Abstract
Interleukin-6 (IL-6) is a pleiotropic cytokine with a broad range of physiological and pathological functions. Because in cancer IL-6 contributes to a microenvironment that promotes tumor cell survival, angiogenesis and inflammation, understanding the mechanism responsible for its production is important. In neuroblastoma, the second most common solid tumor in children, IL-6 is produced not by tumor cells but by stromal cells such as monocytes and bone marrow mesenchymal stem cells (BMMSC). Here we show that the production of IL-6 in BMMSC is in part stimulated by galectin-3 binding protein (Gal-3BP) secreted by neuroblastoma cells. We identified a distal region of the IL-6 promoter that contains 3 CCATT/enhancer binding protein (C/EBP) binding domains involved in the transcriptional upregulation of IL-6 by Gal-3BP.Gal-3BP interacted with Galectin-3 (Gal-3) present in BMMSC, and a Gal-3BP/Gal-3/Ras/MEK/ERK signaling pathway was responsible for the transcriptional upregulation of IL-6 in BMMSC where Gal-3 has a necessary function. In support of the role of this pathway in human neuroblastoma tumors, Gal-3BP was found to be present in tumor cells and in the adjacent extracellular matrix of 96% of 78 primary neuroblastoma tumor samples examined by immunohistochemistry. Considering the protumorigenic function of IL-6 in cancer, this tumor cell-stromal cell interactive pathway could be a target for anticancer therapy.
Keywords: Galectin-3, galectin-3 binding protein, neuroblastoma, interleukin-6, microenvironment
Introduction
It is now well recognized that interactions between tumor cells and stromal cells in the tumor microenvironment play a determinant role in cancer initiation and progression (1–4). Therefore the mechanisms by which the tumor microenvironment influences the behavior of malignant cells have been the subject of intensive investigation over the last decade (5). The production of soluble growth factors, cytokines and chemokines by stromal cells in the presence of tumor cells is one among the several mechanisms by which the tumor microenvironment affects cancer cells (6, 7). Among these soluble factors is interleukin-6 (IL-6) (8, 9). This pleiotropic cytokine has multiple protumorigenic activities including the promotion of tumor cell proliferation and survival, the stimulation of angiogenesis, the induction of a state of immune tolerance, and the activation of osteoclasts to promote osteolytic bone metastasis (10–14). IL-6 also promotes the self-seeding of circulating tumor cells (15) and contributes to a stress response that protects tumor cells from drug action (16). IL-6 is not only produced by tumor cells but also by stromal cells in the tumor microenvironment (17, 18). In neuroblastoma for example, tumor cells do not make IL-6 but upregulate its expression in bone marrow mesenchymal stem cells (BMMSC). Stromal-derived IL-6 then promotes osteoclast activation, the formation of osteolytic bone metastasis (19) and the resistance of tumor cells to cytotoxic drugs (20). The mechanism by which IL-6 is upregulated in the tumor microenvironment is however not entirely understood. We reported that the production of Galectin-3 binding protein (Gal-3BP) (also known as 90 kDa Mac-2 binding protein), by neuroblastoma cells was one mechanism that stimulated the expression of IL-6 in BMMSC (21) and in monocytes/macrophages (22). Gal-3BP is a self-adhesive glycoprotein that forms oligomers of 1,000 to 1,500 kDa in the extracellular milieu and promotes cell adhesion to matrix proteins (23). Its function in cancer is not well defined but it has been suggested that it contributes to an inflammatory reaction to tumors and infections (24). Gal-3BP affects the Th2 cytokine profile in peripheral blood monocytes of patients with asthma with a decrease in IL-4, IL-5 and IL-13 and an increase in IL-6 (25). Its role in regulating IL-6 in cancer is new and the mechanism not known. Gal-3BP interacts with multiple proteins and in particular Galectin-3 (Gal-3), a ubiquitous glycosylated protein present at the surface, in the cytoplasm and in the nucleus of many non-malignant and malignant cells. It acts as a membrane-associated receptor and signal transduction protein (26). Gal-3 is synthesized by MSC, and has a suppressive effect on T-cell proliferation (27, 28).
In this study we have tested the hypothesis that the interaction between Gal-3BP made by neuroblastoma cells and Gal-3 present in BMMSC is responsible for the upregulation of IL-6 in BMMSC in the presence of neuroblastoma cells.
Materials and Methods
Cell culture
Human neuroblastoma cell lines were cultured as previously reported (20). PC3 human prostate cancer cells were cultured in DMEM supplemented with 10% FBS, penicillin and streptomycin. The nature of the cell lines was confirmed by genotype analysis using AmpFISTR Identifier kit PCR Reagents and Gene Mapper ID v3.2 (Applied Biosystems). Human BMMSC were purchased from ALLCELLS and cultured in accordance to the instructions of the manufacturer.
Reagents
Farnesyl thiosalicylic acid (FTS) was purchased from Santa Cruz Biotechnology, Inc. (sc-205322). Recombinant human Gal-3BP (rGal-3BP) was purchased from R&D Systems and GenWay and these preparations had endotoxin levels of less than 1 EU/µg protein.
Conditioned medium
Conditioned medium (CM) from neuroblastoma cell lines was collected as follows. Cells were plated at 5×106 in 150 cm2 tissue culture flasks or at 1×106/well in 6 well plates and cultured for 24–72 hours in FBS containing medium. Cells were then washed with phosphate buffered saline (PBS) (KCl 0.2 g/L; KH2PO4 0.2 g/L, NaCl 8 g/L, NaHPO4 1.15 g/L, pH 7.4) twice and cultured in FBS free medium for 24 hours. The medium was collected, centrifuged to eliminate floating cells and concentrated by ultrafiltration at 4,500 rpm in a Centriplus YM-10 microconcentrator (Millipore).
IL-6 and Gal-3BP measurements
IL-6 and Gal-3BP in the CM were detected by ELISA, using a Quantikine Immunoassay Kit (R&D Systems) for IL-6 and a platinum ELISA kit from eBioscience for Gal-3BP. Readings were obtained in a Synergy HT (BIO-TEK) spectrometer.
Immunodepletion of Gal-3BP
Protein A Sepharose CL-4B (Amersham) was pre-swollen with distilled water and equilibrated with PBS containing a protease and phosphatase Inhibitor Cocktail (Thermo Scientific). Five hundred µl of a 50% (v/v) Protein A slurry was mixed with 15 µg of a goat polyclonal antibody against human Gal-3BP or goat IgG (control) and rotated at 4°C for 3 hours. This slurry was mixed with 1 ml of 10× serum-free CM and rotated at 4°C overnight. After centrifugation, the supernatant was collected and the sepharose beads were washed three times in PBS containing protease inhibitors and 0.05% (v/v) Tween-20.
