Summary
The histone variant H2A.Z is a genome-wide signature of nucleosomes proximal to eukaryotic regulatory DNA. While the multi-subunit chromatin remodeler SWR1 is known to catalyze ATP-dependent deposition of H2A.Z, the mechanism of SWR1 recruitment to S. cerevisiae promoters has been unclear. A sensitive assay for competitive binding of di-nucleosome substrates revealed that SWR1 preferentially binds long nucleosome-free DNA and the adjoining nucleosome core particle, allowing discrimination of gene promoters over gene bodies. Analysis of mutants indicates that the conserved Swc2/YL1 subunit and the ATPase domain of Swr1 are mainly responsible for binding to substrate. SWR1 binding is enhanced on nucleosomes acetylated by the NuA4 histone acetyltransferase, but recognition of nucleosome-free and nucleosomal DNA is dominant over interaction with acetylated histones. Such hierarchical cooperation between DNA and histone signals expands the dynamic range of genetic switches, unifying classical gene regulation by DNA-binding factors with ATP-dependent nucleosome remodeling and post-translational histone modifications.
Introduction
A hallmark of eukaryotic genomes is the organization of DNA in nucleosomes and the folding of nucleosome arrays to enable packaging within the volume of a cell nucleus. Individual nucleosomes are composed of an octameric core of histones around which 147 bp of DNA are wrapped in ~1.7 left-handed superhelical turns (Arents et al., 1991; Luger et al., 1997). The bulk of the nucleosomes in chromatin contain two each of four major histones, H2A, H2B, H3 and H4; however, certain regions of chromatin are marked with nucleosomes containing minor histone variants. Histone H2A has the largest number of variant isoforms, but only one isoform, H2A.Z, is conserved in all eukaryotes. Studies in budding yeast show that H2A.Z is important for regulation of gene expression (Kobor et al., 2004; Mizuguchi et al., 2003), gene silencing boundaries (Meneghini et al., 2003), DNA repair (Kalocsay et al., 2009), cell cycle progression (Dhillon et al., 2006) and chromosome stability (Krogan et al., 2004; Rangasamy et al., 2004). In flies, frogs and mice, H2A.Z is essential for viability, while yeast cells lacking H2A.Z are viable but impaired for growth (Santisteban et al., 2000).
The amino acid sequences of budding yeast and human H2A.Z proteins are 69% identical, indicative of conserved function. Although the atomic structure of H2A.Z-containing nucleosomes has similar overall architecture to conventional nucleosomes (Suto et al., 2000), differences in protein sequence confer distinctive biochemical and biophysical properties. Purified, native yeast and human H2A.Z-containing mono-nucleosomes display greater sensitivity to salt dissociation (Jin and Felsenfeld, 2007; Zhang et al., 2005), and interactions between the extended acidic patch on the H2A.Z surface and the histone H4 amino-terminal tail enhance intra-molecular folding of reconstituted nucleosome arrays (Fan et al., 2002). Moreover, H2A.Z in combination with the histone H3 variant, H3.3, results in the most labile state of nucleosomes in human cells (Jin and Felsenfeld, 2007).
Histone H2A.Z is a stereotypic component of the chromatin landscape at eukaryotic promoters and enhancer elements. Most yeast promoters have a DNase I hypersensitive site relatively depleted or free of nucleosomes (also called the nucleosome-free region, or NFR) interrupting the nucleosome array; the two nucleosomes flanking the NFR are referred to as nucleosome −1 and +1 (Bernstein et al., 2004; Jiang and Pugh, 2009; Yuan et al., 2005). In budding yeast, the +1 nucleosome starts at a position ~13 bp upstream of, and occluding the transcription start site (TSS) (Mavrich et al., 2008a). Positioned nucleosomes also flank NFRs at human promoters and enhancers, although the +1 nucleosome maps 10 or 40 bp downstream of the TSS for inactive and active genes respectively (Schones et al., 2008). A promoter-proximal localization of H2A.Z was first reported for individual genes in budding yeast (Santisteban et al., 2000). Subsequent studies strikingly demonstrated genome-wide localization of H2A.Z to nucleosomes −1 and +1 flanking the NFR (Albert et al., 2007; Barski et al., 2007; Mavrich et al., 2008b).
The incorporation of H2A.Z at yeast promoters in vivo is uniquely dependent on the multi-subunit SWR1 complex, a conserved, ATP-dependent SWI/SNF–related chromatin remodeling enzyme (Kobor et al., 2004; Krogan et al., 2003; Mizuguchi et al., 2003). Upon stimulation by its two substrates - conventional nucleosomes and free H2A.Z-H2B dimers - purified SWR1 utilizes the energy of ATP hydrolysis to evict a H2A-H2B dimer from the nucleosome in a reaction that is coupled to deposition of H2A.Z-H2B (Luk et al., 2010; Mizuguchi et al., 2003). Stepwise replacement leads to a variant nucleosome containing two H2A.Z-H2B dimers. The SWR1 complex is composed of 14 distinct components, at least seven of which (Swr1, the catalytic subunit, and accessory subunits Swc2, Arp6, Swc6, Swc5, Arp4 and Yaf9) are necessary for the core histone replacement reaction in vitro, while three (Swc3, Swc7 and Bdf1) are dispensable and may be involved in enzyme recruitment or regulatory activities in vivo (Wu et al., 2005; Wu et al., 2009). Chromatin immunoprecipitation (ChIP) shows genome-wide SWR1 binding at sites of H2A.Z incorporation, with special preference for the +1 nucleosome and -1 nucleosome of budding yeast genes (Venters and Pugh, 2009).
How is the SWR1 complex (hereafter also called SWR1) recruited to most, if not all, promoters in the yeast genome to catalyze histone replacement? We anticipate that universal chromatin architectures at gene promoters should play a significant role. Previous molecular genetic studies implicated three interrelated aspects of chromatin that lead to preferential incorporation of H2A.Z in promoter-proximal nucleosomes. First, general regulatory factors such as the multifunctional, sequence-specific DNA binding proteins Reb1 and Abf1 populate many yeast promoters, but there is no evidence that they recruit SWR1 directly. Rather, Reb1 recruits the RSC chromatin remodeler (Gavin et al., 2006) and both are required, not only for NFR formation at subsets of promoters but also for efficient H2A.Z deposition (Badis et al., 2008; Hartley and Madhani, 2009; Raisner et al., 2005). Second, there is a correlation between the presence of an NFR and H2A.Z enrichment at yeast and human promoters in vivo (Tirosh and Barkai, 2008). Third, histone acetylation occurs at promoter nucleosomes, albeit not exclusively, and the Bdf1 subunit of SWR1 has two bromodomains that bind acetylated histone H4 (Ladurner et al., 2003; Matangkasombut and Buratowski, 2003).
We have developed biochemical assays to determine whether the NFR and histone acetylation have direct roles and are sufficient for SWR1 recruitment, and to measure their relative contributions. We reconstituted fluorescent di-nucleosomes separated by a long or short linker to measure substrate competition for SWR1 binding. The NFR is sufficient to target SWR1 directly, owing to the Swc2 and the Swr1 and/or Rvb subunits. Acetylated histones also make direct contributions to the genome-wide recruitment of SWR1, but the cooperative relationship between the NFR and histone acetylation is strikingly hierarchical.
