Abstract
Background
Osteochondrosis (OC) is a common developmental orthopedic disease affecting both humans and animals. Despite increasing recognition of this disease among children and adolescents, its pathogenesis is incompletely understood because clinical signs are often not apparent until lesions have progressed to end-stage, and examination of cadaveric early lesions is not feasible. In contrast, both naturally-occurring and surgically-induced animal models of disease have been extensively studied, most notably in horses and swine, species in which OC is recognized to have profound health and economic implications. The potential for a translational model of human OC has not been recognized in the existing human literature.
Objective
The purpose of this review is to highlight the similarities in signalment, predilection sites and clinical presentation of naturally-occurring OC in humans and animals and to propose a common pathogenesis for this condition across species.
Study Design
Review
Methods
The published human and veterinary literature for the various manifestations of OC was reviewed. Peer-reviewed original scientific articles and species-specific review articles accessible in PubMed (US National Library of Medicine) were eligible for inclusion.
Results
A broad range of similarities exists between OC affecting humans and animals, including predilection sites, clinical presentation, radiographic/MRI changes, and histological appearance of the end stage lesion, suggesting a shared pathogenesis across species.
Conclusion
This proposed shared pathogenesis for OC between species implies that naturally-occurring and surgically-induced models of OC in animals may be useful in determining risk factors and for testing new diagnostic and therapeutic interventions that can be used in humans.
Key Terms: osteochondrosis, osteochondritis dissecans, articular-epiphyseal cartilage complex, endochondral ossification, translational model, pathogenesis
INTRODUCTION
Osteochondrosis (OC) is a developmental orthopedic disease characterized by clinical signs of joint pain, effusion, and dysfunction caused by the formation of clefts extending through the articular cartilage into the subchondral bone. Extensive studies evaluating the clinical aspects of this condition are available in both human and veterinary medicine; however, there is limited information available regarding the similarities and differences between OC in humans and animals.
The majority of studies aimed at describing the etiologic factors and pathogenesis of OC in humans focus on osteochondral fragments removed surgically from adolescents or adults presenting with clinical symptoms of OC.[1] By this time, the fragments have been present for months to years. Understandably, it is nearly impossible to determine the pathogenesis of the disease from examination of these end-stage tissues. Obtaining osteochondral samples from juvenile human cadavers is difficult, and currently there is no established method for screening asymptomatic children or adolescents for OC. Both of these factors have hampered the understanding of the pathogenesis of naturally-occurring human disease. In contrast, in the veterinary literature, OC is defined as a focal disturbance of endochondral ossification,[2] the process by which a cartilage template ossifies in the appendicular skeleton of a growing individual. Extensive studies performed in young growing animals of several species have demonstrated early, developing lesions at predilection sites well before the age at which clinical disease manifests.[3–5] We believe that naturally-occurring and surgically-induced OC in animals may provide valuable translational models to help understand the etiology and pathogenesis of human disease. Our review, therefore, aims to highlight the similarities in signalment, predilection sites and clinical presentation of naturally-occurring OC in humans and animals, and by doing so, propose a common pathogenesis for this condition across species.
DISEASE TERMINOLOGY
Evaluation of the literature pertaining to OC is complicated by the variety of terminologies used. In 1887, König proposed the term “osteochondritis dissecans” for an underlying lesion in the joint cartilage facilitating formation of loose bodies in the absence of significant trauma.[6] Subsequent histological studies have not supported a primary inflammatory etiology for the condition, making “osteochondrosis” the more accurate term, as suggested by Howa1d in 1942.[7, 8] However, the original phrase has persisted, and in fact, “osteochondrosis” and “osteochondritis” are often used interchangeably. In the clinical literature, when a fissure or fracture in the overlying articular cartilage is present, the condition is nearly universally referred to as osteochondritis dissecans (OCD), although osteochondrosis dissecans would be more appropriate. In the veterinary medical field, focal abnormalities of endochondral ossification involving the articular-epiphyseal cartilage complex (AECC) are referred to as osteochondrosis (or osteochondrosis dissecans, as appropriate) regardless of anatomical location. Conversely, in the human literature, manifestations of OC at various anatomical sites are given different names (Table 1). Additionally, the phrase “the osteochondroses” includes conditions affecting the AECC, the physis, and various apophyseal locations. This general phrase has also been used to describe diseases of primary osteonecrotic etiology, such as Legg-Calvé-Perthes disease.[9] The present article will specifically focus on articular manifestations of OC.
Table 1.
Disease names for manifestations of osteochondrosis at specific anatomical locations as reported in the human literature. For comparison, location of predilection sites in pigs and horses is also presented.
| Disease Name (Human) | Location | Pig | Horse | |
|---|---|---|---|---|
| Articular | ||||
| Theimann’s Disease | proximal and distal interphalangeal joints (fingers and toes) | |||
| Panner’s Disease | elbow (humeral capitellum) | X | ||
| osteochondritis dissecans (OCD) | elbow (humeral capitellum), knee (medial or lateral femoral condyle), ankle (medial talus) | X | X | |
| Freiberg’s Disease | metatarsophalangeal joint (head of 2nd metatarsal) | X | ||
| Non-articular/apophyseal | ||||
| Sinding-Larsen-Johansson Disease | knee (inferior pole of patella) | X | ||
| Osgood-Schlatter Disease | knee (proximal tibia) | |||
| Sever’s Disease | ankle (proximal calcaneus) | |||
| Köhler’s Disease | ankle (tarsal navicular bone) | |||
| Iselin Disease | ankle (base of 5th metatarsal) | |||
| medial epicondyle apophysitis | elbow (medial epicondyle) | |||
| Physeal | ||||
| Blount Disease (tibia vara) | proximal tibial physis | |||
| Scheuermann’s Disease | vertebrae | X | X | |
CLINICAL ASPECTS OF OSTEOCHONDROSIS IN HUMANS AND ANIMALS
Human OC is typically not recognized in children or adolescents until the onset of clinical symptoms, at which point the disease is advanced.[16] In many cases, a lag time of months to years may exist between the onset of symptoms and diagnosis of the disease.[17] OC diagnosed prior to the age at which physeal closure occurs is known as juvenile OC; however, lesions diagnosed in adulthood also most likely developed prior to physeal closure.[10] Common presenting clinical complaints include joint pain, especially with extreme flexion or extension, swelling, and catching or locking of the joint. These symptoms may be intermittent, especially early in the course of disease, and may be associated with athletic activity. Continuous or more severe symptoms may be indicative of a loose osteochondral fragment within the joint.[10, 18] Bilateral disease is not uncommon, although clinical symptoms are typically worse in one joint than the other.[19] Diagnosis is typically made by radiologic and/or magnetic resonance imaging (MRI) examination of the affected joint. MRI more closely aligns with arthroscopic findings[20] and is also more sensitive for identification of subtle cartilage abnormalities (i.e. prior to formation of overt osteochondral fragments), suggesting that this may be the better imaging modality for OC, especially for early lesions.[10] The preferred initial treatment for OC when the articular surface is intact is non-surgical management, including a combination of non-steroidal anti-inflammatory drugs, physical therapy, and modification of activity, typically with some form of joint immobilization. If conservative therapy fails, or if partially or completely detached osteochondral fragments are present at the time of diagnosis, then surgical intervention via arthroscopy is pursued.[10, 11, 19] Although removal of the fragment/flap followed by debridement is most common, reattachment of large osteochondral flaps using internal fixation has also been described.[21] Lesions that are not treated adequately may lead to development of degenerative joint disease with long-term debilitative consequences for the individual; thus, early intervention is recommended.[16, 21]
In horses, asymptomatic OC is usually identified at an early age due to extensive radiographic screening aimed at facilitating sale of racehorses as yearlings (before two years of age). In more slowly-maturing breeds that usually do not undergo early radiographic screening, OC is most often identified after 3 years of age as clinical signs, including subtle lameness and joint effusion, develop after the commencement of regular training. This latter presentation is strikingly similar to that noted in cases of juvenile OC in humans, which most frequently affects young athletes and usually presents with poorly localized pain that is exacerbated with exercise.[8, 19, 22] In horses, OC lesions are most often treated with arthroscopic removal of loose fragments followed by debridement of the fragment bed with or without microfracture.[23] Although many horses go on to perform in their intended capacity after treatment, the prognosis for future athletic career following surgical debridement of OC lesions diminishes as the size of the lesion increases.[24] Novel treatment modalities attempting to salvage and reattach large osteochondral flaps have recently been introduced to address this concern.[23]
In commercially bred pigs, OC is considered to be an important cause of lameness with profound economic implications.[5, 13] Clinical signs consistent with OC have been associated with decreased longevity of young female swine intended for breeding.[25, 26] Although histological changes characteristic of early articular OC have been described in the femoral condyles of pigs as young as 6–8 weeks of age[27], clinical signs usually do not become apparent before adolescence.[28] Treatment of OC in pigs is usually not economically feasible and severely affected animals are generally sent to slaughter. As a result, greater emphasis is placed on prevention rather than treatment of disease, although arthroscopic removal of an OC lesion affecting the talus has been reported in this species.[29]
It is worth noting that skeletal maturity is reached much more rapidly in the animal species discussed above than in humans. Ossification (“closure”) of the physeal (metaphyseal growth plate) and epiphyseal (AECC) cartilage is the hallmark of skeletal maturity in all species. In young humans, this process occurs between 14 and 25 years of age, depending on anatomical location.[30] In contrast, growth cartilage closure in horses begins around three months of age and is considered complete before 3 years of age.[31] Thus, a yearling horse would be at the approximate maturity of an adolescent human, with the onset of clinically-apparent OC in both species occurring around the time of cessation of growth. The association of athletic activity and onset of clinical signs is also reflected in both humans and horses, although asymptomatic lesions are undoubtedly present earlier.
