INTRODUCTION
Second order neurons in the nucleus tractus solitarius (NTS) play a pivotal role in control of blood pressure (BP) by receiving afferent baroreceptor input and modulating, integrating and transforming this input to determine magnitude, pattern, and duration of the signal that is then transmitted to distal central sites (Andresen et al., 2004; Andresen et al., 1994; Chen et al., 1999). Baroreceptor afferent input has been shown to innervate neurons within subnuclei of the NTS, including the medial, dorsomedial, dorsolateral, and commissural subnuclei (Miura et al., 1994; Saha, 2005). Inhibitory gamma-amino butyric acid (GABA) inputs from interneurons and descending inputs are integrated within these subnuclei with excitatory glutamate inputs from baroreceptor afferent fibers to contribute to the net output of baroreceptive neurons in the NTS (Chen et al., 2005; Fong et al., 2005; Sved, 1994). Previously, we found that activation of neural cannabinoid (CB1) receptors in the NTS by either exogenous or endogenous endocannabinoids (ECBs) did not affect baseline BP or baseline renal sympathetic nerve activity (RSNA), but did prolong baroreflex-evoked sympathoinhibition of RSNA produced by acute increases in BP in Sprague Dawley (SD) rats (Brozoski et al., 2005; Seagard et al., 2004). Endocannabinoids including N-arachidonylethanolamine (anandamide, AEA) and 2-arachidonyl glycerol (2-AG) are synthesized upon demand in depolarized neurons, including those in the NTS (Seagard et al., 2005), and serve as retrograde signaling molecules that presynaptically inhibit the release of neurotransmitters, including GABA (Chevaleyre et al., 2006; Freund et al., 2003). Our previous studies suggest that the prolonged sympathoinhibition produced by ECBs in the NTS was due to CB1 receptor-evoked presynaptic inhibition of GABA release, leading to depolarization-induced suppression of inhibition (DSI), or disinhibition of the second order baroreceptive NTS neurons (Seagard et al., 2005).
The findings of others show that spontaneously hypertensive rats (SHR) have elevated sympathetic activity (Judy et al., 1976; Li et al., 2006; Sato et al., 2002), that blockade of this elevated activity corrects hypertension in this model (Kumagai et al., 1996; Xie et al., 2006), and that baroreflex function is attenuated in this model as compared to normotensive controls (Dickhout et al., 1998; Head, 1994; Ossting et al., 1997). Therefore, we questioned whether dysfunction of ECB signaling in the NTS, leading to less presynaptic inhibition of GABA release may be a mechanism that could lead to decreased baroreflex-evoked sympathoinhibition in SHRs.
To examine this possibility in the present study, AEA or the indirect ECB agonist, N-(4-hydroxyphenyl5Z,8Z,11Z,14Z-eicosatetraenamide) (AM404), were microinjected into the NTS in age-matched SHRs, Wistar Kyoto (WKY) rats, and SD rats to enhance the effects of exogenous and endogenously released ECBs, respectively, during baroreflex activation or direct excitation of NTS neurons which evoked depressor and sympathoinhibitory responses. AM404 was initially considered an ECB transport inhibitor (Beltramo et al., 1997; Calignano et al., 1997) but more recent evidence suggests that AM404 may also inhibit the enzyme that degrades AEA, fatty acid amide hydrolase (FAAH) (Jarrahian et al., 2000; Pertwee, 2008). In either case AM404 prolongs the presence of endogenously released AEA in the synapse, increasing the stimulation of CB1Rs. Results from this study found that rather than enhanced sympathoinhibition as seen in SD and WKY rats, microinjection of AEA and AM404 into the NTS of SHRs had almost no effect on stimulus-evoked changes in RSNA. In addition, specific binding of CB1 receptors in the NTS of SHR rats was significantly reduced as compared to SD and WKY rats. These findings suggest that function of the ECB system in the NTS is blunted in SHRs, due at least in part to reduced density of CB1 receptors, which could possibly contribute to the elevation of sympathetic activity evident in this strain.
MATERIALS AND METHODS
General Methods
The Animal Care and Use Committees at the Medical College of Wisconsin and the Zablocki Department of Veterans Affairs Center approved this protocol. These experiments were performed in 8 – 10 week, age-matched adult male inbred SD rats (344±79 g), WKY rats (278±65 g) and SHRs (266±34 g) from Charles River Laboratories, Inc. The WKY rats were studied as the genetic control for the SHRs. For at least one full week prior to the acute experiment, these rats were given water and food (Laboratory Rodent Diet 5001) ad libitum and maintained on a 12-h light/dark cycle in a temperature-controlled room. For these studies, rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.), supplemented by 4.0–5.5 mg/h i.v. to maintain an even level of anesthesia. While this anesthetic has been found to be vagolytic, we have used it previously for studies examining regulation of differential sympathetic outflow and baroreflex control of sympathetic activity (Dean, 2005), as well as ECB modulation of baroreflex inhibition of sympathetic activity (Seagard et al., 2004). These studies found that it provided the stable level of anesthesia needed for the duration of the experiments while maintaining reflex modulation of sympathetic activity. Throughout the experiments, a heating pad (Fine Science Tools) maintained body temperature at 37°C. In each rat, a catheter was inserted into a femoral vein for supplemental administration of anesthetic and administration of phenylephrine (PE) used for baroreflex testing. Arterial BP was monitored continuously from a femoral arterial cannula connected via a pressure transducer (Statham) to a polygraph (Grass Model 7) and recorded on tape (Vetter PCM recording adapter Model 3000A, Vetter Co., Rebersburg, PA, USA).
RSNA was recorded using flexible silver wire electrodes (Medwire, AG5T) positioned on a renal nerve exposed via a retroperitoneal approach. The electrodes were fixed in position with silastic gel (Sil Gel), allowing body adjustments without disturbing neural recordings. The electrophysiological signal was directed to a high impedance differential preamplifier (gain = 1000; 0.1 – 10 kHz passband), followed by a filter/amplifier (gain up to 400; high and low pass filtering 10 Hz – 3 kHz), and recorded on tape, along with BP, for later analysis. The amplifier output was directed to a precision full-wave rectifier and averaged using a Bessel linear averaging filter (averaging interval = 100 mS) to obtain an online moving time average. Averaged RSNA and BP were displayed on a Grass Model 7 recorder to observe trends during the experiment.
