Abstract
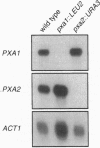
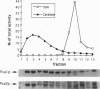
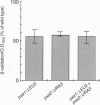
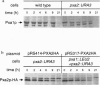
The adrenoleukodystrophy protein (ALDP) and the 70-kDa peroxisomal membrane protein (PMP70) are half ATP-binding cassette (ABC) transporters in the human peroxisome membrane. ALDP and PMP70 share sequence homology and both are implicated in genetic diseases. PXA1 and YKL741 are Saccharomyces cerevisiae genes that encode homologs of ALDP and PMP70. Pxa1p, a putative ortholog of ALDP, is involved in peroxisomal beta-oxidation of fatty acids while YKL741 is an open reading frame found by the yeast genome sequencing project. Here we designate YKL741 as PXA2 and show that its protein product, Pxa2p, like Pxa1p, is associated with peroxisomes but not required for their assembly. Yeast strains carrying gene disruption of PXA1, PXA2, or both have similar and, in the case of the latter, nonadditive phenotypes. We also find that the stability of Pxa1p, but not Pxa2p, is markedly reduced in the absence of the other. Finally, we find that Pxa1p and Pxa2p coimmuno-precipitate. These genetic and physical data suggest that Pxa1p and Pxa2p heterodimerize to form a complete peroxisomal ABC transporter involved in fatty acid beta-oxidation. This result predicts the presence of similar heterodimeric ABC transporters in the mammalian peroxisome membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkower C., Loayza D., Michaelis S. Metabolic instability and constitutive endocytosis of STE6, the a-factor transporter of Saccharomyces cerevisiae. Mol Biol Cell. 1994 Nov;5(11):1185–1198. doi: 10.1091/mbc.5.11.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkower C., Michaelis S. Mutational analysis of the yeast a-factor transporter STE6, a member of the ATP binding cassette (ABC) protein superfamily. EMBO J. 1991 Dec;10(12):3777–3785. doi: 10.1002/j.1460-2075.1991.tb04947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossier P., Fernandes L., Vilela C., Rodrigues-Pousada C. The yeast YKL741 gene situated on the left arm of chromosome XI codes for a homologue of the human ALD protein. Yeast. 1994 May;10(5):681–686. doi: 10.1002/yea.320100512. [DOI] [PubMed] [Google Scholar]
- Braverman N., Dodt G., Gould S. J., Valle D. Disorders of peroxisome biogenesis. Hum Mol Genet. 1995;4(Spec No):1791–1798. doi: 10.1093/hmg/4.suppl_1.1791. [DOI] [PubMed] [Google Scholar]
- Carson M. R., Travis S. M., Welsh M. J. The two nucleotide-binding domains of cystic fibrosis transmembrane conductance regulator (CFTR) have distinct functions in controlling channel activity. J Biol Chem. 1995 Jan 27;270(4):1711–1717. doi: 10.1074/jbc.270.4.1711. [DOI] [PubMed] [Google Scholar]
- Chapman K. B., Boeke J. D. Isolation and characterization of the gene encoding yeast debranching enzyme. Cell. 1991 May 3;65(3):483–492. doi: 10.1016/0092-8674(91)90466-c. [DOI] [PubMed] [Google Scholar]
- Christianson T. W., Sikorski R. S., Dante M., Shero J. H., Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992 Jan 2;110(1):119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- Crane D. I., Kalish J. E., Gould S. J. The Pichia pastoris PAS4 gene encodes a ubiquitin-conjugating enzyme required for peroxisome assembly. J Biol Chem. 1994 Aug 26;269(34):21835–21844. [PubMed] [Google Scholar]
