Abstract
Leukotriene B4 (LTB4) receptor, BLT1 is expressed on variety of immune cells and has been implicated as a mediator of diverse inflammatory diseases. However, whether biological responses initiated via this receptor generate tumor promoting inflammation or anti-tumor immunity remains unexplored. In this study, we investigated the role of BLT1 in antitumor immunity using syngeneic TC-1 cervical cancer model and observed accelerated tumor growth and reduced survival in BLT1−/− mice compared to BLT1+/+ mice. Analysis of the tumor infiltrates by flow cytometry and confocal microscopy revealed a significant decrease in effector immune cells, most notably CD8+-T cells and NK cells in the tumors of the BLT1−/− mice. Gene expression profiling confirmed the dramatic decrease of IFN-γ, granzyme-B and IL-2 in tumors growing in BLT1−/− mice. Furthermore, depletion of CD8+ T cells enhanced the tumor growth in BLT1+/+ but not in BLT1−/− mice. However, similar levels of antigen dependent CD8+ T cell mediated killing activity were observed in spleens of BLT1+/+ and BLT1−/− mice. Adoptive transfer of CD8+ T cells from tumor bearing BLT1+/+ but not BLT1−/− mice significantly reduced tumor growth and increased the survival of Rag2−/− mice. While the homeostatic proliferation and expression profiles of other chemokine receptors of adoptively transferred BLT1+/+ and BLT1−/− CD8+ T cells appears to be similar, BLT1+/+ T-lymphocytes entered the tumors in greater numbers. These results suggest that BLT1 expression on CD8+ T cells plays an important role in their trafficking to tumors.
Keywords: Leukotriene B4, BLT1, cancer, anti-tumor immunity, leukocyte migration
Introduction
The immune system plays a dual role in modulating cancer progression. Chronic inflammation mediated through myeloid cells promotes tumor progression whereas immune surveillance mediated by the CTLs suppresses tumor growth (1-3). In both cases recruitment of diverse immune cell subsets into tumor micro environment is a critical event. Cytotoxic T lymphocytes (CTLs) that directly kill the tumor cells are essential for generation of antitumor immunity (4, 5). As a result, most cancer immunotherapy approaches such as cancer vaccines, cancer gene therapy, dendritic cell based therapy and adoptive T cell transfer therapy have targeted to augment CTL responses and have shown considerable efficacy in preclinical and limited clinical settings (6-8). However, a major obstacle common in these immunotherapies is the poor recruitment of the effector CTLs into the tumor despite the generation of potent CD8+ T cell responses in the periphery (9). In a chimeric antigen receptor (CAR) based adoptive T-cell immunotherapy (ACT) settings less than one to two percent of transferred tumor reactive T cells are recruited to the tumors (9, 10). Given adoptively transferred or vaccine activated tumor reactive T cells must accumulate and function within the tumors and therefore effector T-cell recruitment and retention at the tumor site is essential for optimal efficacy (11). In this regard, CCR5 has been shown to regulate the selective CTL recruitment to tumor site in response to production of CCL5 in a mouse model (12, 13). In addition, CXCR3 ligands CXCL9 and CXCL10 have been shown to be mediators of effector immune cell recruitment. High levels of CXCL9 and CXCL10 have correlated with higher CTL infiltration into tumors, reduced tumor growth and reduced recurrence after surgery in renal cell carcinoma (14). CXCL9 was also shown to promote CTL infiltration of malignant melanoma extending the survival (15).
Leukotriene B4 (LTB4), an eicosanoid derivative of arachidonic acid metabolism produced by the sequential action of 5-lipoxygenase and LTA4-hydrolase, is a potent leukocyte chemoattractant (16). LTB4 signals through two G-protein coupled seven transmembrane domain receptors, BLT1 and BLT2 the high and low affinity receptors, respectively (17, 18). BLT1 is expressed on various immune cells including neutrophils, eosinophils, monocytes, dendritic cells, and activated T cells (19). BLT1 has been implicated in diverse diseases such as asthma, atherosclerosis, arthritis, autoimmune uveitis and diet induced obesity as a mediator of inflammation owing to the protective phenotype of BLT1 knockout mice in aforementioned pro-inflammatory and autoimmune diseases (20-31). Since BLT1 is expressed on majority of immune cell subtypes, it is not clear whether it mediates inflammation promotion or immune surveillance of cancers. Previous studies have outlined an important role for BLT1 in CTL migration in allergic airway hyper responsiveness and autoimmune uveitis models (26, 32, 33). As much as BLT1 mediated T-effector cell recruitment and their pathogenic effects are undesirable in settings of autoimmune and allergic diseases, these very precise responses are essential for generation of cancer immune surveillance and anti-tumor immunity.
Here, we examined the requirement for BLT1 expression for trafficking of the activated CTLs to tumors. The results showed accelerated tumor growth and reduced survival of BLT1−/− mice in a TC-1 cervical cancer model. Using immune cell profiling of tumors, in vivo killing assays, T-cell depletion and adoptive transfers of CTLs into Rag2−/− mice, we demonstrate an important role for BLT1 expression on CD8+ T cells in recruitment of these cells to tumors for generation of anti-tumor immune response and effective immune surveillance.
Materials and Methods
Mice and cell lines
C57BL/6 mice (6–8 wk old) were purchased from The Jackson Laboratory or bred in our animal facility at the University of Louisville. Previously described BLT1−/− mice in C57BL/6 background were also bred in our animal facility at the University of Louisville (34). Rag2−/− mice in C57BL/6 background were purchased from Taconic (Germantown, NY). BLT1−/− Rag2−/− double KO mice were generated by crossing the BLT1−/− with Rag2−/− mice. All animals were cared for in accordance with institutional and National Institute of Health guidelines. TC-1 and B16 cell lines were purchased from American Type Culture Collection (Manassas, VA).
Reagents
Fluorochrome-conjugated Abs (anti-CD45.2-APC-Cy7, anti-CD3-FITC, anti-CD4-APC, anti-CD8-PerCP, anti-CD25-PE, NK1.1-PE, CCR5-PE, CXCR3-FITC, CCR9 FITC, SA-APC) and isotype controls were purchased from BD PharMingen and eBioscience. HPV-16 E7 peptide (E749-57 RAHYNIVTF) was purchased from Peptide 2.O Inc. Anti-mouse BLT1 antibody conjugated to biotin was developed in the lab (unpublished data). RT Primers for IFN-γ, Granzyme-B and IL-2 genes were obtained from Real Time Primers, LLC.
Tumor model and vaccination
To establish tumors, 1×105 live TC-1 cells were resuspended in 200 μl of PBS and injected s.c. into the right flank of naive syngenic WT or BLT1−/− C57BL/6 mice as previously described (7). For sub-lethal dose (survival) experiments, 2×104 TC-1 cells in 200 μl of PBS were injected s.c. For Rag2−/− and Rag2−/−BLT1−/− comparison experiments, 5×104 live TC-1 cells were re-suspended in 200 μl of PBS and injected s.c. into the right flank. Tumor growth was monitored 2-3 times per week and tumor size was measured in mm using a caliper. Average tumor size was calculated by measuring two perpendicular diameters. Animals bearing tumors were euthanized when tumors reached a size of 15 mm in one of the two perpendicular diameters or earlier if tumors ulcerated or animal showed signs of discomfort. For immunization studies, C57BL/6 WT or BLT1−/− mice were immunized s.c. with various vaccine formulations containing 100 μg of E749-57 peptide in PBS or PBS alone as control.