To allow microinjections into the NTS, the rat was placed in a stereotaxic frame and the dorsal surface of the medulla was exposed via an occipital craniotomy. A four-barreled micropipette (50–60µ total tip diameter) was used to microinject test agents into the NTS using a picoejection system designed and constructed in the laboratory. The volumes of injected solutions were visually determined by measuring the change in meniscus height in each barrel using a 50x monocular microscope with a calibrated graticule (7 nl/div). The first barrel was filled with D,L-homocysteic acid (DLH) (4 mM) (Aldrich Chemical Company, Inc.), an excitatory amino acid receptor agonist. DLH (2–5 nl; 8–20 pmoles) was microinjected to ensure that the micropipette was placed in a site in the NTS from which depressor and sympathoinhibitory responses could be evoked. The second barrel contained AM404 (50 µM) (Tocris Cookson, Inc.), dissolved in 0.01% Tocrisolve100 in double distilled (dd) H2O, and the third barrel contained AEA (50 µM) (Tocris Cookson, Inc.), dissolved in 0.01% Tocrisolve100 in ddH2O. Tocrisolve100 (Tocris Cookson, Inc.) is an emulsion of 1:4 soya oil/water, emulsified with Pluronic F68. The fourth barrel contained vehicle with pontamine sky blue dye (BDH Laboratories Supplies Poole) and was used to test for vehicle effects and mark injection sites. In animals in which the effects of 6-Imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid hydrobromide (gabazine, GZ; Tocris Cookson, Inc.), a GABAA receptor antagonist, were to be tested, barrel 3 was filled with GZ (200 mM) dissolved in ddH2O instead of AEA.
Baroreflex Activation
The effects of baroreceptor activation was assessed by quantitating the rate of recovery of RSNA in response to two methods to used activate the baroreflex or baroreflex-like responses. First, a bolus injection of PE (10 mg%; 2–4 µl i.v.) was given to produce a transient increase in BP of approximately 40–50 mmHg to activate baroreceptors (PE pressor test). This PE dose was used for all subsequent PE pressor tests in the rat to produce reproducible increases in BP (Figure 1). However, despite the care taken to ensure that the amplitude of the PE pressor tests were consistent, there was an occasional 8–14 s prolongation of the pressure increase after microinjection of AM404 or AEA. Thus, a second method was also used to evoke baroreflex-like responses in an additional experimental group of rats. In these rats, depressor and sympathoinhibitory responses were evoked by direct stimulation of neurons in the NTS by microinjecting larger volumes of DLH (7–10 nl; 28–40 pmoles) bilaterally into identified depressor/sympathoinhibitory sites in the NTS. This protocol (DLH test) produced a discernable, transient decrease in RSNA and an associated decrease in BP of approximately 30 mmHg. The volume/dose was then used for all subsequent microinjections within that rat to evoke consistent excitation of NTS neurons directly.
Figure 1.
Responses in blood pressure (BP) and averaged renal sympathetic nerve activity (RSNA) to a 2 µl i.v. injection of phenylephrine (10 mg%) in a Sprague Dawley rat, diagramming the method used to analyze recovery of RSNA after baroreflex-induced sympathoinhibition. Superimposed on the tracing of RSNA is the exponential curve fit (white line) of the recovery response of RSNA. The time constant of recovery (TCR) of the exponential function represents the rate at which RSNA recovers following the increase in BP. For each time constant (duration between dotted lines), RSNA will recover 63.2% of the remaining distance to the asymptotic value (baseline RSNA, black line). Thus, after four TCR, RSNA will have recovered to 98% of the baseline level of RSNA. A prolonged TCR indicates a prolongation of baroreflex inhibition of RSNA. a.u. = arbitrary units.
Experimental Protocol
Rats were allowed to stabilize for at least 10 min after preparation prior to the start of acute experiments. Microinjection sites in which baroreflex-like effects could be evoked were confirmed pharmacologically with microinjections of DLH, as explained above, targeting the caudal region of the NTS at 0.5 mm rostral to calamus scriptorius, 0.5 mm lateral to the midline and 0.5 mm beneath the dorsal surface. If depressor and sympathoinhibitory responses were not observed, the micropipette was carefully repositioned to an adjacent site until a depressor response greater than 20 mmHg and an associated sympathoinhibition could be evoked. Once established, corresponding coordinates were utilized to target and confirm a site in the contralateral NTS to allow bilateral microinjections. For each protocol, a control PE pressor or DLH test was performed and time was allowed for parameters to return to baseline levels. Next, a microinjection of vehicle with dye (70 nl, 3–10 sec duration) was made into bilateral NTS sites immediately followed by a PE pressor test or DLH test to serve as a vehicle control. Once control tests were completed and baseline values recovered, AM404 or AEA (70 nl, 3–10 s duration), determined randomly, was then microinjected bilaterally into the NTS and the PE pressor test was repeated at 1, 5, 10, and 20 min or DLH test at 1, 5, 10, 20, 30 and 40 min. These time points allowed for recovery of baseline parameters before the next test was performed. After an additional 20 min to allow for elimination of AM404 or AEA, a second control PE pressor test or DLH test was obtained and after recovery, the remaining AEA or AM404 (70 nl) was microinjected bilaterally into the NTS. The time sequence for baroreflex-like testing was then repeated. In some cases, it was possible to perform two trials in which the order of drugs was reversed in the second trial. To examine whether AM404 in WKY rats was acting through modulation of GABAergic transmission, similar to that previously seen in SD rats, we performed an additional study in which AM404 was microinjected after microinjection of GZ (70 nl, n=8) into the NTS. Administration of GZ was occasionally accompanied by a transient decrease in BP and/or RSNA, which recovered within 3–5 minutes, at which point the effects of gabazine alone were examined by a PE pressor test or DLH microinjection. Following this trial, microinjection of AM404 was performed and a time sequence for baroreflex-like testing was repeated. Following the completion of the studies, the animals were killed with an overdose of anesthetic and the brainstem was removed and frozen. Transverse (25 mm) sections through the medulla were cut, stained with neutral red and examined microscopically to determine the locations of microinjections. Histologically verified microinjection sites were reconstructed on transverse representations of the medulla.
Data Analysis and Statistics
Data analysis was performed as described in an earlier study (Seagard et al., 2004). Briefly, analog-to-digital conversion of recorded parameters was performed using a computer (PC built in house running programs written in HTBasic, TransEra Corporation, East Orem, Utah). Arterial BP and averaged RSNA were sampled at 20 Hz for each control and microinjection procedure and stored on disk files for quantification and statistical analysis. The change in BP for each PE pressor or DLH test for each rat were compared using an analysis of variance to verify the reproducibility of the stimuli. To examine the rate of recovery of RSNA following each evoked sympathoinhibition, averaged RSNA was plotted versus time and nonlinear regression was used to fit the data to a first order exponential recovery equation using methods described previously (Figure 1) (Rademacher et al., 2003; Seagard et al., 2004). This analysis provided a value for the rate of recovery time constant (TCR) for RSNA. TCR is proportional to the rate at which RSNA recovers following the baroreflex or DLH-induced sympathoinhibition. For each TCR, RSNA recovers 63.2% of the remaining distance to the asymptotic value (baseline RSNA), such that after four TCR periods, RSNA recovery is 98.2% of the baseline level of RSNA. TCR for each test following microinjection of vehicle, AM404, AEA or GZ were normalized as a percent of control TCR and responses for each treatment at all time points were compared using a repeated measures analysis of variance.