Results
SWR1 binds a di-nucleosome mimic of promoter chromatin
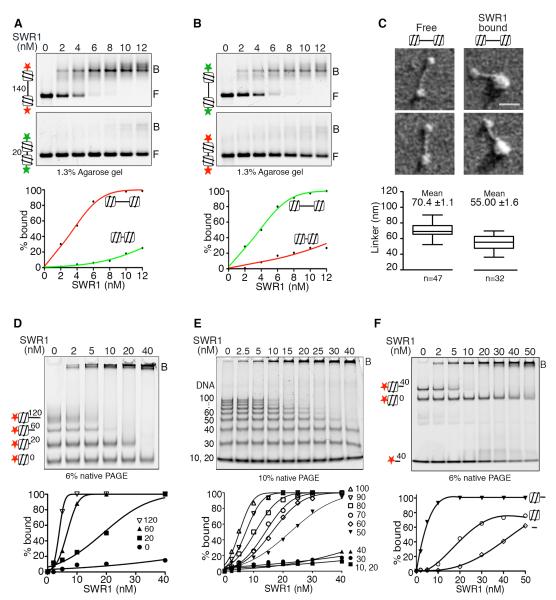
While DNase I hypersensitive regulatory DNA in chromatin may be histone-deficient, it is not entirely protein-free (Neph et al., 2012; Wu, 1984). However, the dynamic nature of transcription factor-DNA interactions at promoters allows for stretches of protein-free DNA as a transient or persistent state of promoter chromatin. We reconstituted a stable nucleosome-free region (NFR) consisting of a 140 bp natural yeast promoter (RPL4A), flanked on both sides by the strong `601' nucleosome positioning sequence (Lowary and Widom, 1998), i.e. a long linker di-nucleosome. For comparison, we also reconstituted a mimic of yeast gene body di-nucleosomes by inserting a 20 bp linker between two 601 positioned nucleosomes (Fig S1A). DNA individually end-labeled with fluorescent dyes Cy5 or Cy3 enabled comparison of an equimolar mixture of long and short linker di-nucleosomes for binding purified SWR1 complex under competitive conditions (Fig 1A). As shown by electrophoretic mobility shift analysis (EMSA) on agarose gels, SWR1 has a strong preference for long linker di-nucleosomes; binding is independent of ATP (data not shown). Interchanging Cy3 and Cy5 indicated that the specific label does not contribute to observed preferences (Fig 1B). Inspection for SWR1 concentrations that give half-maximal binding to the long linker di-nucleosome indicated binding affinity in the nanomolar range. Electron microscopy of samples prepared under conditions where di-nucleosomes are under-saturated by SWR1 shows a mixture of free long linker di-nucleosome (80%) and another species (20%) bearing a larger mass on one end (Fig 1C, S1D). Scanning Transmission Electron Microscopy (STEM) quantified the large mass at ~1.2 MDa (Fig S1E), consistent with one SWR1 complex bound to one nucleosome. Notably, bound SWR1 covers part of the long linker, suggesting that it interacts with both core particle and adjoining extranucleosomal DNA (Fig 1C).
Figure 1. SWR1 binding to di-nucleosomes, nucleosomes, and naked DNA.
(A) EMSA showing SWR1 binding to Cy5 (red) or Cy3 (green) di-nucleosomes (2nM) with 140 bp (long) or 20 bp (short) linker. Free (F) and SWR1 bound (B) di-nucleosomes. Bottom: Binding curves for long- and short-linker di-nucleosome.
(B) Dye-reversal of experiment in (A).
(C) Electron microscopy of free and SWR1-bound long linker di-nucleosomes. Enzyme and substrates (52 nM) cross-linked before EM. Scale bar is 50 nm. Bottom: Box plot of DNA linker lengths for free and SWR1 bound di-nucleosome. (n=number of particles analyzed; error bars are SEM values).
(D) EMSA showing SWR1 binding to Cy5 labeled mono-nucleosomes (2 nM) with 0, 20, 60 and 120 bp linker DNA, resolved by native PAGE. Bottom: Binding curves for mono-nucleosomes with different linker lengths.
(E) EMSA showing SWR1 binding to free DNA fragments of 10 to 100 bp. Bound (B) and free DNA resolved by native PAGE, and stained with SYBR Green 1. Concentration of 40, 50, 60 and 70 bp fragments in reaction were 2.3, 1.9, 1.5 and 1.6 nM respectively. Bottom: Binding curves.
(F) EMSA showing SWR1 binding to Cy5 labeled 40 bp DNA and mono-nucleosomes with 0 or 40 bp linker DNA (2 nM). Bottom: Binding curves for DNA and nucleosome with different linker lengths.
See also Figure S1.
SWR1 binds optimally to mono-nucleosomes with long linker
To evaluate whether both nucleosomes flanking the NFR are essential for preferential binding, we introduced SWR1 to an equimolar mixture of mono-nucleosomes bearing linkers of 0, 20, 60, and 120 bp at one end. Under competitive conditions, SWR1 binds preferentially to nucleosomes with 120 and 60 bp linkers and poorly to nucleosomes carrying 20 bp or 0 bp linkers (Fig 1D). These observations led us to question whether the length of nucleosome-free DNA is an important factor for SWR1 binding. We employed a commercial 10 bp DNA-step ladder of generic sequence and equivalent AT content (60%) (Fig S1F) to show that SWR1 binds more efficiently to long fragments, like any typical DNA binding factor, but displays atypical discrimination against DNAs smaller than 50 bp (Fig 1E).
Moreover, in a direct competition, SWR1 displays superior binding to a nucleosome core particle bearing a 40 bp linker compared to the core particle or to 40 bp DNA alone (Fig 1F). The results suggest that DNA geometries of the nucleosome core particle and extranucleosomal linker, together with linker length, create an optimal substrate for SWR1.
Functional consequences of preferential SWR1 recruitment
We next examined whether preferential binding of SWR1 leads to increased nucleosome remodeling, using a new version of the histone replacement assay (Luk et al., 2010; Mizuguchi et al., 2003). We treated an equimolar mixture of fluorescently labeled di-nucleosomes bearing long and short linkers with limiting SWR1 enzyme, ATP and triple FLAG-tagged H2A.Z3F/H2B dimer. After reaction, restriction enzyme (BamH1) digestion releases mono-nucleosomes, and EMSA resolves products of histone replacement. Mono-nucleosomes incorporated with one or two H2A.Z3F-H2B dimers display step-wise electrophoretic mobility shifts owing to the addition of one or two triple-FLAG tags (Fig 2A). SWR1 preferentially catalyzes H2A.Z replacement on long linker di-nucleosome substrates (~4-fold difference in a 20-minute reaction); similar results are obtained when dyes are reversed (Fig 2B).
Figure 2. SWR1 preferentially remodels nucleosomes with long linker DNA.
(A) Experimental design.
(B)Left Panel. Long (red) and short (green) linker di-nucleosomes (2 nM) were mixed with 1 nM SWR1, H2A.Z-3F/H2B (8 nM) and ATP (1 mM). Histone exchange was stopped at indicated times, and nucleosomes containing 0, 1 or 2 copies of H2A.Z-3F were resolved on native PAGE after BamH1 digestion. Bottom: H2A.Z incorporation curves. Right Panel. Dye-reversal of experiment on left.
(C) Cy5 labeled mono-nucleosomes (5 nM) with 20, 60 or 120 bp linker were mixed with Cy3 labeled mono-nucleosome (5 nM) with 20 bp linker in a histone exchange reaction, analyzed as in (B).
See also Figure S2.
We also investigated the functional consequences of preferential SWR1 binding. Equimolar amounts of mono-nucleosomes with linkers of 20, 60 and 120 bp at one end were incubated with limiting SWR1 enzyme, ATP and H2A.Z3F/H2B dimers (all DNAs labeled with Cy5). In comparison with internal control (Cy3 labeled, 20 bp linker mono-nucleosome) SWR1 mediates a 2.5-fold and 4-fold increase in H2A.Z incorporation for nucleosomes with 60 and 120 bp linkers respectively (Fig 2C). Mono-nucleosomes bearing 20 bp linkers on both ends rather than one end were better substrates for H2A.Z3F incorporation (Fig S2A). Importantly, the nucleosome core particle (without linker DNA) is a highly effective substrate under non-competitive conditions (Fig S2B). Hence, extranucleosomal DNA primarily facilitates SWR1 binding rather than enzyme activity.
