Abstract
Objective
Tumor necrosis factor (TNF)-α, a pleiotropic pro-inflammatory cytokine involved in a variety of biological processes including oxidative stress, has been associated with vascular dysfunction in aged and ovariectomized animals. We determined whether acute inhibition of TNF-α improves vascular endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women.
Methods
Arterial stiffness (carotid artery compliance) and endothelial function (brachial artery flow-mediated dilation [FMD]) were measured in postmenopausal women (n=23; 57±1 years, mean±SE) before and following randomization to two days of either transdermal estradiol (0.05 mg/d, N=12) or placebo (N=11) alone and following a single subcutaneous injection of the TNF-α inhibitor, etanercept (25 mg), and in premenopausal (n=9; 33±2 years) before and following etanercept.
Results and Conclusions
Baseline carotid artery compliance and brachial artery FMD were lower in postmenopausal than premenopausal women (p<0.0001). In postmenopausal women, carotid artery compliance (n=12; 0.59±0.05 to 0.78±0.06 mm2/mmHg × 10−1, P<0.001) and FMD (4.1±0.6 to 6.0±0.7%, P=0.02) increased in response to estradiol but not placebo (n=11). Carotid artery compliance (0.71±0.06 to 0.81±0.06 mm2/mmHg × 10−1, P=0.02) and FMD (5.2±0.7 to 7.5±0.9%, P=0.003) increased with etanercept in the placebo group but had no effect in postmenopausal randomized to estradiol or premenopausal women. These results suggest that TNF-α contributes to impaired endothelial-dependent vasodilation and arterial stiffening in estrogen-deficient postmenopausal women.
Keywords: aging, menopause, endothelium, inflammation, cardiovascular disease, imaging
INTRODUCTION
Even though death rates from cardiovascular disease (CVD) have been declining, CVD is still a major public health concern in women. Vascular aging, featuring endothelial dysfunction and arterial stiffening precede clinical disease and increase the risk for the development of atherosclerosis and other CVD1. Vascular aging in women is unique because of the influence of the decline in estrogen with the menopause transition. Indeed endothelial dysfunction and arterial stiffening appear to worsen with the loss of ovarian function and estrogen deficiency suggesting that declines in estrogen with menopause contributes to endothelial dysfunction and arterial stiffening in postmenopausal women2, 3. With the increasing life expectancy of women, more than one-third of their lifespan will occur after menopause. As such, understanding how the loss of estrogen in addition to aging contributes to endothelial dysfunction and arterial stiffening is of clinical importance for the prevention of CVD.
We and others have previously demonstrated that oxidative stress is a key mechanism mediating large artery stiffening and endothelial dysfunction with aging and with estrogen deficiency in women4, 5. Tumor necrosis factor (TNF)-α is a pleiotropic inflammatory cytokine that is involved in a variety of biological processes including oxidative stress6, 7. Thus, it is plausible that the oxidative-stress mediated arterial stiffening and endothelial dysfunction observed with aging and estrogen-deficiency in women is related, in part to, TNF-α-mediated processes. In animal studies, antagonism of TNF-α with etanercept reduced reactive oxygen species (ROS) and improved endothelial vasodilatory function in carotid arteries from aged male rats7 and in mesenteric arteries from aged and ovariectomized female rats6. Additionally, estrogen treatment was shown to reduce TNF-α levels in uterine arteries obtained from postmenopausal women9. However, whether TNF-α is mechanistically involved in endothelial dysfunction and arterial stiffening with aging and estrogen deficiency in women has not been studied. Accordingly, we tested the hypothesis that acute TNF-α inhibition improves brachial artery flow-mediated dilation (FMD) and carotid artery compliance, measures of endothelial function and arterial stiffness, respectively, in estrogen-deficient postmenopausal women, but not in estrogen-replete women (i.e., postmenopausal women treated with estradiol and eumenorrheic premenopausal controls) because of the potential antagonistic effects of estrogen on TNF-α processes.
MATERIALS AND METODS
Study Population
We recruited healthy premenopausal and postmenopausal women as part of an ongoing investigation on the biological mechanisms mediating arterial stiffening in estrogen-deficient postmenopausal women. Premenopausal and postmenopausal women were included if they were between the ages of 18–40 years or 50–65 years, respectively. Forty-seven women consented to participate, of which 9 women were excluded and 5 women dropped out during screening, leaving 32 women (23 postmenopausal, aged 51–65 years; 9 premenopausal) who completed the study. Postmenopausal women were at least one year past the absence of menses and premenopausal women were eumenorrheic. Participants were included if they met the following eligibility criteria: sedentary or recreationally active (not exercising regularly > 2 days/week), non-smokers, resting blood pressure <140/90 mmHg, fasted plasma glucose concentrations <7.0 mmol/L (126 mg/dL), fasted LDL-cholesterol <4.1 mmol/L (160 mg/dl), BMI < 36 kg/m2, no history of CVD, and free of overt chronic diseases and inflammatory conditions as assessed by medical history, physical examination, standard blood chemistries and hematological evaluation. Participants had not taken any oral contraceptives, hormone therapy, cardiovascular or lipid-lowering medications for at least 6 months or aspirin, non-steroidal anti-inflammatory medications or vitamin supplements for at least 4 weeks. Postmenopausal women with contraindications to estrogen (e.g., history of active estrogen-dependent neoplasms, thromboembolism) or etanercept (e.g., positive Mantoux test, positive hepatitis B surface antigen, active infection) were excluded. All subjects gave their written informed consent to participate. The research was carried out in accordance with the Declaration of Helsinki of the World Medical Association and all procedures were reviewed and approved by the Colorado Multiple Institutional Review Board.