Analysis of tumor infiltrating leukocytes
Tumors were harvested at the end point (15mm) and cut into 2-mm pieces after removal of connective tissue by dissection. To isolate leukocytes, tumors were incubated in an enzyme mixture consisting of collagenase-A (2 mg /ml), DNase I (1 mg /ml), penicillin (10 U /ml), and streptomycin (10 μg /ml) in PBS for 2 hrs at 37°C with occasional vortexing. The digested tissue was passed through a nylon mesh and the resultant cells were washed twice in PBS before being stained for flow cytometric analysis. Cells were stained with appropriate fluorochrome labeled anti-mouse CD3, CD4, CD25, NK1.1, CD11b, and CD8 Abs and PE-conjugated anti-mouse CD45.2 Ab to selectively exclude CD45 negative tumor cells from the analysis. Two million total tumor cells were stained and analyzed using multi parameter flow cytometry. Similarly spleens, lymph nodes were processed into single-cell suspensions, and cells were labeled with saturating concentrations of fluorochrome-conjugated Abs. Intracellular cytokine staining of CD8+ T cells within tumor were performed as described previously (35). Briefly, single-cell suspension from the tumors were stimulated with the cell stimulation cocktail (eBiosciences, 500X used at 1X) containing PMA (40.5μM), Ionomycin (670μM), and protein transport inhibitors Brefeldin A (5.3mM) and Monensin (1mM) for 6 hrs and stained for IFN-γ using anti-IFN-γ Ab (BD Biosciences). In all instances isotype matched Abs with the same fluorochrome were used as controls. Data was analyzed using CellQuest (BD Biosciences) and FlowJo (Tree Star) software.
For immunofluorescence analysis, tumors described above were dissected, washed, embedded in OCT, snap-frozen, and cut into 5.0 μm sections with a cryostat. Sections were fixed with ice cold acetone followed by blocking with PBS supplemented with 1% BSA and 5% goat serum for 30 min. at room temperature to avoid any nonspecific bindings. To assess the presence of tumor infiltrating CD8+ T cells, sections were next incubated with rat anti-mouse CD8 Ab (PharMingen) for 1 hr at room temperature. After 3 washes with PBS, sections were incubated with 1/400 dilution of goat anti-rat Alexa 488 (2 mg/ml, Invitrogen), and counterstained with DAPI, and analyzed using Nikon A1R confocal microscope at 200X magnification. A minimum of 4 fields for each tumor section were analyzed to assess the infiltration of T cells.
In vivo cytotoxicity assay
A standard in vivo killing assay was performed by injecting peptide-pulsed target cells into congenic immunized mice as previously described (7). In brief, a population of C57BL/6 spleen cells were labeled with 2.5 μM fluorescent dye CFSE (CFSEhigh) while a second population was labeled with 0.25 μM CFSE (CFSElow). CFSEhigh cells were then pulsed with 2 μg/ml of E749-57 peptide representing the dominant CD8+ T cell epitope for E7 for 90 min at 37°C in a 5% CO incubator. CFSEhigh and CFSElow 2 cells were then extensively washed to remove free peptide, mixed at 1:1 ratio, and injected i.v. into C57BL/6 WT or BLT1−/− mice, 7 days after vaccination or in tumor (3-4 mm size) bearing C57BL/6 WT or BLT1−/− mice. Spleens were harvested 48 hrs later, processed into single cell suspension, and analyzed by multiparameter flow cytometry to determine the ratio of CFSEhigh/CFSElow target cells. The percentage of in vivo killing was calculated by the following formula: [1-((CFSEhigh/CFSElow for experimental) / (CFSEhigh/CFSElow for naive))] × 100.
CD8+ T cell depletion and adoptive transfer studies
Depletion of CD8+ cells were performed by single i.p. injection 500 μg of CD8 depleting antibodies (BioXCell, NH) a day before tumor challenge in WT and BLT1−/− mice. These mice were then challenged with 1×105 live TC-1 cells re-suspended in 200 μl of PBS into the right back flank. Depletion of CD8+ T-cells was monitored at day 3 and 7 (~ 99% depletion versus 0% depletion with an Isotype control antibody) in the peripheral blood (data not shown). For adoptive transfer studies, Rag2−/− mice were challenged s.c. with 5×104 live TC-1 cells re-suspended in 200 μl of PBS into the right flank. Two days later, CD8+ T cells were isolated from spleens and lymph nodes of small tumor bearing WT or BLT1−/− mice by magnetic sorting using CD8Ly2 beads (Miltenyi Biotec) with >97% purity. Purified CD8+ T cells (8 × 105) in PBS were injected i.v. in these (Rag2−/−) mice and vehicle alone was used as control. Tumor growth was monitored 2-3 times per week and tumor size was measured in mm using a caliper. Average tumor size was calculated by measuring two perpendicular diameters. Animals bearing tumors were euthanized when tumors reached a size of 15 mm in one of the two perpendicular diameters or earlier if tumors ulcerated or animal showed signs of discomfort. At the end point the spleen, tumor draining lymph node (TDLN) and tumor were harvested and CD8+ T cells were analyzed for their frequency and chemokine receptor expression including BLT1.
Real time PCR
Total RNA from the excised tumors was isolated using Trizol followed by RNAse mini prep kit from Qiagen. The RNA was treated with DNAase using Turbo DNAse kit, Ambion Inc. For quantitative real-time PCR, 1 μg of total RNA was reverse transcribed in 50 μl reaction using TaqMan reverse transcription reagents (Applied Biosystems) using random hexamer primers. 2 μl of cDNA and the 1 μM real time PCR primers were used in a final 20 μl qPCR reaction with ‘power SYBR-green master mix’ (Applied Biosystems). The real time primers were purchased from Real Time Primers, LLC, Elkins Park, PA. The sequence of the primers will be provided upon request. Real time qPCR was performed in Bio-Rad CFX-96 Real Time System. Expression of the target genes was normalized to β-actin and displayed as fold change relative to the wild type sample. Data are representative of tumors isolated from at least 5 different mice for each genotype.
Statistical analysis
Statistical analysis was done using the Student’s t-test or ANOVA. The survival assays were analyzed using long-rank test in Prism Graph pad software. For each test, p value less than 0.05 and 0.001 were considered significant (*) and very significant (**), respectively. Error bars represent ± SD.
Results
Poor immune-surveillance and antitumor immunity in BLT1−/− mice
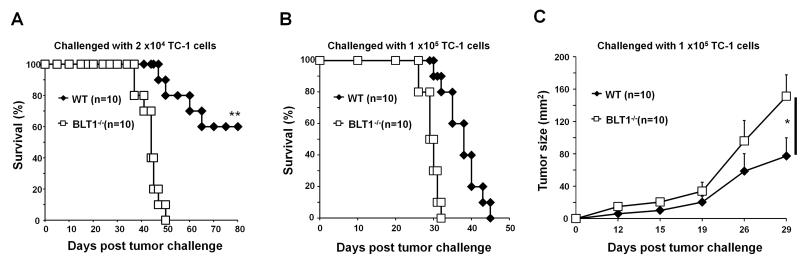
To investigate the role of BLT1 in immune surveillance and antitumor-immunity, the TC-1, a syngeneic tumor model in immune competent C57BL/6 mice was used. Implantation of a sub lethal dose (2.0 ×104) of TC-1 cells resulted in development of tumors in all of the BLT1−/− mice leading to 100% mortality by 50 days. However, under these conditions only 50% of WT mice developed relatively slow growing tumors which led to 60% survival of WT mice over 80 days of observation (Fig. 1A). We also challenged the BLT1−/− and WT mice with 1×105 cells of TC-1 cells that allowed tumor development in 100% of the mice. Enhanced tumor growth and reduced survival in BLT1−/− mice compared to WT mice (Figs. 1B and 1C) was observed in this setting as well. Similar phenotype of accelerated tumor growth was also observed in BLT1−/− mice in a syngenic model of B16 melanoma (data not shown). These results suggest that BLT1 might be important for mediating antitumor immunity.
