Abstract
How does prior experience with action change how we perceive a similar action performed by someone else? Previous research has examined the role of sensorimotor and visual experiences in action mirroring during subsequent observation, but the contribution of somatosensory experiences to this effect has not been adequately examined. The current study tests whether prior somatosensory stimulation experienced during action production modulates brain activity during observation of similar actions being performed by others. Specifically, changes in alpha- and beta-range oscillations in the electroencephalogram (EEG) during observation of reaching actions were examined in relation to the observer’s own prior experience of somatosensory stimulation while carrying out similar actions. Analyses revealed that alpha power over central electrodes was significantly decreased during observation of an action expected to result in somatosensory stimulation. Conversely, beta power was increased when an observed action was expected to result in somatosensory stimulation. These results suggest that somatosensory experiences may uniquely contribute to the way in which we process others people’s actions.
Keywords: alpha, beta, EEG, somatosensory, experience
1. Introduction
1.1 Action observation
A great deal of evidence shows that when we see other people acting, our own sensorimotor systems are activated—a phenomenon that has been suggested to play a role in our understanding and interpretation of others’ actions (Rizzolatti and Sinigaglia, 2010). Recent years have seen a surge of interest in delineating the nature and function of brain systems that show common activation during the observation and execution of action (Iacoboni, 2009; Keysers and Gazzola, 2009). When we observe other people performing actions, a network of brain systems is active that allows us to process and interpret the action we are seeing. Some of the cortical areas active during action observation are also active when we perform actions ourselves, such as the somatosensory and motor cortices, the frontal gyrus, and the inferior parietal lobe (Bonini et al., 2010; Gallese et al., 1996; Kilner et al., 2009). This common activation of sensorimotor systems in response to acting and the observation of action relates to suggestions of a putative human mirroring system, which is thought to include ventral premotor cortex, the anterior inferior parietal lobe, somatosensory areas such as BA2, and the middle temporal gyrus (Gazzola and Keysers, 2009).
Alpha (8-13 Hz) and beta (14-30 Hz) rhythms in the electroencephalogram (EEG) have been extensively studied in the context of action processing, due to mounting evidence that suppression of power within these frequency ranges is related to the activation of sensorimotor cortex (Cheyne, 2012; Gaetz and Cheyne, 2006; McFarland et al., 2000; Muthukumaraswamy et al., 2004). The alpha rhythm generally shows regional decreases in power (i.e., event-related desynchronization, or ERD) during the execution of action (Neuper et al., 2006; van Wijk et al., 2012) as well as during the observation of action (Avanzini et al., 2012; Orgs et al., 2008; Perry and Bentin, 2009; Quandt et al., 2012), suggesting that it may be useful in the study of the linkages between self-movement and the perception of others’ movements. Although the specific role of the beta rhythm in action processing is debated, oscillations in this frequency range are also closely linked to action production and action perception (Gaetz and Cheyne, 2006; Hari and Salmelin, 1997). While the alpha and beta frequency bands often display relatively similar characteristics in relation to action processing, there is also evidence for functional dissociations between the two, which is consistent with their different cortical origins (Shao et al., 2012).
A variant of the alpha rhythm, known as the mu rhythm, oscillates within the typical alpha frequency band (8-13 Hz) and is maximal over central electrode sites at rest (Niedermeyer, 1997). The mu rhythm has been suggested as a possible index of mirror system functioning, given that it is sensitive to somatosensory variations in action execution, motor imagery (McFarland et al., 2000), and observation of movement (Muthukumaraswamy et al., 2004; Perry and Bentin, 2009; Perry et al., 2010). This desynchronization is generally strongest at electrodes contralateral to the hand being used to perform the action (Babiloni et al., 1999; Neuper and Pfurtscheller, 1999; Perry and Bentin, 2009; Perry et al., 2011; Quandt et al., 2011; Quandt et al., 2012). While the precise relation between mu suppression and the activation of sensorimotor cortex is not well understood, combined fMRI/EEG studies have provided evidence that the mu rhythm is associated with activity in the primary somatosensory cortex (Arnstein et al., 2011; Ritter et al., 2009). In contrast, the beta rhythm originates in the pre-central (motor) gyrus (Hari and Salmelin, 1997; Salmelin and Hari, 1994), although the accuracy of this distinction has been questioned (van Ede et al., 2011).
The mu rhythm is functionally distinct from alpha-range rhythms at other scalp regions (e.g., the classical occipital alpha rhythm), and several studies have suggested that it is particularly sensitive to the somatosensory components of action or action observation (Arroyo et al., 1993; Kuhlman, 1978; Muthukumaraswamy et al., 2004). In addition, recent work has shown that the sensorimotor alpha and beta rhythms appear to be modulated by the anticipated sensorimotor outcome of another person’s unfolding action (Fontana et al., 2011; Kilner et al., 2007; van Ede et al., 2010).
1.2 Experience and action processing
A number of recent studies have examined the role of the observer’s own sensorimotor experiences on subsequent neural processing during action observation. Several studies have demonstrated that when an observer is highly trained in performing an action, the action observation network is more activated during observation of that action (Behmer, Jr. and Jantzen, 2011; Calvo-Merino et al., 2005; Orgs et al., 2008). Much of the work in this area compares action experts (e.g., professional dancers) to novices, such that the differences between groups are the result of years of experience, including motor experience, somatosensory experience, visual experience, and possibly different levels of familiarity with or knowledge about the action being observed.
A number of short-term training studies have attempted to identify what specific characteristics of action experience might serve to modulate action processing during subsequent observation. For example, people who rehearsed a set of new dance movements showed greater activation of premotor cortex while they observed the rehearsed dance moves, compared to when they observed unrehearsed dance moves (Cross et al., 2009). In another study, participants who learned novel drawing actions showed greater frontal EEG activation during observation of actions they had previously performed compared to other novel actions they had not been trained on (Marshall et al., 2009). These studies and others in which participants are trained with novel actions (Cross et al., 2012; Quandt et al., 2011) suggest that the somatosensory and motor experiences involved in learning a new action may drive an increase in sensorimotor system engagement during subsequent observation. This notion is supported by recent work showing that learning to associate varied sensorimotor characteristics with actions changes how an observer processes gestures referring to those actions (Quandt et al., 2012). However, one factor common to all of these studies is a lack of differentiation between somatosensory and motor components of action experience, and so it remains unclear to what extent these two aspects of experience are contributing to these effects.
1.3 Somatosensory experience
While it is clear that sensorimotor experience with actions changes neural processing during observation of similar actions, the specific question of how somatosensory experience affects subsequent action processing remains largely unaddressed. The primary somatosensory cortex on the postcentral gyrus is involved in the immediate processing of somatosensation such as tactile, proprioceptive, or nociceptive input. Adjacent to the primary somatosensory cortex on the parietal operculum, the secondary somatosensory cortex is involved in higher-order processing such as integration of nociceptive or non-nociceptive somatosensory input, visceral sensation, and tactile attention (Eickhoff et al., 2006; Hagiwara et al., 2010). Processing somatosensory input is associated with a decrease in the amplitude of the mu rhythm, likely through activation of somatosensory cortex (Arnstein et al., 2011; Babiloni et al., 2006; Babiloni et al., 2010; Haegens and Luther, 2012; Hari, 2006; Proverbio, 2012).