In all cases, normalized values for RSNA and BP for each procedure were tested and confirmed for normal distribution. Hence, all data are presented as mean± standard deviation. Following one-way analyses of variance with repeated measures, differences were identified using the Dunnett’s test which compared all time points versus control, with significance set at p < 0.05.
CB1 Receptor Binding Methods and Analysis
These experiments were performed in 10 week, age-matched adult male SD rats, WKY rats and SHRs from Charles River Laboratories, Inc. The WKY rats were studied as the genetic control for the SHRs. A total of 24 SD rats, 24 WKY rats, and 24 SHRs were used for this complete study. For this study, rats were killed by inhalation of 5% isoflurane in oxygen. Immediately after death, the animal was decapitated and a craniotomy performed to expose the brain, which was extracted and placed on an ice plate. The cerebellum was removed to expose the dorsal surface of the medulla. The dorsal medulla (DM) containing the NTS, (between 1 mm rostral and 1 mm caudal to calamus scriptorius, 1.25 mm lateral from midline, and 1.5 mm ventral) was then dissected out, placed in a plastic centrifuge tube and flash frozen in liquid nitrogen. All tissue samples were stored at −80 °C until binding analysis of CB1 receptors was performed.
Due to the small sample size, tissue from the DM in each rat strain yielded low quantities of membrane fractions. In order to obtain sufficient quantities of membrane fractions to perform analysis, tissue from 3 rats of the same strain were combined, weighed, and then homogenized. This created a total of 8 tissue samples for each strain for the DM.
Membranes from tissue samples were extracted and prepared for a protein assay (Bradford Protein Assay) and a CB1 receptor binding assay using the radioligand [3H]CP55,940. Membrane preparation consisted of homogenizing samples in 10 volumes of cold TME buffer (50mM Tris HCl, 1.0mM EDTA, 3.0mM MgCl2, pH 7.4). This mixture was centrifuged for 20 min at −4 °C at 12,000 rpm (Beckman J2-MI). The pellet containing the membrane fraction was resuspended in cold TME at 1 g/ml and gently homogenized. Then 10 µl of this homogenate was used to assay the protein concentration using the Bradford method (Bio-Rad, Hercules, CA, USA). The remaining homogenate was aliquoted into microcentrifuge tubes and stored at −80 °C to be used in the CB1 radioligand receptor binding assay as was done previously (Hillard et al., 1995). This provided a multi-point assay over a dose range for the ligand from 0.25 nM to 2.5 nM. However, initial analysis of CB1 receptor binding in the DM indicated very low specific binding, particularly for the WKY rats and SHRs. An example of one of the few multi-point assays obtained for a SD rat is shown in Figure 2. It was difficult to obtain a signal above background at the higher concentrations tested. Based on this curve, single point assays for the DM were performed, using a single concentration of [3H]CP55,940 (0.5 nM, shaded area in Figure 2) to compare the level of binding in the DM among all strains.
Figure 2.
Binding curve example for one Sprague Dawley rat in the dorsal medulla (DM) reflecting the low levels of CB1 receptor binding in this region. [3H]CP55,940 at increasing concentrations (0.25 nM, 0.40 nM, 0.50 nM, 0.75 nM, and 1.00 nM) was incubated with membrane preparations (1.2 mg of protein/ml for the DM) for 1 h at 20°C. The amount of binding was reduced even further in Wistar Kyoto and spontaneously hypertensive rats, making construction of complete binding curves impossible. As a result, a single point binding assay at 0.50 nM [3H]CP55,940 (shaded boxed) was performed.
Data analysis and statistics
Data were analyzed using Graph Pad Prism software. The specific binding was obtained by subtracting the nonspecific binding determined by the well containing THC from the total binding in the well without THC at the same radioligand concentration, 0.5 nM [3H]CP55,940. Values for each sample were pooled and summed data for all strains were compared using a one-way analyses of variance with differences identified using the Bonferroni’s multiple comparison test, with significance set at p < 0.05. All data are presented as mean ± one standard deviation.
RESULTS
Histological analysis of microinjection sites marked by blue dye confirmed that the bilateral microinjection sites were located within the commissural and medial subnuclei of the NTS from 0.3 mm caudal to 0.9 mm rostral to obex, clustered around 500µm rostral to obex (Figure 3). The SHRs had significantly higher baseline BP (132.7±26.9 mmHg) than either normotensive strain tested, but there was no significant difference between baseline BP of SD (102.9±25.3 mmHg ) and WKY (93.5±24.4 mmHg) rats. Baseline BP and RSNA remained stable throughout each experiment and were not significantly altered by microinjections of AM404 or AEA in any rat strain tested (Figure 4). The magnitude of the changes in BP produced by the PE pressor test were reproducible within each rat, with no significant differences in the magnitude of the change in peak systolic pressure among all rats (Figure 5). There were no significant differences in duration of the PE test within rat strains, but three trials for SHR rats after AEA microinjections into the NTS had significantly longer durations than some trials in SD rats and WKY rats (Figure 5). For the DLH test, the dose of DLH was determined for each control stimulation and the BP decrease averaged 26±18 mmHg across all rats.
Figure 3.
Diagrammatic representation of a transverse section through the rat dorsal medulla at the level of obex illustrating the centers of microinjection sites in the NTS. All microinjections were made bilaterally, but for clarity, nuclei are shown on the left side of the dorsal medulla. Microinjection sites were located in the commissural and medial subnuclei with a rostrocaudal distribution from 300 µm caudal to 900 µm rostral to obex. Spontaneously hypertensive rats (SHR; n = 19), Wistar Kyoto rats (WKY; n = 24), Sprague Dawley rats (SD; n = 24). Abbreviations: 4V, fourth ventricle; CU, cuneate nucleus; SL, lateral subnucleus; SM, medial subnucleus; TS, tractus solitarius; X, dorsal motor vagal nucleus; XII, hypoglossal nucleus.
Figure 4.
Summed data for baseline mean arterial blood pressure (MAP) and renal sympathetic nerve activity (RSNA) for control and each time period after microinjection of either anandamide (AEA – 50µM, 70 nl) or AM404 (50µM, 70 nl ) for Sprague Dawley (SD), Wistar Kyoto (WKY) and spontaneously hypertensive (SHR) rats. There were no significant changes in MAP or RSNA from control at 1, 5, 10 and 20 minutes post microinjection of AEA or AM404. Data shown as mean±standard deviation. n=number of rats
Figure 5.