PREVALENCE OF OSTEOCHONDROSIS
Global estimates of the prevalence of articular OC are not reported in the human literature, likely due to the tendency to regard manifestations of OC at different anatomical locations as separate diseases. Prevalence of elbow OC was reported as 4.1% in one radiographic survey of 1,000 Danish men over the age of 15,[32] while incidence of knee OC was calculated to be between 15 and 30 per 100,000 in women and men (respectively) between the ages of 10 and 20 in a single Swedish city.[33] In general, OC of the knee is considered to be most common, representing approximately 75% of all cases,[10] with manifestations in the elbow (second most commonly affected location), ankle, and hip occurring relatively uncommonly.[17] However, it is likely that any estimate of OC prevalence in humans is an underestimate, given that diagnosis is generally delayed until the onset of clinical signs[34]; many individuals may be asymptomatically affected and never diagnosed. In contrast, in horses, where survey radiographs are routinely taken in many breeds before the onset of clinical signs, global prevalence estimates range from 20% to 80%, although prevalence varies by joint and breed.[35, 36] Similarly, in pigs, where prevalence is determined based on post-mortem surveys, up to 70% of animals are reportedly affected.[5, 37] Most of these lesions in horses and pigs are subclinical/asymptomatic at the time of diagnosis.
PROPOSED PATHOGENESIS AND RISK FACTORS
The underlying etiology and pathogenesis of OC have long been the subject of controversy and speculation, and a variety of environmental and genetic risk factors have been proposed. Historically, the major schools of thought have been divided into those who propose trauma as the primary cause for OC and those who suggest alternative underlying processes, including inflammation, osteonecrosis, vascular abnormalities, and cartilage extracellular matrix abnormalities.[6, 7] König himself fell into the latter category, describing osteochondritis dissecans as occurring in the absence of any significant trauma.[38] However, the inflammatory etiology suggested by the term “osteochondritis” has not been corroborated by subsequent histological studies.[1, 39] Histological results from osteochondral fragments removed during surgery also fail to support osteonecrosis of subchondral bone as the primary lesion of OC.[1, 39] Instead, necrosis of the subchondral bone is thought to most commonly occur secondary to detachment of the osteochondral fragment, rather than being an inciting cause.[40] The production and accumulation of abnormal extracellular matrix molecules in the endoplasmic reticulum (ER) has been suggested as the underlying cause of abnormal mineralization (failed endochondral ossification) and subsequent OC lesions, based on electron microscopy of surgically removed OC lesions and adjacent “normal” cartilage biopsies from four human patients.[41] The authors hypothesized that an underlying inherited ER storage disorder was responsible for abnormal protein production and accumulation, although no candidate mutation was identified.[41] However, other conditions, including local ischemia, can lead to accumulation of unfolded, but otherwise normal, proteins in the ER.[41] Additionally, the tissues examined were end-stage, making it difficult to determine if the ultrastructural changes were a cause or a consequence of disease. Abnormalities in cartilage extracellular matrix maturation have also been proposed to play a central role in the development of OC. Decreased collagen content and alterations in collagen cross-linking were reported in cartilage samples obtained from foals with OC compared to healthy foals, and this “immature” cartilage was considered potentially more susceptible to external trauma.[42] Similarly, it has been suggested that the marked changes in collagen fibril orientation and density across the epiphyseal cartilage, especially near nutritive cartilage canals in the ossification front may create focal areas of biomechanical weakness.[43] However, there is little evidence that these matrix changes are primary. Indeed, many of the matrix alterations reported by Lecocq et al (2008)[43] were located in or near focal regions of chondronecrosis. Studies performed in animals demonstrate that these focal regions of chondronecrosis form due to interruption of the blood supply to the nutritive cartilage canals within the epiphyseal cartilage of the AECC during endochondral ossification[44], and this pathogenesis can be reproduced experimentally.[27, 45, 46] Areas of chondronecrosis resist normal ossification and degenerate, resulting in tissue that is prone to clefting or collapse under the influence of external forces.
Proponents of an etiology for OC involving major trauma have suggested that the preponderance of disease in young boys compared to girls is related to greater athletic activity and tendency towards overuse injuries and trauma in males.[9, 34] In most cases of human OC there is no history of a single traumatic event; however, repetitive stress could be important in the development of lesions.[10] This latter hypothesis is supported circumstantially by the fact that most patients affected with juvenile OC have a lengthy history of participation in specific exercise regimens or sports.[40] It is also possible, however, that more active individuals are more likely to become symptomatic and are therefore more likely to have OC diagnosed. In naturally-occurring disease in animals, the role of athletic activity is not clear-cut and is thought to be a secondary factor. For example, in one large study in horses, controlled exercise affected the distribution of OC lesions within joints, but not the total number of lesions.[47] Another study found that regular, limited exercise appeared to reduce the risk of OC development in young foals.[48] Osteochondral fragments can be elicited in experimental animal models using either repetitive impacts or acute compression and rotation,[49] but these models cannot replicate the more commonly recognized early OC lesions with intact overlying articular cartilage.[50] The idea of major trauma as the sole etiologic agent is also brought into question by the occurrence of OC at anatomical locations not exposed to increased stress during physical activity, and because it cannot explain early histologic changes seen at OC predilection sites in young animals.[3, 27, 51] Thus, while trauma undoubtedly is a key precipitating factor in the onset of clinical signs of OC (i.e. by resulting in disruption of the articular surface and separation of an osteochondral fragment), it is less likely to be the initiating factor in disease development.
A variety of additional environmental factors have been proposed to play a role in the risk for development of OC in veterinary species, including nutrition, exercise, conformation and other biomechanical factors, stress response, in utero environment, and hormonal interactions.[52, 53] Of these, nutrition has been the subject of the most study. In animal models of disease, dietary factors that have been implicated in OC risk include copper deficiency,[54, 55] excess phosphorus,[56] and excess dietary energy.[57] However, the relationship between disease and nutrition is far from one of straightforward cause-and-effect. For example, in pigs, dietary supplementation of specific amino acids and microminerals reduced the severity, but not the incidence, of OC lesions when compared with a control diet.[58] Similarly, reducing digestible energy and increasing micromineral concentrations reduced the incidence of OC in foals in a prospective study of 17 breeding farms, but did not eliminate the condition.[59] It is likely that “windows of susceptibility” exist during which dietary factors may play key roles in OC manifestation,[60] but these may vary between species and anatomic location, and have yet to be clearly defined. To our knowledge, there are no reports examining a potential relationship between OC development and nutrition in humans.
Genetic risk factors are also thought to play an important role in the development of OC in both humans and animals. Since the initial description of human OC, there have been many reports of families with apparently increased incidence of disease. Many of these initial reports were small, involving a few siblings or a parent and his/her children. [61, 62] However, extended families with high incidence of OC over multiple generations have also been reported in the literature[63, 64], and these suggested an autosomal dominant pattern of inheritance with varied penetrance. Patients in these families were often affected in multiple joints, and an association with short stature was noted.[63, 64] This condition was named Dominant Familial Osteochondritis Dissecans (OMIM 165800), and is caused by a missense mutation in the aggrecan (ACAN) gene that results in an aggrecan protein with a reduced ability to interact with other proteins found in the cartilage extracellular matrix.[65] However, this familial form of disease is rare, and the ACAN mutation is unlikely to underlie other manifestations of OC. The more common, sporadic, form of OC is likely polygenic in nature; that is, the combined effects of multiple gene variants determine the underlying genetic risk of disease. To our knowledge, there are no reports in the human literature attempting to identify genes contributing to sporadic OC. However, several case reports describing identical lesions, including nearly simultaneous timing of clinical presentation, in monozygotic twins have been reported in the literature.[66–68]
Heritability estimates for OC in horses and pigs range from 0.14 to 0.52, depending on location and disease definition.[69–71] Thus, between 14% and 52% of disease risk may be attributed to genetic factors in these species. Up to 70% of foals from a single sire have been reportedly affected with OC,[69] and offspring of affected sires were more than twice as likely to develop OC than offspring of non-affected sires.[72] Similarly, boars with affected half-siblings were highly likely to have OC-affected offspring.[5] Two approaches have been taken to try to identify genetic risk factors in these species. The first is a candidate gene approach, where genes known to play a role in skeletogenesis and related processes are identified and subsequently sequenced to try to identify putative risk alleles/causative mutations.[73, 74] The second approach is genome-wide association (GWA) analysis, which evaluates statistical association between allele frequency at tens of thousands of known sites of variation throughout the genome and disease status.[75–77] GWA studies have been performed examining both the overall occurrence of OC in commercial pigs, and the manifestation of OC in specific locations, including the metacarpophalangeal joint and tibiotarsal joint, in several breeds of horses including Standardbreds, Warmbloods, Thoroughbreds, and French Trotters.[75–79] Both candidate gene and GWA approaches have limitations and, to date, although several promising candidate chromosomal regions have been identified, specific genes and alleles underlying risk have not been definitively identified. Work in this field is ongoing, however, and improvements in next-generation sequencing technology as well as the formation of cross-institutional consortia will aid efforts. Genes and pathways identified in veterinary species will not only provide insight into OC pathophysiology, but will also become compelling biological candidates for further study in humans.
EVIDENCE FOR DISTURBANCES OF ENDOCHONDRAL OSSIFICATION LEADING TO OSTEOCHONDROSIS
Evidence from animal models most strongly supports the theory that the primary pathophysiological process underlying the development of osteochondrosis is a disturbance in endochondral ossification, the process by which the epiphyseal growth cartilage of the AECC is gradually replaced by bone. It is likely that several of the etiologic factors described above contribute to this disturbance.