Quantitative RT-PCR
Total RNA was isolated using TRIzol (Invitrogen). The RNA quality and quantity was measured by NanoDrop spectrometer (ThermoScientific). 100 ng of total RNA was reverse transcribed by SuperScript III reverse transcriptase (Invitrogen) according to manufacturer’s instructions. Quantitative Polymerase Chain reaction (qPCR) was performed in duplicate samples using an ABI 7900HT Fast Real time PCR System (Applied Biosystems) at an annealing temperature of 60°C. The relative change in gene expression was calculated by the relative standard curve method using the mean of GAPDH as an internal control.
Construction of plasmids
To construct the pGL2-IL-6-Luc plasmid, genomic DNA was extracted from normal human whole blood using a Blood and Cell Culture DNA Mini Kit (Qiagen). A fragment of the IL-6 promoter region corresponding to position −2155 to +4 was amplified by PCR using Pfu DNA polymerase (Stratagene) and the primers shown in Supplemental Table 1. The PCR fragment generated was digested with Kpnl and HindIII for 3 hours at 37°C and ligated into the multiple cloning site of pGL2 Luc (Promega) and treated with alkaline phosphatase (Roche) for 1 hour. The ligation reaction was performed using the Quick Ligation Kit (New England Biolabs). IL-6 promoter deletion mutants in the pGL2-IL-6-Luc construct were generated either by Exonuclease III digestion using the Deletion Kit for kilo sequencing (Takara) in accordance with the instructions of the manufacturer (deletion −1041) or by restriction endonuclease digestion with KpnI and NheI (deletion mutant −212) followed by Klenow fragment reaction at 37°C for 15 minutes. Construct −97 was created by PCR reaction using a forward primer: 5’-CCC GGT ACC CAC CCT CAC CCT CCA AC-3’ and the IL-6 reverse primer described above. All constructs were sequenced to verify the absence of replication errors.
Transfection and luciferase reporter assay
Cells cultured at 1×105 per well in 24 well plates for 24 hours in serum-containing medium were co-transfected with a pGL2 or a pGL2-IL-6-Luc plasmid (0.5 µg) and a pRV plasmid (1ng) expressing Renilla luciferase as (transfection efficiency internal control) in the presence of Lipofectamine LTX (Invitrogen). Luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega) in a Lumat LB9501 luminometer (Berthold).
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts of PC3 cells cultured as described above were obtained using a NE-PER Nuclear and Cytoplasmic Extraction Reagent (Thermo Scientific). Extracts (8 µg) were incubated in the presence of the double stranded biotinylated oligonucleotides described in Supplemental Table1. When indicated, cold (non-biotinylated) oligonucleotides (10 fold excess) were added to the nuclear extracts and incubated at 4°C for 30 minutes before the addition of double stranded biotinylated oligonucleotides. Reactions were performed in 10 mM Tris pH 7.5, 50 mM KCl, 1 mM dithiothreitol (DTT), 2.5% (v/v) glycerol, 5 mM MgCl2, 1 µg poly dI-dC and 0.05% (v/v) NP-40. Samples were electrophoresed in a 6% native polyacrylamide gel containing 2.5% (v/v) glycerol and transferred to Immun-Blot PVDF membranes (Bio-Rad). After UV crosslinking, the membranes were blocked with 5% (w/v) skim milk in 50 mM Tris-HCl, pH 7.6, 150 mM NaCl (TBS) and incubated in the presence of horseradish peroxidase (HRP) conjugated streptavidin (1 µg/ml) (Vector Laboratories). The detection was performed by enhanced chemiluminescence (ECL, Amersham).
Western blot
Western blot was performed as previously described (21). Primary antibodies used were a goat polyclonal antibody against human Gal-3BP at a 1:1000 dilution (R&D Systems), a mouse monoclonal antibody against human Gal-3 at a 1:1000 dilution (Santa Cruz Biotechnology, Inc.), rabbit polyclonal antibodies against human ERK1/2, MEK1/2 and their phosphorylated forms, p-ERK1/2 and p-MEK1/2 (Cell Signaling Technology, Inc., dilution 1:1000) and a rabbit antibody against human actin at a 1:3000 dilution (Sigma). Mouse monoclonal antibodies against human Ras (Thermo Scientific), human N-Ras (Calbiochem), human H-Ras (Calbiochem), and human K-Ras (Santa Cruz Biotechnology, Inc) were used at 1:200, 1:200, 1:100 and 1:100 dilutions, respectively. The detection of immune complexes and their quantification was performed using the Odyssey Infrared Imaging Systems (LI-COR Biosciences).
Gal-3 knockdown experiments
A control siRNA against mouse lamin A and 3 siRNA sequences against human Gal-3 (FlexiTube siRNA) were purchased from Qiagen. Sequences are described in Supplemental Table 1. Transfection was performed using Lipofectamine RNAi Max (Invitrogen).
Ras pull down assay
Pull down assay for active Ras was performed using an Active Ras Pull-Down and Detection Kit (Thermo Scientific). BMMSC lysate (80 µg) and GST-Raf1-RBD (20 µg) were mixed and subjected to immunoprecipitation by centrifugation at 6000 g for 30 seconds in the presence of glutathione beads resin. The precipitate was washed three times with lysis/binding/wash buffer (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1% (v/v) NP-40 and 5% (v/v) glycerol) and proteins in the precipitate were eluted in the presence of 125 mM Tris-HCl, pH 6.8, 2% (v/v) glycerol, 4% (v/v) SDS, 0.05% (w/v) bromophenol blue and 0.05% (v/v) and 200 mM dithiothreitol (reducing buffer). The eluates were then electrophoresed in a 4–20% gradient Tris-glycine polyacrylamide gel (Biorad) and examined for the presence of total Ras, K-Ras, H-Ras and N-Ras by Western blot analysis.
Immunofluorescence
For immunofluorescence studies performed on cultured cells, cells were plated at 1×105 in LabTek II (Nunc) chambers for 24 hours. Cells were washed with PBS and fixed in 4% paraformaldehyde or 3% (w/v) formaldehyde. When indicated, cells were permeabilized in 0.1% (v/v) Triton X-100 for 5 minutes followed by blocking with 15% (v/v) FBS in PBS. Cells were then incubated in the presence of a goat polyclonal antibody against human Gal-3BP (1:100 dilution) and an anti-human Gal-3 mouse monoclonal antibody (1:100 dilution) in PBS for 1 hour at 37°C, washed twice with 0.1% (v/v) Triton X-100 in PBS and incubated in the presence of a Cy-3 conjugated anti-mouse IgG antibody (1:300 dilution) and a FITC-conjugated anti-goat IgG for 45 minutes at room temperature. The slides were washed 6 times in 0.1% (v/v) Triton X-100 in PBS and the wells were removed. The slides were mounted in Vectashield mounting medium (Vector Laboratories). For immunofluorescence studies on formalin-fixed paraffin-embedded (FFPE) specimens, 7–10 µm tissue sections were deparaffinized by xylene, hydrated through alcohol gradient and immersed in PBS. The slides were microwaved for 5 minutes 3 times in VECTOR antigen unmasking solution. After blocking with 3% BSA and 0.1% Tween-20 in PBS for 1 hour, slides were incubated in 5% donkey serum in PBS with the primary antibody (1:100 dilution for goat anti-human IL-6 antibody or 1:200 dilution for rabbit anti-human Gal-3BP antibody) overnight at 4°C. After washing by 0.1% Tween-20 in PBS 3 times, slides were incubated with the secondary antibody (FITC-conjugated anti-goat or Cy-3 conjugated anti-rabbit IgG antibody dilution 1:150) in the presence of 3% BSA and 0.1% Tween-20 in PBS for 1 hour at room temperature. Slides were washed 3 times and incubated in 300 nM DAPI (Invitrogen) in PBS for 1 minute, washed in PBS 3 times and mounted with Vectashield mounting medium (VECTOR).