DNA binding components of the SWR1 complex
We next examined which of the 14 hierarchically assembled components of SWR1 are critical for its preferential binding to long linker di-nucleosomes. Previous work indicated that the Swr1 subunit functions not only as the catalytic ATPase but also as a scaffold for assembly of the other components (Wu et al., 2005). Moreover, the Swr1 polypeptide can be essentially split into N- and C-terminal portions, each carrying a distinct subset of components. N-SWR1 (residues 1–681) assembles Swc4, Arp4, Yaf9, Act1, Swc7, and Bdf1, while C-SWR1 (residues 681–1513) assembles Arp6, Swc6, Swc2, Swc3, Rvb1 and Rvb2 (Fig 3A) (Wu et al., 2009). Only the Swc5 subunit requires the full-length Swr1 polypeptide for assembly. We compared binding of mutant SWR1 complexes after affinity- and glycerol gradient purification (Fig 3B). EMSA shows that C-SWR1, like WT enzyme, has a high preference for the long linker di-nucleosome, while N-SWR1 has little detectable binding. The bound C-SWR1 complex(es) display altered mobility, probably owing to mass or conformational changes (Fig 3C). We therefore exclude N-SWR1 with its five associated components from having a major role in NFR targeting.
Figure 3. Identification of subunits required for SWR1 binding to nucleosomes.
(A) Schematic representation of subunits interacting with N-domain of Swr1 (1–681) and C-domain of Swr1 protein (681–1513) (adapted from (Wu et al., 2009)). (The C-SWR1 sub-complex retains N-terminal Swr1 residues 1–277 and associated subunit Swc7).
(B) Purified complexes resolved on SDS-PAGE and stained with Coomassie Blue. Complexes were affinity-purified on anti-FLAG-beads followed by glycerol gradient centrifugation. SWR1 (swc2Δ)* was only affinity-purified. Note: 15 KDa Swc7 protein, which is present in all complexes, runs as a faint band (not shown) at the bottom of gel.
(C–D) EMSA showing WT and mutant SWR1 binding to long and short linker di-nucleosome (2 nM). Free (F) and SWR1 bound (B)
(E) Long linker di-nucleosome binding curves for WT and missing subunit mutants.
(F) EMSA showing SWR1 and mutant complexes binding to 10 bp DNA ladder. Bound (B) and (F) free DNA.
(G) EMSA showing WT and mutant SWR1 complexes binding to Alexa 647 labeled nucleosome core particle (2 nM). Bound (B) and free (F) core particle.
See also Figure S3.
The absence of Swc5 in the C-SWR1 sub-complex suggests that it is not important for nucleosome binding, and a mutant complex purified from the swc5Δ deletion strain (Fig S3A) (Wu et al., 2005) retains preferential binding to long linker di-nucleosomes, albeit with altered mobility shifts (Fig S3B). Thus, only the Swr1 ATPase domain and associated Arp6, Swc6, Swc2, Swc3, Rvb1, and Rvb2 components, individually or combined, are responsible for preferential binding.
Swc3 is neither required for the histone exchange reaction nor for chromatin binding (Wu et al., 2005). However, a strain deleted for the H2A.Z binding subunit Swc2, yields an affinity-purified complex deficient for Swc2 and Swc3 (Fig. 3B, SWR1(swc2Δ)*) (Wu et al., 2005), and this complex displays weak binding to long linker di-nucleosomes, suggesting that Swc2 is important for substrate binding (Fig 3D). But Arp6-Swc6 are also destabilized when Swc2 is deleted, becoming highly deficient on further gradient purification (Fig 3B, SWR1(swc2Δ)). This SWR1(swc2Δ) complex binds substrate slightly better than does SWR1(swc2Δ)*, and complete elimination of Arp6 in the SWR1(arp6Δ) sub-complex, in which Swc2, Swc6 and Swc3 are also eliminated (Wu et al., 2005), gives a clear complex with long linker di-nucleosome (Fig 3D, 3E). This indicates that there are additional binding component(s) unmasked by the loss of Swc2, Arp6, Swc6 and Swc3. The Swr1 ATPase domain itself is a likely candidate, not to exclude the Rvb1, Rvb2 helicases.
We then examined the mutant SWR1 complexes for binding free DNA. WT SWR1 and C-SWR1 have similar affinities for the DNA ladder (Fig 3F and S3D), whereas N-SWR1 is defective for DNA binding (Fig 3F). SWR1(swc2Δ) is also defective, and SWR1(arp6Δ) displays weak binding (Fig 3F). Moreover, N-SWR1 carrying Swc4, Arp4, Yaf9, Act1, Swc7, and Bdf1 shows little binding to the core particle, while C-SWR1 containing Swc2 binds strongly (Fig 3G). SWR1(arp6Δ) does show weak binding to the core particle, consistent with the unmasking of additional DNA binding component(s).
Swc2 is required for SWR1 recruitment in vivo
The role of the Swc2 subunit in binding DNA and nucleosomes prompted us to examine its function in SWR1 recruitment in vivo. Using formaldehyde cross-linking, chromatin immuno-precipitation (ChIP), we mapped Swr1 occupancies chromosome-wide. As shown previously (Venters and Pugh, 2009), SWR1 is enriched at nucleosomes +1 and −1 adjoining the TSS (Fig 4A). Strikingly, Swr1 binding is substantially diminished in swc2Δ cells, (Fig 4A, left), indicating that Swc2 is indeed required for SWR1 recruitment. These chromosome-wide effects confirm and extend observations on individual promoters (Fig S3E) (Morillo-Huesca et al., 2010). The convergence of biochemical and genetic findings indicates that Swc2 functions not only to bind H2A.Z (Wu et al., 2005), but also to target SWR1 to promoter-proximal nucleosomes.
Figure 4. Swc2 is required for SWR1 targeting in vivo and binds DNA directly.
(A) ChIP analysis of Swr1-TAP. DNA hybridization to a tiling microarray covering chromosome 3 and 6. The graphs show geometric mean of SWR1 signal for 226 genes ±1 Kb around TSS for WT and mutant strains. Data shown twice for WT (Left and Center) and htz1Δ (Center and Right).
(B) Map of Swc2 protein. Basic (blue) and acidic (red) amino acids. Grey bars mark evolutionarily conserved domains in Swc2.
(C) EMSA showing binding of bacterially expressed Swc2 segments to free DNA.
(D) Affinity-purified (*) and glycerol gradient purified SWR1 complexes resolved by SDS-PAGE and stained with Coomassie Blue.
(E–F) EMSA showing binding of WT and mutant SWR1 complexes to Alexa 647 labeled DNA and Cy5 labeled mono-nucleosome.
(G) ChIP-PCR analysis of Swr1-TAP binding to FUN12 and SWR1 promoters in WT and swc2 (136–345Δ) mutant cells. Tel: sub-telomeric sequence on chr 6. In mutant cells, reduced Swr1-TAP binding was also seen at RPL4A, SNT1 and NUP1 gene promoters (data not shown).
See also Figure S3 and S4.
Parenthetically, we observe enhanced site-specific Swr1 occupancy in H2A.Z-depleted (htz1Δ) cells for all genes (Fig 4A middle, Fig S3E), and this residency drops dramatically when both H2A.Z and Swc2 are depleted (htz1Δ & swc2Δ) (Fig 4A, right). This provides an explanation for the partial suppression of htz1Δ phenotypes by a second, swr1Δ mutation that dismantles the SWR1 complex (Halley et al., 2010; Morillo-Huesca et al., 2010). Under these circumstances, elimination of SWR1 evidently relieves interference with promoter function by the inactive, bound enzyme.