In both humans and animals, nutrients are normally supplied to the epiphyseal cartilage of the AECC and the physis via vessels running in channels called cartilage canals (Figure 1).[80–83] The majority of these vessels arise from the perichondrium and run parallel to the articular surface. Physiologically, as endochondral ossification progresses and the ossification front advances in the direction of the articular surface, the blood supply to most vessels in the cartilage canals must change from vessels originating from the perichondrium/periosteum to vessels originating from the medullary cavity of the secondary center of ossification. This transfer enables a consistent supply of nutrients to the ever-thinning AECC and involves the formation of anastomoses between vessels in the epiphyseal cartilage and vessels in the advancing ossification front. Studies performed in animals, however, have demonstrated that this blood supply is prone to failure.[44] This failure may be due to physical instability of the newly formed anastomoses, local effects on the vasculature by growth factors at the ossification front, or inadequate mechanical support for the developing vasculature from the surrounding tissue.[44] The impact of mechanical forces may also be especially high at the transition between two tissue types with very different mechanical characteristics.[44] The lack of anastomoses among vessels contained within the cartilage canals[84] means that failure of this transition in blood supply often results in avascular necrosis of a well-defined area of epiphyseal growth cartilage. Failure of vascularization and mineralization of the necrotic cartilage causes focal arrest of endochondral ossification, the hallmark of osteochondrosis.[51, 85] Indeed, lesions resembling OC have been successfully reproduced in pigs and horses by surgical transection of vessels contained within cartilage canals.[27, 45, 46] The retained area of necrotic epiphyseal cartilage is inferior to viable epiphyseal cartilage or subchondral bone in providing support to the overlying articular cartilage, predisposing the site to collapse and/or cleft formation that often results in clinical disease.
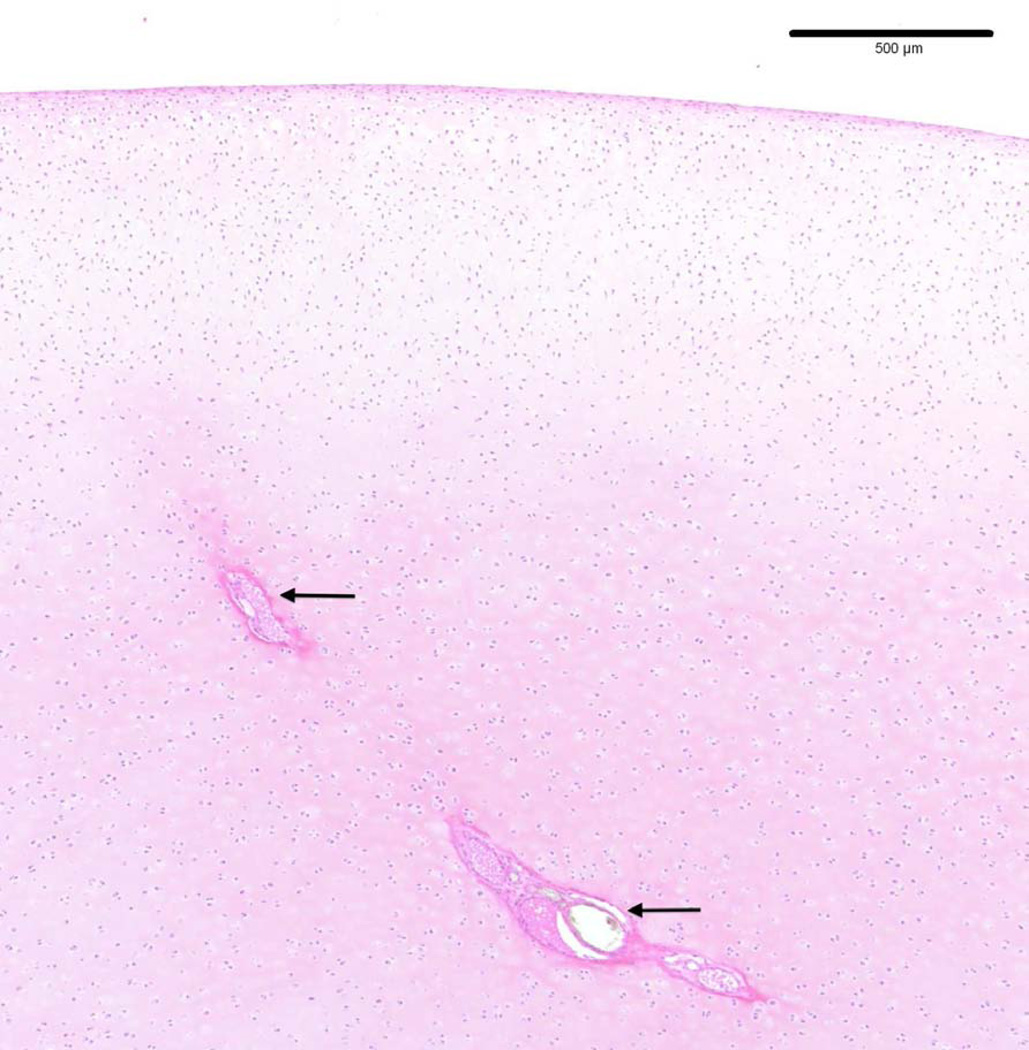
Figure 1.
Photomicrograph depicting normal cartilage canals (arrows) containing blood vessels in the articular-epiphyseal cartilage complex in the medial femoral condyle of a 14-week-old pig (hematoxylin and eosin stain).
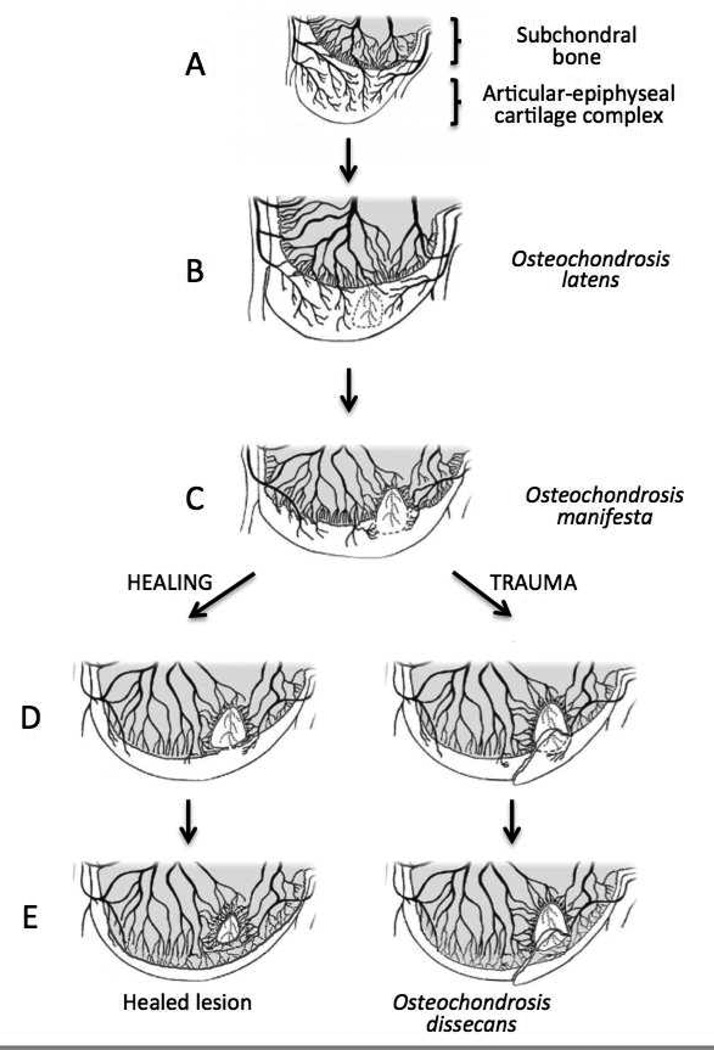
The focal area of cartilage necrosis in the epiphyseal cartilage is the first histologically apparent lesion during the pathogenesis of OC and is termed osteochondrosis latens (Figure 2) in the veterinary literature.[81] This lesion has not been described in humans, most likely due to limited access to appropriate tissues for evaluation and because it is not radiographically evident. As the ossification front reaches the necrotic epiphyseal cartilage, the necrotic cartilage resists ossification and becomes radiographically apparent as a radiolucent defect in the subchondral bone, at which point it is designated as osteochondrosis manifesta (Figure 3).[5] This lesion is observed in human medicine but is not currently recognized as a preclinical form of OC.[86, 87] There are two potential fates for this area of necrotic cartilage. In some cases (likely depending, in part, on the size and location of the lesion), it will eventually undergo ossification and resolve with minimal to no visible remnants.[5] Alternatively, if the area of necrotic epiphyseal cartilage is very large or if the overlying articular cartilage sustains excessive trauma, as may occur during athletic activities, a fissure extending from articular cartilage through the underlying necrotic epiphyseal cartilage may develop, at which point the lesion is described as osteochondrosis dissecans (Figures 4 and 5).[5] This lesion is most frequently termed osteochondritis dissecans in the human literature. In both humans and animals, this stage of the disease results in clinical signs of joint pain/dysfunction and lameness.