Immunohistochemistry
FFPE sections of 78 primary neuroblastoma tumors were collected from the file of Children’s Oncology Group ANBL00B1 Neuroblastoma Biology Study with the approval of the committee. Appropriate consent was obtained from patients/guardians at the individual contributing institutions. These sections were processed as above described and incubated in the presence of a rabbit polyclonal antibody against human Gal-3BP (ProteinTech Group, Inc.) as primary antibody (dilution 1: 300) and biotin conjugated anti-rabbit IgG and streptavidin HRP as secondary antibody. Immunocomplexes were visualized using 3, 3-diaminobenzidine (DAB) (iView DAB detection system, BenchMark, Ventana Medical Systems, Inc). Slides were counterstained with methyl green. The presence of Gal-3BP in these tumor samples was semi-quantified by counting the number of positive cells on a total of 200 cells per sample. A score of 1 was assigned to samples having between 0 and 33% positive cells, 2 to samples having between 34 and 66% positive cells, and 3 to samples with more than 66% positive cells. Slides were blindly scored by 3 investigators (AMS, HS and YAD). Where there was a discrepancy, a second blind analysis was performed. After this second analysis, only 7 samples were differently scored by the 3 observers and were assigned the consensus score of 2 observers. The samples were also classified for the following parameters, age at diagnosis, clinical stage, DNA index, MYCN status, degree of neuroblastic differentiation, mitosis karyorrhexis index (MKI) and favorable vs. unfavorable histology group according to the International Neuroblastoma Pathology Classification (29, 30) and overall and event-free survival (EFS).
Statistics
Data were analyzed using the student’s t test, analysis of variance, or univariate or multivariate linear regression methods, or Spearman rank correlation, as appropriate to the experiment or context. Two-sided p-values are reported with values < 0.05 reported as statistically significant. For studies correlating Gal-3BP scoring and patient survival, the primary endpoint for EFS analysis was time from diagnosis until the first occurrence of progressive disease, disease recurrence, or death from any cause. There were 18 total events in this patient cohort. The primary endpoint for survival analysis was time from diagnosis to death from any cause (total 10 deaths). EFS and survival plots were produced using the Kaplan-Meier estimate. The test of the differences in EFS and survival among the three expression groups were based on the logrank trend test.
Results
Gal-3BP is secreted by several neuroblastoma cell lines that upregulate the expression of IL-6 by mesenchymal stem cells
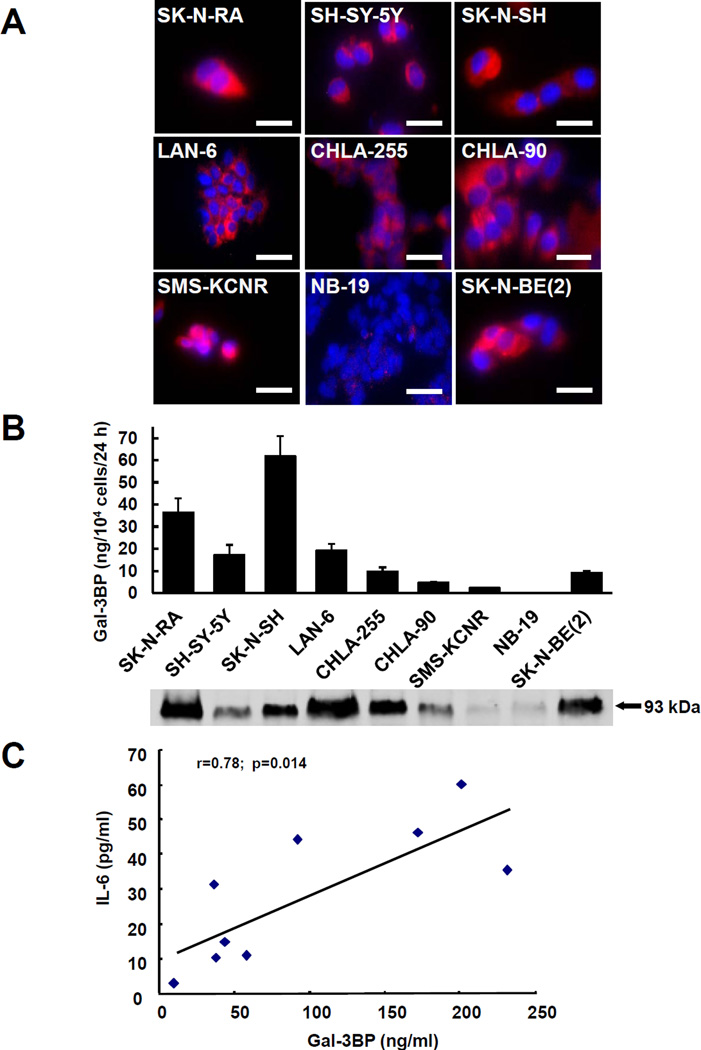
We first examined the production of Gal-3BP in 9 well-characterized human neuroblastoma cell lines derived from patients with advanced disease. The presence of abundant intracellular Gal-3BP was detected by immunocytofluorescence in most cultures examined. In only one cell line (NB-19) the amount detected was very small (Fig. 1A). The cells also secreted Gal-3BP as demonstrated by the amount of Gal-3BP detected by ELISA in the CM which varied from 0 to 60 ng/104 cells over 24 hours. A Western blot analysis of the CM confirmed the presence of a 93 kDa protein band identified by an anti-Gal-3BP antibody (Fig. 1B). BMMSC were then incubated in the presence of the CM of these cell lines and examined after 24 hours for the presence of IL-6. This analysis revealed a direct correlation between the amount of IL-6 produced by BMMSC and the amount of Gal-3BP present in the CM of neuroblastoma cells (Fig. 1C).
Figure 1. Gal-3BP is secreted by several neuroblastoma cell lines that upregulate IL-6 in mesenchymal stem cells.