A conserved N-terminal region of Swc2 binds DNA
The conserved N-terminal residues 1–345 of Swc2 encompasses an acidic and basic domain; this region is known to recruit the H2A.Z-H2B dimer to the SWR1 complex (Wu et al., 2005). To examine whether Swc2 also binds DNA directly, we compared the DNA binding activity of bacterially expressed segments of Swc2. EMSA shows that N-terminal residues 1–345, and 136–345 display binding to the DNA step-ladder, while residues 1–135 and 346–795 have no detectable activity (Fig 4C). Drosophila Swc2/YL1 also shows DNA binding activity (Fig S4B). Our findings are consistent with an early report of DNA binding by YL1, the murine Swc2 (Horikawa et al., 1995), and K. Luger also found DNA binding by segments of Swc2 (personal communication). Deletion of the basic region, swc2(Δ136–345), gives a SWR1 complex depleted only for Swc2-Swc3 (Fig 4D), although as in swc2Δ, the Arp6-Swc6 subunits dissociate on gradient purification. Both purified forms of SWR1 bearing swc2(Δ136–345) show diminished binding to DNA and nucleosomes in vitro (Fig 4E, 4F; Fig S3C), and to promoters in vivo (Fig 4G).
SWR1 binds preferentially to acetylated di-nucleosomes
The NuA4 histone acetyltransferase (HAT) is genetically linked to SWR1 function (Krogan et al., 2004), physically localized to yeast promoters (Brown et al., 2001), and forms a super-complex with SWR1 in Drosophila and vertebrates (Cai et al., 2005; Kusch et al., 2004). NuA4 primarily acetylates histones H2A, H2A.Z and H4 (Keogh et al., 2006; Millar and Grunstein, 2006). We treated long linker di-nucleosomes with purified NuA4 enzyme, acetylating nucleosomes to saturation in vitro (Li et al., 2007) (Fig S5A, Fig S5B). EMSA shows that SWR1 has a clear preference for binding the acetylated, long linker di-nucleosome over non-acetylated substrate (~3-fold at 50% binding) (Fig 5A). In addition, H2A.Z incorporation is enhanced, consistent with an earlier report (Altaf et al., 2010); when SWR1 concentration (2 nM) is limiting, H2A.Z replacement increases ~3-fold in a 20 min reaction for the acetylated di-nucleosome (Fig 5B).
Figure 5. Enhanced SWR1 binding to acetylated di-nucleosomes requires DNA binding modules.
(A) EMSA showing SWR1 binding to NuA4 acetylated (green) or non-acetylated (red) long linker dinucleosomes (2 nM). Bound (B) and free (F) di-nucleosomes. Bottom: Binding curves. Right: Control for experiment in left, using Cy3- and Cy5-labeled di-nucleosomes.
(B) Acetylated (Cy5) and non-acetylated (Cy3) long linker di-nucleosomes (2nM) were mixed with 2 nM SWR1, H2A.Z-3F/H2B and ATP, and analyzed as in Fig 2B. Bottom: H2A.Z incorporation curves.
(C) EMSA showing WT and mutant SWR1 complexes (15 nM) binding to acetylated (green) or non-acetylated (red) long linker di-nucleosomes (2 nM). Bound (B) and free (F) di-nucleosomes Right: Dye control of experiment on left.
See also Figure S5.
The Bdf1 subunit of SWR1 contains two bromodomains that bind acetylated histone H4 peptides (Ladurner et al., 2003). Notably, in the absence of DNA binding components Swc2 and the Swr1 ATPase domain, Bdf1-containing N-SWR1 binds poorly to acetylated di-nucleosomes, even when the mutant SWR1 concentration (15 nM) is elevated several-fold above standard conditions, and similar results obtain for the SWR1(swc2Δ) complex (Fig 5C). However, weak interactions can be observed, as judged by modest depletion of acetylated, unbound substrate, consistent with the low affinity of Bdf1 for acetylated H4. By contrast, C-SWR1 containing DNA binding components, but not Bdf1, binds strongly to acetylated and unacetylated substrates when the sub-complex is either saturating (Fig 5C), or limiting (Fig. S5C). We conclude that nucleosome-free and nucleosomal DNA binding is the dominant recruitment mechanism for SWR1.
Genome-wide SWR1 enrichment in vivo correlates with NFR length
Our biochemical studies suggest a mechanism in which targeting of the SWR1 complex to promoters is primarily caused by the preferential binding of enzyme to an NFR of 50 bp length or greater. Although there have been extensive genome-wide mapping of NFRs and of SWR1 enrichment at promoters (Radman-Livaja and Rando, 2010; Venters and Pugh, 2009; Zhang et al., 2005), the relationship between these two features was not examined.
We aligned 4270 budding yeast genes at the TSS and arranged them in rank order on the y-axis according to increasing NFR length, as defined by the gap between −1 and +1 nucleosomes (Cole et al., 2011) (Fig 6). We then plotted the corresponding occupancy of SWR1 from data obtained by genome-wide ChIP (Cole et al., 2011; Venters and Pugh, 2009; Xu et al., 2009), (Fig 6). As shown previously, most genes possess a distinct NFR in the promoter region, and well-positioned +1 and −1 nucleosomes flanking the NFR (Fig 6). In addition, SWR1 is generally enriched at locations particularly overlapping nucleosome +1 and to some extent −1 (Venters and Pugh, 2009). Of first importance, little or no SWR1 enrichment occurs near the TSS for a small number of genes whose NFRs are less than 50 bp, consistent with our biochemical findings (Fig 6). This subset of the genome is represented by a group of repressed meiotic genes and inducible genes like GAL1, PHO5 and CLN2 that are known to have NFRs further upstream of the TSS, where there is a corresponding increase of SWR1 occupancy. Second, we find a progressive increase of SWR1 occupancy over promoter nucleosomes as NFR length increases from 50 bp to 200 bp. The majority of the 4270 genes examined thus show a positive correlation between NFR length and SWR1 enrichment, notwithstanding differences in transcription factor binding.
Figure 6. Enrichment of SWR1 and other factors at gene promoters with increasing NFR length.
4269 budding yeast genes were aligned by TSS and ordered for length of NFR between −1 and +1 nucleosomes. Indicated NFR sizes (left-end) and distance from TSS (bottom of each panel) are in base pairs. Ribosomal genes shown in blue. Transcription frequency of genes, in log 2 scale, is shown from low (red) to high (green) (Holstege et al., 1998). In `fragile nucleosomes' heat map showing log 2 ratio of complete/partial MNase digestion, the nucleosomes depleted during MNase complete digestion are shown in green. Averaged enrichment for each factor around TSS is shown as a plot below the heat maps. Box Plot. Genes were binned for NFR sizes in increments of 50 bp and plotted for SWR1 enrichment within the highlighted (cyan) peak region. Boxes represent the interquartile ranges (IQR) and whiskers represent minima and maxima.
See also Figure S6 and S7.
For genes possessing extended NFRs greater than 200 bp, SWR1 occupancy is reduced. However, standard nucleosome mapping by vigorous MNase digestion eliminates a population of `fragile nucleosomes' during sample preparation (Weiner et al., 2010; Xi et al., 2011). When the dataset for these fragile nucleosomes (isolated by limited MNase cleavage (Xi et al., 2011)) is included, they are well represented within NFRs greater than 200 bp (Fig 6). The reduced SWR1 occupancy for these extended NFRs could therefore be a consequence of less available DNA than is apparent, due to fragile nucleosomes or to other factors (see below).