- Erdmann R., Blobel G. Giant peroxisomes in oleic acid-induced Saccharomyces cerevisiae lacking the peroxisomal membrane protein Pmp27p. J Cell Biol. 1995 Feb;128(4):509–523. doi: 10.1083/jcb.128.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann R., Veenhuis M., Mertens D., Kunau W. H. Isolation of peroxisome-deficient mutants of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5419–5423. doi: 10.1073/pnas.86.14.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart G. D., Cannell D., Cox G. B., Howells A. J. Mutational analysis of the traffic ATPase (ABC) transporters involved in uptake of eye pigment precursors in Drosophila melanogaster. Implications for structure-function relationships. J Biol Chem. 1994 Apr 8;269(14):10370–10377. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fath M. J., Kolter R. ABC transporters: bacterial exporters. Microbiol Rev. 1993 Dec;57(4):995–1017. doi: 10.1128/mr.57.4.995-1017.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipits M., Simon M. M., Rapatz W., Hamilton B., Ruis H. A Saccharomyces cerevisiae upstream activating sequence mediates induction of peroxisome proliferation by fatty acids. Gene. 1993 Sep 30;132(1):49–55. doi: 10.1016/0378-1119(93)90513-3. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Gärtner J., Moser H., Valle D. Mutations in the 70K peroxisomal membrane protein gene in Zellweger syndrome. Nat Genet. 1992 Apr;1(1):16–23. doi: 10.1038/ng0492-16. [DOI] [PubMed] [Google Scholar]
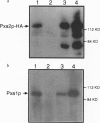
- Hettema E. H., van Roermund C. W., Distel B., van den Berg M., Vilela C., Rodrigues-Pousada C., Wanders R. J., Tabak H. F. The ABC transporter proteins Pat1 and Pat2 are required for import of long-chain fatty acids into peroxisomes of Saccharomyces cerevisiae. EMBO J. 1996 Aug 1;15(15):3813–3822. [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Higgins C. F. The ABC of channel regulation. Cell. 1995 Sep 8;82(5):693–696. doi: 10.1016/0092-8674(95)90465-4. [DOI] [PubMed] [Google Scholar]
- Kamijo K., Taketani S., Yokota S., Osumi T., Hashimoto T. The 70-kDa peroxisomal membrane protein is a member of the Mdr (P-glycoprotein)-related ATP-binding protein superfamily. J Biol Chem. 1990 Mar 15;265(8):4534–4540. [PubMed] [Google Scholar]
- Kelly A., Powis S. H., Kerr L. A., Mockridge I., Elliott T., Bastin J., Uchanska-Ziegler B., Ziegler A., Trowsdale J., Townsend A. Assembly and function of the two ABC transporter proteins encoded in the human major histocompatibility complex. Nature. 1992 Feb 13;355(6361):641–644. doi: 10.1038/355641a0. [DOI] [PubMed] [Google Scholar]
- Kok F., Neumann S., Sarde C. O., Zheng S., Wu K. H., Wei H. M., Bergin J., Watkins P. A., Gould S., Sack G. Mutational analysis of patients with X-linked adrenoleukodystrophy. Hum Mutat. 1995;6(2):104–115. doi: 10.1002/humu.1380060203. [DOI] [PubMed] [Google Scholar]
- Kunau W. H., Beyer A., Franken T., Götte K., Marzioch M., Saidowsky J., Skaletz-Rorowski A., Wiebel F. F. Two complementary approaches to study peroxisome biogenesis in Saccharomyces cerevisiae: forward and reversed genetics. Biochimie. 1993;75(3-4):209–224. doi: 10.1016/0300-9084(93)90079-8. [DOI] [PubMed] [Google Scholar]
- Ligtenberg M. J., Kemp S., Sarde C. O., van Geel B. M., Kleijer W. J., Barth P. G., Mandel J. L., van Oost B. A., Bolhuis P. A. Spectrum of mutations in the gene encoding the adrenoleukodystrophy protein. Am J Hum Genet. 1995 Jan;56(1):44–50. [PMC free article] [PubMed] [Google Scholar]
- Lombard-Platet G., Savary S., Sarde C. O., Mandel J. L., Chimini G. A close relative of the adrenoleukodystrophy (ALD) gene codes for a peroxisomal protein with a specific expression pattern. Proc Natl Acad Sci U S A. 1996 Feb 6;93(3):1265–1269. doi: 10.1073/pnas.93.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo T. W., Clarke D. M. Functional consequences of glycine mutations in the predicted cytoplasmic loops of P-glycoprotein. J Biol Chem. 1994 Mar 11;269(10):7243–7248. [PubMed] [Google Scholar]