A. immunofluorescence analysis of Gal-3BP in 9 neuroblastoma cell lines permeabilized with Triton-X100. Bar=50µm. B. The data represent the mean (±SD) amount of Gal-3BP produced by 104 cells over 24 hours. Aliquots of the medium containing 10 µg of proteins were also examined for the presence of a 93 kDa Gal-3BP protein by Western blot (bottom of graph). C. Serum-free CM of the 9 neuroblastoma cell lines shown in panel A and B was incubated with BMMSC for 24 hours. The graph is a plot of the mean concentrations of Gal-3BP in the neuroblastoma serum-free CM (in ng/ml; x axis) and the mean concentration of IL-6 present after 24 hours of incubation with BMMSC (in pg/ml; y axis) from triplicate samples.
Gal-3BP is one among other regulators of IL-6 expression and increases the transcriptional upregulation of IL-6 in BMMSC
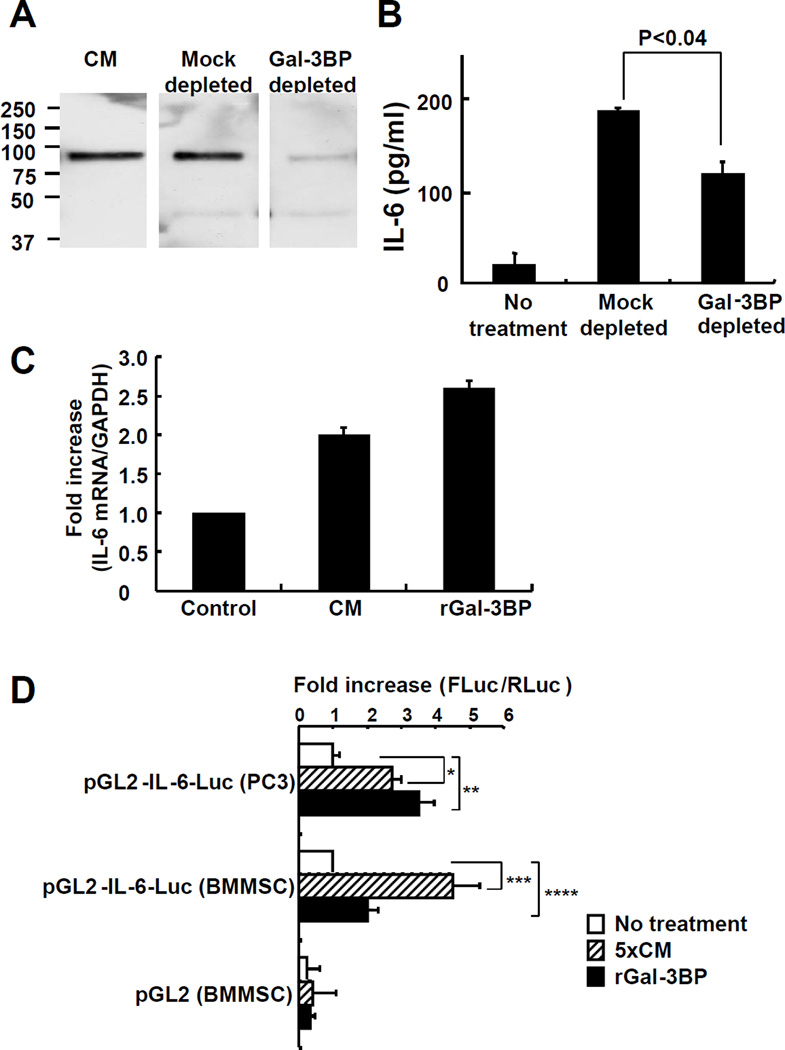
To confirm the stimulatory function of Gal-3BP in neuroblastoma CM on IL-6 production, we immune-depleted Gal-3BP from the CM of CHLA-255 cells and tested this immune-depleted CM on the production of IL-6 by BMMSC. This procedure eliminated 90% of the Gal-3BP (Fig. 2A) but only decreased the IL-6 stimulatory effect by 37% (Fig. 2B). These data indicate that Gal-3BP is only in part responsible for the stimulatory effect of neuroblastoma CM on IL-6 expression by BMMSC and that other factors contribute.
Figure 2. Gal-3BP is one regulator of IL-6 expression and increases its transcriptional regulation in BMMSC.
A. Western blot analysis of 10× serum-free CM from CHLA-255 immunodepleted of Gal-3BP as described in Materials and Methods. B. Concentration of IL-6 in the medium of BMMSC exposed for 24 hours to the CM of CHLA-255 cells treated as described in A. The data represent the mean concentration (± SD) of IL-6 in triplicate samples and are representative of one among two independent experiments showing similar results. C. Increase in IL-6 mRNA expression in BMMSC 4 hours after treatment with CM or rGal-3BP (2.5 µg/ml) determined by qRT-PCR. The data represent the mean fold increase in the ratio IL-6: GAPDH (± SD) of duplicate samples from 2 independent experiments. D. IL-6 promoter Luciferase activity in BMMSC and PC3 cells transfected with pGL2 or pGL2-IL-6-Luc and pRV and incubated in the absence or presence of 5× serum-free CM from CHLA-255 cells or rGal-3BP (2.5 µg/ml) for 24 hours. The data represent the mean fold increase in the FLuc/RLuc ratio (± SD) over control (no treatment) from 5 separate experiments each performed in triplicate (*p<0.002, **p<0.03, ***p<0.004 and ****p<0.025).
We then tested the effect of CHLA-255 CM on the expression of IL-6 mRNA in BMMSC and found a 2-fold increase in IL-6 mRNA by qRT-PCR at 4 hours after exposure to the CM. Consistent with Gal-3BP being a stimulator, we also observed a 2.5 fold increase in IL-6 mRNA upon exposure to rGal-3BP (Fig. 2C). To confirm that Gal-3BP transcriptionally upregulates IL-6 expression, BMMSC and PC3 human prostate cancer cells (as positive control) were transfected with the pGL2-IL6-Luc construct and tested luciferase activity upon treatment with rGal-3BP or CM from CHLA-255 cells. This analysis indicated a 2 to 4.7 fold increase in promoter activity by rGal-3BP or CHLA-255 CM and is consistent with a transcriptional mechanism of upregulation (Fig.2D).