Observing someone else being touched is associated with activation of primary and secondary somatosensory cortex (Bolognini et al, 2013; Gazzola and Keysers, 2009; Keysers et al., 2004; Keysers and Gazzola, 2009; Keysers et al., 2004; Kuehn et al., 2012; Meyer et al., 2011; Rossetti et al., 2012; Schaefer et al., 2009; Schaefer et al., 2012), which may convey to the observer an implicit sense of the tactile and proprioceptive qualities of the touch. However, there is less research concerning the contribution of somatosensory influences during the observation of goal-directed action, rather than passive touch, an issue which is likely due to the complications involved in separating out the effects of afferent and efferent influences from one another (Hommel, 2004).
Despite evidence for the activation of somatosensory cortex during action observation, it remains unknown whether prior somatosensory experiences contribute uniquely to this activation. If somatosensory experience facilitates subsequent vicarious activation of the sensorimotor system, then it is likely that many previous studies demonstrating the effect of sensorimotor experience on subsequent action processing are partially driven by the somatosensory experiences gained during action learning. In the current study we use EEG methods to more specifically address how prior somatosensory experience relates to the activation of the action observation network during subsequent action observation.
1.4 The current study
Prior work has suggested that action experience modulates cortical activity during subsequent action observation, and that somatosensation may play an important role in social perception (e.g., Keysers et al., 2010). Here we aimed to combine these two lines of research to examine whether a person’s first-person experiences with the somatosensory consequences of an action would change EEG patterns during observation of similar actions being performed by another person. Participants were given experience reaching into yellow and blue boxes—one of which was empty and one of which contained feathers to provide tactile stimulation. By repeatedly performing these reaching actions, participants formed an association between the color of the box and the somatosensory consequences of reaching inside of it. Participants then completed an imitative task in which they observed video clips depicting an actor reaching inside a similar-looking yellow or blue box, and they then performed the corresponding action (e.g., they saw the actor reach inside a blue box, and then they reached inside the blue box). There was no information regarding the content of the boxes (i.e., containing feathers or not) visible in the video clips. Thus, any differences in EEG during the observation of these clips would be based solely upon participants’ own experiences with the yellow and blue boxes, and their implicit expectation of the tactile consequences of reaching inside a yellow or blue box.
Initial analyses examined whether participants’ expectations regarding the somatosensory consequences of the observed actions would modulate alpha and beta rhythms, with a focus on activity on scalp regions overlying somatosensory cortex. We also analyzed EEG during the execution of the reaches into the two boxes, to assess differences in alpha (particularly the central mu rhythm) and beta rhythms during two actions differing primarily in their tactile consequences. The study was designed to test the following hypotheses concerning action observation: 1) Alpha-range power (8-13 Hz) will be more suppressed at electrodes overlying somatosensory cortex during observation of a reach into a box that the observer associates with tactile stimulation, compared to a box expected to be empty; and 2) The effects observed for the alpha rhythm based on tactile expectations will be present not only during the time the actor’s hand is inside of the box, but also during observation of reaching toward the box, in line with the predictive or anticipatory properties of the mu rhythm. We also examined whether the beta rhythm (14-30 Hz) would be sensitive to prior somatosensory experiences during the observation of actions. This investigation of beta was somewhat more exploratory in nature, given the lack of consensus concerning the precise role of this rhythm in somatosensory processing.
2. Results
2.1 Observation of Action
2.1.1 Alpha Band (8-13 Hz)
During observation of an actor’s hand resting on the table prior to movement, the hand reaching into a box, and the hand retracting (see Figure 1), alpha power was significantly more suppressed for trials expected to result in tactile stimulation (OT), based on the participant’s own experiences with a similarly-colored box, compared to trials expected to result in no tactile stimulation for the actor (ON trials). This effect was significant at electrodes F3, F7, T7, CP1, P3, Pz, and CP6 at the p<.05 threshold, and at C3 and CP5 at the p<.01 threshold. Figure 2 shows an overview of action observation results, and Figure 3 shows the results from the left central electrode (C3).
Figure 1. Experimental setup and presentation of video stimuli.
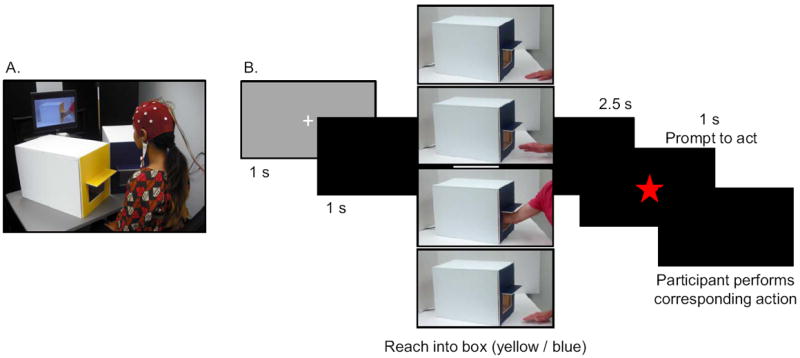
A) The participant was seated in front of a computer screen and the two boxes. Removable front panels on the boxes were used to create one of two conditions: blue – tactile stimulation / yellow- no stimulation, or yellow – tactile stimulation / blue – no tactile stimulation. B) Schematic depicting the structure of each trial. Video clips depicted an actor performing a reach into a yellow or blue box. After a brief delay, the appearance of a star prompted the participant reach into the box of the same color. The boxes visible in the video clips were angled such that it was impossible to see if there was tactile stimulation inside the box.
Figure 2. Differences in alpha and beta power during observation of reaching actions.
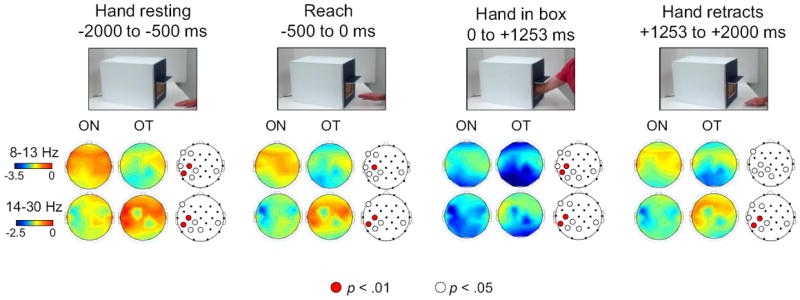
Alpha-(8-13 Hz) and beta-range (14-30 Hz) power during the observation of reaching actions. Scalp maps are shown for each epoch of interest, including the hand resting on the table, reaching into the box, and retracting away from the box (time is shown in ms). For each epoch, the two observation conditions are compared: observation of reaches into the box expected to contain no tactile stimulation, based on the participant’s experiences with the box of the same color (ON), and the observation of reaches into the box expected to result in tactile stimulation, based on the participant’s experiences (OT). The third scalp map in each group depicts the electrodes for which there was any significant (white dots = p<.05, red dots = p<.01, FDR corrected) difference between the two conditions during that epoch. The nose is located at the top of the scalp maps.
