Abstract
Successful expansion of functional CD4+CD25+ regulatory T cells (Treg) ex vivo under good manufacturing practice conditions has made Treg-cell therapy in clinical transplant tolerance induction a feasible possibility. In animals, Treg cells home to both transplanted tissues and local lymph nodes and are optimally suppressive if active at both sites. Therefore, they have the opportunity to suppress both naïve and memory CD4+CD25− T cells (Tresp). Clinical transplantation commonly involves depleting therapy at induction (e.g. anti-CD25), which favors homeostatic expansion of memory T cells. Animal models suggest that Treg cells are less suppressive on memory, compared with naïve Tresp that mediate allograft rejection. As a result, in the context of human Treg-cell therapy, it is important to define the effectiveness of Treg cells in regulating naïve and memory Tresp. Therefore, we compared suppression of peripheral blood naïve and memory Tresp by fresh and ex vivo expanded Treg cells using proliferation, cytokine production and activation marker expression (CD154) as readouts. With all readouts, naïve human Tresp were more suppressible by approximately 30% than their memory counterparts. This suggests that Treg cells may be more efficacious if administered before or at the time of transplantation and that depleting therapy should be avoided in clinical trials of Treg cells.
Keywords: Cell therapy, human, memory T cells, naïve T cells, regulatory T cells, suppression
Introduction
Transplantation is the treatment of choice for most end-stage solid organ diseases, with tangible benefits to survival and quality of life. Immunological recognition of polymorphisms in histocompatibility proteins leads to graft rejection between HLA-mismatched individuals. This necessitates the use of broad-spectrum pharmacological immunosuppression (IS) that renders recipients susceptible to accelerated cardiovascular disease, metabolic complications, life-threatening infections and malignancy (1). In the long-term, immunosuppressive drugs are both directly toxic to transplanted tissues and inefficient at preventing chronic allograft rejection. The establishment of clinical tolerance to engrafted tissues to minimize or eliminate long-term IS is therefore, a key research objective.
Natural regulatory T cells (Treg), expressing high levels of the IL-2 receptor chain α, CD25 are considered by many as ideal candidates for cell therapy for the induction of transplantation tolerance. Although their physiological function is the maintenance of tolerance to self-components, preventing autoimmune disease, Treg cells can regulate CD4+CD25− T cells (Tresp) responses to both cognate antigen and polyclonal stimuli in vitro and mediate allograft tolerance in experimental animals (2). In humans, numbers of graft-infiltrating Treg cells during rejection correlate positively with lower inflammatory responses and better renal outcomes (3). The ability to expand autologous Treg cells ex vivo under good manufacturing practice conditions (4) has made Treg-cell therapy in protocols of tolerance induction a feasible possibility in the near future.
T-cell immunity, in particular, CD4+ T cells plays a central role in allograft rejection. Priming of naïve alloreactive T cells occurs in local lymph nodes (LN) before migration of effector cells to the transplanted tissue (5). In experimental animals, fate-tracking of injected antigen-specific Treg cells reveals that they home to both transplanted tissues and the local draining LN (6) and that they need to act within both the LN and the tissue to effectively induce tolerance (7). In this way, injected Treg cells have the opportunity to suppress both naïve and memory Tresp. Clinical transplantation commonly involves the use of depleting induction agents (e.g. anti-CD25 or Campath; Ref. 1), which favor expansion of memory and prolonged depletion of naïve T cells (8). Animal models suggest that Treg cells have a lower capacity to suppress memory, compared to naïve Tresp that mediate allograft rejection (9). As a result, it is important to define in the context of human Treg-cell therapy how effective Treg cells will be in regulating naïve and memory Tresp. This information will inform the design of clinical trials of Treg-cell therapy, as it will determine whether Treg cells should ideally be administered before or after transplantation and with or without depleting agents. Therefore, we sought to compare the suppressive capacity of human Treg cells, both freshly isolated and expanded ex vivo for cell therapy, on peripheral blood naïve (defined as CD45RA expressing) and memory (defined as CD45RO expressing) Tresp.
Materials and Methods
Cell separation and fluorescence-activated cell sorting
CD25+ and CD25− CD4+ T cells were separated from human buffy coats as previously described (10). Naïve and memory Tresp were negatively selected using anti-CD45RO (Caltag, Burlingame, CA, USA) and anti-CD45RA, respectively (Diaclone Research, Besaucon, France) and anti-mouse magnetic beads (Dynal Biotech, Oslo, Norway). CD4+ T cells stained for CD4,CD25 and CD127 using a Human Regulatory T Cell Sorting Kit™ (BD-Biosciences, San Jose, CA, USA) were FACS sorted to CD4+CD25− and CD4+CD25hiCD127lo cells, respectively, using a FACSAria (BD). Cell sorting efficiency for the populations of interest was routinely over 95%.