Summed data for the magnitude of the change in systolic blood pressure and duration of pressure change for the phenylephrine (PE) pressor tests for Sprague Dawley (SD), Wistar Kyoto (WKY) and spontaneously hypertensive (SHR) rats. There were no significant differences in the magnitude of the change in systolic pressure within or between strains. There were no significant differences in the duration of the change in pressure within strains, but there was a significant prolongation of duration for three trials in SHRs versus SD and WKY rats, as indicated. * Significantly different from SHR C2, AEA1 min and AEA5 min trials. ** Significantly different from SHR AEA 1 min and AEA 5 min trials. + Significantly different from SHR AEA 1 min trial. Data shown as mean±standard deviation. n=number of rats
Elevation of BP by the PE pressor test produced baroreflex-induced decreases in RSNA and examples of the effects of microinjections of AM404 and AEA on baroreflex-evoked inhibition of RSNA are shown in Figure 6. As seen in this figure, at 5 min postinjection of either AM404 and AEA, there is a prolongations of RSNA inhibition in SD rats, with moderate effects in WKY rats and little effect in SHRs. For summed data (Figure 7), microinjections of AM404 or AEA produced strain-specific prolongations of baroreflex-induced sympathoinhibition, i.e. an increase in TCR. For AM404, TCR was increased significantly at 1, 5, and 10 min in SD rats (Figure 7A), while it was only significantly prolonged at the 5 and 20 min time points in WKY rats (Figure 7B). In SHRs, only the TCR at the 10 min time point after AM404 was significantly increased over control ( Figure 7C). For AEA, TCR was increased significantly at 1 and 5 min following AEA for SD rats ( Figure 7A), but AEA did not produce any significant change in TCR in WKY ( Figure 7B) or SHR rats (Figure 7C). The order of administration of AM404 versus AEA did not produce any significantly different results in any strain. Microinjections of vehicle did not have a significant effect on the duration of the baroreflex-induced sympathoinhibition, or TCR, produced by the PE pressor test (Figure 7) within and among strains.
Figure 6.
Examples of baroreflex-evoked decreases in renal sympathetic nerve activity (RSNA) produced by phenylephrine (PE) pressor tests for Sprague Dawley (SD), Wistar Kyoto (WKY) and spontaneously hypertensive (SHR) rats, showing control responses and responses 5 minutes after microinjection of either AM404 (50µM, 70 nl ) or anandamide (AEA- 50µM, 70 nl) into the NTS. Both AM404 and AEA prolonged reflex sympathoinhibition in the SD rat, and to a lesser degree, the WKY rat, but neither had any effect in the SHR.
Figure 7.
Summed data showing the effects of NTS microinjection of AM404 or AEA on the time constant for the rate of the recovery (TCR) of baroreflex inhibition of RSNA obtained by nonlinear regression in response to i.v. injections of phenylephrine (PE pressor test) in Sprague Dawley (SD) rats (Panel A), Wistar Kyoto (WKY) rats (Panel B), and spontaneously hypertensive rats (SHR) (Panel C). Values are normalized as percents of control TCR for each microinjection. AM404 significantly increased TCR at 1, 5, and 10 minutes post AM404 for SD rats and 5 and 20 minutes in WKY rats, reflecting a prolonged inhibition of RSNA. AEA significantly increased TCR at 1 and 5 minutes post AEA microinjections in only SD rats. AM404 significantly increased TCR at only 10 min in SHRs. C1= first control PE test; V = vehicle PE test; C2 = second control PE test. * Significantly different from first control and vehicle at p < 0.05; † Significantly different from second control. Data shown as mean±standard deviation. n = number of rats
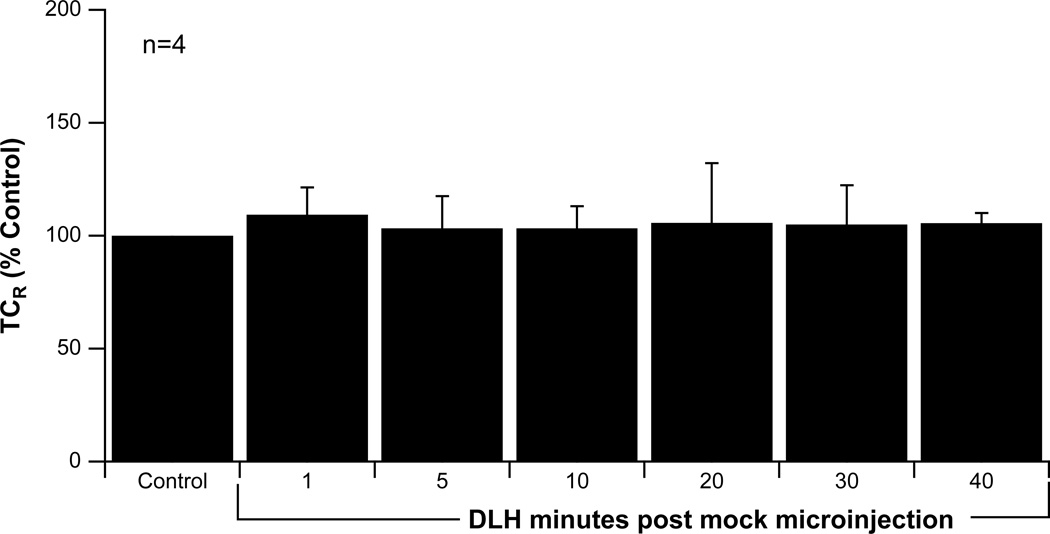
Activation of NTS neurons using the DLH test produced baroreflex-like decreases in RSNA and examples of the effects of microinjections of AM404 and AEA on DLH-evoked inhibition of RSNA are shown in Figure 8. As seen in this figure, at 5 min postinjection of either AM404 and AEA, there is a prolongation of RSNA inhibition in SD rats, with moderate effects in WKY rats, especially for AM404, and little effect in SHRs. To verify that reproducible changes in TCR could be produced by the DLH test, a timed series of microinjections was performed in 4 SD rats (Figure 9). There were no significant differences in the TCR over the test period, verifying that this technique could evoke consistent baroreflex-like stimulations.
Figure 8.
Examples of sympathoinhibition of renal sympathetic nerve activity (RSNA) evoked by microinjection of DLH (DLH test) into the NTS of Sprague Dawley (SD), Wistar Kyoto (WKY) and spontaneously hypertensive (SHR) rats, showing control responses and responses 5 minutes after microinjection of either AM404 (50µM, 70 nl ) or anandamide (AEA- 50µM, 70 nl) into the NTS. Both AM404 and AEA prolonged DLH-evoked sympathoinhibition in the SD rat, and to a lesser degree, the WKY rat, but neither had any effect in the SHR.
Figure 9.
Summed data showing the time constant for the rate of the recovery (TCR) of baroreflex inhibition of RSNA obtained by nonlinear regression following NTS microinjections of DLH at 1, 5, 10, 20, 30 and 40 minutes post vehicle microinjection in Sprague Dawley rats. There were no significant differences in TCR for this timed series of DLH tests, demonstrating the reproducibility of the stimulus. Data shown as mean±standard deviation. n = number of rats.