We extended the in silico comparison to other remodeling enzymes and transcription factors (Venters and Pugh, 2009). By contrast with SWR1, we find little or no correlation between NFR size and the genomic occupancies of the RSC chromatin remodeler (Fig 6). This is likely due in part to the sequence-specific DNA-binding properties of several RSC components (Angus-Hill et al., 2001; Badis et al., 2008; Cairns et al., 1999; Da et al., 2006), which would override NFR size. Interestingly, the enrichment of general transcription factor SuA7/TFIIB, a component of the transcription pre-initiation complex (PIC), is positively associated with NFR length up to and beyond 200 bp (Fig 6), and a similar correlation is found for TBP, another component of the PIC complex (data not shown). Unlike SWR1, the enrichment of SuA7/TFIIB and TBP occurs over the NFR itself rather than the adjoining nucleosome, suggesting that the length of accessible DNA is one important determinant for recognition by the PIC complex.
Discussion
We have elucidated the recruitment mechanism for the SWR1 chromatin remodeling complex. An NFR and adjoining nucleosome core particle have a central and direct role in the ATP-independent recruitment of SWR1 in vitro, with binding affinity in the nanomolar range. However, while the NFR is critical for the targeting of SWR1, it is not required for post-recruitment enzyme activity, which requires binding of the H2A.Z-H2B dimer.
SWR1 exhibits a preference for mono-nucleosomes bearing a long linker. Electron microscopy shows that the complex localizes over the core particle and adjacent DNA on a di-nucleosome substrate. Published in vivo ChIP data also map the enzyme to −1 or +1 nucleosomes flanking the NFR, but not centered on it (Venters and Pugh, 2009) (Fig 6). Thus, DNA geometries of nucleosome core particle and long linker together provide an optimal substrate for SWR1, allowing for discrimination of yeast promoters over gene bodies. Our biochemical findings are fully consistent with genetic studies showing that an NFR programmed in the middle of the inactive PRM1 gene - by insertion of a 22- or 14-bp SNT1 promoter fragment carrying a Reb1 site and poly dA-dT stretch - expands a pre-existing 33 bp linker to 173 bp by the action of recruited RSC, thereby enriching H2A.Z in flanking nucleosomes (Hartley and Madhani, 2009; Raisner et al., 2005).
Amongst the 14 distinct components of SWR1, the evolutionarily conserved Swc2/YL-1 subunit is important for preferential recognition of the NFR in vitro and in vivo. The interaction between Swc2 and DNA is apparently direct, as evinced by the activity of bacterially expressed Swc2. However, closely associating components Swc6 and Arp6 may also contribute, either directly, or more likely indirectly, by presenting Swc2 in macromolecular context as the Arp6-Swc6 module tends to dissociate from complex when Swc2, full length or residues 136–345, are deleted. Moreover, the Swr1 ATPase domain itself (and/or the Rvb components) also appear to be important for DNA binding, because activity can be revealed by complete depletion of Swc2-Arp6-Swc6 in an arp6Δ mutant. We suggest that the spatial organization of DNA binding modules and steric hindrance by non-DNA binding components together restrict SWR1 targeting to gene promoter than to gene body, where linker DNAs are substantially smaller (~20 bp for budding yeast (Radman-Livaja and Rando, 2010)). This discrimination against the gene body is vividly illustrated when genome-wide occupancies of SWR1 are presented in order of gene length (Fig S7).
SWR1 is enriched at 5' but not 3' gene termini when the highly compact yeast genome is subdivided into conventionally defined tandem or convergent genes, notwithstanding the genome-wide landscape of noncoding RNAs at yeast promoters (Neil et al., 2009; Xu et al., 2009). The absence of SWR1 at the 3' end of convergent genes is unequivocal (Fig S7). Consistent with this, an intrinsic NFR is not evident at the 3' end of budding yeast genes, where histone occupancy remains substantial (Fan et al., 2010). The enrichment of SWR1 or H2A.Z at budding yeast promoters agrees well with a similar enrichment at metazoan promoters, enhancers, and insulators (Barski et al., 2007; Thurman et al., 2012), which commonly display nucleosome-depleted DNase hypersensitive sites, individually and genome-wide (Thurman et al., 2012; Wu, 1980). Thus, the NFR may have a role in the recruitment of metazoan SWR1 enzymes for H2A.Z deposition. Furthermore, the strikingly positive correlation between NFR size and occupancy of PIC components suggests that the length of nucleosome-free, accessible DNA also has a prominent role in regulating promoter occupancy by the yeast core transcriptional machinery, in addition to its recruitment by sequence-specific transcription factors and histone modifications.
For a large number of yeast promoters, SWR1 localizes over one of two nucleosomes flanking the NFR, indicating competition between nucleosome +1 and −1 for limiting enzyme. Where the gene upstream is in tandem orientation, SWR1 clearly has preference for nucleosome +1 rather than nucleosome −1 (Fig S6). The biochemical basis of this preference is not clear, but may be related to differences in histone acetylation, adding directionality to SWR1 targeting.
At present, there is no indication that SWR1 is directly recruited by sequence-specific transcription factors in budding yeast, although in human cells, a physical interaction occurs between the p53 family member ΔNp63α and SRCAP, the mammalian SWR1 homolog (Gallant-Behm et al., 2012). Acetylation of nucleosomal histones by NuA4, which modifies H4 residues K5, 8, 12, 16 and H2A K7, has a direct influence on the recruitment of SWR1. But DNA binding is dominant over acetyl-histone binding in vitro, as the affinity of SWR1 for acetylated nucleosome is unable to compensate for the drastically reduced binding of N-SWR1, where Swc2 and the Swr1 ATPase domain are absent from the complex. Thus, there is a striking hierarchical aspect to the recruitment process, with histone acetylation participating effectively only in the context of an adjacent NFR. Such a hierarchical relationship is consistent with the little change in SWR1 occupancy and H2A.Z incorporation at budding yeast promoters in a bdf1Δ mutant (Durant and Pugh, 2007; Raisner et al., 2005; Zhang et al., 2005), or in a strain in which H4 tail lysines were replaced by non-acetylatable arginine (Zhang et al., 2005).
Hierarchical cooperation between DNA and histone signals
Our findings indicate that the mechanism of SWR1 targeting to yeast promoters is dependent on hierarchical cooperation between DNA and acetyl-histone recognition (Fig 7). Recruitment of limiting enzyme is primarily regulated by the nanomolar DNA-binding affinity of SWR1 and the 50 bp minimal preferred length of the NFR - the probability of recruitment rising with increasing NFR length above 50 bp. Effects of DNA composition on SWR1 binding are not excluded. Once bound, SWR1 at high local concentration diffuses to engage nucleosome −1 or +1, utilizing the micromolar affinity of Bdf1 bromodomains for acetylated histones to fine-tune enzyme recruitment and possibly provide directionality. Implicit in this model is that SWR1 binding and histone H2A.Z deposition requires the prior establishment of NFRs (and of histone acetylation).
Figure 7. Model for SWR1 recruitment at promoters.
High affinity DNA binding components of SWR1 allows the complex to preferentially bind promoter regions containing NFR > 50 bp. Nucleosomes flanking the NFR provide additional affinities for SWR1. Low affinity SWR1 binding to acetylated histones may direct binding to +1 nucleosome over −1 nucleosome.