FIGURE 1. Decreased survival and increased tumor growth in BLT1−/− mice.
(A-B) Kaplan-Meier survival plots of BLT1+/+ and BLT1−/− mice. (A) The mice were injected s.c. with a sub-lethal dose (2.0 ×104) of TC-1 cells and monitored their survival up to 80 days. (B) BLT1+/+ and BLT1−/− mice were injected s.c. with a lethal dose (1.0 ×105) of TC-1 cells and survival was followed as described in materials and methods section. (C) Tumor size in lethal dose challenged group was measured and calculated by multiplication of two perpendicular diameters (LxW). Log Rank test were performed for statistical analysis of survival and one-way Anova was used for tumor sizes. The experiment shown is representative of three independent experiments.
Reduced effector immune cell infiltration into tumors of BLT1−/− mice
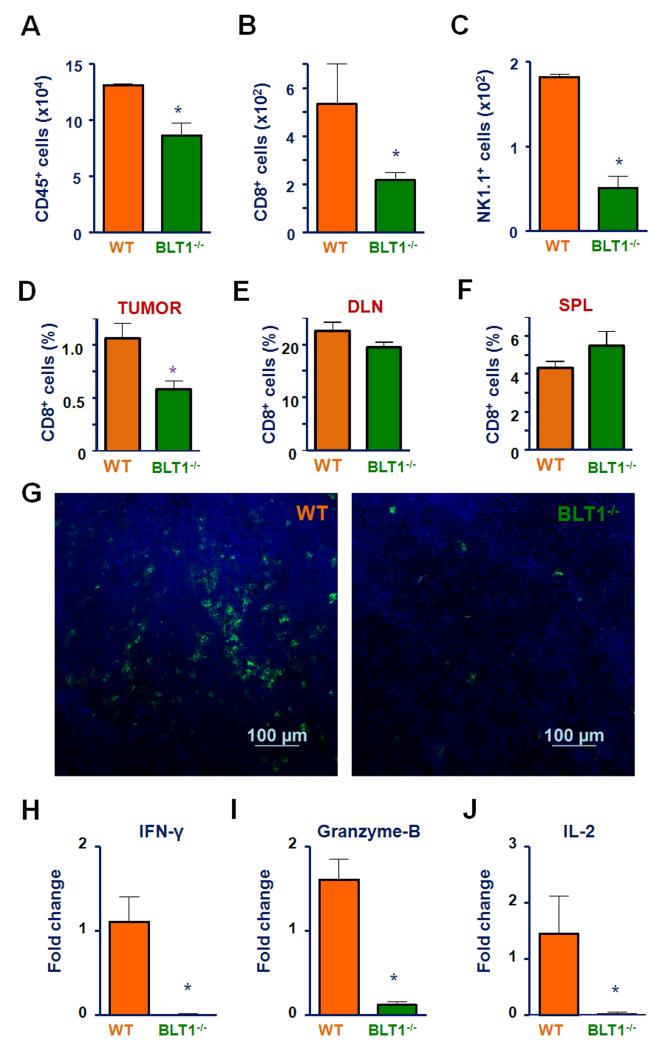
To determine the cellular basis for enhanced tumor growth in BLT1−/− mice, tumor infiltrating leukocyte subpopulations were profiled. Tumors were harvested from WT and BLT1−/− mice and processed into single cell suspension and stained for CD45.2 antibody for analyzing total immune infiltrate into tumors. The immune cell subsets were further analyzed with antibodies specific for CD4+, CD8+ T cells and NK cells using flow cytometry. A marked decrease in infiltration of overall CD45.2+ immune cells with most striking drop in CD8+ T and NK cell population in tumors of BLT1−/− mice compared to WT (Fig. 2A, 2B and 2C) was observed. Since tumors in BLT1−/− mice grew faster compared to WT, to ensure the observed differences were not related to tumor size, similar experiments were performed in size matched tumors which also showed reduced CD8 T-cell infiltration (Fig 2D). However, no differences in the CD8 T-cell numbers were observed in the spleen and lymph nodes of these tumor bearing WT and BLT1−/− mice (Fig 2E and F). Immunofluorescence staining and analysis by confocal microscopy also showed a reduction in the numbers of CD8+ T-cells in the TC-1 tumors derived from BLT1−/− mice compared to WT mice (Fig. 2G). Gene expression analysis by RTPCR showed a significant reduction in effector T-cell markers such as interferon-γ, granzyme B and Il-2 in mRNA from tumors of BLT1−/− mice relative to WT mice (Fig2 H-J).
FIGURE 2. Reduced infiltration of effector anti-tumor immune cells into TC-1 tumors growing in BLT1−/− mice.
(A-C) BLT1+/+ and BLT1−/− mice were injected s.c. with 1.0 ×105 of TC-1 cells and tumors were harvested at 29 days of post tumor challenge for both groups for analysis of tumor infiltrating leukocytes. Absolute numbers of tumor infiltrating total CD45+ immune cells (A), CD8+ T cells (B) and NK cells (C) per million of total tumor cells were analyzed from WT and BLT1−/− mice using standard flow cytometry methods as described. (D-F) CD8+ T cell staining in size matched tumors showing % CD8+ T cells of CD45+ cells within tumor (D), % CD8+ T cells of total cells obtained from DLNs (E) and % CD8+ T cells of total cells obtained from spleen (F) from WT and BLT1−/− mice. (G) Representative images of immunofluorescence staining of CD8+ T cells. Immunofluorescence analysis of CD8+ T-cells in TC-1 tumors harvested from BLT1+/+ and BLT1−/− mice were carried out as described in methods. The images were captured using Nikon A1R confocal at 200X magnification. The scale represents 100 μm. (H-J). Quantitative real time PCR: The levels of IFN-γ (H), granzyme-B (I) and IL-2 (J) mRNA expression in BLT1−/− tumors as compared to WT tumors by RT-PCR were determined as described in methods. The data are representative of 2-3 independent experiments involving at least n=4 mice/group in each experiment.
Similar growth Kinetics of TC-1 tumors in Rag2−/− and BLT1−/− Rag2−/− mice
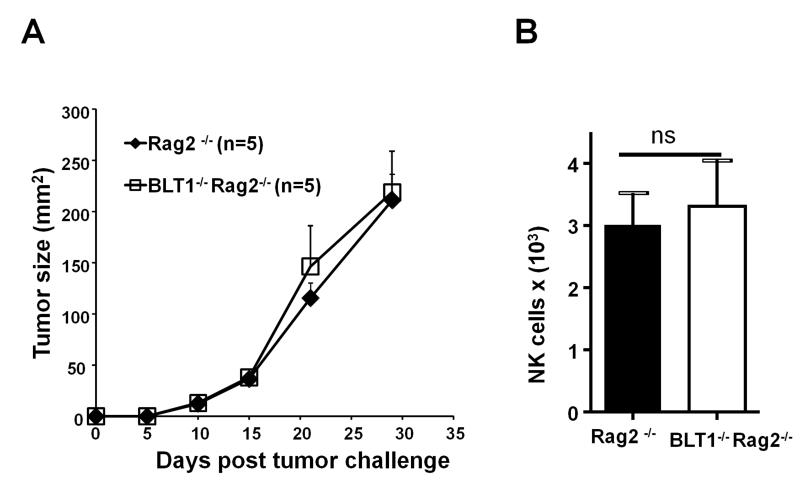
To understand the relative role of BLT1 expression on the cells of innate vs adaptive immunity in this accelerated tumor growth phenotype, BLT1−/− mice was crossed with Rag2−/− mice and Rag2−/−BLT1−/− mice were generated. Comparison of tumor growth curves between Rag2−/− and BLT1−/−Rag2−/− mice revealed no significant differences in tumor growth (Fig. 3A) indicating that BLT1 expression on innate cells including NK cells does not play a dominant role in this model. Moreover, similar numbers of NK cells were found in Rag2−/− and Rag2−/− BLT1−/− tumors (Fig. 3B).