Expansion of Treg-cell lines
Human CD4+CD25+ T cells isolated from peripheral blood mononuclear cell (PBMC) of healthy subjects were plated at 1 × 106/mL in X-vivo 15 (Lonza, Basel, Switzerland) supplemented with 5% human AB serum (HS) (Biosera, Ringer, East Sussex, UK) containing 100 nM Rapamycin (LC Laboratories, Woburn, MA, USA). Cells were activated with anti-CD3 and anti-CD28 coated beads (Dynal) at a bead:cell ratio of 1:1. IL-2 (1000 IU/mL; Proleukin®, Novartis, Surrey, UK) was added at day 2 postactivation and replenished every 2 days. Beads were removed by magnetic adherence every 10 days postactivation and fresh anti-CD3 anti-CD28 beads (1:1 ratio), Rapamycin (100 nM) and IL-2 (1000 IU/mL) were added. Expanded cells were used for further analysis 30 days postactivation.
Cell culture and proliferation assays
Syngeneic Tresp (naïve and memory) and Treg cells were incubated alone and in coculture in 96 well U-bottomed plates (Techno Plastic Products, Zurich, Switzerland) in 250 μL of complete medium (RPMI 1640; Invitrogen, Paisley, UK) with 50 IU/mL penicillin, 50 μg/mL streptomycin and 2 mM L-glutamine (PSG; PAA Laboratories GmbH, Pasching, Austria) with 10% HS. Cells were activated with either anti-CD3/CD28 beads at bead:cell ratios from 0 to 0.8 chosen from previous experience, or by wells coated with anti-CD3 (2 μg/mL) and anti-CD28 (0–2 μg/mL; both R&D, Leeds, UK) monoclonal antibodies. All conditions were set up in triplicate for 3, 5 and 7 days before supernatant recovering and addition of 1 μCi/well 3H-thymidine (GE Healthcare, Bucks, UK). Cells were recovered 20 h later and incorporated thymidine measured using a 1205 Betaplate liquid scintillation counter (LKB Wallac, Turku, Finland). Alternatively, assessment of proliferation by flow cytometry on days 3 and 5 was done by carboxyfluoroscein diacetate succinimidyl ester (CFSE) dilution of Tresp stained with 1 μM (CFSE; Invitrogen). 7-Amino-actinomycin D (7-AAD; BD) was added for 30 min at room temperature before flow cytometry and CFSE dilution of gated 7AAD− cells was assessed. Cells were acquired in a total volume of 250 μL PBS containing 20 μL of Perfect-Count® microspheres (Cytognos, Salamanca, Spain) on a FACScalibur running CellQuest software (BD). Acquisition was limited by gating on Perfect-Count® microspheres, collecting 7500 events for each sample. The number of acquired cells in each 7-AAD− CFSE peak was multiplied by the ratio of acquired to added counting beads prior to analysis. The numbers of nonproliferating (cells in the first peak) and proliferating precursors were calculated using standard formulae (11) (Figure S1) and the former expressed as the percentage of nonproliferating precursors from the total number of cells added. Suppression was expressed as the difference in frequency of nondividing precursors. Cell death was estimated by the sum of the proliferating and nonproliferating CFSE+ precursors subtracted from the number of cells originally added to the culture, expressed as a percentage of the latter.
Suppression of activation markers
FastImmune Regulatory T-cell Function kit™ (BD) was used to estimate FACS sorted Treg suppression of the activation markerCD154 on FACS sorted Tresp at 7 h, according to the manufacturer’s instructions. Tresp were additionally stained with CD45RA-FITC and CD45RO-APC-H7 (both BD) before data acquisition on an LSRII (BD) running FACSDiva software. Percentage suppression of CD154 on gated memory (CD4+CD25−CD45RO+CD45RA−) and naïve (CD4+CD25−CD45RO−CD45RA+) Tresp was calculated according to the protocol from the manufacturer.
Cytokine estimation
IFN-γ, IL-2 and IL-10 in supernatants of cell cultures were estimated using a multiple cytokine assay kit (Meso Scale Discovery, Gaithersburg, MD, USA) according to the manufacturer’s instructions. Data was acquired on a SECTOR™ Imager 2400 (MSD).