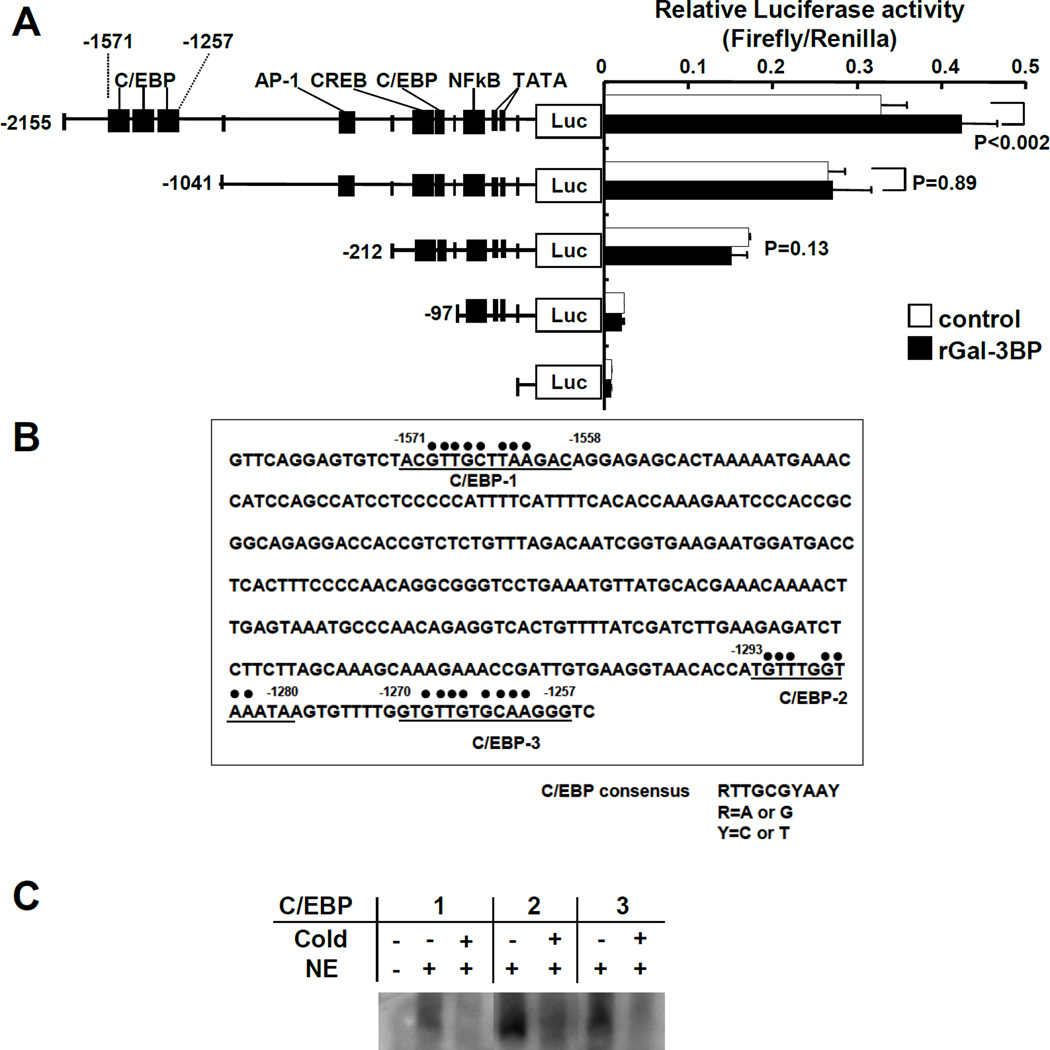
A distal region of the IL-6 promoter containing C/EBP binding domains is involved in Gal-3BP-mediated IL-6 transcription
We then used deletion mutagenesis in the IL-6 promoter to identify region(s) of the promoter responsible for the upregulation of IL-6 by Gal-3BP. For these experiments we used human PC3 prostate cancer cells that had a higher transfection efficiency than BMMSC. The transfection of IL-6 promoter mutants into these cells identified a distal region of the promoter between position −2155 to position −1041 responsible for the stimulatory activity of Gal-3BP. When this region of the promoter was deleted, we observed no changes in baseline activity but a loss of response to treatment with rGal-3BP (Fig. 3A). Sequencing analysis of this region identified 3 C/EBP binding motifs located at positions −1571 to −1558, −1293 to −1280 and −1270 to −1257 (Fig. 3B). By EMSA we identified the presence of a protein-DNA complex when double stranded biotinylated oligonucleotides corresponding to these 3 C/EBP binding domains were incubated with nuclear extracts of rGal-3BP-treated PC3 cells. The specificity of these complexes was confirmed by the addition of a 10 fold excess of non-biotinylated double stranded oligonucleotides that competed for binding (Fig. 3C). Altogether the data indicate that Gal-3BP transcriptionally upregulates IL-6 by a mechanism that involves 3 C/EBP-binding motifs present in the distal region of the IL-6 promoter.
Figure 3. The distal region of the IL-6 promoter contains functional C/EBP motifs.
A. IL-6 promoter Luciferase activity in PC3 cells transfected with the pGL2-IL-6 deletion mutants shown on the left and pRV with and without treatment with rGal-3BP (2.5 µg/ml) for 24 hours. The data represent the mean (±SD) ratio Firefly Luciferase/Renilla Luciferase and are representative of one among three separate experiments each performed in triplicate samples. B. Sequencing analysis of the region of the IL-6 promoter extending from position −1586 to −1255 showing the presence of 3 C/EBP domains. C. EMSA of nuclear extracts from PC3 cells treated with rGAL-3BP (5 µg/ml) using biotinylated oligonucleotides in the presence of 10× fold excess of non-biotinylated oligonucleotides when indicated.
Gal-3 is necessary for Gal-3BP-mediated upregulation of IL-6
We next asked whether Gal-3, known to interact with Gal-3BP (26), was a necessary intermediate in the transcriptional upregulation of IL-6 by Gal-3BP. We had previously demonstrated the presence of Gal-3 in BMMSC by Western blot analysis (21). We therefore used immunofluorescence to confirm its presence in the cytoplasm of cultured BMMSC cells (Fig. 4A, a to c). When rGal-3BP was added to the culture medium, we detected Gal-3BP at the cell surface and also inside the cells where it was associated with Gal-3 (Fig. 4A, d to f) indicating the formation of intracellular Gal-3BP - Gal-3 complexes. We then tested 3 siRNA oligonucleotides (and an anti-mouse lamin A siRNA as control), for their ability to downregulate Gal-3 expression in BMMSC and observed inhibition of Gal-3 expression with the 3 siRNA tested (Fig. 4B). Knock-down of Gal-3 expression in BMMSC prior to exposure to CHLA-255 CM or to rGal-3BP resulted in a 75 to 100% inhibition of IL-6 production (Fig. 4C) indicating that Gal-3 is a necessary intermediate for the upregulation of IL-6 by Gal-3BP. In further support of a necessary role of Gal-3 for Gal-3BP-mediated IL-6 stimulation, we demonstrated that downregulation of Gal-3 by siRNA in PC3 cells transfected with pGL-2-IL-6-Luc resulted in a loss of increase in IL-6 promoter activity upon treatment with rGal-3BP (Fig 4D).
Figure 4. Gal-3 is necessary for Gal-3BP mediated IL-6 expression.
A. Immunocytofluorescence analysis of Gal-3 (red) and Gal-3 BP (green) in BMMSC treated with rGal-3BP (5 µg/ml), fixed without permeabilization as described in Materials and Methods. a to c. Untreated cells. d to f. Cells treated with rGal-3BP. B. Western blot analysis of cell – associated Gal-3 in BMMSC examined 72 hours after transfection with indicated siRNA as described in Materials and Methods. C. Amount of IL-6 produced over 24 hours by BMMSC Parallel transfected with siRNA as shown in B and incubated in the absence or presence of 5× CM from CHLA-255 cells or rGal-3BP (2.5 µg/ml). The data represent the mean IL-6 concentration (±SD) in the supernatant of triplicate samples and are representative of three independent experiments showing similar results. D. IL-6 Luciferase promoter activity in PC3 cells transfected with a Gal-3 siRNA and treated with rGal-3BP (5 µg/ml). The data represent the mean fold increase in the FLuc/RLuc from triplicate samples from 3 separate experiments.