Measurements
Participants were studied in the supine position following an overnight fast with proper hydration (water drinking only) and abstinence from caffeine for ≥ 12 hours. Normal dietary patterns were maintained, including sodium intake, for the 2-day period immediately prior to any measurements. Premenopausal women were tested 7 to 10 days after onset of menstruation (i.e., mid-follicular phase) so that vascular comparisons between premenopausal and postmenopausal women randomly assigned to the estradiol treatment group would be at similar estradiol concentrations. The study took place at the University of Colorado Clinical and Translational Sciences Institute (CCTSI) Clinical and Translational Research Center (CTRC).
Arterial Stiffness
Carotid artery compliance was determined using high-resolution ultrasound imaging, as previously described10. Carotid images were analyzed for arterial distention using semi-automated wall tracking software (Vascular Analysis Tools v. 5.5; Medical Imaging Applications, LLC, Iowa City, IA). The same segment of the carotid artery (~1–2 cm distal to carotid bulb) was analyzed for baseline and follow-up images. All images were coded by number, blinded to group assignment and imaged and analyzed by the same individual. The coefficient of variation (CV) and intra-class correlation coefficient (ICC) for trial-to trial reliability measured in 13 individuals for carotid artery diameter, carotid artery distention, pulse pressure and carotid artery compliance were 0.7% and 0.99, 4.2% and 0.99, 3.7% and 0.97, and 3.1% and 0.99, respectively.
Brachial Artery Blood Pressure
Peripheral arterial blood pressure was measured in triplicate in the seated and the supine positions with a semi-automated device (Dinamap, Johnson & Johnson) over the brachial artery, as previously described10.
Vascular Endothelial-Dependent Vasodilation
Ultrasound measurements of brachial artery flow-mediated dilation (FMD) were performed as previously described11. Briefly, a pediatric cuff was placed on the upper forearm and brachial artery images were acquired ~ 3–6 cm above the antecubital fossa at baseline and following reactive hyperemia produced by inflating the cuff to 250 mmHg of pressure for 5 minutes. After the release of the arterial occlusion, the initial ten Doppler blood flow velocity waveform envelopes were acquired and B-mode ultrasound brachial artery diameter images were measured continuously for 2 minutes. Brachial artery diameter was analyzed using a commercially available semi-automated wall-tracking software package and analyzed by the same individual who was blinded to group assignment (Vascular Analysis Tools 5.5.1, Medical Imaging Applications, LLC, Iowa City, IA). Brachial artery peak hyperaemic shear rate was calculated as the peak mean blood velocity divided by occlusion diameter. All procedures conformed to recently published guidelines for assessing FMD in human subjects12. Placement of the ultrasound probe was measured with a tape measure and landmarks were identified (e.g., branch-points and veins) to ensure that the same location of the brachial artery was analyzed. The CV and ICC for trial-to trial reliability measured in 10 individuals for baseline brachial artery diameter, peak diameter and FMD (%) were 2% and 0.97, 1.5% and 0.99, and 2.2% and 0.99, respectively.
Body Composition, Metabolic Risk Factors, Sex Hormones and Circulating Humoral Factors
Total and trunk fat mass and fat-free mass were determined using dual energy x-ray absorptiometry (Hologic Discovery, version 12.6). Minimal waist and hip circumferences were measured and waist-tohip ratio (WHR) was calculated as previously described11. Fasted plasma concentrations of insulin, and blood lipids and lipoproteins were determined as previously described11. Serum estradiol was measured using chemiluminescense (Beckman Coulter) and plasma endothelin-1 (ET-1), interleukin (IL)-6 and TNF-α were measured using an enzyme-linked immunoassay2, 13. High-sensitivity C-reactive protein (hs-CRP) was measured using immunoturbidimetric method. Oxidized LDL, an indirect measure of oxidative stress, was determined with ELISA plate assays (Alpco Diagnostics, Windham, NH). Total antioxidant status (TAS), a measure of the overall antioxidant defenses, was determined on serum samples using the Randox Laboratories enzymatic kit (Oceanside, CA). All assays were performed by the CTRC core laboratory.
Experimental Design
Vascular assessment was first assessed in premenopausal and postmenopausal women prior to the start of the interventions. Next, postmenopausal women were randomly assigned to either transdermal placebo (n=11) or estradiol (0.05 mg/d, n=12) for two days and vascular assessment was repeated. The randomization scheme was developed by a biostatistician and executed by a research assistant not affiliated with data analyses. All participants were blinded to group assignment (placebo or estradiol patch) and were not told of the type of regimen being administered to them. Additionally, any member of the investigative team involved in the acquisition or analysis of data on any of the key dependent variables were unaware of the treatment status of the subjects. Postmenopausal women remained on their assigned placebo or estradiol treatment and were administered a single 25 mg subcutaneous injection of the TNF-α inhibitor, etanercept (Enbrel®) and vascular measurements were repeated two days later. Premenopausal women were also administered a single dose of 25 mg etanercept and vascular measures were re-assessed two days later. Etanercept is a soluble TNF receptor fusion protein that binds specifically to TNF and blocks its interaction with cell surface TNF receptors, preventing TNF-mediated cellular responses by rendering TNF biologically inactive. Etanercept may also modulate inflammatory responses controlled by other cytokines or adhesion molecules that are regulated and/or induced by TNF. The dose and duration of etanercept was chosen because a single 25 mg subcutaneous injection has been previously shown to increase endothelial-dependent vasodilation within 6 hours in heart failure patients14. Additionally, pharmacokinetic data of etanercept in healthy volunteers demonstrated a time to peak concentration of 51±14 hours (mean±SD) and a half-life of 68±19 hours15. Etanercept has FDA approval for the treatment of rheumatoid arthritis and psoriasis.