As seen in summed data in Figure 10, the TCR for the DLH-induced decreases in RSNA in SD rats was increased at 1, 5, 10 and 20 min following AM404 administration ( Figure 10A). These effects are slightly prolonged over those seen with the PE pressor test (Figure 7A). AM404 microinjections in WKY rats increased the TCR of DLH-evoked sympathoinhibition at all time points except 40 min (Figure 10B), which were earlier in onset compared to those obtained during the PE pressor test (5, 20 min) (Figure 7B). AM404 did not produce any changes in TCR in the SHR during the DLH test (Figure 10C). Following AEA, the TCR for SD rats was significantly prolonged for all time points except 40 min ( Figure 10A). In contrast to the PE pressor tests, AEA also produced significant increases in TCR in the WKY strain at 10 and 30 min time points ( Figure 10B). AEA did not produce any changes in TCR in the SHR during the DLH test ( Figure 10C), a finding similar to results obtained during the PE pressor test (Figure 7C). Microinjections of vehicle did not have a significant effect on the TCR produced by the DLH test (Figure 10).
Figure 10.
Summed data showing the effects of NTS microinjection of AM404 or AEA on the time constant for the rate of the recovery (TCR) of baroreflex inhibition of RSNA obtained by nonlinear regression in response to NTS microinjections of DLH at 1, 5, 10, 20, 30, and 40 minutes following AM404 or AEA in Sprague Dawley (SD) rats (Panel A), Wistar Kyoto (WKY) rats (Panel B), and spontaneously hypertensive rats (SHR) (Panel C). Values are normalized as percents of control TCR for each microinjection. AM404 significantly increased TCR, reflecting a prolonged inhibition of RSNA, at 1, 5, 10, and 20 minutes post AM404 for SD rats and 1, 5, 10, 20 and 30 minutes in WKY rats. AEA significantly increased TCR at 1, 5, 10, 20 and 30 minutes post AEA microinjections in SD rats and 10 and 30 minutes in WKY rats. Neither AM404 nor AEA had an effect in SHRs. C1= first control DLH test; V = vehicle DLH test; C2 = second control DLH test. *Significantly different from first control and vehicle at p < 0.05; † significantly different from second control. Data shown as mean ± standard deviation. n = number of rats.
To examine the role of modulation of GABAergic transmission on ECB effects in the SD and WKY rats, AM404 trials were repeated after NTS microinjection of GZ, similar to that which was done previously (Seagard et al., 2004). An example of DLH-induced RSNA inhibition before and after GZ for a SD and WKY rat is shown in Figure 11. As seen in the figure, NTS microinjection of AM404 prolonged RSNA inhibition at 5 min postinjection for both the SD and WKY rat, and this prolongation was attenuated following NTS microinjection of GZ prior to the AM404 microinjection. In summed data for both strains of rats, GZ alone did not have a significant effect on TCR and AM404 did not produce any significant prolongation of RSNA inhibition at any time point following microinjection of GABAA receptor antagonist GZ (Figure 12). Gabazine was not tested in SHRs due to the lack of response to ECBs in this strain.
Figure 11.
Examples of sympathoinhibition of renal sympathetic nerve activity (RSNA) evoked by microinjection of DLH (DLH test) into the NTS of Sprague Dawley (SD) and Wistar Kyoto (WKY) rats, showing control responses and responses 5 minutes after NTS microinjection of AM404 (50µM, 70 nl ) before and after microinjection of gabazine (GZ – 200µM, 70 nl) into the NTS. AM404 produced a prolongation of DLH-evoked sympathoinhibition of RSNA in both the SD and WKY rat, which was attenuated after prior microinjection of GZ into the NTS to block GABAA receptors. These results suggest that AM404 produces endocannabinoid effects through modulation of GABA release within the NTS.
Figure 12.
Averaged data showing the effects of NTS microinjection of AM404 before and after NTS microinjection of gabazine (GZ) on the time constant for the rate of the recovery (TCR) of baroreflex inhibition of RSNA in response to NTS microinjections of DLH at 1, 5, 10, 20 and 30 minutes in SD (Panel A) and WKY rats (Panel B). Values are normalized as percents of control TCR for each microinjection. AM404 significantly increased TCR at 1, 5, 10, 20, and 30 minutes post AM404 for SD and WKY rats. Prior microinjection of GZ blocked the increase in TCR produced by AM404 in both strains. C1= first control DLH test; V = vehicle DLH test; C2 = second control DLH test. * Significantly different from first control and vehicle at p < 0.05. (n) = number of rats.
In the DM, CB1R binding site density was significantly reduced for the SHRs (0.037 pmol/mg ± .0167) compared to WKY (0.114 pmol/mg ± 0.025) and SD rats (0.106 pmol/mg ± 0.028) at the 0.5 nM concentration of [3H]CP55,940. CB1R binding site density was not significantly different between the normotensive WKY and SD rats
DISCUSSION
Data from this study found that increases in ECBs in the NTS enhanced baroreflex-induced sympathoinhibition in normotensive SD and WKY rats, but not SHRs. The results from SD rats in this study are similar to those found in earlier studies for increases in exogenous AEA (Seagard et al., 2004) and endogenously-released ECBs (Brozoski et al., 2005), which showed that ECBs can prolong baroreflex control of RSNA evoked by the PE pressor test and increase discharge of baroreceptive neurons in the NTS of SD rats (Seagard et al., 2005). In addition, this laboratory previously found that AEA content in the NTS was increased in SD rats subjected to a short period of hypertension (Seagard et al., 2004), indicating that the ECB system is functional in the NTS of this strain of rat. Further, prior application of the CB1 receptor antagonist SR141716 blocked ECB-induced changes in baroreflex-induced sympathoinhibition (Seagard et al., 2004) and discharge of baroreceptor-sensitive NTS neurons (Seagard et al., 2005) indicating that the effects were due to activation specifically of CB1 receptors and not vanilloid receptors that can also be activated by higher concentrations of ECBs. The effects of ECBs on baroreflex function were found to be dependent on functional GABAergic input to NTS neurons, since prior application of GABAA receptor antagonists bicuculline or GZ prevented ECB-induced changes in baroreflex-induced sympathoinhibition (Seagard et al., 2004) or discharge of baroreceptor-sensitive NTS neurons (Seagard et al., 2005). Data from the present study support the involvement of ECB GABAergic modulation in WKY rats, as well as SD rats, since GZ prevented AM404-induced increases in TCR evoked by the DLH test in these rat models. The effects of ECBs in the NTS of SD and WKY rats could be similar to the ECB presynaptic inhibition of GABA release found in many regions of the central nervous system in brain slice studies (Foldy et al., 2006; Freund et al., 2003), which is thought to contribute to DSI in these brain regions. In SD and WKY rats, a decrease in GABA release in the NTS would lead to a disinhibition of second order barosensitive neurons, leading to the prolongation of baroreflex-induced sympathoinhibition.