Gal-3BP activates the Ras/MEK/ERK pathway
We then explored whether IL-6 upregulation in BMMSC by Gal-3BP was dependent on Ras activation. Active Ras pull-down experiments demonstrated that Ras became rapidly activated upon exposure of BMMSC to rGal-3BP as we observed a 3.5 fold increase in the ratio Ras-GTP/total Ras after treatment with Gal-3BP (Fig. 5A). This increase involved N-Ras as K-Ras and H-Ras were not detected in BMMSC. The increase in Ras activation was followed by an increase in MEK and ERK activation that peaked 30 minutes after treatment . Both ERK1 and ERK2 were equally activated after 30 minutes (Fig. 5B). To confirm the involvement of Ras activation in Gal-3BP-mediated IL-6 regulation, we pre-treated BMMSC with the Ras inhibitor FTS (12.5 µM, a concentration that maintained 85% cell viability) prior to exposure to the CM of CHLA-255 cells or rGal-3BP and examined their production of IL-6 (Fig. 6A). The data revealed that pretreatment with FTS inhibited IL-6 production by 92 to 100%. Providing further evidence to support the involvement of Ras, we then demonstrated that treatment of BMMSC cells transfected with pGL2-IL-6-Luc with FTS suppressed the upregulation of the IL-6 promoter activity by rGal-3BP or CHLA-255 CM (Fig. 6B). A similar suppression of IL-6 promoter activity was observed when BMMSC were pre-treated with the MEK inhibitor PD98058 (50 µM) (Fig 6.C).
Figure 5. Gal-3BP signals through the Ras/MEK/ERK pathway.
A. Western blot analysis of lysates of BMMSC treated with rGal-3BP (10 to 25 µg/ml) subjected to pull down assay and examined for Ras-GTP, N, K and H-Ras as described in Materials and Methods. The data are representative of 2 experiments showing similar results (left panel). The bar diagram on the right represents the fold increase in Ras-GTP/total Ras and N-Ras/total Ras from analysis of data by scanning densitometry. B. Western blot analysis of BMMSC treated as in A and examined for pMEK1/2, MEK1/2, pERK1/2 and ERK1/2 expression (left panel). The bar diagrams on the right represents the mean fold increase (±SD) in the ratio phosphorylated protein : total protein over time for pMEK1/2 (10 µg/ml, n=6; 25 µg/ml, n=3) and pERK1/2 (10 µg/ml, n=7; 25 µg/ml, n=5). The bar diagram on the right lower panel represents the ratio pERK1/ERK1 and pERK2/ERK2 obtained by scanning densitometry of the data shown on the Western blot.
Figure 6. Inhibition of Gal-3BP-mediated IL-6 stimulation by Ras and MEK inhibition.
A. Amount of IL-6 produced by BMMSC cultured with 12.5 µM FTS or DMSO for 12 hours before being exposed to 5× CM from CHLA-255 cells or rGal-3BP (5 µg/ml) for 24 hours. The data represent the mean (±SD) concentration from triplicate samples and are representative of one from three independent experiments showing similar results. B. IL-6 Luciferase promoter activity in PC3 cells treated with FTS for 4 hours before exposure to CHLA-255 CM or rGal-3BP. The data represent the mean (±SD) increase from control (no treatment) in the FLuc/RLuc ratio of triplicate samples and is representative of one among 3 independent experiments showing similar results. C. Same as B except that PC3 cells were treated with PD98058 (50 µM). The data represent the mean (±SD) increase from control in triplicate samples and is representative of 2 independent experiments showing similar results.
Gal-3BP is present in primary neuroblastoma tumors
To obtain evidence that Gal-3BP has a similar function in human neuroblastoma tumors in vivo, we semi-quantitatively examined its expression by immunohistochemistry on FFPE sections from 78 samples of primary neuroblastoma tumors as described in Materials and Methods. This analysis (Fig. 7A) revealed a strong (score 3) expression in 25% of the samples, a score of 2 in 40% of the samples and a score of 1 in 31% of the samples. Only 3 samples (4%) were found entirely negative. Gal-3BP was present not only inside the cells but also in the pericellular space and extracellular matrix surrounding neuroblastoma cells and often at the center of clusters of tumor cells forming pseudo-rosettes (Fig. 7A, panel c). Some sections were also examined for the presence of IL-6. This analysis revealed the presence of IL-6 positive cells in close proximity of Gal-3BP expressing cells (Fig. 7B). We performed a univariate analysis of the score of Gal-3BP expression in these tumors and specific tumor characteristics (Fig. 7C). We only detected a significantly lower score in patients with stage 4S (children < 1 year with metastatic disease to skin, liver or bone marrow and good prognosis) when compared to patients with stage 1–3 (non-metastatic) or stage 4 (metastatic) disease. Kaplan-Meier estimate for survival (Fig. 7D) or EFS (Supplemental Data Fig. S1) did not show a difference in survival when patients were stratified according to the Gal-3BP expression score.
Figure 7. Gal-3BP is present in neuroblastoma tumors.
A. Digital photomicrographs of representative neuroblastoma tumor sections stained for Gal-3BP. Panel a: absence of Gal-3BP; panel b: score 1; panel c: score 2; panel d: score 3. Bar=50 µm. B. Sections were stained for Gal-3BP (red) and IL-6 (green). Bar=10 µm, inset: 30 µm. C. Bar diagram showing the mean score (±SD) in 78 neuroblastoma samples according to categories: age at diagnosis (<18 months vs. ≥18 months); DNA index (1 or >1); Diff = grade of neuroblastic differentiation: D (differentiated), P (poorly differentiated), and U (undifferentiated); Histo: F (Favorable Histology) and U (Unfavorable Histology); MKI = mitosis-karyorrhexis index; H (high, ≥200/5,000 cells), I (intermediate, 100–200/5,000 cells), and L (low, < 100/5,000 cells); MYCN: A (amplified) and NA (non-amplified); and Stage: 1 to 3: non-metastatic; 4: metastatic; 4S: metastatic with good prognosis. D. Kaplan-Meier graph of survival probability by time in years from diagnosis within each of the three Gal-3BP expression groups.
In summary, our data demonstrate that Gal-3BP is produced by neuroblastoma cells and present in the tumor microenvironment of neuroblastoma tumors being one among the factors responsible for the stimulation of the production of IL-6 by stromal cells. They provide evidence for the first time for a Gal-3BP/Gal-3/Ras/MEK/ERK1/2 signaling pathway that is responsible for the transcriptional regulation of IL-6 in these cells.