Statistical Analysis
All data elements were examined using descriptive statistics. Parameters with skewed distributions were log transformed and are presented as median and interquartile range. ANOVA was used to assess group differences in subject characteristics, humoral factors, baseline brachial artery FMD and carotid artery compliance. Two-way repeated measures ANOVA (group × time) were used to determine the effects of transdermal and estradiol (in postmenopausal women), and etancercept (in all three groups) on brachial artery FMD and carotid artery compliance. If significant differences were observed, paired t tests were used to determine differences among the mean values. ANCOVA was used to adjust for baseline differences and changes in factors that could confound interpretation of the results. Secondary analyses were performed using ANCOVA to adjust for baseline carotid artery compliance and brachial artery FMD on the changes in carotid artery compliance and FMD with transdermal estradiol or placebo, and with etanercept. The study was powered to test the hypothesis that TNF-α antagonism using etanercept would improve carotid artery compliance and brachial artery FMD in estrogen-deficient postmenopausal women (i.e., those women treated with placebo), and that TNF-α antagonism would not improve vascular function in estrogen-replete women (i.e., postmenopausal women treated with estrogen and premenopausal women). Power and sample size calculations were determined from previously published data on the effects of etancercept and estradiol on brachial artery FMD in rheumatoid arthritis patients16 and postmenopausal women11. No data exist on the effects of etancercept on carotid artery compliance, thus power calculations were estimated from published data from our laboratory on changes in carotid artery compliance with acute interventions of transdermal estradiol, ascorbic acid and tetrahydrobiopterin4, 11, 13. Based on these studies, there would be 80% power at an α of 0.05, with sample sizes of 10–12 postmenopausal women per group to detect anticipated changes in brachial artery FMD and carotid artery compliance of 1.5% and 0.1 mm2/mmHg × 10−1, respectively, with etanercept. Data analysis was performed with SPSS software, version 21.0.
RESULTS
Participant Characteristics
Postmenopausal women randomized to the estradiol treatment group had higher trunk body fat content and systolic blood pressure compared to premenopausal women (Table 1, both P<0.05). There were no other group differences in clinical characteristics among the groups.
Table 1.
Characteristics of estradiol and placebo treated postmenopausal and premenopausal women
| Variable | Estradiol | Placebo | Premenopausal |
|---|---|---|---|
| n | 12 | 11 | 9 |
| Age, years | 57±3 | 59±4 | 33±6 |
| Menopause Duration, yr | 9.2±5.6 | 8.5±6.2 | -- |
| Body Mass, kg | 69.6±12.0 | 64.5±10.2 | 63.1±7.5 |
| BMI | 26.5±4.0 | 25.2±5.1 | 22.7±3.1 |
| Body Fat, % | 39±6 | 35±10 | 31±10 |
| Trunk Fat, % | 38±8* | 34±12 | 25±10 |
| Waist Circumference, cm | 83±22 | 83±15 | 77±8 |
| WHR | 0.85±0.09 | 0.81±0.09 | 0.81±0.06 |
| Systolic BP, mmHg | 122±16* | 117±14 | 103±8 |
| Diastolic BP, mmHg | 75±13 | 69±10 | 67±10 |
| Total-Cholesterol, mmol/L | 4.9±0.8 | 5.1±0.8 | 4.3±0.7 |
| LDL-Cholesterol, mmol/L | 2.8±0.6 | 3.0±0.7 | 2.4±0.7 |
| HDL-cholesterol, mmol/L | 1.6±0.4 | 1.6±0.4 | 1.5±0.3 |
| Fasting Glucose, mmol/L | 4.9±0.3 | 4.9±0.5 | 4.5±0.4 |
Data are mean±SD or median (interquartile range).
P<0.05 vs premenopausal.
BMI, body mass index; WHR=waist to hip ratio; BP, blood pressure; LDL, low density lipoprotein; HDL, high density lipoprotein.
Circulating Humoral Factors
Baseline insulin concentrations were higher in postmenopausal women who were randomized to estradiol treatment compared to premenopausal women and estradiol concentrations were lower in both postmenopausal groups compared to premenopausal women (both P<0.05). There were no group differences in baseline TNF-α or any other circulating humoral factor (Table 2). There was a trend for group differences in hsCRP (P=0.07).
Table 2.
