Haller et al. reported that EPO induced tyrosine phosphorylation of six distinct proteins in endothelial cells, one of which is the transcription factor STAT-5.56 EPO exerted a proliferative effect on endothelial cells which was abolished by genistein, indicating that tyrosine phosphorylation is the intracellular signal responsible for EPO-induced EC growth. Vogel et al. observed that EPO stimulates endothelin-1 synthesis in vascular endothelial cells.35 Genistein suppressed EPO-induced rises in intracellular calcium and cellular endothelin-1 synthesis. Kawakami et al. recently observed that EPO reduced calcium-induced glutamate release from cultured cerebellar granule neurons.31 Inhibition was also produced by EMP1 but not iEMP and genistein was able to antagonize the effects of EPO, including the phosphorylation of JAK2.31 Although our experiments do not permit us to infer the mechanism of the EPO-mediated shift in the Bax/Bcl-2 ratio with absolute certainty, it is plausible that an augmentation of intracellular calcium concentrations and the activation of tyrosine kinase-mediated signaling pathways may be involved since genistein but not genistin was able to reverse the EPO-mediated suppression of apoptosis and the shift in the Bax/Bcl-2 ratio. Since we failed to observed Jak2 or Stat5 phosphorylation, a different kinase must be involved in the EPO-mediated activation of HUVECs. In this context, Ammarguellat et al. have published that pharmacological doses of EPO (up to 250 U/ml) also failed to activate the Jak2/Stat5 signaling pathway but instead activate the MAPK pathway in vascular smooth muscle cells. A variety of proteins have been reported to be phosphorylated in response to EPOR activation, including protein kinase C and MAP kinase (ERK1/2),40 and the signal transduction cascade is likely to be specific to the cell type involved.
Further studies are required to characterize what role, if any, the antiapoptotic activity of EPO has in the pathogenesis of atherosclerosis in the setting of CKD. An obvious limitation of our in vitro studies is the use of LPS as an apoptotic stimulus that may not be relevant in the pathogenesis of atherosclerosis as it occurs in vivo. To date, the effect of chronic EPO treatment on plaque stability has not been explored. Emerging clinical data that demonstrate increased cardiovascular morbidity and mortality in patients receiving EPO underscore the urgent need to understand the effects of EPO on the cardiovascular system.2, 3, 57 In this regard, the development of novel peptide agonists may present a promising strategy to selectively minimize the potentially deleterious effects of EPO on the vascular endothelium while optimizing erythropoietic activity.
ACKNOWLEDGEMENTS
We gratefully acknowledge the technical help of Dr. Hua Yang with the flow cytometric analyses of apoptotic cells.
This work was supported by a Scientist Development Grant from the American Heart Association (A.D.), the University of Michigan Cardiovascular Center (A.D.) and the National Institutes of Health (RY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wheeler DC. Cardiovascular disease in patients with chronic renal failure. Lancet. 1996;348(9043):1673–1674. doi: 10.1016/S0140-6736(05)65816-3. [DOI] [PubMed] [Google Scholar]
- 2.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355(20):2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361(21):2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 4.Desai A, Zhao Y, Lankford HA, Warren JS. Nitric oxide suppresses EPO-induced monocyte chemoattractant protein-1 in endothelial cells: implications for atherogenesis in chronic renal disease. Lab Invest. 2006;86(4):369–379. doi: 10.1038/labinvest.3700396. [DOI] [PubMed] [Google Scholar]
- 5.Wang XQ, Vaziri ND. Erythropoietin depresses nitric oxide synthase expression by human endothelial cells. Hypertension. 1999;33(3):894–899. doi: 10.1161/01.hyp.33.3.894. [DOI] [PubMed] [Google Scholar]