Data analysis and statistics
Flow cytometric data was analyzed with FlowJo (Treestar Inc., Ashland, OR, USA). Statistical analysis was carried out on GraphPad Prism (Graph-Pad software Inc., San Diego, CA, USA) and Microsoft Excel 2008 (Microsoft Corporation, Redmond, WA, USA). Parametric and nonparametric data were calculated as the mean ± SD and median (interquartile range, IQR), respectively. For comparison of parametric data, paired and unpaired t-tests were used (for paired and unpaired data sets). Comparison of central tendency for nonparametric data sets was made by using the Wilcoxon signed rank test for paired data, respectively. For comparisons of trends in proliferation between naïve and memory T cells, a repeated measures ANOVA was used for each condition (volume of expander beads) over the three time points. Regression lines of percentage suppression with increasing stimulation for naive and memory cells were compared using an analysis of covariance (ANCOVA) at an interaction depth of 1. In all cases, p value of less than 0.05 was taken as denoting statistical significance.
Results
CD4+CD25+Treg cells exert a greater suppressive effect on naïve than memory Tresp
Naive (CD45RA+) and memory (CD45RO+) Tresp were activated in parallel with increasing anti-CD3 and anti-CD28 stimulation and dose–response proliferation was measured on days 3, 5 and 7. Proliferation of naïve Tresp was greater than that of memory Tresp at higher anti-CD3/CD28 stimulation (Figure S2), due to greater sensitivity to costimulation in the former and greater cell death in the latter (Figure S3). However, the kinetics of proliferation across the three time-points was the same in naïve and memory Tresp [comparing the two populations by repeated measures ANOVA at each level of stimulus (anti-CD3/CD28 bead:Tresp ratio; p > 0.05, not shown)]. Therefore, like time-point were directly compared for Treg-mediated suppression.
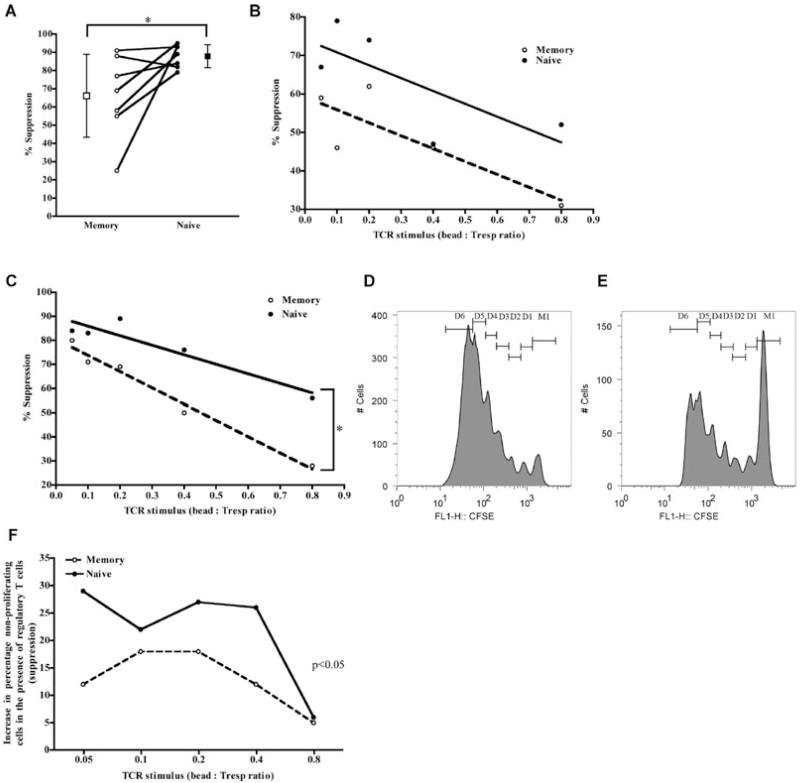
Naïve and memory Tresp were cocultured in the presence and absence of 1:1 ratio of syngeneic Treg cells and activated with increasing anti-CD3 and anti-CD28 stimulation (bead:Tresp ratio 0–0.8). Proliferation and suppression of proliferation was initially measured by 3H-Thymidine incorporation on days 3, 5 and 7. As expected, little suppression was evident on day 3 (2.4 ± 11.9% vs. 16.8 ± 12.7% for memory and naïve Tresp, respectively p NS, data not shown) but significant suppression was seen on days 5 (Figures 1A and B) and 7 (Figure 1C). Regression lines of suppression against stimulus on days 5 (Figure 1B) and 7 (Figure 1C) showed divergence of the regression lines for naïve and memory Tresp, which reached statistical significance on day 7 where on average the proliferation of naïve Tresp was 20–30% more suppressed than memory cells (Figure 1C). To confirm, CFSE-labeled naïve and memory Tresp were activated in the presence and absence of 1:1 syngeneic Treg cells with increasing anti-CD3 and CD28 stimulus and proliferation estimated on days 3 and 5 as previously (Figures 1D-F). As before, suppression was consistently higher in naïve than memory Tresp (Figures 1D-F), being apparent at both days 3 and 5 (although more pronounced at the latter). This difference was not accounted for by an increase in Tresp death in the presence of Treg cells (median increase in cell death −3% and −1% for naïve and memory Tresp, respectively, p NS).