However, in WKY rats, we found that primarily AM404, and not AEA, had an effect on the prolongation of sympathoinhibition, suggesting some differences in the central ECB system between WKY and SD rats. The differences in responses of the WKY rats to increases in endogenous versus exogenous ECBs might be explained by the type of changes seen in ECBs evoked by each type of microinjection. Administration of AM404 could have the effect of prolonging the presence of multiple ECBs in the NTS released in response to activation of the baroreflex, including both AEA and 2-AG. Studies have suggested that AEA and 2-AG share the same uptake transporter, and thus blockade of uptake by AM404 could increase both ECBs (Hajos et al., 2004). Microinjection of AEA would only increase central AEA levels. Therefore, AM404 would have the effect of increasing both ECBs, which may be needed in WKY rats. If the level of activity of the catabolic enzyme fatty-acid amide hydrolase (FAAH), which metabolizes AEA but not 2-AG (Pazos et al., 2005), was greater in WKY versus SD rats, AEA alone may have a reduced effect in the NTS. Preliminary studies in this laboratory have found that 2-AG, but not AEA, is significantly elevated in WKY rats in response to acute increases in BP, supporting an enhanced role for 2-AG in this strain. Therefore, possible differences in specific ECB synthesis or uptake could account for the differences seen between SD and WKY rats.
Data from the present study demonstrated that AEA had no effect on TCR in SHRs and AM404 had only a minimal effect at one time point, suggesting that function of the ECB system in the NTS was depressed in this strain. Results from the CB1 receptor binding study suggest that at least a part of this depressed function is due to a reduced number of CB1 receptors in the NTS of SHRs. In addition, this data could be explained if the activity of the catabolic enzymes FAAH and monoacylglycerol lipase, which metabolize AEA and 2-AG, respectively, are both upregulated in the NTS of SHRs. Interestingly, WKY rats seemed to have responses that spanned those of SD rats and SHR, namely that increases in endogenous but not exogenous ECBs modulated sympathoinhibition of RSNA. If there are genetic alterations in the ECB system in SHRs, these might be present to a lesser degree in the genetic WKY control. The elucidation of the mechanism(s) behind the differential effects of ECBs in all strains will help to understand the importance of the physiological role of ECBs in BP regulation.
The effects of ECBs on BP regulation have been studied previously, but most of these studies involved systemic administration of ECBs or CB1 receptor agonists or antagonists, which did not localize action to central sites. Intravenous administration of AEA in anesthetized SD and WKY rats has been found to have either no effect (Batkai et al., 2004) or produce hypotension (Lake et al., 1997a; Lake et al., 1997b; Niederhoffer et al., 2003; Varga et al., 1995). However, systemic administration of CB1 receptor antagonists have generally been found to have no effect on BP in normotensive rats, leading investigators to suggest that the ECB system is not operating under normal conditions (Batkai et al., 2004; Lake et al., 1997a; Pfitzer et al., 2005a; Varga et al., 1995). Interestingly, in hypertensive rats, i.v. administration of AEA has been found to produce a more pronounced hypotension than in normotensive rats (Batkai et al., 2004). In addition, blockade of CB1 receptors by administration of SR141716 produced a greater increase in BP in hypertensive versus normotensive WKY rats. The results were found to be the same in three models of hypertension – SHRs, angiotensin infusion, and Dahl salt-sensitive rats, which are also known to have elevated levels of sympathetic activity. These effects on BP were accompanied by higher levels of CB1 receptors in heart and aortic endothelium in the hypertensive rats. This has lead investigators to suggest that the ECB system becomes tonically active during hypertension and that this effect is evoked by increased pressure itself versus mechanisms behind the hypertension. The authors propose that the increase in CB1 receptors in the heart and vascular are sites of action leading to enhanced ECB function in hypertension. It is possible that ECBs are acting at peripheral sympathetic nerve endings at these sites, blocking the release of norepinephrine (Niederhoffer et al., 2003; Pfitzer et al., 2005b). Since the level of sympathetic activity is elevated in these models of hypertension, the effects of inhibition of transmitter release would be exaggerated in the hypertensive rats versus normotensive controls. The SHRs used in this earlier study were 8–10 month old rats, and thus represented a much more chronic model of hypertension than those used in the present study (8–10 weeks). The changes seen in the older rats might represent an adaptation to the pressure not seen in the current study. Further, it is not clear what changes had occurred centrally in the chronic hypertensive animals since ECBs were administered systemically and not restricted to specific brain regions.
In several studies, attempts have been made to distinguish central from peripheral effects of ECBs. In one study, i.v. administration of the CB1 receptor agonist WIN 555212-2 (WIN-2) produced hypotension, bradycardia and decreased plasma norepinephrine levels, while microinjection of WIN-2 into the NTS was not found to affect these parameters (Niederhoffer et al., 2003). The lack of a change in baseline BP following activation of CB1 receptors in the NTS was also seen in the current study, suggesting that the primary effects of CB1 receptor activation were only seen when the full baroreflex was evoked. In a study in rabbits, intracisternal administration of two CB1 receptor agonists (WIN-2 and CP 55940) increased baseline levels of RSNA, plasma norepinephrine concentration and BP, effects that were attenuated by SR141716 (Niederhoffer et al., 2000). The central site of action of the drugs could not be determined from the study. In a study by Varga et al. (Varga et al., 1996) intravenous administration of AEA in rats was found to produce an initial brief increase in discharge of baroreceptive, presympathetic neurons in the rostral ventrolateral medulla (RVLM), which preceded transient increases in RSNA and BP. This initial burst of neuronal activity was followed by a more prolonged hypotension and sustained increases in sympathetic and RVLM neuronal activities, possibly due to baroreflex-evoked changes in response to the hypotension. The initial burst in RVLM neuronal activity could be indicative of disinhibition from inhibitory GABA input, similar to that which we saw previously for NTS neurons (Seagard et al., 2005). A second study (Niederhoffer et al., 2003) found that direct microinjection of WIN-2 into the RVLM produced a brief hypotension, without any transient increase in BP. These two studies suggest that the route of administration of the CB1 receptor agonist may influence the response seen in central sites. We attempted to avoid the complications from peripheral versus central effects by making localized, small volume microinjections into barosensitive regions of the NTS. In addition, we selected SHRs at an age where hypertension was just developing, to avoid secondary adaptations to chronic elevated pressure. More work remains to determine if there is a time or age-related difference in the effects of ECBs in the NTS of hypertensive animals, and if the changes in CB1 receptor binding accompany or precede the development of hypertension.