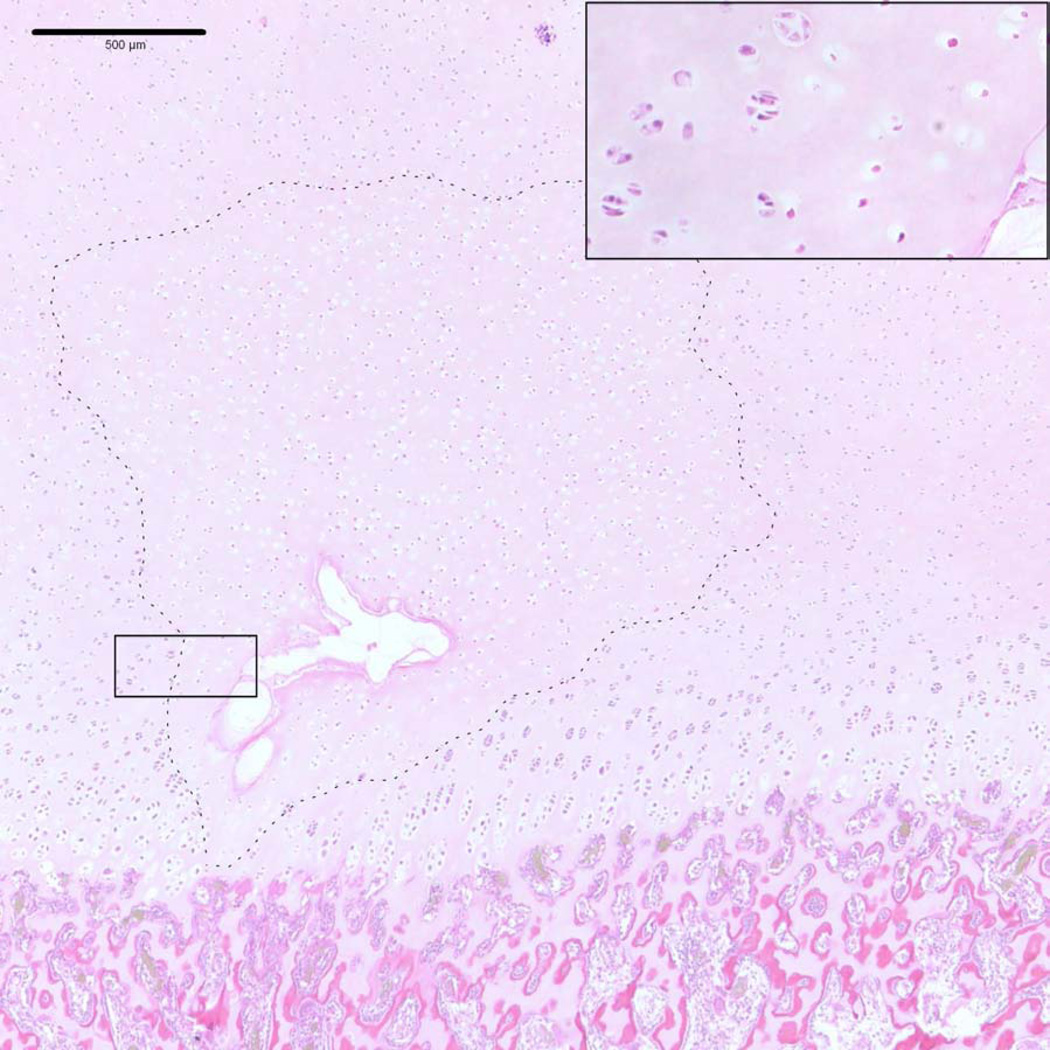
Figure 2.
Photomicrograph of an osteochondrosis latens lesion (dashed line), including necrotic blood vessels and surrounding necrotic cartilage, involving the medial femoral condyle of a 14-week-old pig. Inset: viable chondrocytes are present in the left half of the image whereas in the right half, chondrocytes are eosinopilic and contain no obvious nucleus, consistent with chondrocyte necrosis (hematoxylin and eosin stain).
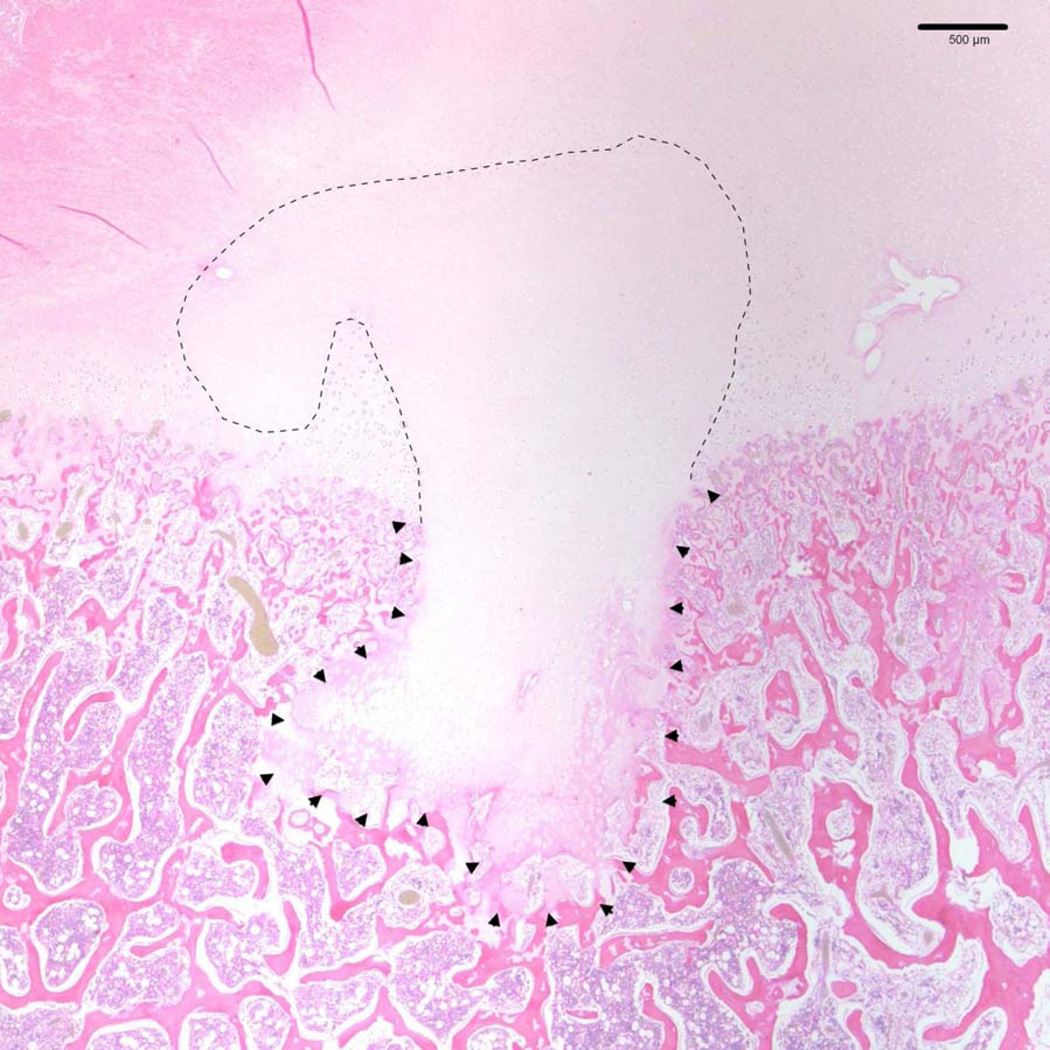
Figure 3.
Photomicrograph depicting an osteochondrosis manifesta lesion in the medial femoral condyle of a 14-week-old pig (same animal as in Figure 2). A large area of necrotic epiphyseal cartilage is present (dashed line) and has resulted in a focal failure of endochondral ossification (arrowheads) (hematoxylin and eosin stain).
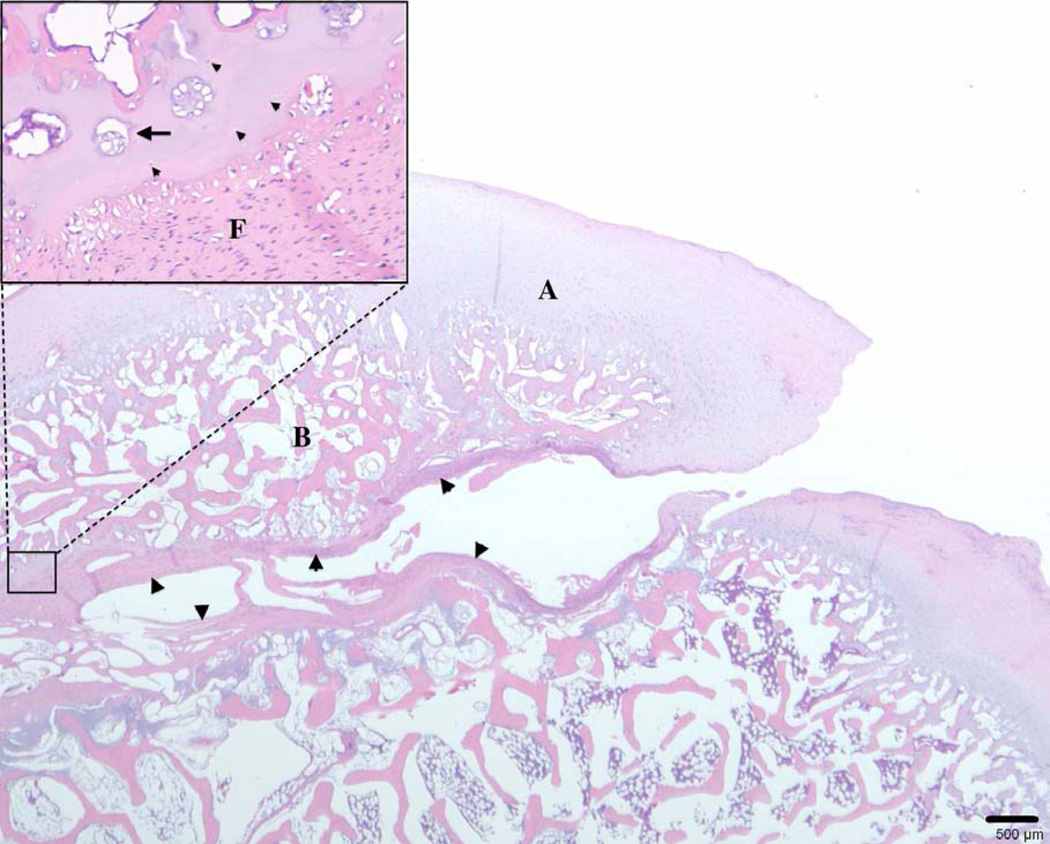
Figure 4.
Photomicrograph showing an osteochondrosis dissecans lesion involving the medial femoral condyle of a 6-month-old pig. A fissure that is partially lined by fibrous connective tissue extends through the articular cartilage to the subchondral bone, resulting in the formation of an osteochondral cleft. A: articular-epiphyseal cartilage complex; B: subchondral bone; arrowheads: fibrous tissue. Inset: Remnants of necrotic cartilage are present adjacent to the cleft and are accompanied by chondrocyte clones. Arrowheads: necrotic chondrocytes; arrow: chondrocyte clone; F: fibrous tissue (hematoxylin and eosin stain).
Figure 5.
Diagram demonstrating the pathogenesis of osteochondrosis dissecans (modified with permission from Figure 7,[2]). Panel A: normal enchondral ossification. Panel B: Development of osteochondrosis latens lesion due to failure of cartilage canal blood supply causing necrosis of the epiphyseal cartilage (circled area). Panel C: Osteochondrosis manifesta lesion appears as a delay in the progression of the ossification front. Panels D and E: healing of osteochondrosis manifesta lesion by incorporation into the subchondral bone. Panels F and G: Development of osteochondrosis dissecans lesion due to trauma causing collapse of the articular cartilage overlying areas of necrotic epiphyseal cartilage.