Figure 1. Tregcells suppress naïve more than memory Tresp.
(A–C) Suppression of 3H-Thymidine incorporation in naïve and memory Tresp by Treg cells at 1:1 ratio. (A), plot showing pooled data from seven independent experiments taken from day 7 with a bead:Tresp ratio of 0.2. Mean percentage suppression against increasing stimulus for days 5 (B) and seven (C). Solid lines show regression for naïve while dashed lines indicate those for memory Tresp; *p < 0.05. (D and E) Suppression of CFSE dilution in Tresp by Treg cells at 1:1 ratio. (D and –E) Representative CFSE profiles of Tresp in the presence (E) and absence (D) of 1:1 Treg cells. (F) Suppression, expressed as the increase in the number of Tresp not dividing. (D and E) are representative examples, from day 5 of culture, from two independent experiments. Due to differences in absolute numbers between the two experiments, representative, rather than pooled data, have been shown.
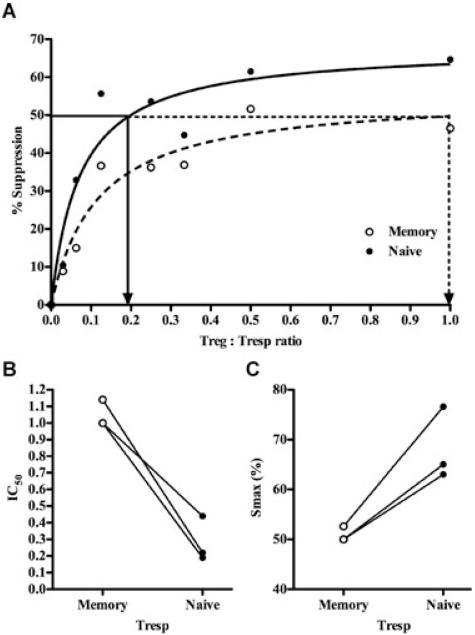
To quantify differences in susceptibility to suppression between naïve and memory Tresp, the two populations were cultured alone and in coculture with Treg cells in 1:1, 1:2, 1:3, 1:4, 1:8, 1:16 and 1:32 ratio (Figure S4) at constant Tresp numbers. Suppression of 3H-Thymidine incorporation on day 5 was calculated as per standard methods (Figure S4). For comparison of suppression, we used the IC50 (Treg cells:Tresp ratio needed to cause 50% suppression) and maximum suppression obtained at any ratio (Smax) were calculated for each population as per the methods described in (Figure 2A; Ref. 12). A clear difference was seen between the suppression of naïve and memory Tresp, with the memory population having a higher IC50 (mean Treg: Tresp ratio of 1.05 ± 0.08 vs. 0.28 ± 0.14; Figure 2B) and lower Smax (mean 50.9 ± 1.5% vs. 68.2 ± 7.3%; Figure 2C) than naïve cells.
Figure 2. Suppression of naïve and memory Tresp by Treg cells in dose–response.
(A) Representative example from three independent experiments of suppression of 3H-Thymidine incorporation by naïve and memory Tresp at different Treg cell:Tresp ratios (but constant bead:Tresp ratio of 0.2) for 5 days. Solid and dashed arrows show the IC50 for naïve and memory Tresp respectively. (B and C) cumulative IC50 (B) and Smax (C) for the three experiments. Lines join paired data.