FIGURE 3. Unaltered tumor growth and intra-tumoral NK cell numbers in Rag2−/− and Rag2−/−BLT1−/−.
(A). Rag2−/− mice and Rag2−/− BLT1−/− mice were challenged s.c. with 5 × 104 TC-1 cells on the right flank and observed for the rate of tumor growth as described in materials and methods section. (B). Absolute numbers of tumor infiltrating NK cells per million of total tumor cells were analyzed from Rag2−/− mice and Rag2−/−BLT1−/− mice using standard flow cytometry methods as described. The data shown are representative of three independent experiments.
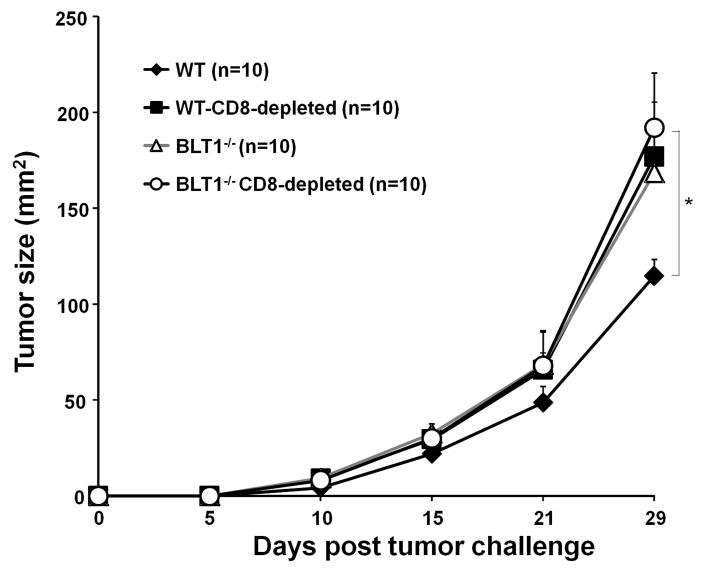
CD8+ T cell depletion accelerates tumor growth in WT but not in BLT1−/− mice
To explore the contribution of BLT1 expression on CD8+ T cells in limiting the tumor growth, CD8+ T cells were depleted in these mice followed by TC-1 tumor challenge. Depletion of CD8+ T cells resulted in a significant acceleration of tumor growth only in WT but not in BLT1−/− mice (Fig. 4). The tumor growth in the CD8 depleted WT mice nearly overlapped with the tumor growth observed in native BLT1−/− mice and/or CD8+ T-cell depleted BLT1−/− mice. Therefore, elimination of CD8+ T cells alone was sufficient for complete loss of the observed phenotype implicating a central role for BLT1 expression on CD8+ T cells (Fig. 4).
FIGURE 4. Depletion of CD8+ T cells accelerates TC-1 tumor growth in BLT1+/+ but not in BLT1−/− mice.
A single dose of 500 μg CD8 depleting antibody was injected i.p. in WT and BLT1−/− mice. The next day 1×105 TC-1 tumor cells were inoculated s.c in the right flank in WT and BLT1−/− mice and the tumor growth was monitored. The data shown are representative of three independent experiments.
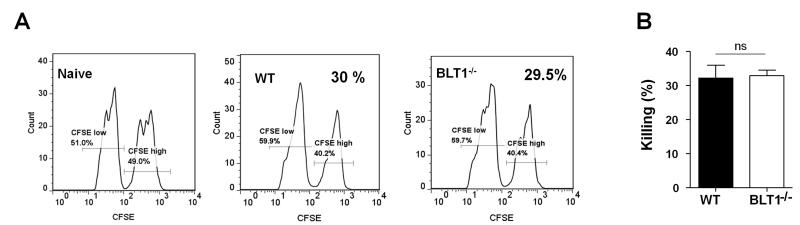
BLT1−/− CD8+ T cells display normal cytotoxic function
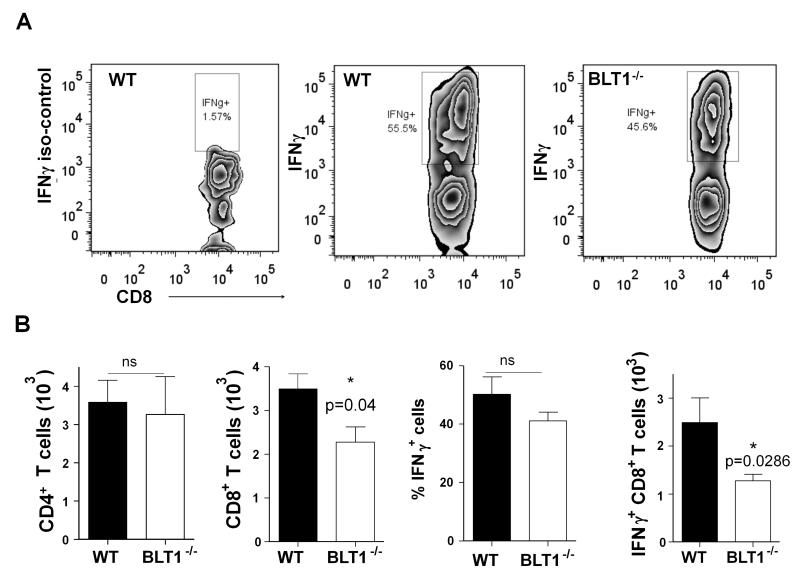
To test if the function of CD8+ T cells in the BLT1−/− is defective, we assessed the killing activity of CD8+ T cells in WT and BLT1−/− mice using an in vivo killing assay. As TC-1 cells express the human papillomavirus (HPV) early antigen E7, generation of E7-antigen specific spontaneous in vivo killing responses were previously shown in animals challenged with TC-1 cells (7). Similarly, immunization of E749-57 peptide (immunodominant epitope of E7) also generates CD8 mediated cytotoxic responses in vivo (7). The killing function of CD8+ T cells in vivo in WT and BLT1−/− mice that were either challenged by TC-1 cells or immunized by an HPV E749-57 peptide using an in vivo cytotoxicity assays was tested as described in methods. No significant differences in the killing activities of the CD8+ T cells in the spleens of WT or BLT1−/− mice either in peptide immunization or in TC-1 challenge settings were observed (data not shown). We next vaccinated the mice with 10μg MPL-A + 50μg HPV E749-57 peptide and assessed killing responses using in vivo cytotoxicity assays. Although peptide specific killing were observed to much higher levels there was no difference in killing activity of CD8+ T cells between BLT1+/+ and BLT1−/− mice (Fig. 5). Thus, a reduction in cytotoxic killing function of CD8+ T-cells seems unlikely to be responsible for the enhanced tumor growth in BLT1−/− mice. To examine the activation profiles of tumor infiltrating CD8+ T cells from the WT or BLT1−/− mice IFN-γ production was detected by intracellular cytokine staining by Flow cytometry (Fig 6). While there is reduced CD8+ T cell infiltration in tumors of BLT1−/− mice there was no significant difference in the percentage of IFN-γ positive cells of the total CD8+ T cells between WT and BLT1−/− tumors indicating similar activation and functional state of these cells (Fig 6B, middle panels). As might be expected from the reduced CD8+ T cell numbers in the BLT1−/− tumors, the total IFN-γ+ CD8+ T cells are also reduced in the BLT1−/− tumors relative to WT tumors (Fig 6B, right panel). The numbers of CD4+ T cells were not significantly different between WT and BLT1−/− tumors (Fig 6B, left panel).