- 6.Filippatos G, Ang E, Gidea C, Dincer E, Wang R, Uhal BD. Fas induces apoptosis in human coronary artery endothelial cells in vitro. BMC Cell Biol. 2004;5:6. doi: 10.1186/1471-2121-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choy JC, Granville DJ, Hunt DW, McManus BM. Endothelial cell apoptosis: biochemical characteristics and potential implications for atherosclerosis. J Mol Cell Cardiol. 2001;33(9):1673–1690. doi: 10.1006/jmcc.2001.1419. [DOI] [PubMed] [Google Scholar]
- 8.Geng YJ, Libby P. Progression of atheroma: a struggle between death and procreation. Arterioscler Thromb Vasc Biol. 2002;22(9):1370–1380. doi: 10.1161/01.atv.0000031341.84618.a4. [DOI] [PubMed] [Google Scholar]
- 9.Dimmeler S, Haendeler J, Zeiher AM. Regulation of endothelial cell apoptosis in atherothrombosis. Curr Opin Lipidol. 2002;13(5):531–536. doi: 10.1097/00041433-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Bombeli T, Schwartz BR, Harlan JM. Endothelial cells undergoing apoptosis become proadhesive for nonactivated platelets. Blood. 1999;93(11):3831–3838. [PubMed] [Google Scholar]
- 11.Tsujimoto Y. Bcl-2 family of proteins: life-or-death switch in mitochondria. Biosci Rep. 2002;22(1):47–58. doi: 10.1023/a:1016061006256. [DOI] [PubMed] [Google Scholar]
- 12.Rosse T, Olivier R, Monney L, Rager M, Conus S, Fellay I, et al. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391(6666):496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- 13.Dong C, Granville DJ, Tuffnel CE, Kenyon J, English D, Wilson JE, et al. Bax and apoptosis in acute and chronic rejection of rat cardiac allografts. Lab Invest. 1999;79(12):1643–1653. [PubMed] [Google Scholar]
- 14.Bannerman DD, Goldblum SE. Mechanisms of bacterial lipopolysaccharide-induced endothelial apoptosis. Am J Physiol Lung Cell Mol Physiol. 2003;284(6):L899–L914. doi: 10.1152/ajplung.00338.2002. [DOI] [PubMed] [Google Scholar]
- 15.Kim KY, Shin HK, Choi JM, Hong KW. Inhibition of lipopolysaccharide-induced apoptosis by cilostazol in human umbilical vein endothelial cells. J Pharmacol Exp Ther. 2002;300(2):709–715. doi: 10.1124/jpet.300.2.709. [DOI] [PubMed] [Google Scholar]
- 16.Susin SA, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, et al. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med. 1996;184(4):1331–1341. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haendeler J, Zeiher AM, Dimmeler S. Vitamin C and E prevent lipopolysaccharide-induced apoptosis in human endothelial cells by modulation of Bcl-2 and Bax. Eur J Pharmacol. 1996;317(2–3):407–411. doi: 10.1016/s0014-2999(96)00759-5. [DOI] [PubMed] [Google Scholar]
- 18.Molostvov G, Morris A, Rose P, Basu S. Modulation of Bcl-2 family proteins in primary endothelial cells during apoptosis. Pathophysiol Haemost Thromb. 2002;32(2):85–91. doi: 10.1159/000065081. [DOI] [PubMed] [Google Scholar]
- 19.Silva M, Grillot D, Benito A, Richard C, Nunez G, Fernandez-Luna JL. Erythropoietin can promote erythroid progenitor survival by repressing apoptosis through Bcl-XL and Bcl-2. Blood. 1996;88(5):1576–1582. [PubMed] [Google Scholar]
- 20.Carlini RG, Alonzo EJ, Dominguez J, Blanca I, Weisinger JR, Rothstein M, et al. Effect of recombinant human erythropoietin on endothelial cell apoptosis. Kidney Int. 1999;55(2):546–553. doi: 10.1046/j.1523-1755.1999.00266.x. [DOI] [PubMed] [Google Scholar]
- 21.Sekiguchi N, Inoguchi T, Kobayashi K, Nawata H. Effect of erythropoietin on endothelial cell apoptosis induced by high glucose. Diabetes Res Clin Pract. 2004;66(Suppl 1):S103–S107. doi: 10.1016/j.diabres.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Sekiguchi N, Inoguchi T, Kobayashi K, Sonoda N, Nawata H. Erythropoietin attenuated high glucose-induced apoptosis in cultured human aortic endothelial cells. Biochem Biophys Res Commun. 2005;334(1):218–222. doi: 10.1016/j.bbrc.2005.06.072. [DOI] [PubMed] [Google Scholar]