As noted earlier, the majority of histological studies evaluating OC/OCD in humans have focused on osteochondral fragments removed during surgery, which are easily accessible but represent the end stage of the disease and rarely include evidence of the early changes affecting the endochondral ossification process. Indeed, human studies regard fibrous/fibro-cartilaginous tissue present at areas of separation of osteochondral fragments from the parent bone as areas of delayed or nonunion.[1] However, the complete absence of a calcified cartilage layer and subchondral bone plate in osteochondral fragments removed from adolescents affected by juvenile OCD[1] indicates that juvenile OCD develops while the endochondral ossification is still ongoing, thus it is unlikely to be a primary disease of the subchondral bone.[88, 89] Instead, we believe that this fibrous/fibro-cartilaginous tissue is the remnant of necrotic epiphyseal cartilage and accompanying reactive fibrous tissue which has been present as osteochondrosis manifesta well before the development of clinical signs (Figure 4). This theory is supported by histological studies in horses and pigs using specimens from young animals undergoing active endochondral ossification and in osteochondral samples obtained from adult animals affected by clinically apparent OCD. Histological studies in foals with ongoing endochondral ossification demonstrated lesions consistent with subclinical OC (osteochondrosis latens) at predilection sites of clinically relevant OC.[4, 85] Conversely, studies examining the osteochondral fragments removed from adult horses affected with OCD revealed histological changes consistent with those noted in human studies, including fibrous tissue at the separation border.[90] The similar appearance of end-stage lesions across species suggests that the continuum of disease demonstrated in animals is also likely present in humans.
SHARED ASPECTS OF HUMAN AND ANIMAL OSTEOCHONDROSIS
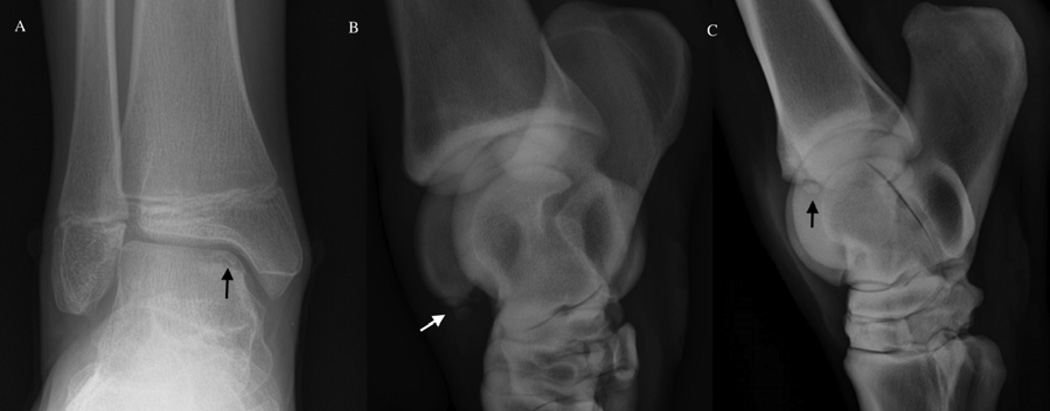
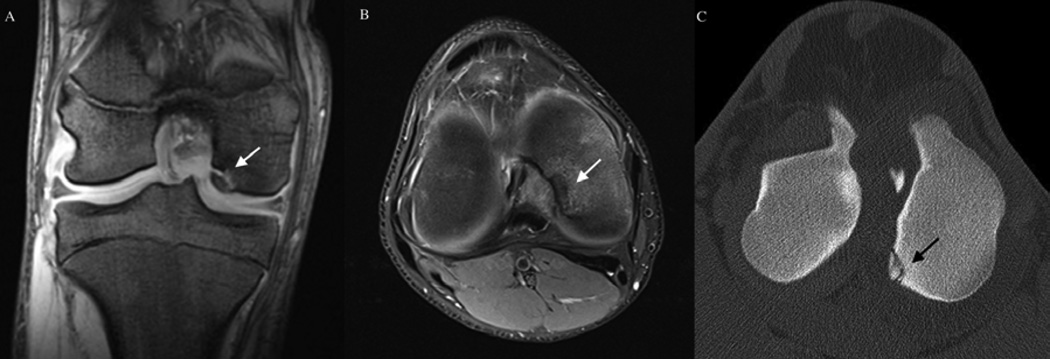
Several factors are suggestive of a shared pathogenesis of OC between humans and veterinary species. In addition to histologically identical end-stage disease as described previously, humans and animals share common predilection sites for development of disease (Table 1). In humans, OC is diagnosed in the knee, elbow, and ankle joints with decreasing frequency.[91] Within the knee, the medial femoral condyle is the most commonly affected area, whereas in the ankle, OC affects the talus[92] more commonly than the tibial plafond.[93] OC of the elbow joint usually involves the humeral capitellum.[88] Similarly, in swine, the disease is most commonly seen in the medial condyle of the femur and the medial aspect of the sagittal ridge of the humeral condyle.[2] Lesions are also found, at a lower frequency, in the shoulder, hip, and tibiotarsal (ankle) joints of swine. The most commonly affected sites in horses are the tibiotarsal, stifle (knee), and metacarpo/metatarsophalangeal joints.[14, 35] Similar to humans, the most commonly affected sites in the tibiotarsal joint in the horse are the distal intermediate ridge of the tibia and the lateral trochlear ridge of the talus (Figure 6).[94] In the stifle, the lateral trochlear ridge is the most commonly affected structure in the horse[35, 95], but involvement of the medial femoral condyle has been described as well (Figure 7).[96] OC also infrequently affects the shoulder, carpal, and hip joints of both humans and horses.[14, 97]
Figure 6.
Osteochondrosis dissecans lesion involving the ankle (tibiotarsal joint). Panel A: posterio-anterior radiographic image of an osteochondrosis dissecans lesion (black arrow) of the talus in a juvenile human subject. Panel B: dorsomedial-plantarolateral oblique radiographic image of an osteochondrosis dissecans lesion (white arrow) involving the lateral trochlear ridge in a horse. Panel C: lateromedial radiographic image of an Ooteochondrosis dissecans lesion (black arrow) involving the distal intermediate ridge of the tibia in a horse.
Figure 7.
Osteochondrosis dissecans lesion of the medial femoral condyle. Panels A (coronal plane) and B (transverse plane) depict MRI findings from a human subject with osteochondrosis dissecans of the medial femoral condyle (white arrows). Panel C: CT image of an osteochondrosis dissecans lesion (black arrow) of the medial femoral condyle of a horse obtained in the transverse plain. (Image is courtesy of Dr. Bergman, VetCT-Lingehoeve Diergeneeskunde, Netherlands)
Clinically apparent bilateral involvement in both humans and animals is widely reported, although the frequency of occurrence varies between joints. Bilateral juvenile OC of the knee is reported in 13 – 30% of human patients,[22] and 10% of human subjects are affected bilaterally with osteochondral lesions of the talus.[98] Similarly, in Thoroughbred racehorses, bilateral involvement of the stifle (knee) occurs in 17.5 – 20.5% of affected animals, while incidence of bilateral disease in the tibiotarsal joint is 6 – 10%.[35, 95] Many more human patients may have clinically silent lesions in the contralateral joint visible on MRI, although the importance of these lesions has been recently been called into question.[86] However, based on studies in animals, it is highly likely that the majority of the “ossification variants” identified in the MRI studies in patients under the age of 8[87] were, in fact, actually osteochondrosis manifesta lesions. The lack of progression of these “ossification variants” into clinically apparent OC, and their occurrence in young patients, corresponds with observations made in animals, where the high ratio of subclinical (osteochondrosis manifesta) to clinical (OCD) lesions suggest that the majority of lesions undergo complete healing (Figure 5).[5, 37] Indeed, healing of radiographically apparent juvenile OCD is reported to occur in approximately 50% of human patients.[40]
The apparent potential for healing of subclinical OC lesions has been demonstrated in swine and horses as well. In young swine, subclinical osteochondrosis manifesta lesions affecting the trochlea of the humerus and/or the distal femur were found in up to 70% of animals, whereas clinically apparent OCD lesions were noted in only 7%.[5] Similarly, in horses, resolution of subclinical but radiographically apparent changes consistent with osteochondrosis manifesta of the distal intermediate ridge of the tibia, the lateral trochlear ridge of the talus, and the lateral trochlear ridge of the distal femur may occur before 5, 5, and 8 months of age, respectively. Lesions which remain present beyond these ages, however, become permanent.[14] Experimental studies, in which chondral fractures were created in the cartilage of the femoral condyles in skeletally immature rabbits demonstrated that cartilage flaps are capable of healing if they are stable and have a wide pedicle containing abundant cartilage canals. However, unstable fragments, attached only by a narrow isthmus devoid of cartilage canals, are unlikely to heal completely and result in a fragment that closely resembles OCD.[99]
The gender distribution of OC is similar between humans and swine. In humans, females are affected less frequently than males, accounting for approximately 20–40% of all cases of OC.[22, 86] Similarly, in pigs the incidences of osteochondrosis manifesta and osteochondrosis dissecans are significantly lower in females.[5] Conversely, the incidence of OC in horses does not appear to differ between females and males.[48, 72, 100] The reason for this difference in gender predilection in the horse compared to other species is unknown.
CONCLUSION
A broad range of similarities exists between OC affecting humans and animals, including predilection sites, clinical presentation, radiographic/MRI changes, and histological appearance of end-stage (OCD) lesions, suggesting a shared pathogenesis among the various species. Histological findings from examination of sequential early naturally-occurring and experimentally-induced OC lesion in young animals strongly supports that this pathogenesis is characterized by localized avascular necrosis of the epiphyseal cartilage of the AECC leading to focal retardation and/or failure of endochondral ossification. Further investigation of early, subclinical OC in human subjects using in vivo imaging and post mortem histological evaluation of predilection sites should confirm or refute the role of vascular compromise and necrosis of epiphyseal cartilage of the AECC in the development of OC affecting humans. If indeed, this is the case, then naturally-occurring or surgically-induced cases of OC in animals will provide an excellent opportunity to develop and test diagnostic and treatment modalities for this increasingly recognized condition.