FIGURE 5. Similar CTL mediated killing responses in spleens of vaccinated BLT1+/+ or BLT1−/− animals.
The CTL in vivo killing assay was performed in groups of WT and BLT1−/−mice as described in methods. The mice were vaccinated with 50 μg of E749-57 peptide + 10 μg of MPL-A in 200 μl of PBS. A group of naïve mice was also used as control. (A). Representative histograms of CFSE labeled targets. The killing levels of target cells in WT and BLT1−/− mice immunized with the E749-57 peptide+ MPL-A vaccine as described in methods. Cumulative levels of killing activity (n=4) of peptide immunized (B). No functional defect was observed in killing activity of CTLs in BLT1−/− mice.
FIGURE 6. Tumor Infiltrating CD8+ T cells from BLT1+/+ and BLT1−/− mice showed similar fraction of IFN-γ+ cells.
WT and BLT1−/− mice were challenged with TC-1 cells and the tumors were harvested at 6-8mm diameter and analyzed for IFN-γ as described in methods. (A) Representative image of flow cytometry analysis of IFN-γ positive cells gated on tumor resident total CD8+ cells from WT, BLT1−/− mice. Left panel represents isotype control. (B) Cumulative graphical representation (n=4) of tumor infiltrating CD4+ cells (left), tumor infiltrating CD8+ cells, the percentage of IFN-γ producing cells of total CD8+ cells and total number of CD8+ and IFN-γ+ double positive cells from the WT and BLT1−/− mice. The data shown are representative of two independent experiments.
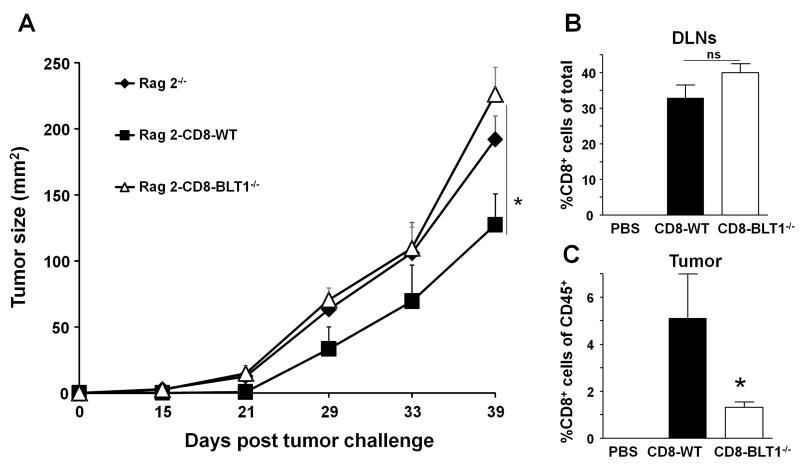
Adoptive transfer of CD8+ T cells from WT but not BLT1−/− mice slows tumor growth
To further assess that BLT1 expression on CD8+ T cells is critical; we used a gain of function approach. The Rag 2−/− mice that lack adaptive immune system were challenged with 5×104 TC-1 cells and 48 hrs later were adoptively transferred with tumor primed sorted CD8+ T cells (>97% purity) either from WT or BLT1−/− mice. Tumor primed cells were derived from mice with low tumor burden as these mice have shown to have enhanced CTL activity (7). CD8+ T cells from tumor primed WT but not from tumor primed BLT1−/− mice delayed tumor growth in Rag 2−/−mice (Fig 7A) reinforcing that BLT1 expression on the CD8+ T cells is crucial for effective antitumor immunity. The adoptive transfer of the naïve CD8+ T cells from WT or BLT1−/− mice in the Rag2−/− setting displayed a similar increase in tumor growth in BLT1−/− CD8+ T cell transfer group as compared to WT CD8+ T cells (data not shown). Analysis of tumor draining lymph nodes (TDLNs) from these mice revealed no differences in homeostatic proliferation and accumulation of transferred WT or BLT1−/− CD8+ T cells (Fig 7B). In contrast, WT CD8+ T cells entered Rag2−/− tumors in greater numbers as compared to BLT1−/− CD8+ T cells (Fig. 7C). Thus, a major defect in BLT1−/− CD8+ T-cells appears to be their reduced homing to the tumors.
FIGURE 7. Adoptive transfer of tumor primed CD8+ T cells from BLT1+/+ but not from BLT1−/− mice retards tumor growth.
Rag2−/− mice were challenged with 5×104 TC-1 tumor cells in the right flank. Two days later CD8+ T cells were isolated from the spleens and LN of tumor bearing (3-5mm) WT or BLT1−/− mice by magnetic sorting. 8×105 CD8 T cells (>97% purity) or PBS were injected i.v. in the tumor inoculated Rag2−/− mice. (A) Tumor growth curve of Rag2−/−, Rag2−/− transferred either with BLT1+/+ or BLT1−/− CD8+ cells. (B-C) The percentage of CD8+ T-cells of total cells recovered from tumor draining lymph nodes (B) and % CD8+ T cells of total CD45+ cells within tumors (C) are shown. The data shown are representative of three independent experiments (n=5 each time).
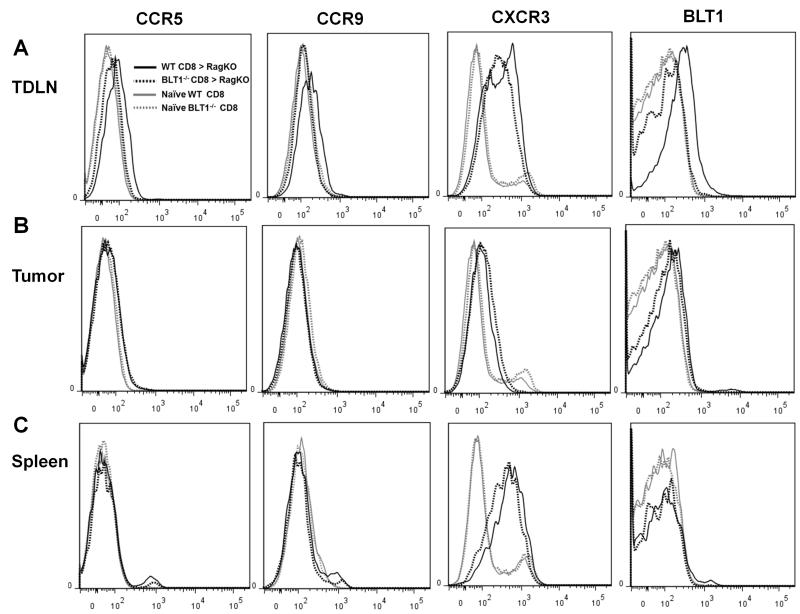
Chemokine receptor expression profiles in adoptively transferred CD8+ Cells
Chemokine receptor profiling of transferred CD8+ T cells from WT and BLT1−/− mice by flow cytometry revealed no striking differences beside the significantly enhanced expression of BLT1 on WT CTLs in TDLNs compared to the absence of its expression in naïve WT and BLT1−/− CTLs (Fig. 8A). A significant increase in CXCR3 expression was observed in adoptively transferred WT or BLT1−/− CD8+ T-cells relative to the CD8+ T cells from the naïve mice (Fig. 8A). Analysis of expression of all these receptors on CD8+ T-cells from the tumors showed complete down regulation of BLT1 expression on WT CD8+ T-cells. Similarly, CXCR3 expression was also down regulated on tumor infiltrating CD8+ T-cells independent of their origin from WT or BLT1−/− mice (Fig 8B). In contrast, CD8+ T-cells from the spleen showed similar levels of CXCR3 expression but no detectable BLT1 expression (Fig 8C).
FIGURE 8. Chemokine receptor expression on the adoptively transferred CD8+ T cells from spleen, draining lymph nodes and tumors.