In principle, hierarchical cooperation within chromatin-based regulators between high affinity nucleic acid binding modules physically linked to low affinity histone modification-binding (or histone-binding) modules expands the dynamic range for local recruitment by increasing affinity up to several-fold. For example, Drosophila and mammalian NURF301/BPTF, a component of the ISWI-type ATP-dependent nucleosome remodeling complex NURF has multiple domains that bind sequence-specific transcription factors, nucleosomes and DNA, all likely contributing to high affinity recruitment (Alkhatib and Landry, 2011; Xiao et al., 2001). In addition, NURF301/BPTF has multiple bromodomains and PHD fingers that bind acetyl-lysine and methyl-lysine histone marks (Ruthenburg et al., 2011; Wysocka et al., 2006). A NURF301 mutant deleted for bromodomains and two PHD fingers shows no effect on viability or expression of many NURF-dependent genes in the larva and adult fly, but these modules are indispensible for spermatogenesis (Kwon et al., 2009). Thus, hierarchical cooperation of DNA-and histone modification-binding modules could underpin NURF's role in activating and repressing specific groups of genes at different developmental stages (Kwon et al., 2009; Landry et al., 2011). Likewise, the SAGA histone acetyltransferase complex is recruited through interaction of the Tra1 subunit with sequence-specific factors and further stabilized by interactions with acetyl-and methyl-histone marks (Weake and Workman, 2012). Consistent with recent perspectives on the role of DNA recognition by gene regulators (Ptashne, 2013; Rando, 2012), an appreciation of the hierarchical nature of chromatin-based recruitment unites classical mechanisms of gene control by DNA-binding transcription factors with ATP-dependent nucleosome remodeling and post-translational histone modifications.
Experimental Procedures
Reconstitution of nucleosomes
Nucleosomes were reconstituted by mixing recombinant histone proteins and Widom 601 DNA, followed by salt gradient dialysis. Excess Lambda DNA was included in reconstitution mix to remove excess histones and allow efficient reconstitution of nucleosomes with long linker DNA. Reconstituted nucleosomes were purified by sucrose gradient sedimentation.
EMSA for SWR1 interaction with various substrates
Purified SWR1 and nucleosome/DNA were mixed and incubated at RT for 15 min. 10% Sucrose was added for loading. Free and SWR1-bound substrates were resolved on agarose gel (for di-nucleosome) or native PAGE (for mono-nucleosome and DNA fragments). All gels were run in 0.2 × TB (18 mM Tris, 18 mM Boric acid) at RT.
SWR1 mediated histone exchange assay
Histone exchange assay (Luk et al., 2010; Mizuguchi et al., 2003) was modified for fluorescently labeled nucleosomes (see supplement).
ChIP and microarrays
ChIP follows (Venters and Pugh, 2009). Briefly, cells were grown in YPD at 25° C to A600 =0.8 and fixed with 1% formaldehyde. Chromatin was sheared by sonication and immunoprecipitated with IgG sepharose beads. DNA was purified PCR amplified by LM-PCR and fluorescent labeled as described (Luk et al., 2010). The Agilent 4×180K tiling microarray covered chromosomes 3 and 6 at 10 bp resolution. The GEO accession number for microarray data is GSE49183.
In vitro nucleosome acetylation using purified HATs
Nucleosome acetylation follows Li et al (2007). Briefly, long linker di-nucleosomes were incubated with purified NuA4 and Acetyl CoA (50 μM) in acetylation buffer (50 mM Tris pH 8.0, 50 mM NaCl, 1 mM EDTA, 1 mM MgCl2, 1 mM DTT, 0.1 mg/ml BSA) for 2 hours at 30° C. Acetylated di-nucleosomes were further purified by sucrose gradient sedimentation.
Supplementary Material
Paper highlights
SWR1 recognizes universal nucleosome free architecture at gene promoters
DNA binding is dominant over histone acetylation for SWR1 targeting
Swc2 subunit is responsible for DNA recognition in addition to H2A.Z binding
NFR length correlates with SWR1 occupancy in vivo
Acknowledgements
We thank Dr. Joseph Wall, STEM facility, Biology Department, Brookhaven Laboratories, Upton, NY and his staff for conducting STEM mass measurements, Karolin Luger's laboratory for sharing unpublished data on Swc2, Sameet Mehta for bioinformatics, and Michael Lichten, Sean Eddy, members of our laboratory, and reviewers for helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert I, Mavrich TN, Tomsho LP, Qi J, Zanton SJ, Schuster SC, Pugh BF. Translational and rotational settings of H2A.Z nucleosomes across the Saccharomyces cerevisiae genome. Nature. 2007;446:572–576. doi: 10.1038/nature05632. [DOI] [PubMed] [Google Scholar]
- Alkhatib SG, Landry JW. The nucleosome remodeling factor. FEBS Lett. 2011;585:3197–3207. doi: 10.1016/j.febslet.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altaf M, Auger A, Monnet-Saksouk J, Brodeur J, Piquet S, Cramet M, Bouchard N, Lacoste N, Utley RT, Gaudreau L, et al. NuA4-dependent acetylation of nucleosomal histones H4 and H2A directly stimulates incorporation of H2A.Z by the SWR1 complex. J Biol Chem. 2010;285:15966–15977. doi: 10.1074/jbc.M110.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus-Hill ML, Schlichter A, Roberts D, Erdjument-Bromage H, Tempst P, Cairns BR. A Rsc3/Rsc30 zinc cluster dimer reveals novel roles for the chromatin remodeler RSC in gene expression and cell cycle control. Mol Cell. 2001;7:741–751. doi: 10.1016/s1097-2765(01)00219-2. [DOI] [PubMed] [Google Scholar]
- Arents G, Burlingame RW, Wang BC, Love WE, Moudrianakis EN. The nucleosomal core histone octamer at 3.1 A resolution: a tripartite protein assembly and a left-handed superhelix. Proc Natl Acad Sci U S A. 1991;88:10148–10152. doi: 10.1073/pnas.88.22.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badis G, Chan ET, van Bakel H, Pena-Castillo L, Tillo D, Tsui K, Carlson CD, Gossett AJ, Hasinoff MJ, Warren CL, et al. A library of yeast transcription factor motifs reveals a widespread function for Rsc3 in targeting nucleosome exclusion at promoters. Mol Cell. 2008;32:878–887. doi: 10.1016/j.molcel.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Liu CL, Humphrey EL, Perlstein EO, Schreiber SL. Global nucleosome occupancy in yeast. Genome Biol. 2004;5:R62. doi: 10.1186/gb-2004-5-9-r62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CE, Howe L, Sousa K, Alley SC, Carrozza MJ, Tan S, Workman JL. Recruitment of HAT complexes by direct activator interactions with the ATM-related Tra1 subunit. Science. 2001;292:2333–2337. doi: 10.1126/science.1060214. [DOI] [PubMed] [Google Scholar]
- Cai Y, Jin J, Florens L, Swanson SK, Kusch T, Li B, Workman JL, Washburn MP, Conaway RC, Conaway JW. The mammalian YL1 protein is a shared subunit of the TRRAP/TIP60 histone acetyltransferase and SRCAP complexes. J Biol Chem. 2005;280:13665–13670. doi: 10.1074/jbc.M500001200. [DOI] [PubMed] [Google Scholar]
- Cairns BR, Schlichter A, Erdjument-Bromage H, Tempst P, Kornberg RD, Winston F. Two functionally distinct forms of the RSC nucleosome-remodeling complex, containing essential AT hook, BAH, and bromodomains. Mol Cell. 1999;4:715–723. doi: 10.1016/s1097-2765(00)80382-2. [DOI] [PubMed] [Google Scholar]