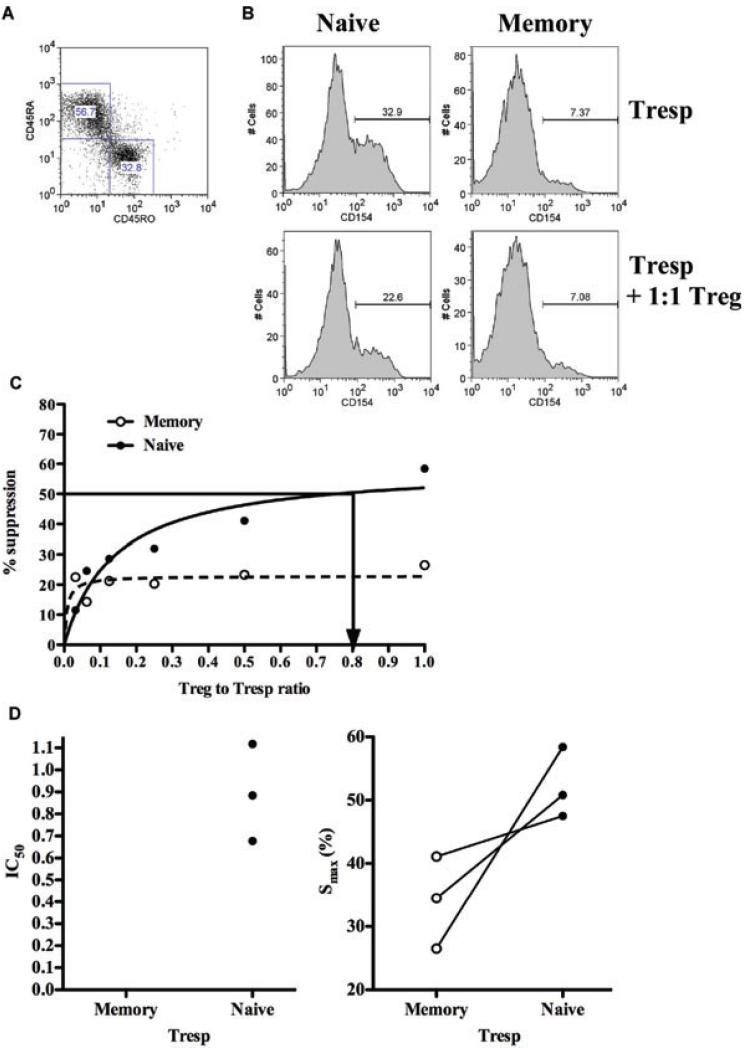
Both the 3H-Thymidine incorporation and CFSE dilution methods assess suppression at “late” time points and are, therefore susceptible to confounding variables such as Treg-cell death and exhaustion of culture medium. To remove these confounders, we measured suppression through a nonproliferation dependent assay, upregulation of the activation markers CD154 at 7 h. FACS-sorted Tresp were activated in the presence or absence of cell-sorted syngeneic Treg cells at Tresp: Treg cell ratio from 1:1 to 32:1 and suppression of CD154 on naïve and memory Tresp was analyzed by flow cytometry (Figures 3A and B). Similarly, in the absence of Treg cells expression of CD154 on naïve Tresp was greater than on their memory counterparts (both had negligible expression at baseline; Figure 3B). Treg cells suppressed expression of this marker on naïve, compared to memory Tresp to a greater extent (Figure 3B). In dose–response, the Smax was again consistently higher for naïve compared to memory Tresp (mean 52.2 ± 5.6 vs. 34.0 ± 7.3); the IC50 for naïve Tresp was 0.89 ± 0.2 whereas that for memory Tresp could not be calculated as 50% suppression was not achieved in any experiment (Figures 3C and D).
Figure 3. T/reg–cell-mediated suppression of early activation markers on naïve Tresp is greater than on memory Tresp.
(A) CD45RA and CD45RO gating strategy to identify naïve and memory Tresp respectively in cocultures of FACS-sorted Tresp and Treg cells. (B), CD154 expression on naïve and memory Tresp after 7 h of stimulation (bead:Tresp ratio of 0.2) in the presence and absence of Treg cells. (C), percentage suppression of CD154 expression on Tresp by Treg cells at increasing Treg cell:Tresp ratios. (A–C) are representative examples from three independent experiments. (D), cumulative IC50 (left panel) and Smax from the three experiments.
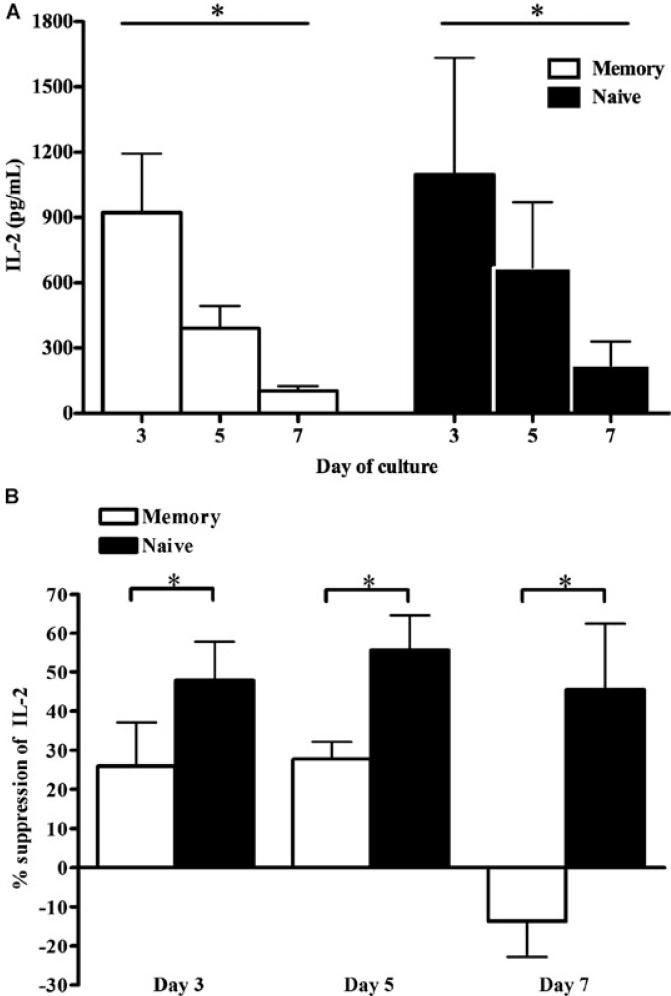
In addition to proliferation, effector function is an important consequence of T-cell activation. Therefore, the Treg-cell suppression of cytokine production by naïve and memory Tresp activated with anti-CD3/CD28 beads for 3, 5 and 7 days in the presence or absence of 1:1 ratio of Treg cells was also assessed. In the absence of Treg cells, IL-2 concentrations in supernatants of naïve and memory Tresp were comparable at each time point, with peaks on day 3 (Figure 4A). IL-2 production from both populations was suppressed by Treg cells. That from naïve Tresp was consistently more suppressed than