Discussion
Here we describe a novel mechanism by which malignant cells upregulate the production of IL-6 by stromal cells. In this study we used BMMSC as a source of stromal cells because we had previously shown that in the bone marrow microenvironment BMMSC produce IL-6 when in the presence of neuroblastoma cells (19). However we had also shown that peripheral blood monocytes/macrophages behave similarly and respond to tumor-derived Gal-3BP by increasing their production of IL-6 (22). Although a similar IL-6 stimulatory function of Gal-3BP on peripheral blood monocytes was previously reported in patients with asthma (25), this is the first report of such a function in cancer. Thus the Gal-3BP/Gal-3/IL-6 interactive axis between tumor cells and stromal cells described here likely plays a role not only in the bone marrow microenvironment where BMMSC are an important source of IL-6 but also in primary tumors where tumor-associated macrophages are the source of this cytokine among others. This is consistent with the observation of an abundant presence of Gal-3BP in primary human tumor specimens in proximity of IL-6 expressing cells.
Although our data provide evidence that Gal-3BP is involved in the regulation of IL-6 by stromal cells, it is clearly not the only factor secreted by neuroblastoma cells that upregulates IL-6. Immunodepletion experiments (Fig. 2) demonstrate that removal of most of Gal-3BP in the CM of neuroblastoma cells only partially suppresses the stimulatory effect on IL-6 production. Consistently we had previously shown that the knock-down of Gal-3BP in neuroblastoma cells, only partially (40–50%) suppressed the stimulatory effect on IL-6 production by BMMSC (21). The data thus indicate the presence of Gal-3BP-independent mechanism(s) of interaction between neuroblastoma cells and BMMSC responsible for the upregulation of IL-6 in BMMSC. One such mechanism previously reported by us is the COX-2-mediated production of prostaglandin E-2 by neuroblastoma cells that provides an amplification loop (20). The presence of other Gal-3BP-independent mechanisms is the subject of further investigation in our laboratory.
Gal-3BP is expressed in many solid tumors. It is present in tumors of the ovary, in particular serous, and mucinous tumors and clear cell carcinoma compared to benign tumors (31). It is expressed in gastric cancer (32), and in human colon cancer (33). Its function is not entirely defined although it has been shown to promote tumor cell adhesion to ECM proteins (33), the expression of MMP in prostate cancer cells (34), the formation of metastasis in lung cancer (35), and to contribute to an inflammatory reaction to tumor cells (24). We provide here evidence that it is expressed in neuroblastoma (cell lines and primary tumors) and that it contributes to the production of IL-6.
The mechanism by which Gal-3BP upregulates IL-6 has not previously been explored. Here we provide evidence that it involves a transcriptional effect. The transcriptional regulation of IL-6 expression has been well characterized and several key regulatory elements present in the proximal region of the promoter (position −271 to −54) have been identified (40–41). Little attention has however been placed on the more distal region of the promoter. Here we have identified a region of the promoter extending from −1571 to −1257 not previously associated with the transcriptional expression of IL-6. This region contains 3 C/EBP binding motifs.
We also provide evidence that the transcriptional upregulation of IL-6 by Gal-3BP is mediated by one of its binding partners, Gal-3, and that upregulation of IL-6 involves activation of the Ras/MEK/ERK pathway, a pathway that is activated by Gal-3 in breast cancer cells (36). In tumor cells, overexpression of Gal-3 coincided with an increase in K-Ras activation with a loss in N-Ras-GTP. Our data in BMMSC indicate an activation of N-Ras-GTP in the absence of K-Ras and H-Ras which were not expressed in BMMSC. The reason for this discrepancy is not entirely clear but may be due to differences between normal and malignant cells. We also used rGal-3BP as an activator of Gal-3 rather than Gal-3 overexpression, as was the case in breast cancer cells.
Our analysis of primary tumor neuroblastoma specimens shows the expression in the majority of the samples analyzed, which is consistent with similar reports in lymphoma and ovarian cancer (31, 37). In our analysis in a cohort of 78 samples of primary neuroblastoma however we did not find any correlation between the amount of Gal-3BP in tissue and other biological markers of outcome and survival. Only samples from patients with Stage 4S disease, who have a favorable outcome, showed a significantly lower Gal-3BP score (p<0.001). Considering the complexity of the interactions between neuroblastoma cells and stromal cells and the presence of other pathways upregulating stromal IL-6, the absence of correlation between Gal-3BP expression and outcome is not entirely unanticipated.
Supplementary Material
Acknowledgments
Financial support: This work was supported by NIH grant P01 CA084103 to YAD and HS, and by the Bogart Foundation Pediatric Cancer Research Program and the T.J. Martell Foundation for Leukemia, Cancer, and AIDS Research to YAD.
The authors thank J. Rosenberg for her excellent assistance in preparing the manuscript and Dr. L. Blavier Sarte for her technical assistance in the laboratory. We also thank Dr. A. Sakurai, Dr. R. Suganuma and S.A. Phung for assistance with the immunohistochemistry study of Gal-3BP, and K. Engell and E. Fernandez for acquiring immunofluorescence images by confocal microscopy. The authors thank the members of the Children’s Oncology Group ANBL00B1 Committee for providing the tumor specimens.