Acknowledgments
FUNDING
This research received no specific funding for scientific objectives. AMM and FT were supported by a NIH institutional training grant (T32OD10993); CSC was supported by NIH K18OD010468.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Attribute Work: Veterinary Population Medicine Department, University of Minnesota
CONTRIBUTORSHIP STATEMENT
All authors contributed significantly to the article formulation. AMM and FT drafted the primary manuscript with input from CSC. All authors were involved in critical revision of the manuscript and approved the final version to be published. Responsibility for the integrity of this work as a whole is assumed by McCoy (mccoy134umn.edu), Toth (ftothumn.edu), and Carlson (carls099umn.edu).
COMPETING INTERESTS
There are no competing interests to report.
Contributor Information
Annette M McCoy, Diplomate American College of Veterinary Surgeons; Postdoctoral Fellow, Veterinary Population Medicine Department, University of Minnesota, St. Paul, MN 55108, USA; mccoy134@umn.edu.
Ferenc Toth, Diplomate American College of Veterinary Surgeons; Postdoctoral Fellow, Veterinary Population Medicine Department, University of Minnesota, St. Paul, MN, USA; ftoth@umn.edu.
Nils I Dolvik, Department of Companion Animal Clinical Sciences, Equine Section, Norwegian School of Veterinary Science, Oslo, Norway; Nils.Dolvik@nvh.no.
Stina Ekman, Department of Biomedicine and Veterinary Public Health, Division of Pathology, Swedish University of Agricultural Sciences, Uppsala, Sweden; Stina.Ekman@slu.se.
Jutta Ellermann, Department of Radiology, The Center for Magnetic Resonance Imaging Research, University of Minnesota, Minneapolis, MN, USA; eller001@umn.edu.
Kristin Olstad, Department of Companion Animal Clinical Sciences, Equine Section, Norwegian School of Veterinary Science, Oslo, Norway; Kristin.Olstad@nvh.no.
Bjornar Ytrehus, Section for Wildlife Diseases, Division of Pathology, National Veterinary Institute, Oslo, Norway; bjornar.ytrehus@vetinst.no.
Cathy S Carlson, Diplomate American College of Veterinary Pathologists; Professor, Veterinary Population Medicine Department, University of Minnesota, St. Paul, MN, USA; carls099@umn.edu.
REFERENCES
- 1.Yonetani Y, Nakamura N, Natsuume T, Shiozaki Y, Tanaka Y, Horibe S. Histological evaluation of juvenile osteochondritis dissecans of the knee: a case series. Knee Surg Sports Traumatol Arthrosc. 2010;18:723–730. doi: 10.1007/s00167-009-0898-6. [published Online First:4 September 2009]. [DOI] [PubMed] [Google Scholar]
- 2.Ytrehus B, Carlson CS, Ekman S. Etiology and pathogenesis of osteochondrosis. Vet Pathol. 2007;44:429–448. doi: 10.1354/vp.44-4-429. [DOI] [PubMed] [Google Scholar]
- 3.Olstad K, Ytrehus B, Ekman S, Carlson CS, Dolvik NI. Early lesions of osteochondrosis in the distal tibia of foals. J Orthop Res. 2007;25:1094–1105. doi: 10.1002/jor.20375. [published Online First: 5 April 2007]. [DOI] [PubMed] [Google Scholar]
- 4.Olstad K, Ytrehus B, Ekman S, Carlson CS, Dolvik NI. Early lesions of articular osteochondrosis in the distal femur of foals. Vet Pathol. 2011;48:1165–1175. doi: 10.1177/0300985811398250. [published Online First: 14 February 2011]. [DOI] [PubMed] [Google Scholar]
- 5.Ytrehus B, Grindflek E, Teige J, Stubsjøen E, Grøndalen T, Carlson CS, et al. The effect of parentage on the prevalence, severity and location of lesions of osteochondrosis in swine. J Vet Med A Physiol Pathol Clin Med. 2004;51:188–195. doi: 10.1111/j.1439-0442.2004.00621.x. [published Online First: 16 July 2004]. [DOI] [PubMed] [Google Scholar]
- 6.Wagoner G, Cohn BNE. Osteochondritis dissecans: a résumé of the theories of etiology and the consideration of heredity as an etiologic factor. Arch Surg. 1931;23:1–25. [Google Scholar]
- 7.Edmonds EW, Polousky J. A Review of Knowledge in Osteochondritis Dissecans: 123 Years of Minimal Evolution from Konig to the ROCK Study Group. Clin Orthop Relat Res. 2013;471:1118–1126. doi: 10.1007/s11999-012-2290-y. Published Online First: 24 February 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howald H. Zur kenntnis der osteochondrosis dissecans (osteochondrotos dissecans) Archiv für orthopädische und Unfall-Chirurgie. 1942;41:730–788. [Google Scholar]
- 9.Atanda A, Jr, Shah SA, O'Brien K. Osteochondrosis: common causes of pain in growing bones. Am Fam Physician. 2011;83:285–291. [PubMed] [Google Scholar]
- 10.Schenck RC, Jr, Goodnight JM. Osteochondritis dissecans. J Bone Joint Surg Am. 1996;78:439–456. [PubMed] [Google Scholar]
- 11.Doyle SM, Monahan A. Osteochondroses: a clinical review for the pediatrician. Curr Opin Pediatr. 2010;22:41–46. doi: 10.1097/MOP.0b013e328334579e. [DOI] [PubMed] [Google Scholar]
- 12.Siffert RS. Classification of the osteochondroses. Clin Orthop Relat Res. 1981;158:10–18. [PubMed] [Google Scholar]
- 13.Grøndalen T. Osteochondrosis and arthrosis in pigs. I. Incidence in animals up to 120 kg live weight. Acta Vet Scand. 1974;15:1–25. doi: 10.1186/BF03547490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Weeren PR. Osteochondrosiseds. In: Auer JA, Stick JA, editors. Equine Surgery. 4th ed. St. Louis: Elsevier; 2012. pp. 1239–1255. [Google Scholar]
- 15.Rejno S, Stromberg B. Osteochondrosis in the horse. II. Pathology. Acta Radiol Suppl. 1978;358:153–178. [PubMed] [Google Scholar]
- 16.Pappas AM. Osteochondrosis dissecans. Clin Orthop Relat Res. 1981;158:59–69. [PubMed] [Google Scholar]
- 17.Lindholm TS, Osterman K, Vankka E. Osteochondritis dissecans of elbow, ankle and hip: a comparison survey. Clin Orthop Relat Res. 1980;148:245–253. [PubMed] [Google Scholar]
- 18.Omer GE., Jr Primary articular osteochondroses. Clin Orthop Relat Res. 1981;158:33–40. [PubMed] [Google Scholar]
- 19.Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current concepts review. Am J Sports Med. 2006;34:1181–1191. doi: 10.1177/0363546506290127. [DOI] [PubMed] [Google Scholar]
- 20.Dipaola JD, Nelson DW, Colville MR. Characterizing osteochondral lesions by magnetic resonance imaging. Arthroscopy. 1991;7:101–104. doi: 10.1016/0749-8063(91)90087-e. [DOI] [PubMed] [Google Scholar]
- 21.Kocher MS, Czarnecki JJ, Andersen JS, Micheli LJ. Internal fixation of juvenile osteochondritis dissecans lesions of the knee. Am J Sports Med. 2007;35:712–718. doi: 10.1177/0363546506296608. [published Online First: 2 March 2007] [DOI] [PubMed] [Google Scholar]
- 22.Wall E, Von Stein D. Juvenile osteochondritis dissecans. Orthop Clin North Am. 2003;34:341–353. doi: 10.1016/s0030-5898(03)00038-5. [DOI] [PubMed] [Google Scholar]
- 23.Fortier LA, Nixon AJ. New surgical treatments for osteochondritis dissecans and subchondral bone cysts. Vet Clin North Am Equine Pract. 2005;21:673–690. doi: 10.1016/j.cveq.2005.07.005. vii. [DOI] [PubMed] [Google Scholar]
- 24.Foland JW, McIlwraith CW, Trotter GW. Arthroscopic surgery for osteochondritis dissecans of the femoropatellar joint of the horse. Equine Vet J. 1992;24:419–423. doi: 10.1111/j.2042-3306.1992.tb02870.x. [DOI] [PubMed] [Google Scholar]
- 25.Jorgensen B. Longevity of breeding sows in relation to leg weakness symptoms at six months of age. Acta Vet Scand. 2000;41:105–121. doi: 10.1186/BF03549643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jorgensen B. Osteochondrosis/osteoarthrosis and claw disorders in sows, associated with leg weakness. Acta Vet Scand. 2000;41:123–138. doi: 10.1186/BF03549644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlson CS, Meuten DJ, Richardson DC. Ischemic necrosis of cartilage in spontaneous and experimental lesions of osteochondrosis. J Orthop Res. 1991;9:317–329. doi: 10.1002/jor.1100090303. [published Online First: 18 February 2005]. [DOI] [PubMed] [Google Scholar]
- 28.Dewey C. Diseases of the nervous and locomotor systems. In: Straw B, D'Allaire S, Mengeling W, et al., editors. Diseases of Swine. 8th ed. Oxford: Blackwell Science Ltd; 1999. pp. 861–883. [Google Scholar]
- 29.Devine DV, VanPelt SR, Boileau MJ. What is your diagnosis? Osteochondrosis dissecans. J Am Vet Med Assoc. 2011;238:39–40. doi: 10.2460/javma.238.1.39. [DOI] [PubMed] [Google Scholar]
- 30.Bullough PG. Atlas of Orthopedic Pathology with Clinical and Radiologic Correlations. 2nd ed. New York: Gower Medical Publishing; 1992. [Google Scholar]
- 31.Jansson N, Ducharme NG. Angular limb deformities in foals: Treatment and prognosis. Comp Cont Educ Pract. 2005;27:134-+. [Google Scholar]
- 32.Nielsen NA. Osteochondritis dissecans capituli humeri. Acta Orthop Scand. 1933;4:307–418. [Google Scholar]