that seen in supernatants from memory Tresp. At each time point, naïve Tresp IL-2 production was at least 25% more suppressed by Treg cells (Figure 4B). IFN-γ and IL-10 were almost exclusively produced from memory Tresp (data not shown), making direct comparison of suppression invalid. As expected, Treg cells suppressed IFN-γ but not IL-10 production from memory Tresp (data not shown).
Figure 4. IL-2 production from naïve Tresp is more regulated by Treg cells than those from memory Tresp.
(A) IL-2 concentrations in supernatants of naïve and memory Tresp polyclonally stimulated with anti-CD3/CD28 beads (bead:Tresp ratio of 0.2) in vitro at days 3, 5 and 7. (B) Percentage suppression of IL-2 production from naïve and memory Tresp by Treg cells at 1:1 ratio on days 3, 5 and 7. All graphs show pooled (mean ± s.d.) results from three independent experiments; *p < 0.05.
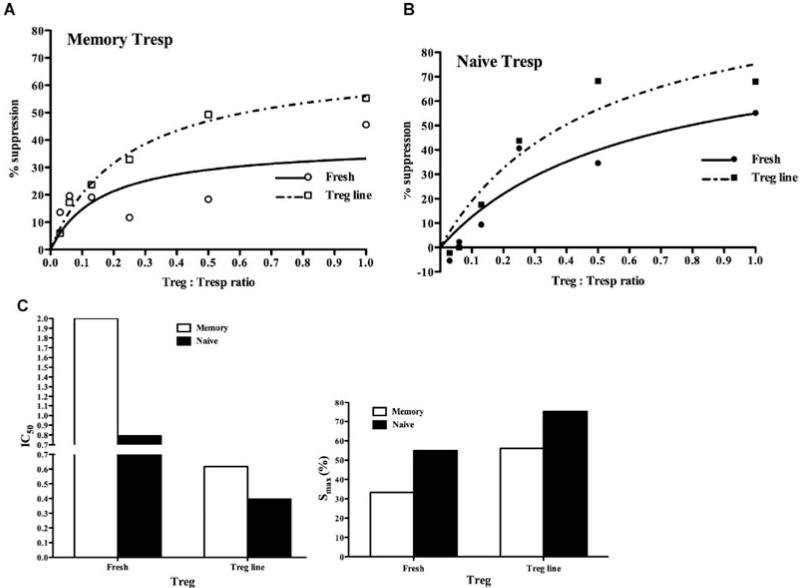
Tregcell lines expanded for cell therapy have greater suppressive effect on naïve, compared to memory Tresp
In the above series of experiments, Treg cells were freshly isolated from PBMC. However, for clinical cell therapy Treg cells must first be expanded in vitro and then infused into patients as part of a tolerance induction protocol. Large-scale expansion of Treg cells that retain suppressive ability and stably express FOXP3 in vitro is possible through expansion in the presence of the mTOR inhibitor Rapamycin (13). To test whether expanded Treg cells retain the same suppressive profile on naïve and memory Tresp as freshly isolated Treg cells, the latter were expanded in vitro in the presence of Rapamycin. These cells retained high expression of CD25 and FOXP3 and were more potently suppressive than freshly isolated Treg cells (data not shown). CFSE-labeled naïve and memory Tresp were activated in vitro alone and in the presence of dose–response titration of either freshly isolated or expanded Treg-cell lines. Suppression was calculated on day 5 and the IC50 and Smax determined as before (Figures 5A and B). Both the freshly isolated and expanded Treg-cell line showed a reduced ability to suppress memory when compared to naïve Tresp, although both were suppressible (IC50 2.0 vs. 0.79 for fresh and 0.61 vs. 0.39 for Treg-cell line; Smax 33.2 vs. 55.0% for fresh and 56 vs. 75% for Treg-cell line; Figure 5C). Further-more, Treg cells expanded in the presence of Rapamycin demonstrated an increased suppression of both naïve and memory Tresp compared to freshly isolated Treg cells.