- 23.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106(23):2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 24.Bieber E. Erythropoietin, the biology of erythropoiesis and epoetin alfa. An overview. J Reprod Med. 2001;46(5 Suppl):521–530. [PubMed] [Google Scholar]
- 25.Wrighton NC, Farrell FX, Chang R, Kashyap AK, Barbone FP, Mulcahy LS, et al. Small peptides as potent mimetics of the protein hormone erythropoietin. Science. 1996;273(5274):458–464. doi: 10.1126/science.273.5274.458. [DOI] [PubMed] [Google Scholar]
- 26.Hardee ME, Kirkpatrick JP, Shan S, Snyder SA, Vujaskovic Z, Rabbani ZN, et al. Human recombinant erythropoietin (rEpo) has no effect on tumour growth or angiogenesis. Br J Cancer. 2005;93(12):1350–1355. doi: 10.1038/sj.bjc.6602846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz O, Barzilay E, Skaat A, Herman A, Mittelman M, Neumann D. Erythropoietin induced tumour mass reduction in murine lymphoproliferative models. Acta Haematol. 2005;114(3):177–179. doi: 10.1159/000087896. [DOI] [PubMed] [Google Scholar]
- 28.Mittelman M, Neumann D, Peled A, Kanter P, Haran-Ghera N. Erythropoietin induces tumor regression and antitumor immune responses in murine myeloma models. Proc Natl Acad Sci U S A. 2001;98(9):5181–5186. doi: 10.1073/pnas.081275298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desai A, Zhao Y, Warren JS. Human recombinant erythropoietin augments serum asymmetric dimethylarginine concentrations but does not compromise nitric oxide generation in mice. Nephrol Dial Transplant. 2008;23(5):1513–1520. doi: 10.1093/ndt/gfm869. [DOI] [PubMed] [Google Scholar]
- 30.Livnah O, Stura EA, Johnson DL, Middleton SA, Mulcahy LS, Wrighton NC, et al. Functional mimicry of a protein hormone by a peptide agonist: the EPO receptor complex at 2.8 A. Science. 1996;273(5274):464–471. doi: 10.1126/science.273.5274.464. [DOI] [PubMed] [Google Scholar]
- 31.Kawakami M, Iwasaki S, Sato K, Takahashi M. Erythropoietin inhibits calcium-induced neurotransmitter release from clonal neuronal cells. Biochem Biophys Res Commun. 2000;279(1):293–297. doi: 10.1006/bbrc.2000.3926. [DOI] [PubMed] [Google Scholar]
- 32.Desai A, Miller MJ, Gomez HF, Warren JS. Loxosceles deserta spider venom induces NFkappaB-dependent chemokine production by endothelial cells. J Toxicol Clin Toxicol. 1999;37(4):447–456. doi: 10.1081/clt-100102435. [DOI] [PubMed] [Google Scholar]
- 33.Desai A, Zhao Y, Warren JS. Development of atherosclerosis in Balb/c apolipoprotein Edeficient mice. Cardiovasc Pathol. 2008;17(4):233–240. doi: 10.1016/j.carpath.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Desai A, Miller MJ, Huang X, Warren JS. Nitric oxide modulates MCP-1 expression in endothelial cells: implications for the pathogenesis of pulmonary granulomatous vasculitis. Inflammation. 2003;27(4):213–223. doi: 10.1023/a:1025036530605. [DOI] [PubMed] [Google Scholar]
- 35.Vogel V, Kramer HJ, Backer A, Meyer-Lehnert H, Jelkmann W, Fandrey J. Effects of erythropoietin on endothelin-1 synthesis and the cellular calcium messenger system in vascular endothelial cells. Am J Hypertens. 1997;10(3):289–296. doi: 10.1016/s0895-7061(96)00410-4. [DOI] [PubMed] [Google Scholar]
- 36.Anagnostou A, Liu Z, Steiner M, Chin K, Lee ES, Kessimian N, et al. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci U S A. 1994;91(9):3974–3978. doi: 10.1073/pnas.91.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kondyli M, Gatzounis G, Kyritsis A, Varakis J, Assimakopoulou M. Immunohistochemical detection of phosphorylated JAK-2 and STAT-5 proteins and correlation with erythropoietin receptor (EpoR) expression status in human brain tumors. J Neurooncol. 2010 doi: 10.1007/s11060-010-0156-2. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez PE, Navarro FP, Fares RP, Nadam J, Georges B, Moulin C, et al. Erythropoietin receptor expression is concordant with erythropoietin but not with common beta chain expression in the rat brain throughout the life span. J Comp Neurol. 2009;514(4):403–414. doi: 10.1002/cne.22020. [DOI] [PubMed] [Google Scholar]