Circulating inflammatory, oxidative stress and humoral factors before and after estradiol, placebo and etanercept treatments
| Variable | Premenopausal | Postmenopausal Estradiol |
Postmenopausal Placebo |
|---|---|---|---|
| TNFα, pg/mL | |||
| Baseline | 0.8(0.6–1.4) | 1.3 (0.8–1.8) | 1.0 (0.8–1.2) |
| Estrogen or Placebo | -- | 1.0 (0.8–1.6) | 1.0 (0.9–1.2) |
| Etanercept§ | 109.7 (103.9–132.8)† | 108.5 (95.3–145.2)† | 135.8 (89.9–192.8)† |
| IL6, pg/mL | |||
| Baseline | 0.7(0.4–1.6) | 1.4 (1.0–2.0) | 1.3 (0.7–2.6) |
| Estrogen or Placebo | -- | 1.2 (0.8–1.9) | 1.5 (0.7–1.9) |
| Etanercept§ | 0.8(0.4–1.0) | 1.2 (0.9–1.4) | 1.0 (0.6–1.8) |
| CRP, mg/L | |||
| Baseline | 0.5 (1.7) | 1.4 (0.8–3.6) | 1.9 (1.1–3.7) |
| Estrogen or Placebo | -- | 1.4 (0.5–4.1) | 1.6 (0.9–3.1) |
| Etanercept§ | 0.4 (0.3–1.2) | 1.3 (0.5–2.7)‡ | 1.7 (0.7–3.7) |
| Oxidized LDL, U/L | |||
| Baseline | 47.3±16.5 | 53.3±11.5 | 58.4±21.1 |
| Estrogen or Placebo | -- | 56.2±12.6 | 56.7±16.4 |
| Etanercept§ | 47±15.1 | 55.5±11.0 | 55.4±18.0 |
| TAS, mmol/L | |||
| Baseline | 1.36±0.10 | 1.44±0.12 | 1.35±0.16 |
| Estrogen or Placebo | -- | 1.40±0.13 | 1.41±0.14 |
| Etanercept§ | 1.40±0.14 | 1.43±0.13 | 1.38±0.12 |
| Endothelin-1, pg/mL | |||
| Baseline | 5.8±0.8 | 6.5±1.7 | 7.0±1.5 |
| Estrogen or Placebo | -- | 6.2±1.0 | 7.6±0.9 |
| Etanercept§ | 5.7±1.3 | 6.3±1.6 | 7.1±1.6 |
| Glucose, mmol/L | |||
| Baseline | 4.7±02 | 5.1±0.4 | 5.0±0.4 |
| Estrogen or Placebo | -- | 5.0±0.5 | 5.0±0.7 |
| Etanercept§ | 4.7±0.4 | 5.1±0.5 | 4.9±0.5 |
| Insulin, pmol/L | |||
| Baseline | 63 (31–70) | 76 (70–115)* | 76 (63–90) |
| Estrogen or Placebo | -- | 63 (56–87)† | 76 (63–83) |
| Etanercept§ | 49 (38–83) | 70 (57–102) | 70 (65–83) |
| Estradiol, pmol/L | |||
| Baseline | 286±160 | 66±27* | 63±27* |
| Estrogen or Placebo | -- | 297±116† | 63±27 |
| Etanercept§ | -- | 231±92 | 62±26 |
All data are mean±SD or median (interquartile range).
TNFα=tumor necrosis factor-α; CRP, C-reactive protein; IL-6, interleukin-6; TAS, total antioxidant status.
Estrogen/placebo co-administered with etanercept in postmenopausal women, and etanercept alone in premenopausal women;
P<0.05 vs premenopausal;
P<0.05 vs Baseline of the same group;
P<0.05 vs Follow-Up.
In estradiol-treated women, insulin concentrations decreased and, as expected, estradiol concentrations increased (both P<0.05, Table 2). Also as expected, etanercept increased TNF-α concentrations by ~ 100 fold in all three groups (P<0.001, Table 2). In estradiol-treated postmenopausal women, hsCRP decreased following etanercept (P<0.05). There were no other changes in circulating inflammatory or oxidative stress markers or ET-1 following estradiol, placebo or etanercept.
Arterial Stiffness and Endothelial Function
Baseline carotid artery compliance and brachial artery FMD (both relative and absolute change) were lower in postmenopausal than premenopausal women (Figure 1a and 1b, P<0.001). Postmenopausal women had a smaller carotid artery distention and larger brachial artery diameters than premenopausal women (P<0.05, Supplemental Table 1). In postmenopausal women randomized to estradiol, baseline mean arterial and pulse pressure were higher compared to premenopausal women (P<0.05, Supplemental Table 1).
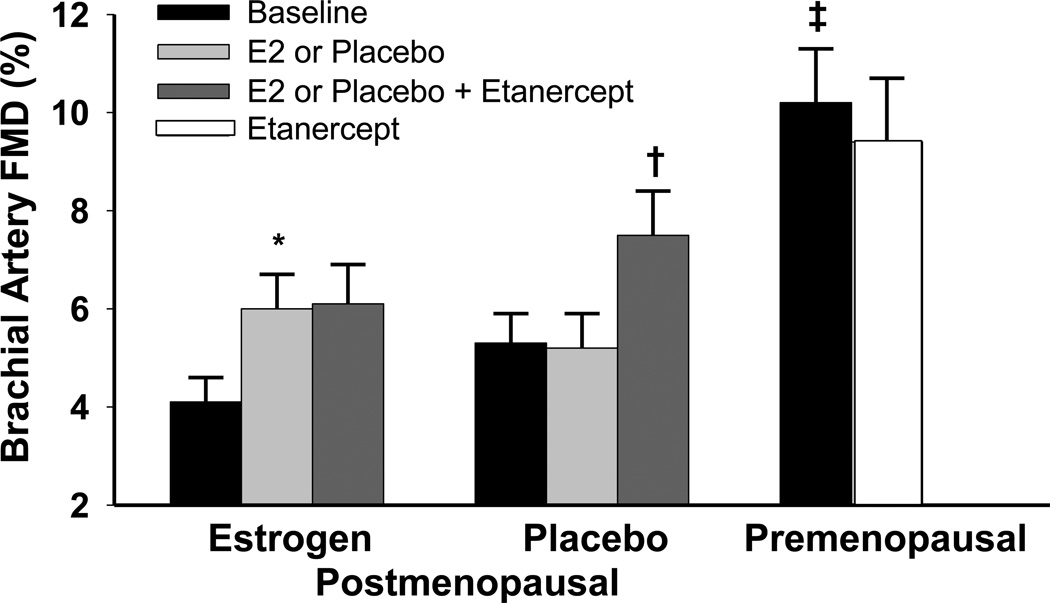
Figure 1.
a) Carotid artery compliance, and b) brachial artery FMD at baseline and following transdermal placebo or estradiol, and after anti-TNF-α treatment in postmenopausal women, and at baseline and after anti-TNF-α in premenopausal women. Data are means±SE. *P<0.005 vs. baseline of the same group; † P<0.005 vs. after placebo treatment; ‡P<0.001 vs. postmenopausal women of either group.