References
- 1.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 2.van Kempen LC, Ruiter DJ, van Muijen GN, Coussens LM. The tumor microenvironment: a critical determinant of neoplastic evolution. Eur J Cell Biol. 2003;82:539–548. doi: 10.1078/0171-9335-00346. [DOI] [PubMed] [Google Scholar]
- 3.Roskelley CD, Bissell MJ. The dominance of the microenvironment in breast and ovarian cancer. Semin Cancer Biol. 2002;12:97–104. doi: 10.1006/scbi.2001.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witz IP. Tumor-microenvironment interactions: dangerous liaisons. Adv Cancer Res. 2008;100:203–229. doi: 10.1016/S0065-230X(08)00007-9. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Nefedova Y, Landowski TH, Dalton WS. Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia. 2003;17:1175–1182. doi: 10.1038/sj.leu.2402924. [DOI] [PubMed] [Google Scholar]
- 7.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 8.Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther. 2006;8(Suppl 2):S2. doi: 10.1186/ar1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheller J, Ohnesorge N, Rose-John S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol. 2006;63:321–329. doi: 10.1111/j.1365-3083.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- 10.Ara T, DeClerck YA. Interleukin-6 in bone metastasis and cancer progression. Eur J Cancer. 2010;46:1223–1231. doi: 10.1016/j.ejca.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for Translational Therapeutics. Cancer. 2007;110:1911–1928. doi: 10.1002/cncr.22999. [DOI] [PubMed] [Google Scholar]
- 12.Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM. Interleukin-6 is a potent growth factor for ER-{alpha}-positive human breast cancer. FASEB J. 2007 doi: 10.1096/fj.07-8832com. [DOI] [PubMed] [Google Scholar]
- 13.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Pu YS, Hour TC, Chuang SE, Cheng AL, Lai MK, Kuo ML. Interleukin-6 is responsible for drug resistance and anti-apoptotic effects in prostatic cancer cells. Prostate. 2004;60:120–129. doi: 10.1002/pros.20057. [DOI] [PubMed] [Google Scholar]
- 15.Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert LA, Hemann MT. DNA damage-mediated induction of a chemoresistant niche. Cell. 2010;143:355–366. doi: 10.1016/j.cell.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas X, Anglaret B, Magaud JP, Epstein J, Archimbaud E. Interdependence between cytokines and cell adhesion molecules to induce interleukin-6 production by stromal cells in myeloma. Leuk Lymphoma. 1998;32:107–119. doi: 10.3109/10428199809059251. [DOI] [PubMed] [Google Scholar]
- 18.Sohara Y, Shimada H, DeClerck YA. Mechanisms of bone invasion and metastasis in human neuroblastoma. Cancer Lett. 2005;228:203–209. doi: 10.1016/j.canlet.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 19.Sohara Y, Shimada H, Minkin C, Erdreich-Epstein A, Nolta JA, DeClerck YA. Bone marrow mesenchymal stem cells provide an alternate pathway of osteoclast activation and bone destruction by cancer cells. Cancer Res. 2005;65:1129–1135. doi: 10.1158/0008-5472.CAN-04-2853. [DOI] [PubMed] [Google Scholar]
- 20.Ara T, Song L, Shimada H, Keshelava N, Russell HV, Metelitsa LS, et al. Interleukin-6 in the bone marrow microenvironment promotes the growth and survival of neuroblastoma cells. Cancer Res. 2009;69:329–337. doi: 10.1158/0008-5472.CAN-08-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukaya Y, Shimada H, Wang LC, Zandi E, DeClerck YA. Identification of Gal-3 binding protein as a factor secreted by tumor cells that stimulates interleukin-6 expression in the bone marrow stroma. J Biol Chem. 2008;283:18573–18581. doi: 10.1074/jbc.M803115200. [DOI] [PubMed] [Google Scholar]
- 22.Song L, Asgharzadeh S, Salo J, Engell K, Wu HW, Sposto R, et al. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest. 2009;119:1524–1536. doi: 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasaki T, Brakebusch C, Engel J, Timpl R. Mac-2 binding protein is a cell-adhesive protein of the extracellular matrix which self-assembles into ring-like structures and binds beta1 integrins, collagens and fibronectin. EMBO J. 1998;17:1606–1613. doi: 10.1093/emboj/17.6.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ullrich A, Sures I, D'Egidio M, Jallal B, Powell TJ, Herbst R, et al. The secreted tumor-associated antigen 90K is a potent immune stimulator. J Biol Chem. 1994;269:18401–18407. [PubMed] [Google Scholar]
- 25.Kalayci O, Birben E, Tinari N, Oguma T, Iacobelli S, Lilly CM. Role of 90K protein in asthma and TH2-type cytokine expression. Ann Allergy Asthma Immunol. 2004;93:485–492. doi: 10.1016/S1081-1206(10)61417-2. [DOI] [PubMed] [Google Scholar]
- 26.Nakahara S, Raz A. On the role of galectins in signal transduction. Methods Enzymol. 2006;417:273–289. doi: 10.1016/S0076-6879(06)17019-6. [DOI] [PubMed] [Google Scholar]
- 27.Haudek KC, Spronk KJ, Voss PG, Patterson RJ, Wang JL, Arnoys EJ. Dynamics of galectin-3 in the nucleus and cytoplasm. Biochim Biophys Acta. 2010;1800:181–189. doi: 10.1016/j.bbagen.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sioud M, Mobergslien A, Boudabous A, Floisand Y. Evidence for the involvement of galectin-3 in mesenchymal stem cell suppression of allogeneic T-cell proliferation. Scand J Immunol. 2010;71:267–274. doi: 10.1111/j.1365-3083.2010.02378.x. [DOI] [PubMed] [Google Scholar]
- 29.Shimada H, Chatten J, Newton W, Jr., Sachs N, Hamoudi AB, Chiba T, et al. Histopathologic prognostic factors in neuroblastic tumors: definition of subtypes of ganglioneuroblastoma and an age-linked classification of neuroblastoma. J Natl Cancer Inst. 1984;73:405–416. doi: 10.1093/jnci/73.2.405. [DOI] [PubMed] [Google Scholar]
- 30.Shimada H, Ambros IM, Dehner LP, Hata J-I, Joshi VV, Roald B. Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee. Cancer. 1999;86:348–362. [PubMed] [Google Scholar]
- 31.Lee JH, Zhang X, Shin BK, Lee ES, Kim I. Mac-2 binding protein and galectin-3 expression in mucinous tumours of the ovary: an annealing control primer system and immunohistochemical study. Pathology. 2009;41:229–233. doi: 10.1080/00313020902756279. [DOI] [PubMed] [Google Scholar]
- 32.Park YP, Choi SC, Kim JH, Song EY, Kim JW, Yoon DY, et al. Up-regulation of Mac-2 binding protein by hTERT in gastric cancer. Int J Cancer. 2007;120:813–820. doi: 10.1002/ijc.22369. [DOI] [PubMed] [Google Scholar]
- 33.Ulmer TA, Keeler V, Loh L, Chibbar R, Torlakovic E, Andre S, et al. Tumor-associated antigen 90K/Mac-2-binding protein: possible role in colon cancer. J Cell Biochem. 2006;98:1351–1366. doi: 10.1002/jcb.20784. [DOI] [PubMed] [Google Scholar]
- 34.Bair EL, Nagle RB, Ulmer TA, Laferte S, Bowden GT. 90K/Mac-2 binding protein is expressed in prostate cancer and induces promatrilysin expression. Prostate. 2006;66:283–293. doi: 10.1002/pros.20343. [DOI] [PubMed] [Google Scholar]
- 35.Marchetti A, Tinari N, Buttitta F, Chella A, Angeletti CA, Sacco R, et al. Expression of 90K (Mac-2 BP) correlates with distant metastasis and predicts survival in stage I non-small cell lung cancer patients. Cancer Res. 2002;62:2535–2539. [PubMed] [Google Scholar]
- 36.Shalom-Feuerstein R, Cooks T, Raz A, Kloog Y. Galectin-3 regulates a molecular switch from N-Ras to K-Ras usage in human breast carcinoma cells. Cancer Res. 2005;65:7292–7300. doi: 10.1158/0008-5472.CAN-05-0775. [DOI] [PubMed] [Google Scholar]
- 37.Kim SJ, Lee SJ, Sung HJ, Choi IK, Choi CW, Kim BS, et al. Increased serum 90K and Galectin-3 expression are associated with advanced stage and a worse prognosis in diffuse large B-cell lymphomas. Acta Haematol. 2008;120:211–216. doi: 10.1159/000193223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.