- Cole HA, Howard BH, Clark DJ. The centromeric nucleosome of budding yeast is perfectly positioned and covers the entire centromere. Proc Natl Acad Sci U S A. 2011;108:12687–12692. doi: 10.1073/pnas.1104978108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da G, Lenkart J, Zhao K, Shiekhattar R, Cairns BR, Marmorstein R. Structure and function of the SWIRM domain, a conserved protein module found in chromatin regulatory complexes. Proc Natl Acad Sci U S A. 2006;103:2057–2062. doi: 10.1073/pnas.0510949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon N, Oki M, Szyjka SJ, Aparicio OM, Kamakaka RT. H2A.Z functions to regulate progression through the cell cycle. Mol Cell Biol. 2006;26:489–501. doi: 10.1128/MCB.26.2.489-501.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant M, Pugh BF. NuA4-directed chromatin transactions throughout the Saccharomyces cerevisiae genome. Mol Cell Biol. 2007;27:5327–5335. doi: 10.1128/MCB.00468-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JY, Gordon F, Luger K, Hansen JC, Tremethick DJ. The essential histone variant H2A.Z regulates the equilibrium between different chromatin conformational states. Nat Struct Biol. 2002;9:172–176. doi: 10.1038/nsb767. [DOI] [PubMed] [Google Scholar]
- Fan X, Moqtaderi Z, Jin Y, Zhang Y, Liu XS, Struhl K. Nucleosome depletion at yeast terminators is not intrinsic and can occur by a transcriptional mechanism linked to 3'-end formation. Proc Natl Acad Sci U S A. 2010;107:17945–17950. doi: 10.1073/pnas.1012674107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant-Behm CL, Ramsey MR, Bensard CL, Nojek I, Tran J, Liu M, Ellisen LW, Espinosa JM. {Delta}Np63alpha represses anti-proliferative genes via H2A.Z deposition. Genes Dev. 2012;26:2325–2336. doi: 10.1101/gad.198069.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin AC, Aloy P, Grandi P, Krause R, Boesche M, Marzioch M, Rau C, Jensen LJ, Bastuck S, Dumpelfeld B, et al. Proteome survey reveals modularity of the yeast cell machinery. Nature. 2006;440:631–636. doi: 10.1038/nature04532. [DOI] [PubMed] [Google Scholar]
- Halley JE, Kaplan T, Wang AY, Kobor MS, Rine J. Roles for H2A.Z and its acetylation in GAL1 transcription and gene induction, but not GAL1-transcriptional memory. PLoS Biol. 2010;8:e1000401. doi: 10.1371/journal.pbio.1000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley PD, Madhani HD. Mechanisms that specify promoter nucleosome location and identity. Cell. 2009;137:445–458. doi: 10.1016/j.cell.2009.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege FC, Jennings EG, Wyrick JJ, Lee TI, Hengartner CJ, Green MR, Golub TR, Lander ES, Young RA. Dissecting the regulatory circuitry of a eukaryotic genome. Cell. 1998;95:717–728. doi: 10.1016/s0092-8674(00)81641-4. [DOI] [PubMed] [Google Scholar]
- Horikawa I, Tanaka H, Yuasa Y, Suzuki M, Oshimura M. Molecular cloning of a novel human cDNA on chromosome 1q21 and its mouse homolog encoding a nuclear protein with DNA-binding ability. Biochem Biophys Res Commun. 1995;208:999–1007. doi: 10.1006/bbrc.1995.1433. [DOI] [PubMed] [Google Scholar]
- Jiang C, Pugh BF. A compiled and systematic reference map of nucleosome positions across the Saccharomyces cerevisiae genome. Genome Biol. 2009;10:R109. doi: 10.1186/gb-2009-10-10-r109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Felsenfeld G. Nucleosome stability mediated by histone variants H3.3 and H2A.Z. Genes Dev. 2007;21:1519–1529. doi: 10.1101/gad.1547707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalocsay M, Hiller NJ, Jentsch S. Chromosome-wide Rad51 spreading and SUMO-H2A.Z-dependent chromosome fixation in response to a persistent DNA double-strand break. Mol Cell. 2009;33:335–343. doi: 10.1016/j.molcel.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Keogh MC, Mennella TA, Sawa C, Berthelet S, Krogan NJ, Wolek A, Podolny V, Carpenter LR, Greenblatt JF, Baetz K, et al. The Saccharomyces cerevisiae histone H2A variant Htz1 is acetylated by NuA4. Genes Dev. 2006;20:660–665. doi: 10.1101/gad.1388106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobor MS, Venkatasubrahmanyam S, Meneghini MD, Gin JW, Jennings JL, Link AJ, Madhani HD, Rine J. A protein complex containing the conserved Swi2/Snf2-related ATPase Swr1p deposits histone variant H2A.Z into euchromatin. PLoS Biol. 2004;2:E131. doi: 10.1371/journal.pbio.0020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan NJ, Baetz K, Keogh MC, Datta N, Sawa C, Kwok TC, Thompson NJ, Davey MG, Pootoolal J, Hughes TR, et al. Regulation of chromosome stability by the histone H2A variant Htz1, the Swr1 chromatin remodeling complex, and the histone acetyltransferase NuA4. Proc Natl Acad Sci U S A. 2004;101:13513–13518. doi: 10.1073/pnas.0405753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan NJ, Keogh MC, Datta N, Sawa C, Ryan OW, Ding H, Haw RA, Pootoolal J, Tong A, Canadien V, et al. A Snf2 family ATPase complex required for recruitment of the histone H2A variant Htz1. Mol Cell. 2003;12:1565–1576. doi: 10.1016/s1097-2765(03)00497-0. [DOI] [PubMed] [Google Scholar]
- Kusch T, Florens L, Macdonald WH, Swanson SK, Glaser RL, Yates JR, 3rd, Abmayr SM, Washburn MP, Workman JL. Acetylation by Tip60 is required for selective histone variant exchange at DNA lesions. Science. 2004;306:2084–2087. doi: 10.1126/science.1103455. [DOI] [PubMed] [Google Scholar]
- Kwon SY, Xiao H, Wu C, Badenhorst P. Alternative splicing of NURF301 generates distinct NURF chromatin remodeling complexes with altered modified histone binding specificities. PLoS Genet. 2009;5:e1000574. doi: 10.1371/journal.pgen.1000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladurner AG, Inouye C, Jain R, Tjian R. Bromodomains mediate an acetyl-histone encoded antisilencing function at heterochromatin boundaries. Mol Cell. 2003;11:365–376. doi: 10.1016/s1097-2765(03)00035-2. [DOI] [PubMed] [Google Scholar]
- Landry JW, Banerjee S, Taylor B, Aplan PD, Singer A, Wu C. Chromatin remodeling complex NURF regulates thymocyte maturation. Genes Dev. 2011;25:275–286. doi: 10.1101/gad.2007311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Gogol M, Carey M, Lee D, Seidel C, Workman JL. Combined action of PHD and chromo domains directs the Rpd3S HDAC to transcribed chromatin. Science. 2007;316:1050–1054. doi: 10.1126/science.1139004. [DOI] [PubMed] [Google Scholar]
- Lowary PT, Widom J. New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J Mol Biol. 1998;276:19–42. doi: 10.1006/jmbi.1997.1494. [DOI] [PubMed] [Google Scholar]
- Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- Luk E, Ranjan A, Fitzgerald PC, Mizuguchi G, Huang Y, Wei D, Wu C. Stepwise histone replacement by SWR1 requires dual activation with histone H2A.Z and canonical nucleosome. Cell. 2010;143:725–736. doi: 10.1016/j.cell.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matangkasombut O, Buratowski S. Different sensitivities of bromodomain factors 1 and 2 to histone H4 acetylation. Mol Cell. 2003;11:353–363. doi: 10.1016/s1097-2765(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Mavrich TN, Ioshikhes IP, Venters BJ, Jiang C, Tomsho LP, Qi J, Schuster SC, Albert I, Pugh BF. A barrier nucleosome model for statistical positioning of nucleosomes throughout the yeast genome. Genome Res. 2008a;18:1073–1083. doi: 10.1101/gr.078261.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrich TN, Jiang C, Ioshikhes IP, Li X, Venters BJ, Zanton SJ, Tomsho LP, Qi J, Glaser RL, Schuster SC, et al. Nucleosome organization in the Drosophila genome. Nature. 2008b;453:358–362. doi: 10.1038/nature06929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneghini MD, Wu M, Madhani HD. Conserved histone variant H2A.Z protects euchromatin from the ectopic spread of silent heterochromatin. Cell. 2003;112:725–736. doi: 10.1016/s0092-8674(03)00123-5. [DOI] [PubMed] [Google Scholar]