The results from activation of the baroreflex by the PE pressor tests versus direct activation of baroreflex-like responses via DLH tests were similar. Primary differences were related to the timing and duration of their effects. The use of bolus injections of PE to evoke the baroreflex is a traditional method used to examine the effects of activation of the baroreceptors in many studies. However, the increase in BP produced by PE would activate all barosensitive receptors, including arterial baroreceptors and cardiac receptors, which have second order neurons in the NTS. Similarly, the DLH test would directly activate these neurons as well as second order neurons in the same or adjacent areas of the NTS that receive chemoreceptor and other pulmonary receptor inputs. We attempted to minimize the extent of activation of non-baroreceptive neurons by targeting regions of the NTS known to receive primary afferent input from arterial baroreceptors (Erickson et al., 1991; Miura et al., 1994) and produce a decrease in BP and sympathoinhibition in response to microinjection of DLH. Activation of chemoreceptor-sensitive neurons would evoke the opposite responses, namely increases in BP and sympathoexcitatory responses. Thus, while the use of DLH is not selective for activation of only barosensitive inputs, the results suggest that careful, localized microinjection of DLH allowed a fairly discrete activation of the desired neurons and verified the results from activation of baroreceptive neurons via the PE pressor test. While neither test can claim exclusive activation of the baroreceptors, both suggest that baroreflex-like responses can be modulated by activation of CB1 receptors in SD and WKY rats. The changes in blood pressure produced by the PE pressor test were similar for all animals, and thus could not be a source of differences seen in endocannabinoid-modulated sympathoinhibition within or among strains. There was no significant difference in duration of the PE tests within strains, but there was a significant prolongation in duration of the PE test in three trials for SHRs versus some trials in SD and WKY rats, which could have induced changes in the amount of sympathoinhibition at these points. However, the lack of differences seen overall using the PE versus DLH test to determine the effects of endocannabinoids in the NTS on evoked sympathoinhibition suggests that differences in duration were not sufficient to alter the degree of sympathoinhibition directly.
In summary, increases in endogenous ECBs induced by NTS microinjections of AM404 prolonged baroreflex-induced sympathoinhibition in SD and WKY rats, but not SHRs. Exogenous AEA enhanced sympathoinhibition primarily in the SD rat strain. There was reduced specific binding of CB1 receptors in SHR versus SD and WKY rats, and this difference could contribute to the lack of responses seen in SHRs to increases in both exogenous and endogenous ECB in the NTS. Although SHRs have both elevated sympathetic nerve activity and increased GABAergic function in the NTS, the ECB system is less functional in this model. The data from this study suggests that depressed ECB modulation of GABAergic neurotransmission in the NTS of SHRs could be either altered by the hypertensive condition or could possibly contribute to the generation of hypertension and sympathoexcitation in SHRs. However, specific mechanisms behind altered ECB modulation of baroreflex-induced sympathoinhibition in the SHR remain to be determined.
ACKNOWLEDGMENTS
The authors acknowledge the excellent technical assistance of Claudia Hermes and Victoria Woyach. Supported by VA Medical Research Funds (J.L.S.), Advancing a Healthier Wisconsin Award from the Medical College of Wisconsin (J.L.S.), American Heart Grant-in-Aid 0750010Z (C.D.), NSF Award IOS 0751613 (C.D.) and NIDA Award DA09155 (C.J.H.)
REFERENCES
- Andresen MC, Doyle MW, Bailey TW, Jin Y-H. Differentiation of autonomic reflex control begins with cellular mechanisms at the first synapse within the nucleus tractus solitarius. Brazilian Journal of Medical and Biological Research. 2004;37:549–558. doi: 10.1590/s0100-879x2004000400012. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Kunze DL. Nucleus tractus solitarius - gateway to neural circulatory control. Ann. Rev. Physiol. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- Batkai S, Pacher P, Osei-Hyiaman D, Radeaeva S, Liu J, Harvey-White J, Offertaler L, Mackie K, Rudd A, Bukoski RD, Kunos G. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation. 2004;110:1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science (New York, N.Y. 1997;277:1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- Brozoski DT, Dean C, Hopp FA, Seagard JL. Uptake blockade of endocannabinoids in the NTS modulates baroreflex-evoked sympathoinhibition. Brain Res. 2005;1059:197–202. doi: 10.1016/j.brainres.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Beltramo M, Makriyannis A, Piomelli D. Potentiation of anandamide hypotension by the transport inhibitor, AM404. Eur J Pharmacol. 1997;337:R1–R2. doi: 10.1016/s0014-2999(97)01297-1. [DOI] [PubMed] [Google Scholar]
- Chen C-Y, Bonham AC. Glutamate suppresses GABA release via presynaptic metabotropic glutamate receptors at baroreceptor neurones in rats. J. Physiol. 2005;562.2:535–551. doi: 10.1113/jphysiol.2004.076885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-Y, Horowitz JM, Bonham AC. A presynaptic mechanism contributes to depression of autonomic signal transmission. Am. J. Physiol. (Heart Circ. Physiol.) 1999;277:H1350–H1360. doi: 10.1152/ajpheart.1999.277.4.H1350. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu. Rev. Neurosoci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Dean C. Sympathoinhibition from ventrolateral periaqueductal gray mediated by the caudal midline medulla. American journal of physiology. 2005;289:R1477–R1481. doi: 10.1152/ajpregu.00326.2005. [DOI] [PubMed] [Google Scholar]
- Dickhout JG, Lee RM. Blood pressure and heart rate development in young spontaneously hypertensive rats. Am. J Physiol. Heart Circ Physiol. 1998;274:H794–H800. doi: 10.1152/ajpheart.1998.274.3.H794. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Fos-like protein is induced in neurons of the medulla oblongata after stimulation of the carotid sinus nerve in awake and anesthetized rats. Brain Res. 1991;567:11–24. doi: 10.1016/0006-8993(91)91430-9. [DOI] [PubMed] [Google Scholar]