Figure 5. Rapamycin-expanded Treg-cell lines suppress naïve Tresp more than memory Tresp.
(A) Percentage suppression of CFSE dilution in memory (A) and naïve (B) Tresp by fresh Treg cells and a Treg-cell line generated in vitro in the presence of Rapamycin. (C) IC50 (left panel) and Smax (right panel).
Discussion
Treg cells are promising agents for cell therapy in the clinical induction of tolerance to transplanted tissues. Although Treg cells certainly inhibit naïve T cell responses in vitro and in vivo and can prevent allograft rejection in naïve mice (2) studies of skin transplantation in animal models suggest that they have a lower capacity to suppress memory than naive CD4+ T cells mediating graft rejection (14). Here we have shown that human Treg cells also have greater capacity to suppress naïve, compared to memory Tresp proliferation. This pattern was true of both freshly isolated and in vitro expanded, therapeutically relevant Treg cells, although Treg cells expanded in the presence of Rapamycin were more suppressive on both populations than freshly isolated Treg cells.
To remove confounders relating to length of cultures or purity of bead-selected Treg cells, we confirmed these findings by cell sorting and measuring surface expression of the early activation marker CD154 that is upregulated on naïve and memory Tresp 7 h after activation. In this setting, Treg cells also exerted greater suppression of naïve than memory Tresp. To our knowledge, although there is a previous report on this subject, the definition of memory and naïve T cells in the publication were based on in vitro activation of CD4+CD25− with alloantigen and no comparisons of the two were made (15).
Naïve and memory Tresp produce effector cytokines upon activation and reactivation, respectively (Th1, Th2 and Th17) whose activity determines outcomes. In these series of experiments, cytokine concentrations were used as surrogate markers for effector function. The only cytokine directly comparable between naïve and memory Tresp IL-2 was also more suppressed in the naïve population than the memory one by Treg cells indicating that Treg cells may have greater ability to suppress naïve Tresp cytokine production.
Although naïve andy memor Tresp were enriched by negative selection, it is unlikely that cross-contamination could explain the differences observed between the populations. This is because the purities were generally greater than 90% for each population (data not shown) and the results for the two populations were clearly different. In addition, the FastImmune assay was carried out on FACS sorted cells.
Memory CD4+ T cells are clearly important in allograft rejection (16), having the ability to circulate through both secondary and nonlymphoid tissues, mount immune responses within the latter and survive independently of peripheral low-grade stimulation by MHC-self peptide complexes (17). Naïve T cells (defined as CD45RA expressing) may also be directly involved in allograft rejection. The pre-transplant frequency of alloreactivity between naïve and memory T cells are equal (18,19) and CD45RA+ T cells can not only be identified, but sometimes dominate in biopsy materials from acutely rejecting human organs (20). Because injected tissue-specific Treg cells home to both transplanted tissue and local draining LN (6) and are optimally suppressive when functioning within both LN and tissue (7) they have the opportunity to regulate both naïve and memory Tresp. Greater suppressive ability of Treg cells on naïve compared to memory Tresp has potential implications for clinical cell therapy given that induction therapy for human transplantation routinely involves protocols of depleting antibodies, resulting in expansion of memory T cells during reconstitution (8). The efficacy of expanded Treg cells following transplantation may, therefore be less than anticipated. These findings suggest that one possibility to maximize the efficiency of Treg-cell therapy is to consider Treg-cell infusion either before or concurrently with transplantation without the use of depleting therapy for induction. Arguably, regulating the priming of naïve Tresp may also have a greater benefit on long-term allograft survival than regulating memory Tresp at a later time point. In addition, the difference in susceptibility to suppression may indicate a therapeutic window for adoptive Treg-cell therapy in which naïve alloreactive responses could be inhibited but pathogen-specific memory T cell responses might be preserved.
Supplementary Material
Acknowledgments
The authors acknowledge financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust. The authors acknowledge the support of the MRC Centre for Transplantation.
Funding source: This work was funded by grants from the Medical Research Council (to BA, PM, SJ, MH-F and RIL), National Institute for Health Research (JC), the British Heart Foundation and Guy’s and St Thomas’ Charity Trust (MH-F, RIL and GL).