Figure 3. EEG power at C3 during observation of action.
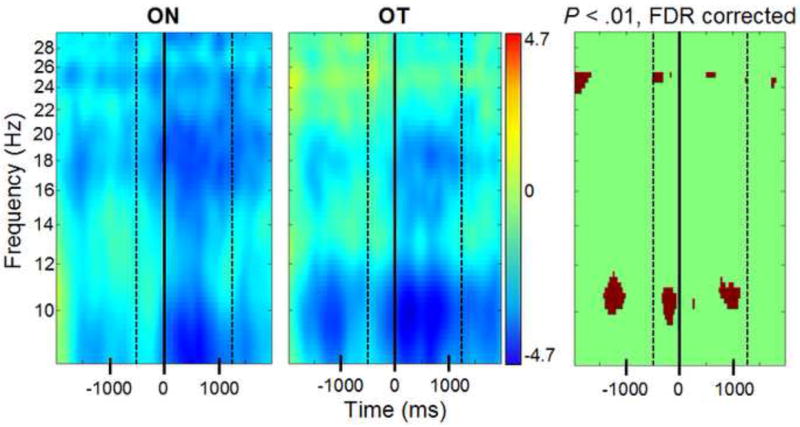
Time-frequency plot at electrode C3 during observation of reaches expected to result in no tactile stimulation (ON) and reaches expected to result in tactile stimulation (OT). Time zero is the time at which the actor’s hand entered the box. The first dashed line (-500 ms) is the onset of the actor’s reaching action, and the second dashed line (+1253 ms) is the time at which the actor’s hand was no longer in the box. Blue colors indicate a decrease in power relative to the baseline (measured in dB). The third panel depicts the results of a significance test between the two plots. Dark red signifies a significant difference between power at a specific time and frequency, as tested by bootstrap significance testing with FDR corrections applied (p<.01).
2.1.2 Beta Band (14-30 Hz)
During observation of the actor’s reaches, beta power was significantly more suppressed during observation of ON trials compared to OT trials at electrodes F3, F7, CP1, P3, Pz, T7, and CP6 at the p<.05 threshold, and C3 and CP5 at the p<.01 threshold (see Figures 2 and 3).
2.2 Execution of Action
2.2.1 Behavioral Results
The mean duration of participant’s reaches into the boxes was significantly longer when they were reaching into the box containing tactile stimulation (ET condition, mean = 1.94 s, SD = .38) compared to reaching into an empty box (EN condition, mean = 1.82 s, SD = .37; t(27) = 3.15, p = .004). Inter-individual variability also varied between conditions, such that the standard deviation of reaching duration for the EN trials (mean SD = .46, SD = .33) was significantly greater than for ET trials (mean SD = .40, SD = .24; t(27) = 2.19, p = .038).
2.2.2 EEG Results for Action Execution
EEG analyses for the execution of the reaching actions into the box yielded no significant differences between the ET condition, in which the participant executed a reach into the box containing tactile stimulation, and the EN condition, in which the participant reached into a box containing no stimulation. An example of the ERSP for each execution condition from electrode C3 is shown in Figure 4.
Figure 4. EEG power at C3 during execution of action.
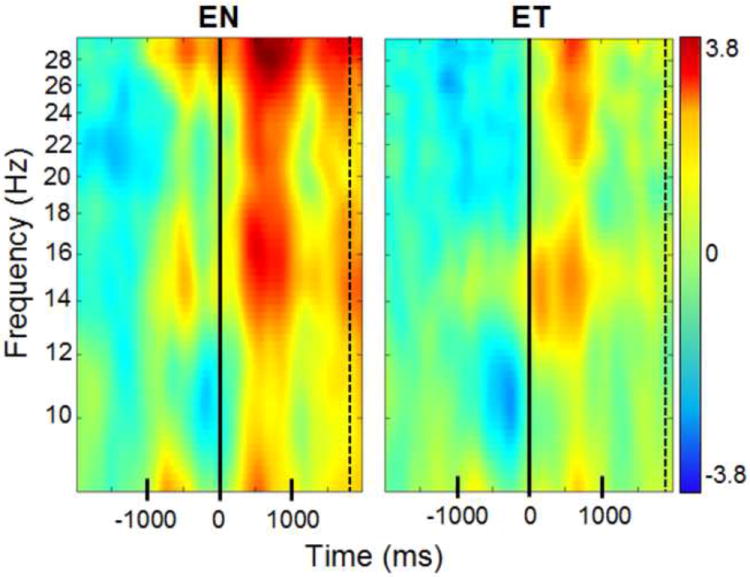
Time-frequency plot at electrode C3 during the participant’s reaches into boxes that either did not contain tactile stimulation (EN) or boxes that did contain tactile stimulation (ET). Time zero is the time at which the hand entered the box. The dashed line is the average time at which the participant’s hand was no longer in the box (see Section 2.2.1 for details). Blue colors indicate a decrease in power relative to the baseline (measured in dB); red colors indicate an increase in power relative to the baseline. There were no significant differences between the two conditions.
3. Discussion
3.1 Alpha-range desynchronization
When participants observed an actor reaching inside the box expected to contain tactile stimulation, the upper alpha rhythm (10-12 Hz) showed greater desynchronization than during the observation of reaches expected to result in no tactile stimulation. While the scalp distribution of this effect varied slightly between epochs, the differences between the ON and OT conditions were most reliable at central and centro-parietal electrodes in the left hemisphere (C3 and CP5). This finding may reflect differential mu rhythm activity between the two conditions, which would be consistent with studies showing that the amplitude of the central mu rhythm is particularly sensitive to somatosensory information (Cheyne, 2012; Hari, 2006) and is linked to activity in primary somatosensory cortex (Arnstein et al., 2011; Gaetz and Cheyne, 2006; Ritter et al., 2009). Given these associations, our findings suggest the possibility that somatosensory cortex was more strongly activated during observation of a reach into the box expected to contain feathers than a reach into the box expected to be empty, with these expectations resulting from the participant’s own prior experiences. This novel finding further suggests that somatosensory experiences alone (without substantive variation in motor or visual experience) may therefore contribute uniquely to the effects of action experience on subsequent action processing.