Adoptively transferred CD8+ T cells from WT and BLT1−/− mice into Rag2−/− mice were analyzed the for the expression levels of chemokine receptors as described in methods. Naïve WT and BLT1−/− CD8+ T cells were used as controls. Chemokine receptors CCR5, CCR9, CXCR3 and BLT1 were stained and analyzed on the transferred CD8+ WT or BLT1−/− CD8+ T cells from TDLNs (A), Tumor (B) and Spleen (C). The data shown are representative of two independent experiments.
Discussion
Leukotriene B4 receptor 1, BLT1 controls the migration of various immune cell types mediating inflammation in many diseases and host response to infections. Migration of CD8+ T cells into the tumors is a critical event for effective anti-tumor immunity (11). Using an implantable cervical cancer model, we herein demonstrated that expression of BLT1 on CD8+ T cells plays a crucial role in mediating their recruitment to tumors thereby initiating and sustaining anti-tumor immunity.
Development of cancer is proposed to be the end result of a malignant transformation that has passed through Elimination, Equilibrium and Escape phases (36). The results with sub lethal and lethal challenge of TC-1 cells presented here (Fig. 1) suggest an important function for BLT1 on CD8+ T-cells in controlling both the elimination and equilibrium phases of tumor development, respectively. The sub-lethal dose (2×104) of TC-1 cells resulted in tumor only in 50% of the WT mice but in 100% of the BLT1−/− mice showing that BLT1 mediates immune surveillance and the lethal dose results in more rapidly growing tumors in BLT1−/− mice indicating its function in antitumor immunity.
Studies on the tumor microenvironment has demonstrated that poor recruitment of CD8+ T cells into the tumor as a limiting factor to achieving antitumor immunity and clinical responses (9, 10). In the context of cancer, little is known about the mechanisms controlling migration of CTLs to the tumor site. CCR5 was the first receptor shown to enhance the infiltration of CTLs in tumors and local production of CCL5 or CCL3 enhanced recruitment of these cells to tumors (13). However, the role of CCL5 remains controversial due to its divergent prognosis/survival outcomes in patents of non-small-cell lung carcinoma (NSCLC) verses breast and cervical carcinoma (37). The CXC chemokine receptor, CXCR3 and its ligands CXCL9/10 has been demonstrated to facilitate the recruitment of CTLs in a variety of inflammatory and infectious diseases (38, 39). CXCR3 expression on CTLs is very important as shown in RCC and melanoma (14, 15). In addition, CX3CL1 and CXCL16 have also been associated with high frequency of intra-tumoral T cells and better prognosis in colorectal cancer (40). The results presented here for the first time demonstrate a critical function for BLT1 expression on CTLs in controlling the progression of syngenic TC-1 tumor growth as evidenced by greatly reduced CTL infiltration and the intratumoral T-cell effector molecules (Fig 2). Although BLT1 has been repeatedly shown to be a critical homing receptor for diverse immune cell types including CTLs to their target organs, the biological significance of this receptor in antitumor immunity has not been explored. Majority of work carried out in the context of inflammatory, autoimmune diseases demonstrated that CD8+ T cells inducibly express BLT1 upon activation and the receptor expression is essential for their recruitment to target organs and disease development (41). In a model of auto reactive T cell induced uveitis, BLT1 expression on both T cells and innate immune cells was found critical for full disease development and absence of BLT1 is highly protective in ocular inflammation (32). However, in the context of cancer, lack or delay in recruitment of effector immune cells such as T cells may delay generation of immune response to tumor antigens that can lead to breach of immune surveillance and poor antitumor immunity.
While BLT1 is expressed on a variety of immune cells, selective immune cell subsets are preferentially recruited under different inflammatory conditions such as neutrophils in the joints of arthritis, T cells in uveitis and pulmonary hypersensitivity, M2 Macrophages in diet induced obesity and atherosclerosis (26, 32, 42, 43). The current studies demonstrate that absence of BLT1 leads to reduced CTL infiltration in to TC-1 tumors. Comparison of tumor growth curves between Rag2−/− and BLT1−/−Rag2−/− mice revealed no significant differences in tumor growth (Fig. 3) indicating that BLT1 expression on innate cells including NK cells does not play a dominant role in this model. Furthermore, given all other cell types still harbor the BLT1 in CD8 depleted WT mice, complete overlap of WT phenotype to BLT1−/− phenotype demonstrate that BLT1 expression on the CD8+ T cells accounts for the entire enhancement of TC-1 tumor growth (Fig. 4). Interestingly, Rag2−/− and BLT1−/−Rag2−/− mice revealed no significant differences in intra-tumoral NK cell numbers. While NK-cells by themselves may not hinder tumor growth in this model, it is still possible through CD8-NK cross talk (44) they contribute to effective antitumor immunity.
Although the recruitment of the CD8+ T cells into the tumors was significantly impaired in BLT1−/− mice, the cytolytic function of the BLT1−/− CD8+ T cells was intact as demonstrated by the killing ability in the spleen (Fig 5) and also by the IFN-γ staining of the CD8+ T cells in the tumors from WT and BLT1−/− mice (Fig 6). Moreover, the in vivo killing experiments suggest that the contribution of BLT1 expression on antigen presenting cells in generating effective CTL responses is very limited, if at all. Our previous studies with bone marrow derived dendritic cells (BMDCs) from BLT1−/− mice showed reduced levels of CCR7 and transient delay in migration of DCs into draining lymph nodes (DLNs) for antigen presentation (45). While we cannot rule out a delay of antigen presentation in the context of tumor development in the BLT1−/− mice, it does not appear to influence the generation of a peripheral CTL response. Instead, the failure of these T-cells in reaching the tumor appears to play a dominant role in the end phenotype.
In Rag2−/− mice, the CTLs from WT mice are capable of entering tumors in greater numbers compared to CTLs from BLT1−/− mice despite their similar frequency in TDLNs. Given the recruitment of BLT1+/+ cells are LTB4 dependent; it is likely that tumors have higher gradient of LTB4 than TDLN. Due to rapid growth, tumor cells undergo apoptosis that might lead to the recruitment myeloid cells including neutrophils and macrophages. This might set up a cascade where leukocytes in the tumor micro environment produce LTB4 for preferential recruitment of CTLs in this model. In this regard, recent studies have shown that LTB4/BLT1 axis plays a critical role in amplifying local cell death signals by neutrophil recruitment to the sites of sterile inflammation (46). It is not known which factors in the context of tumor environment contribute to selectively recruit CD8+ T cells to tumors compared to other immune cell types. The enhanced expression of BLT1 in TDLN was anticipated as activated antigen specific T cells are proposed to express the BLT1 for their migration to inflamed tissues (23, 26, 41). In this context the tumor is the inflamed site that attracts antigen primed CTLs through BLT1/LTB4 axis. The loss of BLT1, CCR5, and CXCR3 expression within tumor is consistent with our previous studies and many others showing that chemokine receptors are down regulated due to receptor internalization upon ligand binding at the target organ (42, 47). Future studies will be required to address the cooperative interactions between BLT1 and other chemokine receptors in orchestrating CTL migration into tumors. Indeed, such interactions between BLT1 and chemokine receptors CCR1 and CXCR2 were recently demonstrated in recruitment of neutrophils into arthritic joints (42).