- Millar CB, Grunstein M. Genome-wide patterns of histone modifications in yeast. Nat Rev Mol Cell Biol. 2006;7:657–666. doi: 10.1038/nrm1986. [DOI] [PubMed] [Google Scholar]
- Mizuguchi G, Shen X, Landry J, Wu WH, Sen S, Wu C. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science. 2003;303:343–348. doi: 10.1126/science.1090701. [DOI] [PubMed] [Google Scholar]
- Morillo-Huesca M, Clemente-Ruiz M, Andujar E, Prado F. The SWR1 histone replacement complex causes genetic instability and genome-wide transcription misregulation in the absence of H2A.Z. PLoS One. 2010;5:e12143. doi: 10.1371/journal.pone.0012143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil H, Malabat C, d'Aubenton-Carafa Y, Xu Z, Steinmetz LM, Jacquier A. Widespread bidirectional promoters are the major source of cryptic transcripts in yeast. Nature. 2009;457:1038–1042. doi: 10.1038/nature07747. [DOI] [PubMed] [Google Scholar]
- Neph S, Vierstra J, Stergachis AB, Reynolds AP, Haugen E, Vernot B, Thurman RE, John S, Sandstrom R, Johnson AK, et al. An expansive human regulatory lexicon encoded in transcription factor footprints. Nature. 2012;489:83–90. doi: 10.1038/nature11212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. Epigenetics: Core misconcept. Proc Natl Acad Sci U S A. 2013;110:7101–7103. doi: 10.1073/pnas.1305399110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman-Livaja M, Rando OJ. Nucleosome positioning: how is it established, and why does it matter? Dev Biol. 2010;339:258–266. doi: 10.1016/j.ydbio.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisner RM, Hartley PD, Meneghini MD, Bao MZ, Liu CL, Schreiber SL, Rando OJ, Madhani HD. Histone variant H2A.Z marks the 5' ends of both active and inactive genes in euchromatin. Cell. 2005;123:233–248. doi: 10.1016/j.cell.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando OJ. Combinatorial complexity in chromatin structure and function: revisiting the histone code. Curr Opin Genet Dev. 2012;22:148–155. doi: 10.1016/j.gde.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy D, Greaves I, Tremethick DJ. RNA interference demonstrates a novel role for H2A.Z in chromosome segregation. Nat Struct Mol Biol. 2004;11:650–655. doi: 10.1038/nsmb786. [DOI] [PubMed] [Google Scholar]
- Ruthenburg AJ, Li H, Milne TA, Dewell S, McGinty RK, Yuen M, Ueberheide B, Dou Y, Muir TW, Patel DJ, et al. Recognition of a mononucleosomal histone modification pattern by BPTF via multivalent interactions. Cell. 2011;145:692–706. doi: 10.1016/j.cell.2011.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santisteban MS, Kalashnikova T, Smith MM. Histone H2A.Z regulats transcription and is partially redundant with nucleosome remodeling complexes. Cell. 2000;103:411–422. doi: 10.1016/s0092-8674(00)00133-1. [DOI] [PubMed] [Google Scholar]
- Schones DE, Cui K, Cuddapah S, Roh TY, Barski A, Wang Z, Wei G, Zhao K. Dynamic regulation of nucleosome positioning in the human genome. Cell. 2008;132:887–898. doi: 10.1016/j.cell.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto RK, Clarkson MJ, Tremethick DJ, Luger K. Crystal structure of a nucleosome core particle containing the variant histone H2A.Z. Nat Struct Biol. 2000;7:1121–1124. doi: 10.1038/81971. [DOI] [PubMed] [Google Scholar]
- Thurman RE, Rynes E, Humbert R, Vierstra J, Maurano MT, Haugen E, Sheffield NC, Stergachis AB, Wang H, Vernot B, et al. The accessible chromatin landscape of the human genome. Nature. 2012;489:75–82. doi: 10.1038/nature11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirosh I, Barkai N. Two strategies for gene regulation by promoter nucleosomes. Genome Res. 2008;18:1084–1091. doi: 10.1101/gr.076059.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venters BJ, Pugh BF. A canonical promoter organization of the transcription machinery and its regulators in the Saccharomyces genome. Genome Res. 2009;19:360–371. doi: 10.1101/gr.084970.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weake VM, Workman JL. SAGA function in tissue-specific gene expression. Trends Cell Biol. 2012;22:177–184. doi: 10.1016/j.tcb.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A, Hughes A, Yassour M, Rando OJ, Friedman N. High-resolution nucleosome mapping reveals transcription-dependent promoter packaging. Genome Res. 2010;20:90–100. doi: 10.1101/gr.098509.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980;286:854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]
- Wu C. Two protein-binding sites in chromatin implicated in the activation of heat-shock genes. Nature. 1984;309:229–234. doi: 10.1038/309229a0. [DOI] [PubMed] [Google Scholar]
- Wu WH, Alami S, Luk E, Wu CH, Sen S, Mizuguchi G, Wei D, Wu C. Swc2 is a widely conserved H2AZ-binding module essential for ATP-dependent histone exchange. Nat Struct Mol Biol. 2005;12:1064–1071. doi: 10.1038/nsmb1023. [DOI] [PubMed] [Google Scholar]
- Wu WH, Wu CH, Ladurner A, Mizuguchi G, Wei D, Xiao H, Luk E, Ranjan A, Wu C. N terminus of Swr1 binds to histone H2AZ and provides a platform for subunit assembly in the chromatin remodeling complex. J Biol Chem. 2009;284:6200–6207. doi: 10.1074/jbc.M808830200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocka J, Swigut T, Xiao H, Milne TA, Kwon SY, Landry J, Kauer M, Tackett AJ, Chait BT, Badenhorst P, et al. A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature. 2006;442:86–90. doi: 10.1038/nature04815. [DOI] [PubMed] [Google Scholar]
- Xi Y, Yao J, Chen R, Li W, He X. Nucleosome fragility reveals novel functional states of chromatin and poises genes for activation. Genome Res. 2011;21:718–724. doi: 10.1101/gr.117101.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Sandaltzopoulos R, Wang HM, Hamiche A, Ranallo R, Lee KM, Fu D, Wu C. Dual functions of largest NURF subunit NURF301 in nucleosome sliding and transcription factor interactions. Mol Cell. 2001;8:531–543. doi: 10.1016/s1097-2765(01)00345-8. [DOI] [PubMed] [Google Scholar]
- Xu Z, Wei W, Gagneur J, Perocchi F, Clauder-Munster S, Camblong J, Guffanti E, Stutz F, Huber W, Steinmetz LM. Bidirectional promoters generate pervasive transcription in yeast. Nature. 2009;457:1033–1037. doi: 10.1038/nature07728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan GC, Liu YJ, Dion MF, Slack MD, Wu LF, Altschuler SJ, Rando OJ. Genome-scale identification of nucleosome positions in S. cerevisiae. Science. 2005;309:626–630. doi: 10.1126/science.1112178. [DOI] [PubMed] [Google Scholar]
- Zhang H, Roberts DN, Cairns BR. Genome-wide dynamics of Htz1, a histone H2A variant that poises repressed/basal promoters for activation through histone loss. Cell. 2005;123:219–231. doi: 10.1016/j.cell.2005.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.