- Meyer T. H., van Endert P. M., Uebel S., Ehring B., Tampé R. Functional expression and purification of the ABC transporter complex associated with antigen processing (TAP) in insect cells. FEBS Lett. 1994 Sep 12;351(3):443–447. doi: 10.1016/0014-5793(94)00908-2. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Berkower C. Sequence comparison of yeast ATP-binding cassette proteins. Cold Spring Harb Symp Quant Biol. 1995;60:291–307. doi: 10.1101/sqb.1995.060.01.034. [DOI] [PubMed] [Google Scholar]
- Monaco J. J. A molecular model of MHC class-I-restricted antigen processing. Immunol Today. 1992 May;13(5):173–179. doi: 10.1016/0167-5699(92)90122-N. [DOI] [PubMed] [Google Scholar]
- Mosser J., Douar A. M., Sarde C. O., Kioschis P., Feil R., Moser H., Poustka A. M., Mandel J. L., Aubourg P. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature. 1993 Feb 25;361(6414):726–730. doi: 10.1038/361726a0. [DOI] [PubMed] [Google Scholar]
- Mosser J., Lutz Y., Stoeckel M. E., Sarde C. O., Kretz C., Douar A. M., Lopez J., Aubourg P., Mandel J. L. The gene responsible for adrenoleukodystrophy encodes a peroxisomal membrane protein. Hum Mol Genet. 1994 Feb;3(2):265–271. doi: 10.1093/hmg/3.2.265. [DOI] [PubMed] [Google Scholar]
- Pollard H., Moreau J., Aubourg P. Localization of mRNAs for adrenoleukodystrophy and the 70 kDa peroxisomal (PMP70) proteins in the rat brain during post-natal development. J Neurosci Res. 1995 Oct 15;42(3):433–437. doi: 10.1002/jnr.490420318. [DOI] [PubMed] [Google Scholar]
- Shani N., Sapag A., Valle D. Characterization and analysis of conserved motifs in a peroxisomal ATP-binding cassette transporter. J Biol Chem. 1996 Apr 12;271(15):8725–8730. doi: 10.1074/jbc.271.15.8725. [DOI] [PubMed] [Google Scholar]
- Shani N., Watkins P. A., Valle D. PXA1, a possible Saccharomyces cerevisiae ortholog of the human adrenoleukodystrophy gene. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):6012–6016. doi: 10.1073/pnas.92.13.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzman E. E., Viswanathan M. N., Thorner J. The PAL1 gene product is a peroxisomal ATP-binding cassette transporter in the yeast Saccharomyces cerevisiae. J Cell Biol. 1996 Feb;132(4):549–563. doi: 10.1083/jcb.132.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyers M., Tokiwa G., Nash R., Futcher B. The Cln3-Cdc28 kinase complex of S. cerevisiae is regulated by proteolysis and phosphorylation. EMBO J. 1992 May;11(5):1773–1784. doi: 10.1002/j.1460-2075.1992.tb05229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle D., Gärtner J. Human genetics. Penetrating the peroxisome. Nature. 1993 Feb 25;361(6414):682–683. doi: 10.1038/361682a0. [DOI] [PubMed] [Google Scholar]
- Watkins P. A., Gould S. J., Smith M. A., Braiterman L. T., Wei H. M., Kok F., Moser A. B., Moser H. W., Smith K. D. Altered expression of ALDP in X-linked adrenoleukodystrophy. Am J Hum Genet. 1995 Aug;57(2):292–301. [PMC free article] [PubMed] [Google Scholar]
- Wilson G. N., Bryant D. D. Structure and expression of mammalian peroxisome assembly factor-1 (PMP35) genes. Biochem Med Metab Biol. 1994 Apr;51(2):140–148. doi: 10.1006/bmmb.1994.1018. [DOI] [PubMed] [Google Scholar]
- Zielenski J., Tsui L. C. Cystic fibrosis: genotypic and phenotypic variations. Annu Rev Genet. 1995;29:777–807. doi: 10.1146/annurev.ge.29.120195.004021. [DOI] [PubMed] [Google Scholar]