- 33.Linden B. The incidence of osteochondritis dissecans in the condyles of the femur. Acta Orthop Scand. 1976;47:664–667. doi: 10.3109/17453677608988756. [DOI] [PubMed] [Google Scholar]
- 34.Duthie RB, Houghton GR. Constitutional aspects of the osteochondroses. Clin Orthop Relat Res. 1981;158:19–27. [PubMed] [Google Scholar]
- 35.Kane AJ, Park RD, McIlwraith CW, Rantanen NW, Morehead JP, Bramlage LR. Radiographic changes in Thoroughbred yearlings. Part 1: Prevalence at the time of the yearling sales. Equine Vet J. 2003;35:354–365. doi: 10.2746/042516403776014280. [DOI] [PubMed] [Google Scholar]
- 36.Kroll A, Hertsch B, Hoppner S. Entwicklung osteochondraler veränderungen in den fesselund talokruralgelenken im röntgenbild beim fohlen. Pferdeheilkunde. 2001;17:489–500. [Google Scholar]
- 37.Carlson CS, Hilley HD, Meuten DJ, Hagan JM, Moser RL. Effect of reduced growth rate on the prevalence and severity of osteochondrosis in gilts. Am J Vet Res. 1988;49:396–402. [PubMed] [Google Scholar]
- 38.Barrie HJ. Osteochondritis dissecans 1887–1987: a centennial look at König’s memorable phrase. J Bone Joint Surg Br. 1987;69:693–695. doi: 10.1302/0301-620X.69B5.3316236. [DOI] [PubMed] [Google Scholar]
- 39.Uozumi H, Sugita T, Aizawa T, Takahashi A, Ohnuma M, Itoi E. Histologic findings and possible causes of osteochondritis dissecans of the knee. Am J Sports Med. 2009;37:2003–2008. doi: 10.1177/0363546509346542. [published Online First 8 September 2009]. [DOI] [PubMed] [Google Scholar]
- 40.Cahill BR. Osteochondritis Dissecans of the Knee: Treatment of Juvenile and Adult Forms. J Am Acad Orthop Surg. 1995;3:237–247. doi: 10.5435/00124635-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Skagen PS, Horn T, Kruse HA, Staergaard B, Rapport MM, Nicolaisen T. Osteochondritis dissecans (OCD), an endoplasmic reticulum storage disease?: a morphological and molecular study of OCD fragments. Scand J Med Sci Sports. 2011;21:e17–e33. doi: 10.1111/j.1600-0838.2010.01128.x. [DOI] [PubMed] [Google Scholar]
- 42.van de Lest CH, Brama PA, van El B, DeGroot J, van Weeren PR. Extracellular matrix changes in early osteochondrotic defects in foals: a key role for collagen? Biochim Biophys Acta. 2004;1690:54–62. doi: 10.1016/j.bbadis.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Lecocq M, Girard CA, Fogarty U, Beauchamp G, Richard H, Laverty S. Cartilage matrix changes in the developing epiphysis: early events on the pathway to equine osteochondrosis? Equine Vet J. 2008;40:442–454. doi: 10.2746/042516408X297453. [DOI] [PubMed] [Google Scholar]
- 44.Ytrehus B, Ekman S, Carlson CS, Teige J, Reinholt FP. Focal changes in blood supply during normal epiphyseal growth are central in the pathogenesis of osteochondrosis in pigs. Bone. 2004;35(6):1294–1306. doi: 10.1016/j.bone.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 45.Olstad K, Hendrickson EHS, Carlson CS, Ekman S, Dolvik NI. Transection of vessels in epiphyseal cartilage canals leads to osteochondrosis and osteochondrosis dissecans in the femoro-patellar joint of foals; a potential model of juvenile osteochondritis dissecans. Osteoarthritis Cart. 2013;21:730–738. doi: 10.1016/j.joca.2013.02.005. [published Online First: 18 February 2013]. [DOI] [PubMed] [Google Scholar]
- 46.Ytrehus B, Andreas Haga H, Mellum CN, Mathisen L, Carlson CS, Ekman S, et al. Experimental ischemia of porcine growth cartilage produces lesions of osteochondrosis. J Orthop Res. 2004;22(6):1201–1209. doi: 10.1016/j.orthres.2004.03.006. [published Online First: 1 January 2006]. [DOI] [PubMed] [Google Scholar]
- 47.van Weeren PR, Barneveld A. The effect of exercise on the distribution and manifestation of osteochondrotic lesions in the Warmblood foal. Equine Vet J Suppl. 1999;31:16–25. doi: 10.1111/j.2042-3306.1999.tb05309.x. [DOI] [PubMed] [Google Scholar]
- 48.Lepeule J, Bareille N, Robert C, Ezanno P, Valette JP, Jacquet S, et al. Association of growth, feeding practices and exercise conditions with the prevalence of Developmental Orthopaedic Disease in limbs of French foals at weaning. Prev Vet Med. 2009;89:167–177. doi: 10.1016/j.prevetmed.2009.02.018. [published Online First: 28 March 2009]. [DOI] [PubMed] [Google Scholar]
- 49.Douglas G, Rang M. The role of trauma in the pathogenesis of the osteochondroses. Clin Orthop Relat Res. 1981;158:28–32. [PubMed] [Google Scholar]
- 50.Campbell CJ, Ranawat CS. Osteochondritis dissecans: the question of etiology. J Trauma. 1966;6:201–221. [PubMed] [Google Scholar]
- 51.Carlson CS, Cullins LD, Meuten DJ. Osteochondrosis of the articular-epiphyseal cartilage complex in young horses: evidence for a defect in cartilage canal blood supply. Vet Pathol. 1995;32:641–647. doi: 10.1177/030098589503200605. [DOI] [PubMed] [Google Scholar]
- 52.Hintz HF. Factors which influence developmental orthopedic disease. Proceedings of the American Association of Equine Practitioners. 1987;33:159–162. [Google Scholar]
- 53.McIlwraith CW, McIlwraith CW, editors. American Quarter Horse Association. Summary of panel findings. Proceedings Panel on Developmental Orthopedic Disease, AQHA Developmental Orthopedic Symposium; Amarillo, TX. Amarillo, TX: The American Quarter Horse Association; 1986. p. 58. [Google Scholar]
- 54.Cymbaluk NF, Smart ME. A review of possible metabolic relationships of copper to equine bone disease. Equine Vet J Suppl. 1993;16:19–26. [Google Scholar]
- 55.Hurtig M, Green SL, Dobson H, Mikuni-Takagaki Y, Choi J. Correlative study of defective cartilage and bone growth in foals fed a low copper diet. Equine Vet J Suppl. 1993;16:66–73. [Google Scholar]
- 56.Savage CJ, McCarthy RN, Jeffcott LB. Effects of dietary phosphorus and calcium on induction of dyschondroplasia in foals. Equine Vet J Suppl. 1993;16:80–83. [Google Scholar]
- 57.Savage CJ, McCarthy RN, Jeffcott LB. Effects of dietary energy and protein on induction of dyschondroplasia in foals. Equine Vet J Suppl. 1993;16:74–79. [Google Scholar]
- 58.Frantz NZ, Andrews GA, Tokach MD, Nelssen JL, Goodband RD, Derouchey JM, et al. Effect of dietary nutrients on osteochondrosis lesions and cartilage properties in pigs. Am J Vet Res. 2008;69:617–624. doi: 10.2460/ajvr.69.5.617. [DOI] [PubMed] [Google Scholar]
- 59.Gabel AA, Knight DA, Reed SM. Comparison of incidence and severity of developmental orthopedic disease on 17 farms before and after adjustment of ration. Proceedings of the American Association of Equine Practitioners. 1987;33:163–170. [Google Scholar]
- 60.van Grevenhof EM, Heuven HCM, van Weeren PR, Bijma P. The relationship between growth and osteochondrosis in specific joints in pigs. Livestock Sci. 2012;143:85–90. [Google Scholar]
- 61.Novotny H. Osteochondrosis dissecans in two brothers; the pre- and developed state. Acta Radiol. 1952;37:493–497. doi: 10.3109/00016925209138672. [DOI] [PubMed] [Google Scholar]
- 62.Pick MP. Familial osteochondritis dissecans. J Bone Joint Surg Br. 1955;37-B:142–145. doi: 10.1302/0301-620X.37B1.142. [DOI] [PubMed] [Google Scholar]
- 63.Mubarak SJ, Carroll NC. Familial osteochondritis dissecans of the knee. Clin Orthop Relat Res. 1979;140:131–136. [PubMed] [Google Scholar]
- 64.Phillips HO, Grubb SA. Familial multiple osteochondritis dissecans. Report of a kindred. J Bone Joint Surg Am. 1985;67:155–156. [PubMed] [Google Scholar]
- 65.Stattin EL, Wiklund F, Lindblom K, Onnerfjord P, Jonsson BA, Tegner Y, et al. A missense mutation in the aggrecan C-type lectin domain disrupts extracellular matrix interactions and causes dominant familial osteochondritis dissecans. Am J Hum Genet. 2010;86:126–137. doi: 10.1016/j.ajhg.2009.12.018. [published Online First: 4 February 2010]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsirikos AI, Riddle EC, Kruse R. Bilateral Kohler's disease in identical twins. Clin Orthop Relat Res. 2003;409:195–198. doi: 10.1097/01.blo.0000057993.41099.d5. [DOI] [PubMed] [Google Scholar]
- 67.Mackie T, Wilkins RM. Case report: Osteochondritis dissecans in twins: treatment with fresh osteochondral grafts. Clin Orthop Relat Res. 2010;468:893–897. doi: 10.1007/s11999-009-1017-1. [published Online First: 7 August 2009]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woods K, Harris I. Osteochondritis dissecans of the talus in identical twins. J Bone Joint Surg Br. 1995;77:331. [PubMed] [Google Scholar]