While the effect of somatosensory experience was most reliable at centro-parietal sites, the frontal, central, and parietal distribution of electrodes showing a broader sensitivity of alpha-range power to somatosensory experience is consistent with some prior work (Dowman et al., 2008; Stančák et al., 2005, Shao et al., 2012). Additionally, the pattern of results clearly shows that the effect of expected somatosensory input was most evident over the left hemisphere, which is consistent with recent work showing that alpha-range modulation based on prior experience during action observation is strongest over the hemisphere contralateral to the observed hand (Quandt et al., 2011; Quandt et al., 2012). However, we also note that most studies in this area have not systematically manipulated spatial factors which would help distinguish between alternative explanations of hemispheric effects.
Effects in the alpha frequency band were present during the first three epochs of the observed actions—including the time the hand was resting in front of the box, the reach toward the box, and while the hand was inside the box. The lack of specificity in timing of this effect is somewhat surprising, since that sensorimotor activation during action observation might be expected to be tied to the onset of action, rather than the observation of a scene in which action is implied (i.e., seeing a hand resting on a table in front of a box). However, while desynchronization of the alpha rhythm was closely linked to the onset of the hand entering the box for both conditions (see Figure 3), the differences between conditions were apparent before the reach began, which suggests that changes in neural activation may broadly reflect the consequences of upcoming actions. This finding is consistent with work suggesting that changes in the alpha rhythm have a predictive component, desynchronizing in anticipation of the onset of observed action (Hari, 2006; Pineda, 2005).
It is important to note that since the protocol required participants to reproduce the actions they were observing shortly after each video clip ended, the experiment involved an imitative paradigm. Thus, we cannot be certain whether the effects seen during action observation are related solely to processing of others’ actions, or to the preparation of the action they were preparing to perform. The observed actions were also highly predictable—every time a participant saw the hand resting in front of a box, the hand reached into the box following a short delay, which may have increased the anticipation or expectation about the presumed somatosensory consequences of the action. Future work should consider implementing some variation in predictability in order to see whether anticipatory action processing differs under less predictable circumstances, as demonstrated by Haegens at al. (2011).
One other related interpretation of our results concerns the relation between alpha suppression and anticipatory attention directed towards upcoming tactile stimulation. Recent work suggests that attention to upcoming tactile stimuli is associated with suppression of oscillatory activity over contralateral somatosensory cortex (Bauer et al., 2012; van Ede et al., 2012). It is possible that our findings may reflect similar processes and that such a difference between conditions was based upon the participant’s prior experience with reaching into the boxes and the associations that each participant had formed with the yellow and blue boxes. Future work in this area should further explore how attentional mechanisms may modulate cortical activity during action observation, and what implications this has for the understanding of embodied action processing. In addition, in order to localize the source of the effects found in the current study, future work could consider using functional imaging techniques.
3.2 Beta-range synchronization
At left centro-parietal electrodes (and to a lesser extent, at some frontal and bilateral parietal electrodes), analyses revealed significantly greater beta-range synchronization during observation of reaches expected to result in tactile stimulation than during observation of reaches expected to result in no stimulation. While it is common to see beta rhythm desynchronization in response to tactile stimulation (Avanzini et al., 2012; McFarland et al., 2000; van Ede et al., 2012), our results are not consistent with those findings. However, there is also evidence of synchronization in the beta band in response to tactile stimulation (Cheyne et al., 2003; Neuper and Pfurtscheller, 2001; Pfurtscheller et al., 2001). Furthermore, it is important to note that the ~20 Hz beta rhythm is generally dominant in the primary motor cortex (Hari, 2006; Stančák et al., 2006), in contrast to the somatosensory sources of the mu rhythm, which likely explains why the two rhythms are often functionally dissociated (Haegens et al., 2012; Hari and Salmelin, 1997; Shao et al., 2012).
The beta-range effects seen here are notable because the expectation of tactile stimulation modulated beta power during all epochs of observation. As seen in Figures 2 and 3, differences in beta power were evident during observation of the hand resting on table, and throughout the remaining duration of the video clips. This suggests that the EEG correlates of the expectation regarding the tactile consequences of action have a predictive component—occurring in advance of the actual action, much as was seen for the alpha-range findings. This anticipation is consistent with prior work showing prestimulus modulation of the beta rhythm in anticipation of tactile stimulation (van Ede et al., 2010).
3.3 Execution of action
We predicted that there would be greater alpha and beta desynchronization during the execution of reaches into the box containing tactile stimulation than during reaches into the empty box, but this hypothesis was not supported. Instead, there was no significant difference in the EEG patterns between the types of execution epochs. However, the overall changes in power during the execution of reaching actions are consistent with other studies reporting brief desynchronization followed by sustained synchronization in the alpha and beta range (Hari, 2006). Similar rebound effects following movement have been reliably observed in other studies (Avanzini et al., 2012; Cheyne, 2012; Hari, 2006; Neuper et al., 2006).
One candidate explanation for why the EEG analyses showed no differences between the ET and EN conditions is that the participants’ reaching actions varied significantly between the conditions in mean duration and in the variability of duration. Since the reaches performed by participants were longer when they were reaching into a box with tactile stimulation, but more variable when they were reaching into an empty box, it is possible that the variation in action timing blurred any differences in EEG activation that may have been present between conditions.
Another possible explanation for why the two execution conditions did not differ lies in the dynamics of the actions that the participants were performing. In the majority of experiments examining alpha and beta power during action execution, the actions being performed are very small-scale—for example, lifting the index finger (Hari, 2006) or performing pincer grasps (Muthukumaraswamy et al., 2004). In the current study, participants were performing actions that required movement (at a minimum) of the hand, arm, and shoulder. Although participants were instructed to keep their bodies as still as possible, the actions necessarily caused some degree of movement throughout the body, putting our actions on a different scale than most existing work. A recent study by McGarry et al. (2012) found significant alpha and beta synchronization while participants picked up a piece of paper and ripped it, and the authors suggested the effect might be due to the large-scale nature of the movement. In addition, the predictability of the sensory effects of the participants’ reaching actions may have also contributed to the presence of sensory attenuation, as suggested in other recent studies (Hughes et al., 2013; Roussel et al., 2013).
4. Experimental Procedures
4.1 Participants
Twenty-seven undergraduates (17 females; mean age = 23.08 years; SD = 5.68) participated in the experiment in exchange for course credit. All were right handed according to the Edinburgh Handedness Inventory (Oldfield, 1971), had no history of neurological abnormality, and had normal or corrected-to-normal vision. All participants gave informed consent prior to participation, and the University’s Institutional Review Board approved the study protocol prior to data collection.
4.2 Apparatus
Two boxes were created for use in the video clips and during the experimental session. Each box was 31 cm (width) by 36 cm (height) by 50 cm (length) in size, with opaque white sides and interchangeable colored front panels. There was one yellow front panel, and one blue front panel, and each panel could be attached to either of the boxes. The front panels had openings measuring 18 cm (width) by 15 cm (height), and a perpendicular panel extending 7 cm over the opening that was designed to obscure the participant’s view into the box. One box was empty, such that when reaching inside the box, a person’s hand would not come into contact with any objects. The other box contained two feather dusters arranged such that a hand could easily pass through them, and a person reaching into the box would feel the feathers brush against all sides of the fingers, hand, and wrist.