In postmenopausal women, carotid artery compliance and brachial artery FMD increased by 35.5 ± 6% and 65.8 ± 20.8%, respectively, in response to transdermal estradiol (Figure 1a, P<0.001; 1b, P=0.019) but remained unchanged with placebo (P=0.33 and P=0.63; respectively). Although mean arterial blood pressure and pulse pressure decreased with transdermal estradiol (both P<0.05; Supplemental Table 1), the changes in carotid artery compliance and brachial artery FMD with estradiol were still significant after ANCOVA adjustment for changes in MAP, pulse pressure and baseline carotid artery compliance and FMD (P<0.05). There were no changes in carotid or brachial artery diameters, brachial artery peak hyperemic shear rates or heart rate with estradiol or placebo treatment (Supplemental Table 1).
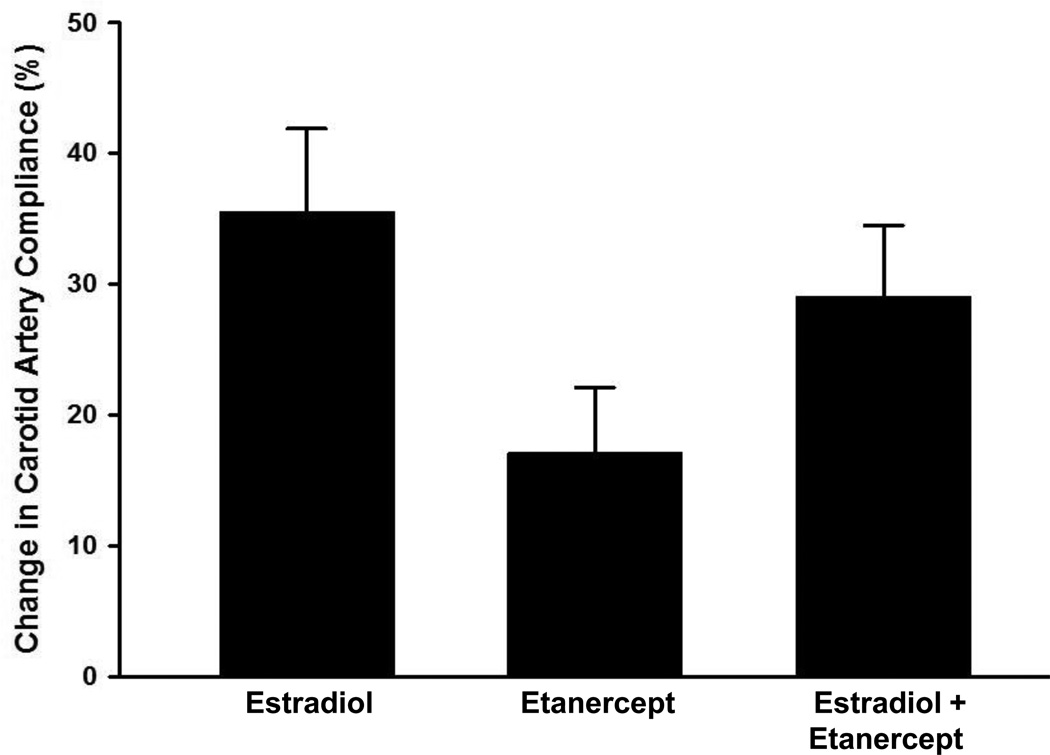
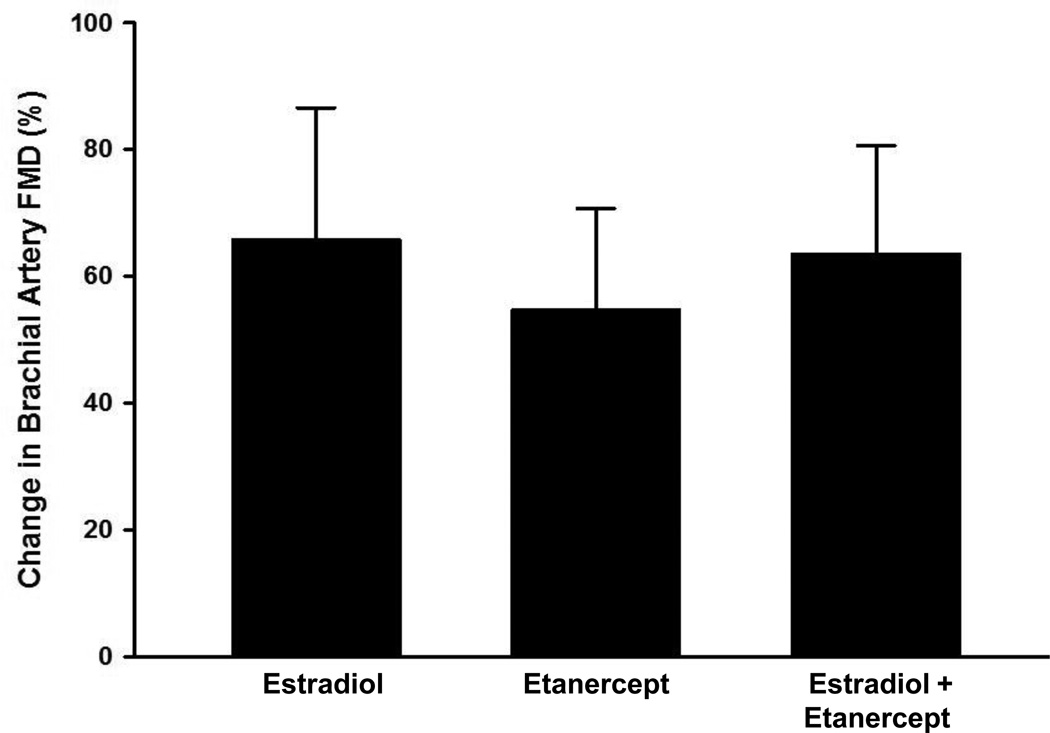
Carotid artery compliance (P=0.004) and brachial artery FMD (P=0.003, Figure 1a and 1b) increased by 17.1±4.3% and 54.7±15.6% following etanercept administration in the placebo-treated women, but did not change significantly in premenopausal or estradiol-treated postmenopausal women (all P>0.50). Adjusting for baseline values did not alter the findings. The magnitude of change in carotid artery compliance tended to be greater (P=0.08, Figure 2a) with estradiol treatment alone compared to etanercept treatment alone but there was no greater magnitude in change with the co-administration of etanercept with estradiol. There were no differences in the magnitude of change in brachial artery FMD in response to either estradiol or etanercept alone or the co-administration of the two (Figure 2b). Carotid and brachial artery diameter, brachial artery peak hyperemic shear rates and heart rate did not change with etanercept administration in any group (Supplemental Table 1).