- 39.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293(1):90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jelkmann W, Bohlius J, Hallek M, Sytkowski AJ. The erythropoietin receptor in normal and cancer tissues. Crit Rev Oncol Hematol. 2008;67(1):39–61. doi: 10.1016/j.critrevonc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Aiello RJ, Bourassa PA, Lindsey S, Weng W, Natoli E, Rollins BJ, et al. Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19(6):1518–1525. doi: 10.1161/01.atv.19.6.1518. [DOI] [PubMed] [Google Scholar]
- 42.Terkeltaub R, Boisvert WA, Curtiss LK. Chemokines and atherosclerosis. Curr Opin Lipidol. 1998;9(5):397–405. doi: 10.1097/00041433-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Vaziri ND, Zhou XJ, Naqvi F, Smith J, Oveisi F, Wang ZQ, et al. Role of nitric oxide resistance in erythropoietin-induced hypertension in rats with chronic renal failure. Am J Physiol. 1996;271(1 Pt 1):E113–E122. doi: 10.1152/ajpendo.1996.271.1.E113. [DOI] [PubMed] [Google Scholar]
- 44.Siren AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98(7):4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siren AL, Fasshauer T, Bartels C, Ehrenreich H. Therapeutic potential of erythropoietin and its structural or functional variants in the nervous system. Neurotherapeutics. 2009;6(1):108–127. doi: 10.1016/j.nurt.2008.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1997;76(1):105–116. doi: 10.1016/s0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- 47.Genc S, Kuralay F, Genc K, Akhisaroglu M, Fadiloglu S, Yorukoglu K, et al. Erythropoietin exerts neuroprotection in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated C57/BL mice via increasing nitric oxide production. Neurosci Lett. 2001;298(2):139–141. doi: 10.1016/s0304-3940(00)01716-x. [DOI] [PubMed] [Google Scholar]
- 48.Hardee ME, Rabbani ZN, Arcasoy MO, Kirkpatrick JP, Vujaskovic Z, Dewhirst MW, et al. Erythropoietin inhibits apoptosis in breast cancer cells via an Akt-dependent pathway without modulating in vivo chemosensitivity. Mol Cancer Ther. 2006;5(2):356–361. doi: 10.1158/1535-7163.MCT-05-0196. [DOI] [PubMed] [Google Scholar]
- 49.Solar P, Koval J, Mikes J, Kleban J, Solarova Z, Lazur J, et al. Erythropoietin inhibits apoptosis induced by photodynamic therapy in ovarian cancer cells. Mol Cancer Ther. 2008;7(8):2263–2271. doi: 10.1158/1535-7163.MCT-08-0483. [DOI] [PubMed] [Google Scholar]
- 50.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 51.Li D, Yang B, Mehta JL. Ox-LDL induces apoptosis in human coronary artery endothelial cells: role of PKC, PTK, bcl-2, and Fas. Am J Physiol. 1998;275(2 Pt 2):H568–H576. doi: 10.1152/ajpheart.1998.275.2.H568. [DOI] [PubMed] [Google Scholar]
- 52.Salvayre R, Auge N, Benoist H, Negre-Salvayre A. Oxidized low-density lipoproteininduced apoptosis. Biochim Biophys Acta. 2002;1585(2–3):213–221. doi: 10.1016/s1388-1981(02)00343-8. [DOI] [PubMed] [Google Scholar]
- 53.Napoli C, Quehenberger O, De Nigris F, Abete P, Glass CK, Palinski W. Mildly oxidized low density lipoprotein activates multiple apoptotic signaling pathways in human coronary cells. FASEB J. 2000;14(13):1996–2007. doi: 10.1096/fj.99-0986com. [DOI] [PubMed] [Google Scholar]
- 54.Saxena A, McMeekin JD, Thomson DJ. Expression of Bcl-x, Bcl-2, Bax, and Bak in endarterectomy and atherectomy specimens. J Pathol. 2002;196(3):335–342. doi: 10.1002/path.1040. [DOI] [PubMed] [Google Scholar]
- 55.Neusser M, Tepel M, Zidek W. Erythropoietin increases cytosolic free calcium concentration in vascular smooth muscle cells. Cardiovasc Res. 1993;27(7):1233–1236. doi: 10.1093/cvr/27.7.1233. [DOI] [PubMed] [Google Scholar]
- 56.Haller H, Christel C, Dannenberg L, Thiele P, Lindschau C, Luft FC. Signal transduction of erythropoietin in endothelial cells. Kidney Int. 1996;50(2):481–488. doi: 10.1038/ki.1996.339. [DOI] [PubMed] [Google Scholar]
- 57.Drueke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355(20):2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]