4.3 Trial structure
We created video clips depicting an actor using his right hand to reach into the yellow and blue boxes. A total of four video clips were created (two for each color), and one clip was included in each trial (see Figure 1). Trials started with a fixation point (1 s), followed by a black screen (1 s). The video clip then began, showing the actor’s right hand resting on a table, in front of either the yellow or blue box. After 1.5 s, the actor initiated movement and reached into the box until most of his forearm was in the box, at which point he retracted his arm and replaced his hand on the table. The duration of the actor’s movements varied slightly between each of the four video clips. The duration of the reach varied between 433 ms and 600 ms (mean = 500 ms; SD = 72 ms), and the hand was in the box for between 1160 and 1350 ms (mean = 1253 ms; SD = 86 ms). Participants then saw a black screen for 2.5 s, and a red star appeared on the screen (1 s duration) as a prompt for them to perform a reach into the same-colored box in front of them. Following the participant’s action, the next trial started. At no time was the inside of the box in the video clip visible to participants, so participants were unaware of whether or not the boxes in the videos contained any tactile stimulation. Video clips were recorded at 30 frames per second. The protocol included three blocks of 20 trials each, for a total of 60 trials. In each block, half the trials depicted reaches into yellow boxes, and half depicted reaches into blue boxes. Three randomized trial orders were created. Two “distracter” trials were also created (one yellow, one blue) which were not included in any analysis and were designed to keep the participant’s attention directed toward the video clips. The distracter trials were identical to the other trials, except that at the frame when the arm was furthest into the box, the clip froze for 2 s. Four of these trials (two of each color) were included in each block, for a total of 12 during the experiment. The protocol regarding these trials is described below.
4.4 Experimental Session
Prior to their arrival, each participant was randomly assigned to a color-somatosensation association (e.g., the yellow box contains tactile stimuli, the blue box is empty). The interchangeable colored front panels were then placed on the corresponding boxes, and remained in place for the entirety of the experimental session. The boxes were covered with a sheet at the time of the participant’s arrival, in order to limit their visual experience with the boxes and prevent them from looking inside them. Each participant was also randomly assigned to one of three trial orders. Upon arrival, participants were seated at a table with the two boxes in front of them, and a video screen (43.5 cm diagonal) visible above the boxes, approximately 85 cm away from the participant’s head. Participants were first familiarized with the two boxes, and were asked to reach once into each box. They were then instructed to watch the videos carefully, and when prompted by the appearance of the red star on the screen, to perform the corresponding action (i.e., after watching the actor reach into the blue box, the participant should reach into the blue box on the table in front of her). They were instructed to reach with their right hand, with fingers together and palm facing to the left, and to try and perform the action at the same speed as the actor in the videos. On distracter trials, participants were told to not perform any action, and simply wait for the next trial to begin. An experimenter viewing a live video feed of the testing room checked performance on each trial to ensure that the participants followed all instructions. Prior to data collection, participants completed a practice block of ten trials (including two distracter trials) to ensure that they understood the task.
4.5 EEG Acquisition
EEG was collected from 27 electrode sites using a Lycra stretch cap (Electro-Cap, Eaton, OH, USA) with Electro-Gel conducting gel. The sites recorded from were Fp1, Fp2, F3, F4, Fz, F7, F8, FC1, FC2, C3, C4, Cz, CP1, CP2, T7, T8, P3, P4, Pz, P7, P8, O1, O2 and the left and right mastoids. Vertical electrooculogram (EOG) activity was collected from electrodes placed above and below the left eye. The EEG signal was amplified by optically isolated, high input impedance (>1 GΩ) bioamplifiers from SA Instrumentation (San Diego, CA, USA). Scalp electrode impedances were kept under 25 kΩ. Bioamplifier gain was 4000 for the EEG channels, 1000 for the EOG channels, and the hardware filter settings were .1 Hz (high-pass) and 100 Hz (low-pass), with a 12 dB/octave rolloff. The EEG signal was collected referenced to Cz with an AFz ground.
4.6 Event Mark Generation
The video clips used in the observation trials contained brief audio tones denoting the onset and offset of each phase of interest. Specifically, audio tones were placed at the video frames corresponding to the following events: initiation of the reaching action, the fingertips entering the box, and the fingertips exiting the box as the actor retracted his hand. These tones (which were not audible) triggered the precise placement of event markers in the digitized EEG record, and were later used to extract epochs for the action observation analysis (see Section 4.8). A device monitoring the audio track of the video clips generated an immediate TTL pulse at tone onset, which was sent to the acquisition device to be digitized alongside the EEG. In order to record the timing of the participant’s own action, the boxes were equipped with a custom infrared beam device (James Long Company, Armonk, NY). On one side of each box, the infrared emitter sent an infrared beam to a sensor on the other side of the box (23 cm distance). When the hand entered the box, the beam was broken and an event mark was placed, and when the beam was restored as the hand left the box, another event mark was placed. These event marks were used to extract epochs from the EEG for execution analysis (as described in Section 4.8).
4.7 Data Processing
EEG signals were analyzed using MATLAB (The Mathworks, Inc., Natick, MA) and the open source toolbox EEGLAB (Swartz Center for Computational Neuroscience, La Jolla, CA; http://www.sccn.ucsd.edu/eeglab). The raw EEG signal was re-referenced offline to the average of the left and right mastoids, and independent components identified as reflecting blink activity were removed from the EEG (Delorme, 2004; Hoffmann and Falkenstein, 2008).
4.8 Data reduction
For each trial, two epochs were extracted, one encompassing the observation of the actor’s reach, and one encompassing the execution of the corresponding action. Two types of observation epoch were created, with conditions based on the color-stimulation association formed by the participants’ own experiences with the boxes (e.g., yellow box is empty, blue box contains feathers):
Observation of action with expected tactile stimulation (OT condition), from −2000 to +2000 ms surrounding the zero-point at which the actor’s hand entered the box expected to contain stimulation.
Observation of action with no expected tactile stimulation (ON condition), from −2000 to +2000 ms surrounding the zero-point when the actor’s hand entered the box expected to be empty. These observation epochs encompassed the observation of the hand resting on the table, the hand reaching toward the box, the hand moving within the box, and the retraction of the hand from the box (see Figure 2 for precise timing).
Two types of execution epochs were also created based upon the presence of tactile stimulation in the box that the participant was reaching into:
-
(3)
Execution of the reach inside the box with tactile stimulation (ET condition), from −2000 to +2000 ms surrounding the zero-point at which the participant’s hand entered the box containing stimulation;
-
(4)
Execution of the reach inside the box containing no tactile stimulation (EN condition), from −2000 to +2000 ms surrounding the zero-point at which the participant’s hand entered the empty box. The execution epochs encompassed the time when the participants were waiting for the prompt to perform the action, their reach toward the box, the movement of their hand inside the box, and the retraction of their hand from the box. All epochs were visually inspected, and any epochs containing gross artifacts from biological or environmental noise were rejected from further analysis.