A recent article by Yokota et al (2012; Ref) used GM-CSF-based tumor vaccine setting in BALB/c leukemia model to evaluate the vaccine and secondary/recall immune responses. In contrast to the current study, their results showed similar or better primary and recall immune responses in the BLT1−/− mice. Several differences that might account for the divergent results include 1) different mouse strains (BALB/c) 2) different cancer type (leukemia) and 3) GM-CSF transformed cancer cell lines. In addition, Yokota et al study found differences WT and BLT1−/− mice only in the recall responses with CD4+ T-cells playing a dominant role. In our studies (Fig 6, lower left panel) the numbers of tumor infiltrating CD4+ T cells in WT and BLT1−/− mice were similar indicating limited if any direct role of CD4+ T cells in controlling the tumor growth. Furthermore, the depletion of CD8+ T-cells alone completely accounts for the observed phenotypic differences between WT and BLT1−/− mice eliminating a direct role of BLT1 on CD4+ T cells. It is possible that in tumors of epithelial origin where CTL responses play major role in controlling the disease outcome, BLT1 expression on CD8+ T cells might facilitate the anti-tumor immunity. In this regard, we have confirmed that lack of BLT1 on CD8+ T cells causes defective migration and antitumor immunity in B16 melanoma and spontaneous APCMin+ intestinal tumor models (unpublished results). In human cervical cancers, melanoma and intestinal cancers CTL numbers correlate with better prognosis and survival (48-50) suggesting that BLT1 expression on CTLs might have significant therapeutic relevance.
These findings have important implications in the context of adoptive T cell therapies (ACT) where recruitment of T cells to the tumor site is the primary requirement and a major hurdle for achieving clinical efficacy (9, 51). It is conceivable that perhaps forced expression of BLT1 on tumor reactive T cells in chimeric antigen receptor (CAR) or adoptive transfer T cell therapies may enhance the tumor infiltration of T cells and thus the efficacy of such treatment regimens. Furthermore, selective and high affinity agonists of BLT1 released at the tumor site in conjunction with immunotherapies and cancer vaccines may facilitate the tumor infiltration of CTLs for better therapeutic outcome.
Acknowledgements
We thank Michelle Elisabeth Smith for technical help and support during the studies.
This work was supported in part by the NIH R01 CA138623 grants and The James Graham Brown Cancer Center.
Footnotes
Disclosures: Authors have no financial conflicts of interest.
REFERENCES
- 1.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 2.Swann JB, Vesely MD, Silva A, Sharkey J, Akira S, Schreiber RD, Smyth MJ. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci U S A. 2008;105:652–656. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui Y, Kelleher E, Straley E, Fuchs E, Gorski K, Levitsky H, Borrello I, Civin CI, Schoenberger SP, Cheng L, Pardoll DM, Whartenby KA. Immunotherapy of established tumors using bone marrow transplantation with antigen gene--modified hematopoietic stem cells. Nature medicine. 2003;9:952–958. doi: 10.1038/nm882. [DOI] [PubMed] [Google Scholar]
- 5.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlom J. Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst. 2012;104:599–613. doi: 10.1093/jnci/djs033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma RK, Elpek KG, Yolcu ES, Schabowsky RH, Zhao H, Morgan L, Bandura-Shirwan H. Costimulation as a platform for the development of vaccines: a peptide-based vaccine containing a novel form of 4-1BB ligand eradicates established tumors. Cancer Res. 2009;69:4319–4326. doi: 10.1158/0008-5472.CAN-08-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nature reviews. Immunology. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher DT, Chen Q, Appenheimer MM, Skitzki J, Wang WC, Odunsi K, Evans SS. Hurdles to lymphocyte trafficking in the tumor microenvironment: implications for effective immunotherapy. Immunol Invest. 2006;35:251–277. doi: 10.1080/08820130600745430. [DOI] [PubMed] [Google Scholar]
- 10.Moon EK, Carpenito C, Sun J, Wang LC, Kapoor V, Predina J, Powell DJ, Jr., Riley JL, June CH, Albelda SM. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clinical cancer research. 2011;17:4719–4730. doi: 10.1158/1078-0432.CCR-11-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franciszkiewicz K, Boissonnas A, Boutet M, Combadiere C, Mami-Chouaib F. Role of chemokines and chemokine receptors in shaping the effector phase of the antitumor immune response. Cancer Res. 2012;72:6325–6332. doi: 10.1158/0008-5472.CAN-12-2027. [DOI] [PubMed] [Google Scholar]
- 12.Johrer K, Pleyer L, Olivier A, Maizner E, Zelle-Rieser C, Greil R. Tumour-immune cell interactions modulated by chemokines. Expert Opin Biol Ther. 2008;8:269–290. doi: 10.1517/14712598.8.3.269. [DOI] [PubMed] [Google Scholar]
- 13.Mule JJ, Custer M, Averbook B, Yang JC, Weber JS, Goeddel DV, Rosenberg SA, Schall TJ. RANTES secretion by gene-modified tumor cells results in loss of tumorigenicity in vivo: role of immune cell subpopulations. Hum Gene Ther. 1996;7:1545–1553. doi: 10.1089/hum.1996.7.13-1545. [DOI] [PubMed] [Google Scholar]
- 14.Kondo T, Ito F, Nakazawa H, Horita S, Osaka Y, Toma H. High expression of chemokine gene as a favorable prognostic factor in renal cell carcinoma. J Urol. 2004;171:2171–2175. doi: 10.1097/01.ju.0000127726.25609.87. [DOI] [PubMed] [Google Scholar]
- 15.Mullins IM, Slingluff CL, Lee JK, Garbee CF, Shu J, Anderson SG, Mayer ME, Knaus WA, Mullins DW. CXC chemokine receptor 3 expression by activated CD8+ T cells is associated with survival in melanoma patients with stage III disease. Cancer Res. 2004;64:7697–7701. doi: 10.1158/0008-5472.CAN-04-2059. [DOI] [PubMed] [Google Scholar]
- 16.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 17.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 18.Yokomizo T, Kato K, Terawaki K, Izumi T, Shimizu T. A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J Exp Med. 2000;192:421–432. doi: 10.1084/jem.192.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tager AM, Luster AD. BLT1 and BLT2: the leukotriene B(4) receptors. Prostaglandins Leukot Essent Fatty Acids. 2003;69:123–134. doi: 10.1016/s0952-3278(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 20.Kim ND, Chou RC, Seung E, Tager AM, Luster AD. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J Exp Med. 2006;203:829–835. doi: 10.1084/jem.20052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li RC, Haribabu B, Mathis SP, Kim J, Gozal D. Leukotriene B4 receptor-1 mediates intermittent hypoxia-induced atherogenesis. Am J Respir Crit Care Med. 2011;184:124–131. doi: 10.1164/rccm.201012-2039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathis SP, Jala VR, Lee DM, Haribabu B. Nonredundant roles for leukotriene B4 receptors BLT1 and BLT2 in inflammatory arthritis. J Immunol. 2010;185:3049–3056. doi: 10.4049/jimmunol.1001031. [DOI] [PubMed] [Google Scholar]
- 23.Medoff BD, Seung E, Wain JC, Means TK, Campanella GS, Islam SA, Thomas SY, Ginns LC, Grabie N, Lichtman AH, Tager AM, Luster AD. BLT1-mediated T cell trafficking is critical for rejection and obliterative bronchiolitis after lung transplantation. J Exp Med. 2005;202:97–110. doi: 10.1084/jem.20042481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medoff BD, Tager AM, Jackobek R, Means TK, Wang L, Luster AD. Antibody-antigen interaction in the airway drives early granulocyte recruitment through BLT1. Am J Physiol Lung Cell Mol Physiol. 2006;290:L170–178. doi: 10.1152/ajplung.00212.2005. [DOI] [PubMed] [Google Scholar]