Figure 2.
a) Relative change in carotid artery compliance, and b) brachial artery FMD in response to transdermal estradiol alone, etanercept alone, and estradiol co-administered with etanercept in postmenopausal women. Data are means±SE.
Adverse Events
Both transdermal estradiol and etanercept were well tolerated. One estradiol-treated postmenopausal and 1 premenopausal woman reported local irritation or bruising/swelling at the injection site and 3 women reported headaches following etanercept administration. One placebo-treated postmenopausal woman reported a fever and flu-like symptoms that resulted in an upper respiratory tract infection 1 week after completion of the study. Finally, 1 woman who was in the estrogen-treated group developed a headache, injection site redness and swelling, and rash on the chest and arms 12 days after the administration of etanercept.
DISCUSSION
The findings of the present study provide novel insight into the effects of TNF-α on vascular function in estrogen-deficient postmenopausal women. Specifically, our findings support the idea that TNF-α is a mediator of endothelial dysfunction and arterial stiffening in estrogen-deficient postmenopausal women and that the favorable influence of estradiol on endothelial function and arterial compliance may be related, in part, by altering TNF-α effects.
TNF-α-mediated Endothelial Dysfunction and Arterial Stiffening in Estrogen-deficient Postmenopausal Women
In the present study, brachial artery FMD and carotid artery compliance were lower in postmenopausal women compared to premenopausal women, consistent with previous observations2–4, 10, 11. The mechanisms mediating endothelial dysfunction and arterial stiffening with aging and estrogen deficiency in women has been an important area of study. To our knowledge, the present study was the first to examine the direct influence of the pro-inflammatory cytokine TNF-α on vascular function in healthy postmenopausal women. The key new findings were that acute inhibition of the effects of TNF-α with etanercept increased brachial artery FMD and carotid artery compliance in postmenopausal women randomized to placebo treatment, but remained unchanged in eumenorrheic premenopausal women. Additionally, although brachial artery FMD and carotid artery compliance increased in postmenopausal women randomized to transdermal estradiol, there were no further improvements in either measurement with the co-administration of etanercept, possibly because of antagonism of TNF-α mediated biological processes with estradiol treatment. It is also possible that estradiol and etanercept have similar mechanisms of action on vascular function. Indeed there were no differences in the magnitude of improvements in response to etanercept and estradiol alone or combined. Additionally, although, circulating TNF-α concentrations were augmented ~100 fold after administration of etanercept, consistent with previous investigations17, the increase in TNF-α concentrations appear to be attenuated in estrogenreplete women (i.e., premenopausal and estradiol-treated postmenopausal women). The mechanism for elevations of circulating TNF-α levels after treatment with etanercept are unknown; however, they are most likely due to prolongation of TNF-α half-life by etanercept, despite inhibition of TNF activity. Thus, the assay may have measured TNF-α that was bound to etanercept18. Our results are consistent with data reported in ovariectomized rats demonstrating that the impaired endothelial vasodilatory response with estrogen deficiency is prevented with etanercept treatment6. Additionally, acute and chronic TNF-α inhibition has been reported previously to decrease arterial stiffness, and improve endothelial vasodilatory function in men and women with advanced heart failure and those with rheumatoid arthritis14, 16, 19.
Potential Mechanisms of the Modulatory Influence of TNF-α on Endothelial Function and Arterial Stiffening
We can only speculate as to the mechanisms by which TNF-α modulates endothelial function and arterial stiffness in estrogen-deficient postmenopausal women. TNF-α is a pleotropic factor that can work in a paracrine manner. TNF-α stimulates the production of acute phase reactants (e.g., CRP), and activates endothelial cells to express adhesion molecules, chemoattractants and promotes leukocyte accumulation in the vascular wall20. Additionally, TNF-α can down-regulate and inactivate the NO producing enzyme, endothelial nitric oxide synthase (eNOS)21, up-regulate other inflammatory and vasoconstrictors (e.g., ET-1, angiotensin II)7, 22, and induce oxidative stress by increasing the production of ROS, which can scavenge NO and decrease endothelial vasodilatory function6, 7. Finally, inflammation may reduce tetrahydrobiopterin (BH4), an essential co-factor for eNOS, either directly or through the production of ROS, leading to eNOS uncoupling, and consequently decreasing NO and increasing ROS production23. Because NO is a potent regulator of arterial stiffness24, inflammation-induced endothelial dysfunction may mediate at least part of the arterial stiffening in estrogen-deficient postmenopausal women. In this regard, we previously demonstrated that carotid artery compliance improves in response to systemic infusion of ascorbic acid, an antioxidant, and to oral supplementation of BH4 in estrogen-deficient postmenopausal women4, 11. Future investigations will need to examine these TNF-α mediated biological processes further.