4.9 Data Analysis
4.9.1 Behavioral Analysis
The amount of time each participant’s hand was inside the box for each trial was calculated using the event marks described in section 2.6. These durations were averaged across conditions (ET and EN), and SPSS Statistics (IBM, Armonk, NY) was used to test for significant differences in action timing between the conditions.
4.9.2 Time-frequency Analysis
The study function in EEGLAB was used to analyze changes in EEG power across time and frequency (Delorme, 2004). The baseline for all observation epochs was defined as -2999 to -2000 ms, relative to time zero as defined in Section 2.8. For the observation epochs, this baseline corresponded to presentation of a fixation point and a black screen. The baseline for all execution epochs was -3499 to -2499 ms, corresponding to a time when the participant was viewing a black screen and waiting for the prompt to perform the reaching action. Event-related spectral perturbation (ERSP; Makeig, 1993) was then computed to visualize the dynamics of the power spectra during the epochs in two dimensions (time and frequency). ERSP was computed using 200 overlapping windows that were 800 ms wide (overlap = 760 ms), starting with a 4-cycle wavelet at the lowest frequency. The ERSP images show mean changes in spectral power (expressed as dB) for alpha and beta bands relative to the defined baseline. Analyses were performed to assess statistical significance between conditions (OT vs. ON and ET vs. EN) across the alpha (8-13 Hz) and beta (14-30 Hz) frequency ranges.
4.9.3 Statistical Analysis
Significant differences between conditions were assessed by comparing ERSPs. We used a non-parametric bootstrap statistical method to determine the significance between differences in ERSPs at each electrode channel depending on the expectation of tactile stimulation (OT vs. ON conditions) or the presence of actual tactile stimulation (ET vs. EN conditions). We performed tests at two different statistical thresholds, p<.05 and p<.01, and all analyses were corrected for multiple comparisons using the False Discovery Rate correction (Benjamini & Hochberg, 1995). Conditions were determined to differ significantly from one another at a certain time and frequency if there was any significant difference found using the bootstrap comparison.
5. Conclusions
Taken together, the results revealed that action processing is modified by the observer’s previous tactile experiences with the action they are observing. The increased suppression of alpha range frequencies over contralateral somatosensory cortex during observation of actions expected to result in tactile stimulation suggests greater activity of the somatosensory system compared to the observation of an action that is not expected to result in tactile stimulation. Additionally, findings reveal beta-range increases in power when tactile stimulation is expected, suggesting a functional dissociation between the alpha and beta frequency bands. Overall, these results demonstrate the contribution of somatosensation to the facilitation of action mirroring processes during action observation, and provide support for further investigating the somatosensory properties of the EEG mu rhythm.
We examined the effect of somatosensory experience on action processing.
Participants viewed actions that varied in expected somatosensory consequences.
Mu power was lower when observed actions had more tactile consequences.
Beta power was greater when the actions had more tactile consequences.
Acknowledgments
The authors are grateful to Gulnaz Khan, Nhi Tran, Todd Woldoff, Reanna Serafine, Maria Langan, and Hayley Haaf for their assistance with data collection and processing. The work was supported by funding from the National Institutes of Health (HD-68734) to PJM and TFS and a PICS award from the Centre National de la Recherche Scientifique to CAB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnstein D, Cui F, Keysers C, Maurits NM, Gazzola V. Mu suppression during action observation and execution correlates with BOLD in dorsal premotor, inferior parietal, and SI cortices. J Neurosci. 2011;31:14243–14249. doi: 10.1523/JNEUROSCI.0963-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo S, Lesser R, Gordon B, Uematsu S, Jackson D, Webber R. Functional significance of the mu rhythm of human cortex: An electrophysiologic study with subdural electrodes. Electroen Clin Neuro. 1993;87:76–87. doi: 10.1016/0013-4694(93)90114-b. [DOI] [PubMed] [Google Scholar]
- Avanzini P, Fabbri-Destro M, Dalla Volta R, Daprati E, Rizzolatti G, Cantalupo G. The dynamics of sensorimotor cortical oscillations during the observation of hand movements: An EEG study. PLoS ONE. 2012;7:e37534. doi: 10.1371/journal.pone.0037534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Brancucci A, Del Percio C, Capotosto P, Arendt-Nielsen L, Chen ACN, Rossini PA. Anticipatory electroencephalography alpha rhythm predicts subjective perception of pain intensity. J Pain. 2006;7:709–717. doi: 10.1016/j.jpain.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Capotosto P, Del Percio C, Babiloni F, Petrini L, Buttiglione M, Arendt-Nielsen L, et al. Sensorimotor interaction between somatosensory painful stimuli and motor sequences affects both anticipatory alpha rhythms and behavior as a function of the event side. Brain Res Bull. 2010;81:398–405. doi: 10.1016/j.brainresbull.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Carducci F, Cincotti F, Rossini PM, Neuper C, Pfurtscheller G, Babiloni F. Human movement-related potentials vs desynchronization of EEG alpha rhythm: A high-resolution EEG study. Neuroimage. 1999;10:658–665. doi: 10.1006/nimg.1999.0504. [DOI] [PubMed] [Google Scholar]
- Bauer M, Kennett S, Driver J. Attentional selection of location and modality in vision and touch modulates low-frequency activity in associated sensory cortices. J Neurophysiol. 2012;107:2342–2351. doi: 10.1152/jn.00973.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behmer LP, Jr, Jantzen KJ. Reading sheet music facilitates sensorimotor mu-desynchronization in musicians. Clin Neurophysiol. 2011;122:1342–1347. doi: 10.1016/j.clinph.2010.12.035. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- Bolognini N, Rossetti A, Convento S, Vallar G. Understanding others’ feelings: The role of the right primary somatosensory cortex in encoding the affective valence of others’ touch. J Neurosci. 2013;33:4201–4205. doi: 10.1523/JNEUROSCI.4498-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini L, Rozzi S, Serventi FU, Simone L, Ferrari PF, Fogassi L. Ventral premotor and inferior parietal cortices make distinct contribution to action organization and intention understanding. Cereb Cortex. 2010;20:1372–1385. doi: 10.1093/cercor/bhp200. [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B, Glaser D, Grezes J, Passingham R, Haggard P. Action observation and acquired motor skills: An fMRI study with expert dancers. Cereb Cortex. 2005;15:1243–1249. doi: 10.1093/cercor/bhi007. [DOI] [PubMed] [Google Scholar]