- 25.Miyahara N, Ohnishi H, Matsuda H, Miyahara S, Takeda K, Koya T, Matsubara S, Okamoto M, Dakhama A, Haribabu B, Gelfand EW. Leukotriene B4 receptor 1 expression on dendritic cells is required for the development of Th2 responses and allergen-induced airway hyperresponsiveness. J Immunol. 2008;181:1170–1178. doi: 10.4049/jimmunol.181.2.1170. [DOI] [PubMed] [Google Scholar]
- 26.Miyahara N, Takeda K, Miyahara S, Taube C, Joetham A, Koya T, Matsubara S, Dakhama A, Tager AM, Luster AD, Gelfand EW. Leukotriene B4 receptor-1 is essential for allergen-mediated recruitment of CD8+ T cells and airway hyperresponsiveness. J Immunol. 2005;174:4979–4984. doi: 10.4049/jimmunol.174.8.4979. [DOI] [PubMed] [Google Scholar]
- 27.Serezani CH, Perrela JH, Russo M, Peters-Golden M, Jancar S. Leukotrienes are essential for the control of Leishmania amazonensis infection and contribute to strain variation in susceptibility. J Immunol. 2006;177:3201–3208. doi: 10.4049/jimmunol.177.5.3201. [DOI] [PubMed] [Google Scholar]
- 28.Shao WH, Del Prete A, Bock CB, Haribabu B. Targeted disruption of leukotriene B4 receptors BLT1 and BLT2: a critical role for BLT1 in collagen-induced arthritis in mice. J Immunol. 2006;176:6254–6261. doi: 10.4049/jimmunol.176.10.6254. [DOI] [PubMed] [Google Scholar]
- 29.Soares EM, Mason KL, Rogers LM, Serezani CH, Faccioli LH, Aronoff DM. Leukotriene B4 Enhances Innate Immune Defense against the Puerperal Sepsis Agent Streptococcus pyogenes. J Immunol. 2013;190:1614–1622. doi: 10.4049/jimmunol.1202932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terawaki K, Yokomizo T, Nagase T, Toda A, Taniguchi M, Hashizume K, Yagi T, Shimizu T. Absence of leukotriene B4 receptor 1 confers resistance to airway hyperresponsiveness and Th2-type immune responses. J Immunol. 2005;175:4217–4225. doi: 10.4049/jimmunol.175.7.4217. [DOI] [PubMed] [Google Scholar]
- 31.Subbarao K, Jala VR, Mathis S, Suttles J, Zacharias W, Ahamed J, Ali H, Tseng MT, Haribabu B. Role of leukotriene B4 receptors in the development of atherosclerosis: potential mechanisms. Arterioscler Thromb Vasc Biol. 2004;24:369–375. doi: 10.1161/01.ATV.0000110503.16605.15. [DOI] [PubMed] [Google Scholar]
- 32.Liao T, Ke Y, Shao WH, Haribabu B, Kaplan HJ, Sun D, Shao H. Blockade of the interaction of leukotriene b4 with its receptor prevents development of autoimmune uveitis. Invest Ophthalmol Vis Sci. 2006;47:1543–1549. doi: 10.1167/iovs.05-1238. [DOI] [PubMed] [Google Scholar]
- 33.Miyahara N, Takeda K, Miyahara S, Matsubara S, Koya T, Joetham A, Krishnan E, Dakhama A, Haribabu B, Gelfand EW. Requirement for leukotriene B4 receptor 1 in allergen-induced airway hyperresponsiveness. Am J Respir Crit Care Med. 2005;172:161–167. doi: 10.1164/rccm.200502-205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haribabu B, Verghese MW, Steeber DA, Sellars DD, Bock CB, Snyderman R. Targeted disruption of the leukotriene B(4) receptor in mice reveals its role in inflammation and platelet-activating factor-induced anaphylaxis. J Exp Med. 2000;192:433–438. doi: 10.1084/jem.192.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmadzadeh M, Felipe-Silva A, Heemskerk B, Powell DJ, Jr., Wunderlich JR, Merino MJ, Rosenberg SA. FOXP3 expression accurately defines the population of intratumoral regulatory T cells that selectively accumulate in metastatic melanoma lesions. Blood. 2008;112:4953–4960. doi: 10.1182/blood-2008-06-163048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 37.Niwa Y, Akamatsu H, Niwa H, Sumi H, Ozaki Y, Abe A. Correlation of tissue and plasma RANTES levels with disease course in patients with breast or cervical cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7:285–289. [PubMed] [Google Scholar]
- 38.Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nature immunology. 2008;9:970–980. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- 39.Sung JH, Zhang H, Moseman EA, Alvarez D, Iannacone M, Henrickson SE, de la Torre JC, Groom JR, Luster AD, von Andrian UH. Chemokine guidance of central memory T cells is critical for antiviral recall responses in lymph nodes. Cell. 2012;150:1249–1263. doi: 10.1016/j.cell.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohta M, Tanaka F, Yamaguchi H, Sadanaga N, Inoue H, Mori M. The high expression of Fractalkine results in a better prognosis for colorectal cancer patients. International journal of oncology. 2005;26:41–47. [PubMed] [Google Scholar]
- 41.Tager AM, Bromley SK, Medoff BD, Islam SA, Bercury SD, Friedrich EB, Carafone AD, Gerszten RE, Luster AD. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nature immunology. 2003;4:982–990. doi: 10.1038/ni970. [DOI] [PubMed] [Google Scholar]
- 42.Chou RC, Kim ND, Sadik CD, Seung E, Lan Y, Byrne MH, Haribabu B, Iwakura Y, Luster AD. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity. 2010;33:266–278. doi: 10.1016/j.immuni.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spite M, Hellmann J, Tang Y, Mathis SP, Kosuri M, Bhatnagar A, Jala VR, Haribabu B. Deficiency of the leukotriene B4 receptor, BLT-1, protects against systemic insulin resistance in diet-induced obesity. J Immunol. 2011;187:1942–1949. doi: 10.4049/jimmunol.1100196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan Z, Yu P, Wang Y, Fu ML, Liu W, Sun Y, Fu YX. NK-cell activation by LIGHT triggers tumor-specific CD8+ T-cell immunity to reject established tumors. Blood. 2006;107:1342–1351. doi: 10.1182/blood-2005-08-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DelPrete A, Shao WH, Mitola S, Santoro G, Sozzani S, Haribabu B. Regulation of dendritic cell migration and adaptive immune response by leukotriene B4 receptors: a role for LTB4 in up-regulation of CCR7 expression and function. Blood. 2007;109:626–631. doi: 10.1182/blood-2006-02-003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, Germain RN. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–375. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jala VR, Haribabu B. Real-time imaging of leukotriene B(4) mediated cell migration and BLT1 interactions with beta-arrestin. J Vis Exp. 2010 doi: 10.3791/2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Vos van Steenwijk PJ, Ramwadhdoebe TH, Goedemans R, Doorduijn EM, van Ham JJ, Gorter A, van Hall T, Kuijjer ML, van Poelgeest MI, van der Burg SH, Jordanova ES. Tumor-infiltrating CD14 positive myeloid cells and CD8 positive T cells prolong survival in patients with cervical carcinoma. International journal of cancer. 2013 doi: 10.1002/ijc.28309. [DOI] [PubMed] [Google Scholar]
- 49.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 50.van Houdt IS, Sluijter BJ, Moesbergen LM, Vos WM, de Gruijl TD, Molenkamp BG, van den Eertwegh AJ, Hooijberg E, van Leeuwen PA, Meijer CJ, Oudejans JJ. Favorable outcome in clinically stage II melanoma patients is associated with the presence of activated tumor infiltrating T-lymphocytes and preserved MHC class I antigen expression. International journal of cancer. 2008;123:609–615. doi: 10.1002/ijc.23543. [DOI] [PubMed] [Google Scholar]
- 51.Mondino A, Dardalhon V, Michelini R. Hess, Loisel-Meyer S, Taylor N. Redirecting the immune response: role of adoptive T cell therapy. Hum Gene Ther. 2010;21:533–541. doi: 10.1089/hum.2010.033. [DOI] [PMC free article] [PubMed] [Google Scholar]