Glossary
Abbreviations
- 7AAD
7-amino-actinomycin D
- ANOVA
Analysis of Variance
- ANCOVA
Analysis of Covariance
- CFSE
Carboxyfluoroscein diacetate succinimidyl ester
- FOXP3
Forkhead box P3
- HLA
Human Leukocyte Antigen
- HS
Human Serum
- IC50
Half maximal Inhibitory Concentration
- IS
Immunosuppression
- LN
Lymph node
- mTOR
Mammalian Target of Rapamycin
- PBMC
Peripheral Blood Mononuclear Cells
- Th
helper
- Treg
Regulatory T cell
- Tresp
Responder T cells
Footnotes
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 2.Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ trasplantation and autoimmune disease. Clin Exp Immunol. 2007;148:32–46. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taflin C, Nochy D, Hill G, et al. Regulatory T cells in kidney allograft infiltrates correlate with initial inflammation and graft function. Transplantation. 2010;89:194–199. doi: 10.1097/TP.0b013e3181c3ca11. [DOI] [PubMed] [Google Scholar]
- 4.Sagoo P, Lombardi G, Lechler RI. Regulatory T cells as therapeutic cells. Curr Opin Organ Transplant. 2008;13:645–653. doi: 10.1097/MOT.0b013e328317a476. [DOI] [PubMed] [Google Scholar]
- 5.Afzali B, Lombardi G, Lechler RI. Pathways of major histocompatibility complex allorecognition. Curr Opin Organ Transplant. 2008;13:438–444. doi: 10.1097/MOT.0b013e328309ee31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golshayan D, Jiang S, Tsang J, Garin MI, Mottet C, Lechler RI. In vitro-expanded donor alloantigen-specific CD4+CD25+ reguatory T cells promote experimental transplantation tolerance. Blood. 2007;109:827–835. doi: 10.1182/blood-2006-05-025460. [DOI] [PubMed] [Google Scholar]
- 7.Zhang N, Schroppel B, Lal G, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearl JP, Parris J, Hale DA, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 9.Yang J, Brook MO, Carvalho-Gaspar M, et al. Allograft rejection mediated by memory T cells is resistant to regulation. Proc Natl Acad Sci USA. 2007;104:19954–19959. doi: 10.1073/pnas.0704397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yates J, Rovis F, Mitchell P, et al. The maintenance of human CD4+ CD25+ regulatory T cell function: IL-2, IL-4, IL-7 and IL-15 preserve optimal suppressive potency in vitro. Int Immunol. 2007;19:785–799. doi: 10.1093/intimm/dxm047. [DOI] [PubMed] [Google Scholar]
- 11.Wells AD, Gudmundsdottir H, Turka LA. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J Clin Invest. 1997;100:3173–3183. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monk CR, Spachidou M, Rovis F, et al. MRL/Mp CD4+,CD25−T cells show reduced sensitivity to suppression by CD4+,CD25 + regulatory T cells in vitro: A novel defect of T cell regulation in systemic lupus erythematosus. Arthritis Rheum. 2005;52:1180–1184. doi: 10.1002/art.20976. [DOI] [PubMed] [Google Scholar]
- 13.Strauss L, Czystowska M, Szajnik M, Mandapathil M, Whiteside TL. Differential responses of human regulatory T cells (Treg) and effector T cells to rapamycin. PLoS One. 2009;4:e5994. doi: 10.1371/journal.pone.0005994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang XO, Panopoulos AD, Nurieva R, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 15.Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–1302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Heeger PS, Valujskikh A. In vivo helper functions of alloreactive memory CD4+ T cells remain intact despite donor-specific transfusion and anti-CD40 ligand therapy. J Immunol. 2004;172:5456–5466. doi: 10.4049/jimmunol.172.9.5456. [DOI] [PubMed] [Google Scholar]
- 17.Swain SL, Hu H, Huston G. Class II-independent generation of CD4 memory T cells from effectors. Science. 1999;286:1381–1383. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 18.Macedo C, Orkis EA, Popescu I, et al. Contribution of naive and memory T-cell populations to the human alloimmune response. Am J Transplant. 2009;9:2057–2066. doi: 10.1111/j.1600-6143.2009.02742.x. [DOI] [PubMed] [Google Scholar]
- 19.Lombardi G, Sidhu S, Daly M, Batchelor JR, Makgoba W, Lechler RI. Are primary alloresponses truly primary? Int Immunol. 1990;2:9–13. doi: 10.1093/intimm/2.1.9. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim S, Dawson DV, Van Trigt P, Sanfilippo F. Differential infiltration by CD45RO and CD45RA subsets of T cells associated with human heart allograft rejection. Am J Pathol. 1993;142:1794–1803. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.