- Cheyne DO. MEG studies of sensorimotor rhythms: A review. Exp Neurol. 2012 doi: 10.1016/j.expneurol.2012.08.030. online preprint. [DOI] [PubMed] [Google Scholar]
- Cheyne DO, Gaetz W, Garnero L, Lachaux JP, Ducorps A, Schwartz D, Varela FJ. Neuromagnetic imaging of cortical oscillations accompanying tactile stimulation. Cognitive Brain Res. 2003;17:599–611. doi: 10.1016/s0926-6410(03)00173-3. [DOI] [PubMed] [Google Scholar]
- Cross ES, Cohen NR, C Hamilton AFC, Ramsey R, Wolford G, Grafton ST. Physical experience leads to enhanced object perception in parietal cortex: Insights from knot tying. Neuropsychologia. 2012 doi: 10.1016/j.neuropsychologia.2012.09.028. online preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross ES, Hamilton AFC, Kraemer DJM, Kelley WM, Grafton ST. Dissociable substrates for body motion and physical experience in the human action observation network. Eur J Neurosci. 2009;30:1383–1392. doi: 10.1111/j.1460-9568.2009.06941.x. [DOI] [PubMed] [Google Scholar]
- Delorme A. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Meth. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dowman R, Rissacher D, Schuckers S. EEG indices of tonic pain-related activity in the somatosensory cortices. Clin Neurophysiol. 2008;119:1201–1212. doi: 10.1016/j.clinph.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Amunts K, Mohlberg H, Zilles K. The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex. 2006;16:268–279. doi: 10.1093/cercor/bhi106. [DOI] [PubMed] [Google Scholar]
- Fontana AP, Kilner JM, Rodrigues EC, Joffily M, Nighoghossian N, Vargas CD, Sirigu A. Role of the parietal cortex in predicting incoming actions. Neuroimage. 2011;59:556–564. doi: 10.1016/j.neuroimage.2011.07.046. [DOI] [PubMed] [Google Scholar]
- Gaetz W, Cheyne D. Localization of sensorimotor cortical rhythms induced by tactile stimulation using spatially filtered MEG. Neuroimage. 2006;30:899–908. doi: 10.1016/j.neuroimage.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Keysers C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: Single-subject analyses of unsmoothed fMRI data. Cereb Cortex. 2009;19:1239–1255. doi: 10.1093/cercor/bhn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Handel BF, Jensen O. Top-down controlled alpha band activity in somatosensory areas determines behavioral performance in a discrimination task. J Neurosci. 2011;31:5197–5204. doi: 10.1523/JNEUROSCI.5199-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Luther L, Jensen O. Somatosensory anticipatory alpha activity increases to suppress distracting input. J Cognitive Neurosci. 2012;24:677–685. doi: 10.1162/jocn_a_00164. [DOI] [PubMed] [Google Scholar]
- Hagiwara K, Okamoto T, Shigeto H, Ogata K, Somehara Y, Matsushita T, Tobimatsu S, et al. Oscillatory gamma synchronization binds the primary and secondary somatosensory areas in humans. Neuroimage. 2010;51:412–420. doi: 10.1016/j.neuroimage.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Hari R. Action-perception connection and the cortical mu rhythm. Prog Brain Res. 2006;159:253–260. doi: 10.1016/S0079-6123(06)59017-X. [DOI] [PubMed] [Google Scholar]
- Hari R, Salmelin R. Human cortical oscillations: A neuromagnetic view through the skull. Trends Neurosci. 1997;20:44–49. doi: 10.1016/S0166-2236(96)10065-5. [DOI] [PubMed] [Google Scholar]
- Hoffmann S, Falkenstein M. The correction of eye blink artefacts in the EEG: a comparison of two prominent methods. PLoS ONE. 2008:e3004. doi: 10.1371/journal.pone.0003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel B. Event files: feature binding in and across perception and action. Trends Cogn Sci. 2004;8:494–500. doi: 10.1016/j.tics.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Hughes G, Desantis A, Waszak F. Mechanisms of intentional binding and sensory attenuation: The role of temporal prediction, temporal control, identity prediction, and motor prediction. Psychol Bull. 2012 doi: 10.1037/a0028566. online preprint. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Imitation, empathy, and mirror neurons. Ann Rev Psychol. 2009;60:653–670. doi: 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Expanding the mirror: Vicarious activity for actions, emotions, and sensations. Curr Opin Neurobiol. 2009;19:666–671. doi: 10.1016/j.conb.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Keysers C, Kaas J, Gazzola V. Somatosensation in social perception. Nat Rev Neurosci. 2010;11:417–428. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- Keysers C, Wicker B, Gazzola V, Anton J-L, Fogassi L, Gallese V. A touching sight: SII/PV activation during the observation and experience of touch. Neuron. 2004;42:335–346. doi: 10.1016/s0896-6273(04)00156-4. [DOI] [PubMed] [Google Scholar]
- Kilner J, Friston KJ, Frith CD. Predictive coding: An account of the mirror neuron system. Cogn Process. 2007;8:159–166. doi: 10.1007/s10339-007-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner J, Neal A, Weiskopf N, Friston K, Frith C. Evidence of mirror neurons in human inferior frontal gyrus. J Neurosci. 2009;29:10153–10159. doi: 10.1523/JNEUROSCI.2668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn E, Trampel R, Mueller K, Turner R, Schütz-Bosbach S. Judging roughness by sight: A 7-tesla fMRI study on responsivity of the primary somatosensory cortex during observed touch of self and others. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22031. online preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman WN. Functional topography of the human mu rhythm. Electroen Clin Neuro. 1978;44:83–93. doi: 10.1016/0013-4694(78)90107-4. [DOI] [PubMed] [Google Scholar]
- Makeig S. Auditory event-related dynamics of the EEG spectrum and effects of exposure to tones. Electroen Clin Neuro. 1993;86:283–293. doi: 10.1016/0013-4694(93)90110-h. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Bouquet CA, Shipley TF, Young T. Effects of brief imitative experience on EEG desynchronization during action observation. Neuropsychologia. 2009;47:2100–2106. doi: 10.1016/j.neuropsychologia.2009.03.022. [DOI] [PubMed] [Google Scholar]
- McFarland DJ, Miner LA, Vaughan TM, Wolpaw JR. Mu and beta rhythm topographies during motor imagery and actual movements. Brain Topogr. 2000;12:177–186. doi: 10.1023/a:1023437823106. [DOI] [PubMed] [Google Scholar]
- McGarry LM, Russo FA, Schalles MD, Pineda JA. Audio-visual facilitation of the mu rhythm. Exp Brain Res. 2012;218:527–538. doi: 10.1007/s00221-012-3046-3. [DOI] [PubMed] [Google Scholar]