- Foldy C, Neu A, Jones MV, Soltesz I. Presynaptic, activity-dependent modulation of cannabinoid type 1 receptor-mediated inhibition of GABA release. J. Neurosci. 2006;26:1465–1469. doi: 10.1523/JNEUROSCI.4587-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong AY, Stornetta RA, Foley CM, Potts JT. Immunohistochemical localization of GAD67-expressing neurons and processes in the rat brainstem: Subregional distribution in the nucleus tractus solitarius. J. Comp. Neurol. 2005;493:274–290. doi: 10.1002/cne.20758. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol.. Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Hajos N, Kathuria S, Dinh T, Piomelli D, Freund TF. Endocannabinoid transport tightly controls 2-arachidonoyl glycerol action in the hippocampus: effects of low temperature and the transport inhibitor AM404. Eur. J. Neurosci. 2004;19:2991–2996. doi: 10.1111/j.0953-816X.2004.03433.x. [DOI] [PubMed] [Google Scholar]
- Head GA. Cardiac baroreflexes and hypertension. Clinical and experimental pharmacology & physiology. 1994;21:791–802. doi: 10.1111/j.1440-1681.1994.tb02448.x. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Edgemond WS, Campbell WB. Characterization of ligand binding to the cannabinoid receptor of rat brain membranes using a novel method: application to anandamide. J. Neurochem. 1995;64:677–683. doi: 10.1046/j.1471-4159.1995.64020677.x. [DOI] [PubMed] [Google Scholar]
- Jarrahian A, Manna S, Edgemond WS, Campbell WB, Hillard CJ. Structure-activity relationships among N-arachidonylethanolamine (Anandamide) head group analogues for the anandamide transporter. J Neurochem. 2000;74:2597–2606. doi: 10.1046/j.1471-4159.2000.0742597.x. [DOI] [PubMed] [Google Scholar]
- Judy WV, Watanabe AM, Henry DP, Besch HR, Jr, Murphy WR, Hockel GM. Sympathetic nerve activity: role in regulation of blood pressure in the spontaneously hypertensive rat. Circ. Res. 1976;38:21–29. doi: 10.1161/01.res.38.6.21. [DOI] [PubMed] [Google Scholar]
- Kumagai K, Suzuki H, Ichikawa M, Jinmbo M, Nishizawa M, Ryuzaki M, Saruta T. Comparison of early and late start of antihypertensive agents and baroreceptor reflexes. Hypertension. 1996;27:209–218. doi: 10.1161/01.hyp.27.2.209. [DOI] [PubMed] [Google Scholar]
- Lake KD, Compton DR, Varga K, Martin BR, Kunos G. Cannabinoid-induced hypotension and bradycardia in rats mediated by CB1-like cannabinoid receptors. J. Pharmacol. Exp. Ther. 1997a;281:1030–1037. [PubMed] [Google Scholar]
- Lake KD, Martin BR, Kunos G, Varga K. Cardiovascular effects of anandamide in anesthetized and conscious normotensive and hypertensive rats. Hypertension. 1997b;29:1204–1210. doi: 10.1161/01.hyp.29.5.1204. [DOI] [PubMed] [Google Scholar]
- Li D-P, Pan H-L. Plasticity of GABAergic control of hypothalamic presympathetic neurons in hypertension. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H1110–H1119. doi: 10.1152/ajpheart.00788.2005. [DOI] [PubMed] [Google Scholar]
- Miura M, Takayama K, Okada J. Neuronal expression of Fos protein in the rat brain after baroreceptor stimulation. J. Auton. Nerv. Syst. 1994;50:31–43. doi: 10.1016/0165-1838(94)90120-1. [DOI] [PubMed] [Google Scholar]
- Niederhoffer N, Schmid K, Szabo B. The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn-Schmiedeberg's Arch. Pharmacol. 2003;367:434–443. doi: 10.1007/s00210-003-0755-y. [DOI] [PubMed] [Google Scholar]
- Niederhoffer N, Szabo B. Cannabinoids cause central sympathoexcitation and bradycardia in rabbits. J. Pharmacol. Exp. Ther. 2000;294:707–713. [PubMed] [Google Scholar]
- Ossting J, Struijker-Boudier HA, Janssen BJ. Autonomic control of ultradian and circadian rhythms of blood pressure, heart rate, and baroreflex sensitivity in spontaneously hypertensive rats. J. Hypertens. 1997;15:401–410. doi: 10.1097/00004872-199715040-00011. [DOI] [PubMed] [Google Scholar]
- Pazos MR, Nunez E, Benito C, Tolon RM, Romero J. Functional neuroanatomy of the endocannabinoid system. Pharmacol. Biochem. and Behavior. 2005;81:239–247. doi: 10.1016/j.pbb.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoid receptor ligands. Tocris Bioscience Scientific Review Series. 2008;27:1–15. [Google Scholar]
- Pfitzer T, Niederhoffer N, Szabo B. Search for an endogenous cannabinoid-mediated effect in the sympathetic nervous system. Naunyn-Schmiedeberg's Arch. Pharmacol. 2005a;371:9–17. doi: 10.1007/s00210-004-1003-9. [DOI] [PubMed] [Google Scholar]
- Pfitzer T, Niederhoffer N, Szabo B. Search for an endogenous cannabinoid-mediated effect in the sympathetic nervous system. Naunyn Schmiedebergs Arch Pharmacol. 2005b;371:9–17. doi: 10.1007/s00210-004-1003-9. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Patel S, Hopp FA, Dean C, Hillard CJ, Seagard JL. Microinjection of a cannabinoid receptor antagonist into the NTS increases baroreflex duration in dogs. Am. J. Physiol. 2003;284:H1570–H1576. doi: 10.1152/ajpheart.00772.2002. [DOI] [PubMed] [Google Scholar]
- Saha S. Role of the central nucleus of the amygdala in the control of blood pressure: descending pathways to medullary cardiovascular nuclei. Clinical and experimental pharmacology & physiology. 2005;32:450–456. doi: 10.1111/j.1440-1681.2005.04210.x. [DOI] [PubMed] [Google Scholar]
- Sato MA, Colombari E, Morrison SF. Inhibition of neurons in commissural nucleus of solitary tract reduces sympathetic nerve activity in SHR. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H1679–H1684. doi: 10.1152/ajpheart.00619.2001. [DOI] [PubMed] [Google Scholar]
- Seagard JL, Dean C, Patel S, Rademacher DJ, Hopp F, Schmeling WT, Hillard CJ. Anandamide content and interaction of endocannabinoid/GABA modulatory effects in the NTS on baroreflex-evoked sympathoinhibition. Am J Physiol Heart Circ Physiol. 2004;286:H992–H1000. doi: 10.1152/ajpheart.00870.2003. [DOI] [PubMed] [Google Scholar]
- Seagard JL, Hopp FA, Hillard CJ, Dean C. Effect of endocannabinoids on discharge of baroreceptive NTS neurons. Neurosci. Lett. 2005;381:334–339. doi: 10.1016/j.neulet.2005.02.044. [DOI] [PubMed] [Google Scholar]
- Sved AF. GABA-mediated neural transmission in mechanisms of cardiovascular control. In: Barraco IRA, editor. Nucleus of the Solitary Tract. Boca Raton: CRC Press; 1994. pp. 245–254. [Google Scholar]
- Varga K, Lake K, Martin BR, Kunos G. Novel antagonist implicates the CB1 cannabinoid receptor in the hypotensive action of anandamide. Eur J Pharmacol. 1995;278:279–283. doi: 10.1016/0014-2999(95)00181-j. [DOI] [PubMed] [Google Scholar]
- Varga K, Lake KD, Huangfu D, Guyenet PG, Kunos G. Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension. 1996;28:682–686. doi: 10.1161/01.hyp.28.4.682. [DOI] [PubMed] [Google Scholar]
- Xie H-H, Shen F-M, Zhang X-F, Jiang Y-Y, Su D-F. Blood pressure variability, baroreflex sensitivity and organ damage in spontaneously hypertensive rats. Eur. J. Pharmacol. 2006;543:77–82. doi: 10.1016/j.ejphar.2006.05.034. [DOI] [PubMed] [Google Scholar]