Possible TNF-α Inhibitory Mechanisms Associated with Estrogen-mediated Improvements in Endothelial Function and Arterial Stiffness
It is unclear as to why there were no additional improvements in vascular function with the administration of etanercept to the estradiol-treated postmenopausal women. Although beyond the scope of this pilot investigation, it is plausible to speculate that the improvements in endothelial function and arterial stiffness in response to transdermal estradiol may have been related to antagonizing TNF-α mediated biological processes. Estrogen has previously been shown to antagonize the pro-inflammatory effects of TNF-α by inhibiting nuclear factor-kappa-B (NK-κB), an inducible nuclear transcription factor, consequently reducing adhesion molecules and the release of other inflammatory markers and ROS25. Additionally, estrogen suppresses TNF-α and pro-inflammatory cytokines through reducing ROS-stimulated pro-inflammatory cytokine expression, and by enhancing NO release, which itself is anti-inflammatory6, 26.
Considerations and Experimental Limitations
Our findings can only be generalized to apparently healthy women without overt disease. We recognize that because our postmenopausal women had higher levels of trunk fat, blood pressure, insulin and cholesterol compared to premenopausal women that these factors may have contributed to the TNF-α effects. The finding that brachial artery FMD and carotid artery compliance did not reach levels observed in premenopausal women with etanercept and/or estradiol indicates that other mechanisms are involved including local factors, as well as structural alterations within the arterial wall that cannot be modified with short-term etanercept or estrogen. It is possible that a higher dose of etanercept and/or longer duration of treatment may have resulted in a greater improvement in or restoration of endothelial function and arterial compliance.
Two important limitations to our study include the small sample size and the fact that we did not conduct a randomized placebo controlled study of etanercept. Because of the small sample size the randomization of postmenopausal women to either placebo or estradiol was not fully successful because of the apparent, albeit not statistically significant, differences in baseline brachial artery FMD and carotid artery compliance. Importantly, etanercept improved vascular function in the placebo-treated postmenopausal women, who we hypothesized would demonstrate improvements with TNF-α inhibition, and not in the estrogen-treated postmenopausal or premenopausal women, who we hypothesized would not demonstrate improvements with TNF-α inhibition. These findings remained after adjusting for baseline levels of brachial artery FMD and carotid artery compliance.
It is important to emphasize that the aim of the present investigation was to acquire preliminary insight into the role of TNF-α in mediating the endothelial dysfunction and arterial stiffening with estrogen deficiency in postmenopausal women, not to determine the efficacy of etanercept and transdermal estradiol for improving vascular function and reducing CVD events in women. Although etanercept was well tolerated in our study population, there were some minor to moderate adverse events, and thus, it would not be appropriate to administer etanercept to healthy postmenopausal women for the prevention and treatment of vascular dysfunction. Nonetheless, a better understanding of the mechanisms underlying aging and estrogen action in the vasculature will help inform the development of future prevention strategies or other therapies for CVD prevention in women.
Conclusions
Our findings support the accumulating evidence in humans and animals suggesting an important role for TNF-α in vascular dysfunction and increased risk for the development of atherosclerosis. Specifically, our data suggest that TNF-α is mechanistically involved in endothelial dysfunction and arterial stiffening in estrogen-deficient postmenopausal women, and that the improvement in vascular function with acute transdermal estradiol may be related to altering or antagonizing the effects of TNF-α.
Supplementary Material
Highlights.
Vascular dysfunction is observed in estrogen-deficient postmenopausal women
Transdermal estrogen improves vascular function in postmenopausal women
TNFα blockade improves vascular function in estrogen-deficient postmenopausal women
TNFα blockade does not improve vascular function in estrogen-replete women
Acknowledgements
We thank Lauren Tobin, Chelsea Bergman and Kristina Bishard for their technical assistance.
Funding. This study was supported by the National Institutes of Health [grant numbers R01AG027678, K01AG020683, Colorado Clinical and Translational Sciences Institute UL1-RR-025780, Colorado Nutrition and Obesity Research Center P30 DK048520], and University of Colorado Center for Women’s Health Research and University of Colorado Office of Interdisciplinary Research in Women’s Health. None of these sponsors had any involvement in the study design, the collection, and analysis and interpretation of the data, the writing of the manuscript or the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors Conflicts of Interest
None
References
- 1.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a"set up" for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 2.Moreau KL, Hildreth KL, Meditz AL, et al. Endothelial Function Is Impaired across the Stages of the Menopause Transition in Healthy Women. J Clin Endocrinol Metab. 2012;97:4692–4700. doi: 10.1210/jc.2012-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagai Y, Earley CJ, Kemper MK, et al. Influence of age and postmenopausal estrogen replacement therapy on carotid arterial stiffness in women. Cardiovasc Res. 1999;41:307–311. doi: 10.1016/s0008-6363(98)00219-3. [DOI] [PubMed] [Google Scholar]