- Meyer K, Kaplan JT, Essex R, Damasio H, Damasio A. Seeing touch is correlated with content-specific activity in primary somatosensory cortex. Cereb Cortex. 2011;21:2113–2121. doi: 10.1093/cercor/bhq289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy S, Johnson B, McNair N. Mu rhythm modulation during observation of an object-directed grasp. Cogn Brain Res. 2004;19:195–201. doi: 10.1016/j.cogbrainres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G. Handbook of Electroencephalography and Clinical Neurophysiology. Elsevier; Amsterdam: 1999. Motor imagery and ERD: Event-related desynchronization; pp. 303–325. [Google Scholar]
- Neuper C, Pfurtscheller G. Evidence for distinct beta resonance frequencies in human EEG related to specific sensorimotor cortical areas. Clin Neurophysiol. 2001;112:2084–2097. doi: 10.1016/s1388-2457(01)00661-7. [DOI] [PubMed] [Google Scholar]
- Neuper C, Wörtz M, Pfurtscheller G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog Brain Res. 2006;159:211–222. doi: 10.1016/S0079-6123(06)59014-4. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E. Alpha rhythms as physiological and abnormal phenomena. Int J Psychophysiol. 1997;26:31–49. doi: 10.1016/s0167-8760(97)00754-x. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Orgs G, Dombrowski J-H, Heil M, Jansen-Osmann P. Expertise in dance modulates alpha/beta event-related desynchronization during action observation. Euro J Neurosci. 2008;27:3380–3384. doi: 10.1111/j.1460-9568.2008.06271.x. [DOI] [PubMed] [Google Scholar]
- Perry A, Bentin S. Mirror activity in the human brain while observing hand movements: A comparison between EEG desynchronization in the mu-range and previous fMRI results. Brain Res. 2009;1282:126–132. doi: 10.1016/j.brainres.2009.05.059. [DOI] [PubMed] [Google Scholar]
- Perry A, Stein L, Bentin S. Motor and attentional mechanisms involved in social interaction—Evidence from mu and alpha EEG suppression. Neuroimage. 2011;58:895–904. doi: 10.1016/j.neuroimage.2011.06.060. [DOI] [PubMed] [Google Scholar]
- Perry A, Troje N, Bentin S. Exploring motor system contributions to the perception of social information: Evidence from EEG activity in the mu/alpha frequency range. Soc Neurosci. 2010;5:272–284. doi: 10.1080/17470910903395767. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Krausz G, Neuper C. Mechanical stimulation of the fingertip can induce bursts of beta oscillations in sensorimotor areas. J Clin Neurophysiol. 2001;18:559–564. doi: 10.1097/00004691-200111000-00006. [DOI] [PubMed] [Google Scholar]
- Pineda JA. The functional significance of mu rhythms: Translating “seeing” and “hearing” into “doing”. Brain Res Rev. 2005;50:57–68. doi: 10.1016/j.brainresrev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Proverbio AM. Tool perception suppresses 10–12 Hz μ rhythm of EEG over the somatosensory area. Biol Psych. 2012;91:1–7. doi: 10.1016/j.biopsycho.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Quandt LC, Marshall PJ, Bouquet CA, Young T, Shipley TF. Experience with novel actions modulates frontal alpha EEG desynchronization. Neurosci Lett. 2011;499:37–41. doi: 10.1016/j.neulet.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Quandt LC, Marshall PJ, Shipley TF, Beilock SL, Goldin-Meadow S. Sensitivity of alpha and beta oscillations to sensorimotor characteristics of action: An EEG study of action production and gesture observation. Neuropsychologia. 2012;50:2745–2751. doi: 10.1016/j.neuropsychologia.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter P, Moosmann M, Villringer A. Rolandic alpha and beta EEG rhythms’ strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Hum Brain Mapp. 2009;30:1168–1187. doi: 10.1002/hbm.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nature Reviews Neuroscience. 2010;11:264–274. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- Rossetti A, Miniussi C, Maravita A, Bolognini N. Visual perception of bodily interactions in the primary somatosensory cortex. Euro J Neurosci. 2012;36:2317–2323. doi: 10.1111/j.1460-9568.2012.08137.x. [DOI] [PubMed] [Google Scholar]
- Roussel C, Hughes G, Waszak F. A preactivation account of sensory attenuation. Neuropsychologia. 2013 doi: 10.1016/j.neuropsychologia.2013.02.005. online preprint. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hari R. Spatiotemporal characteristics of sensorimotor neuromagnetic rhythms related to thumb movement. Neurosci. 1994;60:537–550. doi: 10.1016/0306-4522(94)90263-1. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Heinze H-J, Rotte M. Embodied empathy for tactile events: Interindividual differences and vicarious somatosensory responses during touch observation. Neuroimage. 2012;60:952–957. doi: 10.1016/j.neuroimage.2012.01.112. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Xu B, Flor H, Cohen LG. Effects of different viewing perspectives on somatosensory activations during observation of touch. Hum Brain Mapp. 2009;30:2722–2730. doi: 10.1002/hbm.20701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao S, Shen K, Yu K, Wilder-Smith EPV, Li X. Frequency-domain EEG source analysis for acute tonic cold pain perception. Clin Neurophysiol. 2012;123:2042–2049. doi: 10.1016/j.clinph.2012.02.084. [DOI] [PubMed] [Google Scholar]
- Stančák A, Mlynář J, Poláček H, Vrána J. Source imaging of the cortical 10 Hz oscillations during cooling and warming in humans. Neuroimage. 2006;33:660–671. doi: 10.1016/j.neuroimage.2006.06.049. [DOI] [PubMed] [Google Scholar]
- Stančák A, Poláček H, Vrána J, Rachmanová R, Hoechstetter K, Tintra J, Scherg M. EEG source analysis and fMRI reveal two electrical sources in the fronto-parietal operculum during subepidermal finger stimulation. Neuroimage. 2005;25:8–20. doi: 10.1016/j.neuroimage.2004.10.025. [DOI] [PubMed] [Google Scholar]
- van Ede F, de Lange F, Jensen O, Maris E. Orienting attention to an upcoming tactile event involves a spatially and temporally specific modulation of sensorimotor alpha-and beta-band oscillations. J Neurosci. 2011;31:2016–2024. doi: 10.1523/JNEUROSCI.5630-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede F, Jensen O, Maris E. Tactile expectation modulates pre-stimulus β-band oscillations in human sensorimotor cortex. Neuroimage. 2010;51:867–876. doi: 10.1016/j.neuroimage.2010.02.053. [DOI] [PubMed] [Google Scholar]
- van Ede F, Köster M, Maris E. Beyond establishing involvement: quantifying the contribution of anticipatory alpha-and beta-band suppression to perceptual improvement with attention. J Neurophysiol. 2012;108:2352–2362. doi: 10.1152/jn.00347.2012. [DOI] [PubMed] [Google Scholar]
- van Wijk BC, Beek PJ, Daffertshofer A. Differential modulations of ipsilateral and contralateral beta (de)synchronization during unimanual force production. Euro J Neurosci. 2012;36:2088–2097. doi: 10.1111/j.1460-9568.2012.08122.x. [DOI] [PubMed] [Google Scholar]