- 69.Grondahl AM, Dolvik NI. Heritability estimations of osteochondrosis in the tibiotarsal joint and of bony fragments in the palmar/plantar portion of the metacarpo- and metatarsophalangeal joints of horses. J Am Vet Med Assoc. 1993;203:101–104. [PubMed] [Google Scholar]
- 70.Reiland S, Ordell N, Lundeheim N, Olsson SE. Heredity of osteochondrosis, body constitution and leg weakness in the pig. A correlative investigation using progeny testing. Acta Radiol Suppl. 1978;358:123–137. [PubMed] [Google Scholar]
- 71.van Grevenhof EM, Schurink A, Ducro BJ, van Weeren PR, van Tartwijk JM, Bijma P, et al. Genetic variables of various manifestations of osteochondrosis and their correlations between and within joints in Dutch warmblood horses. J Anim Sci. 2009;87(6):1906–1912. doi: 10.2527/jas.2008-1199. [published Online First: 11 February 2009]. [DOI] [PubMed] [Google Scholar]
- 72.Philpsson J, Andreasson E, Sandgren B, Dalin G, Carlsten J. Osteochondrosis in the tarsocrural joint and osteochondral fragments in the fetlock joints in Standardbred trotters. II. Heritability. Equine Vet J Suppl. 1993;16:38–41. [Google Scholar]
- 73.Fan B, Onteru SK, Mote BE, Serenius T, Stalder KJ, Rothschild MF. Large-scale association study for structural soundness and leg locomotion traits in the pig. Genet Sel Evol. 2009;41:14. doi: 10.1186/1297-9686-41-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wittwer C, Hamann H, Distl O. The candidate gene XIRP2 at a quantitative gene locus on equine chromosome 18 associated with osteochondrosis in fetlock and hock joints of South German Coldblood horses. J Hered. 2009;100:481–486. doi: 10.1093/jhered/esp006. [published Online First: 20 March 2009]. [DOI] [PubMed] [Google Scholar]
- 75.Andersson-Eklund L, Uhlhorn H, Lundeheim N, Dalin G, Andersson L. Mapping quantitative trait loci for principal components of bone measurements and osteochondrosis scores in a wild boar x large white intercross. Genet Res. 2000;75:223–230. doi: 10.1017/s0016672399004371. [DOI] [PubMed] [Google Scholar]
- 76.Dierks C, Lohring K, Lampe V, Wittwer C, Drögemüller C, Distl O. Genome-wide search for markers associated with osteochondrosis in Hanoverian warmblood horses. Mamm Genome. 2007;18:739–747. doi: 10.1007/s00335-007-9058-9. [published Online First: 28 September 2007]. [DOI] [PubMed] [Google Scholar]
- 77.Lykkjen S, Dolvik NI, McCue ME, Rendahl AK, Mickelson JR, Roed KH. Genome-wide association analysis of osteochondrosis of the tibiotarsal joint in Norwegian Standardbred trotters. Anim Genet. 2010;41(Suppl: 2):111–120. doi: 10.1111/j.1365-2052.2010.02117.x. [published Online First: 10 November 2010]. [DOI] [PubMed] [Google Scholar]
- 78.Corbin LJ, Blott SC, Swinburne JE, Sibbons C, Fox-Clipsham LY, Helwegen M, et al. A genome-wide association study of osteochondritis dissecans in the Thoroughbred. Mamm Genome. 2012;23:294–303. doi: 10.1007/s00335-011-9363-1. [published Online First 4 November 2011]. [DOI] [PubMed] [Google Scholar]
- 79.Teyssèdre S, Dupuis MC, Guérin G, Schibler L, Denoix JM, Elsen JM, et al. Genome-wide association studies for osteochondrosis in French Trotter horses. J Anim Sci. 2012;90:45–53. doi: 10.2527/jas.2011-4031. [published Online First 12 August 2011]. [DOI] [PubMed] [Google Scholar]
- 80.Olstad K, Ytrehus B, Ekman S, Carlson CS, Dolvik NI. Epiphyseal cartilage canal blood supply to the distal femur of foals. Equine Vet J. 2008;40:433–439. doi: 10.2746/042516408X300269. [published Online First: 5 January 2010]. [DOI] [PubMed] [Google Scholar]
- 81.Ytrehus B, Carlson CS, Lundeheim N, Mathisen L, Reinholt FP, Teige J, et al. Vascularisation and osteochondrosis of the epiphyseal growth cartilage of the distal femur in pigs--development with age, growth rate, weight and joint shape. Bone. 2004;34:454–465. doi: 10.1016/j.bone.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 82.Barnewolt CE, Shapiro F, Jaramillo D. Normal gadolinium-enhanced MR images of the developing appendicular skeleton: Part I. Cartilaginous epiphysis and physis. AJR Am J Roentgenol. 1997;169:183–189. doi: 10.2214/ajr.169.1.9207522. [DOI] [PubMed] [Google Scholar]
- 83.Blumer MJ, Longato S, Richter E, Pérez MT, Konakci KZ, Fritsch H. The role of cartilage canals in endochondral and perichondral bone formation: are there similarities between these two processes? J Anat. 2005;206(4):359–372. doi: 10.1111/j.1469-7580.2005.00404.x. [published Online First: 30 March 2005]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Visco DM, Van Sickle DC, Hill MA, Kincaid SA. The vascular supply of the chondroepiphyses of the elbow joint in young swine. J Anat. 1989;163:215–229. [PMC free article] [PubMed] [Google Scholar]
- 85.Olstad K, Ytrehus B, Ekman S, Carlson CS, Dolvik NI. Epiphyseal cartilage canal blood supply to the tarsus of foals and relationship to osteochondrosis. Equine Vet J. 2008;40(1):30–39. doi: 10.2746/042516407X239836. [published Online First: 5 January 2010]. [DOI] [PubMed] [Google Scholar]
- 86.Jans LB, Jaremko JL, Ditchfield M, Verstraete KL. Evolution of femoral condylar ossification at MR imaging: frequency and patient age distribution. Radiology. 2011;258:880–888. doi: 10.1148/radiol.10101103. [published Online First: 21 December 2010]. [DOI] [PubMed] [Google Scholar]
- 87.Jans LB, Jaremko JL, Ditchfield M, Huysse WC, Verstraete KL. MRI differentiates femoral condylar ossification evolution from osteochondritis dissecans. A new sign. Eur Radiol. 2011;21:1170–1179. doi: 10.1007/s00330-011-2058-x. [published Online First: 26 January 2011]. [DOI] [PubMed] [Google Scholar]
- 88.Baker CL, 3rd, Romeo AA, Baker CL., Jr Osteochondritis dissecans of the capitellum. Am J Sports Med. 2010;38:1917–1928. doi: 10.1177/0363546509354969. [DOI] [PubMed] [Google Scholar]
- 89.Hixon AL, Gibbs LM. Osteochondritis dissecans: a diagnosis not to miss. Am Fam Physician. 2000;61:151–156. 158. [PubMed] [Google Scholar]
- 90.Theiss F, Hilbe M, Furst A, Klein K, von Rechenberg B. Histological evaluation of intraarticular osteochondral fragments. Pferdeheilkunde. 2010;26:541–552. [Google Scholar]
- 91.Fisher DR, De Smet AA. Radiologic analysis of osteochondritis dissecans and related osteochondral lesions. Contemp Diag Rad. 1993;16:1–5. [Google Scholar]
- 92.Thacker MM, Dabney KW, Mackenzie WG. Osteochondritis dissecans of the talar head: natural history and review of literature. J Pediatr Orthop B. 2012;21:373–376. doi: 10.1097/BPB.0b013e328346c076. [DOI] [PubMed] [Google Scholar]
- 93.Bui-Mansfield LT, Kline M, Chew FS, Rogers LF, Lenchik L. Osteochondritis dissecans of the tibial plafond: imaging characteristics and a review of the literature. AJR Am J Roentgenol. 2000;175:1305–1308. doi: 10.2214/ajr.175.5.1751305. [DOI] [PubMed] [Google Scholar]
- 94.Relave F, Meulyzer M, Alexander K, Beauchamp G, Marcoux M. Comparison of radiography and ultrasonography to detect osteochondrosis lesions in the tarsocrural joint: a prospective study. Equine Vet J. 2009;41:34–40. doi: 10.2746/042516408x343019. [DOI] [PubMed] [Google Scholar]
- 95.Oliver LJ, Baird DK, Baird AN, Moore GE. Prevalence and distribution of radiographically evident lesions on repository films in the hock and stifle joints of yearling Thoroughbred horses in New Zealand. N Z Vet J. 2008;56:202–209. doi: 10.1080/00480169.2008.36834. [published Online First: 18 February 2011]. [DOI] [PubMed] [Google Scholar]
- 96.Crijns CP, Gielen IM, van Bree HJ, Bergman EH. The use of CT and CT arthrography in diagnosing equine stifle injury in a Rheinlander gelding. Equine Vet J. 2010;42:367–371. doi: 10.1111/j.2042-3306.2010.00082.x. [published Online First: 15 April 2010]. [DOI] [PubMed] [Google Scholar]
- 97.Bojanic I, Smoljanovic T, Kubat O. Osteochondritis dissecans of the first metatarsophalangeal joint: arthroscopy and microfracture technique. J Foot Ankle Surg. 2011;50:623–625. doi: 10.1053/j.jfas.2011.04.028. [published Online First: 1 June 2011]. [DOI] [PubMed] [Google Scholar]
- 98.Hermanson E, Ferkel RD. Bilateral osteochondral lesions of the talus. Foot Ankle Int. 2009;30:723–727. doi: 10.3113/FAI.2009.0723. [DOI] [PubMed] [Google Scholar]
- 99.Bravo C, Kawamura H, Yamaguchi T, Hotokebuchi T, Sugioka Y. Experimental osteochondritis dissecans--the role of cartilage canals in chondral fractures of young rabbits. Fukuoka Igaku Zasshi. 1996;87:133–141. [PubMed] [Google Scholar]
- 100.Hoppe F. Radiological investigations of osteochondrosis dissecans in Standardbred Trotters and Swedish Warmblood horses. Equine Vet J. 1984;16:425–429. doi: 10.1111/j.2042-3306.1984.tb01964.x. [DOI] [PubMed] [Google Scholar]