- 4.Moreau KL, Gavin KM, Plum AE, et al. Ascorbic Acid Selectively Improves Large Elastic Artery Compliance in Postmenopausal Women. Hypertension. 2005;45:1107–1112. doi: 10.1161/01.HYP.0000165678.63373.8c. [DOI] [PubMed] [Google Scholar]
- 5.Virdis A, Ghiadoni L, Pinto S, et al. Mechanisms responsible for endothelial dysfunction associated with acute estrogen deprivation in normotensive women. Circulation. 2000;101:2258–2263. doi: 10.1161/01.cir.101.19.2258. [DOI] [PubMed] [Google Scholar]
- 6.Arenas IA, Armstrong SJ, Xu Y, et al. Chronic Tumor Necrosis Factor-{alpha} Inhibition Enhances NO Modulation of Vascular Function in Estrogen-Deficient Rats. Hypertension. 2005;46:76–81. doi: 10.1161/01.HYP.0000168925.98963.ef. [DOI] [PubMed] [Google Scholar]
- 7.Csiszar A, Labinskyy N, Smith K, et al. Vasculoprotective Effects of Anti-Tumor Necrosis Factor-{alpha} Treatment in Aging. Am J Pathol. 2007;170:388–698. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donato AJ, Black AD, Jablonski KL, et al. Aging is Associated with Greater Nuclear NFkappaB, Reduced IkappaBalpha and Increased Expression of Proinflammatory Cytokines in Vascular Endothelial Cells of Healthy Humans. Aging Cell. 2008;7:805–812. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novella S, Heras M, Hermenegildo C, et al. Effects of estrogen on vascular inflammation: a matter of timing. Arterioscler Thromb Vasc Biol. 2012;32:2035–2042. doi: 10.1161/ATVBAHA.112.250308. [DOI] [PubMed] [Google Scholar]
- 10.Moreau KL, Donato AJ, Seals DR, et al. Regular exercise, hormone replacement therapy and the age-related decline in carotid arterial compliance in healthy women. Cardiovasc Res. 2003;57:861–868. doi: 10.1016/s0008-6363(02)00777-0. [DOI] [PubMed] [Google Scholar]
- 11.Moreau KL, Meditz A, Deane KD, et al. Tetrahydrobiopterin improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Am J Physiol Heart Circ Physiol. 2012;302:H1211–H1218. doi: 10.1152/ajpheart.01065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 13.Moreau KL, Gavin KM, Plum AE, et al. Oxidative stress explains differences in large elastic artery compliance between sedentary and habitually exercising postmenopausal women. Menopause. 2006;13:951–958. doi: 10.1097/01.gme.0000243575.09065.48. [DOI] [PubMed] [Google Scholar]
- 14.Fichtlscherer S, Rossig L, Breuer S, et al. Tumor Necrosis Factor Antagonism With Etanercept Improves Systemic Endothelial Vasoreactivity in Patients With Advanced Heart Failure. Circulation. 2001;104:3023–3025. doi: 10.1161/hc5001.101749. [DOI] [PubMed] [Google Scholar]
- 15.Korth-Bradley JM, Rubin AS, Hanna RK, et al. The pharmacokinetics of etanercept in healthy volunteers. Ann Pharmacother. 2000;34:161–164. doi: 10.1345/aph.19126. [DOI] [PubMed] [Google Scholar]
- 16.Bilsborough W, Keen H, Taylor A, et al. Anti-tumour necrosis factor-alpha therapy over conventional therapy improves endothelial function in adults with rheumatoid arthritis. Rheumatology International. 2006;26:1125–1131. doi: 10.1007/s00296-006-0147-y. [DOI] [PubMed] [Google Scholar]
- 17.Tsimberidou A-M, Waddelow T, Kantarjian HM, et al. Pilot study of recombinant human soluble tumor necrosis factor (TNF) receptor (p75) fusion protein (TNFR:Fc; Enbrel) in patients with refractory multiple myeloma: increase in plasma TNFα levels during treatment. Leukemia Research. 2003;27:375–380. doi: 10.1016/s0145-2126(02)00082-6. [DOI] [PubMed] [Google Scholar]
- 18.Madhusudan S, Foster M, Muthuramalingam SR, et al. A Phase II Study of Etanercept (Enbrel), a Tumor Necrosis Factor α Inhibitor in Patients with Metastatic Breast Cancer. Clinical Cancer Research. 2004;10:6528–6534. doi: 10.1158/1078-0432.CCR-04-0730. [DOI] [PubMed] [Google Scholar]
- 19.Maki-Petaja KM, Hall FC, Booth AD, et al. Rheumatoid Arthritis Is Associated With Increased Aortic Pulse-Wave Velocity, Which Is Reduced by Anti-Tumor Necrosis Factor-{alpha} Therapy. Circulation. 2006;114:1185–1192. doi: 10.1161/CIRCULATIONAHA.105.601641. [DOI] [PubMed] [Google Scholar]
- 20.Blake GJ, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res. 2001;89:763–771. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- 21.Yoshizumi M, Perrella MA, Burnett JC, Jr, et al. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ Res. 1993;73:205–209. doi: 10.1161/01.res.73.1.205. [DOI] [PubMed] [Google Scholar]
- 22.Madge LA, Pober JS. TNF Signaling in Vascular Endothelial Cells. Experimental and Molecular Pathology. 2001;70:317–325. doi: 10.1006/exmp.2001.2368. [DOI] [PubMed] [Google Scholar]
- 23.Mittermayer F, Pleiner J, Schaller G, et al. Tetrahydrobiopterin corrects Escherichia coli endotoxin-induced endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H1752–H1757. doi: 10.1152/ajpheart.00057.2005. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson IB, Qasem A, McEniery CM, et al. Nitric oxide regulates local arterial distensibility in vivo. Circulation. 2002;105:213–217. doi: 10.1161/hc0202.101970. [DOI] [PubMed] [Google Scholar]
- 25.Ghisletti S, Meda C, Maggi A, et al. 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol Cell Biol. 2005;25:2957–2968. doi: 10.1128/MCB.25.8.2957-2968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majmudar NG, Robson SC, Ford GA. Effects of the menopause, gender, and estrogen replacement therapy on vascular nitric oxide activity. J Clin Endocrinol Metab. 2000;85:1577–1583. doi: 10.1210/jcem.85.4.6530. [DOI] [PubMed] [Google Scholar]
- 27.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 28.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. Jama. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 29.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. Jama. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.